B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance (original) (raw)
Abstract
T-cell tolerance is the central program that prevents harmful immune responses against self-antigens, in which inhibitory PD-1 signal given by B7-H1 interaction plays an important role. Recent studies demonstrated that B7-H1 binds CD80 besides PD-1, and B7-H1/CD80 interaction also delivers inhibitory signals in T cells. However, a role of B7-H1/CD80 signals in regulation of T-cell tolerance has yet to be explored. We report here that attenuation of B7-H1/CD80 signals by treatment with anti–B7-H1 monoclonal antibody, which specifically blocks B7-H1/CD80 but not B7-H1/PD-1, enhanced T-cell expansion and prevented T-cell anergy induction. In addition, B7-H1/CD80 blockade restored Ag responsiveness in the previously anergized T cells. Experiments using B7-H1 or CD80-deficient T cells indicated that an inhibitory signal through CD80, but not B7-H1, on T cells is responsible in part for these effects. Consistently, CD80 expression was detected on anergic T cells and further up-regulated when they were re-exposed to the antigen (Ag). Finally, blockade of B7-H1/CD80 interaction prevented oral tolerance induction and restored T-cell responsiveness to Ag previously tolerized by oral administration. Taken together, our findings demonstrate that the B7-H1/CD80 pathway is a crucial regulator in the induction and maintenance of T-cell tolerance.
Introduction
B7-H1 (CD274, PD-L1), a transmembrane glycoprotein belonging to immunoglobulin (Ig) superfamily molecule, plays an integral role in the regulation of immune tolerance and homeostasis.1 Mice deficient of B7-H1 gene or wild-type mice treated with anti–B7-H1 blocking monoclonal antibody (mAb) exhibit exacerbated autoimmune phenotypes associated with an activation of self-reactive CD4+ and CD8+ T cells.2–5 Tolerogenic functions of B7-H1 are dependent on its expression on hematopoietic or parenchymal cells, and mediated by its interaction with PD-1 receptor.6–8 PD-1 is inducibly expressed on T cells after activation and delivers coinhibitory signals via immunoreceptor tyrosine-based switch motif in the cytoplasmic domain.9,10 PD-1 signal interferes with phosphatidylinositol-3-kinase (PI3K) activity and subsequently inhibits interleukin-2 (IL-2) production, which eventually renders T cells anergic.11 The mice deficient of PD-1 gene spontaneously develop autoimmune phenotypes, and single nucleotide polymorphisms of human PD-1 gene are associated with an increased risk of autoimmune diseases.12–16
Recent studies by Butte et al discovered that B7-H1 interacts with CD80 (B7-1) in addition to PD-1.17,18 In vitro studies using CD4+ T cells deficient of PD-1, CD28, and/or CTLA-4 indicated that B7-H1/CD80 interaction delivers bidirectional inhibitory signals to T cells.17 These findings are consistent with previous observations implicating the presence of non–PD-1 receptor(s) of B7-H1. For instance, when the B7-H1/PD-1 interaction is blocked in models of T-cell tolerance, the effects of anti–B7-H1 antagonistic mAb in restoring T-cell functions were more vigorous than that mediated by anti–PD-1 antagonistic mAb.19–21 These results have been observed in multiple experimental systems using distinct clones of anti–B7-H1 and anti–PD-1 mAbs. Thus, a potential role of non–PD-1 inhibitory receptor of B7-H1 has been suggested in T-cell tolerance regulation. However, it remains unknown whether CD80 interaction with B7-H1 is responsible for these observations and, if so, how this interaction affects T-cell tolerance in physiologic or pathologic conditions in vivo.
Potential difficulties of functional studies of the B7-H1/CD80 pathway reside in its complexity of the ligand-receptor interactions. B7-H1 binds both PD-1 and CD80, while CD80 interacts with CD28 and CTLA-4 in addition to B7-H1. Thus, genetic ablation of B7-H1 or CD80 results in a loss of multiple receptor interactions and hardly addresses selective functions of B7-H1/CD80 pathway. Because of this, we selected an approach to generate anti–B7-H1 mAb that specifically interferes with B7-H1/CD80 but not B7-H1/PD-1 interaction. By capitalizing on this mAb, we explored a crucial role of B7-H1/CD80 pathway in the induction and maintenance of peripheral T-cell tolerance in vivo.
Methods
Mice
Female C57BL/6 (B6) and B6-background CD80-knockout (KO) mice were purchased from the National Cancer Institute and The Jackson Laboratory, respectively. OTA-specific CD8 (OT-I) T-cell receptor (TCR)–transgenic mice were purchased from Taconic. B6-background B7-H1-KO mice were originally generated by L.C. B7-H1-KO OT-I mice and CD80-KO OT-I mice were generated by backcrossing OT-I transgenic mice with B7-H1-KO and CD80-KO mice, respectively. The genotypes of these mice were validated by a flow cytometry using H-2Kb/OVA tetramer and PCR of genomic DNA. All mice were maintained under specific pathogen-free conditions and were used at 6–10 weeks of age. All animal experiments were approved by the Animal Care and Use Committee at the Johns Hopkins University and the University of Maryland Baltimore.
Peptide, tetramer, and antibodies
The OVA257-264 peptide (SIINFEKL), an H-2Kb-restricted cytotoxic T lymphocytes (CTLs) epitope derived from chicken ovalbumin (OVA), was purchased from GenScript. Anti–mouse B7-H1 mAb clone 43H12 was generated by immunizing Lewis rats with mouse B7-H1-Ig fusion protein according to our established method.22 Anti–mouse B7-H1 mAb clone 10B5 was established as described.23 Isotype-matched control rat IgG or hamster IgG were purchased from Rockland. Allophycocyanin-conjugated anti–mouse CD8 mAb and fluorescein isothiocyanate (FITC)–conjugated anti–mouse CD80 mAb were purchased from eBioscience. Phycoerythrin (PE)–conjugated H-2Kb/OVA tetramer was purchased from Beckman Coulter. FITC-conjugated anti–human IgG, anti–rat IgG, and anti–mouse IgG Abs were purchased from Invitrogen.
Fusion proteins
Recombinant proteins of mouse B7-H1 or PD-1 extracellular domain fused with human IgG1 Fc region were purchased from R&D Systems. Chimeric genes of the extracellular domain of mouse CD80 or B7-DC (PD-L2, CD273) fused with mouse IgG2a Fc were constructed in plasmid containing mouse IgG2a Fc (pMIgV) vector, as previously reported.24 Proteins were expressed in CHO cells by gene transfection and isolated by protein A affinity column. Similarly, fusion proteins of mouse B7-H3 or B7-H4 extracellular domains linked with human IgG1 Fc region were constructed in plasmid containing human IgG1 Fc (pHIgV) vector, followed by expression and isolation.24 Purity of the isolated proteins was assessed by enzyme-linked immunosorbent assay (ELISA) and sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Biotin conjugation of fusion proteins was performed using EZ-link Sulfo-NHS-Biotin reagents purchased from Thermo Scientific.
Flow cytometric analysis and ELISA
Specific binding of 43H12 with mouse B7-H1 and its capability of selectively blocking B7-H1/CD80 interaction was assessed by flow cytometric analysis and ELISA, according to our previous studies.25 In flow cytometry, Ab binding was detected by LSR II (BD Biosciences) and analyzed by FlowJo Version 8.8.6 software (TreeStar). In ELISA, Ab interaction with fusion proteins immobilized on ELISA plates was visualized by tetramethylbenzidine-based chromogenic assay and optical density (O.D.) at 450 nm was measured by Biotrak II plate reader (Amersham Biosciences).
In vitro T-cell proliferation assay
In vitro T-cell costimulatory assay was conducted as previously reported.26 Briefly, 96-well culture plates were first coated with 0.5 μg/mL anti-CD3 mAb and then with 10 μg/mL mouse B7-H1-human Fc fusion or control human Fc protein. Naive T cells isolated from spleen and lymph nodes (LN) of CD80-KO mice were cultured in these wells at 1.5 × 106 cells/mL in the presence of 10 μg/mL 43H12 or control rat IgG. Proliferative activity of T cells was assessed by an incorporation of 3H-thymidine during the last 6 hours of 2 days of culture.
In vivo anergy model of OVA-reactive OT-I T cells
OT-I T cells were anergized by intravenous injection of OVA peptide, according to our previous studies with some modifications.21,27 First, CD8+ T cells were isolated from the spleen and LN of wild-type (WT) OT-I, B7-H1-KO OT-I, or CD80-KO OT-I mice by negative selection using MACS (Miltenyi Biotech). Purity of the isolated OT-I CD8+ T cells was confirmed by a flow cytometry, and was constantly more than 85%. The purified cells were injected intravenously into WT B6 mice or CD80-KO mice at a dose of 1 × 106 cells/mouse. After 24 hours, the recipient mice were injected intravenously with 0.5 mg of OVA257-264 peptide. For mAb treatment, mice were given intraperitoneally with 200 μg of 43H12 or control rat IgG at the indicated time points. Spleen or peripheral blood mononuclear cells were harvested later, and the percentage of OT-I CD8+ T cells was assessed by flow cytometry.
Assays of in vivo BrdU uptake and annexin V staining
CD8+ T cells from OT-I transgenic mice were transferred intravenously into B6 mice. After 24 hours, mice were given 0.5 mg of OVA257-264 peptide intravenously and treated with 200 μg of 43H12 or control rat IgG intraperitoneally on the day of peptide injection and 3 days later. Two or 4 days after peptide injection, the mice were treated with BrdU (100 μg/mouse; Sigma-Aldrich) intraperitoneally, and spleens was harvested 24 hours after BrdU injection. BrdU incorporation of OT-I CD8+ T cells was assessed by BrdU flow kit (BD Biosciences) along with staining by allophycocyanin-conjugated anti-CD8 mAb and PE-conjugated H-2Kb/OVA tetramer, according to the manufacturer's instructions.
For annexin V staining, 1 × 106 OT-I cells were transferred intravenously into B6 mice, which were then given 0.5 mg of OVA257-264 peptide intravenously and treated with 200 μg of 43H12 or control rat IgG intraperitoneally, as described in the previous paragraph. Four days after peptide administration, spleens were harvested and the percentage of annexin V–positive cells in OT-I T cells was assessed by flow cytometry using FITC-conjugated annexin V (BD Biosciences), allophycocyanin-conjugated anti-CD8 mAb, and PE-conjugated H-2Kb/OVA tetramer, according to the manufacturer's instructions.
Induction and assessment of oral tolerance
B6 mice were given drinking water supplemented with 0.2 mg/mL OVA (Grade 5; Sigma-Aldrich) from day 0 to day 7. OVA-containing drinking water was replenished every other day. On day 14, the mice were immunized subcutaneously with 50 μg of OVA emulsified in 50 μL of Complete Freund Adjuvant (CFA) (Sigma-Aldrich). The mice were treated intraperitoneally with 150 μg of 43H12 or control rat IgG on days 0, 4, 8, and 12 for prevention model or on days 14 and 17 for recovery model. In both models, draining axillary and inguinal LNs were harvested on day 21 and cultured in vitro at 2 × 106 cells/mL in the presence of indicated doses of OVA protein (EndoGrade; Profos AG). The culture supernatants were harvested at indicated time points, and the concentrations of interferon-γ (IFN-γ), IL-2, IL-4, and IL-17 were measured by ELISA kits (eBioscience). Proliferative activity of the incubated cells was assessed by 3H-thymidine incorporation during the last 10 hours of 3 days of culture.
Statistical analysis
The 2-tailed student t test was used to compare 2 groups. P values less than .05 were considered significant.
Results
Anti–B7-H1 mAb 43H12 attenuates B7-H1/CD80 but not B7-H1/PD-1 interaction
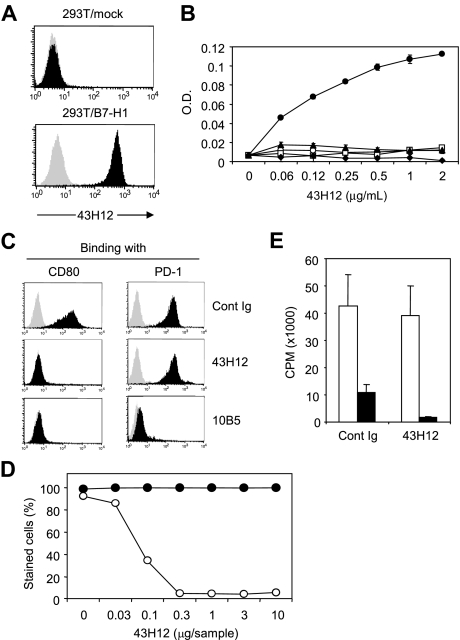
To elucidate immunologic functions of B7-H1/CD80 pathway in vivo, we generated 43H12, a clone of anti–mouse B7-H1 mAb that selectively interferes with B7-H1/CD80 but not B7-H1/PD-1 interaction. First, staining of B7-H1-expressing cells by 43H12 was confirmed by a flow cytometric assay (Figure 1A). Specificity of 43H12 was assessed by ELISA, in which 43H12 showed a dose-dependent interaction with mouse B7-H1 protein but not with other B7 family proteins including CD80, B7-DC, B7-H3, and B7-H4 (Figure 1B). Selective interaction of 43H12 with B7-H1 but not other B7 family molecules was also confirmed by a flow cytometry using cell lines expressing CD80 or CD86 (data not shown).
Figure 1.
Selective blockade of B7-H1/CD80 interaction by anti–B7-H1 mAbý clone 43H12. (A) 293T cells transfected with mock or mouse B7-H1–encoding plasmids were stained with 1 μg/mL anti–B7-H1 monoclonal antibody (mAb) clone 43H12 (black histogram) or control rat immunoglobulin (IgG; gray histogram) followed by fluorescein isothiocyanate (FITC)–conjugated anti–rat IgG. Binding of 43H12 to B7-H1 was analyzed by flow cytometry. (B) ELISA plate was coated with 2 μg/mL mouse B7-H1-Fc (●), mouse CD80-Fc (○), mouse B7-DC-Fc (□), mouse B7-H3-Fc (▴), or mouse B7-H4-Fc (♦) fusion proteins. Indicated doses of 43H12 were added into wells and its binding with the coated proteins were detected by horseradish peroxidase (HRP)–conjugated anti–rat IgG antobody (Ab). Average ± SD of optical density (O.D.) from triplicate wells are shown. (C) 293T cells transfected with plasmids encoding mock (gray histogram) or B7-H1 (black histogram) were incubated with 2 μg/mL biotin-conjugated CD80-Fc (left panels) or PD-1-Fc (right panels) fusion proteins in the presence of 2 μg/mL 43H12, 10B5, or control rat IgG. The staining of fusion proteins were detected by streptavidin-PE in flow cytometry. (D) 293T cells transfected with plasmids encoding B7-H1 were stained with CD80-Fc (○) or PD-1-Fc (●) fusion proteins in the presence of indicated doses of 43H12. Percentage of positively stained cells was assessed by flow cytometry. (E) T cells isolated from CD80-knockout (KO) mice were stimulated with anti-CD3 mAb together with immobilized B7-H1-Fc (■) or control human Fc (□) in the presence of soluble 43H12 or control rat IgG. Proliferation of the culture cells were assessed by 3H-thymidine incorporation. All experiments were repeated at least 3 times and representative data are shown.
Next, a potential of 43H12 to block interactions between B7-H1 and its receptors was examined by flow cytometry. Inclusion of 43H12 completely abolished staining of mouse B7-H1–positive cells with mouse CD80-Fc fusion protein, but not mouse PD-1-Fc protein (Figure 1C). In contrast, other clones of anti–mouse B7-H1 mAb including 10B5 and MIH5, which were previously developed by our group or others,23,28 blocked both B7-H1/CD80 and B7-H1/PD-1 interactions (Figure 1C and data not shown). The specificity of 43H12 to block B7-H1/CD80 was further tested by titration assay, in which as low as 0.3 μg of 43H12 was sufficient to completely attenuate a binding of CD80-Fc with B7-H1–expressing cells, while PD-1-Fc binding with the same cells was not interfered at all even with 10 μg of 43H12 (Figure 1D). In addition, coinhibitory effect of B7-H1-Fc protein on the proliferation of CD80-KO T cells was not abrogated in the presence of 43H12 (Figure 1E), further indicating a negligible effect of 43H12 on the functions of B7-H1/PD-1 pathway. Thus, 43H12 is antagonistic highly selectively to B7-H1/CD80 interaction, endorsing its capacity as a means to exploring B7-H1/CD80 functions. Interaction of 43H12 with B7-H1 did not modify expression levels of B7-H1 on cell surface (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), indicating that the effects of 43H12 are not caused by nonspecific down-regulation or internalization of B7-H1.
Blockade of B7-H1/CD80 interaction enhances Ag-specific T-cell expansion
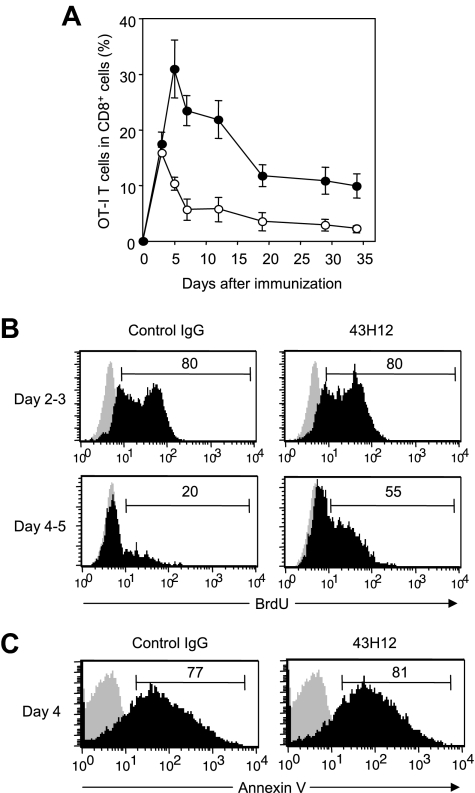
To explore in vivo functions of B7-H1/CD80 interaction, we employed a model in which OVA-reactive OT-I T cells undergo activation in response to intravenous injection of high-dose OVA257-264 peptide. In this model, OT-I T cells show transient expansion followed by activation-induced apoptosis (ie, contraction phase), and eventually undergo anergic status in chronic phase.27 Our recent studies using this model revealed that treatment with 10B5, anti–B7-H1 blocking mAb, accelerated expansion of OT-I T cells in early priming phase and resulted in prevention and recovery from T-cell anergy.21 However, because 10B5 interferes with both B7-H1/PD-1 and B7-H1/CD80 interactions (Figure 1C), a selective role of B7-H1/CD80 in T-cell priming and anergy induction remains unclear. Therefore, we addressed this question by applying 43H12 to this model. 43H12 treatment significantly prolonged the expansion period of OT-I T cells, by which expansion peak shifted from day 3 to day 5 (Figure 2A). 43H12 treatment induced OT-I T-cell expansion up to 30% of total CD8+ T cells, which was twice as high as that in control mice. Expansion level of OT-I T cells by 43H12 treatment was less drastic compared with the effect of 10B5 in the same model,21 demonstrating distinct features of these mAbs, that is, blockade of B7-H1/CD80 alone versus dual blockade of B7-H1/CD80 and B7-H1/PD-1. In 43H12-treated mice, OT-I T cells gradually contracted after initial expansion, while the numbers of OT-I T cells were constantly higher than those in control Ig-treated mice (Figure 2A). Thus, our result demonstrated an enhanced T-cell response in the presence of 43H12, indicating an inhibitory function of B7-H1/CD80 interaction in T-cell activation in vivo.
Figure 2.
Enhanced expansion of Ag-reactive CD8+ T cells by blockade of B7-H1/CD80 interaction. B6 mice were transferred intravenously with OTA-specific CD8 (OT-I) T cells and injected intravenously with 0.5 mg of OVA257-264 peptide. On day of peptide injection and 3 days later, the mice were treated intraperitoneally with 200 μg of 43H12 or control rat IgG. (A) PBMCs were harvested at the indicated time points, and a percentage of OT-I T cells in total CD8-positive cells was assessed in the 43H12-treated (●) or control IgG-treated (○) mice by flow cytometry. The data are shown as mean ± SEM. (B) Mice were given 100 μg of BrdU intraperitoneally on day 2 (top panels) or day 4 (bottom panels) after OVA peptide injection. Twenty-four hours after BrdU injection, spleen cells were harvested and BrdU incorporation in CD8/OVA-tetramer double-positive OT-I T cells was analyzed by flow cytometry (black histogram). As background level, OT-I T cells in the mice without BrdU administration were stained similarly (gray histogram). (C) Spleen cells were harvested 4 days after OVA peptide injection and annexin V staining in CD8/OVA-tetramer double-positive OT-I T cells was analyzed by flow cytometry (black histogram). Background level without annexin V staining is also shown (gray histogram). All experiments were independently repeated at least 3 times, and the representative data are shown. The numbers in the histogram indicate the percentage of positively stained cells.
To explore the immunologic mechanisms of the effects of 43H12, we next assessed in vivo proliferation and apoptosis of OT-I T cells by BrdU uptake and annexin V staining assays. 43H12 treatment did not affect BrdU incorporation in OT-I T cells in the early expansion phase during day 2 to day 3 after OVA injection (Figure 2B). In control mice, OT-I T cells contracted its proliferation after day 3, and only 20% of them showed BrdU positive during day 4 to day 5. In contrast, OT-I T cells in 43H12-treated mice sustained BrdU incorporation in which 55% of cells remained BrdU-positive in this time. These results were concordant with the finding that 43H12 treatment had no effects on OT-I T-cell number until day 3, but it induced continuous expansion of OT-I T cells until day 5 after OVA injection (Figure 2A). On the other hand, percentages of annexin V–positive cells in OT-I T cells were comparable between 43H12- and control Ig-treated mice (Figure 2C), suggesting a negligible role of 43H12 in T-cell apoptosis. These results together indicate that signals delivered by B7-H1/CD80 interaction mediate inhibitory effects on late expansion phase of antigen (Ag)–induced T-cell responses.
Effects of 43H12 are mediated by blockade of CD80 signal in T cells
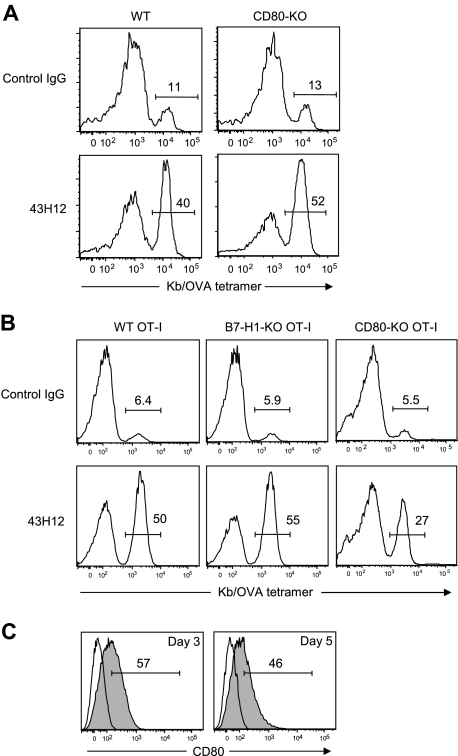
Previous studies indicated that B7-H1 and CD80 delivers inhibitory signals into T cells in bidirectional fashion.17 Therefore, we next explored which of B7-H1 or CD80, or both, are responsible as inhibitory receptor(s) on OT-I T cells in our model. First, we employed CD80-KO mice as hosts of OT-I T-cell transfer. In these conditions, expression of CD80 was ablated in all immune and nonimmune cells other than donor OT-I T cells. 43H12 treatment induced profound expansion of OT-I T cells even in CD80-KO hosts at a level comparable with that observed in WT hosts (Figure 3A). These results suggest that CD80 on non-T cells including antigen-presenting cells (APC) plays a dispensable role in the effects of 43H12. Because CD80-KO mice ablate its functions not only through B7-H1 but also CD28/CTLA-4 interactions, we further tested the effects of 43H12 in the presence or absence of anti-CD80 mAb 16-10A1, a clone that blocks CD80 binding to CD28/CTLA-4 but not B7-H1.18,29 OT-I T-cell expansion induced by 43H12 treatment was not affected by an coadministration of 16-10A1 (supplemental Figure 2). These results indicate that loss of CD80-CD28/CTLA-4 interaction does not manipulate the effects of 43H12, thus validating our findings in the model using CD80-KO mice. We further attempted to use B7-H1-KO mice as hosts of OT-I T-cell transfer to explore a role of B7-H1 on APC. However, OT-I T cells transferred into B7-H1-KO mice profoundly expanded without any treatments due to a loss of PD-1 signal as we previously reported.21 Although 43H12 injection in such condition did not induce further expansion of OT-I T cells (data not shown), the high background number of OT-I T cells hindered our assessment on a role of B7-H1 associated with APC.
Figure 3.
A role of T cell–associated CD80 in the inhibitory effects of B7-H1/CD80 interaction. (A) WT B6 mice or CD80-KO mice were transferred intravenously with OT-I T cells. In panel B, B6 mice were transferred intravenously with WT, B7-H1-KO, or CD80-KO background OT-I T cells. In both settings, the recipient mice were injected intravenously with 0.5 mg of OVA257-264 peptide, and treated intraperitoneally with 200 μg of 43H12 or control rat IgG on day of peptide injection and 3 days later. Splenocytes were harvested 5 days after peptide injection, and the percentage of OT-I T cells in CD8-positive population was assessed by flow cytometry. (C) B6 mice were transferred intravenously with OT-I T cells and injected intravenously with 0.5 mg of OVA257-264 peptide. On day 3 and day 5, CD8/OVA-tetramer double-positive OT-I T cells was stained with anti-CD80 mAb and analyzed by flow cytometry (gray histogram). Nonstained background levels of the same cells are also shown (open histogram). All experiments were repeated at least 3 times, and the representative data are shown. The numbers in the histogram indicate the percentage of positively stained cells.
Therefore, we next applied distinct approaches, in which OT-I T cells deficient of B7-H1 or CD80 were transferred as donor cells into WT hosts. Treatment with 43H12 induced profound expansion of transferred B7-H1-KO OT-I T cells at a level comparable with WT OT-I donor T cells (Figure 3B). On the other hand, expansion of CD80-KO OT-I T cells induced by 43H12 treatment was significantly lower than that of 43H12-treated WT OT-I T cells (27% vs 50%), while it was still higher than control IgG treatment (27% vs 5.5%). These results together suggest that the effects of B7-H1/CD80 blockade by 43H12 are dependent, at least in part, on CD80 expressed on donor OT-I T cells, but not B7-H1. To bolster this notion, we further assessed CD80 expression on OT-I T cells in our model. Three and 5 days after OVA injection, CD80 was detected on approximately 50% of OT-I T cells (Figure 3C), also supporting a potential role of CD80 in transmitting inhibitory signal to Ag-stimulated T cells.
B7-H1/CD80 interaction is required for induction and maintenance of T-cell anergy
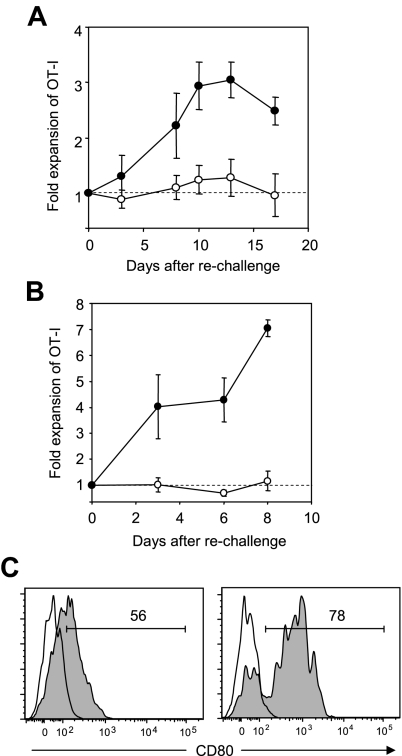
We next explored a regulatory role of B7-H1/CD80 interaction in T-cell tolerance. We previously reported that OT-I T cells undergo anergy following initial expansion and subsequent contraction in response to intravenous injection of OVA peptide in our model.21,27 Consistently, OT-I T cells in the host that was injected with OVA on day 0 and treated with control Ig on day 0 and day 3 showed no detectable proliferation upon rechallenge of OVA on day 34 (Figure 4A). In sharp contrast, OT-I T cells in the host that was treated with 43H12 on the day and 3 days after OVA injection expanded significantly in response to OVA rechallenge. These results indicate that blockade of B7-H1/CD80 interaction during T-cell priming by tolerogenic Ag immunization prevents subsequent T-cell anergy induction.
Figure 4.
Prevention and restoration of CD8+ T-cell anergy by blockade of B7-H1/CD80 interaction. B6 mice were transferred intravenously with OT-I T cells and injected intravenously with 0.5 mg of OVA257-264 peptide. (A) On day of peptide injection and 3 days later, the mice were treated intraperitoneally with 200 μg of 43H12 (●) or control rat IgG (○). Thirty-four days after initial peptide injection, the mice were rechallenged intravenously with 0.5 mg of OVA257-264 peptide, and percentages of CD8/OVA-tetramer double-positive OT-I T cells in PBMCs were assessed by flow cytometry at the indicated time points. Fold expansion of OT-I T cells was calculated by dividing OT-I T-cell percentages after rechallenge by that before rechallenge in individual mice. (B) Twenty days after the initial OVA peptide injection, the mice were rechallenged with 0.5 mg of OVA257-264 peptide and treated intraperitoneally with 200 μg of 43H12 (●) or control rat IgG (○) on day of peptide rechallenge and 3 days later. Fold expansion of OT-I T cells in PBMC was assessed as in panel A at the indicated time points. (C) Twenty days after the initial OVA peptide injection, the mice were left untreated (left panel) or rechallenged with 0.5 mg of OVA257-264 peptide (right panel). Twenty-four hours later, CD8/OVA-tetramer double-positive OT-I T cells in the spleen were stained with anti-CD80 mAb and analyzed by flow cytometry (gray histogram). Nonstained background levels of the same cells are also shown (open histogram). All experiments were repeated at least 3 times and the representative data are shown. The numbers in the histogram indicate the percentage of positively stained cells.
Next, we explored whether blockade of B7-H1/CD80 interaction could also reverse pre-established T-cell anergy. Twenty days after initial OVA peptide injection, the mice harboring anergized OT-I T cells were rechallenged with OVA and simultaneously treated with either control Ig or 43H12 treatment. As expected, OT-I T cells in control Ig-treated mice did not show proliferative responses upon OVA rechallenge (Figure 4B). In sharp contrast, 43H12 treatment together with OVA peptide rechallenge resulted in a profound expansion of OT-I T cells. These results suggest that blockade of B7-H1/CD80 interaction at the time of Ag re-encounter is capable of breaking pre-established T-cell anergy. To further support this conclusion, we examined CD80 expression on anergic T cells with or without Ag rechallenge. Twenty days after initial OVA injection, CD80 was expressed on approximately 50% of anergic OT-I T cells (Figure 4C). This level of expression was comparable with those observed on day 3 and day 5 after OVA injection (Figure 3C), implicating that CD80 is continuously expressed on T cells after priming. When anergic OT-I T cells were exposed to OVA peptide rechallenge, CD80 expression was up-regulated up to 80% within 24 hours (Figure 4C). Taken together, these results suggest that B7-H1/CD80 interaction plays a crucial role in induction and maintenance of anergic T cells, and that blockade of this interaction can prevent and reverse T-cell anergy.
A crucial role of B7-H1/CD80 interaction in induction and maintenance of oral tolerance
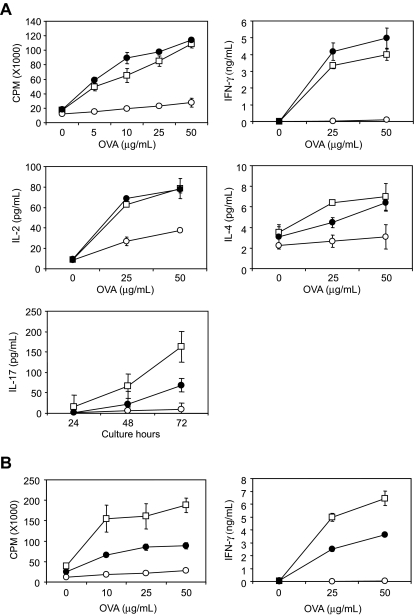
When Ags are given orally, mucosal immune system in gastrointestinal tract does not make productive responses but rather undergo Ag-specific tolerant condition, a process known as oral tolerance.30 This mechanism is essential for preventing deleterious immune reactions to self and exogenous dietary and environmental Ags such as food proteins. Although numbers of studies have investigated the mechanisms of oral tolerance, molecular checkpoints necessary for oral tolerance induction and maintenance are largely unknown. We here examined whether B7-H1/CD80 interaction has regulatory function in oral tolerance. As previously reported,31,32 oral administration of OVA protein significantly diminished T-cell responses including proliferation and cytokine productions of IFN-γ, IL-2, IL-4, and IL-17 that were induced by in vivo OVA/CFA immunization and subsequent in vitro restimulation with OVA protein (Figure 5A). Treatment of mice with 43H12 during oral OVA administration restored OVA-reactive T-cell proliferation and IFN-γ/IL-2 productions almost completely to the level without oral tolerance. Production of IL-4 and IL-17 was partially but significantly restored by 43H12 treatment. These results indicate that B7-H1/CD80 interaction is essential for the induction of oral tolerance in our model. Treatment with 43H12 did not affect cellular compartment of intestinal intraepithelial lymphocytes (IELs), including CD8αα, TCRγδ T cells (supplemental Figure 3), suggesting that the regulatory functions of B7-H1/CD80 pathway in oral tolerance are unlikely associated with its direct effects on gut-specific T cells.
Figure 5.
Prevention and restoration of oral tolerance by blockade of B7-H1/CD80 interaction. B6 mice were given drinking water supplemented with OVA protein (○ or ●) or without OVA (□) from day 0 to day 7. On day 14, the mice were immunized subcutaneously with OVA protein emulsified in CFA. The mice were also treated intraperitoneally with 150 μg of 43H12 (●) or control rat IgG (○) on days 0, 4, 8, and 12 (A) or on days 14 and 17 (B). On day 21, draining LN cells were harvested from the mice and cultured with the indicated doses of OVA protein. After 48 hours, production of IFN-γ, IL-2, and IL-4 in culture supernatant was measured by ELISA. IL-17 level was measured 24, 48, and 72 hours after culture with 25 μg/mL OVA protein. Proliferative activity was assessed by an incorporation of 3H-thymidine. All experiments were repeated at least 3 times. Representative data are shown as mean ± SD of triplicate wells in each group.
Next, we examined whether blockade of B7-H1/CD80 interaction could reverse T-cell responses in the condition of pre-established oral tolerance. The mice that had been given oral OVA administration were treated with 43H12 or control Ig at the time of OVA/CFA immunization. T-cell proliferation and IFN-γ production by in vitro OVA restimulation was partially but significantly restored by 43H12 injections (Figure 5B). Taken together, our findings suggest that B7-H1/CD80 interaction is a crucial regulator for the induction and maintenance of T-cell tolerance induced by oral Ag administration, and blockade of this pathway results in prevention and reversal of oral tolerance.
Discussion
Recent studies revealed that B7-H1 binds CD80 besides PD-1, and the B7-H1/CD80 interaction delivers bidirectional coinhibitory signals to T cells.17,18 However, due to a lack of in vivo studies, a role of the B7-H1/CD80 interaction in T-cell tolerance in physiologic and pathologic conditions remains unexplored. Our current studies address this question by applying 43H12, a mAb that selectively attenuates B7-H1/CD80 but not B7-H1/PD-1 interaction, to in vivo models of T-cell activation and tolerance. Treatment with 43H12 enhanced T-cell responses and consequently hindered induction and maintenance of T-cell tolerance related to intravenous or oral administration of Ag. In our model, CD80, but not B7-H1, on Ag-reactive T cells is responsible at least in part for transmitting coinhibitory signal. Thus, our findings revealed a regulatory mechanism of B7-H1/CD80 interaction in T-cell immunity including peripheral tolerance.
Previous studies using chemical cross-linking analysis and molecular modeling approaches revealed that the binding site of B7-H1 with CD80 partially overlaps with that of PD-1.17 In addition, binding affinity of B7-H1/CD80 (KD ∼ 1.7μM) is weaker than that of B7-H1/PD-1 (KD ∼ 0.5μM).17 These findings suggest that biologic reagents or B7-H1 mutants that preferentially abrogate B7-H1/CD80 interaction while sparing B7-H1/PD-1 interaction are reasonable approaches to explore B7-H1/CD80 functions. Indeed, mAbs with such a selective blocking capacity against B7-H1 has been reported.17 In the present study, we generated a novel clone of anti–B7-H1 mAb, 43H12, which blocks B7-H1/CD80 interaction with at least 30-fold higher specificity than B7-H1/PD-1 (Figure 1D). In addition, binding of 43H12 does not induce internalization or down-regulation of cell surface B7-H1 (supplemental Figure 1). In functional levels, 43H12 does not interfere with T-cell inhibition caused by B7-H1/PD-1 interaction (Figure 1E), further supporting its credibility as a means to exploring selective functions of B7-H1/CD80 pathway.
According to our results of B7-H1-KO or CD80-KO mice used as hosts or the source of donor T cells, inhibitory signals mediated by B7-H1/CD80 interaction are dependent in part on CD80 expressed on Ag-reactive T cells but not on non-T cells such as APC (Figure 3A-B). Consistently, CD80 expression on the primed and anergic T cells was detected in our models (Figures 3C, 4C). A role of CD80 on T cells as an inhibitory receptor to deliver outside-in signal is concordant with previous findings, including: (1) increased cytokine productions in CD80-KO T cells, (2) an enhanced severity of graft-versus-host disease by CD80/CD86-KO donor T cells, and (3) resistance of CD80-KO T cells to inhibitory effects of T regulatory cells (Tregs).33–35 In addition, a cross-link of CD80 by anti-CD80 mAb induces growth retardation and up-regulated expressions of proapoptotic molecules in lymphoma,36 providing more direct evidence of inhibitory signal transduction through CD80. Interestingly, 43H12 treatment induced a partial stimulation even in CD80-KO OT-I T cells (Figure 3B), implicating a possibility of currently unknown non-CD80/non–PD-1 inhibitory receptor(s) of which interaction with B7-H1 is susceptible to blockade by 43H12.
In contrast to CD80, B7-H1 on Ag-reactive T cells plays a negligible role in our models (Figure 3B). Possible cellular sources of B7-H1 on non-T cells include APC, Tregs, myeloid-derived suppressor cells, and nonhematopoietic parenchymal cells. B7-H1 is ubiquitously expressed on these types of cells and recognized to induce immune tolerance via direct inhibition of T cells or generation of adaptive/induced Tregs, while it has yet to be fully explored whether PD-1, CD80, or both receptors play a responsible role in these effects.6,37–41 In addition, B7-H1 expressed on non-T cells may also deliver outside-in signal as previously reported.42,43 Taken together, a role of B7-H1/CD80 signals in T-cell tolerance is likely dependent on both T-cell intrinsic and extrinsic mechanisms. Although it is currently unclear why T cell–associated B7-H1 is dispensable in our models in spite of its capability of delivering T-cell inhibitory signal by CD80 ligation,17 this discrepancy is probably due to some crucial differences in experimental systems (in vitro vs in vivo) and target cells (CD4+ vs CD8+ T cells) between these studies.
Presentation of high-dose Ag without adjuvants or tolerogenic APC leads to transient expansion of Ag-specific T cells and subsequent contraction, followed by generation of long-term T-cell anergy. Negative cosignaling molecules including CTLA-4 and PD-1 play a crucial role in these processes of T-cell tolerance.21,37 Our current findings suggest that B7-H1/CD80 interaction also contributes to T-cell tolerance generation, although its physiologic role and mechanism are distinct from that of B7-H1/PD-1 interaction. First, as previously reported, B7-H1/PD-1 signaling showed regulatory effects on early phase (∼ 48 hours) T-cell responses after Ag encounter.20,21 In contrast, our findings revealed that blockade of B7-H1/CD80 interaction has negligible effects on T-cell responses until 3 days after Ag stimulation, but rather continuously stimulates T-cell expansion after 3 days (Figure 2A-B). Thus, B7-H1/CD80 signal has inhibitory effects on the late stage of T-cell responses that could regulate phase transition from T-cell expansion to contraction. Second, CD80 expression is maintained on anergic T cells for relatively long period and quickly up-regulated by Ag re-exposure to the level higher than that on primed T cells (Figures 3C, 4C). Furthermore, B7-H1/CD80 interaction is prerequisite for maintenance of anergic phenotype of T cells (Figure 4B). Thus, CD80 expression may serve as a biomarker and functional checkpoint for T-cell anergy, while similar features have been suggested with lymphocyte activation gene-3 (LAG-3) and B and T lymphocyte attenuator (BTLA).44,45 On the other hand, B7-H1/PD-1 interaction plays a crucial role in the induction and maintenance of T-cell exhaustion.19
Oral tolerance is the physiologic mechanism by which the mucosal immune system prevents adverse T-cell responses against self and exogenous dietary Ag.30 Among cosignal pathways, CD80/CD86-CTLA-4 and B7-DC (PD-L2)-PD-1 have been shown to contribute to oral tolerance regulation.31,46–49 Our current studies demonstrated that B7-H1/CD80 interaction also plays a crucial role in the induction and maintenance of oral tolerance (Figure 5). This notion could be supported by recent reports that B7-H1 is highly expressed on CD11c+ CD8α− DC in mesenteric LN, which are a vital mediator for oral tolerance.31,50,51 While various mechanisms have been reported in oral tolerance of Ag-reactive CD4+ T cells, one of the primary determinants is quantity of orally administered Ag. High doses of Ag induce T-cell anergy, while low doses of Ag favor suppression-type tolerance caused by Tregs or suppressor T cells that produce inhibitory cytokines such as transforming growth factor-β, IL-10, and IL-4.30 Because the dose used in our study falls within low dose range, B7-H1/CD80 interaction may regulate suppression mechanisms of CD4+ T cells.
Blockade of B7-H1 functions is expected to have significant clinical value as a novel immunotherapy for diseases including cancer and chronic infection. The current studies give an insight into the complexity of these approaches that could affect B7-H1/PD-1, B7-H1/CD80, or both of them according to the reagents to be employed. For example, while selective attenuation of B7-H1/CD80 may have weaker effects compared with nonselective B7-H1 blockade, it could be advantageous in terms of minimizing a risk of autoimmune responses. In addition, our studies implicate that blockade of B7-H1/CD80 inhibitory signal could be used as adjuvant for oral vaccine. In summary, our studies revealed a crucial role of B7-H1/CD80 pathway in the induction and maintenance of T-cell tolerance and propose a therapeutic potential of blocking this pathway for prevention and restoration of peripheral T-cell tolerance.
Supplementary Material
[Supplemental Figures]
Acknowledgments
We thank Ms Youn Son Kim and Dr Takatoshi Chinen for their technical help.
This work was supported by National Institutes of Health grants CA97085 and HL088954, and a Young Investigator Award from the Alliance for Cancer Gene Therapy.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.-J.P., R.O., and Y.M. designed and performed the experiments; Y.S., A.K., M.M.A., S.Y., F.T., H.N., S.A., and Y.L. prepared vital new reagents and performed the experiments; and S.E.S., L.C. and K.T. designed the research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: S.E.S. gets royalties through the Mayo Clinic College of Medicine for intellectual property related to B7-H1. The remaining authors declare no competing financial interests.
Correspondence: Koji Tamada, MD, PhD, 655 W Baltimore St, BRB 9-051, Baltimore, MD 21201; e-mail: ktamada@som.umaryland.edu.
References
- 1.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4(5):336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 2.Dong H, Zhu G, Tamada K, et al. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20(3):327–336. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 3.Latchman YE, Liang SC, Wu Y, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101(29):10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu B, Guleria I, Khosroshahi A, et al. Differential role of programmed death-ligand 1 [corrected] and programmed death-ligand 2 [corrected] in regulating the susceptibility and chronic progression of experimental autoimmune encephalomyelitis. J Immunol. 2006;176(6):3480–3489. doi: 10.4049/jimmunol.176.6.3480. [DOI] [PubMed] [Google Scholar]
- 5.Reynoso ED, Elpek KG, Francisco L, et al. Intestinal tolerance is converted to autoimmune enteritis upon PD-1 ligand blockade. J Immunol. 2009;182(4):2102–2112. doi: 10.4049/jimmunol.0802769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170(3):1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 8.Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur J Immunol. 2008;38(10):2706–2717. doi: 10.1002/eji.200838137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98(24):13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–954. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 11.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prokunina L, Castillejo-Lopez C, Oberg F, et al. A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet. 2002;32(4):666–669. doi: 10.1038/ng1020. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen C, Hansen D, Husby S, Jacobsen BB, Lillevang ST. Association of a putative regulatory polymorphism in the PD-1 gene with susceptibility to type 1 diabetes. Tissue Antigens. 2003;62(6):492–497. doi: 10.1046/j.1399-0039.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- 14.Kong EK, Prokunina-Olsson L, Wong WH, et al. A new haplotype of PDCD1 is associated with rheumatoid arthritis in Hong Kong Chinese. Arthritis Rheum. 2005;52(4):1058–1062. doi: 10.1002/art.20966. [DOI] [PubMed] [Google Scholar]
- 15.Newby PR, Roberts-Davies EL, Brand OJ, et al. Tag SNP screening of the PDCD1 gene for association with Graves' disease. Clin Endocrinol (Oxf) 2007;67(1):125–128. doi: 10.1111/j.1365-2265.2007.02848.x. [DOI] [PubMed] [Google Scholar]
- 16.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27(4):195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butte MJ, Pena-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol Immunol. 2008;45(13):3567–3572. doi: 10.1016/j.molimm.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110(1):186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsushima F, Yao S, Shin T, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110(1):180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109(5):651–659. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–1096. [PubMed] [Google Scholar]
- 24.Chapoval AI, Zhu G, Chen L. Immunoglobulin fusion proteins as a tool for evaluation of T-cell costimulatory molecules. Mol Biotechnol. 2002;21(3):259–264. doi: 10.1385/MB:21:3:259. [DOI] [PubMed] [Google Scholar]
- 25.Anand S, Wang P, Yoshimura K, et al. Essential role of TNF family molecule LIGHT as a cytokine in the pathogenesis of hepatitis. J Clin Invest. 2006;116(4):1045–1051. doi: 10.1172/JCI27083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamada K, Shimozaki K, Chapoval AI, et al. Modulation of T-cell-mediated immunity in tumor and graft-versus-host disease models through the LIGHT co-stimulatory pathway. Nat Med. 2000;6(3):283–289. doi: 10.1038/73136. [DOI] [PubMed] [Google Scholar]
- 27.Wilcox RA, Tamada K, Flies DB, et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood. 2004;103(1):177–184. doi: 10.1182/blood-2003-06-2184. [DOI] [PubMed] [Google Scholar]
- 28.Aramaki O, Shirasugi N, Takayama T, et al. Programmed death-1-programmed death-L1 interaction is essential for induction of regulatory cells by intratracheal delivery of alloantigen. Transplantation. 2004;77(1):6–12. doi: 10.1097/01.TP.0000108637.65091.4B. [DOI] [PubMed] [Google Scholar]
- 29.Razi-Wolf Z, Freeman GJ, Galvin F, et al. Expression and function of the murine B7 antigen, the major costimulatory molecule expressed by peritoneal exudate cells. Proc Natl Acad Sci U S A. 1992;89(9):4210–4214. doi: 10.1073/pnas.89.9.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Chung Y, Bishop C, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci U S A. 2006;103(31):11695–11700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnaboldi PM, Roth-Walter F, Mayer L. Suppression of Th1 and Th17, but not Th2, responses in a CD8(+) T cell-mediated model of oral tolerance. Mucosal Immunol. 2009;2(5):427–438. doi: 10.1038/mi.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweitzer AN, Sharpe AH. Mutual regulation between B7-1 (CD80) expressed on T cells and IL-4. J Immunol. 1999;163(9):4819–4825. [PubMed] [Google Scholar]
- 34.Taylor PA, Lees CJ, Fournier S, et al. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions [corrections]. J Immunol. 2004;172(1):34–39. doi: 10.4049/jimmunol.172.1.34. [DOI] [PubMed] [Google Scholar]
- 35.Paust S, Lu L, McCarty N, Cantor H. Engagement of B7 on effector T cells by regulatory T cells prevents autoimmune disease. Proc Natl Acad Sci U S A. 2004;101(28):10398–10403. doi: 10.1073/pnas.0403342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suvas S, Singh V, Sahdev S, Vohra H, Agrewala JN. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem. 2002;277(10):7766–7775. doi: 10.1074/jbc.M105902200. [DOI] [PubMed] [Google Scholar]
- 37.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005;6(3):280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 38.Kitazawa Y, Fujino M, Wang Q, et al. Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation. 2007;83(6):774–782. doi: 10.1097/01.tp.0000256293.90270.e8. [DOI] [PubMed] [Google Scholar]
- 39.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 40.Wang L, Pino-Lagos K, de Vries VC, et al. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105(27):9331–9336. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azuma T, Yao S, Zhu G, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111(7):3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong H, Strome SE, Matteson EL, et al. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest. 2003;111(3):363–370. doi: 10.1172/JCI16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21(4):503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Hurchla MA, Sedy JR, Gavrieli M, et al. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174(6):3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 46.Desvignes C, Etchart N, Kehren J, et al. Oral administration of hapten inhibits in vivo induction of specific cytotoxic CD8+ T cells mediating tissue inflammation: a role for regulatory CD4+ T cells. J Immunol. 2000;164(5):2515–2522. doi: 10.4049/jimmunol.164.5.2515. [DOI] [PubMed] [Google Scholar]
- 47.Zheng Y, Manzotti CN, Liu M, et al. CD86 and CD80 differentially modulate the suppressive function of human regulatory T cells. J Immunol. 2004;172(5):2778–2784. doi: 10.4049/jimmunol.172.5.2778. [DOI] [PubMed] [Google Scholar]
- 48.Samoilova EB, Horton JL, Zhang H, et al. CTLA-4 is required for the induction of high dose oral tolerance. Int Immunol. 1998;10(4):491–498. doi: 10.1093/intimm/10.4.491. [DOI] [PubMed] [Google Scholar]
- 49.Fowler S, Powrie F. CTLA-4 expression on antigen-specific cells but not IL-10 secretion is required for oral tolerance. Eur J Immunol. 2002;32(10):2997–3006. doi: 10.1002/1521-4141(2002010)32:10<2997::AID-IMMU2997>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 50.Chung Y, Chang JH, Kweon MN, Rennert PD, Kang CY. CD8alpha-11b+ dendritic cells but not CD8alpha+ dendritic cells mediate cross-tolerance toward intestinal antigens. Blood. 2005;106(1):201–206. doi: 10.1182/blood-2004-11-4240. [DOI] [PubMed] [Google Scholar]
- 51.Goubier A, Dubois B, Gheit H, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29(3):464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplemental Figures]