The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation (original) (raw)
. Author manuscript; available in PMC: 2011 Jan 29.
Abstract
Interleukin-1 (IL-1)-mediated signaling in T cells is essential for T helper 17 (Th17) cell differentiation. We showed here that SIGIRR, a negative regulator of IL-1 receptor and Toll-like receptor signaling, was induced during Th17 cell lineage commitment and governed Th17 cell differentiation and expansion through its inhibitory effects on IL-1 signaling. The absence of SIGIRR in T cells resulted in increased Th17 cell polarization in vivo upon myelin oligodendrocyte glycoprotein (MOG35–55) peptide immunization. Recombinant IL-1 promoted a marked increase in the proliferation of SIGIRR-deficient T cells under an in vitro Th17 cell-polarization condition. Importantly, we detected increased IL-1-induced phosphorylation of JNK and mTOR kinase in SIGIRR-deficient Th17 cells compared to wild-type Th17 cells. IL-1-induced proliferation was abolished in mTOR-deficient Th17 cells, indicating the essential role of mTOR activation. Our results demonstrate an important mechanism by which SIGIRR controls Th17 cell expansion and effector function through the IL-1-induced mTOR signaling pathway.
Introduction
Interleukin-17 (IL-17) is a proinflammatory cytokine that plays an important role in host defense against infections and is involved in the pathogenesis of multiple human and animal autoimmune disease as well as allergen-specific immune responses (Bettelli et al., 2006; Dong, 2008; Harrington et al., 2005; Iwakura and Ishigame, 2006; Kolls and Linden, 2004; Mangan et al., 2006; McGeachy and Cua, 2008; Nakae et al., 2002; Park et al., 2005; Veldhoen et al., 2006). IL-17 concentrations are elevated in patients with rheumatoid arthritis (RA), multiple sclerosis (MS), inflammatory bowel disease (IBD), and asthma (Tzartos et al., 2008; Matusevicius et al., 1999; Teunissen et al., 1998). Anti-IL-17 inhibition can lead to substantial reduction of chemokine production in the central nervous system (CNS), markedly reduce experimental autoimmune encephalomyelitis (EAE) severity and reverse the progression of active EAE (Kroenke et al., 2008). The pathogenic role of IL-17 has also been demonstrated in experiments with IL-17- and IL-17R-deficient mice, in which various autoimmune disorders, including EAE and collagen-induced arthritis (CIA), were suppressed (Taylor, 2003; Hofstetter et al., 2005; Gonzalez-Garcia et al., 2009). The main function of IL-17 is to coordinate local tissue inflammation via the interaction with IL-17 receptor, inducing the expression of pro-inflammatory and neutrophilmobilizing mediators (including IL-6, G-CSF, TNFα and IL-1, CXCL1, CCL2, CXCL2, CCL7, and CCL20), as well as matrix metalloproteases (MMPs) that allow activated T cells to penetrate extracellular matrix (Fossiez et al., 1996; Yao et al., 1995; Andoh et al., 2005; Laan et al., 1999; Kao et al., 2005; Chabaud et al., 2000; Bamba et al., 2003; Gaffen, 2008; Qian et al., 2007).
CD4+ T helper (Th) lymphocytes are essential in regulating immune responses and autoimmune and inflammatory diseases. Upon activation, naïve CD4+ T helper (Th) cells differentiate into three effector subsets: Th1 cells are characterized by production of IFN-γ which mediates cellular immunity; and Th2 cells, which synthesize IL-4, IL-5 and IL-13 and are important in humoral immunity and allergic responses (McGuirk and Mills, 2002; Murphy and Reiner, 2002). Th17 cells belong to a third lineage of CD4+ Th cells, which produce IL-17, IL-17F, IL-21 and IL-22 (Langrish et al., 2005; Bettelli et al., 2006; Harrington et al., 2005; Iwakura and Ishigame, 2006; Kolls and Linden, 2004; Mangan et al., 2006; Nakae et al., 2002; Park et al., 2005; Veldhoen et al., 2006). Several cytokines including TGF-β and IL-6 are required for Th17 cell differentiation upon T cell receptor (TCR) activation. Transcription factors including STAT3 together with RORα and RORγt determine Th17 cell lineage specific differentiation program through induction of a set of signature cytokines and cytokine receptors, including IL-23R and IL-1R (Korn et al., 2007; Wei et al., 2007; Yang et al., 2008; Ivanov et al., 2006; McGeachy et al., 2007). Although the detailed molecular mechanism is still unclear, IL-1 and IL-23 have major influences on Th17 cell differentiation, possibly through cell proliferation, and survival to maintain the differentiated state of Th17 cells (Korn et al., 2009). Importantly, mice lacking either IL-1 or IL-23 are resistant to disease induction in Th17 cell-dependent CIA, EAE, and IBD diseases models (Ben Sasson et al., 2009; Sutton et al., 2006; Murphy et al., 2003; Thakker et al., 2007; Langrish et al., 2005; Chung et al., 2009; Brereton et al., 2009). While much effort has been focusing on how cytokines promote Th17 cell differentiation, it is equally critical to investigate how Th cell effector functions are regulated. Multiple cytokines including IFNγ, IL-4 (Harrington et al., 2005; Park et al., 2005), IL-2 and IL-27 (Laurence et al., 2007; Stumhofer et al., 2006) have been shown to down-regulate Th17 cell differentiation through direct and/or indirect impact on the transcriptional program mediated by STAT3, RORγt and RORα. Here, we demonstrate that SIGIRR, a negative regulator of IL-1 receptor and Toll-like receptor, suppressed Th17 cell expansion and IL-17-dependent disease.
SIGIRR (also referred as TIR8) is a negative regulator for IL-1R, TLR4 and TLR9 signaling. SIGIRR contains a single immunoglobulin (Ig) extracellular domain and a TIR (Toll-IL-1R) intracellular domain (Garlanda et al., 2004; Wald et al., 2003), inhibiting IL-1 and lipopolysaccharide (LPS) signaling through its interaction with the TLR4 and IL-1R complexes (Qin et al., 2005). In addition to the role of SIGIRR in mucosal immunity (Garlanda et al., 2004; Xiao et al., 2007), recent studies have shown the critical role of SIGIRR in modulating autoimmunity and inflammatory responses associated with infections (Bozza et al., 2008; Garlanda et al., 2007). SIGIRR is highly expressed in intestinal epithelial cells, contributing to immune tolerance to commensal bacteria. Importantly, SIGIRR is also expressed in T cells and DCs, exerting the fine-tuning modulation in inflammatory responses, manifested by the hypersusceptibility of SIGIRR-deficient mice to pathogen infection or autoimmune lupus (Lech et al., 2008).
Here, we report that whereas SIGIRR expression was induced in differentiated Th17 cells, SIGIRR-deficient mice were more susceptible to experimental autoimmune encephalomyelitis (EAE) due to hyper activation of Th17 cells upon immunization with MOG (myelin oligodendrocyte glycoprotein) peptide. IL-1 treatment resulted in increased TGF-β and IL-6-mediated Th17 cell differentiation in SIGIRR-deficient T cells compared to that in wild-type T cells, suggesting the impact of SIGIRR on Th17 cells is probably through its modulation on IL-1 signaling during Th17 differentiation. We also examined IL-1 signaling mechanism in Th17 cells and detected increased IL-1-induced phosphorylation of JNK and mTOR kinase in SIGIRR-deficient Th17 cells compared to wild-type Th17 cells. Rapamycin, an inhibitor of mTOR, specifically inhibited SIGIRR-regulated IL-1-induced Th17 cell proliferation and IL-17 production. Taken together, our study suggests that SIGIRR plays an important regulatory role in Th17 differentiation and proliferation and IL-17-dependent EAE pathogenesis, probably through its modulation on IL-1 signaling in Th17 cells.
Results
SIGIRR expression is induced during Th17 cell differentiation
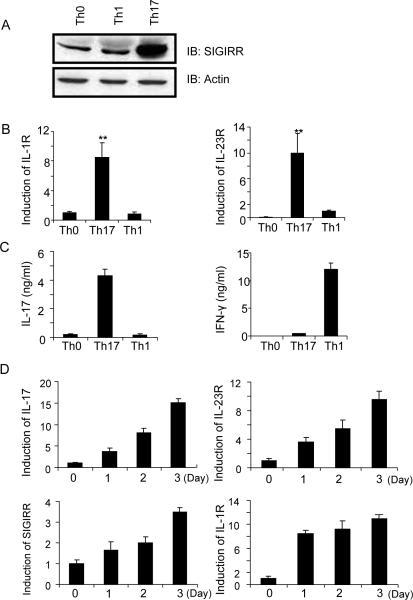
Although SIGIRR is expressed in splenic T cells, its physiological function in T cell is unclear. SIGIRR-deficient mice are highly susceptible to DSS-induced colitis and DSS-treated SIGIRR-deficient colon tissues produce high amounts of IL-17 (Xiao et al., 2007), implicating a possible regulatory role of SIGIRR in Th17 cell function. Therefore, we sought to determine the potential role of SIGIRR in modulation of Th17 cell function. Interestingly, we found that SIGIRR and the known Th17 cell-associated molecules (IL-17, IL-1R1 and IL-23R) were expressed at a much higher amount in Th17 cells compared to IFNγ-producing Th1 or naïve CD4+ T cells, indicating a possible functional role of SIGIRR in Th17 cell subset (Fig. 1A–C). A time-course was performed to examine the kinetics of SIGIRR and IL-1R1 expression by real-time PCR during Th17 cell differentiation. Although IL-1R1 expression was highly induced on day 1 of polarization, SIGIRR, IL-17 and IL-23R expression was induced more gradually during polarization, suggesting a possible feedback regulatory role of SIGIRR for Th17 cell function (Fig. 1D).
Figure 1. SIGIRR expression is induced during Th17 cell differentiation.
(A–C) Naïve CD4+ T cells (CD4+CD44lo) were sorted by flow cytometry and polarized under Th1 (IL-12 and anti-IL-4) and Th17 (TGF-β, IL-6, anti-IFN-γ and anti-IL-4) cell-inducing conditions (on anti-CD3 and anti-CD28-coated plates) for 3 days, followed by (A) immunoblot analysis for the expression of SIGIRR, (B) real-time PCR analysis for the expression of IL-1R1 and IL-23R, and (C) ELISA assay for the production of IL-17 and IFN-γ. (D) Real-time PCR analysis for the expression of IL-17, SIGIRR, IL-1R1 and IL-23R over the time course of Th17 cell polarization. The presented data represent three independent experiments. Error bars, s.d.; *, p<0.05; **.p<0.01 (two tailed t-test).
SIGIRR deficiency leads to increased susceptibility to Th17 cell-dependent EAE
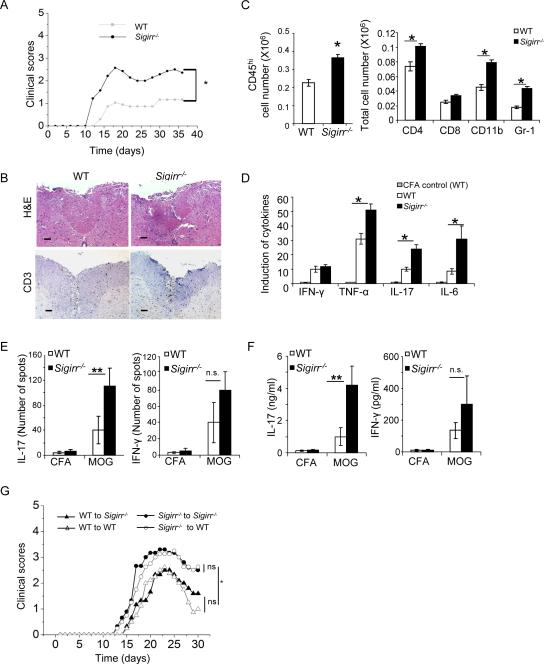
IL-1 signaling in T cells is essential in Th17 cell responses in vivo (Sutton et al., 2006; Ben Sasson et al., 2009). To determine the regulatory role of SIGIRR in IL-1-mediated signaling in Th17 cells, we first examined the impact of SIGIRR deficiency on Th17 cell effector function in vivo. Compared to WT mice, _Sigirr_−/− mice showed an earlier onset of neurologic impairment and markedly increased disease severity compared to WT littermates (Fig. 2A). These data show that SIGIRR is critical for the modulation of MOG35–55-induced EAE in mice. Consistent with the clinical signs, mononuclear cell infiltrates were more prominent in the white matter of spinal cords of MOG35–55 immunized _Sigirr_−/− mice compared to that in WT mice (Fig. 2B–C). Compared with the brain tissues from WT mice, _Sigirr_−/− mice showed substantially more infiltrating CD45+, CD4+, CD11b+ and Gr-1+ cells. In addition, the amount of TNF, IL-17 and IL-6 was increased in the spinal cords of _Sigirr_−/− mice compared to that in control mice (Fig. 2D). Thus, the increased susceptibility of _Sigirr_−/− mice to MOG35–55-induced EAE is associated with increased mononuclear cell infiltration in the CNS during EAE induction and elevated inflammatory cytokines in the spinal cords.
Figure 2. SIGIRR deficiency leads to increased susceptibility of Th17-dependent EAE and hyper activation of MOG-specific Th17 cells.
(A) EAE was induced by MOG35–55 immunization. Mean clinical scores were calculated each day for WT (n = 13) and _Sigirr_−/− mice (n = 10). (B) Hematoxylin and eosin and anti-CD3 staining of spinal cord of WT and _Sigirr_−/−mice 15 days after immunization with MOG35–55. (C) Immune cell infiltration in the brain of MOG35–55 immunized WT and _Sigirr_−/− mice (n=3, 7 days after disease onset) was analyzed by flow cytometry. (D) Real-time PCR analysis of relative expression of IFN-γ, IL-17, TNF-α and IL-6 in spinal cords of MOG35–55 immunized WT and _Sigirr_−/− mice (n=3, 7 days after disease onset) as compared to CFA treated WT control mice. Error bars, s.d.; *, p<0.05; **.p<0.01 (two tailed t-test). Data are representative of three independent experiments. (E–F) Draining lymph node cells from wild-type mice and _Sigirr_−/− mice were collected 10 days after immunization with either MOG35–55 emulsified in complete Freund's adjuvant or complete Freund's adjuvant alone and were re-stimulated with MOG35–55 in vitro for 4 days, followed by ELISPOT analysis (E) and ELISA (F) of IL-17 and IFN-γ. Error bars, s.d.; n = 10 mice per group. p<0.05; **.p<0.01 (two tailed t-test). (G) Primed MOG35–55 specific T cells (10 days) were re-stimulated with MOG35–55 in vitro in the presence of recombinant IL-23 for 4 days, and then transferred to naïve wild-type and _Sigirr_−/− mice. Graph represents the average clinical score after T-cell transfer. n=5. *, p<0.05; (ANOVA). Data are representative of three independent experiments.
To determine the effect of SIGIRR deficiency on the activation of MOG35–55-specific T cells in EAE, we examined responses of _Sigirr_−/− and WT lymph node cells to MOG35–55 in vitro for 10 days after immunization of mice with MOG35–55. Lymph node cells from _Sigirr_−/− mice showed higher frequencies of T cells secreting IL-17, but similar numbers of IFNγ producing cells, compared to WT mice (Fig. 2E). In the recall responses to MOG35–55, supernatants from 10-day primed lymph node cells from _Sigirr_−/− mice showed elevated production of IL-17, but not IFNγ, compared to that in WT mice (Fig. 2F). These results suggest that SIGIRR deficiency enhances Th17 polarization in vivo. The functionality of MOG-specific Th17 from the WT and _Sigirr_−/− mice was further compared by adoptive transfer to WT and _Sigirr_−/− recipient mice to induce EAE. Adoptive transfer of MOG35–55-specific _Sigirr_−/− Th17 cells induced more severe EAE in either WT or _Sigirr_−/− recipient mice compared to WT Th17 cells (Fig. 2G). Thus, SIGIRR deficiency enhances Th17 polarization in vivo, leading to increased severity of IL-17-dependent EAE.
SIGIRR directly regulates Th17 but not Th1 cell development
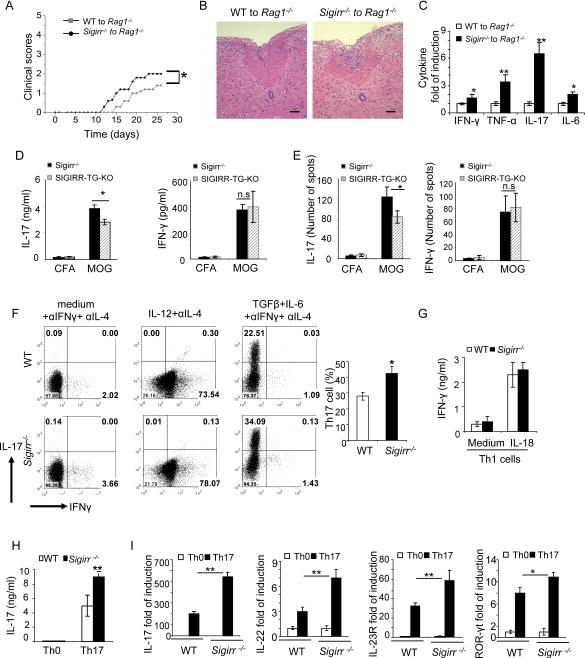
To test the intrinsic role of SIGIRR in T cells, we sorted naïve T cells (CD4+CD44lo) from WT and _Sigirr_−/− mice and transferred them into _Rag1_−/− mice. Upon immunization with MOG35–55, mice with _Sigirr_−/− T cells developed more severe EAE than the mice with WT T cells (Fig 3A). Consistent with the clinical scores, inflammatory cell infiltration and secretion of inflammatory cytokines were significantly increased in the spinal cord of the _Rag1_−/− recipient mice transferred with _Sigirr_−/− T cells compared to that in the mice transferred with WT T cells (Fig 3B–C). Elevated IL-17 mRNA expression in the spinal cord of mice with _Sigirr_−/− T cells supports the regulatory role of SIGIRR on Th17 cell differentiation and expansion (Fig. 3C). To confirm T cell-derived SIGIRR has an intrinsic impact on Th17 cell development, we examined whether T cell-specific expression of SIGIRR is able to rescue the hyper Th17 cell activation in _Sigirr_−/− mice. We generated T cell-specific SIGIRR-transgenic mice using the CD2 promoter to drive the over-expression of SIGIRR, and crossed CD2-SIGIRR onto _Sigirr_−/− mice to generate SIGIRR-TG-KO mice (Lang et al., 1988) (Figure S1). In the recall responses to MOG35–55, supernatants from 10-day primed lymph node cells from SIGIRR-TG-KO mice showed reduced production of IL-17 compared to _Sigirr_−/− mice (Fig 3D). Consistent with ELISA data, the number of MOG35–55 specific IL-17 producing cells, but not IFNγ producing cells, was decreased in SIGIRR-TG-KO mice as compared to that in _Sigirr_−/− mice (Fig 3E). Thus, T cell-specific expression of SIGIRR is able to rescue the hyper Th17 cell activation in _Sigirr_−/− mice.
Figure 3. T-cell-derived SIGIRR regulates Th17 cell development in vivo.
Wild-type and _Sigirr_−/− naïve T cells were intravenousely transferred into _Rag1_−/− mice and EAE was induced by MOG35–55 immunization. (A) Mean clinical scores were calculated each day for _Rag1_−/− mice transferred with wild-type and _Sigirr_−/− T cells. n=5 *, p<0.05; (ANOVA). (B) Hematoxylin and eosin staining of spinal cord of _Rag1_−/− mice transferred with wild-type and _Sigirr_−/− T cells 15 days after immunization with MOG35–55. (C) Real-time PCR analysis of relative expression of IFN-γ, IL-17, TNF-α and IL-6 in spinal cords of MOG35–55 immunized _Rag1_−/− mice transferred with wild-type and _Sigirr_−/− T cells (n=3, 10 days after disease onset). (D–E) Draining lymph node cells from SIGIRR-TG-KO (TG-KO) and littermate _Sigirr_−/− mice were collected 10 days after immunization with MOG35–55 emulsified in complete Freund's adjuvant and were re-stimulated with MOG35–55 in vitro for 4 days, followed by ELISA (D) and ELISPOT analysis (E) (See also Figure S1), Error bars, s.d.; *, p<0.05; **.p<0.01 (two tailed t-test). Data are representative of three independent experiments. (F–H) Naïve T cells (CD4+CD44low) from wild-type and _Sigirr_−/− mice were co-cultured with wild-type splenic antigen-presenting cells (APCs) in the presence of anti-CD3 and polarized them under Th1 (IL-12 and anti-IL-4), or Th17 (TGF-β, IL-6, anti-IFN-γ and anti-IL-4) conditions, followed by intracellular cytokine staining for IL-17 and IFN-γ (F) and ELISA for IL-17 (H). (G) Wild-type and _Sigirr_−/− mice Th1 cells were treated with 5ng/ml IL-18 for 48 h, followed by ELISA for IFN-γ. (I) Real-time PCR analysis of relative expression of IL-17, IL-22, IL-23R, ROR-γt and IL-10 in wild-type and _Sigirr_−/− Th17 cells as compared to naïve T cells. Data are representative of at least three (A–C) independent experiments. Error bars (A–C), s.d. *, p<0.05; **.p<0.01 (two tailed t-test).
To further determine whether SIGIRR has direct impact on Th17 cells, we co-cultured naïve T cells from WT and _Sigirr_−/− mice with WT splenic DCs and differentiated them into Th1 and Th17 cell lineages. The frequency of IFNγ-positive cells as well as amount of secreted IFNγ from Th1 cell differentiated cultures was indistinguishable between WT and _Sigirr_−/− cells (Fig. 3F), suggesting that SIGIRR does not have a substantial impact on Th1 cell differentiation. Furthermore, similar amounts of IFN-γ were detected in WT and _Sigirr_−/− Th1 cells in response to IL-18 stimulation (Fig. 3G). In contrast, the frequency of IL-17-positive cells and total IL-17 production were significantly increased in _Sigirr_−/− Th17 cells compared to WT (Fig. 3F and 3H). In addition to IL-17, the expression of other Th17-associated molecules (including IL-17, IL-22, and IL-23R) was also higher in _Sigirr_−/− Th17 cells than that in WT cells (Fig. 3I). Thus, SIGIRR directly regulates Th17 but not Th1 cell development.
SIGIRR suppresses Th17 cell differentiation and proliferation through IL-1R signaling
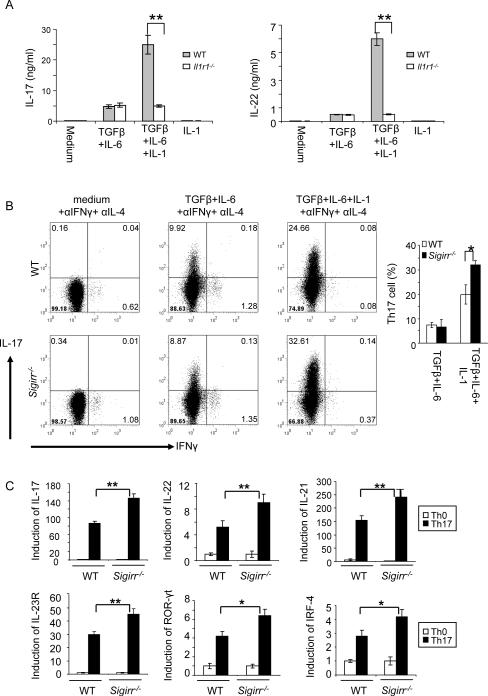
IL-1 signaling in T cells synergizes with IL-6 to regulate TH17 cell differentiation and maintain cytokine production in effector Th17 cells (Chung et al., 2009). To confirm the role of IL-1 signaling in Th17 cell differentiation and maintenance, we purified naïve T cells from WT and _Il1r1_−/− mice and differentiated them into Th17 cells (TGFβ+IL-6) with or without IL-1β. IL-1β indeed enhanced Th17 cell differentiation, which was abolished in the absence of IL-1R (Fig. 4A). SIGIRR inhibits IL-1R-TLR signaling through its interaction with the receptor complexes. We hypothesize that the impact of SIGIRR on Th17 cell differentiation and proliferation is due to enhanced IL-1R signaling in these cells. To test this hypothesis, WT and _Sigirr_−/− naïve T cells were differentiated into Th17 cells with or without IL-1β stimulation. Similar numbers of IL-17-positive cells (~8–10%) were detected in WT and _Sigirr_−/− T cells under TGFβ+IL-6-mediated differentiation condition (in the absence of IL-1β stimulation) (Fig. 4B). Importantly, although IL-1β promoted Th17 cell differentiation in both WT and _Sigirr_−/− T cells, addition of IL-1β resulted in a significantly higher IL-17-positive population in _Sigirr_−/− T cells than that in WT T cells (Fig. 4B). Consistent with flow cytometry analysis, the expression of other Th17 cell-associated molecules (including IL-17, IL-21, IL-22, and IL-23R) was also much higher in _Sigirr_−/− T cells than that in WT cells in the presence of IL-1β stimulation (Fig. 4C). In addition, consistent with IL-1 induction of transcription factor IRF4 and RORγt expression, SIGIRR deficiency significantly enhanced the expression of IRF4 and RORγt during Th17 cell differentiation (Fig. 4C). Thus, SIGIRR negatively regulates Th17 cell differentiation and proliferation by suppressing IL-1R signaling during differentiation of Th17 cells.
Figure 4. SIGIRR suppresses Th17 differentiation and expansion through IL-1R signaling.
(A) Naïve wild-type and _Il1r1_−/− CD4+ T cells (CD4+CD44low) were polarized to Th17 cells (TGFβ+IL-6 on anti-CD3 and anti-CD28 coated plates) in the presence and absence of IL-1β, followed by ELISA for IL-17 and IL-22. (B) Naïve wild-type and _Sigirr_−/− CD4+ T cells (CD4+CD44low) were polarized to Th17 cells (TGFβ+IL-6 on anti-CD3 and anti-CD28 coated plates) in the presence and absence of IL-1β, followed by intracellular cytokine staining for IL-17 and IFN-γ. While IL-1β promoted Th17 cell differentiation in both WT and _Sigirr_−/− T cells, addition of IL-1β resulted in a significantly higher IL-17-positive population in _Sigirr_−/− T cells than that in WT T cells. (C) Real-time PCR analysis of relative expression of IL-17, IL-22, IL-21, IL-23R, ROR-γt, IL-10 and IRF4 in wild-type and _Sigirr_−/− Th17 cells as compared to the naïve T cells. Data are representative of at least three (A–C) separate experiments. Error bars (AC), s.e.m. *, p<0.05; **.p<0.01 (two tailed t-test).
SIGIRR deficiency leads to increased IL-1-induced JNK and mTOR phosphorylation
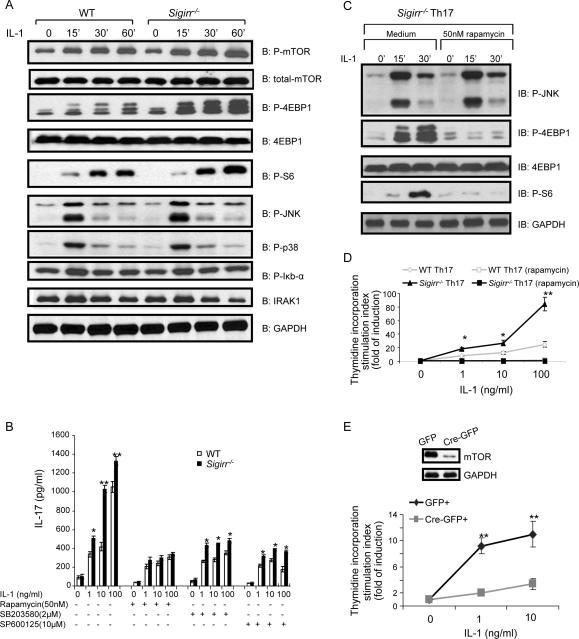
To understand the molecular mechanism by which SIGIRR regulates IL-1-dependent Th17 cell differentiation and proliferation, we examined IL-1 signaling in WT and _Sigirr_−/− Th17 cells. Although IL-1 stimulation induced p38 and IκBα phosphorylation in both WT and _Sigirr_−/− Th17 cells, IL-1 treatment leads to increased JNK and mTOR phosphorylation in _Sigirr_−/− Th17 cells compared to that in WT cells (Fig. 5A). Consistent with this, IL-1-dependent phosphorylation of 4E-BP1 (eukaryotic initiation factor 4E-binding protein, an mTOR substrate) and S6 (a downstream component of mTOR signaling) were also increased in _Sigirr_−/− Th17 cells compared to that in WT cells. Thus, SIGIRR deficiency markedly enhances at least some of the IL-1-mediated signaling cascades in Th17 cells.
Figure 5. SIGIRR suppresses Th17 expansion through IL-1-induced mTOR-mediated cell proliferation.
(A) Cell lysates from wild-type and _Sigirr_−/− Th17 cells untreated or treated with IL-1 (10ng/ml) for different time points were analyzed by western blot analysis using antibodies as indicated. (B) Wild-type and _Sigirr_−/− Th17 cells were rested for two days, followed by incubation with different doses of IL-1 as indicated in the presence or absence of 50nM rapamycin, 2μM SB203580 or 10μM SP600125 for three days. The treated cells were analyzed for production of IL-17 by ELISA. (C) Cell lysates from _Sigirr_−/− Th17 cells pre-treated with rapamycin for 2h and then treated IL-1 (10ng/ml) treatment for 15, 30 and 60 min were analyzed by western blot analysis using antibodies as indicated. (D) Wild-type and _Sigirr_−/− Th17 cells were rested for two days, followed by incubation with different doses of IL-1. The treated cells were incubated one additional day with 3H for thymidine incorporation experiment. (E) _Frap1_fl/fl naïve CD4+ T cells were differentiated into Th17 cells and infected with retrovirus expressing GFP or Cre/GFP. Infected CD4+GFP+ cells were isolated, analyzed for mTOR expression (left), and cultured in the presence and absence of IL-1. GFP+ cells were analyzed by thymidine incorporation assay for proliferation (right). Error bars, s.d.; *, p<0.05; **.p<0.01 (two tailed t-test). Data are representative of three independent experiments.
To determine the impact of SIGIRR-modulated IL-1-induced signaling events on Th17 effector function, we used specific inhibitors to block these IL-1-induced signaling cascades in WT and _Sigirr_−/− TH17 cells, including inhibitors for mTOR (rapamycin), p38 (SB203580) and JNK (SP600125). All three inhibitors significantly reduced IL-1-induced IL-17 production in both WT and _Sigirr_−/− Th17 cells, indicating the importance of mTOR, JNK and p38 activation for IL-1-induced Th17 effector function (Fig. 5B). Although the inhibitors of p38 and JNK decreased IL-1-induced IL-17 production in both WT and _Sigirr_−/− Th17 cells, _Sigirr_−/− Th17 cells still produced more IL-17 in the presence of either inhibitor compared to WT cells. In contrast, the impact of SIGIRR deficiency on Th17 cell effector function was abolished after rapamycin treatment (Fig. 5B). Similar residual amounts of IL-17 production were detected in IL-1-stimulated WT and _Sigirr_−/− Th17 cells after rapamycin treatment, suggesting that the mTOR-mediated signaling is critical for SGIRR to modulate IL-1-dependent Th17 cell effector function. The inhibitory effect of rapamycin is specific for the mTOR pathway because rapamycin treatment inhibited the IL-1-induced 4E-BP1 and S6 but not JNK phosphorylation (Fig. 5C).
Because mTOR signaling is known to have a major impact on cell growth and proliferation, we examined WT and _Sigirr_−/− TH17 cell proliferation in response to IL-1 stimulation. Whereas IL-1 induced proliferation in both WT and _Sigirr_−/− Th17 cells, the IL-1-induced cell proliferation was much higher in _Sigirr_−/− Th17 cells than that in WT cells (Fig. 5D). These results strongly suggest that SIGIRR deficiency has a substantial impact on Th17 cell proliferation, which leads to increased production of cytokines associated with Th17 cells. Importantly, rapamycin abolished IL-1-induced cell proliferation in both WT and _Sigirr_−/− Th17 cells. Thus, one important mechanism for SIGIRR to suppress Th17 proliferation and effector function is through IL-1-induced mTOR-mediated cell proliferation. To exclude the possible non-specific effect of rapamycin, we examined the responsiveness of WT and mTOR-deficient Th17 cells to IL-1 stimulation. Because _Frap1_−/− T cells are unable to differentiate into Th17 cells, we first differentiated _Frap1_fl/fl (wild-type) naïve T cells into Th17 cells. The differentiated Th17 cells (_Frap1_fl/fl) were infected with hCre-GFP-RV to delete mTOR (Delgoffe et al., 2009; Zhu et al., 2004) (Fig. 5E). Whereas IL-1 stimulation increased the proliferation of WT Th17 cells, the proliferation of mTOR-deficient cells was abolished in response to IL-1 treatment (Fig 5E). Thus, IL-1-induced Th17 expansion is mTOR-dependent.
SIGIRR-modulated IL-1-activated mTOR pathway signals through the IRAK proteins
We next investigate how SIGIRR modulates IL-1-mediated mTOR activation. Upon IL-1 stimulation, adaptor MyD88 is recruited to the IL-1 receptor, followed by the recruitment of serine and threonine kinases IRAK4 and IRAK1 (interleukin-1 receptor-associated kinases) (Yao et al., 2007). At the receptor complex, IRAK4 mediates the phosphorylation of IRAK1, followed by the ubiquitination and degradation of IRAK1, which are crucial for the activation of downstream signaling cascades (Yao et al., 2007). We have previously shown that SIGIRR negatively regulates IL-1 signaling through its interaction with the receptor complex, attenuating the recruitment of these receptor proximal signaling components to the receptor (including MyD88, IRAK4 and IRAK1) (Qin et al., 2005). Consistent with this, we found that IRAK1 degradation was accelerated in _Sigirr_−/− Th17 cells in response to IL-1 stimulation, demonstrating the impact of SIGIRR deficiency on IL-1-induced IRAK1 activation in Th17 cells (Fig. 5A).
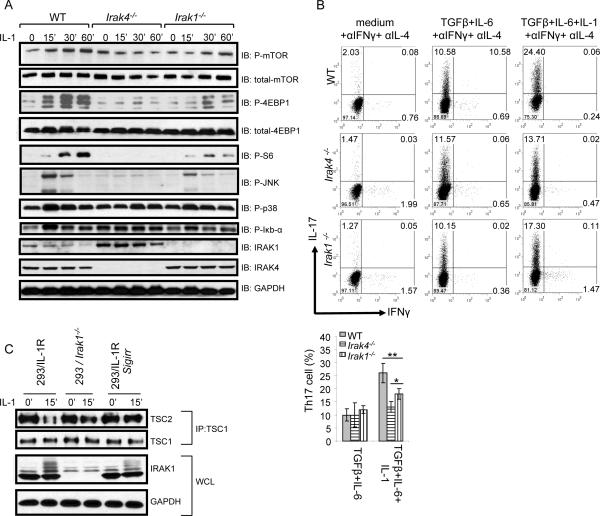
We and others have recently reported that IRAK4 and IRAK1 are required for Th17 cell polarization and development of EAE (Staschke et al., 2009). Therefore, we wondered whether SIGIRR modulates IL-1-mediated mTOR activation and the IL-1-dependent Th17 cell proliferation through the IRAK proteins. To test this hypothesis, we examined the impact of IRAK4 and IRAK1 deficiency on IL-1-induced mTOR signaling and IL-1-dependent Th17 cell development. IL-1-induced phosphorylation of mTOR, 4EBP, and S6 were all abolished in IRAK4-deficient Th17 cell and greatly reduced in IRAK1-defcient Th17 cells (Fig. 6A), indicating that IRAK4 and IRAK1 are indeed required for IL-1-induced activation of mTOR and its downstream signaling components. Consistent with this, IL-1-induced Th17 cell proliferation was significantly reduced in IRAK4- and IRAK1-deficient Th17 cells, implicating the importance of IRAK4 and IRAK1-mediated mTOR activation in IL-1-induced Th17 cell proliferation (Fig. 6B). Taken together, these results suggest that SIGIRR modulates Th17 cell expansion through the IRAK4-IRAK1-mTOR pathway. Interestingly, IRAK4 and IRAK1 were required for IL-1-induced mTOR and JNK but not p38 activation (Fig. 6A). Consistent with this, SIGIRR preferentially affected mTOR and JNK activation, but not p38 in response to IL-1 stimulation, which further supports the notion that SIGIRR modulates IL-1-mediated downstream signaling through its impact on the activation of IRAK4 and IRAK1 (Fig. 6A)
Figure 6. IL-1 activates mTOR pathway through IRAK proteins.
(A) Cell lysates from wild-type, _Irak4_−/− and _Irak1_−/− Th17 cells untreat ed or treated with IL-1 (10ng/ml) for different time points were analyzed by western blot analysis using antibodies as indicated. (B) Naïve wild-type, _Irak4_−/− and _Irak1_−/− CD4+ T cells (CD4+CD44low) were polarized to Th17 cells (TGFβ+IL-6 on anti-CD3 and anti-CD28 coated plates) in the presence and absence of IL-1β, followed by intracellular cytokine staining for IL-17 and IFN-γ, Error bars, s.d.; *, p<0.05; **.p<0.01 (two tailed t-test). (C) 293 cells transfected with IL-1R (293-IL-1R) cells, IRAK1-deficient cells and 293-IL-1R transfected with SIGIRR were treated or untreated with IL-1 for 15 min. TSC1/TSC2 protein complex was immunoprecipitated and analyzed by western blot analysis using antibodies as indicated. Data are representative of at least three independent experiments.
The tuberous sclerosis 1 (TSC1)-TSC2 tumor suppressor complex serves as a repressor of the mTOR pathway, and the disruption of TSC1-TSC2 complex leads to mTOR activation(Lee et al., 2007). We found that IL-1 stimulation can also lead to disruption of TSC1-TSC2 complex in human 293 cells transfected with IL-1R (293-IL-1R). Importantly, IL-1-mediated disruption of TSC1-TSC2 complex was greatly reduced in IRAK1-deficient cells derived from 293-IL-1R cells (Li et al., 1999), suggesting that IRAK1-dependent mTOR activation is through the disruption of TSC1-TSC2 complex (Fig. 6C). Overexpression of SIGIRR suppressed the IL-1-induced disruption of TSC1-TSC2 complex, which is likely due to the inhibitory effect of SIGIRR on IRAK1 activation (as evident by reduced IRAK1 modification upon SIGIRR overexpression) (Fig. 6C). Taken together, these results suggest that SIGIRR modulates IL-1-induced mTOR activation through its impact on IRAK1 activation and consequent effect on IRAK1-mediated disruption of TSC1-TSC2 complex.
Discussion
We report here a mechanism for the regulation of Th17 cell differentiation and expansion. IL-1-mediated signaling in T cells is essential for in vivo Th17 cell differentiation and IL-17-dependent autoimmune diseases. We showed here that SIGIRR is induced during Th17 cell differentiation and it suppresses Th17 cell differentiation and proliferation through direct inhibition of multiple IL-1-dependent signaling pathways. IL-1-induced phosphorylation of JNK and mTOR is enhanced in _Sigirr_−/− Th17 cells. Rapamycin, an inhibitor of mTOR specifically blocked SIGIRR-regulated IL-1-induced Th17 cell proliferation and cytokine production. Importantly, IL-1-dependent proliferation was abolished in mTOR-deficient Th17 cells, confirming the essential role of mTOR activation in IL-1-dependent Th17 cell proliferation. We also showed that SIGIRR is critical for the control of Th17 cell-dependent development of CNS autoimmune inflammation. In the absence of this IL-1 regulatory mechanism, Th17 cells infiltrated the CNS in greater numbers and showed enhanced pathogenic functions. Thus, SIGIRR belongs to a class of immune modulators, including IL-2, IL-4, IL-25, IL-27, IFNγ, IFNα, and ATAR, capable of suppressing the development and function of IL-17-producing T cells.
Both IL-1R1 and IL-23R are induced by IL-6-dependent RORγt expression (Chung et al., 2009). Although stimulation with TGF-β and IL-6 is sufficient to drive the initial development of IL-17-producing T cells in vitro, _Il1r1_−/− T cells can not differentiate into inflammatory Th17 cells in vivo. Accordingly, IL-1 signaling in T cells is absolutely essential for the induction of autoimmune encephalomyelitis (Sutton et al., 2006; Chung et al., 2009). In support of this, IRAK4 (a key kinase in IL-1 signaling) kinase-inactive (IRAK4 KI) Th17 cells fail to induce EAE in either WT or IRAK4 KI recipient mice (Staschke et al., 2009). In contrast, WT Th17 cells are able to induce EAE in both WT and IRAK4 KI recipient mice, indicating that IL-1 signaling in the CNS resident cells is not essential for IL-1-dependent induction of EAE disease. Further evidence supporting a direct effect of IL-1 on T cells came from adoptive transfer of OVA-TCR transgenic T cells into _Il1r1_−/− recipients, which demonstrate that T cells are a major target of IL-1 in vivo (Ben Sasson et al., 2009). In addition, deficiency of IL-1R1 on T cells but not on DCs blocked Th17 cell polarization(Chung et al., 2009). Consistent with these previous results, we found that the lack of SIGIRR on T cells leads to increased autoantigen-induced Th17 cell polarization in vivo. Importantly, Th17 cell differentiation and cytokine production were significantly increased in _Sigirr_−/− T cells after they were polarized in the presence of WT DCs, indicating that deficiency of SIGIRR on T cells—not DCs—results in increased Th17 cell development. It is also noteworthy that WT Th17 cells were able to induce similar EAE severity in both WT and _Sigirr_−/− recipients, demonstrating that SIGIRR deficiency does not have a major impact on the responses of APCs and CNS resident cells to autoantigen-specific Th17 cells.
Recent studies have shown that IL-1 stimulation enhances the expression of transcription factors IRF4 and RORγt, indicating that IL-1 is capable of directly influencing Th17 cell differentiation (Chung et al., 2009). It is thought that naïve T cell-derived TGF-β in the presence of exogenous IL-6 is sufficient to initiate the RORγt-dependent gene expression program of Th17 cells. However, further stabilization of the Th17 cell program is required for their in vivo function. We and others have demonstrated that two additional signaling pathways are essential for this stabilizing effect; these include the IL-1R and IL-23R pathways (Chung et al., 2009; McGeachy and Cua, 2007; McGeachy and Cua, 2008). Consistent with the concept that SIGIRR has a central role in Th17 cell differentation through the regulation of IL-1 signaling, SIGIRR deficiency did not impact Th17 cell differentiation induced by TGFβ+IL-6 in vitro. It is only in the presence of exogenous IL-1 (TGFβ+IL-6+IL-1) that we observed enhanced Th17 cell proliferation and cytokine production in _Sigirr_−/− T cells. Our findings also suggest that SIGIRR negatively regulates Th17 cel differentiation and proliferation by suppressing IL-1 signaling during initial differentiation of Th17 cells as well as in differentiated effector Th17 cells. Furthermore, the absence of SIGIRR regulation enabled the overexpression of IRF4 and RORγt that is mediated by IL-1 in the presence of TGFβ and IL-6, which might account for the increased expression of IL-17 and other Th17 cell -associated cytokines in _Sigirr_−/− T cells. Hence our findings are consistent with the known effects of IL-1 on Th17 cell transcriptional regulation, where SIGIRR is a key regulator to prevent over-activation of Th17-mediated pathogenic effects.
What specific IL-1-mediated signaling events are critical for Th17 differentiation and effector function and how they are modulated by SIGIRR? Although IL-1 induced similar amounts of p38 phosphorylation in WT and _Sigirr_−/− Th17 cells, SIGIRR deficiency allowed greater IL-1-induced phosphorylation of JNK, mTOR and 4E-BP1 (a substrate of mTOR). We showed that the inhibitors of p38, JNK and mTOR all decreased IL-1-induced IL-17 production in both WT and _Sigirr_−/− Th17 cells. However, only rapamycin abolished the impact of SIGIRR deficiency on Th17 cell effector function, indicating the critical role of mTOR pathway for SIGIRR-mediated modulation on Th17 cell function. In addition, the mTOR activated pathways have been linked to cell cycle progression and indeed, IL-1 induced more Th17 cell proliferation in _Sigirr_−/− Th17 cells than that in WT cells. Consistent with our findings, a recent study also reported that IL-1 signaling maintains Th17 cells in the absence of TCR stimuli (Chung et al., 2009). Thus, whereas IL-1 signaling contributes to tissue immunity mediated by Th17 cells by promoting Th17 cell differentiation and maintenance, SIGIRR-modulated IL-1-induced mTOR pathway is essential to ensure a stringent control for the proliferation of potentially pathogenic Th17 cells. The fact that p38 and JNK inhibitors reduced IL-1-induced IL-17 production in WT and _Sigirr_−/− Th17 cells indicates that these MAPKs are also required for IL-1-induced Th17 effector function. Future studies are required to identify the specific substrates of p38 and JNK that are critical for Th17 cell function.
We found that IL-1-mediated disruption of TSC1-TSC2 complex was greatly reduced in IRAK1-deficient cells derived from 293-IL-1R cells, indicating that IL-1-induced IRAK1-dependent mTOR activation is also through the disruption of TSC1-TSC2 complex. Consistent with the negative regulation of IRAK1 activation by SIGIRR, SIGIRR overexpression suppressed IL-1-induced IRAK1 modification and as well as the disruption of TSC1-TSC2. IKKβ interacts with and phosphorylates TSC1, resulting in suppression of TSC1, which plays a critical role in IL-1-mediated mTOR activation (Lee et al., 2007). Because IRAK4 and IRAK are known to mediate the IL-1-induced IKKβ activation, it is possible that IRAK4 and IRAK1 mediate mTOR activation through the activation of IKKβ. Future studies are required to elucidate the detailed signaling cascade for IL-1-induced IRAK4 and IRAK1-mediated mTOR activation.
We have defined the molecular mechanisms for Th17 cell survival and competition for resources in vivo, which explains why Th17 cells are completely absent in IL-1R-deficient mice (Sutton et al., 2006). Compared to in vitro cultures, activating CD4+ T helper cells in lymph nodes are exposed to relatively low nutrient and antigen load. In this restrictive in vivo environment, IL-1 activation of the mTOR (nutrient sensor and cell cycle regulator), p38, and JNK pathways enable Th17 cells to divide faster and dominate an immune response compared to Th1 cells that might have the same antigenic specificity. Hence, presence of IL-1 during an inflammatory response will give Th17 cells important survival and functional advantages over other T cell subsets. In this study, we also identified SIGIRR as the key regulator of IL-1 function in Th17 cells. Our earlier studies have shown that SIGIRR can regulate innate immunity; however, as demonstrated in the present study, SIGIRR is expressed on Th17 cells and directly regulates adaptive immunity. In support of this, recent studies have demonstrated critical roles of SIGIRR in modulating lupus autoantigen priming andantigen-specific anti-microbial responses (Bozza et al., 2008; Lech et al., 2008). Based on these observations, we propose that SIGIRR is an important negative “feedback switch” that prevents detrimental autoimmune and chronic inflammatory responses. Given the potency of rapamycin for blockade of SIGIRR-IL-1-regulated TH17 cell proliferation and cytokine production, it may be feasible to consider this well-developed drug and related compounds for treatment of autoimmune and chronic inflammatory diseases.
Experimental Procedures
Mice
_Il1r1_−/−, and _Rag1_−/− mice were purchased from Jackson Laboratory. _Sigirr_−/− mice were generated as described (Wald et al., 2003). The CD2-SIGIRR construct contains an _EcoRIBam_HI cDNA fragment, coding for the SIGIRR protein, inserted between 5 kilobase (kb) upstream and 5.5 kb downstream flanking sequences of the human CD2 gene (Lang et al., 1988). A Flag tag was included at the N-terminus of SIGIRR to distinguish the transgene from the endogenous gene. The founders that carry the SIGIRR transgene were identified by genomic Southern analysis with SIGIRR cDNA as probes. SIGIRR transgenic mice (CD2-SIGIRR) were bred to _Sigirr_−/− mice to generate SIGIRR-TG-KO mice. Mice were housed in animal facility (SPF condition) at the Cleveland Clinic Foundation in compliance with the guidelines set by Institutional Animal Care and Use Committee.
T cell differentiation
Naïve CD4+CD44lo T cells from _Sigirr_−/−, C57BL/6 and _Il1r1_−/−mice were sorted by flow cytometry and activated with 1 mg/ml anti-CD3 and splenic APCs in the presence of 5 ng/ml TGF-β and 10 ng/ml IL-6 (Peprotech). For anti-CD3 and anti-CD28-stimulated differentiation, naïve sorted CD4+CD44lo T cells were activated with plate-bound 1 mg/ml anti-CD3 and 1 mg/ml anti-CD28 and in the presence of 5 ng/ml TGF-β (Peprotech), 20 ng/ml IL-6 (Peprotech), anti-IL-4 (11B11), 5 mg/ml anti-IFN-γ (XMG 1.2), 10 ng/ml IL-1β (National Cancer Institute) or combination of these stimuli. Three days after activation, cytokines in the supernatant were measured by ELISA. For intracellular staining, cells were stimulated with PMA and ionomycin in the presence of Golgi-stop for 4 hr, after which IL-17- and IFN-γ-producing cells were analyzed with intracellular staining.
Transfection, coimmunoprecipitations and Western analysis
Procedures for transfection, coimmunoprecipitations and western analysis using 293 cells were previously described (Yao et al., 2007). Antibodies used in this study include primary anti-mouse SIGIRR antibody (R&D systems), β-actin, IRAK1 (Santa Cruz), p-4EBP1, 4EBP1, p-IκBα, p-JNK, p-p38, p-mTOR, mTOR, p-S6, TSC1, and TSC2 (Cell Signaling) and GAPDH (Ambion).
EAE Induction and Adoptive transfer experiment
Procedures for EAE induction and adoptive transfer experiment were previously described (Qian et al., 2007). For EAE induction of _Rag1_−/− mice, CD4+ T cells from wild type and _Sigirr_−/−mice were sorted by AutoMacs and intravenousely and transferred into _Rag1_−/− mice (7×106 cells/transfer). One day later, the recipient mice were immunized with MOG(35–55) as described above.
Quantitative Real-Time RT-PCR
Expression of the genes encoding IL-17A, IL-23R, IL-22, IL-21, IL-10 and IRF-4 were quantified with the SYBER Green PCR Master Mix kit (Applied Biosystems) using primer pairs selected for amplification of each individual cytokine: Il17a: 5'-CTCCACCGCAATGAAGAC-3' and 5'- CTTTCCCTCCGCATTGAC-3': Il23r: 5'-TTTTGTCGGGAATGGTCTTC-3' and 5'- GCCACTTTGGGATCATCAGT-3'; Il22: 5'-TCATCGGGGAGAAACTGTTC-3' and 5'- CATGTAGGGCTGGAACCTGT-3'; Il10: 5'-TGAATTCCCTGGGTGAGAAG-3' and 5'-TTCATGGCCTTGTAGACACCT-3'; Il21: 5'-CGCCTCCTGATTAGACTTCG-3' and 5'-TTGAGTTTGGCCTTCTGAAAA-3'; β-actin: 5'-GGTCATCACTATTGGCAACG-3' and 5'- ACGGATGTCAACGTCACACT-3'; Irf4: 5'-TCCTCTGGATGGCTCCAGATGG-3', and, 5'-CACCAAAGCA CAGAGTCACCTG-3'. Relative gene expression was determined as the ratio of cytokine to β-actin gene-expression levels for each sample.
Proliferation
Th17 cells were cultured in 96-well flat-bottom microtiter Falcon plates (BD Labware) at 20×103 cells/well in DMEM (Mediatech CellGro) supplemented with 10% FBS (HyClone), 5% HEPES buffer, 2% L-glutamine, and 1% penicillin/streptomycin (Invitrogen Life Technologies). IL-1β was added in serial dilutions to triplicate wells with negative control wells (without IL-1β). Cells were cultured at a final volume of 200 μl/well. In some wells, rapmaycin (50nM) (Sigma) was added. After incubation for 96 h, wells were pulsed with [_methyl_-3H]thymidine (1.0 μCi/well, specific activity 6.7 Ci/mM; New England Nuclear) and harvested 16 h later by aspiration onto glass fiber filters. For proliferation assay, levels of incorporated radioactivity were determined by scintillation spectrometry. Results are expressed as mean cpm of experimental cultures with IL-1β divided by mean cpm of cultures without IL-1β (stimulation index).
Retroviral infection
Frap1fl/fl naïve T cells were sorted and differentiated into Th17 cells on anti-CD3 and anti-28 coated plate as described above. After differentiation, Th17 cells were rested for two days and re activated with 0.2 mg/ml anti-CD3 and splenic APCs in the presence of 5ng/ml TGF-β (Peprotech) and 10 ng/ml IL-6 (Peprotech). Twenty-four hours after activation, the cells were infected by retroviruses expressing either Cre–GFP (a gift from Dr. William Paul at National Institute of Health), or control empty vector (containing only IRES-GFP). Four days after infection, the cells were washed; CD4+GFP+ cells were sorted and cultured in the presence and absence of IL-1 for three days. At the end of three days GFP+ cells were analyzed for cell proliferation by thymidine incorporation assay.
Statistics
ANOVA was used for EAE clinical score studies. The Student's t test was used to assess all other statistical values. p values were determined, and error bars represent standard error of the mean (SEM).
Acknowledgement
This work was supported by grants from NIH (RO1 Al060632 to X.L.) and a grant from National Multiple Sclerosis Society (X.L.). The plasmid p5' _CD2/coding_- CD2 and p3' CD2 was provided by D. Kioussis of the National Institute for Medical Research, London, UK. Cre-GFP-RV was a generous gift from Dr. William Paul at National Institute of Health. We thank Dr. James Thomas (UT Southwestern) for providing us with the IRAK1−/− mice and Dr. Wen-chen Yeh (Amgen) for providing us with the IRAK4−/− mice.
Reference List
- Andoh A, Yasui H, Inatomi O, Zhang Z, Deguchi Y, Hata K, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-17 augments tumor necrosis factor-alpha-induced granulocyte and granulocyte/macrophage colony-stimulating factor release from human colonic myofibroblasts. J. Gastroenterol. 2005;40:802–810. doi: 10.1007/s00535-005-1632-x. [DOI] [PubMed] [Google Scholar]
- Bamba S, Andoh A, Yasui H, Araki Y, Bamba T, Fujiyama Y. Matrix metalloproteinase-3 secretion from human colonic subepithelial myofibroblasts: role of interleukin-17. J. Gastroenterol. 2003;38:548–554. doi: 10.1007/s00535-002-1101-8. [DOI] [PubMed] [Google Scholar]
- Ben Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D'Angelo C, Giovannini G, Garlanda C, Boon L, Bistoni F, Puccetti P, Mantovani A, Romani L. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J. Immunol. 2008;180:4022–4031. doi: 10.4049/jimmunol.180.6.4022. [DOI] [PubMed] [Google Scholar]
- Brereton CF, Sutton CE, Lalor SJ, Lavelle EC, Mills KH. Inhibition of ERK MAPK suppresses IL-23- and IL-1-driven IL-17 production and attenuates autoimmune disease. J. Immunol. 2009;183:1715–1723. doi: 10.4049/jimmunol.0803851. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Fossiez F, Djossou O, Chomarat P, Flores-Romo L, it-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das MB, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Di LD, Vecchi A, La Manna MP, Buracchi C, Caccamo N, Salerno A, Dieli F, Mantovani A. Damping excessive inflammation and tissue damage in Mycobacterium tuberculosis infection by Toll IL-1 receptor 8/single Ig IL-1-related receptor, a negative regulator of IL-1/TLR signaling. J. Immunol. 2007;179:3119–3125. doi: 10.4049/jimmunol.179.5.3119. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, Muzio M, Bergottini R, Scanziani E, Vecchi A, Hirsch E, Mantovani A. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garcia I, Zhao Y, Ju S, Gu Q, Liu L, Kolls JK, Lu B. IL-17 signaling-independent central nervous system autoimmunity is negatively regulated by TGF-beta. J. Immunol. 2009;182:2665–2671. doi: 10.4049/jimmunol.0802221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, Gold R. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell Immunol. 2005;237:123–130. doi: 10.1016/j.cellimm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J. Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, Skoogh BE, Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- Lang G, Wotton D, Owen MJ, Sewell WA, Brown MH, Mason DY, Crumpton MJ, Kioussis D. The structure of the human CD2 gene and its expression in transgenic mice. EMBO J. 1988;7:1675–1682. doi: 10.1002/j.1460-2075.1988.tb02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Lech M, Kulkarni OP, Pfeiffer S, Savarese E, Krug A, Garlanda C, Mantovani A, Anders HJ. Tir8/Sigirr prevents murine lupus by suppressing the immunostimulatory effects of lupus autoantigens. J. Exp. Med. 2008;205:1879–1888. doi: 10.1084/jem.20072646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC, He X, Hung JY, Lai CC, Ding Q, Su JL, Yang JY, Sahin AA, Hortobagyi GN, Tsai FJ, Tsai CH, Hung MC. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440–455. doi: 10.1016/j.cell.2007.05.058. [DOI] [PubMed] [Google Scholar]
- Li X, Commane M, Burns C, Vithalani K, Cao Z, Stark GR. Mutant cells that do not respond to interleukin-1 (IL-1) reveal a novel role for IL-1 receptor-associated kinase. Mol. Cell Biol. 1999;19:4643–4652. doi: 10.1128/mcb.19.7.4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin. Immunol. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- McGuirk P, Mills KH. Pathogen-specific regulatory T cells provoke a shift in the Th1/Th2 paradigm in immunity to infectious diseases. Trends Immunol. 2002;23:450–455. doi: 10.1016/s1471-4906(02)02288-3. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat. Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- Qin J, Qian Y, Yao J, Grace C, Li X. SIGIRR inhibits interleukin-1 receptor- and toll-like receptor 4-mediated signaling through different mechanisms. J. Biol. Chem. 2005;280:25233–25241. doi: 10.1074/jbc.M501363200. [DOI] [PubMed] [Google Scholar]
- Staschke KA, Dong S, Saha J, Zhao J, Brooks NA, Hepburn DL, Xia J, Gulen MF, Kang Z, Altuntas CZ, Tuohy VK, Gilmour R, Li X, Na S. IRAK4 kinase activity is required for Th17 differentiation and Th17-mediated disease. J. Immunol. 2009;183:568–577. doi: 10.4049/jimmunol.0802361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O'Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PC. Antibody therapy for rheumatoid arthritis. Curr. Opin. Pharmacol. 2003;3:323–328. doi: 10.1016/s1471-4892(03)00032-8. [DOI] [PubMed] [Google Scholar]
- Teunissen MB, Koomen CW, de Waal MR, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- Thakker P, Leach MW, Kuang W, Benoit SE, Leonard JP, Marusic S. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J. Immunol. 2007;178:2589–2598. doi: 10.4049/jimmunol.178.4.2589. [DOI] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wald D, Qin J, Zhao Z, Qian Y, Naramura M, Tian L, Towne J, Sims JE, Stark GR, Li X. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 2003;4:920–927. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, Tuohy VK, Fairchild RL, de la MC, Cua D, Vallance BA, Li X. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Kim TW, Qin J, Jiang Z, Qian Y, Xiao H, Lu Y, Qian W, Gulen MF, Sizemore N, DiDonato J, Sato S, Akira S, Su B, Li X. Interleukin-1 (IL-1)-induced TAK1-dependent Versus MEKK3-dependent NFkappaB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J. Biol. Chem. 2007;282:6075–6089. doi: 10.1074/jbc.M609039200. [DOI] [PubMed] [Google Scholar]
- Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr., Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat. Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]