Ubiquitination regulates MHC class II-peptide complex retention and degradation in dendritic cells (original) (raw)
Abstract
The expression and turnover of MHC class II-peptide complexes (pMHC-II) on the surface of dendritic cells (DCs) is essential for their ability to activate CD4 T cells efficiently. The half-life of surface pMHC-II is significantly greater in activated (mature) DCs than in resting (immature) DCs, but the molecular mechanism leading to this difference remains unknown. We now show that ubiquitination of pMHC-II by the E3 ubiquitin ligase membrane-associated RING-CH 1 (March-I) regulates surface expression, intracellular distribution, and survival of pMHC-II in DCs. DCs isolated from March-I–KO mice express very high levels of pMHC-II on the plasma membrane even before DC activation. Although ubiquitination does not affect the kinetics of pMHC-II endocytosis from the surface of DCs, the survival of pMHC-II is enhanced in DCs obtained from March-I–deficient and MHC-II ubiquitination-mutant mice. Using pMHC-II–specific mAb, we show that immature DCs generate large amounts of pMHC-II that are remarkably stable under conditions in which pMHC-II ubiquitination is blocked. Thus, the cellular distribution and stability of surface pMHC-II in DCs is regulated by ubiquitin-dependent degradation of internalized pMHC-II.
The initiation of an acquired immune response requires coordinated activation of antigen-specific T cells to provide both immune cell help (in the form of cytokines secreted from CD4 T cells) and immune cell effector function (in the form of cytotoxic CD8 T-cell responses and antigen-specific antibody secretion). This cascade of events is regulated primarily by antigen-presenting cells (APCs) in peripheral tissues that take up foreign antigens, process these antigens into immunogenic peptides, and display these antigenic peptides bound to MHC class II molecules on the APC surface (1). Dendritic cells (DCs) are professional APCs that function to prime naïve T cells. In their resting (or immature) state, DCs are relatively poor stimulators of naïve T cells; however, DC activation by a variety of signals induces a DC maturation cascade that up-regulates expression of peptide-loaded MHC-II complexes (pMHC-II), costimulatory molecules, and chemokine receptors that promote DC migration to secondary lymphoid organs and efficient T-cell activation.
Given the central role that pMHC-II expressed on the surface of DCs play in the initiation of immune responses, there is intense interest in understanding the mechanisms leading to immunogenic peptide loading onto MHC-II, pMHC-II transport to the cell surface, and turnover of pMHC-II in DCs. Immature DCs express relatively small amounts of specific pMHC-II on their surface after exposure to antigen (2, 3), and large amounts of MHC-II are retained in intracellular antigen-processing compartments (4). Upon activation of these cells with inflammatory cytokines or Toll-like receptor (TLR) ligands (such as LPS or dsRNA), additional pMHC-II are generated (2, 5), and these pMHC-II are “released” from intracellular stores and traffic to the plasma membrane (6). Curiously, pMHC-II that are expressed on the surface of immature DCs have a short half-life, whereas pMHC-II expressed on the surface of mature DCs are long-lived (4, 7). Immature DCs possess a robust endocytic capacity that is down-regulated upon DC activation (8, 9), a finding that has led to the proposal that the preferential intracellular accumulation and rapid turnover of MHC-II in immature DCs results primarily from the rapid endocytosis of MHC-II in immature, but not mature, DCs (10, 11). The finding that MHC-II is selectively ubiquitinated by the E3 ubiquitin ligase membrane-associated RING-CH 1 (March-I) in immature, but not mature, DCs has increased speculation that ubiquitination regulates MHC-II surface expression by modulating the kinetics of MHC-II endocytosis in DCs (12–14).
We now show that selective ubiquitination of MHC-II in immature DCs by the E3 ubiquitin ligase March-I results in the selective degradation of internalized pMHC-II in immature, but not mature, DCs. Ubiquitination enhances the kinetics of degradation of internalized pMHC-II without affecting the rate of pMHC-II endocytosis from the plasma membrane. Finally, by using mAb that recognize specific pMHC-II, we show the immature DCs efficiently generate pMHC-II; however, ubiquitination of these complexes by the E3 ubiquitin ligase March-I promotes their turnover in immature DCs, revealing a direct role for ubiquitination in regulating the stability of pMHC-II DCs.
Results
Ubiquitination by March-I Regulates pMHC-II Surface Expression and Intracellular Localization.
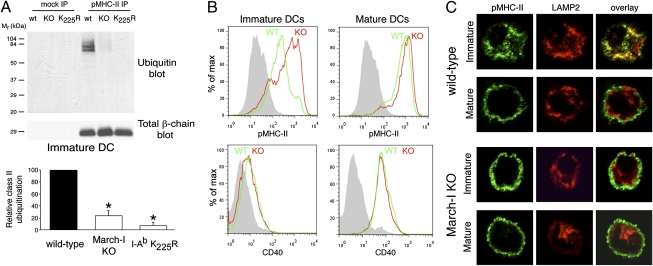
The molecular mechanism by which ubiquitination regulates surface expression of MHC-II remains unknown. The E3 ubiquitin ligase March-I is solely responsible for MHC-II ubiquitination in B cells (15), and we therefore generated DCs from bone marrow of March-I–deficient (KO) mice and their wild-type littermates to investigate the role of March-I in pMHC-II ubiquitination and surface expression in both immature and mature DCs. Ubiquitination of pMHC-II isolated using the conformation-sensitive pMHC-II mAb Y3P (16) was profoundly reduced, but not abolished, in immature DCs obtained from March-I–KO mice (Fig. 1_A_). By contrast, mutation of the sole site of ubiquitination in the MHC-II β-chain from K225 to R225 completely prevented MHC-II ubiquitination in immature DCs. The expression of pMHC-II was significantly higher on immature DCs isolated from March-I–KO mice than on DCs isolated from wild-type mice (Fig. 1_B_). This higher level of expression did not result from the presence of spontaneously matured DCs in the immature DC preparation, because these cells did not express the DC activation marker CD40 (Fig. 1_B_). By contrast, there was little difference in pMHC-II expression in LPS-activated mature DCs isolated from wild-type or March-I–KO mice, a finding that is in excellent agreement with data showing that March-I is not expressed in mature DCs (14, 17). Essentially identical results were obtained when DCs isolated from MHC-II K225R ubiquitination-mutant mice were analyzed (Fig. S1). Furthermore, although large amounts of pMHC-II were present in lysosomal-associated membrane protein 2 (LAMP-2)-positive antigen-processing compartments in immature mouse DCs isolated from wild-type mice (Fig. 1_C_), these complexes were localized primarily on the plasma membrane in March-I–KO immature DCs in a pattern that was indistinguishable from that observed in mature DCs. These data show that ubiquitination of pMHC-II by March-I profoundly affects surface expression and the intracellular distribution of pMHC-II in immature DCs.
Fig. 1.
Ubiquitination regulates pMHC-II expression in DCs. (A) Immature DCs isolated from wild-type, March-I–KO, or MHC-II I-Ab K225R knock-in ubiquitination-mutant mice were lysed in Triton X-100, and aliquots of the lysate were subjected to immunoprecipitation using an isotype control IgG (mock IP) or the anti-pMHC-II mAb Y3P. (Upper panel) The immunoprecipitates were analyzed by immunoblotting with antibodies recognizing ubiquitin or total MHC-II β-chain. (Lower panel) The relative amount of pMHC-II ubiquitination in immature DCs isolated from March-I–KO and MHC-II I-Ab K225R mice is expressed as a percentage of that observed in wild-type DCs. The data shown are the mean ± SD obtained from three independent experiments. *P < 0.05 (relative to wild-type DCs). (B) DCs isolated from wild-type mice (green histograms) or March-I–KO mice (red histograms) were analyzed by FACS analysis for expression of cell-surface pMHC-II (Upper Row) and CD40 (Lower Row) after overnight incubation in the absence of LPS (immature DCs, Left) or in the presence of LPS (mature DCs, Right). The staining of an isotype control mAb is shown in gray. (C) DCs also were fixed, permeabilized, and stained with antibodies that recognize pMHC-II (mAb Y3P; green) or LAMP-2 (red). Overlays show regions of colocalization (yellow).
Ubiquitination Does Not Alter the Kinetics of pMHC-II Endocytosis.
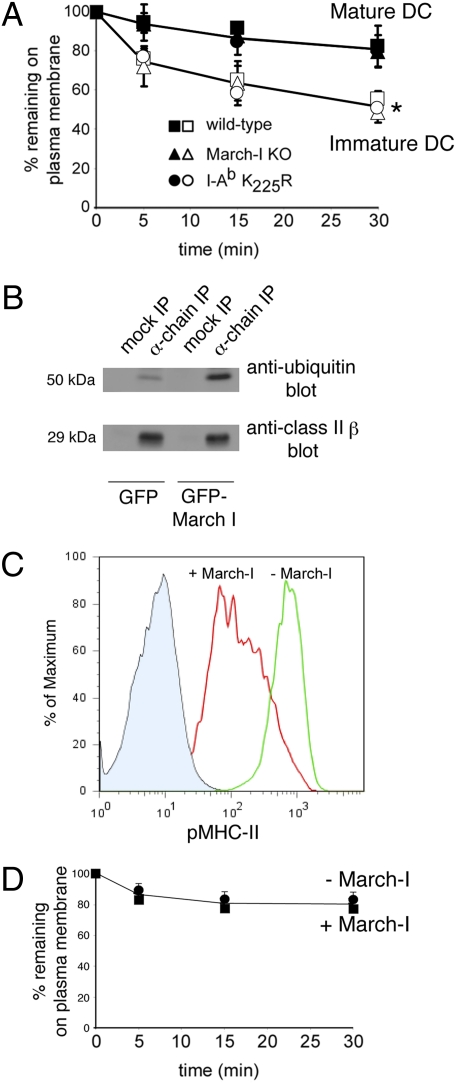
It has been proposed that the differential distribution of MHC-II in immature and mature DCs can be explained by the selective rapid endocytosis of MHC-II in immature DCs (7, 10, 11) and that pMHC-II endocytosis is regulated by ubiquitination (12–14). To investigate the relationship among pMHC-II expression pattern, ubiquitination, and endocytosis in DCs we performed pMHC-II endocytosis assays using DCs from wild-type or ubiquitination-defective mice. This antibody-based assay has been validated previously using an antibody-independent endocytosis assay (18). Although the rate of endocytosis of pMHC-II was indeed more rapid in immature DCs than in mature DCs, we found no statistically significant differences in the initial rate of endocytosis of pMHC-II in DCs isolated from wild-type, March-I–KO, or MHC-II K225R ubiquitination-mutant mice (Fig. 2_A_).
Fig. 2.
Ubiquitination does not affect the kinetics of pMHC-II endocytosis. (A) DCs were isolated from wild-type mice (squares), March-I–KO mice (triangles), or MHC-II I-Ab K225R transgenic mice (circles). After overnight incubation in the absence of LPS (open symbols) or in the presence of LPS (filled symbols), the cells were incubated on ice with mAb Y3P recognizing pMHC-II, and pMHC-II endocytosis rates were measured. The mean fluorescence intensity (MFI) after culture at 37 °C was expressed as a fraction of that present on cells kept on ice. The data shown are the mean ± SD obtained from at least three independent experiments. There was a statistically significant decrease in surface MHC-II survival in immature DCs as compared with mature DCs in each DC preparation at each time point (*P < 0.05). (B) HeLa-CIITA cells transfected with GFP alone or with GFP-March-I were lysed in Triton X-100, and aliquots of the lysate were subjected to immunoprecipitation using an isotype control IgG (mock IP) or the anti-MHC-II α-chain mAb DA6.147 (α-chain IP). The immunoprecipitates were analyzed by immunoblotting with antibodies recognizing ubiquitin (Upper Panel) or total MHC-II β-chain using mAb XD5.A11 (Lower Panel). (C) Aliquots of each transfection were analyzed by FACS for expression of pMHC-II on the cell surface. Representative histograms showing staining of isotype control antibody (filled blue area), pMHC-II (L243) expression on control GFP-expressing cells (green line), or GFP-March-I–expressing cells (red line) are shown. (D) HeLa-CIITA transfectants were incubated on ice with mAb recognizing pMHC-II (L243). The cells were washed extensively and recultured at 37 °C. The percentage of pMHC-II remaining on the surface of cells expressing GFP alone (circles) and on cells expressing GFP-March-I (squares) at each time point was determined as described in Materials and Methods. The data shown are the mean ± SD obtained from more than three independent experiments.
pMHC-II complexes spontaneously internalize in class II transactivator (CIITA)-expressing HeLa cells (18, 19). Because March-I is not expressed in HeLa cells (14), we were able to examine directly the importance of ubiquitination on pMHC-II endocytosis by expressing March-I in HeLa-CIITA cells. Overexpression of GFP-March-I in HeLa-CIITA cells resulted in a fourfold increase in MHC-II ubiquitination (Fig. 2_B_) and marked down-modulation of pMHC-II from the plasma membrane (Fig. 2_C_). Unlike the oligoubiquitination of MHC-II observed in mouse DCs, human MHC-II in transfected HeLa-CIITA cells is primarily di-ubiquitinated (Fig. 2_B_ and ref. 14). Despite the dramatic effects of March-I on MHC-II ubiquitination and pMHC-II surface expression, there were no differences in the rate of pMHC-II endocytosis in HeLa-CIITA cells expressing either GFP alone or GFP-March-I (Fig. 2_D_). Taken together, these data demonstrate that ubiquitination of MHC-II does not affect the kinetics of MHC-II endocytosis from the plasma membrane. Furthermore, these data show that the observed differences in the kinetics of MHC-II endocytosis in immature and mature DCs are unrelated to MHC-II ubiquitination and demonstrate that other activation-induced changes in DC biology underlie the differences in MHC-II endocytosis in immature and mature DCs.
Ubiquitination Regulates Degradation of Surface-Expressed pMHC-II in DCs.
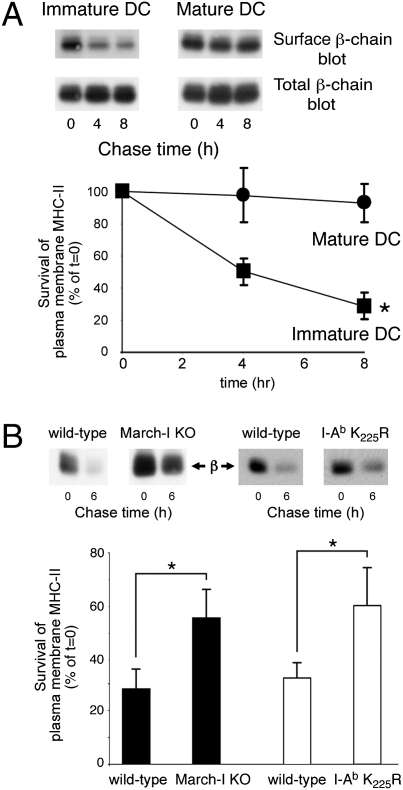
We next set out to investigate whether March-I regulates surface expression of pMHC-II by promoting the degradation of pMHC-II complexes that internalize from the plasma membrane. In these studies we biotinylated cell-surface proteins on either immature or mature wild-type mouse DCs and recultured the labeled cells for 8 h at 37 °C. Cell lysis and immunoprecipitation using pMHC-II mAb Y3P revealed that most pMHC-II present on the plasma membrane of immature DCs was lost during the 8-h chase period, whereas plasma membrane pMHC-II on mature DCs was not (Fig. 3_A_). To determine whether ubiquitination by March-I regulated the rapid pMHC-II turnover in immature DCs, we examined pMHC-II degradation in wild-type and March-I–KO DCs. Although 70% of cell-surface pMHC-II was degraded after wild-type immature DCs were cultured for 6 h at 37 °C, surface pMHC-II on March-I–KO DCs or MHC-II I-Ab K225R ubiquitination-mutant DCs was far more stable, and only 40% of the entire pool of pMHC-II was degraded within the 6-h period (Fig. 3_B_). It should be noted that pMHC-II degradation is not completely blocked in immature DCs obtained from these ubiquitination-mutant mice, revealing a ubiquitin-independent pathway of pMHC-II degradation in immature DCs. These data show that ubiquitination by March-I regulates the stability and degradation of surface pMHC-II in immature DCs.
Fig. 3.
Ubiquitination by March-I regulates surface pMHC-II degradation. (A) Immature or LPS-matured wild-type mouse DCs were surface biotinylated on ice and returned to culture in complete medium at 37 °C for the indicated times. (Upper Panel) Following cell lysis, MHC-II was isolated by immunoprecipitation and analyzed by SDS/PAGE, and biotin-labeled surface MHC-II present in the immunoprecipitate was revealed using HRP-conjugated avidin. Total MHC-II present in the same sample was revealed using an MHC-II β-chain-specific antibody and served as a loading control. (Lower Panel) The amount of biotinylated pMHC-II isolated at each time point from both immature DCs (filled squares) and LPS-matured DCs (filled circles) was determined and expressed as a percentage of the total amount of biotinylated pMHC-II present in aliquots of cells that remained on ice after biotinylation (t = 0, representing the maximum amount of MHC-II obtainable in the assay). The data shown are the mean ± SD obtained from three independent experiments. There was a statistically significant decrease in surface MHC-II survival in immature DCs as compared with mature DCs at each time point. *P < 0.05. (B) Immature DCs were obtained from March-I–KO mice and their wild-type littermates (filled bars) or from MHC-II K225R knock-in mice and wild-type C57BL/6 control mice (open bars) and were biotinylated on ice. An aliquot of cells was harvested immediately and maintained on ice (t = 0 h of chase), and the remaining cells were returned to culture in complete medium at 37 °C for 6 h. The amount of biotinylated pMHC-II isolated after culture of the cells at 37 °C was determined as described above. The data shown are the mean ± SD obtained from three independent experiments. *P < 0.05.
Ubiquitination Promotes Degradation of Specific pMHC-II in Immature DCs.
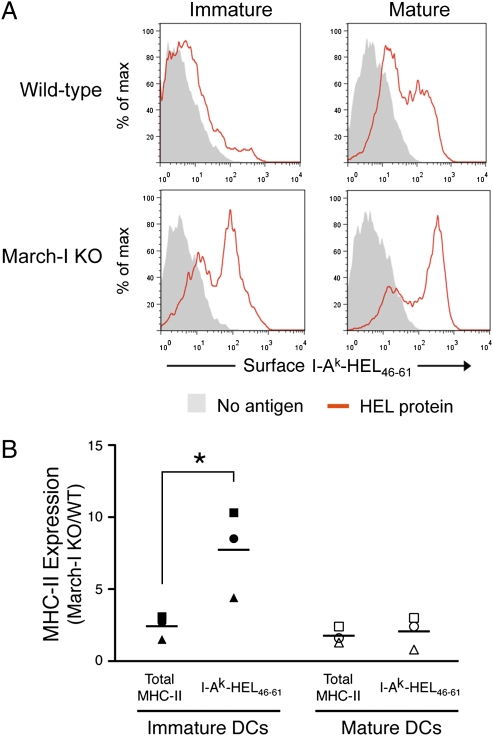
We next asked whether March-I regulates MHC-II expression in immature DCs by promoting the degradation of specific pMHC-II expressed on these cells. To address this question, we used mAb (C4H3 and Aw3.18.14) that recognize I-Ak-HEL46–61 complexes generated after processing of the model antigen hen egg-white lysozyme (HEL) (20, 21). In agreement with previous reports (2, 5), we found that immature DCs expressed small amounts of surface I-Ak-HEL46–61 complexes after overnight incubation with HEL protein antigen, and stimulation with LPS during an overnight antigen-free chase increased I-Ak-HEL46–61 complex expression on the matured DCs (Fig. 4_A_). Curiously, deletion of March-I dramatically increased expression of I-Ak-HEL46–61 complexes on immature DCs; this increase was disproportionate to the modest increase in expression of total MHC-II on the cells as monitored using the pan-I-Ak mAb 10.2.16 (Fig. 4_B_). This enhancement was not observed in mature DCs, a finding that is consistent with the fact that activated DCs do not express March-I (14, 17). These results demonstrate that deletion of March-I specifically enhances expression of newly generated pMHC-II in immature DCs.
Fig. 4.
Ubiquitination regulates degradation of internalized pMHC-II complexes in immature DCs. (A and B). Immature DCs from March-I–KO or wild-type H-2k littermate mice were treated with HEL protein overnight, washed, and then cultured in the presence or absence of LPS overnight to mature the DCs. (A) Expression of I-Ak-HEL46–61 complexes on CD11c-positive cells was analyzed by flow cytometry using mAb Aw3.18.14. Cells that were not pulsed with HEL protein also were stained with mAb Aw3.18.14 as a control (no antigen, gray fill). (B) Surface expression of total MHC-II on CD11c-positive cells was determined using mAb 10.2.16, and expression of I-Ak-HEL46–61 complexes was determined using mAb Aw3.18.14. The MFI of MHC-II expression using each mAb from March-I–KO DCs was compared with the MFI from wild-type littermates. Each symbol (circle, triangle, and square) represents data obtained from three independent pairs of March-I–KO and wild-type littermate mice, and the horizontal line indicates the average value from each group. *P < 0.05. The enhancement in the expression of I-Ak-HEL46–61 complexes relative to total MHC-II (average value I-Ak-HEL46–61/average value total MHC-II) was 3.2 ± 0.2 for immature DCs and 1.1 ± 0.4 for mature DCs (P < 0.005).
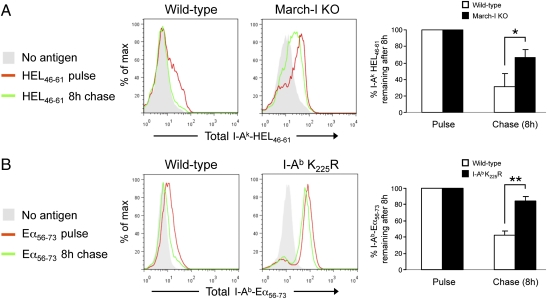
Our studies using March-I–deficient DCs demonstrate that immature DCs are indeed capable of generating pMHC-II from internalized protein antigens and suggest that this generation normally is masked by the expression of March-I and rapid turnover of these complexes in immature DCs. To explore this possibility directly, we (i) used preprocessed peptides as the source of antigen, thereby negating the role of antigen degradation in pMHC-II expression, and (ii) fixed and permeabilized the DCs before mAb staining, thereby allowing us to monitor total expression of complexes and not restrict our analysis to surface pMHC-II. Overnight pulsing of wild-type immature DCs with HEL46–61 peptide resulted in low-level expression of I-Ak-HEL46–61 complexes in immature DCs. This expression was eliminated almost completely following an 8-h peptide-free chase at 37 °C (Fig. 5_A_). By contrast, in immature DCs obtained from March-I–KO mice this same 37 °C chase had only a modest effect on the expression of I-Ak-HEL46–61 complexes. Similar results were obtained when we examined the stability of I-Ab-Eα56–73 complexes in immature DCs obtained from MHC-II K225R ubiquitination-mutant mice using the I-Ab-Eα56–73 complex-specific mAb YAe (22). Although the majority of I-Ab-Eα56–73 complexes generated after an overnight Eα56–73 peptide pulse were lost during the 8-h chase period in wild-type immature DCs, these complexes were remarkably stable in immature DCs obtained from MHC-II K225R ubiquitination-mutant mice cultured under identical conditions (Fig. 5_B_). Taken together, these data demonstrate that ubiquitination regulates pMHC-II expression by promoting the rapid turnover of specific pMHC-II formed in immature DCs.
Fig. 5.
Ubiquitination regulates degradation of I-Ak-HEL46–61 and I-Ab-Eα56–73 complexes in immature DCs. (A and B). Immature DCs from March-I–KO and wild-type littermate H-2k mice (A) or MHC-II I-Ab K225R-transgenic or wild-type H-2b mice (B) were treated overnight with HEL46–61 peptide or Eα56–73 peptide, respectively. Cells were harvested immediately (pulse, red line) or left in culture in the absence of antigen for 8 additional hours (chase, green line). After being fixed and permeabilized, cells were stained for the detection of total (surface plus intracellular) I-Ak-HEL46–61 or I-Ab-Eα56–73 complexes using mAb C4H3 or YAe, respectively. Cells that were not pulsed with peptides also were stained with each mAb as a control (no antigen, gray fill). The amount of I-Ak-HEL46–61 or I-Ab-Eα56–73 complex remaining after 8 h of chase was determined and expressed as a percentage of the total amount of complex present on the pulsed DCs. The data shown are the mean ± SD from three independent experiments. *P = 0.05 and **, P = 0.001.
Discussion
The molecular mechanisms controlling MHC-II expression in DCs have been investigated intensively and hotly debated. It is widely believed that immature DCs express relatively small amounts of surface pMHC-II because pMHC-II generation is inefficient in these cells (2, 5) and that any pMHC-II that are exported to the cell surface are rapidly internalized and degraded (10, 11). We now demonstrate that the rapid turnover of pMHC-II in immature DCs is regulated by ubiquitin-dependent pMHC-II degradation by the E3 ubiquitin ligase March-I and that generation of pMHC-II complexes actually is efficient in immature DCs. The enhanced recovery of pMHC-II from March-I–KO DCs does not result from a reduced loss of MHC-II secreted on exosomes, because ubiquitination does not affect MHC-II sorting into exosomes (23). Although the idea that immature DCs efficiently generate pMHC-II complexes that turn over rapidly in a ubiquitination-dependent manner is at odds with studies arguing that DCs do not generate pMHC-II unless activated (2, 5), this concept is entirely consistent with studies showing that immature DCs constitutively express self–pMHC-II complexes (11) and that DCs can stimulate naïve CD4 T cells over a wide range of activation states (3).
Although it has been proposed that selective ubiquitination of MHC-II on Lys225 is responsible for the relatively rapid rate of endocytosis observed in immature DCs (12–14), we now show that ubiquitination clearly regulates pMHC-II surface expression and intracellular distribution without affecting the kinetics of pMHC-II endocytosis. This finding is in agreement with data showing that the E3 ubiquitin ligase March-I is located primarily in early endosomes and not on the plasma membrane (14). Although the failure of MHC-II to accumulate in lysosome-like compartments in MHC-II ubiquitination-defective DCs is clearly observed in our and others studies (12, 13), we attribute this failure not to an alteration in MHC-II endocytosis per se but to an alteration in MHC-II retention and degradation in these cells. Like many other surface receptors whose expression is modulated by ubiquitination (24), it is likely that ubiquitination regulates surface expression of pMHC-II by targeting internalized pMHC-II for degradation in lysosomes. Our findings are reminiscent of those showing that ubiquitination regulates postendocytic sorting and lysosomal degradation of internalized EGF and FGF receptors without altering the kinetics of receptor endocytosis (25, 26). Data showing that internalized MHC-II traffics inefficiently to lysosomes in March-I–deficient B cells (15) are in agreement with this hypothesis.
Because immature conventional DCs continuously synthesize MHC-II molecules (7, 11), the March-I–dependent degradation of internalized pMHC-II in immature DCs will ensure that a relatively diverse repertoire of pMHC-II is expressed on the DC surface. Such a process is likely to be an important molecular mechanism for enhancing the role of the immature DC as a “sampling sentinel” of the immune system until the DC becomes stimulated, at which time March-I expression ceases, ubiquitin-dependent MHC-II degradation is halted, and the DC becomes an effective antigen-specific immune system activator.
Materials and Methods
Cells and Reagents.
Murine DCs were prepared by differentiating bone marrow progenitors in medium containing GM-CSF using standard protocols (27). The cells were cultured and activated using 1 μg/mL LPS for 24 h. HeLa cells expressing CIITA (a gift from Peter Cresswell, Yale University School of Medicine, New Haven, CT) were maintained as described previously (28).
The following antibodies were used in this study: anti-human pMHC-II mAb L243 (Becton Dickinson), anti-human MHC-II α-chain mAb DA6.147 (29), anti-mouse pMHC-II mAb Y3P (16), biotinylated anti-ubiquitin mAb P4D1 (Covance), and anti-LAMP-2 mAb H4B4 (Developmental Studies Hybridoma Bank). The anti-I-Ak β-chain rabbit serum has been described previously (30). I-Ak-HEL46–61 complexes were detected using mAb C4H3 (20) (a gift from Ron Germain, National Institutes of Health, Bethesda, MD) and Aw3.18.14 (21) (a gift from Emil Unanue, Washington University School of Medicine, St. Louis, MO). I-Ab-Eα56–73 complexes were detected using mAb YAe (22) (a gift from Sasha Rudensky, Memorial Sloan-Kettering Cancer Center, New York, NY). Alexa Fluor-conjugated secondary antibodies were obtained from Molecular Probes, and HRP-conjugated reagents were obtained from Southern Biotech. Plasmids encoding GFP alone or March I in the internal ribosome entry site (IRES) GFP vector pTracer (Invitrogen) have been described previously (15).
Mice.
March-I heterozygous mice on a C57BL/6 background (15) were bred, and March-I–KO mice and their wild-type littermates were used at 6–12 wk of age. March-I heterozygous mice were generated on the H-2k background by breeding with B10.BR mice and used as above. MHC-II I-Ab K225R-transgenic mice were generated by cloning the cDNA for the I-Ab β-chain containing a lysine (Lys)-to-arginine (Arg) mutation in the codon for Lys225 into the vector pDOI-5 (provided by Diane Mathis, Harvard Medical School, Boston). This vector uses the MHC-II I-Eα promoter to drive transgene expression (31). Transgene-positive mice on a C57BL/6 background were bred onto MHC-II I-Ab β-chain–deficient mice [strain Abb (H2-ab1); Taconic Farms] such that the only MHC-II molecule expressed contained the K225R mutation. Where indicated, MHC-II I-Ab K225R-knockin mice were used (32). All mice were cared for in accordance with National Institutes of Health guidelines with the approval of the National Cancer Institute Animal Care and Use Committee.
Intracellular Staining.
Immature DCs were generated from March-I–KO mice on the H-2k background or from MHC-II I-Ab K225R transgenic mice and their wild-type littermates as described above. When supplied, 68 μM of HEL46–61 peptide or Eα56–73 peptide were added overnight at day 6. At day 7, the antigen was removed. Half of the cells were harvested and prepared for intracellular staining (pulse), and half of the cells were cultured further at 37 °C with 3 mL of fresh medium for 8 h (chase) and subsequently prepared for intracellular staining. Immediately after being harvested, DCs were washed twice with HBSS, fixed with 4% paraformaldehyde (PFA) for 30 min on ice, quenched twice with 50 mM NH4Cl, and finally washed with HBSS and kept at 4 °C to perform intracellular staining of pulsed and chased DCs simultaneously. Cells were permeabilized with 0.01% saponin in PBS with 3% goat serum for at least 1 h at room temperature, incubated with primary mAb C4H3 or YAe for 40 min at room temperature, and washed three times with HBSS containing 2% FBS (FACS buffer). DCs then were incubated with the appropriate Alexa Fluor 633- or Cy5-conjugated secondary antibody for 40 min at room temperature and washed three times with FACS buffer before being finally fixed with 1% PFA. Cells were analyzed in a FACSCalibur flow cytometer (Becton Dickinson) using antigen-untreated DCs stained with C4H3 or YAe mAb as a negative control.
Surface Staining of I-Ak and I-Ak-HEL46–61 Complexes.
Immature DCs were generated from March-I–KO mice on the H-2k background and their wild-type littermates as described above. When supplied, 68 μM of HEL protein was added at day 5. On day 6, cells were activated with LPS at 1 μg/mL or were left untreated. DCs were harvested, washed once with FACS buffer, and incubated with the primary mAb Aw3.18.14 or 10.2.16 for 45 min on ice. Cells were washed extensively with FACS buffer before being incubated with Cy5-labeled goat anti-mouse secondary antibody for 40 min on ice. Cells subsequently were washed three times with FACS buffer and incubated for 25 min on ice with the phycoerythrin-labeled CD11c mAb. DCs were washed twice with FACS buffer and finally fixed with 1% PFA before being analyzed in a FACSCalibur flow cytometer using HEL-untreated DCs stained with Aw3.18.14 mAb as a negative control or with mouse IgG2b as a negative control for mAb 10.2.16.
Immunoprecipitation, Immunoblotting, and Transfection.
Cells were lysed in Triton X-100 and analyzed by immunoprecipitation and immunoblot analysis for expression of MHC-II α-chains and β-chains as described previously (18). All cell lysis and immunoprecipitation buffers contained 25 mM _N_-ethylmaleimide to inhibit de-ubiquitination activity. Immunoblots were probes for ubiquitinated proteins using biotinylated anti-ubiquitin mAb P4D1 and HRP-streptavidin. HeLa-CIITA cells were transfected using Lipofectamine and Plus reagent (Invitrogen) according to manufacturer's instructions and were assayed 24 h posttransfection.
Immunofluorescence Microscopy.
DCs were incubated for 30 min at room temperature on poly-lysine–coated glass slides before fixation and immunostaining. Cells were fixed in 4% PFA in PBS for 12 min at room temperature and permeabilized with 0.1% saponin in PBS at room temperature. Primary antibodies were applied to the cells for 30 min at room temperature, and the cells were thoroughly washed in PBS/0.1% saponin before the incubation with Alexa Fluor 488- or Alexa Fluor 546-conjugated secondary antibodies in the same buffer. All cells were imaged using a Zeiss LSM 510 META confocal microscope with a 63× oil-immersion objective lens (NA 1.4).
FACS-Based MHC-II Internalization Assays.
Cells were briefly trypsinized and washed twice in FACS buffer. Primary mAb were incubated with cells (≈5 × 106 cells/mL) for 20 min on ice and washed twice in ice-cold FACS buffer. The labeled cells were incubated with Alexa Fluor-conjugated specific secondary antibodies for 20 min on ice, washed twice, and then fixed in 1% PFA in PBS. For all internalization experiments, the cells were stained with the primary mAb on ice, washed twice in ice-cold FACS buffer, and recultured at 37° for various times. After two more washes in ice-cold buffer, the amount of primary antibody remaining on the cell surface was identified by staining cells with Alexa Fluor-conjugated secondary antibodies on ice. The cells were washed in ice-cold FACS buffer and then were fixed in 1% PFA in PBS at room temperature. Expression of each antibody was determined by flow cytometry using a FACSCalibur. The fluorescence intensity (geometric mean) was determined for each FACS profile and expressed as a percentage of the value present on cells kept on ice for the duration of the internalization assay.
Cell-Surface Biotinylation.
Plasma membrane proteins of immature and mature DCs were surface biotinylated by incubating ≈10 × 106 cells/mL using the membrane-impermeable biotinylation reagent sulfo-NHS-biotin (1 mg/mL in HBSS for 30 min on ice) according to the manufacturer's protocol (Pierce). After biotinylation, cells were washed extensively in ice-cold HBSS, resuspended in complete medium, and incubated for various times at 37 °C. In experiments using DCs from March-I–KO mice (or their wild-type littermates), equivalent portions of each cell population were lysed, pMHC-II was immunoprecipitated with anti-pMHC-II mAb Y3P, and biotinylated pMHC-II was detected by blotting with streptavidin-HRP conjugate.
In experiments using DCs from MHC-II K225R ubiquitination-mutant mice (or C57BL/6 control mice), surface proteins were isolated using streptavidin-agarose beads as described previously (18), and MHC-II was detected using an anti-I-A β-chain rabbit serum.
Supplementary Material
Supporting Information
Acknowledgments
We thank Alfred Singer and Richard Hodes for extensive discussions and critical reading of this manuscript. We thank Peter Cresswell (Yale University School of Medicine), Ron Germain (National Institutes of Health), Emil Unanue (Washington University School of Medicine), Sasha Rudensky (Memorial Sloan-Kettering Cancer Center), and Diane Mathis (Harvard Medical School) for the gifts of reagents used in this study. This work is supported by the Norwegian Research Council (E.W. and O.B.), the Ministry of Education, Culture, Sports, Science and Technology of Japan (S.I.), the Japan Society for the Promotion of Science (S.I.), and the Intramural Research Program of the National Institutes of Health (P.A.R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 2.Inaba K, et al. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J Exp Med. 2000;191:927–936. doi: 10.1084/jem.191.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veeraswamy RK, Cella M, Colonna M, Unanue ER. Dendritic cells process and present antigens across a range of maturation states. J Immunol. 2003;170:5367–5372. doi: 10.4049/jimmunol.170.11.5367. [DOI] [PubMed] [Google Scholar]
- 4.Pierre P, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 5.Turley SJ, et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science. 2000;288:522–527. doi: 10.1126/science.288.5465.522. [DOI] [PubMed] [Google Scholar]
- 6.Mellman I, Steinman RM. Dendritic cells: Specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 7.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 8.Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: Downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrett WS, et al. Developmental control of endocytosis in dendritic cells by Cdc42. Cell. 2000;102:325–334. doi: 10.1016/s0092-8674(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 10.Villadangos JA, et al. MHC class II expression is regulated in dendritic cells independently of invariant chain degradation. Immunity. 2001;14:739–749. doi: 10.1016/s1074-7613(01)00148-0. [DOI] [PubMed] [Google Scholar]
- 11.Wilson NS, El-Sukkari D, Villadangos JA. Dendritic cells constitutively present self antigens in their immature state in vivo and regulate antigen presentation by controlling the rates of MHC class II synthesis and endocytosis. Blood. 2004;103:2187–2195. doi: 10.1182/blood-2003-08-2729. [DOI] [PubMed] [Google Scholar]
- 12.van Niel G, et al. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity. 2006;25:885–894. doi: 10.1016/j.immuni.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Shin JS, et al. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature. 2006;444:115–118. doi: 10.1038/nature05261. [DOI] [PubMed] [Google Scholar]
- 14.De Gassart A, et al. MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc Natl Acad Sci USA. 2008;105:3491–3496. doi: 10.1073/pnas.0708874105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuki Y, et al. Novel regulation of MHC class II function in B cells. EMBO J. 2007;26:846–854. doi: 10.1038/sj.emboj.7601556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janeway CA, Jr., et al. Monoclonal antibodies specific for Ia glycoproteins raised by immunization with activated T cells: Possible role of T cellbound Ia antigens as targets of immunoregulatory T cells. J Immunol. 1984;132:662–667. [PubMed] [Google Scholar]
- 17.Young LJ, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 18.Walseng E, Bakke O, Roche PA. Major histocompatibility complex class II-peptide complexes internalize using a clathrin- and dynamin-independent endocytosis pathway. J Biol Chem. 2008;283:14717–14727. doi: 10.1074/jbc.M801070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick PJ, Martina JA, Bonifacino JS. Involvement of clathrin and AP-2 in the trafficking of MHC class II molecules to antigen-processing compartments. Proc Natl Acad Sci USA. 2005;102:7910–7915. doi: 10.1073/pnas.0502206102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong G, Reis e Sousa C, Germain RN. Production, specificity, and functionality of monoclonal antibodies to specific peptide-major histocompatibility complex class II complexes formed by processing of exogenous protein. Proc Natl Acad Sci USA. 1997;94:13856–13861. doi: 10.1073/pnas.94.25.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dadaglio G, Nelson CA, Deck MB, Petzold SJ, Unanue ER. Characterization and quantitation of peptide-MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity. 1997;6:727–738. doi: 10.1016/s1074-7613(00)80448-3. [DOI] [PubMed] [Google Scholar]
- 22.Rudensky AYu, Rath S, Preston-Hurlburt P, Murphy DB, Janeway CA., Jr. On the complexity of self. Nature. 1991;353:660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- 23.Buschow SI, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10:1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 24.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 25.Huang F, Goh LK, Sorkin A. EGF receptor ubiquitination is not necessary for its internalization. Proc Natl Acad Sci USA. 2007;104:16904–16909. doi: 10.1073/pnas.0707416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haugsten EM, Malecki J, Bjørklund SM, Olsnes S, Wesche J. Ubiquitination of fibroblast growth factor receptor 1 is required for its intracellular sorting but not for its endocytosis. Mol Biol Cell. 2008;19:3390–3403. doi: 10.1091/mbc.E07-12-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 28.Ackerman AL, Cresswell P. Regulation of MHC class I transport in human dendritic cells and the dendritic-like cell line KG-1. J Immunol. 2003;170:4178–4188. doi: 10.4049/jimmunol.170.8.4178. [DOI] [PubMed] [Google Scholar]
- 29.Guy K, Van Heyningen V, Cohen BB, Deane DL, Steel CM. Differential expression and serologically distinct subpopulations of human Ia antigens detected with monoclonal antibodies to Ia alpha and beta chains. Eur J Immunol. 1982;12:942–948. doi: 10.1002/eji.1830121109. [DOI] [PubMed] [Google Scholar]
- 30.Muntasell A, Berger AC, Roche PA. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. EMBO J. 2007;26:4263–4272. doi: 10.1038/sj.emboj.7601842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kouskoff V, Fehling HJ, Lemeur M, Benoist C, Mathis D. A vector driving the expression of foreign cDNAs in the MHC class II-positive cells of transgenic mice. J Immunol Methods. 1993;166:287–291. doi: 10.1016/0022-1759(93)90370-m. [DOI] [PubMed] [Google Scholar]
- 32.Ohmura-Hoshino M, et al. Cutting edge: Requirement of MARCH-I-mediated MHC II ubiquitination for the maintenance of conventional dendritic cells. J Immunol. 2009;183:6893–6897. doi: 10.4049/jimmunol.0902178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information