β-Adrenergic–regulated phosphorylation of the skeletal muscle CaV1.1 channel in the fight-or-flight response (original) (raw)
Abstract
CaV1 channels initiate excitation–contraction coupling in skeletal and cardiac muscle. During the fight-or-flight response, epinephrine released by the adrenal medulla and norepinephrine released from sympathetic nerves increase muscle contractility by activation of the β-adrenergic receptor/cAMP-dependent protein kinase pathway and up-regulation of CaV1 channels in skeletal and cardiac muscle. Although the physiological mechanism of this pathway is well defined, the molecular mechanism and the sites of protein phosphorylation required for CaV1 channel regulation are unknown. To identify the regulatory sites of phosphorylation under physiologically relevant conditions, CaV1.1 channels were purified from skeletal muscle and sites of phosphorylation on the α1 subunit were identified by mass spectrometry. Two phosphorylation sites were identified in the proximal C-terminal domain, serine 1575 (S1575) and threonine 1579 (T1579), which are conserved in cardiac CaV1.2 channels (S1700 and T1704, respectively). In vitro phosphorylation revealed that CaV1.1-S1575 is a substrate for both cAMP-dependent protein kinase and calcium/calmodulin-dependent protein kinase II, whereas CaV1.1-T1579 is a substrate for casein kinase 2. Treatment of rabbits with isoproterenol to activate β-adrenergic receptors increased phosphorylation of S1575 in skeletal muscle CaV1.1 channels in vivo, and treatment with propranolol to inhibit β-adrenergic receptors reduced phosphorylation. As S1575 and T1579 in CaV1.1 channels and their homologs in CaV1.2 channels are located at a key regulatory interface between the distal and proximal C-terminal domains, it is likely that phosphorylation of these sites in skeletal and cardiac muscle is directly involved in calcium channel regulation in response to the sympathetic nervous system in the fight-or-flight response.
Keywords: adrenalin, calcium channels, cyclic AMP, mass spectometry, protein kinase A
The voltage-gated calcium channels CaV1.1 and CaV1.2 initiate excitation–contraction coupling in skeletal and cardiac muscle, respectively (1–4), and up-regulation of L-type calcium currents through these channels by epinephrine and norepinephrine via activation of β-adrenergic receptors, G proteins, adenylyl cyclase, and cAMP-dependent protein kinase (PKA) phosphorylation increases contractility in the fight-or-flight response (1, 5–8). In skeletal muscle, CaV1.1 channels initiate excitation–contraction coupling by direct protein–protein interactions with the ryanodine-sensitive calcium release channels in the sarcoplasmic reticulum (3). CaV1.1 channels also conduct slowly activated, sustained calcium currents (9), which do not participate directly in initiation of excitation–contraction coupling in single muscle twitches (10). However, tetanic stimulation of skeletal muscle is required for development of substantial contractile force (11), and slow calcium currents through CaV1.1 channels are necessary for the increase in contractile force with increasing frequency of stimulation of skeletal muscle fibers (12, 13). Calcium currents conducted by CaV1.1 channels are up-regulated by β-adrenergic regulation and PKA phosphorylation, resulting in increased contractile force (14–16). Remarkably, despite decades of work, the molecular mechanism of calcium channel up-regulation by this crucial physiological pathway and the sites of phosphorylation that regulate CaV1.1 and CaV1.2 channels by cAMP-dependent protein kinase remain undefined.
The intracellular C terminus of CaV1 channels is an important region for regulation of channel activity. Skeletal muscle CaV1.1 channels and cardiac CaV1.2 channels are both posttranslationally processed in vivo by proteolysis near the center of the 600-amino acid C-terminal domain (17–23), but proteolytic processing is not observed in transfected nonmuscle cells (24). The C terminus is a substrate for PKA, and the sites of most rapid phosphorylation of purified CaV1.1 and CaV1.2 in vitro are located in the distal C-terminal domain (19, 25, 26). Despite proteolytic processing, the distal C terminus (dCT) beyond the point of proteolytic truncation is required for interaction with A Kinase Anchoring Protein 15 (AKAP15) (27–29), which in turn is required for β-adrenergic regulation of CaV1.1 and CaV1.2 channels in intact skeletal and cardiac myocytes (28–31). Coexpression of the dCT as a separate protein by transfection in nonmuscle cells leads to binding to the proximal C-terminal domain of truncated CaV1.1 and CaV1.2 channels (23, 24) and to dramatic autoinhibition of CaV1.2 channel activity (24). We have previously proposed that the autoinhibitory complex of the CaV1.2 channel with its proteolytically processed dCT noncovalently bound is the natural substrate for regulation by the β-adrenergic pathway, which relieves the autoinhibition by the dCT (24). Unfortunately, the only confirmed site of cAMP-dependent phosphorylation of the CaV1.2 channel [S1928 in the dCT (19, 32)] is not conserved in CaV1.1 and is not required for regulation of CaV1.2 channels in vivo (33, 34). Therefore, we set out to identify additional sites of cAMP-dependent phosphorylation that may be responsible for regulation of CaV1.1 and CaV1.2 channels by the β-adrenergic pathway.
Research on the mechanisms of regulation of the cardiac and skeletal calcium channels has been linked by their similarities of structure and regulation. Biochemical studies are most easily performed on CaV1.1 channels, which can be isolated in pure form but are not easily studied by in vitro functional expression. On the other hand, CaV1.2 channel activity is easily studied by in vitro expression, but CaV1.2 cannot be isolated in high yield and purity for biochemical studies. In the experiments reported here, we have exhaustively mapped the sites of phosphorylation on the C terminus of purified CaV1.1 channel using mass spectrometry (MS) to identify sites that may be required for regulation by the β-adrenergic signaling pathway in vivo. We identified two phosphorylation sites within the proximal C terminus of the pore-forming α1 subunit and demonstrated β-adrenergic regulation of phosphorylation of S1575 in rabbit skeletal muscle in vivo. This site and an adjacent casein kinase II phosphorylation site at T1579 lie at the interface for noncovalent interaction between the dCT and the proximal C terminus in a position where site-directed mutations disrupt the regulatory interactions between these domains (24). The rapid phosphorylation of S1575 in intact skeletal muscle, the conservation of both of these sites in cardiac CaV1.2 channels, and their position at a key regulatory interface in both CaV1.1 and CaV1.2 channels make phosphorylation of S1575 and T1579 in CaV1.1 channels, or S1700 and T1704 in CaV1.2 channels, prime candidates for β-adrenergic-mediated regulation of skeletal muscle and cardiac calcium channels.
Results
Identification of Endogenous Phosphorylation Sites on the C Terminus of CaV1.1 Channels.
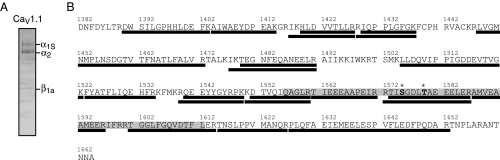
To identify potential sites of regulation of CaV1.1 channels under physiologically relevant conditions, we developed methods to examine the phosphorylation state of CaV1.1 purified from rabbit skeletal muscle tissue using MS. Established procedures were used to purify CaV1.1 from rabbit skeletal muscle tissue (35), and the calcium channel subunits were separated by SDS/PAGE, visualized with Coomassie blue staining (Fig. 1_A_), excised from the gel, and subjected to in-gel digestion with trypsin. Tryptic peptides were analyzed by electrospray ionization-liquid chromatography-MS/MS on an LCQ Classic (Thermo Electron) ion trap mass spectrometer. All observable tryptic peptides predicted for the proximal C terminus were detected, giving 80% sequence coverage (Fig. 1_B_). This enabled detection of 25 of 31 potentially phosphorylated serine, threonine, and tyrosine residues present in the proximal C-terminal domain.
Fig. 1.
LC-MS/MS sequence coverage and identified phosphorylation sites in the proximal C terminus of CaV1.1. (A) CaV1.1 subunits were purified from rabbit skeletal muscle, separated by SDS/PAGE, and visualized with Coomassie staining. Identity of indicated subunits was confirmed by tryptic digest and LC-MS/MS analysis. (B) Observed tryptic peptides within the proximal C terminus of the CaV1.1 α1S subunit are indicated by black bars (amino acid sequence coverage, 79.8%). Identified endogenous phosphorylation sites are indicated by an asterisk (*). PCRD is shaded in gray.
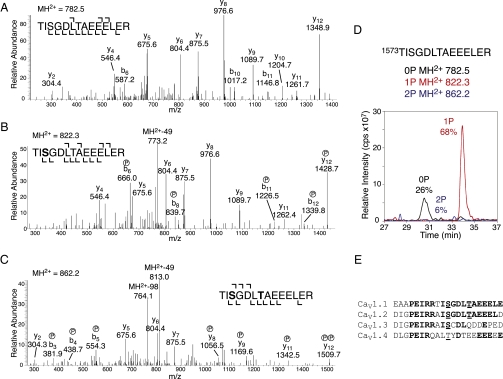
Two previously uncharacterized phosphorylation sites were identified on the C terminus of CaV1.1: S1575 and T1579 (Fig. 2). These phosphorylation sites reside in the proximal C-terminal regulatory domain (PCRD), which is responsible for interaction with and regulation by the proteolytically processed dCT of CaV1.1 (23). Both phosphorylation sites are present on a single tryptic peptide (Fig. 2_A_). The MS/MS spectra reveal the presence of monophosphorylated pS1575 peptide (Fig. 2_B_) and diphosphorylated pS1575+pT1579 peptide (Fig. 2_C_). The monophosphorylated pT1579 peptide was not observed. The relative abundance of the phosphorylated forms was examined by monitoring the total ion current of extracted ions corresponding to the tryptic peptide 1573TISGDLTAEEELER. Approximately 26% of the total peptide ions were unphosphorylated, 68% were monophosphorylated at S1575, and 6% were diphosphorylated (pS1575+pT1579) (Fig. 2_D_). Thus, S1575 was the primary site of phosphorylation of the proximal C-terminal domain of CaV1.1 channels in skeletal muscle.
Fig. 2.
LC-MS/MS confirms phosphorylation of CaV1.1 at S1575 and T1579, and sequence alignment reveals that sites are conserved in CaV1.2. (A) MS/MS spectra of the unphosphorylated MH2+ peptide ion (782.5 Da). (B) MS/MS spectra of monophosphorylated MH2+ peptide ion (822.3 Da). (C) MS/MS spectra of diphosphorylated MH2+ peptide ion (862.2 Da) corresponding to tryptic peptide 1573TISGDLTAEEELER. Fragment ions (y8 and y12) confirm phosphorylation at S1575 and T1579. For clarity, only a portion of each spectrum is shown. (D) LC/MS analysis shows MH2+ extract ions corresponding to unphosphorylated (0P), monophosphorylated (1P, +80 Da), and diphosphorylated (2P,+160 Da) forms of the indicated peptide. (E) Sequence alignment of CaV1 family members; rabbit CaV1.1 is aligned with mouse CaV1.2, CaV1.3, and CaV1.4. Boldface type indicates identical residues; and underlining indicates conserved phosphorylation sites.
If phosphorylation of S1575 or T1579 is important in β-adrenergic regulation of calcium channels, we would expect conservation of these amino acid residues in CaV1.1 and CaV1.2 channels, but not necessarily in other CaV1 family calcium channels that have different modes of regulation. S1575 and T1579 are indeed conserved in CaV1.1 and CaV1.2 channels, and the amino acid sequence surrounding them has only a single substitution in 18 residues (Fig. 2_E_). S1575 is also conserved in CaV1.3 channels, but T1579 and the surrounding amino acid sequence are not highly conserved (Fig. 2E). Perfusion of PKA into transfected cells expressing CaV1.3 channels gives a small (≤30%) increase in calcium current, which is partially dependent on phosphorylation of S1743, the homolog of S1575 in CaV1.1 channels (36). However, this increase is far smaller than the three- to fourfold increase in calcium currents typically recorded in skeletal or cardiac myocytes stimulated with β-adrenergic agonists. S1575 is replaced by a Thr residue in CaV1.4 channels, which are specifically expressed in the rod and cone photoreceptors in the retina; but the changes in amino acid sequence context make it less likely that this residue would be phosphorylated by PKA (Fig. 2_E_). Rods and most types of cones respond to activation of PKA with a decrease in calcium current, but a small (≤20%) increase in calcium current was observed upon activation of PKA in red-sensitive single large cones from salamander retina (37). The weaker PKA regulation of calcium currents in cells expressing CaV1.3 and CaV1.4 channels, compared with the robust regulation in skeletal and cardiac myocytes, is consistent with the differences in amino acid sequences at these phosphorylation sites and suggests that phosphorylation of amino acid residues homologous to S1575 may contribute to the low level of regulation of calcium channels by PKA in cells expressing these channel subtypes.
Identification of Protein Kinases Responsible for Phosphorylation of S1575 and T1579.
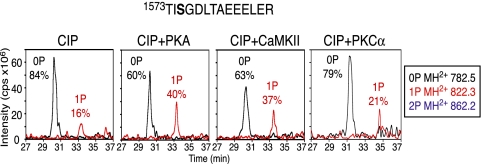
Programs for prediction of sites of protein phosphorylation were used to deduce which enzymes might be responsible for phosphorylation of the identified endogenous phosphorylation sites on CaV1.1 channels. The basic amino acids at positions -3 and -4 from S1575 suggested that this site could be phosphorylated by PKA (consensus RRXS) and/or CaMKII (consensus RRXXS). To determine whether S1575 is a substrate for these predicted kinases, in vitro phosphorylation assays were performed. Purified CaV1.1 was dephosphorylated with calf intestinal alkaline phosphatase (CIP), and CIP was then inhibited by addition of sodium orthovanadate. This dephosphorylated preparation of CaV1.1 was incubated with the protein kinase of interest in the presence of Mg•ATP. Phosphate incorporation was assessed by LC-MS and MS/MS as described for Fig. 2. Fig. 3 shows that the majority of phosphate was removed from the S1575-containing peptide after CIP treatment, with only 16% of the pS1575 remaining. PKA treatment resulted in an increase in phosphorylation of the S1575-containing peptide by 24%, and CaMKII treatment resulted in a 20% increase. The site of phosphorylation at S1575 was reconfirmed by MS/MS sequencing for both PKA and CaMKII as in Fig. 2, indicating that both kinases are capable of phosphorylating CaV1.1-S1575 in vitro. Treatment with PKCα, another basic residue-directed kinase (consensus RRXXS(X)0–2(R/K)1–3), did not result in substantial phosphorylation of S1575 in vitro (Fig. 3). These results indicate that PKA and CaMKII selectively phosphorylate S1575 in the context of the intact CaV1.1 channel in vitro.
Fig. 3.
Identification of protein kinases responsible for phosphorylation of CaV1.1-S1575. LC-MS analysis shows MH2+ extract ions corresponding to unphosphorylated (0P), monophosphorylated (1P), and diphosphorylated (2P) forms of the indicated peptide. CaV1.1 purified from rabbit skeletal muscle was pretreated with CIP for 2 h at 30 °C, quenched with sodium orthovanadate, and treated with PKA, CaMKII, or PKCα, as indicated, in the presence of Mg•ATP for 2.5 h at 30 °C. Sites of phosphorylation were confirmed by MS/MS sequencing in all cases.
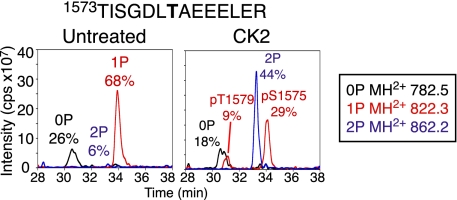
The acidic amino acid residues at positions +3 and +4 from T1579 suggested this site could be a substrate for Casein Kinase 2 (CK2, consensus TXXEE). To test this prediction, in vitro phosphorylation assays were performed. Treatment with CK2 and Mg•ATP resulted in a 47% increase in phosphorylation at T1579 (Fig. 4), and this site of phosphorylation was reconfirmed by MS/MS sequencing as in Fig. 2. Therefore, T1579 is a substrate for CK2 in the context of the intact CaV1.1 channel in vitro.
Fig. 4.
Identification of protein kinase responsible for phosphorylation of CaV1.1-T1579. An experiment similar to that described in Fig. 3 was carried out with CK2. LC-MS analysis shows MH2+ extract ions corresponding to unphosphorylated (0P), monophosphorylated (1P), and diphosphorylated (2P) forms of the indicated peptide. CaV1.1 purified from rabbit skeletal muscle was treated with CK2 in the presence of Mg•ATP for 2.5 h at 30 °C. Sites of phosphorylation were confirmed by MS/MS sequencing.
β-Adrenergic Stimulation of CaV1.1-S1575 Phosphorylation in Vivo.
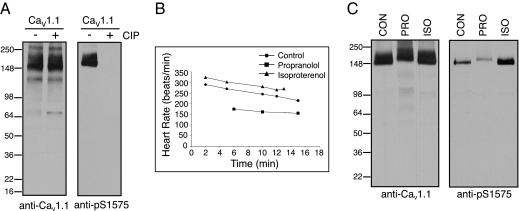
Because S1575 is a substrate for PKA, we examined whether phosphorylation at this site is regulated in vivo during β-adrenergic stimulation. To do this, we developed a phosphospecific antipeptide antibody directed against this site. Immunoblots of purified CaV1.1 channels revealed strong labeling of the native protein (Fig. 5_A_), as expected from the high level of phosphorylation of S1575 in vivo (Fig. 2). In contrast, after dephosphorylation with CIP, immunolabeling with an antibody against CaV1.1 was retained, whereas labeling with the phosphospecific antibody against pS1575 was completely lost (Fig. 5_A_). These results establish that this phosphospecific antibody is specific for pS1575.
Fig. 5.
Regulation of phosphorylation of CaV1.1-S1575 in vivo in response to β-adrenergic stimulation. (A) CaV1.1 purified from rabbit skeletal muscle was treated with CIP for 2 h at 30 °C where indicated, separated by SDS/PAGE, and immunoblots were probed with either anti-CaV1.1 or the phosphospecific anti-pS1575 antibody. (B) Rabbits were injected with control saline, 1 mg/kg isoproterenol, or 4 mg/kg propranolol, and heart rate was monitored postinjection. (C) Protein from T-tubule membranes prepared from rabbits treated with saline (CON), the β-adrenergic blocker propranolol (PRO), or the β-adrenergic agonist isoproterenol (ISO) were solubilized, separated by SDS/PAGE, and examined by immunoblot as in A.
To study the effects of β-adrenergic stimulation, New Zealand White rabbits were treated with the β-adrenergic receptor agonist isoproterenol, the β-adrenergic receptor antagonist propranolol, or saline as a control. Heart rate was monitored to confirm the effectiveness of drug treatment. Because handling of the rabbits causes excitement and anxiety, the basal heart rate of our experimental animals was typically above normal. For example, in the group of three rabbits illustrated in Fig. 5_B_, the beating rate at 5 min after treatment was 252 beats/min under control conditions. Isoproterenol increased heart rate to 305 beats/min, and propranolol reduced heart rate to 170 beats/min. Following these treatments, the animals were killed, and skeletal muscle was harvested and rapidly frozen in liquid nitrogen. T-tubule membranes were prepared, and proteins were solubilized in 1% digitonin and separated by SDS/PAGE. Immunoblot analysis showed that comparable amounts of CaV1.1 channel protein were recovered following all three treatments (Fig. 5_C_). In contrast, the level of phosphorylation of S1575 was substantially increased by treatment with isoproterenol and substantially reduced by treatment with propranolol (Fig. 5_C_). These results demonstrate that the level of phosphorylation of S1575 in vivo is high during β-adrenergic stimulation and low when β-adrenergic stimulation is blocked. These results are consistent with an important role for phosphorylation of this site in β-adrenergic stimulation of CaV1 channel activity in the fight-or-flight response.
Discussion
S1575 and T1579 Are Phosphorylated in CaV1.1 Channels in Vivo.
Numerous protein kinases have been shown to phosphorylate CaV1.1 channels in this laboratory and others by incubating the purified protein in vitro with protein kinases and [32P]ATP and measuring incorporation of 32P into the α1 subunits (19, 25, 26, 38–42). By this approach, phosphorylation by PKA, PKC, PKG, and CaMKII has been measured, and specific sites of phosphorylation have been identified for PKA and other kinases. Unfortunately, none of these sites has been convincingly shown to be required for regulation of CaV1.1 channels by phosphorylation in vitro or in vivo. As an alternative approach, we have identified sites that are phosphorylated under physiologically relevant conditions in vivo by isolation of the CaV1.1 channel protein from skeletal muscle and identification of sites of endogenous phosphorylation using MS. Because regulation of skeletal muscle contraction is continuous in an actively moving animal, dynamic phosphorylation and dephosphorylation of regulatory sites on CaV1.1 channels would be expected to result in measurable steady-state phosphorylation of key amino acid residues. Isolation of the CaV1.1 channel under conditions that prevent dephosphorylation would therefore reveal sites of regulatory phosphorylation. Identification of S1575 and T1579 as sites of phosphorylation in vivo by MS of purified CaV1.1 channels marks these sites as important candidates for regulation of CaV1.1 channel activity in vivo.
CaV1.1-S1575 Is Selectively Phosphorylated by PKA and CaMKII.
Our results demonstrate that S1575 can be phosphorylated by PKA and CaMKII in vitro. The activity of CaV1.1 channels is greatly increased by PKA phosphorylation in skeletal muscle cells, dissociated skeletal muscle fibers, and reconstituted phospholipid vesicles and bilayers (14–16, 43–45), and the activity of CaV1.2 channels is increased by both PKA and CaMKII (2, 5, 6). Phosphorylation of CaV1.1-S1575 and CaV1.2-S1700, the corresponding site in CaV1.2 channels, could contribute to both basal and β-adrenergic–stimulated calcium channel regulation by these protein kinases. However, recent studies have suggested a dominant role for phosphorylation of two other sites in the proximal C-terminal domain of CaV1.2 channels by CaMKII in voltage-dependent facilitation of cardiac calcium currents in transfected cells and in murine heart in vivo (46, 47). In light of those results, phosphorylation of CaV1.2-S1700 may be primarily involved in PKA regulation in response to activation of the sympathetic nervous system and β-adrenergic signaling.
CaV1.1-T1579 Is Selectively Phosphorylated by CK2.
Our results also show that T1579 is phosphorylated in rabbit skeletal muscle and is phosphorylated by CK2 in vitro in purified CaV1.1 channels. CK2 has not previously been implicated in regulation of CaV1.1 or CaV1.2 channels in physiological studies, but its phosphorylation of CaV1.1 channels has previously been observed in biochemical studies of purified CaV1.1 channels (42). Our results showing that T1579 is phosphorylated on CaV1.1 channels in vivo in rabbit skeletal muscle suggest a potential role in calcium channel regulation because this site is located at the interface between the PCRD and DCRD, well-positioned to modulate the autoinhibition of CaV1 channel activity by the distal C-terminal domain (24). CK2 is thought to phosphorylate its target sites constitutively, often through direct binding to its substrates, and control of phosphorylation of those sites is thought to be dependent on second messenger-regulated phosphoprotein phosphatases (48). Phosphorylation at this site may serve as a priming mechanism that controls the amplitude of the β-adrenergic response and its reversal by second messenger-activated phosphoprotein phosphatases.
β-Adrenergic Regulation Increases Phosphorylation of S1575 in CaV1.1 Channels in Vivo.
We obtained direct evidence for a possible role of phosphorylation of S1575 in β-adrenergic stimulation of CaV1.1 channels in vivo. Stimulation of β-adrenergic receptors in vivo with isoproterenol increased phosphorylation of S1575 in rabbit skeletal muscle, whereas inhibition with propranolol reduced phosphorylation, in parallel with regulation of heart rate as a monitor of the sympathetic response. Because poor expression of CaV1.1 channels in vitro has prevented reconstitution of regulation by the PKA pathway in nonmuscle cells, further analysis of regulation of CaV1.1 channels by phosphorylation of S1575 may require mutation of this amino acid residue in vivo and studies of muscle fibers isolated from genetically altered mice.
Important Roles for Phosphorylation of S1700 and T1704 in Regulation of Cardiac Calcium Channels.
In contrast to CaV1.1 channels, expression of CaV1.2 channels in vitro in nonmuscle cells is robust and can be used for functional analysis of mutant CaV1.2 channels. In recent studies, we have reconstituted PKA-dependent regulation of CaV1.2 channels in nonmuscle cells with a dynamic range of up to fourfold (49) under conditions that require formation of an autoinhibitory signaling complex containing the noncovalently associated distal C-terminal domain, AKAP15, and PKA, as in intact ventricular myocytes (24, 31). In this reconstituted system, we have found that phosphorylation of CaV1.2-S1700 and CaV1.2-T1704 are required for PKA-dependent regulation, with phosphorylation of S1700 and T1704 both contributing to basal regulation and phosphorylation of S1700 playing a dominant role in stimulation by the β-adrenergic/PKA signaling pathway (49). These results reveal a key role for phosphorylation of these amino acid residues in CaV1.2 channels in mediating the fight-or-flight response in cardiac myocytes. By analogy, it is likely that phosphorylation of S1575 and T1579 is required for regulation of CaV1.1 channels in skeletal muscle as well.
Materials and Methods
Antibodies and cDNA Constructs.
Anti-CaV1.1: rabbit polyclonal antibodies (anti-CP11) were generated against peptides corresponding to rabbit CaV1.1–1601-1618. Anti-CaV1.2-pS1700 phosphospecific antibody (which also recognizes CaV1.1-pS1575) was generated against residues 1694EIRRAIpSGDLTAEEEL in the proximal C terminus (Pacific Immunology).
Rabbit Drug Treatment, Protein Purification, and Sample Preparation.
All animal procedures were conducted in compliance with the recommendations of the Institutional Animal Care and Use Committee of the University of Washington. Rabbit skeletal muscle CaV1.1 channels were purified as previously described (35). Male New Zealand White rabbits (2.5 kg, Western Oregon Rabbit Co.) were drug treated with saline control, 1 mg/kg isoproterenol, or 4 mg/kg propranolol, and heart rate was monitored to ensure drug effectiveness. Animals were killed by lethal injection of pentobarbital; skeletal muscle was rapidly harvested, rinsed briefly in PBS (PBS, pH 7.4), snap frozen in liquid nitrogen, and stored at −80 °C. All purification steps were carried out at 4 °C in the presence of the following protease and phosphatase inhibitors: 1 μM aprotinin, 1 μM pepstatin, 10 μM leupeptin, 100 μM benzamidine, 1 mM phenanthroline, 10 μM E-64, 20 μg/μL soybean trypsin inhibitor, 0.5 mM NaVO4, 100 nM microcystin, and 10 nM cyclosporin A. CaV1.1 was purified using wheat germ agglutinin-linked agarose (Vector Labs) and anion exchange DEAE Sephadex A-25 (GE Healthcare), chromatography (35, 50). Pure protein was snap frozen in liquid nitrogen and stored at −80 °C in single-use aliquots.
For mass spectrometric analysis, 1–2.5 μg of pure protein was separated by SDS/PAGE, the band of interest was excised and subjected to in-gel trypsin (Gold, Promega) digestion by standard procedures (http://donatello.ucsf.edu/ingel.html). A 1- to 3-pmol quantity of tryptic peptides was analyzed by MS.
In Vitro Phosphorylation.
Pure CaV1.1 was first dephosphorylated with 5 U CIP (NEB) for 2 h at 30 °C in assay buffer (20 mM Tris, pH 7.5, 10 mM MgCl2, 2 mM DTT, 10 mM NaCl, and 200 mM ATP), and phosphatase was quenched with 10 mM NaVO4. Then CaV1.1 was phosphorylated with 100 U PKA from bovine heart (Sigma), 100 U CaMKII (NEB), or 25 U CK2 (NEB) for 2.5 h at 30 °C in assay buffer (20 mM Tris pH 7.5, 10 mM MgCl2, 2 mM DTT, 10 mM NaCl, and 200 mM ATP). Reactions were quenched, proteins were resolved by SDS/PAGE and subjected to in-gel digestion, and phosphate incorporation was assessed by LC-MS and LC-MS/MS.
MS.
MS was performed using an Agilent 1100 series HPLC (Agilent Technologies) coupled to an LCQ Classic ion trap mass spectrometer (ThermoElectron). Peptides were loaded onto a Paradigm Platinum Peptide Nanotrap (Microm) and then separated on a reverse-phase capillary column (10 cm × 75 μm, Jupiter Proteo C12, Phenomenex) with a linear gradient from 2% to 40% acetonitrile in 40 min. One full mass scan was acquired (300–2,000 Da), and then the four most intense peaks were selected for MS/MS analysis. The dynamic exclusion limit was set to exclude a given m/z after it had been sequenced twice during a 90-s interval. Mass spectra were analyzed using TurboSequest configured with the following parameters: a peptide mass tolerance of 2.5 Da (average), a fragment ion mass tolerance of 1.0 Da (average), differential modification on S/T/Y +80 Da, and allowance of two incomplete cleavages. All MS/MS peak assignments were manually validated. The relative abundance of the modified forms for each peptide was determined using the ICIS peak detection method (Xcalibur, Thermo Scientific) for the indicated extract ions. The percentages were calculated based on the sum of integrated peak areas for all detected modified forms of the peptide.
Acknowledgments
We thank the late Elizabeth M. Sharp for technical assistance and Dr. Joanne Hulme for cDNA constructs and valuable experimental advice. This work was funded by research grants from the Muscular Dystrophy Association (to W.A.C.), National Institutes of Health Research Grant R01 HL085372 (to W.A.C.), National Research Service Award T32 HL007312 (Dr. Stephen Schwartz, PI), and a pilot project grant from the National Heart, Lung and Blood Institute (NHLBI/N01-HV-29179; Dr. Ruedi Aebersold, PI) (to M.A.E).
Footnotes
The authors declare no conflict of interest.
References
- 1.Catterall WA. Excitation-contraction coupling in vertebrate skeletal muscle: A tale of two calcium channels. Cell. 1991;64:871–874. doi: 10.1016/0092-8674(91)90309-m. [DOI] [PubMed] [Google Scholar]
- 2.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 3.Tanabe T, Beam KG, Powell JA, Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988;336:134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- 4.Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- 5.Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- 6.Tsien RW, et al. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol. 1986;18:691–710. doi: 10.1016/s0022-2828(86)80941-5. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann F, Lacinová L, Klugbauer N. Voltage-dependent calcium channels: From structure to function. Rev Physiol Biochem Pharmacol. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 8.Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez JA, Stefani E. Inward calcium current in twitch muscle fibres of the frog. J Physiol. 1978;283:197–209. doi: 10.1113/jphysiol.1978.sp012496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong CM, Bezanilla FM, Horowicz P. Twitches in the presence of ethylene glycol bis(-aminoethyl ether)-N,N′-tetracetic acid. Biochim Biophys Acta. 1972;267:605–608. doi: 10.1016/0005-2728(72)90194-6. [DOI] [PubMed] [Google Scholar]
- 11.Kernell D, Eerbeek O, Verhey BA. Relation between isometric force and stimulus rate in cat's hindlimb motor units of different twitch contraction time. Exp Brain Res. 1983;50:220–227. doi: 10.1007/BF00239186. [DOI] [PubMed] [Google Scholar]
- 12.Oz M, Frank GB. Decrease in the size of tetanic responses produced by nitrendipine or by extracellular calcium ion removal without blocking twitches or action potentials in skeletal muscle. J Pharmacol Exp Ther. 1991;257:575–581. [PubMed] [Google Scholar]
- 13.Kotsias BA, Muchnik S, Obejero Paz CA. Co2+, low Ca2+, and verapamil reduce mechanical activity in rat skeletal muscles. Am J Physiol. 1986;250:C40–C46. doi: 10.1152/ajpcell.1986.250.1.C40. [DOI] [PubMed] [Google Scholar]
- 14.Arreola J, Calvo J, García MC, Sánchez JA. Modulation of calcium channels of twitch skeletal muscle fibres of the frog by adrenaline and cyclic adenosine monophosphate. J Physiol. 1987;393:307–330. doi: 10.1113/jphysiol.1987.sp016825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993b;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 16.Schmid A, Renaud JF, Lazdunski M. Short term and long term effects of beta-adrenergic effectors and cyclic AMP on nitrendipine-sensitive voltage-dependent Ca2+ channels of skeletal muscle. J Biol Chem. 1985;260:13041–13046. [PubMed] [Google Scholar]
- 17.De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: A 212-kDa form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci USA. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Jongh KS, Warner C, Colvin AA, Catterall WA. Characterization of the two size forms of the α 1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci USA. 1991;88:10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Jongh KS, et al. Specific phosphorylation of a site in the full-length form of the alpha-1 subunit of the cardiac L-type calcium channel by cAMP-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- 20.Lai Y, Seagar MJ, Takahashi M, Catterall WA. Cyclic AMP-dependent phosphorylation of two size forms of α1 subunits of L-type calcium channels in rat skeletal muscle cells. J Biol Chem. 1990;265:20839–20848. [PubMed] [Google Scholar]
- 21.Brawley RM, Hosey MM. Identification of two distinct proteins that are immunologically related to the α1 subunit of the skeletal muscle dihydropyridine-sensitive calcium channel. J Biol Chem. 1992;267:18218–18223. [PubMed] [Google Scholar]
- 22.Gao TY, et al. Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J Biol Chem. 1997b;272:19401–19407. doi: 10.1074/jbc.272.31.19401. [DOI] [PubMed] [Google Scholar]
- 23.Hulme JT, et al. Sites of proteolytic processing and noncovalent association of the distal C-terminal domain of CaV1.1 channels in skeletal muscle. Proc Natl Acad Sci USA. 2005;102:5274–5279. doi: 10.1073/pnas.0409885102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulme JT, Yarov-Yarovoy V, Lin TW-C, Scheuer T, Catterall WA. Autoinhibitory control of the Cav1.2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rotman EI, De Jongh KS, Florio V, Lai Y, Catterall WA. Specific phosphorylation of a COOH-terminal site on the full-length form of the α1 subunit of the skeletal muscle calcium channel by cAMP-dependent protein kinase. J Biol Chem. 1992;267:16100–16105. [PubMed] [Google Scholar]
- 26.Rotman EI, Murphy BJ, Catterall WA. Sites of selective cAMP-dependent phosphorylation of the L-type calcium channel α1 subunit from intact rabbit skeletal muscle myotubes. J Biol Chem. 1995;270:16371–16377. doi: 10.1074/jbc.270.27.16371. [DOI] [PubMed] [Google Scholar]
- 27.Gray PC, Tibbs VC, Catterall WA, Murphy BJ. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem. 1997;272:6297–6302. doi: 10.1074/jbc.272.10.6297. [DOI] [PubMed] [Google Scholar]
- 28.Gray PC, et al. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998a;20:1017–1026. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 29.Fraser IDC, et al. A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J Biol Chem. 2002;277:4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 31.Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. Beta-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci USA. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci USA. 2006;103:16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganesan AN, Maack C, Johns DC, Sidor A, O'Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circ Res. 2006;98:e11–e18. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemke T, et al. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Cav1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–34744. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Florio V, Striessnig J, Catterall WA. Purification and reconstitution of skeletal muscle calcium channels. Methods Enzymol. 1992;207:529–546. doi: 10.1016/0076-6879(92)07037-o. [DOI] [PubMed] [Google Scholar]
- 36.Liang Y, Tavalin SJ. Auxiliary beta subunits differentially determine PKA utilization of distinct regulatory sites on Cav1.3 L type Ca2+ channels. Channels (Austin) 2007;1:102–112. doi: 10.4161/chan.4284. [DOI] [PubMed] [Google Scholar]
- 37.Stella SL, Jr, Thoreson WB. Differential modulation of rod and cone calcium currents in tiger salamander retina by D2 dopamine receptors and cAMP. Eur J Neurosci. 2000;12:3537–3548. doi: 10.1046/j.1460-9568.2000.00235.x. [DOI] [PubMed] [Google Scholar]
- 38.Nastainczyk W, et al. Phosphorylation of the purified receptor for calcium channel blockers by cAMP kinase and protein kinase C. Eur J Biochem. 1987;169:137–142. doi: 10.1111/j.1432-1033.1987.tb13590.x. [DOI] [PubMed] [Google Scholar]
- 39.O'Callahan CM, Ptasienski J, Hosey MM. Phosphorylation of the 165-kDa dihydropyridine/phenylalkylamine receptor from skeletal muscle by protein kinase C. J Biol Chem. 1988;263:17342–17349. [PubMed] [Google Scholar]
- 40.O'Callahan CM, Hosey MM. Multiple phosphorylation sites in the 165-kilodalton peptide associated with dihydropyridine-sensitive calcium channels. Biochemistry. 1988;27:6071–6077. doi: 10.1021/bi00416a036. [DOI] [PubMed] [Google Scholar]
- 41.Mitterdorfer J, et al. Identification of PK-A phosphorylation sites in the carboxyl terminus of L-type calcium channel α1 subunits. Biochemistry. 1996;35:9400–9406. doi: 10.1021/bi960683o. [DOI] [PubMed] [Google Scholar]
- 42.Jahn H, Nastainczyk W, Röhrkasten A, Schneider T, Hofmann F. Site-specific phosphorylation of the purified receptor for calcium-channel blockers by cAMP- and cGMP-dependent protein kinases, protein kinase C, calmodulin-dependent protein kinase II and casein kinase II. Eur J Biochem. 1988;178:535–542. doi: 10.1111/j.1432-1033.1988.tb14480.x. [DOI] [PubMed] [Google Scholar]
- 43.Nunoki K, Florio V, Catterall WA. Activation of purified calcium channels by stoichiometric protein phosphorylation. Proc Natl Acad Sci USA. 1989;86:6816–6820. doi: 10.1073/pnas.86.17.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flockerzi V, et al. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature. 1986;323:66–68. doi: 10.1038/323066a0. [DOI] [PubMed] [Google Scholar]
- 45.Johnson BD, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels in skeletal muscle cells requires anchored cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1994;91:11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blaich A, et al. Facilitation of murine cardiac L-type Ca(v)1.2 channel is modulated by calmodulin kinase II-dependent phosphorylation of S1512 and S1570. Proc Natl Acad Sci USA. 2010;107:10285–10289. doi: 10.1073/pnas.0914287107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee TS, et al. Calmodulin kinase II is involved in voltage-dependent facilitation of the L-type Cav1.2 calcium channel: Identification of the phosphorylation sites. J Biol Chem. 2006;281:25560–25567. doi: 10.1074/jbc.M508661200. [DOI] [PubMed] [Google Scholar]
- 48.Litchfield DW. Protein kinase CK2: Structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010 doi: 10.1126/scisignal.2001152. 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Curtis BM, Catterall WA. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984;23:2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]