T-Cell Lineage Determination (original) (raw)
. Author manuscript; available in PMC: 2011 Nov 1.
Summary
T cells originate from hematopoietic stem cells (HSCs) in the bone marrow but complete their development in the thymus. HSCs give rise to a variety of non-renewing hematopoietic progenitors, among which a rare subset migrates to the thymus via the bloodstream. The earliest T-cell progenitors identified in the thymus are not T-lineage restricted but possess the ability to give rise to cells of many different lineages. Alternative lineage potentials are gradually lost as progenitors progress towards later developmental stages. Here, we review the early developmental events that might be involved in T-cell lineage fate determination, including the properties of possible thymus settling progenitors, their homing into the thymus, and their T-cell lineage specification and commitment.
Keywords: lineage commitment/specification, thymus settling progenitors, early thymic progenitors, Notch signal, transcription factors
Properties of thymus-settling progenitors
The thymus does not contain self-renewing progenitors, and therefore long-term thymopoiesis depends on the recruitment of thymus-settling progenitors (TSPs) throughout life (1–3). The specific identity of thymic-settling progenitors remains unclear. Many hematopoietic progenitors possess T-lineage potential, but among them only a small subset is able to enter the thymus (4–7). In this section, we discuss the lineage potentials of various bone marrow and blood progenitors and their ability to settle the adult mouse thymus.
Hematopoietic progenitors with T-lymphoid potential
A variety of bone marrow progenitors, including HSCs and the downstream progenitors with different degrees of lineage restriction, have been found to possess T-cell potential. Long-term self-renewing HSCs, functionally defined by their capability to generate all blood lineages, demonstrate potent long-term T-lineage differentiation potential when placed inside the thymus (7–12). Immediately downstream of HSCs, the multipotent progenitors (MPPs) have lost self-renewal potential but maintain multi-lineage differentiation capability (13, 14). Further downstream, lymphoid-primed MPPs (LMPPs) in adult mice, characterized by high expression of the fms-like tyrosine kinase receptor-3 (Flt3), have lost megakaryocyte/erythroid potential and are biased towards lymphoid and granulocyte/macrophage lineages (15, 16). Common lymphoid progenitors (CLPs), originally thought to be committed towards the B and T-lymphoid lineages, are now shown to be a rather heterogeneous population in terms of lineage potential. The surface marker Ly6D separates CLPs into two functionally distinct subsets: Ly6D− CLPs possess B cell, T cell, natural killer (NK) cell, dendritic cell (DC), and some degree of myeloid potential; whereas Ly6D+ CLPs are largely restricted towards the B-cell lineage (17). In another study using recombination-activating gene-1 (Rag-1) and λ5 reporter mice, CLPs were further divided into three functionally distinct subsets: Rag-1low λ5− CLPs which display B cell, T cell, NK cell and a degree of myeloid cell potential; Rag-1high λ5− CLPs which show a bias towards the B-lymphoid lineage and decreased potential in the other lineages; and Rag-1high λ5+ CLPs which are completely B-lineage committed (18, 19). Furthermore, fate mapping also suggests that CLPs with a history of Rag-1 expression are heavily biased towards the B lineage and are lineage-stable after exposure to lipopolysaccharide (LPS), whereas CLPs without a history of Rag-1 expression are directed to the DC fate in response to Toll-like receptor (TLR) ligation (20). Together, these findings demonstrate that many types of progenitors possess T-cell potential with varying combinations of myeloid and other lymphoid lineage potentials (Fig. 1).
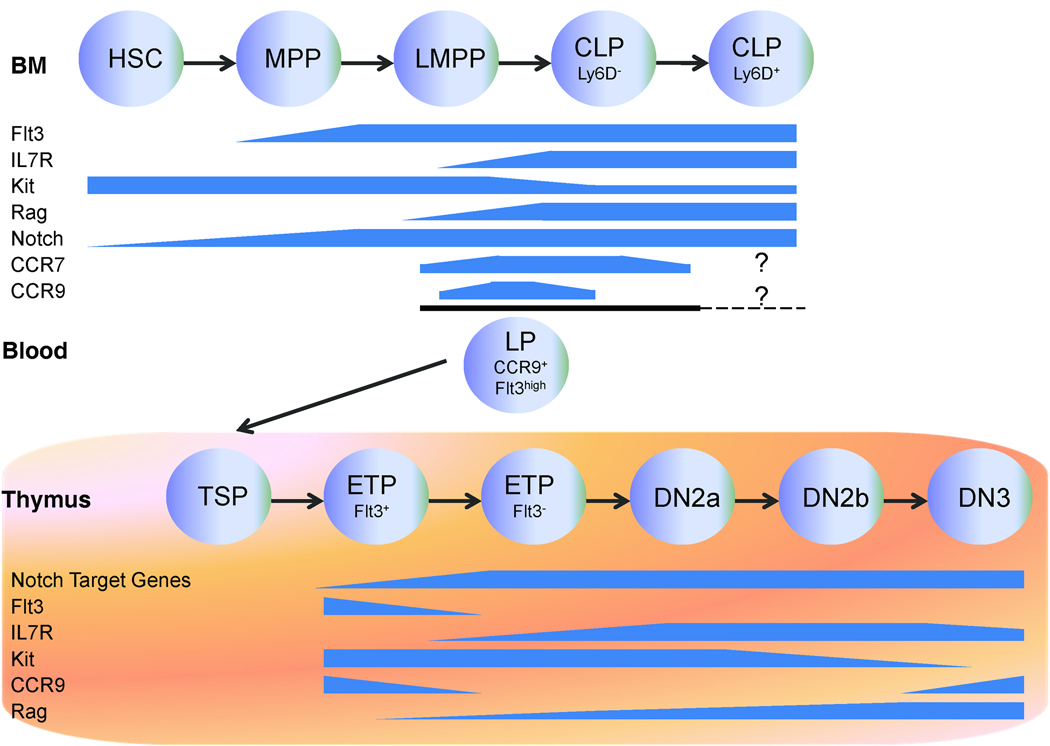
Figure 1. Molecular signals in early T-cell development.
Among the many types of bone marrow progenitors, a subset of lymphoid progenitors (LP) expressing CCR9 enters the thymus via the blood. The non-T-lineage potentials are gradually lost through multiple prethymic and intrathymic developmental steps, and T-cell lineage commitment is completed at the intrathymic DN3 stage. The expression patterns of the key cytokine receptors, chemokine receptors, Notch signaling molecules, and Rag recombinase during T-cell lineage commitment are indicated (14, 15, 19, 20, 30, 42, 45–49, 116, 125–129). Abbreviations: HSC, hematopoietic stem cell; MPP, multipotent progenitor; LMPP, lymphoid-primed multipotent progenitor; CLP, common lymphoid progenitor; LP, lymphoid progenitor; TSP, thymus settling progenitor; ETP, early thymic progenitor; DN, double-negative.
In humans, multiple prethymic progenitor types with T-cell lineage potential have also been identified in neonatal cord blood and in adult bone marrow. The most efficient T-lineage progenitors appear to be contained within a population possessing mixed myeloid and lymphoid potentials (21). Interestingly, these progenitors, termed multilymphoid progenitors (MLPs), give rise to T cells, B cells, NK cells, DCs, monocytes, and macrophages, but not granulocytes, suggesting a separation of granulocyte fate from the other myeloid and lymphoid lineage fates in early hematopoiesis (21). T-lymphoid potential has also been identified in human cord blood granulocyte and monocyte progenitors (GMPs) (21). The presence of progenitors with different combinations of myeloid and lymphoid lineage potentials in both humans and mice indicates that the expression or repression of genes regulating a specific lineage fate may occur in a gradual rather than abrupt process (22).
Identifying thymus-settling progenitors
It is thought that only a very small number of progenitors settle the thymus (23, 24). Considerable progress has been made in recent years in revealing the identity of these thymus-settling progenitors. Despite their potent T-cell differentiation potential when placed inside the thymus, HSCs and Flt3low MPPs do not enter the thymus in physiologic conditions (7). The most efficient thymus-settling progenitors appear to be contained within a small subset of LMPPs and CLPs, progenitors which express high levels of the cytokine receptor Flt3 (7, 25) (Fig. 1). At least some LMPPs are likely to home to the thymus via the blood, as suggested by the emergence of donor derived thymocytes within a short period of time following intravenous injection into unirradiated mice (7, 25). Ly6D−CLPs are also thought to enter the thymus, and after intravenous transfer, they generate thymocytes at an earlier time point than LMPPs (6, 17).
The ability of specific hematopoietic progenitors with T-cell potential to settle the thymus is due to their expression of the required homing molecules. Circulating progenitors are thought to enter the thymus via a similar three-step process as occurs in the trans-endothelial migration of mature leukocytes: the cells first roll along the endothelial monolayer by interacting with adhesion molecules, then are activated by chemokine receptor signaling, and finally adhere firmly to the endothelium (26). In support of this model, the P-selectin/PSGL-1 axis has a demonstrated role in recruiting progenitors to the thymus, perhaps by mediating the rolling step (27, 28). Chemokine receptors CCR9 and CCR7 also each support thymus settling (7, 25, 29, 30). Bone marrow cells lacking either CCR9 or CCR7 are impaired in the ability to generate thymocytes after intravenous transfer (7, 25, 30). Yet, these defects are lost when CCR7−/− or CCR9−/− bone marrow progenitors are transferred intrathymically, indicating that both CCR7 and CCR9 are important for thymus settling rather than intrathymic development (7, 25, 30). More severe thymus homing defects are observed in the progenitors deficient for both CCR9 and CCR7 (25, 30). These cells are almost completely prevented from thymus settling in competitive scenarios, indicating CCR9 and CCR7 are the two major chemokine receptors acting in the recruitment of hematopoietic progenitors to the thymus (25, 30). The expression of CCR9 on early bone marrow progenitors is dependent on Flt3 ligand (FL)/Flt3 signaling, as CCR9+ LMPPs are greatly reduced in mice lacking Flt3 or FL (7). Thus, Flt3 signaling controls the expression of homing molecules such as CCR9. Together, these findings suggest that efficient thymus settling under physiologic condition depends on the coordinated actions of an ensemble of cytokines, adhesion molecules, and chemoattractants.
Increasing evidence suggests that extramedullary maturation is not unique to T cells. A small number of circulating hematopoietic stem cells and progenitors enter organs such as the spleen and lungs, and expand and differentiate therein (31, 32). The expansion and extramedullary maturation of these tissue-resident progenitors appears to be enhanced by challenges such as inflammation or infection (31, 33, 34). Interestingly, IL-25, a member of the proinflammatory IL-17 cytokine family, has been found to induce the accumulation of Lineage−Sca-1+Kitint progenitors in the gut-associated lymph tissue that give rise to a variety of myeloid lineages and promote T-helper 2 (Th2) responses (35). Lineage-marker negative cells that express IL-7R have also been identified in the mesenteric lymph nodes of IL-25-treated mice and in the fat-associated lymphoid clusters (FALC) of unmanipulated mice, and these cells mediate Type-2 immune responses mimicking the function of T cells (36, 37). Together, these observations suggest an association between extramedullary hematopoiesis and the generation of innate type-2 effector cells (38). The mechanisms responsible for the migration of tissue-resident progenitors remain unclear. The recruitment of hematopoietic stem cells and progenitors into peripheral sites of inflammation and injury has been found to be dependent on CCR2, the cognate receptor of the monocyte chemoattractant proteins (MCPs) (39). It is possible that lymphoid progenitors bearing the essential homing molecules for thymus settling may have important extramedullary biological functions other than settling the thymus to initiate T-cell development.
Intrathymic T-lineage maturation of TSPs
Identity of the earliest intrathymic T-lineage progenitors
Past work found that the DN1 (CD4−CD8−CD3−CD44+CD25−) progenitors proliferate extensively and can generate both αβ and γδ T-cell subsets (40, 41). Many studies have showed that this DN1 population is heterogeneous, and only cells within this population expressing high levels of Kit efficiently generate T-lineage progeny (42–44). These Kithi cells are referred to as early thymic progenitors (ETPs), phenotypically defined as linlowCD44+cKithighCD25− thymocytes, and are presently the earliest known and most efficient intrathymic T progenitors. ETPs constitute a rare population comprising about 0.01% of total thymocytes and can expand extensively to repopulate thymocyte populations after intrathymic transfer (42).
Despite their rarity, ETPs are still a heterogeneous population and can be divided into functionally distinct subsets by the expression of the cytokine receptor Flt3. Flt3+ETPs are 10 to 20-fold more efficient in generating CD4+CD8+ double positive (DP) thymocytes than Flt3−ETPs (45). The Flt3+ETP pool also contains rare cells with B-cell lineage potential, whereas Flt3−ETPs appear devoid of B-cell progenitors (45). The frequency of B-cell progenitors among Flt3+ETPs decreases sharply with age. About 5% of Flt3+ETPs possess B-cell potential at birth, and this frequency drops by around fifty fold in adult mice (46). Studies using CCR9-green fluorescence protein (GFP) reporter mice suggest that ETPs with B-cell potential also express high level of CCR9 (47). This extremely rare subset of CCR9+Flt3+ETPs that can give rise to B cells has been suggested to represent the most immature intrathymic T lineage progenitors (46, 48) (Fig. 1). However, it remains unclear whether all downstream thymocytes are derived through a CCR9+Flt3+ETP obligate intermediate.
Lineage potential of ETPs
Far from being T-lineage restricted, ETPs are a multipotent population (45, 49). In mice, the majority of ETPs possess T cell, myeloid, and NK cell potential, but the vast majority do not give rise to B cells in vitro (49). The myeloid and NK cell potentials of ETPs are gradually lost as cells progress towards the DN2 (Kit+CD25+) and DN3 (Kit−CD25+) stages. Yet a considerable percentage of DN2 cells can still generate myeloid and NK progeny, and T-lineage commitment is not completed until the DN3 stage (Fig. 1). In human thymus, progenitors with B lymphoid, myeloid, and even erythroid potentials have been identified (50, 51). Single cell analysis reveals that CD34+lineage−CD7− progenitors maintain T cell, NK cell, B cell, and myeloid cell potentials (50). The myeloid and B-cell potentials are lost as these immature thymic progenitors differentiate into CD7+ cells (50). Together, these findings suggest that the earliest T-lineage progenitors in both mice and humans are multipotent, with myeloid and lymphoid lineage potentials. Signals within the thymus must promote the development of incoming progenitors down the T-lineage pathway and also ensure the concomitant loss of the alternative lineage potentials.
Early T-cell fate determination under Notch signaling
Early T-cell development depends on the highly conserved Notch signaling pathway (45, 52–55). Notch receptors are single-pass transmembrane glycoproteins (56). There are four Notch receptors named Notch1–4 in mammals, among which Notch 1 has been found to be both necessary and sufficient for T-cell development (52, 53). Two families of Notch ligands, Delta-like (DL) and Jagged, have been identified in mammals (57). Upon interaction with Notch ligands, the Notch receptors are cleaved sequentially by metalloprotease and γ-secretase, releasing the intracellular domain of Notch (ICN) (58–60). ICN moves to the nucleus, binds the transcription factor CSL, and recruits co-activators such as mastermind-like proteins (MAML) (61). The activated transcriptional complex then regulates the expression of a variety of Notch target genes.
Notch plays an essential role in the intrathymic maturation of ETPs. There is strong evidence that Notch signaling inhibits the development of the non-T cell lineages. The Notch ligands DL1 and DL4 have been shown to suppress the generation of Mac-1+ myeloid cells from ETPs in vitro (49, 62, 63). Furthermore, deletion of Notch in ETPs results in their differentiation into both conventional DCs and plasmacytoid DCs in the thymus, suggesting a requirement of Notch in suppressing the DC fates during early T-cell development (49, 64, 65). Notch signaling is also known to potently inhibit B-cell development (52, 55, 66–68). Expression of a constitutively activated form of Notch results in extrathymic T-cell development and an early block in B-cell differentiation in the bone marrow (52). Conversely, deletion of Notch1 in bone marrow progenitors or ETPs leads to accumulation of intrathymic B cells at the expense of T-cell development (55). Recent evidence suggests that DL4 is the essential Notch ligand in thymic T-cell commitment (69, 70). The deletion of DL4 in thymic epithelial cells results in a complete block of T-cell development and ectopic accumulation of immature B cells in the thymus, reminiscent of the effect of Notch 1 deletion (69, 70). Together, these observations suggest that Notch signaling plays a critical role in inhibiting non-T lineage fates to promote T-cell commitment.
In contrast with the essential role of Notch signaling in intrathymic development, less evidence exists to suggest an involvement of Notch signaling in prethymic development in adult mice. Inactivation of Notch signaling by overexpression of a dominant negative mutant of Mastermind-like 1 (DNMAML 1) does not dramatically impair the self-renewal or lineage differentiation of HSCs in the bone marrow of adult recipient mice (71). Also, the frequencies of downstream MPPs and LMPPs remain undisturbed in the absence of Notch signaling. Furthermore, loss of Notch signaling does not prevent primitive LSK progenitors from mobilizing into the blood (45). Thus, Notch signaling seems to be largely dispensable for the development, maintenance, and mobilization of adult prethymic progenitors with T-cell potential.
Despite the relative dispensability of Notch signaling in prethymic development, a low level of Notch receptor and signaling molecules have been detected in bone marrow progenitors, and Notch ligands are present in the bone marrow (71–74). It has been suggested that mechanisms exist to suppress Notch signaling in the bone marrow. Such mechanisms may be important in preventing the premature T-cell specification of the prethymic progenitors. The transcriptional suppressor LRF or Pokemon, encoded by the Zbtb7a gene, plays a critical role in countering the Notch effects in the bone marrow (75). Deletion of Zbtb7a results in substantial extrathymic T-cell development in the bone marrow at the expense of B lymphopoiesis, strikingly reminiscent of the effect of constitutive activation of Notch signaling by ICN expression (52, 75). Conversely, aberrant lymphoid lineage commitment in Zbtb7a-deficient mice is rescued by inhibition of Notch signaling with γ-secretase inhibitor (GSI) treatment (75). Together these observations suggest that LRF prevents the premature T-cell specification by opposing Notch signaling in the bone marrow, whereas the roles of LRF are probably overridden by a high dose of Notch signaling in the thymus to allow for T-cell development.
A yet unresolved question is whether attenuation or extinction of a specific non-T-cell lineage potential may involve multiple developmental steps at both prethymic and intrathymic sites. The vast majority of ETPs do not possess B-cell potential. It is still unknown whether the majority of thymus settling progenitors lose B-cell potential prior to thymic entry, early in the thymus, or both. The earliest intrathymic T-cell progenitors possess myeloid and NK potentials, and these lineage potentials are not completely lost until the intrathymic DN3 stage (49, 65). However, attenuation of the myeloid lineage potential likely initiates prethymically. Both LMPPs and CLPs, the two prethymic subsets containing CCR9-expressing thymus settling progenitors, show decreased myeloid differentiation ability as compared to the more immature HSCs and Flt3low MPPs (17, 19, 76). Fate mapping studies using IL-7R-Cre mice indicate that most ETPs and DN2 cells are marked with a history of IL-7R expression, and that once IL-7R is expressed, progenitor cells are unlikely to adopt the myeloid fate inside the thymus (77). However, the specific sites where T-lineage progenitors are marked with a history of IL-7R expression remain to be further investigated, which may shed light on whether the myeloid versus T-lineage fate is determined prethymically, early in the thymus, or both. If both prethymic and intrathymic sites are involved in suppressing the alternative lineage potentials, multiple molecular mechanisms, i.e. both Notch-dependent and Notch-independent mechanisms, are likely to contribute to T-cell lineage commitment.
Transcription factors regulating T-lineage fate determination
A number of transcription factors have been recognized to play critical roles in T-cell lineage fate determination. These transcription factors function in different stages of T-cell lineage development, and together control T-cell lineage commitment and specification.
Bcl11b, a zinc finger transcription factor, plays an essential role in intrathymic T-lineage commitment. Expression of Bcl11b is upregulated from ETP to DN2 cells, and Bcl11b has been shown to be required for the survival of double positive thymocytes (78, 79). Recent studies find that Bcl11b is also important for T-cell lineage commitment (80–82). Conditional deletion of Bcl11b results in a block of T-cell development at the Kithigh DN2 stage both in vivo and in vitro (80, 81). This developmental arrest appears to be associated with a critical role of Bcl11b in repressing myeloid and NK cell fates. The arrested Bcl11b-deficient DN2 cells express NK cell-associated genes and Bcl11b-deficient fetal liver cells readily differentiate into NK cells and macrophages in supportive in vitro conditions (80, 81). Furthermore, deletion of Bcl11b results in the production of NK cells from T-lineage progenitors at multiple stages of development, including ETP/DN1, DN2, and DN3 progenitors, as well as from DP and even mature CD8+ thymocytes (82). Single-cell analysis suggests that the reprogramming of committed T-lineage progenitors to NK cells requires deletion of both Bcl11b alleles, and that once both alleles are deleted, the efficiency of reprogramming reaches nearly 100% (82). Together, these observations suggest that Bcl11b is required for maintaining the T-cell lineage commitment of T-lineage progenitors. Analogous to Bcl11b, the B-cell lineage-specific transcription factor PAX5 plays an essential role in maintaining the B-cell identity of the B-lineage progenitors, as deletion of PAX5 causes committed pro-B cells to develop into T cells (83). However, Bcl11b-deficient T cells and T-committed DN3 progenitors do not reciprocally adopt the B-cell fate but appear to develop chiefly into NK cells (82). Bcl11b-deficient uncommitted DN2 progenitors differentiate additionally into myeloid cells (80). The results are consistent with the suggestion that T cells and NK cells share a committed progenitor (44) but raise the question as to why fates distinct from the NK fate are not accessed by T-committed cells when Bcl11b is deleted. One possibility is that transcription factors distinct from Bcl11b are responsible for the active repression of other non-T-cell fates and remain to be discovered.
PU.1 is another transcription factor which plays an important role in regulating early T-cell commitment and development. The expression of PU.1 in thymocytes is restricted within the DN progenitors, and deletion of PU.1 results in complete or nearly complete loss of all thymocytes (84). The earliest stage of T-cell development that requires PU.1 remains unclear, as PU.1 plays critical roles at prethymic stages. It regulates the expression of the cytokine receptors Flt3 and IL-7R and is important for segregation of the myeloerythroid from myelolymphoid fates (85). While a physiological dose of PU.1 is required to generate the earliest T-lineage progenitors, a high dose by enforced expressed of PU.1 dedifferentiates even T-lineage committed DN3 progenitors and induces them to differentiate into cells with macrophage and DC phenotypes (86, 87). DL1/Notch interaction is able to counter the lineage diversion effects of PU.1 and to restore T-cell commitment. Thus, Notch signaling might inhibit myeloid lineage differentiation of early T-lineage progenitors at least partly through countering the effects of PU.1.
E2A/HEB heterodimers are critical transcriptional regulators in early T-cell development (88). Loss of either E2A or HEB results in a partial block in early T-cell development at DN1 or immature single positive (ISP) stages respectively, and expression of a dominant-negative mutant of HEB which forms non-functional dimers with E2A results in a much stronger block within the DN stages. E2A has been found to cooperate with Notch signaling in promoting early thymopoiesis (89). Inducible overexpression of E2A increases the expression of Notch receptors and canonical Notch target genes such as Hes1 and Deltex1. Conversely, constitutive activation of Notch signaling by ICN expression rescues the developmental block of E2A deficient thymus. Furthermore, five canonical E-box sites conserved for both humans and mice have been identified in the promoter region of the Notch1 gene, and E2A has been shown to directly bind these E-boxes to induce Notch1 transcription in DN3 thymocytes (90). In addition to its essential role in intrathymic development, E2A has also been suggested to contribute to prethymic developmental steps that are important for the generation of thymus settling progenitors. In the absence of E2A, the expression of the cytokine receptor Flt3 in bone marrow LSKs is severely reduced, and the expression of the chemokine receptor CCR9 is almost absent (76, 91, 92). E2A may also suppress myeloid development by LMPPs, as indicated by increased size of the myeloid colonies produced by E2A deficient progenitors (76). Together, these observations suggest that E2A plays important roles in both intrathymic and prethymic steps of T-cell development.
The Runx1/CBFβ complex is another important transcriptional regulatory complex in early T-cell development (93, 94). CBFβ, the cofactor for Runx proteins, is the non-DNA-binding subunit of the core binding factor (CBF). Mice with reduced level of CBFβ by a hypomorphic mutant allele display profound defects in thymopoiesis (94). CBFβ insufficiency impairs the generation of ETPs and the downstream DN2 and DN3 progenitors, leading to complete absence of mature T cells. The mechanisms through which CBFβ promotes early T-cell development remain unclear. DN thymocytes with the CBFβ hypomorphic mutation do not contain intracellular TCRβ and TCRγ, suggesting CBFβ is required for the expression of these T-lineage genes (93). Constitutive activation of Notch signaling by ICN expression does not overcome the T-cell development defects of CBFβ insufficient fetal liver cells, nor does overexpression of CBFβ rescue the differentiation defects of DN2 and DN3 cells induced by GSI, the inhibitor of Notch signaling (93). These data indicate that CBFβ is necessary for normal T-cell development and that CBFβ and Notch each regulate required pathways in early T-cell development.
The basic helix-loop-helix (bHLH) transcriptional repressor Hes1, a canonical Notch target gene, is expressed at high levels in ETPs and the downstream DN progenitors (95–97). Adoptively transfer of Hes1-deficient fetal liver cells into Rag-deficient mice generates very limited number of DN progenitors and almost no CD4+CD8+ DP thymocytes, suggesting Hes1 is required for early T-cell development (96). The specific mechanisms by which Hes1 promotes T-cell development remain unclear. Overexpression of Hes1 inhibits the development of the B and myeloid cells, indicating a possible role of Hes1 in suppressing the B and myeloid lineage potential (95, 97). However, B-lineage cells do not expand in the absence of Hes1, which does not support a critical role of Hes1 in inhibiting the B-cell lineage (96). Furthermore, overexpression of Hes1 alone is not sufficient to promote T-cell differentiation in the absence of other Notch signaling molecules (98). Therefore, other major transcriptional factors must be required for Notch-mediated T-lineage commitment and/or specification.
GATA3, another direct target gene of Notch signaling, is also required for early T-cell development (99). GATA3 is expressed in ETPs, DN2, and DN3 progenitors, and its absence results in the elimination of almost the entire T lineage (29, 45, 100, 101). GATA3 null fetal liver cells give rise to very few ETPs and almost no downstream T-lineage progenitors after transferred into irradiated adult mice (101). However, normal numbers of LMPPs and CLPs are derived from GATA3 fetal liver cells (101). GATA3-deficient ETPs exhibits neither increased apoptosis nor disturbed cell cycle progression, suggesting GATA3 likely regulates the differentiation, but not the survival or proliferation, of early T lineage progenitors (101).
Evidence has suggested that many other transcription factors, such as Ikaros, Myb, and TCF1 (102–104), also play important roles in the generation and/or differentiation of the earliest T-lineage progenitors. Whether different transcription factors are responsible for the loss of specific lineage potentials within the distinct prethymic and intrathymic environments remains unclear. The interaction of these transcription factors with each other and their relations with Notch signaling pathway also remain to be further defined. Which transcription factors are important for the establishment and maintenance of the expression of T-lineage-specific genes and whether these are distinct from molecules important for inhibiting alternative fates such as Bcl11b are very poorly understood at this time.
Trophic factors promoting the survival and proliferation of early T-lineage progenitors T-cell development requires not only the repression of the alternative lineages but also the survival and expansion of T-lineage progenitors. The interactions between several secreted proteins and their membrane-bound receptors have been shown to contribute to the survival, proliferation, and differentiation of the T-lineage progenitors in early thymopoiesis.
BMP, Hedgehog, and Wnt are three important signaling mediators in early T-cell development. Sonic hedgedog (SHH) signaling has been found to regulate the survival and differentiation of early T-lineage progenitors in a manner that is not cell-autonomous, possibly by functioning in the stromal cell compartment (105). BMP 2/4 signaling pathway acts as a negative regulator in early T-cell differentiation by inhibiting the proliferation and maturation of the earliest T-lineage progenitors (106, 107).
The Wnt proteins play a critical role in the development of multiple hematopoietic lineages, among which Wnt1 and Wnt4 are essential for early thymopoiesis. Loss of Wnt1 or Wnt4 results in dramatically reduced cellularity of the fetal thymus, and the defects are more severe in Wnt1 and Wnt4 double knockout mice (108). Recent evidence also shows that Wnt4 promotes the survival of LMPPs, and the expansion of ETPs and DN2 progenitors, mainly through mechanisms that are not cell-autonomous, and that may depend on a non-canonical Wnt signaling pathway (109). The canonical Wnt signaling cascade has been suggested to result in the accumulation and nuclear translocation of β-catenin which binds with and activates TCF1 and LEF (110–113). TCF1, a T-cell specific transcription factor, is an essential transcription factor in the early T-lineage differentiation (100, 104, 114). TCF1-deficient mice display drastically reduced thymic cellularity, and a partial block of T-cell development at ISP to DP transition (104). The defects of T-cell development in these mice are exacerbated with age. At 6 months, only a small number of DN1 cells but no other T-lineage cells are present in the TCF1-deficient thymus. The N-terminal sequence of TCF1 containing the β-catenin interaction site is required for restoring the survival, proliferation, and differentiation of TCF1-deficient early T-lineage progenitors (112). However, mice lacking both β and γ catenin have very little identifiable defects in T-cell development (115, 116). Thus, TCF1 might predominantly act independently of the canonical Wnt signaling pathway; the nature of such alternative mechanisms is unknown at this time.
The proliferation and survival of early T-lineage progenitors depend on an ensemble of cytokines, including stem cell factor (SCF), Flt3 ligand, and IL-7. ETPs and downstream progenitors are significantly reduced in IL-7R-deficient mice and FL-deficient mice (117). More severe defects have been observed in IL-7R and FL double knockout mice in which ETPs are almost absent, suggesting a synergistic role for FL/Flt3 and IL-7/IL-7R signaling pathways in early T-cell development (117, 118). In addition to its role as a trophic factor for lymphocyte development, IL-7R is also essential for γδ T-cell commitment by controlling the accessibility of TCRγ locus to V(D)J recombinase (54, 119). Kit, the receptor for the cytokine stem cell factor (SCF), is expressed at high level on ETPs. Studies using viable Kit (W/W) mutant mice show that Kit plays a critical role in the development and/or maintenance of early T-lineage progenitors (120). In these Kit-deficient mice, CD25+ thymic progenitors are severely reduced in young mice and totally absent in aged mice. The more immature Thy-1lowHSA+CD25−/−CD44+ DN progenitors are not detectable in the absence of Kit. Together, these data suggest that several cytokines act together to promote early T-cell development. However, these cytokines are also involved in prethymic development, such as the generation of CLPs and LMPPs, and/or the maintenance of HSCs (15, 16, 121, 122). Understanding their functions will require further investigation of their separate roles in each prethymic and intrathymic environment.
Extrathymic T-cell commitment
The majority of T lymphocytes mature in the thymus. However, T-lineage-committed progenitors have also been detected in the spleens of unmanipulated B6 mice, indicating extrathymic T-cell commitment occurs under physiologic conditions (123). Abundant extrathymic T-cell progenitors have been found in athymic mice and following bone marrow transplantation of irradiated mice, possibly as a result of lymphopenia in these mice (73, 124–126). The developmental fate of these extrathymic T-cell progenitors is not clear. The splenic T progenitors resemble but are not identical to thymic DN3 cells, as they express lower level of Notch1, Notch3, and Notch target genes (73, 123–126). Interestingly, at the same time as the emergence of splenic T progenitors after irradiation and bone marrow transplantation, substantial donor-derived DN3 cells, but almost no donor-derived ETPs, are found in the thymus of the recipient mice, which may suggest an ETP-independent T-cell developmental pathway in this scenario (73). However, the absence of ETPs may also be due to their exhaustion after differentiation into DN3 progenitors in response to irradiation-induced thymocytopenia (115, 116). Therefore, the origins of extrathymic T progenitors and their relationship to intrathymic progenitors remain to be defined by future studies.
The development of the splenic T progenitors in irradiated mice is dependent on Notch, since inhibition of Notch ablates this population (73). However, it differs from intrathymic T-cell development in that the generation of splenic T progenitors can use either Notch1 or Notch2. Loss of either Notch1 or Notch2 does not affect the development of these extrathymic T-lineage progenitors, but loss of both Notch receptors results in the absence of these progenitors. (127). The splenic T-lineage progenitors in unmanipulated wildtype mice have also been found to be IL-7R dependent (123). Also, irradiated mice have increased level of the cytokines FL and IL-7 (128, 129). The roles of these cytokines and Notch signaling molecules in extrathymic T-cell development remain to be further defined.
Conclusions
Significant progress has been made in understanding the prethymic and intrathymic mechanisms governing T-cell lineage determination. A selective subset of progenitors settles the thymus and initiates T-lineage development in the thymus. Alternative lineage potentials are gradually lost under the combined effects of environmental signals and transcription factors, via multiple prethymic and intrathymic steps. Commitment to the T-cell fate is eventually completed intrathymically.
Many questions remain unresolved. What signals allow the prethymic progenitors to leave the bone marrow and to enter the thymus in physiologic conditions and under hematopoietic stresses? What are the mechanisms underlying each stage of T-lineage commitment and the loss of each specific lineage fate? Do the same mechanisms inhibit multiple lineage potentials, or do distinct mechanisms exist for each lineage? Are different mechanisms responsible for the loss of a specific non-T potential in different anatomical sites? And do recirculating progenitors in periphery tissues have functions distinct from their described roles in T-cell development, perhaps contributing to immune responses against infection? Answers to many of these questions are likely to be forthcoming in the near future.
Acknowledgments
This work is supported by NIH grants AI080091 (J.J.B.), AI059621, HL086900, and RC1HL099758 (A.B.), and a Scholar Award from the Leukemia and Lymphoma Society (A.B.). We thank members of the Bhandoola lab for helpful discussions and critical comments.
References
- 1.Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148:1604–1612. [PubMed] [Google Scholar]
- 2.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193:365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldschneider I, Komschlies KL, Greiner DL. Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J Exp Med. 1986;163:1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5:953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 5.Bhandoola A, Sambandam A. From stem cell to T cell: one route or many? Nat Rev Immunol. 2006;6:117–126. doi: 10.1038/nri1778. [DOI] [PubMed] [Google Scholar]
- 6.Serwold T, Ehrlich LI, Weissman IL. Reductive isolation from bone marrow and blood implicates common lymphoid progenitors as the major source of thymopoiesis. Blood. 2009;113:807–815. doi: 10.1182/blood-2008-08-173682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz BA, Sambandam A, Maillard I, Harman BC, Love PE, Bhandoola A. Selective thymus settling regulated by cytokine and chemokine receptors. J Immunol. 2007;178:2008–2017. doi: 10.4049/jimmunol.178.4.2008. [DOI] [PubMed] [Google Scholar]
- 8.Ikuta K, Weissman IL. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A. 1992;89:1502–1506. doi: 10.1073/pnas.89.4.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 10.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 11.Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin- Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. J Exp Med. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vicente R, Adjali O, Jacquet C, Zimmermann VS, Taylor N. Intrathymic transplantation of bone marrow-derived progenitors provides long-term thymopoiesis. Blood. 2010;115:1913–1920. doi: 10.1182/blood-2009-06-229724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc Natl Acad Sci U S A. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adolfsson J, et al. Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity. 2001;15:659–669. doi: 10.1016/s1074-7613(01)00220-5. [DOI] [PubMed] [Google Scholar]
- 15.Adolfsson J, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythromegakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med. 2006;203:1867–1873. doi: 10.1084/jem.20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inlay MA, et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell JJ, Bhandoola A. There's many a CLP on the path to B. Blood. 2010;115:2562–2563. doi: 10.1182/blood-2009-12-260463. [DOI] [PubMed] [Google Scholar]
- 19.Mansson R, et al. Single-cell analysis of the common lymphoid progenitor compartment reveals functional and molecular heterogeneity. Blood. 2010;115:2601–2609. doi: 10.1182/blood-2009-08-236398. [DOI] [PubMed] [Google Scholar]
- 20.Welner RS, et al. Asynchronous RAG-1 expression during B lymphopoiesis. J Immunol. 2009;183:7768–7777. doi: 10.4049/jimmunol.0902333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doulatov S, Notta F, Eppert K, Nguyen LT, Ohashi PS, Dick JE. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat Immunol. 2010;11:585–593. doi: 10.1038/ni.1889. [DOI] [PubMed] [Google Scholar]
- 22.Dorshkind K. Not a split decision for human hematopoiesis. Nat Immunol. 2010;11:569–570. doi: 10.1038/ni0710-569. [DOI] [PubMed] [Google Scholar]
- 23.Kadish JL, Basch RS. Hematopoietic thymocyte precursors. I. Assay and kinetics of the appearance of progeny. J Exp Med. 1976;143:1082–1099. doi: 10.1084/jem.143.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spangrude GJ, Scollay R. Differentiation of hematopoietic stem cells in irradiated mouse thymic lobes. Kinetics and phenotype of progeny. J Immunol. 1990;145:3661–3668. [PubMed] [Google Scholar]
- 25.Krueger A, Willenzon S, Lyszkiewicz M, Kremmer E, Forster R. CC chemokine receptor 7 and 9 double-deficient hematopoietic progenitors are severely impaired in seeding the adult thymus. Blood. 2010;115:1906–1912. doi: 10.1182/blood-2009-07-235721. [DOI] [PubMed] [Google Scholar]
- 26.Scimone ML, Aifantis I, Apostolou I, von Boehmer H, von Andrian UH. A multistep adhesion cascade for lymphoid progenitor cell homing to the thymus. Proc Natl Acad Sci U S A. 2006;103:7006–7011. doi: 10.1073/pnas.0602024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gossens K, et al. Thymic progenitor homing and lymphocyte homeostasis are linked via S1P-controlled expression of thymic P-selectin/CCL25. J Exp Med. 2009;206:761–778. doi: 10.1084/jem.20082502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi FM, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6:626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 29.Lai AY, Kondo M. Identification of a bone marrow precursor of the earliest thymocytes in adult mouse. Proc Natl Acad Sci U S A. 2007;104:6311–6316. doi: 10.1073/pnas.0609608104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zlotoff DA, Sambandam A, Logan TD, Bell JJ, Schwarz BA, Bhandoola A. CCR7 and CCR9 together recruit hematopoietic progenitors to the adult thymus. Blood. 2010;115:1897–1905. doi: 10.1182/blood-2009-08-237784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;132:612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 33.Nagai Y, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welner RS, et al. Lymphoid precursors are directed to produce dendritic cells as a result of TLR9 ligation during herpes infection. Blood. 2008;112:3753–3761. doi: 10.1182/blood-2008-04-151506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saenz SA, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c- Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 37.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 38.Eberl G. Immunology: Close encounters of the second type. Nature. 2010;464:1285–1286. doi: 10.1038/4641285a. [DOI] [PubMed] [Google Scholar]
- 39.Si Y, Tsou CL, Croft K, Charo IF. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest. 2010;120:1192–1203. doi: 10.1172/JCI40310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 41.Wu L, Antica M, Johnson GR, Scollay R, Shortman K. Developmental potential of the earliest precursor cells from the adult mouse thymus. J Exp Med. 1991;174:1617–1627. doi: 10.1084/jem.174.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allman D, et al. Thymopoiesis independent of common lymphoid progenitors. Nat Immunol. 2003;4:168–174. doi: 10.1038/ni878. [DOI] [PubMed] [Google Scholar]
- 43.Matsuzaki Y, et al. Characterization of c-kit positive intrathymic stem cells that are restricted to lymphoid differentiation. J Exp Med. 1993;178:1283–1292. doi: 10.1084/jem.178.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Sambandam A, et al. Notch signaling controls the generation and differentiation of early T lineage progenitors. Nat Immunol. 2005;6:663–670. doi: 10.1038/ni1216. [DOI] [PubMed] [Google Scholar]
- 46.Ceredig R, Bosco N, Rolink AG. The B lineage potential of thymus settling progenitors is critically dependent on mouse age. Eur J Immunol. 2007;37:830–837. doi: 10.1002/eji.200636728. [DOI] [PubMed] [Google Scholar]
- 47.Benz C, Bleul CC. A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med. 2005;202:21–31. doi: 10.1084/jem.20050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zediak VP, Maillard I, Bhandoola A. Closer to the source: notch and the nature of thymus-settling cells. Immunity. 2005;23:245–248. doi: 10.1016/j.immuni.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Bell JJ, Bhandoola A. The earliest thymic progenitors for T cells possess myeloid lineage potential. Nature. 2008;452:764–767. doi: 10.1038/nature06840. [DOI] [PubMed] [Google Scholar]
- 50.Hao QL, et al. Human intrathymic lineage commitment is marked by differential CD7 expression: identification of CD7- lympho-myeloid thymic progenitors. Blood. 2008;111:1318–1326. doi: 10.1182/blood-2007-08-106294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weerkamp F, et al. Human thymus contains multipotent progenitors with T/B lymphoid, myeloid, and erythroid lineage potential. Blood. 2006;107:3131–3137. doi: 10.1182/blood-2005-08-3412. [DOI] [PubMed] [Google Scholar]
- 52.Pui JC, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 53.Radtke F, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 55.Wilson A, MacDonald HR, Radtke F. Notch 1-deficient common lymphoid precursors adopt a B cell fate in the thymus. J Exp Med. 2001;194:1003–1012. doi: 10.1084/jem.194.7.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D'Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 59.Fortini ME, Rebay I, Caron LA, Artavanis-Tsakonas S. An activated Notch receptor blocks cell-fate commitment in the developing Drosophila eye. Nature. 1993;365:555–557. doi: 10.1038/365555a0. [DOI] [PubMed] [Google Scholar]
- 60.Struhl G, Fitzgerald K, Greenwald I. Intrinsic activity of the Lin-12 and Notch. Cell. 1993;74:331–345. doi: 10.1016/0092-8674(93)90424-o. [DOI] [PubMed] [Google Scholar]
- 61.Wu L, Sun T, Kobayashi K, Gao P, Griffin JD. Identification of a family of mastermind-like transcriptional coactivators for mammalian notch receptors. Mol Cell Biol. 2002;22:7688–7700. doi: 10.1128/MCB.22.21.7688-7700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Pooter RF, Schmitt TM, de la Pompa JL, Fujiwara Y, Orkin SH, Zuniga-Pflucker JC. Notch signaling requires GATA-2 to inhibit myelopoiesis from embryonic stem cells and primary hemopoietic progenitors. J Immunol. 2006;176:5267–5275. doi: 10.4049/jimmunol.176.9.5267. [DOI] [PubMed] [Google Scholar]
- 63.Zhou L, et al. Notch-dependent control of myelopoiesis is regulated by fucosylation. Blood. 2008;112:308–319. doi: 10.1182/blood-2007-11-115204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feyerabend TB, et al. Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity. 2009;30:67–79. doi: 10.1016/j.immuni.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 65.Wada H, et al. Adult T-cell progenitors retain myeloid potential. Nature. 2008;452:768–772. doi: 10.1038/nature06839. [DOI] [PubMed] [Google Scholar]
- 66.Heinzel K, Benz C, Martins VC, Haidl ID, Bleul CC. Bone marrow-derived hemopoietic precursors commit to the T cell lineage only after arrival in the thymic microenvironment. J Immunol. 2007;178:858–868. doi: 10.4049/jimmunol.178.2.858. [DOI] [PubMed] [Google Scholar]
- 67.Izon DJ, et al. Deltex1 redirects lymphoid progenitors to the B cell lineage by antagonizing Notch1. Immunity. 2002;16:231–243. doi: 10.1016/s1074-7613(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 68.Schmitt TM, Ciofani M, Petrie HT, Zuniga-Pflucker JC. Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J Exp Med. 2004;200:469–479. doi: 10.1084/jem.20040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hozumi K, et al. Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med. 2008;205:2507–2513. doi: 10.1084/jem.20080134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koch U, et al. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med. 2008;205:2515–2523. doi: 10.1084/jem.20080829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maillard I, et al. Canonical notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2:356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 73.Maillard I, et al. Notch-dependent T-lineage commitment occurs at extrathymic sites following bone marrow transplantation. Blood. 2006;107:3511–3519. doi: 10.1182/blood-2005-08-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milner LA, Kopan R, Martin DI, Bernstein ID. A human homologue of the Drosophila developmental gene, Notch, is expressed in CD34+ hematopoietic precursors. Blood. 1994;83:2057–2062. [PubMed] [Google Scholar]
- 75.Maeda T, et al. Regulation of B versus T lymphoid lineage fate decision by the proto-oncogene LRF. Science. 2007;316:860–866. doi: 10.1126/science.1140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dias S, Mansson R, Gurbuxani S, Sigvardsson M, Kee BL. E2A proteins promote development of lymphoid-primed multipotent progenitors. Immunity. 2008;29:217–227. doi: 10.1016/j.immuni.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schlenner SM, et al. Fate mapping reveals separate origins of T cells and myeloid lineages in the thymus. Immunity. 2010;32:426–436. doi: 10.1016/j.immuni.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 78.Albu DI, et al. BCL11B is required for positive selection and survival of double-positive thymocytes. J Exp Med. 2007;204:3003–3015. doi: 10.1084/jem.20070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tydell CC, David-Fung ES, Moore JE, Rowen L, Taghon T, Rothenberg EV. Molecular dissection of prethymic progenitor entry into the T lymphocyte developmental pathway. J Immunol. 2007;179:421–438. doi: 10.4049/jimmunol.179.1.421. [DOI] [PubMed] [Google Scholar]
- 80.Ikawa T, et al. An essential developmental checkpoint for production of the T cell lineage. Science. 2010;329:93–96. doi: 10.1126/science.1188995. [DOI] [PubMed] [Google Scholar]
- 81.Li L, Leid M, Rothenberg EV. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science. 2010;329:89–93. doi: 10.1126/science.1188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li P, et al. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science. 2010;329:85–89. doi: 10.1126/science.1188063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mikkola I, Heavey B, Horcher M, Busslinger M. Reversion of B cell commitment upon loss of Pax5 expression. Science. 2002;297:110–113. doi: 10.1126/science.1067518. [DOI] [PubMed] [Google Scholar]
- 84.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 85.Arinobu Y, et al. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1:416–427. doi: 10.1016/j.stem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 86.Franco CB, et al. Notch/Delta signaling constrains reengineering of pro-T cells by PU.1. Proc Natl Acad Sci U S A. 2006;103:11993–11998. doi: 10.1073/pnas.0601188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–744. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Barndt RJ, Dai M, Zhuang Y. Functions of E2A-HEB heterodimers in T-cell development revealed by a dominant negative mutation of HEB. Mol Cell Biol. 2000;20:6677–6685. doi: 10.1128/mcb.20.18.6677-6685.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ikawa T, Kawamoto H, Goldrath AW, Murre C. E proteins and Notch signaling cooperate to promote T cell lineage specification and commitment. J Exp Med. 2006;203:1329–1342. doi: 10.1084/jem.20060268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yashiro-Ohtani Y, et al. Pre-TCR signaling inactivates Notch1 transcription by antagonizing E2A. Genes Dev. 2009;23:1665–1676. doi: 10.1101/gad.1793709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Semerad CL, Mercer EM, Inlay MA, Weissman IL, Murre C. E2A proteins maintain the hematopoietic stem cell pool and promote the maturation of myelolymphoid and myeloerythroid progenitors. Proc Natl Acad Sci U S A. 2009;106:1930–1935. doi: 10.1073/pnas.0808866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Q, et al. E47 controls the developmental integrity and cell cycle quiescence of multipotential hematopoietic progenitors. J Immunol. 2008;181:5885–5894. doi: 10.4049/jimmunol.181.9.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo Y, Maillard I, Chakraborti S, Rothenberg EV, Speck NA. Core binding factors are necessary for natural killer cell development and cooperate with Notch signaling during T-cell specification. Blood. 2008;112:480–492. doi: 10.1182/blood-2007-10-120261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Talebian L, et al. T-lymphoid, megakaryocyte, and granulocyte development are sensitive to decreases in CBFbeta dosage. Blood. 2007;109:11–21. doi: 10.1182/blood-2006-05-021188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kawamata S, Du C, Li K, Lavau C. Overexpression of the Notch target genes Hes in vivo induces lymphoid and myeloid alterations. Oncogene. 2002;21:3855–3863. doi: 10.1038/sj.onc.1205487. [DOI] [PubMed] [Google Scholar]
- 96.Tomita K, Hattori M, Nakamura E, Nakanishi S, Minato N, Kageyama R. The bHLH gene Hes1 is essential for expansion of early T cell precursors. Genes Dev. 1999;13:1203–1210. doi: 10.1101/gad.13.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Varnum-Finney B, Dallas MH, Kato K, Bernstein ID. Notch target Hes5 ensures appropriate Notch induced T- versus B-cell choices in the thymus. Blood. 2008;111:2615–2620. doi: 10.1182/blood-2007-03-079855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoebeke I, et al. Overexpression of HES-1 is not sufficient to impose T-cell differentiation on human hematopoietic stem cells. Blood. 2006;107:2879–2881. doi: 10.1182/blood-2005-05-1815. [DOI] [PubMed] [Google Scholar]
- 99.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hattori N, Kawamoto H, Fujimoto S, Kuno K, Katsura Y. Involvement of transcription factors TCF-1 and GATA3 in the initiation of the earliest step of T cell development in the thymus. J Exp Med. 1996;184:1137–1147. doi: 10.1084/jem.184.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hosoya T, et al. GATA3 is required for early T lineage progenitor development. J Exp Med. 2009;206:2987–3000. doi: 10.1084/jem.20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Emambokus N, Vegiopoulos A, Harman B, Jenkinson E, Anderson G, Frampton J. Progression through key stages of haemopoiesis is dependent on distinct threshold levels of c- Myb. EMBO J. 2003;22:4478–4488. doi: 10.1093/emboj/cdg434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Georgopoulos K, et al. The Ikaros gene is required for the development of all lymphoid lineages. Cell. 1994;79:143–156. doi: 10.1016/0092-8674(94)90407-3. [DOI] [PubMed] [Google Scholar]
- 104.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC. Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol. 1998;161:3984–3991. [PubMed] [Google Scholar]
- 105.Uhmann A, et al. The Hedgehog receptor Patched controls lymphoid lineage commitment. Blood. 2007;110:1814–1823. doi: 10.1182/blood-2007-02-075648. [DOI] [PubMed] [Google Scholar]
- 106.Hager-Theodorides AL, et al. Bone morphogenetic protein 2/4 signaling regulates early thymocyte differentiation. J Immunol. 2002;169:5496–5504. doi: 10.4049/jimmunol.169.10.5496. [DOI] [PubMed] [Google Scholar]
- 107.Tsai PT, Lee RA, Wu H. BMP4 acts upstream of FGF in modulating thymic stroma and regulating thymopoiesis. Blood. 2003;102:3947–3953. doi: 10.1182/blood-2003-05-1657. [DOI] [PubMed] [Google Scholar]
- 108.Mulroy T, McMahon JA, Burakoff SJ, McMahon AP, Sen J. Wnt-1 and Wnt-4 regulate thymic cellularity. Eur J Immunol. 2002;32:967–971. doi: 10.1002/1521-4141(200204)32:4<967::AID-IMMU967>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 109.Louis I, Heinonen KM, Chagraoui J, Vainio S, Sauvageau G, Perreault C. The signaling protein Wnt4 enhances thymopoiesis and expands multipotent hematopoietic progenitors through beta-catenin-independent signaling. Immunity. 2008;29:57–67. doi: 10.1016/j.immuni.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 110.Behrens J, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 111.Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of beta-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Staal FJ, et al. Wnt signaling is required for thymocyte development and activates Tcf-1 mediated transcription. Eur J Immunol. 2001;31:285–293. doi: 10.1002/1521-4141(200101)31:1<285::AID-IMMU285>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 113.Xu Y, Banerjee D, Huelsken J, Birchmeier W, Sen JM. Deletion of beta-catenin impairs T cell development. Nat Immunol. 2003;4:1177–1182. doi: 10.1038/ni1008. [DOI] [PubMed] [Google Scholar]
- 114.Verbeek S, et al. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- 115.Goux D, et al. Cooperating pre-T-cell receptor and TCF-1-dependent signals ensure thymocyte survival. Blood. 2005;106:1726–1733. doi: 10.1182/blood-2005-01-0337. [DOI] [PubMed] [Google Scholar]
- 116.Koch U, Wilson A, Cobas M, Kemler R, Macdonald HR, Radtke F. Simultaneous loss of beta- and gamma-catenin does not perturb hematopoiesis or lymphopoiesis. Blood. 2008;111:160–164. doi: 10.1182/blood-2007-07-099754. [DOI] [PubMed] [Google Scholar]
- 117.Sitnicka E, et al. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–2964. doi: 10.1182/blood-2006-10-054726. [DOI] [PubMed] [Google Scholar]
- 118.Sitnicka E, Bryder D, Theilgaard-Monch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 119.Ye SK, et al. The IL-7 receptor controls the accessibility of the TCRgamma locus by Stat5 and histone acetylation. Immunity. 2001;15:813–823. doi: 10.1016/s1074-7613(01)00230-8. [DOI] [PubMed] [Google Scholar]
- 120.Waskow C, Paul S, Haller C, Gassmann M, Rodewald HR. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity. 2002;17:277–288. doi: 10.1016/s1074-7613(02)00386-2. [DOI] [PubMed] [Google Scholar]
- 121.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 122.Thoren LA, et al. Kit regulates maintenance of quiescent hematopoietic stem cells. J Immunol. 2008;180:2045–2053. doi: 10.4049/jimmunol.180.4.2045. [DOI] [PubMed] [Google Scholar]
- 123.Gautreau L, et al. Identification of an IL-7-dependent pre-T committed population in the spleen. J Immunol. 2007;179:2925–2935. doi: 10.4049/jimmunol.179.5.2925. [DOI] [PubMed] [Google Scholar]
- 124.Arcangeli ML, et al. Extrathymic hemopoietic progenitors committed to T cell differentiation in the adult mouse. J Immunol. 2005;174:1980–1988. doi: 10.4049/jimmunol.174.4.1980. [DOI] [PubMed] [Google Scholar]
- 125.Dejbakhsh-Jones S, Garcia-Ojeda ME, Chatterjea-Matthes D, Zeng D, Strober S. Clonable progenitors committed to the T lymphocyte lineage in the mouse bone marrow; use of an extrathymic pathway. Proc Natl Acad Sci U S A. 2001;98:7455–7460. doi: 10.1073/pnas.131559798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guy-Grand D, et al. Extrathymic T cell lymphopoiesis: ontogeny and contribution to gut intraepithelial lymphocytes in athymic and euthymic mice. J Exp Med. 2003;197:333–341. doi: 10.1084/jem.20021639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Besseyrias V, et al. Hierarchy of Notch-Delta interactions promoting T cell lineage commitment and maturation. J Exp Med. 2007;204:331–343. doi: 10.1084/jem.20061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chung B, Barbara-Burnham L, Barsky L, Weinberg K. Radiosensitivity of thymic interleukin-7 production and thymopoiesis after bone marrow transplantation. Blood. 2001;98:1601–1606. doi: 10.1182/blood.v98.5.1601. [DOI] [PubMed] [Google Scholar]
- 129.Kenins L, Gill JW, Boyd RL, Hollander GA, Wodnar-Filipowicz A. Intrathymic expression of Flt3 ligand enhances thymic recovery after irradiation. J Exp Med. 2008;205:523–531. doi: 10.1084/jem.20072065. [DOI] [PMC free article] [PubMed] [Google Scholar]