Differentiation of neuroepithelia from human embryonic stem cells (original) (raw)
. Author manuscript; available in PMC: 2010 Oct 1.
Abstract
We describe the method for in vitro differentiation of neuroepithelial cells from human embryonic stem cells under a chemically defined condition. The protocol is established following the fundamental principle of in vivo neuroectodermal specification. The primitive neuroepithelial cells generated by this protocol can be further induced into neuronal and glia cells with forebrain, mid/hind brain, and spinal cord identities.
Keywords: Human embryonic stem cells, Embryoid body, Neuroepithelia, Neuronal differentiation, Chemically defined condition
1. Introduction
Directed differentiation of human embryonic stem cells (hESCs) is key to dissecting early human development as well as producing lineage and stage specific cells for pharmaceutical screening and potentially cell therapy. To that end, we have devised a generic protocol for directing hESCs to synchronized neuroepithelial cells efficiently in a chemically defined system. This protocol is built upon the fundamental principle of neuroctodermal specification and the basic timeline of human embryo development. Technically, it avoids the harsh cell dissociation approach employed in mouse ESC differentiation technique which is not amenable for hESCs. It also stays away from a co-culture with stroma cells to prevent biasing the differentiated cells to a particular regional progenitor (e.g., mid/hind brain progenitors) and being contaminated by carryover of tumorigenic stroma cells (1). The protocol described below is chemically defined and typically yields over 90% of neuroepithelial cells among total differentiated progenies, defined by immunostaining for the neuroepithelial transcription factors Pax6, Sox1 and Sox2 (2). More importantly, this method allows control of developmental stages and generation of primitive neuroepithelial cells which can be further induced to neuronal and glial progenitors with forebrain, mid/hind brain, and spinal cord identities (3, 4). Thus, this neuroepithelial differentiation method can be used as generic approach for generating neural progenitors and mature neural subtypes, as well as adapted to the needs of individual investigators who intend to differentiate hESCs to specific classes of neurons and glial cells (5).
The protocol is a simplified and optimized version of a previous reported adherent colony culture (6). It comprises three major steps: aggregation of ESCs (“embryoid body” formation), differentiation of multipotential primitive neuroepithelial cells, and generation of region-specific definitive neuroepithelial cells. Each step is morphologically distinct and is readily identifiable under a regular phase contrast microscope and typical photos have been provided as a guideline. The protocol has been followed by many amateur cell culture practitioners with consistent results. The key is that the hESC culture is free of partially differentiated cells.
2. Materials
2.1 Supplies
- Polystyrene flask with polyethylene filter cap, T25 and T75 (Fisher Scientific, Pittsburgh, PA; cat. No. 12-565-57 and 12-565-31; or Nunc, Roskilde, Denmark; cat. No. 136196 and 178891).
- Polystyrene plate, 6-well and 24-well (Fisher Scientific; cat. No. 12-565-73 and 12-565-75; or Nunc; cat. No. 140675 and 143982).
- Polystyrene petri dish, 60mm (Fisher scientific; cat. No. 08-757-13A).
- Polystyrene conical tube, 15 and 50ml (Fisher scientific; cat. No. 05-527-90 and 14-432-23; or BD Bioscience, Bedford, MA; cat. No. 352095 and 352073).
2.2 Stock Solutions
- Dulbecco’s modified Eagle’s medium (DMEM): nutrient mixture F-12 1:1 (DMEM/F12) (Gibco-BRL; cat. No. 11330-032).
- L-glutamine solution (200mM) (Sigma, St. Louis, MO; cat. No. G7513). Make aliquots of 2.5ml and store at −20°C.
- MEM nonessential amino acids solution (Gibco-BRL, Rockville, MD; cat. No. 11140-050).
- Knockout serum replacer (Gibco-BRL; cat. No. 10828-028). Make aliquots of 50ml and store at −20°C.
- Fetal bovine serum (Gibco-BRL; cat. No. 16000-044).
- N2 supplement (Gibco-BRL; cat. No. 17502-048).
- β-mercaptoethanol (14.3M) (Sigma; cat. No. M7522)
- Recombinant human FGF basic (bFGF, R&D systems, Minneapolis, MN, cat. No. 233-FB) is dissolved in sterile PBS with 0.1% bovine serum albumin (Sigma; cat. No. A-7906) at a final concentration of 100µg/ml. Make Aliquots of 50ul and store at −80°C.
- Dispase solution (1mg/ml): dissolve 50mg dispase (Gibco-BRL; cat. No. 17105-041) in 50ml DMEM/F12 in a water bath for 15min and filter-sterilize the dispase solution with a 50ml steri-flip (Fisher Scientific; cat. No. SCGP00525).
- Heparin (1mg/ml); dissolve 10mg heparin (Sigma; cat. No. H3149) in 10ml DMEM/F12 medium. Make aliquots of 0.5ml into and store at −80°C.
- Laminin from human placenta (Sigma; cat. No. L6274).
2.3 Media
- The hESC growth medium. First add 3.5µl β-mercaptoethanol to 2.5ml of L-glutamine solution, then combine it with 392.5ml DMEM/F12, 100ml Knockout serum replacer and 5ml MEM non-essential amino acids solution. Sterilize by filtering through a 500ml filter unit (0.22µm sterilizing low protein binding membrane) (Corning Incorporated, Corning, NY; cat. No. 430513). The medium can be stored at 4°C for up to 10 days. Add bFGF to final 4ng/ml prior to use (see Note 1).
- The neural induction medium. Sterilely combine: 489.5ml DMEM/F12, 5ml N2 supplement, 5ml MEM nonessential amino acids solution, and 0.5ml of 1mg/ml heparin. Medium can be stored at 4°C for up to 2 weeks. bFGF (10 ng/ml) may be added prior to use.
3. Methods
3.1 Making ES aggregates/”Embryoid bodies” (Day 1–4)
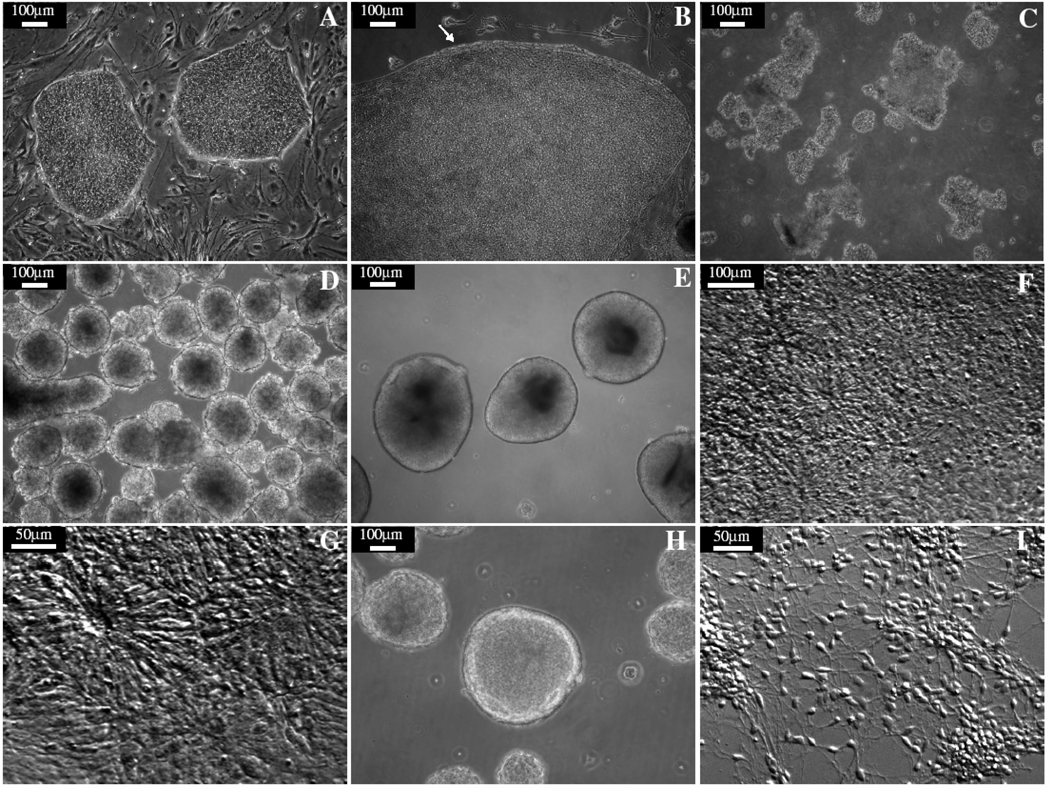
- ESCs (Fig. 1A) are grown to confluent in a 6-well plate. Aspirate the ESC growth medium off and add 1ml of Dispase to each well (The volumes used below are all for 1 well of a 6-well plate).
- Check the cells every 2 minutes until the edges of cell colonies begin to curl off of the plate (Fig. 1B). This usually takes 2 to 5 minutes when fresh dispase solution is used (see Note 2).
- Add 3ml ESC growth medium and gently detach the ESC colonies by pipetting using a 1000 µl tip. Transfer the cells to a 15-ml conical tube. Gently triturate 3–5 times to break cell colonies into smaller clusters of about 100–200µm in diameter using a 10-ml pipette (Fig. 1C).
- Spin the ESC clusters at 200g for 2 minutes to settle them to the bottom of the tube. Aspirate off the medium.
- Wash the cells once by adding 5ml fresh ESC growth medium and then centrifuge for 2 minutes at 200g.
- Aspirate off supernatant and resuspend cells in 5ml of the ESC growth medium and transfer to a 60-mm Petri dish or a T25 flask (see Note 3).
- Next day, undifferentiated ESCs will form aggregates and mostly float in the medium (Fig. 1D). Release those loosely attached aggregates by gently swirling the dish. If there are MEF and/or differentiated ESCs, simply transfer the ESC aggregates to a new dish to remove the contaminated cells (see Note 4).
- Feed the cells with the ESC growth medium every day for the first 4 days. When feeding, use a 5-ml pipette to gently pull aggregates up and then blow them back into the medium 2–3 times. This will help clean dead cells off the aggregate surface. Let the clusters settle to the bottom in a standing flask and aspirate off the medium.
Fig. 1. Differentiation of neuroepithelia from hESCs.
(A) hESC colonies. (B) A hESC colony was treated with dispase. The edge of the colony curled up as indicated by the arrow. (C) hESC were detached and pipetted into fragments of a couple of hundred m in diameter. (D) hESC aggregated as spheres and floated in the medium. (E) After 3 days in the neural induction medium, cell aggregates became bright and clear. (F) Cell aggregates formed monolayer after attachment, “rosette” structures were observed about 4–5 days after attachment. (G) The columnar neuroepithelia cells formed neural tube-like rosettes. (H) Neuroepithelial cells were collected and grew as neuroepithelial clusters in suspension. (I) Neuroepithelial cells differentiated into neurons after attachment.
3.2 Differentiating to primitive and definitive Neuroepithelia (Day 5–17)
- Collect the ESC aggregates by centrifuging for 2 min at 200g and wash once with 5 mls of neural induction medium.
- Resuspend cells in 5 mls of neural induction medium and transfer to a new T25 flask.
- Feed the cells with neural Induction medium every other day. Aggregates should become bright and clear after a few days in neural induction medium, healthy cell aggregates should be round and have smooth edges (Fig. 1E).
- Prepare the laminin coated plates 2 days after cells in the neural induction medium (day 6). To coat, use 20 µg/ml mouse or human laminin in neural induction medium and leave the coated plates in 37°C incubator overnight (see Note 5).
- Cells should be ready for attachment after 3 days in the neural induction medium. 30–50 aggregates are deposited in fresh neural nnduction medium in each well of a 6-well plate. Distribute the aggregates evenly over the plate to prevent contacting one another. This can be done by shaking the plate on the incubator shelf up and down twice and then left and right twice gently when placing the plate into the incubator (see Note 6).
- Attached aggregates will collapse to form a monolayer colony after 1–2 days. Continue feeding with the neural induction medium every other day (see Note 7).
- After 10–11 total days of differentiation (4–5 days following attachment), over 95% of the colonies should take on a morphology in which the center cells exhibit an elongated, columnar morphology (Fig. 1F). We call these columnar epithelial cells primitive neuroepithelial because they express a range of early neuroectodermal markers including Pax6, Sox2 and a host of anterior transcription factors. Cells at this stage are multipotential and can be used to differentiate to neuronal and glial types with distinct regional specificities.
- Neuroepithelial cells should be fed with the same neural induction medium every other day and cultured for another 7 days. During this period starting at day 14–15 the columnar neuroepithelia cells will further proliferate, often forming ridges or rings of cells outlining a distinct lumen (Fig. 1G). The overall morphology is reminiscent of the neural tube and the structures at this stage are often referred to as neural tube-like rosettes.
- After 17–18 days of differentiation under these conditions the neuroepithelia that makes up the rosettes will stain positive for the definitive neural tube stage marker Sox1.
3.3 Isolating Definitive Neuroepithelia (day 17–18)
- Scratch off any colonies that do not contain any neuroepithelial cells (this should be less than 10% of colonies). The “bad” colonies can be marked by an objective marker lens under a phase contrast scope and then scraped away with a pipette tip in a sterile hood.
- Dislodge the neuroepithelia by gentle pipetting with a 1000-µl tip. Neuroepithelial cells are denser in the center of colonies so that they are easily detached as opposed to the flat, tightly bound cells at the periphery.
- Collect the detached neuroepithelial cells in a 15-ml conical tube and spin them down at 100g for 2 min. Wash the cells once with fresh neural induction medium.
- Aspirate off the medium and resuspend the clusters of neuroepithelia in 5 ml of neural induction medium, supplement with B27 to improve cell survival. Transfer the cells to a new T25 flask.
- Over the next 24 hours the rosette aggregates will roll up to form round spheres (Fig. 1H) while any flat non-rosette peripheral cells will usually attach to the culture vessel. After this period rosette aggregates should be switched to a new flask and grown in neural induction medium (see Note 8).
Neuroepithelial cells can be maintained in the neural induction medium until they are used for further transplantation purpose or in vitro differentiation to neuronal and glial cells (Fig. 1I). Aggregates will stop growing when a maximal size is reached. To help cell proliferation, aggregates should be broken down to smaller sizes using the Pasteur pipette technique described in Fig. 2. In this way the total number of cells can be amplified and neuroepithelia can be propagated for several months in the neural induction medium with bFGF or other growth factors. However the types of neural cells generated will inevitably change with long term culture.
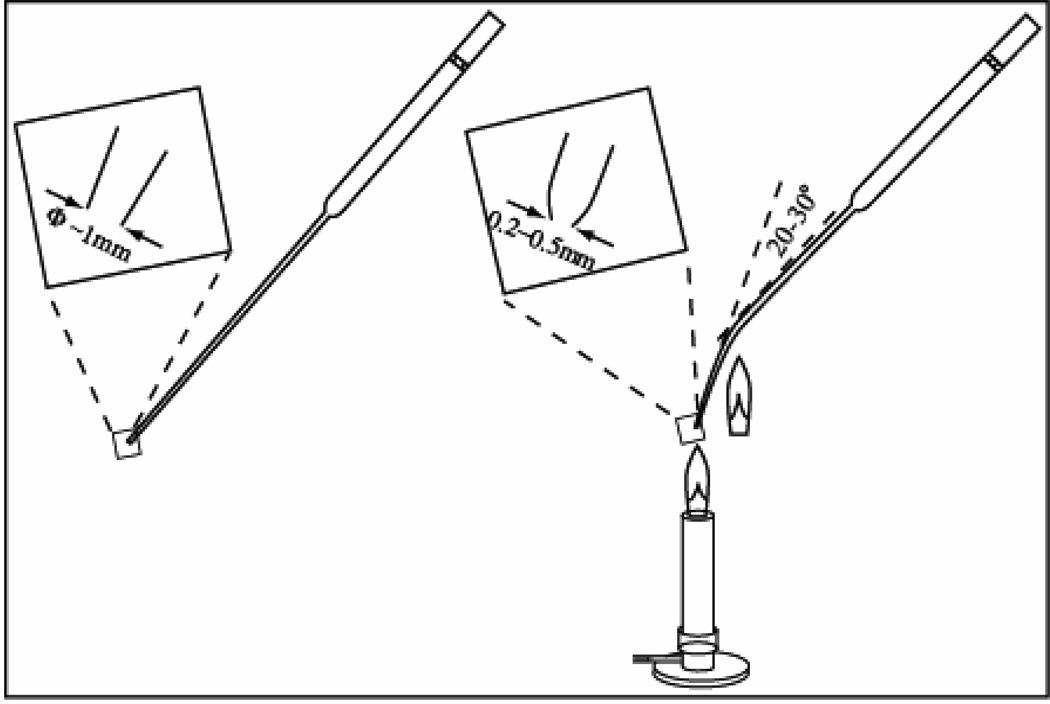
Fig. 2. Pasteur pipette technique for breaking up neuroepithelial clusters.
Flame the end of cotton filtered Pasteur pipette to round the edges and also narrow the opening to about 0.2–0.5mm in diameter. Flame the narrow part of the shaft to introduce a 20–30° bend. Rinse the pipette wall with the culture medium three times. Pipette neuroepithelial clusters using the treated Pasteur pipette by pull the cells in and push them out for 3 times. The narrow opening and bend in the pipette will help shear the clusters into smaller pieces of roughly uniform size.
Footnotes
1
Recombinant growth factors lose activities quickly in diluted condition. Do not add them into the medium until use.
2
If the colonies do not start to curl off in 5 minutes, old dispase solution should be discarded and change to fresh solution.
3
Before put the dish or flash into the incubator, shake to disperse the cell clumps to avoid aggregation.
4
Do not pipette to release the flat attached cells. They are residual MEFs or differentiated cells that should be avoided in the following steps.
5
We have found 3 hours coating to be sufficient, coating for less than 3 hours is not recommended.
6
Do NOT swirl the plate. Swirling will almost inevitably lead to aggregation in the center of the well. Cell differentiation will be affected due to lack of space in between.
7
Cells attach loosely in the first 1 to 2 days. Be gentle not the detach them when changing medium.
8
The rosette aggregates are usually called neurospheres. Neurosphres are generally brighter than the spherical cell aggregates made from ES cells in the previous step, although they are morphologically identical.
References
- 1.Du ZW, Zhang SC. Neural differentiation from embryonic stem cells: which way? Stem Cells Dev. 2004;13:372–381. doi: 10.1089/scd.2004.13.372. [DOI] [PubMed] [Google Scholar]
- 2.Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed Neural Differentiation of hESCs via an Obligated Primitive Anterior Stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 4.Yan Y, Yang D, Zarnowska ED, Du Z, Werbel B, Valliere C, Pearce RA, Thomson JA, Zhang SC. Directed differentiation of dopaminergic neuronal subtypes from human embryonic stem cells. Stem Cells. 2005;23:781–790. doi: 10.1634/stemcells.2004-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang SC. Neural subtype specification from embryonic stem cells. Brain Pathol. 2006;16:132–142. doi: 10.1111/j.1750-3639.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]