Genome packaging in viruses (original) (raw)
. Author manuscript; available in PMC: 2011 Feb 1.
Published in final edited form as: Curr Opin Struct Biol. 2010 Jan 8;20(1):114–120. doi: 10.1016/j.sbi.2009.12.006
Summary of recent advances
Genome packaging is a fundamental process in a viral life cycle. Many viruses assemble preformed capsids into which the genomic material is subsequently packaged. These viruses use a packaging motor protein that is driven by the hydrolysis of ATP to condense the nucleic acids into a confined space. How these motor proteins package viral genomes had been poorly understood until recently, when a few X-ray crystal structures and cryo-electron microscopy structures became available. Here we discuss various aspects of genome packaging and compare the mechanisms proposed for packaging motors based on structural information.
Introduction
There are two main strategies for viral genome packaging. Many viruses assemble their capsids around the viral genomes, such as the ssRNA helical tobacco mosaic virus [1], the ssRNA icosahedral bacteriophage R17 [2], the ssRNA conical HIV [3], and the dsRNA unsegmented icosahedral Totiviridae [4] viruses (e.g. L-A virus of yeast). Similarly small dsDNA viruses, with a less than 20 kb genome, assemble the shell around condensed DNA [5,6].
On the other hand, some dsDNA viruses (e.g. tailed bacteriophages and herpesviruses) and dsRNA viruses (e.g. φ6 and φ12 bacteriophages) form protein capsid shells first and then package their genomes into the procapsids. For the latter viruses, a motor is required to perform the packing with energy supplied by hydrolysis of ATP [7].
The final genome organization varies independently of the mode of packaging. In most icosahedral viruses there is no apparent order in the packaged genome, whereas in some viruses the genome takes on partial icosahedral symmetry in its secondary structure. Examples are the plant ssRNA bean-pod mottle virus [8], ssRNA satellite tobacco mosaic virus [9], to a lesser extent the ssDNA bacteriophage φX174 [10] and the ssDNA canine parvovirus [11]. Many viruses, especially DNA bacteriophages, show systematic layers of packaged DNA [12–14]. Yet other, mostly filamentous, viruses package their genome as helices surrounded by proteins [15,16]. Some viruses neutralize the negative charge with polyamines (e.g. some RNA plant viruses) [17] and/or cations (e.g. bacteriophages) [18], whereas other viruses neutralize the charge with a positively charged domain of the capsid protein [19–21].
Here we review recent structural studies on certain well-characterized viral packaging motor proteins. A variety of methods have been used, including x-ray crystallography, cryo-electron microscopy (cryo-EM) and single-molecule measurements using optical tweezers. We will compare models of packaging mechanisms derived from these structural studies for both dsRNA and dsDNA viruses. We will not discuss in detail the genome organization inside the viruses and the way genomes are unpackaged after infection has been initiated.
Packaging initiation
Viral genome packaging must differentiate between host and viral nucleic acid. A strategy employed by many viruses is that the viral capsid protein contains a binding site which recognizes a specific sequence of the genome. For example, in unsegmented Totiviridae dsRNA viruses, such as the L-A virus of yeast, there is a secondary structure (stem-loop) and a specific sequence at the 5' end of the genome [22] that are used for recognition by the polymerase-group antigen (pol-gag) fusion protein [23]. However, segmented dsRNA viruses such as Reoviridae, Orthomyxoviridae (influenza), Bunyaviruses and Arenaviruses need to package one of each of the dsRNA segments. It has been proposed that there is complementarity between regions of the segments which helps the virus to include one of each of the genomic RNA molecules. Alternatively, for the segmented dsRNA bacteriophage φ6, the procapsid may have specific binding sites for each of the different segments [22,24].
Double-stranded DNA viruses have evolved yet other strategies for packaging their own genome. Most bacteriophages and herpesviruses replicate their genomes as head-to-tail concatemers. A “terminase” complex of two proteins, which is also a part of the packaging motor, recognizes a specific sequence or structure on the concatemeric DNA and makes the initial cut to generate the free end at which packaging is initiated. After one or slightly more than one genome length of DNA has been packaged into the head, the same nuclease makes another cut to terminate packaging. Hence, historically, these proteins that are required for the generation of genome termini were named “terminases”. Certain dsDNA viruses such as bacteriophage φ29 and adenoviruses employ protein-primed DNA replication and do not produce concatemers. These viruses recognize their own DNAs through the terminal primer proteins that are covalently linked to the genome ends [25].
Mechanism for dsRNA packaging
In dsRNA viruses such as φ6 and φ12, the positive-sense strand is packaged and then replicated to form dsRNA inside the capsid. Therefore, the substrate for the packaging motor in these dsRNA viruses is ssRNA. Structurally, the best studied packaging motor protein for dsRNA viruses is the P4 ATPase in bacteriophage φ12. The hexameric P4 is a multi-functional molecular unit, which is involved in procapsid assembly [26], actively packages ssRNA molecules [27], and acts as a passive channel when newly synthesized mRNA molecules are extruded from the virus during infection [28]. The φ12 genome contains three segmented dsRNAs. The positive strand of each RNA molecule is recognized sequentially by the procapsid [24,29]. During the packaging process, the procapsid undergoes conformational changes and expands successively to accommodate all RNA molecules [30].
The P4 ATPase is structurally similar to RecA/F1-ATPase-like motors [31]. For multi-subunit motors there are two fundamental questions. How is the ATP hydrolysis coupled to translocation and how do the motor subunits communicate with each other to achieve efficient packaging? In hexameric P4, the central channel is lined with a portion of a helix α6 and loops L1 and L2. Mutagenesis and hydrogen-deuterium exchange experiments have shown that L1 and L2 are essential for RNA binding and translocation [32]. The phosphate-binding P-loop, where the conserved Walker A motif resides, is in a “down” conformation before the bound ATP is hydrolyzed, and adopts an “up” conformation after ATP has been hydrolyzed. This is accompanied by the transition of α6 and loop L2 from an “up” position to a “down” position by about 6 Å, which was proposed to be the driving force for RNA translocation [31]. The efficient coordination between the substrate and the motor requires a symmetry match. The ssRNA was proposed to have a structure similar to the A form dsRNA, which has about 11 bases per turn with a rise of 2.6–2.8 Å per base. It was proposed that the slight symmetry mismatch of 11 bases per turn ssRNA to six motor subunits is alleviated by the flexible loop L1 which acts as a “grommet” to correct the position of the substrate ssRNA [31]. The sequential hydrolysis of ATP by neighboring P4 subunits is achieved through an “arginine” finger that is commonly seen in hexameric ATPase motors [33–35]. ATP hydrolysis in one ATPase subunit induces a conformational change that places the “_trans_” arginine finger into the ATPase active center of the neighboring subunit, triggering the subsequent ATP hydrolysis (Figure 1).
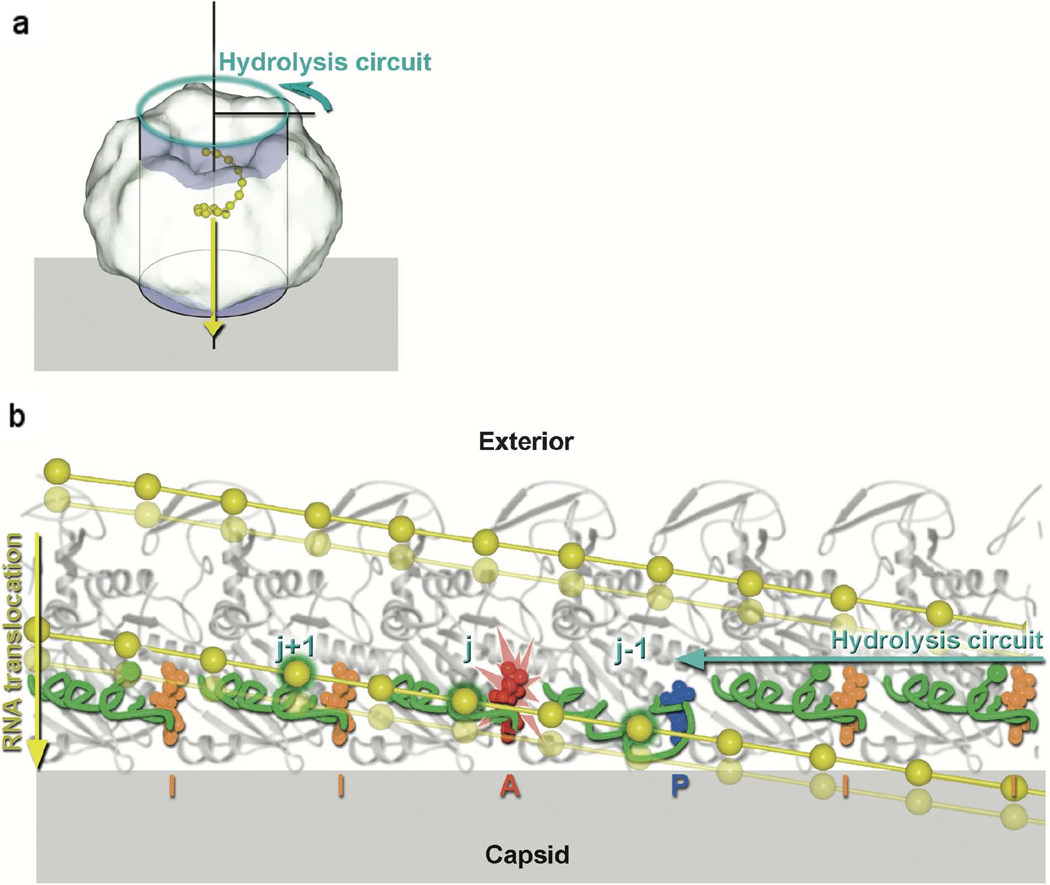
Figure 1.
Model for translocation along P4 by the ssRNA in bacteriophage φ12 [31]. The relative movements of the RNA binding loops in six adjacent subunits as the ATP hydrolysis-induced conformational changes ripple around the ring. (a) Diagram showing the hexameric molecule represented as a cylinder associated with the viral capsid shell (gray). The direction of ssRNA (yellow) translocation is depicted by a yellow arrow, while the cyan arrow shows the direction of sequential ATP hydrolysis. To obtain the view shown in b, the coordinates are projected onto the cylinder shown and the cylinder unwrapped to lie across the page. The effect of this is that the P4 hexamer is peeled open and viewed from inside the ring looking outward. (b) Cylindrical polar projection showing a snapshot of the hexamer just prior to hydrolysis at subunit j (marked with the flash). The RNA binding loops are colored green and the ssRNA yellow with phosphates represented by balls. Phosphates interacting with the RNA binding loops are identified by a green outer glow. Diphosphate and triphosphate moieties are colored according to their conformation: AMPcPP inactive “I” (orange), AMPcPP active “A” (red), and product “P” (blue). The power stroke associated with the hydrolysis step about to occur will translocate RNA downward (from the solid RNA position to the semitransparent position). Two turns of the RNA spiral are shown. [Reprinted from Cell, Volume 118, Mancini EJ, Kainov DE, Grimes JM, Tuma R, Bamford DH, and Stuart DI: Atomic snapshots of an RNA packaging motor reveal conformational changes linking ATP hydrolysis to RNA translocation, pages 743–755, copyright 2004, with permission from Elsevier].
Mechanism for dsDNA packaging
Genome packaging motors in dsDNA viruses are powerful molecular machines. The density of DNA genomes in bacteriophages is near crystalline and the resultant pressure inside the viral capsid is about 60 atmospheres [36]. Thus, dsDNA packaging motors are required to generate enough force to counter this pressure towards the end of the packaging process [36–39]. The most powerful molecular motor known to date is the bacteriophage T4 genome packaging motor, which can generate a force of up to 60 piconewtons and package DNA at an average rate of about 700 bp/sec [37].
The packaging machine in dsDNA viruses usually consists of a dodecameric portal protein at one special 5-fold vertex [40–46] and a pentameric motor protein (historically named the large terminase) which hydrolyzes ATP to package DNA [40,47–49]. For viruses that replicate their genomes as concatemers, a regulator protein, historically named the small terminase, works together with the large terminase in the packaging initiation process and fine tunes the activity of the large terminase during packaging [50,51]. Unlike the dsRNA motor, the dsDNA packaging motor is a transient component of the viral particle and does not remain with the final virion. Rather, the motor complex binds to the empty procapsid at the portal and dissociates once packaging is completed. For bacteriophage φ29, a viral genome encoded RNA component (pRNA) forms a bridge between the capsid and the motor protein [40,47].
Rotary motor mechanism
Much of the early structural work on DNA packaging motors was obtained from bacteriophage φ29. The first atomic structure of a packaging machine component is that of the dodecameric portal protein gp10 of φ29 [40]. The portal has a wider end that is inside the capsid and a narrower end that is protruding from the capsid. It has a central channel formed by α-helices and lined with negative charges, which is suitable for the smooth passage of DNA. Cryo-EM reconstruction of the procapsid showed that five pRNA molecules bind to the procapsid [40]. The symmetry mismatch of DNA (101 screw), portal (12-fold) and procapsid/pRNA/ATPase (5-fold) would be consistent with an earlier proposed rotary mechanism for DNA packaging [52]. In this mechanism the portal rotates using the energy from ATP hydrolysis, driving the DNA into the procapsid. However, single molecule fluorescence spectroscopy experiments show no portal rotation, making this mechanism unlikely [53].
Linear motor mechanism based on electrostatic forces
The packaging motor protein in dsDNA viruses generally has two domains, an N-terminal ATPase domain and a C-terminal domain that has nuclease activity [54]. The two domains need to be physically linked together to retain packaging activity [55]. The linker between the two domains consists of small amino acids, presumably providing the flexibility the motor requires to accomplish packaging. Recent crystal structures of the bacteriophage T4 packaging ATPase gp17 showed that the N-terminal domain has a nucleotide binding fold and is structurally more similar to monomeric helicases than hexameric ATPases [48,49], whereas the C-terminal domain belongs to the RnaseH/resolvase/intergrase superfamily [49,56]. The interactions between the N- and C-terminal domains are quite extensive that involve six charge pairs and a hydrophobic core. An in vitro active packaging complex [57] that is composed of T4 proheads and gp17 was examined by cryo-EM which showed five gp17 molecules bound below the special vertex [49]. In contrast to the crystal structure, where the two domains are in close contact with each other, the cryo-EM reconstruction showed that the two domains are well-separated. It was proposed that the two structures represent two states of the motor [49]. Comparison of the crystal structure (post-translocation state) with the structure fitted into the EM density (pre-translocation state) showed an about 7 Å translation of the C-terminal domain of gp17 along the central axis of the phage head. This is consistent with the bulk measurement in phages φ29 and T3, where each ATP consumed packages two base pairs of DNA [58,59]. Therefore, a mechanism based on electrostatic interactions was proposed for the bacteriophage T4 packaging motor. Upon binding of dsDNA to the C-terminal domain of one gp17 subunit, a cis “arginine” finger in the N-terminal domain is properly positioned into the ATPase active center which triggers ATP hydrolysis. The subsequent conformation change aligns the opposing charges in the N- and C-terminal domains, causing the C-terminal domain to be pulled 7 Å towards the N-terminal domain by the electrostatic forces, packaging two base pairs of dsDNA. Because of the symmetry match between the five gp17s and the 101 B-form DNA, the neighboring gp17 is now aligned with dsDNA substrate for the next round of translocation (Figure 2).
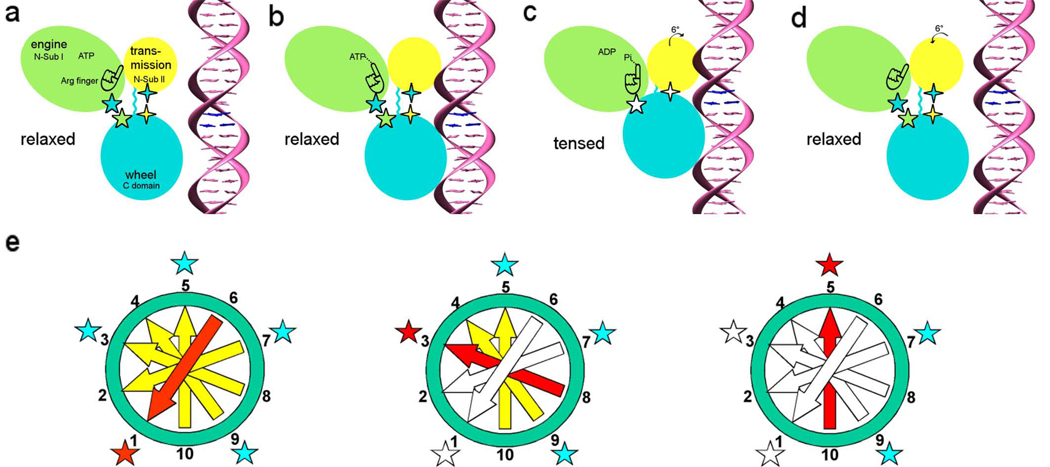
Figure 2.
DNA packaging mechanism in bacteriophage T4 [49]. Panels (a)–(d) relate to the sequence of events that occur in a single gp17 molecule. The gp17 N-terminal subdomain I, subdomain II, and C-terminal domain are represented as green, yellow, and cyan ovals, respectively. The five-pointed stars show the charge interactions between the N-terminal subdomain I and the C-terminal domain. The four-pointed stars show the charge interaction between the N-terminal subdomain II and the C-terminal domain. The flexible linker between N- and C-terminal domain is represented by a wiggly cyan line.(a) The gp17 C-terminal domain is ready to bind DNA.(b) The C-terminal domain, when bound to the DNA, brings the DNA closer to the N-terminal domain of the same subunit. Conformational change in the N-terminal domain causes Arg162 to be placed into the ATPase active center in preparation for hydrolysis.(c) Hydrolysis of ATP has rotated the N-terminal subdomain II by about 6°, thereby aligning the charge pairs resulting in an electrostatic attraction that moves the C-terminal domain and the DNA 6.8 Å (equivalent to the distance between two base pairs) closer to the N-terminal domain and into the capsid.(d) ADP and Pi are released and the C-terminal domain returns to its original position. DNA is released and is aligned to bind the C-terminal domain of the neighboring subunit. (e) This panel relates to the synchronization of the five gp17 molecules located around the special vertex of the procapsid. Successive DNA base pairs are indicated by yellow arrows outside the procapsid, red entering the procapsid, and white inside the procapsid. The surrounding five gp17 molecules are shown as stars. The red star represents gp17 ATPase hydrolyzing ATP, the blue star represents ATPase that is ready to hydrolyze ATP, and the white star represents ATPase that has already hydrolyzed ATP. Left: Hydrolysis of ATP at position 1 translocating two base pairs into the procapsid. Middle: Hydrolysis of ATP at position 3 causing the translocation of further two base pairs into the procapsid. Right: The ATP at position 5 is ready to be hydrolyzed. [Reprinted from Cell, Volume 135, Sun S, Kondabagil K, Draper B, Alam TI, Bowman VD, Zhang Z, Hegde S, Fokine A, Rossmann MG, and Rao VB, The structure of the phage T4 DNA packaging motor suggests a mechanism dependent on electrostatic forces, pages 1251–1262, copyright 2008.]
Non-integer step size motor mechanism
Although it would be logical to assume that each motor subunit would package an integral number of base pairs for each ATP hydrolyzed, a recent publication by Moffitt et al. reported a step size of 2.5 bps/ATP for the φ29 packaging motor using high resolution optical tweezer measurements [60]. The φ29 procapsid/pRNA/ATPase and DNA were tethered to microbeads held in two optical traps. Packaging was measured by the distance change between the two traps. External forces were applied to slow down the motor so that individual steps of packaging could be resolved. Bursts of 10 base pairs packaging were observed separated by dwells. Each burst consisted of four steps, which was interpreted as four ATP hydrolysis and DNA translocation events. The dwells between bursts were interpreted as the time required to reload the ATP molecules onto the motor subunits that had “fired”. The presence of five motor subunits and four ATP hydrolysis events during each burst demands some asymmetry within the pentameric motor. Two models were proposed, a piston-like model and an inchworm-like model. The piston-like model assumes that four of the subunits hydrolyze ATP while the fifth one retains its ATP and also holds onto the DNA while the other subunits are being reloaded. This model would require that each motor subunit binds DNA in a non-specific manner or has multiple binding sites on the DNA. However, it is not clear how the motor would “know” which subunit needs to be special at a certain time. The inchworm-like model proposes that only two subunits make contact with the DNA while conformational changes from all subunits create a distortion that drives the packaging of 10bps. This model requires the subunits in the pentameric motor to change its organization from a symmetrical ring to a staircase during each burst of 10bps. This seems unlikely since the motor would be attached to the pRNA in the case of φ29 or to the capsid/portal in the case of T4. Changing the organization of the subunits from a ring to a one-turn staircase with a 34 Å pitch would dramatically reduce the contact surface of the motor with the procapsid, which might result in dissociation of the motor (Figure 3). A very recent paper [61] discusses the type of chemical contact between the DNA and the motor parts that are required for successful packaging for bacteriophage φ29.
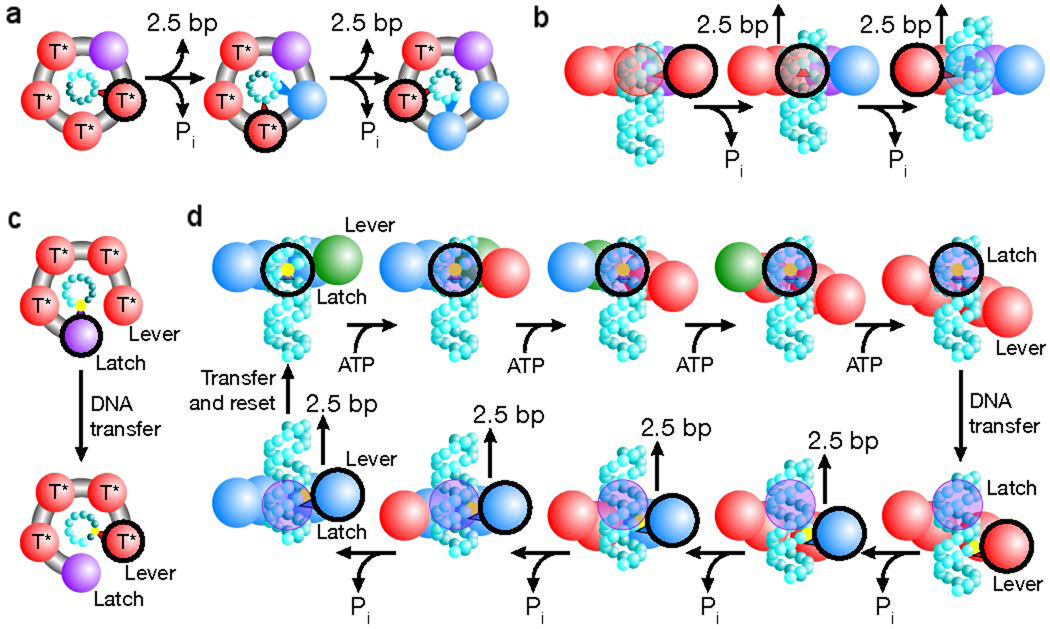
Figure 3.
Packaging models that produce a non-integer step size in bacteriophage φ29 [60]. (a) Depiction of a translocation model in which all subunits eventually contact the DNA (cyan spheres). The contacting subunit is outlined in black (top view). (b) In such a model the size of internal conformational changes set the step size (side view). (c) Depiction of a translocation model in which only two subunits contact the DNA (black outline). (d) In such a model, one subunit maintains contact with the DNA (the latch) while the loading of each ATP introduces relative subunit–subunit rotations which distort the ring. This distortion extends one subunit (the lever) along the DNA by ~10 bp. The DNA contact point is then transferred from the latch to the lever, and the release of hydrolysis products relaxes the ring, retracting the lever and the DNA. The DNA contact is then transferred back to the latch, the ring resets and the cycle begins again. Because there are four subunits, the ring is retracted in four steps, dividing a 10 bp step into four ~2.5 bp substeps. The subunit is colored based on its substrate binding state as follows: ATP docking (green, T), tight ATP binding and activation (red, T*), ADP bound (blue) and apo (purple). [Reprinted by permission from Macmillan Publishers Ltd: Moffitt JR, Chemla YR, Aathavan K, Grimes S, Jardine PJ, Anderson DL, Bustamante C: **Intersubunit coordination in a homomeric ring ATPase**. _Nature_ 2009, 457:446–450., www.nature.com]
Closing remarks
A few models with common themes for virus genome packaging have emerged based on results from structural studies. In these models packaging occurs by linear movement of the viral nucleic acid driven by conformational changes in the ATPase motor protein. In addition, the subunits of the motor are all proposed to function in a highly coordinated manner. However, the details of the mechanisms are quite different, partly due to the structural difference of the ligands that are translocated, e.g. ssRNA vs dsDNA. For dsDNA packaging motors, the recent finding that the packaging step could be non-integral has severely challenged other proposed mechanisms. Although this is only observed in the phage φ29 packaging system, the only system that has an RNA component, it would be evolutionarily more likely that all phages share similar packaging mechanisms. Hopefully, future experiments will shed light on this puzzle.
Acknowledgements
We are grateful to Sheryl Kelly for assistance in the preparation of this manuscript. The work was supported by National Science Foundation grants to MGR (MCB-0443899) and VDB (MCB-0923873) as well as National Institutes of Health grant R56AI081726 to MGR, VDB and SS.
References
- 1.Buck KW. Replication of tobacco mosaic virus RNA. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:613–627. doi: 10.1098/rstb.1999.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valegård K, Murray JB, Stockley PG, J SN, Liljas L. Crystal structure of an RNA bacteriophage coat protein-operator complex. Nature. 1994;371:623–626. doi: 10.1038/371623a0. [DOI] [PubMed] [Google Scholar]
- 3.Russell RS, Liang C, Wainberg MA. Is HIV-1 RNA dimerization a prerequisite for packaging? Yes, no probably? Retrovirology. 2004;1:23. doi: 10.1186/1742-4690-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimura T, Esteban R, Esteban LM, Wickner RB. Portable encapsidation signal of the L-A double-stranded RNA virus of S. cerevisiae. Cell. 1990;62:819–828. doi: 10.1016/0092-8674(90)90125-x. [DOI] [PubMed] [Google Scholar]
- 5.Burroughs AM, Iyer LM, Aravind L. Comparative genomics and evolutionary trajectories of viral ATP dependent DNA-packaging system. Genome Dyn. 2007;3:48–65. doi: 10.1159/000107603. [DOI] [PubMed] [Google Scholar]
- 6.Roitman-Shemer V, Stokrova J, Forstova J, Oppenheim A. Assemblages of simian virus 40 capsid proteins and viral DNA visualized by electron microscopy. Biochem. Biophys. Res. Commun. 2007;353:424–430. doi: 10.1016/j.bbrc.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 7.Rao VB, Feiss M. The bacteriophage DNA packaging motor. Annu. Rev. Genet. 2008;42:642–681. doi: 10.1146/annurev.genet.42.110807.091545. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Stauffacher C, Li Y, Schmidt T, Bomu W, Kamer G, Shanks M, Lomonossoff G, Johnson JE. Protein-RNA interactions in an icosahedral virus at 3.0 Å resolution. Science. 1989;245:154–159. doi: 10.1126/science.2749253. [DOI] [PubMed] [Google Scholar]
- 9.Larson SB, Koszelak S, Day J, Greenwood A, Dodds JA, McPherson A. Three-dimensional structure of satellite tobacco mosaic virus at 2.9 Å resolution. J. Mol. Biol. 1993;231:375–391. doi: 10.1006/jmbi.1993.1289. [DOI] [PubMed] [Google Scholar]
- 10.McKenna R, Xia D, Willingmann P, Ilag LL, Rossmann MG. Structure determination of the bacteriophage φX174. Acta Crystallogr. sect. B. 1992;48:499–511. doi: 10.1107/s0108768192001344. [DOI] [PubMed] [Google Scholar]
- 11.Chapman MS, Rossmann MG. Structural refinement of the DNA-containing capsid of canine parvovirus using RSRef, a resolution-dependent stereochemically restrained real-space refinement method. Acta Crystallogr D Biol. Crystallogr. 1996;52:129–142. doi: 10.1107/S0907444995007268. [DOI] [PubMed] [Google Scholar]
- 12.Fokine A, Kostyuchenko VA, Efimov AV, Kurochkina LP, Sykilinda NN, Robben J, Volckaert G, Hoenger A, Chipman PR, Battisti AJ, et al. A three-dimensional cryo-electron microscopy structure of the bacteriophage φKZ head. J. Mol. Biol. 2005;352:117–124. doi: 10.1016/j.jmb.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Fokine A, Chipman PR, Leiman PG, Mesyanzhinov VV, Rao VB, Rossmann MG. Molecular architecture of the prolate head of bacteriophage T4. Proc. Natl. Acad. Sci. U.S.A. 2004;101:6003–6008. doi: 10.1073/pnas.0400444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi KH, McPartland J, Kaganman I, Bowman VD, Rothman-Denes LB, Rossmann MG. Insight into DNA and protein transport in double-stranded DNA viruses: the structure of bacteriophage N4. J. Mol. Biol. 2008;378:726–736. doi: 10.1016/j.jmb.2008.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachse C, Chen JZ, Coureux P-D, Stroupe ME, Fändrich M, Grigorieff N. High-resolution electron microscopy of helical specimens: a fresh look at tobacco mosaic virus. J. Mol. Biol. 2007;371:812–835. doi: 10.1016/j.jmb.2007.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goulet A, Blangy S, Redder P, Prangishvili D, Felisberto-Rodrigues C, Forterre P, Campanacci V, Cambillau C. Acidianus filamentous virus 1 coat proteins: a helical fold spanning the filamentous archaeal viruses lineage. Proc. Natl. Acad. Sci. U.S.A. 2009 doi: 10.1073/pnas.0909893106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balint R, Cohen SS. The incorporation of radiolabeled polyamines and methionine into turnip yellow mosaic virus in protoplasts from infected plants. Virology. 1985;144:181–193. doi: 10.1016/0042-6822(85)90316-2. [DOI] [PubMed] [Google Scholar]
- 18.Yu T-Y, Schaefer J. REDOR NMR characterization of DNA packaging in bacteriophage T4. J. Mol. Biol. 2008;382:1031–1042. doi: 10.1016/j.jmb.2008.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warrier R, Linger BR, Golden BL, Kuhn RJ. Role of Sindbis virus capsid protein region II in nucleocapsid core assembly and encapsidation of genomic RNA. J. Virol. 2008;82:4461–4470. doi: 10.1128/JVI.01936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hacker DL. Identification of a coat protein binding site on southern bean mosaic virus RNA. Virology. 1995;210:562–565. doi: 10.1006/viro.1995.1117. [DOI] [PubMed] [Google Scholar]
- 21.Hillman BI, Hearne P, Rochon D, Morris TJ. Organization of tomato bushy stunt virus genome: characterization of the coat protein gene and the 3' terminus. Virology. 1989;169:42–50. doi: 10.1016/0042-6822(89)90039-1. [DOI] [PubMed] [Google Scholar]
- 22.Wickner RB. Double-stranded RNA virus replication and packaging. J. Biol. Chem. 1993;268:3797–3800. [PubMed] [Google Scholar]
- 23.Fujimura T, Ribas JC, Makhov AM, Wickner RB. Pol of gag-pol fusion protein required for encapsidation of viral RNA of yeast L-A virus. Nature. 1992;359:746–749. doi: 10.1038/359746a0. [DOI] [PubMed] [Google Scholar]
- 24.Olkkonen VM, Gottlieb P, Strassman J, Qiao XY, Bamford DH, Mindich L. In vitro assembly of infectious nucleocapsids of bacteriophage φ6: formation of a recombinant double-stranded RNA virus. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9173–9177. doi: 10.1073/pnas.87.23.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez I, Lázaro JM, Salas M, de Vega M. φ29 DNA polymerase-terminal protein interaction. Involvement of residues specifically conserved among protein-primed DNA polymerases. J. Mol. Biol. 2004;337:829–841. doi: 10.1016/j.jmb.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Poranen MM, Paatero AO, Tuma R, Bamford DH. Self-assembly of a viral molecular machine from purified protein and RNA constituents. Mol. Cell. 2001;7:845–854. doi: 10.1016/s1097-2765(01)00228-3. [DOI] [PubMed] [Google Scholar]
- 27.Juuti JT, Bamford DH. RNA binding, packaging and polymerase activities of the different incomplete polymerase complex particles of dsRNA bacteriophage φ6. J. Mol. Biol. 1995;249:545–554. doi: 10.1006/jmbi.1995.0317. [DOI] [PubMed] [Google Scholar]
- 28.Kainov DE, Lísal J, Bamford DH, Tuma R. Packaging motor from double-stranded RNA bacteriophage φ12 acts as an obligatory passive conduit during transcription. Nucleic Acids Res. 2004;32:3515–3521. doi: 10.1093/nar/gkh680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao X, Casini G, Qiao J, Mindich L. In vitro packaging of individual genomic segments of bacteriophage φ6 RNA: serial dependence relationships. J. Virol. 1995;69:2926–2931. doi: 10.1128/jvi.69.5.2926-2931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butcher SJ, Dokland T, Ojala PM, Bamford DH, Fuller SD. Intermediates in the assembly pathway of the double-stranded RNA virus φ6. EMBO J. 1997;16:4477–4487. doi: 10.1093/emboj/16.14.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancini EJ, Kainov DE, Grimes JM, Tuma R, Bamford DH, Stuart DI. Atomic snapshots of an RNA packaging motor reveal conformational changes linking ATP hydrolysis to RNA translocation. Cell. 2004;118:743–755. doi: 10.1016/j.cell.2004.09.007. **This work described the packaging mechanism for ssRNA packaging in a dsRNA virus.
- 32.Lísal J, Lam TT, Kainov DE, Emmett MR, Marshall AG, Tuma R. Functional visualization of viral molecular motor by hydrogen-deuterium exchange reveals transient states. Nat. Struct. Mol. Biol. 2005;12:460–466. doi: 10.1038/nsmb927. [DOI] [PubMed] [Google Scholar]
- 33.Sawaya MR, Guo S, Tabor S, Richardson CC, Ellenberger T. Crystal structure of the helicase domain from the replicative helicase-primase of bacteriophage T7. Cell. 1999;99:167–177. doi: 10.1016/s0092-8674(00)81648-7. [DOI] [PubMed] [Google Scholar]
- 34.Hishida T, Han Y-W, Fujimoto S, Iwasaki H, Shinagawa H. Direct evidence that a conserved arginine in RuvB AAA+ ATPase acts as an allosteric effector for the ATPase activity of the adjacent subunit in a hexamer. Proc. Natl. Acad. Sci. U.S.A. 2004;101:9573–9577. doi: 10.1073/pnas.0403584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abrahams JP, Leslie AGW, Lutter R, Walker JE. Structure at 2.8 Å resolution of F1-ATPase from bovine heart mitochondria. Nature. 1994;370:621–628. doi: 10.1038/370621a0. [DOI] [PubMed] [Google Scholar]
- 36.Smith DE, Tans SJ, Smith SB, Grimes S, Anderson DL, Bustamante C. The bacteriophage φ29 portal motor can package DNA against a large internal force. Nature. 2001;413:748–752. doi: 10.1038/35099581. * This work provides background towards single molecule investigations of packaging motors using optical tweezers.
- 37.Fuller DN, Raymer DM, Kottadiel VI, Rao VB, Smith DE. Single phage T4 DNA packaging motors exhibit large force generation, high velocity, and dynamic variability. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16868–16873. doi: 10.1073/pnas.0704008104. * Examines the properties of the T4 DNA packaging motor using single molecule techniques.
- 38.Fuller DN, Raymer DM, Rickgauer JP, Robertson RM, Catalano CE, Anderson DL, Grimes S, Smith DE. Measurements of single DNA molecule packaging dynamics in bacteriophage λ reveal high forces, high motor processivity, and capsid transformations. J. Mol. Biol. 2007;373:1113–1122. doi: 10.1016/j.jmb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuller DN, Rickgauer JP, Jardine PJ, Grimes S, Anderson DL, Smith DE. Ionic effect on viral DNA packaging and portal motor function in bacteriophage φ29. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11245–11250. doi: 10.1073/pnas.0701323104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simpson AA, Tao Y, Leiman PG, Badasso MO, He Y, Jardine PJ, Olson NH, Morais MC, Grimes S, Anderson DL, et al. Structure of the bacteriophage φ29 DNA packaging motor. Nature. 2000;408:745–750. doi: 10.1038/35047129. * The first structure of a phage portal protein.
- 41.Lebedev AA, Krause MH, Isidro AL, Vagin AA, Orlova EV, Tavares JT, Antson AA. Structural framework for DNA translocation via the viral portal protein. EMBO J. 2007;26:1984–1994. doi: 10.1038/sj.emboj.7601643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orlova EV, Gowen B, Dröge A, Stiege A, Weise F, Lurz R, van Heel M, Tavares P. Structure of a viral DNA gatekeeper at 10 Å resolution by cryo-electron microscopy. EMBO J. 2003;22:1255–1262. doi: 10.1093/emboj/cdg123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leiman PG, Chipman PR, Kostyuchenko VA, Mesyanzhinov VV, Rossmann MG. Three-dimensional rearrangement of proteins in the tail of bacteriophage T4 on infection of its host. Cell. 2004;118:419–429. doi: 10.1016/j.cell.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 44.Chang J, Weigele P, King J, Chiu W, Jiang W. Cryo-EM asymmetric reconstruction of bacteriophage P22 reveals organization of its DNA packaging and infection machinery. Structure. 2006;14:1073–1082. doi: 10.1016/j.str.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Lander GC, Tang L, Casjens SR, Gilcrease EB, Prevelige P, Poliakov A, Potter CS, Carragher B, Johnson JE. The structure of an infectious P22 virion shows the signal for headful DNA packaging. Science. 2006;312:1791–1795. doi: 10.1126/science.1127981. [DOI] [PubMed] [Google Scholar]
- 46.Jiang W, Chang J, Jakana J, Weigele P, King J, Chiu W. Structure of epsilon15 bacteriophage reveals genome organization and DNA packaging/injection apparatus. Nature. 2006;439:612–616. doi: 10.1038/nature04487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morais MC, Koti JS, Bowman VD, Reyes-Aldrete E, Anderson DL, Rossmann MG. Defining molecular and domain boundaries in the bacteriophage φ29 DNA packaging motor. Structure. 2008;16:1267–1274. doi: 10.1016/j.str.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun S, Kondabagil K, Gentz PM, Rossmann MG, Rao VB. The structure of the ATPase that powers DNA packaging into bacteriophage T4 procapsids. Mol. Cell. 2007;25:943–949. doi: 10.1016/j.molcel.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Sun S, Kondabagil K, Draper B, Alam TI, Bowman VD, Zhang Z, Hegde S, Fokine A, Rossmann MG, Rao VB. The structure of the phage T4 DNA packaging motor suggests a mechanism dependent on electrostatic forces. Cell. 2008;135:1251–1262. doi: 10.1016/j.cell.2008.11.015. ** Describes the mechanism of the T4 packaging motor based on crystallographic and cryo-EM structures.
- 50.Němeček D, Lander GC, Johnson JE, Casjens SR, Thomas GJ., Jr Assembly architecture and DNA binding of the bacteriophage P22 terminase small subunit. J. Mol. Biol. 2008;383:494–501. doi: 10.1016/j.jmb.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Zahrani AS, Kondabagil K, Gao S, Nelly N, Ghosh-Kumar M, Rao VB. The small terminase, gp16, of bacteriophage T4 is a regulator of the DNA packaging motor. J. Biol. Chem. 2009;284:24490–24500. doi: 10.1074/jbc.M109.025007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hendrix RW. Symmetry mismatch and DNA packaging in large bacteriophages. Proc. Natl. Acad. Sci. U.S.A. 1978;75:4779–4783. doi: 10.1073/pnas.75.10.4779. * The original basis for considering a rotary motion for the portal protein.
- 53.Hugel T, Michaelis J, Hetherington CL, Jardine PJ, Grimes S, Walter JM, Falk W, Anderson DL, Bustamante C. Experimental test of connector rotation during DNA packaging into bacteriophage φ29 capsids. PLoS Biology. 2007;5:e59. doi: 10.1371/journal.pbio.0050059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell MS, Matsuzaki S, Imai S, Rao VB. Sequence analysis of bacteriophage T4 DNA packaging/terminase genes 16 and 17 reveals a common ATPase center in the large subunit of viral terminases. Nucleic Acids Res. 2002;30:4009–4021. doi: 10.1093/nar/gkf524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanamaru S, Kondabagil K, Rossmann MG, Rao VB. The functional domains of bacteriophage T4 terminase. J. Biol. Chem. 2004;279:40795–40801. doi: 10.1074/jbc.M403647200. [DOI] [PubMed] [Google Scholar]
- 56.Alam TI, Draper B, Kondabagil K, Rentas FJ, Ghosh-Kumar M, Sun S, Rossmann MG, Rao VB. The headful packaging nuclease of bacteriophage T4. Mol. Microbiol. 2008;69:1180–1190. doi: 10.1111/j.1365-2958.2008.06344.x. [DOI] [PubMed] [Google Scholar]
- 57.Kondabagil KR, Zhang Z, Rao VB. The DNA translocating ATPase of bacteriophage T4 packaging motor. J. Mol. Biol. 2006;363:786–799. doi: 10.1016/j.jmb.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 58.Guo P, Peterson C, Anderson D. Prohead and DNA-gp3-dependent ATPase activity of the DNA packaging protein gp16 of bacteriophage φ29. J. Mol. Biol. 1987;197:229–236. doi: 10.1016/0022-2836(87)90121-5. [DOI] [PubMed] [Google Scholar]
- 59.Morita M, Tasaka M, Fujisawa H. DNA packaging ATPase of bacteriophage T3. Virology. 1993;193:748–752. doi: 10.1006/viro.1993.1183. [DOI] [PubMed] [Google Scholar]
- 60.Moffitt JR, Chemla YR, Aathavan K, Grimes S, Jardine PJ, Anderson DL, Bustamante C. Intersubunit coordination in a homomeric ring ATPase. Nature. 2009;457:446–450. doi: 10.1038/nature07637. ** A mechanistic interpretation of the bursts and dwells that occur during DNA packaging in φ29, concluding that there is a non-integral number of bases packaged for each ATP hydrolyzed.
- 61.Aathavan K, Politzer AT, Kaplan A, Moffitt JR, Chemla YR, Grimes S, Jardine PJ, Anderson DL, Bustamante C. Substrate interactions and promiscuity in a viral DNA packaging motor. Nature. 2009 doi: 10.1038/nature08443. in press. ** An investigation of the types of interactions between the packaging motor and dsDNA in φ29.