CD3/CD28 Costimulation-Induced NF-κB Activation Is Mediated by Recruitment of Protein Kinase C-θ, Bcl10, and IκB Kinase β to the Immunological Synapse through CARMA1 (original) (raw)
Abstract
CARMA1 (also known as CARD11) is a scaffold molecule and contains a caspase-recruitment domain (CARD) and a membrane-associated guanylate kinase-like (MAGUK) domain. It plays an essential role in mediating CD3/CD28 costimulation-induced NF-κB activation. However, the molecular mechanism by which CARMA1 mediates costimulatory signals remains to be determined. Here, we show that CARMA1 is constitutively associated with the cytoplasmic membrane. This membrane association is essential for the function of CARMA1, since a mutant of CARMA1, CARMA1(L808P), that is defective in the membrane association cannot rescue CD3/CD28 costimulation-induced NF-κB activation in JPM50.6 CARMA1-deficient T cells. Although CD3/CD28 costimulation effectively induces the formation of the immunological synapse in CARMA1-deficient T cells, the recruitment of protein kinase C-θ (PKC-θ), Bcl10, and IκB kinase β (IKKβ) into lipid rafts of the immunological synapse is defective. Moreover, expression of wild-type CARMA1, but not CARMA1(L808P), restores the recruitment of PKC-θ, Bcl10, and IKKβ into lipid rafts in CARMA1-deficient T cells. Consistently, expression of a mutant CARMA1, CARMA1(ΔCD), that cannot associate with Bcl10 failed to restore CD3/CD28 costimulation-induced NF-κB activation in JPM50.6 cells, whereas expression of Bcl10-CARMA(ΔCD) fusion protein effectively restored this NF-κB activation. Together, these results indicate that CARMA1 mediates CD3/CD28 costimulation-induced NF-κB activation by recruiting downstream signaling components into the immunological synapse.
Activation of T cells is triggered through simultaneous stimulation of the T-cell receptor (TCR)/CD3 complexes and costimulatory receptors such as CD28 by antigen-presenting cells (APC). Stimulation of TCR/CD3 leads to activation of cytosolic tyrosine kinases, such as Lck, ZAP70, and Syk (28). These tyrosine kinases in turn phosphorylate various adapter proteins, including LAT, SLP76, Vav, and Grb2 (5, 21, 32). The phosphorylated adapter proteins further recruit effector proteins, such as small GTPases, phospholipase C-γ1 (PLC-γ1), and protein kinases/phosphatases, leading to activation of multiple transcription factors, including NF-AT, AP-1, and NF-κB, which ultimately control transcription of cytokines and T-cell proliferation. Importantly, stimulation of TCR/CD3 complexes alone is not sufficient to activate NF-κB in T cells. Costimulation of CD28 through its ligand, B7, is required for optimal activation of NF-κB leading to activation of T cells, leading to optimal production of interleukin-2 (IL-2) and other cytokines (13).
NF-κB is a family of transcription factors that contain Rel homology DNA-binding domains that exist as various homo- or heterodimers (1). Their function is regulated through interactions with a series of cytoplasmic inhibitory proteins, termed IκB. IκB proteins mask the nuclear localization signal of NF-κB, thereby sequestering NF-κB in the cytoplasm. Treatment of cells with various stimuli, including tumor necrosis factor alpha (TNF-α), IL-1β, phorbol myristate acetate (PMA), or costimulation of TCR/CD3 and CD28 (CD3/CD28 costimulation) initiates signal transduction cascades leading to activation of IκB kinase (IKK). IKK then phosphorylates IκB, triggering rapid ubiquitination and proteolysis of IκB in the 26S proteasome complex (14). The degradation of IκB then unmasks the nuclear localization signal of NF-κB. NF-κB dimers then rapidly translocate into the nucleus, where they engage cognate κB enhancer elements and modulate the transcription of numerous genes involved in immune and inflammatory responses (12).
Although how signaling pathways induced by CD3/CD28 costimulation lead to activation of NF-κB is not fully understood, recent studies indicate that PLC-γ1 is essential for the CD3/CD28 costimulation-induced NF-κB activation (7). PLC-γ1 induces activation of protein kinase C-θ (PKC-θ), which subsequently leads to activation of the IKK (6, 17, 24) through a CARMA1-Bcl10-dependent pathway (10, 20, 22, 27). CARMA1 (11), also known as CARD11 (2), contains a caspase-recruiting domain (CARD) and a membrane-associated guanylate kinase-like (MAGUK) domain, and plays an essential role in the CD3/CD28 costimulation-induced NF-κB activation (10, 20, 27). When overexpressed in HEK293 cells, CARMA1 associates with Bcl10 (2, 11), another CARD-containing adapter protein (16, 23, 25, 29-31) that links to the IKK complex by an unknown mechanism. Therefore, the molecular mechanism by which PKC-θ connects to and activates IKK complexes remains to be determined.
CD3/CD28 costimulation induces formation of a large multicomponent complex at the site of contact between the T cell and the APC, termed supramolecular activation complex (SMAC) or immunological synapse (9). This contact area of the T cell is highly enriched in cholesterol and glycosphingolipids, also termed lipid rafts. Some signaling molecules, including LAT and Ras, are constitutively associated with the lipid rafts, while other key signaling molecules, such as ZAP70, Vav, SLP-76, PKC-θ, and IκB kinase β (IKKβ), are recruited into the lipid rafts following CD3/CD28 costimulation (4). It has been shown that CARMA1 is associated with the lipid rafts in the immunological synapse (10). However, how CARMA1 mediates CD3/CD28 costimulation-induced NF-κB activation remains to be determined. In this study, we show that CARMA1 functions as a scaffold protein to recruit PKC-θ, Bcl10, and IKKβ to the lipid rafts of the immunological synapse, which ultimately leads to activation of NF-κB.
MATERIALS AND METHODS
Cell culture, transfection, and cell stimulation.
Jurkat E6.1 and JPM50.6 cells (27) were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum at 37°C in 5% CO2. The JPM50.6/WT cell line was established by stably transfecting CARMA1 wild-type cDNA into JPM50.6 cells. Cells were transfected with a Gene Pulser (Bio-Rad, Hercules, Calif.) at 250 V (950 μF) with 20 μg of the plasmid DNA. Costimulation of Jurkat cells was performed in a final volume of 1 ml by addition of anti-CD3 (10 μg/ml) and anti-CD28 (5 μg/ml) antibodies, together with 5 μg of goat anti-mouse immunoglobulin G (IgG) antibody per ml (Signal Transduction Laboratories).
Plasmids and antibodies.
Plasmids encoding PKC-θ, Myc-tagged CARMA1 and Bcl10, and Flag-tagged CARMA1 were described previously (27). The CARMA1 deletion mutants were generated by PCR. Antibodies specific for PKC-θ (clone 27) were obtained from BD Transduction Laboratories (San Diego, Calif.), and antibodies specific for Bcl10 (H197) and Flag epitope tag were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.).
Lipid raft purification.
Detergent-insoluble fractions were separated as described previously (3). Briefly, Jurkat or JPM 50.6 T cells (3 × 106 to ∼5 × 106) were lysed in 1 ml of MNE buffer (25 mM morpholineethanesulfonic acid [MES; pH 6.5], 150 mM NaCl, 5 mM EDTA, 30 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 10 μg of each protease inhibitor per ml) with 1%Triton X-100 for 20 min on ice and Dounce homogenized for 15 times. Samples were centrifuged at 1,000 × g for 10 min at 4°C. The supernatants were mixed with 1 ml of 80% sucrose in MNE buffer and transferred to a Beckman Ultracentrifuge tube. Two milliliters of 30% sucrose followed by 1 ml of 5% sucrose in MNE buffer were overlaid. Samples were ultracentrifuged in an STi55 rotor (200,000 × g for 20 h). Fractions (400 μl per fraction) were collected from the top of the gradient. Proteins from each fraction were precipitated with trichloroacetic acid before separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE [10% polyacrylamide]).
Coimmunoprecipitation.
Flag-tagged CARMA1 and Myc-tagged PKC-θ were cotransfected into HEK293 cells by calcium phosphate precipitation. Twenty hours later, CARMA1 was immunoprecipitated with anti-Flag M2 affinity gel (Sigma, St Louis, Mo.). Samples were washed with lysis buffer four times and eluted with Flag peptide (Sigma). The eluted samples were subjected to SDS-PAGE and Western blotting.
Detection of the immunological synapse and immunofluorescence staining.
Detection of immunological synapse formation was performed as described previously (26). Briefly, polystyrene beads (Polysciences, Warrington, Pa.) were coated with anti-CD3 and anti-CD28 antibodies at 10 μg/ml in phosphate-buffered saline (PBS) by incubation at 4°C overnight. Jurkat cells (5 × 104) were cultured with antibody-coated beads (1 × 105) in 200 μl of RPMI 1640 with 10% fetal calf serum in a round-bottom microplate for 45 min. The mixture of cells and beads was settled onto polylysine coated slides, fixed in 3.7% formaldehyde, stained with fluorescein isothiocyanate (FITC)-conjugated cholera toxin B (CTxB) (8 μg/ml [Sigma]), and analyzed by confocal microscopy.
Raji cell-Staphylococcus exotoxin E (SEE) conjugation and immunofluorescence staining were performed as described previously (19). Briefly, Raji cells were stained by incubation in serum-free RPMI 1640 containing 10 μg of CMAC cell tracker blue for 30 min at 37°C and washed and incubated in RPMI 1640 with or without 2 μg of SEE per ml (Toxin Technology, Inc., Sarasota, Fla.) at 37°C for 1.5 h. Cells were then washed and resuspended in serum-free RPMI 1640 at 106 cells per ml. A total of 0.5 ml of Raji cells was mixed with an equal volume of Jurkat or JPM50.6 cells at 106 cells per ml, and the cells were mixed thoroughly by pipetting and then pelleted at 500 rpm for 5 min at room temperature. A total of 0.5 ml of supernatant was removed, and pellets were incubated for 15 min at 37°C. Tubes were resuspended by vortexing. Cells were attached to coverslips precoated with polylysine by cytospin.
Immunostaining was carried out with appropriate primary antibodies and Alexa-labeled secondary antibodies (Molecular Probes, Eugene, Oreg.). Briefly, cells or cell-bead conjugates settled on coverslips were fixed in 3.7% formaldehyde for 10 min, washed and permeabilized in 0.1% Triton X-100 for 5 min, and washed then blocked in 1% bovine serum albumin (BSA) in PBS. Cells were then stained with primary antibody at 4°C overnight, followed by staining with secondary Alexa-labeled antibodies.
RESULTS
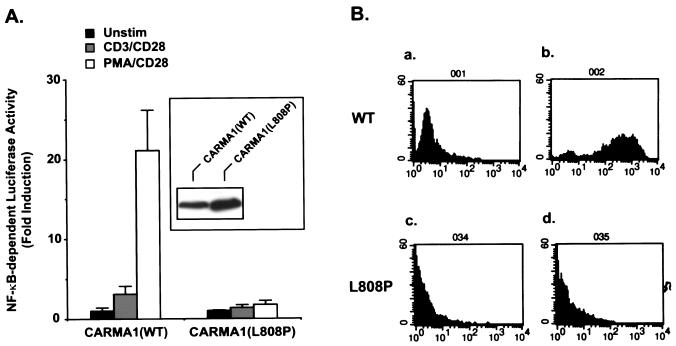
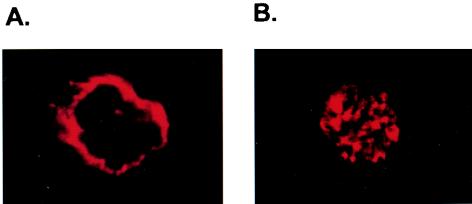
The cDNAs encoding CARMA1 and CARD11 were identified independently by two research groups (2, 11). Interestingly, although both CARMA1 and CARD11 associate with Bcl10 and effectively activate NF-κB when they are overexpressed (2, 11), only CARMA1 can rescue the defect of CD3/CD28 costimulation- or PMA/CD28 costimulation-induced NF-κB activation in JPM50.6 cells—a CARMA1-deficient Jurkat T cell line (Fig. 1) —suggesting that the CARD11 cDNA is somehow defective in connecting to upstream signaling components. After comparing the sequences of the two cDNAs originally deposited in GenBank and the Swiss Protein database, we found that they differ at codon 808, which encodes Leu in CARMA1 and Pro in CARD11. Since the sequence data of human genomic DNA also indicate that residue 808 is Leu in the CARMA1 gene, The Pro 808 in CARD11 is likely a mutation generated by cloning or a natural polymorphism. Therefore, we now refer to the CARD11 cDNA as CARMA1(L808P). To determine the role of CARMA1 in the TCR signaling pathway, we next examined the subcellular localization of CARMA1 by immunofluorescence. We found that CARMA1 is mainly associated with the cytoplasmic membrane (Fig. 2). In contrast, CARMA1(L808P) was more uniformly distributed in the cells (Fig. 2). Together, these data suggest that the subcellular localization of CARMA1 may be essential for its function.
FIG. 1.
CARMA1(L808P) is functionally defective. (A) JPM50.6 cells were transfected with plasmids encoding an NF-κB-dependent luciferase gene in the presence of expression plasmids encoding the wild-type CARMA1 [CARMA1(WT)] or L808P mutant [CARMA1(L808P)]. Twenty-four hours after transfection, the transfected cells were stimulated with (i) media, (ii) 1 μg of anti-CD3 and 1 μg of anti-CD28 per ml, or (iii) 10 ng of PMA and 1 μg of anti-CD28 antibodies per ml for 6 h. The cells were lysed, and luciferase activity was determined. All of the samples were also transfected with an EF1α promoter-dependent Renilla luciferase. The constitutively expressed Renilla luciferase activity was used to normalize the transfection efficiency. All data are presented as fold induction. The expression levels of the transfected CARMA1 were detected by Western blotting with anti-Myc antibodies (inset). Unstim, unstimulated. (B) JPM50.6 cells stably expressing Myc-tagged wild-type CARMA1 or CARMA1(L808P) were unstimulated (a and c) or stimulated with PMA (10 ng/ml) plus anti-CD28 antibodies (1 μg/ml) (b and d) for 6 h. The cells were collected and examined for the expression of NF-κB-dependent GFP in these cells by fluorescence-activated cell sorting (FACS). The y axis indicates the cell numbers (104), and the x axis indicates the fluorescence intensity.
FIG. 2.
CARMA1(L808P) is defective in its subcellular localization. JPM50.6 cells stably expressing Myc-tagged wild-type CARMA1 (A) or CARMA1(L808P) (B) were labeled with anti-Myc antibodies, followed by staining with secondary Alexa-labeled antibodies. Subcellular localization of wild-type CARMA1 or CARMA1(L808P) was visualized by immunofluorescence with a confocal fluorescent microscope.
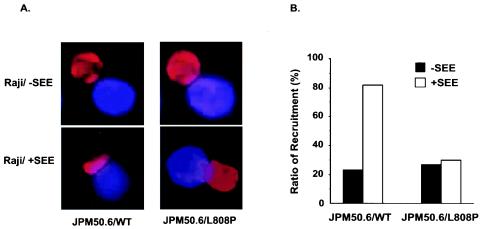
It has been shown that CARMA1 is recruited into the detergent-insoluble membrane (lipid raft) fractions, a part of the immunological synapse, following CD3/CD28 costimulation (10). To examine whether the recruitment of CARMA1(L808P) to the immunological synapse is defective, we incubated JPM50.6 cells that express either the wild-type (Fig. 3A, left panels) or L808P mutant (Fig. 3A, right panels) version of CARMA1 with Raji cells in the presence or absence of a superantigen (SEE), in which Raji cells presented superantigen to TCR complexes (19) of JPM50.6 cells. The subcellular localization of CARMA1 was examined by immunofluorescence staining. The recruitment of CARMA1(L808P), but not wild-type CARMA1, to the immunological synapse was defective (Fig. 3A). Because about 30% of the Raji-JPM50.6 conjugates displayed synapse-associated distribution of both wild-type CARMA1 and CARMA1(L808P) even in the absence of SEE, we counted 25 to 30 random Raji-JPM50.6 conjugates under each condition and calculated the percentage of cells in which CARMA1 was found at the membrane. We found that recruitment of wild-type CARMA1 to the site of contact between Raji and JPM50.6 cells was increased from 30% to about 80% after addition of SEE, whereas the percentages of CARMA1(L808P) recruitment were very similar in the absence and presence of SEE (Fig. 3B). This result suggests that subcellular localization of CARMA1(L808P) is defective.
FIG. 3.
CARMA(L808P) is defective in its recruitment to the immunological synapse. (A) JPM50.6 cells expressing Myc-tagged wild-type CARMA1 (JPM50.6/WT) or CARMA1(L808P) (JPM50.6/L808P) were stimulated with SEE-primed or nonprimed Raji cells (blue) at 37°C for 15 min. The cells were labeled with anti-Myc monoclonal antibodies and Alexa-labeled rabbit anti-mouse antibodies (red). The subcellular localization of CARMA1 (red) was visualized by a confocal fluorescent microscope. (B) The percentage of CARMA1 recruitment to the immunological synapse was calculated by counting the number of the recruitment-like cells among 25 to 30 JMP50.6-Raji conjugates (blue-plus-red cells) in random fields.
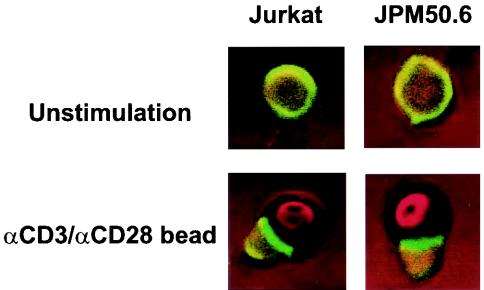
To determine the molecular mechanism by which CARMA1 mediates TCR-induced NF-κB activation, we next examined whether CARMA1 is required for the formation of the immunological synapse following CD3/CD28 costimulation. Jurkat and JPM50.6 cells were stimulated with or without anti-CD3 and anti-CD28 antibodies conjugated to polystyrene beads. The formation of the immunological synapse in these cells was determined by labeling lipid rafts with CTxB, which associates with glycosphingomyelin (GM). The cells were visualized by confocal fluorescence microscopy. The immunological synapse was formed equally well in both Jurkat T and JPM50.6 cells following CD3/CD28 costimulation (Fig. 4). This result indicates that CARMA1 is not required for the formation of the immunological synapse in T cells.
FIG. 4.
Formation of the immunological synapse is independent of CARMA1. Jurkat or JPM50.6 cells were stimulated with or without anti-CD3 and anti-CD28 antibody-coated polystyrene beads for 45 min. The mixtures of cells and beads were labeled with CTxB and visualized by confocal fluorescent microscopy.
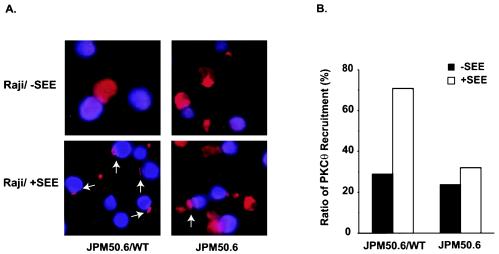
Since CARMA1 contains multiple protein-protein interaction domains, we next investigated whether CARMA1 is required for the recruitment of signaling components into the immunological synapse following CD3/CD28 costimulation. Previous studies suggested that PKC-θ functions as an upstream activator of CARMA1, and PKC-θ was shown to be recruited into the immunological synapse following CD3/CD28 costimulation (10, 27). To examine whether the recruitment of PKC-θ into the immunological synapse is dependent on CARMA1, we incubated JPM50.6 cells or JPM50.6 cells that express wild-type CARMA1 (JPM50.6/WT) with Raji cells in the presence or absence of a superantigen (SEE). The recruitment of PKC-θ to the immunological synapse in JPM50.6 cells (Fig. 5A, right panels) or JPM50.6/WT cells (Fig. 5A, left panels) was examined by immunofluorescence staining. Although PKC-θ was recruited into the contact site between the JPM50.6/WT cells and Raji cells in the presence of SEE, its recruitment occurred in significantly fewer Raji-JPM50.6 conjugates in the absence of CARMA1 (Fig. 5A). PKC-θ localization to the contact site between Raji and JPM50.6 cells (Fig. 5A, white arrows) occurred in about 70% of Raji-JPM50.6 conjugates when CARMA1 was expressed. In contrast, background levels (30%) of the synapse-associated PKC-θ were observed in the absence of either CARMA1 or SEE (Fig. 5B). Together, these data suggest that CD3/CD28 costimulation-induced recruitment of PKC-θ into the immunological synapse is dependent on CARMA1.
FIG. 5.
Recruitment of PKC-θ to the immunological synapse is defective in JPM50.6 cells. (A) JPM50.6 cells or JPM50.6 cells expressing wild-type CARMA1 (JPM50.6/WT) were stimulated with SEE-primed or nonprimed Raji cells (blue) at 37°C for 15 min. The cells were labeled with mouse anti-PKC-θ monoclonal antibodies and Alexa-labeled rabbit anti-mouse antibodies (red). The subcellular localization of PKC-θ (red) was visualized by confocal fluorescent microscopy. (B) The percentage of PKC-θ recruitment to the immunological synapse was calculated by counting the number of the synapse-associated cells among 25 to 30 JPM50.6-Raji conjugates (blue-plus-red cells) in random fields.
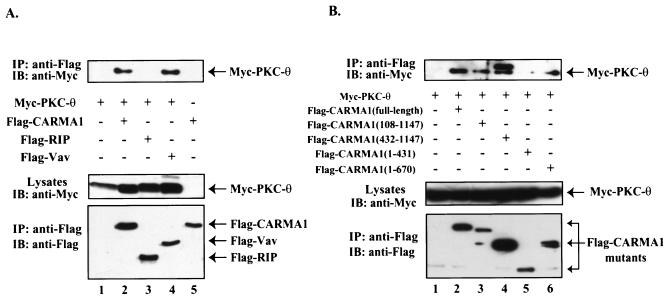
To assess the molecular mechanism of CARMA1-dependent recruitment of PKC-θ to the immunological synapse, we examined whether PKC-θ is associated with a molecular complex containing CARMA1. Myc-tagged PKC-θ was expressed in HEK293 cells in the presence or absence of Flag-tagged CARMA1. CARMA1 was effectively coprecipitated with PKC-θ (Fig. 6A), suggesting that these two molecules coassociate in cells. In addition, this association appears to be dependent on residues 431 to 670 in CARMA1 (Fig. 6B), indicating that PKC-θ associates with the junction region between the coiled-coil domain and the PDZ domain of CARMA1.
FIG. 6.
PKC-θ forms a complex with CARMA1. (A) HEK293 cells were transfected with plasmids encoding Myc-tagged PKC-θ together with Flag-tagged CARMA1, Flag-tagged RIP, Flag-tagged Vav, and the vector control (A) or Flag-tagged CARMA1 and its deletion mutants (B). The transfected cells were lysed and immunoprecipitated (IP) with anti-Flag antibody-conjugated agarose. The resulting immunoprecipitates were subjected to SDS-PAGE and analyzed by Western blotting (immunoblotting [IB]) with antibodies specific for Myc tag (top panel) or Flag tag (bottom panel).
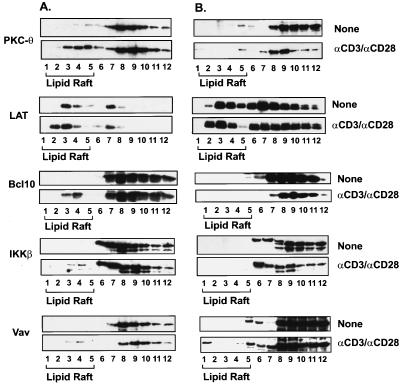
To further confirm that the recruitment of PKC-θ into the immunological synapse depends on CARMA1, the detergent-insoluble membrane (lipid raft) fractions of Jurkat T and JPM50.6 cells were prepared by centrifugation of cellular components in a discontinuous sucrose gradient. The recruitment of PKC-θ into lipid rafts was examined by immunoblotting the individual fractions of a sucrose gradient. Consistent with the immunofluorescence results presented above, the presence of PKC-θ in lipid rafts was significantly reduced in JPM50.6 cells following CD3/CD28 costimulation (Fig. 7B, fractions 1 to 5), whereas CD3/CD28 costimulation efficiently recruited PKC-θ into lipid rafts (Fig. 7A, fractions 1 to 5) in Jurkat T cells. In contrast, the recruitment of Vav to the lipid rafts was the same in both Jurkat T and JPM50.6 cells (Fig. 7A and B). These data further demonstrate that the recruitment of PKC-θ into the lipid rafts of immunological synapse is dependent on CARMA1.
FIG. 7.
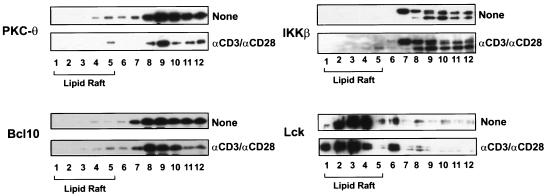
The recruitment of PKC-θ, Bcl10, and IKKβ to lipid rafts is defective in JPM50.6 cells. Jurkat (A) or JPM50.6 (B) cells (∼3 × 106 to 5 × 106) were stimulated with or without anti-CD3 antibodies (1 μg/ml) and anti-CD28 antibodies (1 μg/ml). The cells were then lysed with 1% Triton X-100 and subjected to sucrose density gradient centrifugation to isolate lipid rafts. Proteins from equal volumes of representative collected fractions were separated by SDS-PAGE and analyzed by immunoblotting with antibodies specific for PKC-θ, LAT, Bcl10, or IKKβ.
Previous studies suggested that the IKK complex is also recruited into lipid rafts following CD3/CD28 costimulation (15). Therefore, we examined whether CD3/CD28 costimulation-induced recruitment of IKK to lipid rafts is dependent on CARMA1. The recruitment of IKKβ was also examined by immunoblotting. Although CD3/CD28 costimulation induced the recruitment of IKKβ into lipid rafts in Jurkat T cells (Fig. 7A), the recruitment was defective in JPM50.6 cells (Fig. 7B). Since Bcl10 is a signaling component upstream of IKKβ and potentially downstream of CARMA1, we next examined whether Bcl10 is recruited into the lipid rafts. As expected, CD3/CD28 costimulation triggered the recruitment of Bcl10 into lipid rafts in Jurkat T cells, whereas costimulation failed to recruit Bcl10 into the lipid rafts in JPM50.6 cells (Fig. 7).
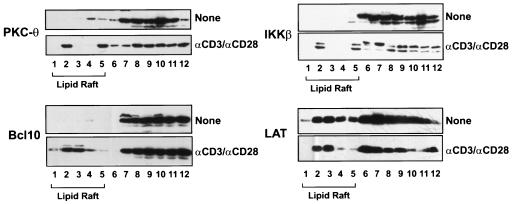
To confirm that the failure to recruit PKC-θ, Bcl10, and IKKβ to the lipid rafts in JPM50.6 cells is due to the deficiency of CARMA1, we examined the subcellular localization of PKC-θ, Bcl10, and IKKβ in JPM50.6 cells that were stably transfected with Myc-tagged CARMA1. The results showed that the expression of wild-type CARMA1 fully rescued the defect of CD3/CD28 costimulation-induced recruitment of PKC-θ, Bcl10, and IKKβ into lipid rafts (Fig. 8), whereas expression of CARMA1(L808P) failed to rescue the recruitment of PKC-θ, Bcl10, and IKKβ (Fig. 9). Together, these results indicate that CARMA1 functions as a scaffold molecule that recruits PKC-θ, Bcl10, and IKKβ into the lipid rafts of the immunological synapse following CD3/CD28 costimulation.
FIG. 8.
Expression of CARMA1 rescued the defect of the localization of signaling components to lipid rafts in JPM50.6 cells. CARMA1-reconstituted JPM50.6 cells (3 × 106 to ∼5 × 106) were stimulated with or without anti-CD3 antibodies (1 μg/ml) and anti-CD28 antibodies (1 μg/ml). The lipid rafts were prepared as described in the legend to Fig. 7. Proteins from equal volumes of representative collected fractions were separated by SDS-PAGE and analyzed by Western blotting with antibodies specific for PKC-θ, LAT, Bcl10, or IKKβ.
FIG. 9.
Expression of CARMA1(L808P) failed to rescue the defect of the localization of signaling components to lipid rafts in JPM50.6 cells. CARMA1(L808P)-reconstituted JPM50.6 cells (JPM50.6/L808P [3 × 106 to ∼5 × 106]) were stimulated with or without anti-CD3 antibodies (1 μg/ml) and anti-CD28 antibodies (1 μg/ml). The lipid rafts were prepared as described in the legend to Fig. 7. Proteins from equal volumes of representative collected fractions were separated by SDS-PAGE and analyzed by Western blotting with antibodies specific for PKC-θ, Lck, Bcl10, or IKKβ.
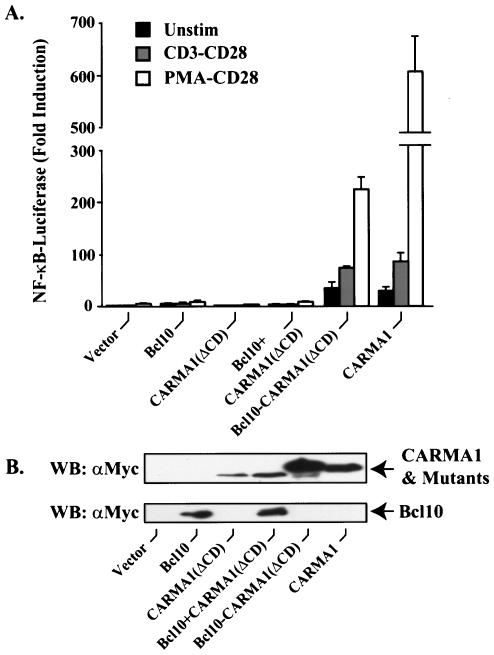
The results presented above indicated that one of roles of CARMA1 is to recruit Bcl10 and downstream IKK complexes in the TCR signaling pathway. Since CARMA1 associates with Bcl10 through its CARD, a deletion mutant of CARMA1 that lacks the CARD failed to rescue CD3/CD28 or PMA/CD28 costimulation-induced NF-κB activation (Fig. 10). This result suggests that recruitment of Bcl10 through the CARD of CARMA1 is essential for TCR-induced NF-κB activation. To prove this hypothesis, we replaced the CARD of CARMA1 with Bcl10. Expression of this chimeric protein, termed Bcl10-CARMA1(ΔCD), in JPM50.6 cells was able to effectively rescue the defect in CD3/CD28 or PMA/CD28 costimulation-induced NF-κB activation (Fig. 10). Together, these results demonstrate that the role of CARMA1 is to recruit Bcl10 to the immunological synapse and consequently lead to activation of NF-κB.
FIG. 10.
Bcl10-CARMA1(ΔCD) fusion protein rescues the functional defect in JPM50.6 cells. (A) JPM50.6 cells were transfected with plasmids encoding a NF-κB-dependent luciferase gene in the presence of expression constructs encoding Bcl10, CARMA1(ΔCD), Bcl10-CARMA1(ΔCD), CARMA1, or the vector control. The transfected cells were stimulated with (i) medium, (ii) 1 μg (each) of anti-CD3 and anti-CD28 antibodies per ml, or (iii) 10 ng of PMA per ml and 1 μg of anti-CD28 antibodies per ml for 6 h. The cells were lysed, and luciferase activity was determined as described in the legend to Fig. 1. Unstim, unstimulated. (B) The expression of transfected constructs was examined by Western blotting (WB) with anti-Myc or anti-Bcl10 antibodies.
DISCUSSION
CARMA1, a MAGUK domain-containing protein, plays an essential role in CD3/CD28 costimulation-induced NF-κB activation. MAGUK domain-containing proteins are generally involved in organization of multiprotein complexes on the interface of cytoplasm membrane and cytoskeleton (8). Consistent with this notion, we find that CARMA1 is constitutively associated with the cytoplasmic membrane. A single-amino-acid mutation (L808P) in the SH3 domain of CARMA1 results in a defect of the localization of CARMA1 to the cytoplasmic membrane, indicating that the SH3 domain of CARMA1 likely interacts with an unknown membrane component. Expression of the CARMA1(L808P) mutant fails to rescue the CD3/CD28 costimulation-induced NF-κB activation. Together, these data indicate that the membrane localization of CARMA1 is essential for CD3/CD28 costimulation-induced NF-κB activation. Although we have not determined whether the L808P mutation found in CARD11 (CARMA1/L808P) cDNA represents a polymorphism in the human genome or a mutation from the original cloning procedure, we predict that such mutations in the CARMA1 gene would likely be associated with immunodeficiency disease.
Previous studies show that PKC-θ is recruited into the immunological synapse following antigen presentation to T cells (18). However, the molecular mechanism of this recruitment remains to be determined. In this study, we found that PKC-θ is associated with CARMA1, and the recruitment of PKC-θ to the immunological synapse is significantly reduced in CARMA1-deficient cells. These findings suggest that PKC-θ is recruited to the immunological synapse through a direct or indirect interaction with CARMA1. Alternatively, the recruitment of PKC-θ to the immunological synapse initially is through a CARMA1-independent process following CD3/CD28 costimulation, but the formation of a stable complex between PKC-θ and other components in the immunological synapse may be dependent on CARMA1. Together, these findings provide the first evidence of how PKC-θ is selectively recruited into immunological synapse.
In this study, we also found that Bcl10 and IKKβ are recruited to the lipid raft of the immunological synapse following CD3/CD28 costimulation. This recruitment of Bcl10 and IKKβ is dependent on CARMA1. Previous studies indicated that the interaction between CARMA1 and Bcl10 is mediated through their CARDs (2, 11). Consistent with these studies, expression of a CARMA1 mutant lacking the CARD cannot restore CD3/CD28 or PMA/CD28 costimulation-induced NF-κB activation, whereas expression of a Bcl10-CARMA1(ΔCD) fusion protein effectively rescues the defect. These data indicate that a function of CARMA1 is to recruit Bcl10 to the immunological synapse, which ultimately leads to recruitment of IKK complex to the immunological synapse. However, the molecular mechanism by which Bcl10 recruits the IKK complex remains to be defined.
The finding that CD3/CD28 costimulation-induced recruitment of PKC-θ to the immunological synapse depends on CARMA1 is consistent with our previous data indicating PKC-θ is functionally upstream of CARMA1 (27). In the previous study, we found that expression of a constitutively active version of PKC-θ failed to activate NF-κB in the CARMA1-deficient cells. These results suggest that CARMA1 is required for the formation of a multicomponent signaling complex, which contains at least PKC-θ, Bcl10, and IKKβ, in the immunological synapse. The formation of such a signaling complex may bring PKC-θ and its substrate or substrates into proximity, where PKC-θ phosphorylates its substrate and subsequently activates IKKβ through an unknown mechanism. However, the substrate or substrates of PKC-θ in the immunological synapse has yet to be defined. Therefore, identification of the substrate or substrates of PKC-θ will be a key step toward understanding how CD3/CD28 costimulation induces activation of the IKK complex in T cells.
Acknowledgments
D.W. is a visiting graduate student from Sun Yet-Sen University, China.
Y.Y. is supported by a Buswell Fellowship from University at Buffalo, SUNY. This work was supported by Public Health Service grants to X.L. (AI50848 and GM65899) and S.L.G (AI49329) from the National Institutes of Health.
REFERENCES
- 1.Baldwin, A. J. 1996. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14**:**649-683. [DOI] [PubMed] [Google Scholar]
- 2.Bertin, J., L. Wang, Y. Guo, M. D. Jacobson, J. L. Poyet, S. M. Srinivasula, S. Merriam, P. S. DiStefano, and E. S. Alnemri. 2001. CARD11 and CARD14 are novel caspase recruitment domain (CARD)/membrane-associated guanylate kinase (MAGUK) family members that interact with BCL10 and activate NF-κB. J. Biol. Chem. 276**:**11877-11882. [DOI] [PubMed] [Google Scholar]
- 3.Bi, K., Y. Tanaka, N. Coudronniere, K. Sugie, S. Hong, M. J. van Stipdonk, and A. Altman. 2001. Antigen-induced translocation of PKC-θ to membrane rafts is required for T cell activation. Nat. Immunol. 2**:**556-563. [DOI] [PubMed] [Google Scholar]
- 4.Bromley, S. K., W. R. Burack, K. G. Johnson, K. Somersalo, T. N. Sims, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 2001. The immunological synapse. Annu. Rev. Immunol. 19**:**375-396. [DOI] [PubMed] [Google Scholar]
- 5.Clements, J. L., N. J. Boerth, J. R. Lee, and G. A. Koretzky. 1999. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Annu. Rev. Immunol. 17**:**89-108. [DOI] [PubMed] [Google Scholar]
- 6.Coudronniere, N., M. Villalba, N. Englund, and A. Altman. 2000. NF-κB activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-θ. Proc. Natl. Acad. Sci. USA 97**:**3394-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dienz, O., A. Moller, A. Strecker, N. Stephan, P. H. Krammer, W. Droge, and M. L. Schmitz. 2003. Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa and phospholipase C gamma 1 are required for NF-kappa B activation and lipid raft recruitment of protein kinase C theta induced by T cell costimulation. J. Immunol. 170**:**365-372. [DOI] [PubMed] [Google Scholar]
- 8.Dimitratos, S. D., D. F. Woods, D. G. Stathakis, and P. J. Bryant. 1999. Signaling pathways are focused at specialized regions of the plasma membrane by scaffolding proteins of the MAGUK family. BioEssays 21**:**912-921. [DOI] [PubMed] [Google Scholar]
- 9.Dustin, M. L., M. W. Olszowy, A. D. Holdorf, J. Li, S. Bromley, N. Desai, P. Widder, F. Rosenberger, P. A. van der Merwe, P. M. Allen, and A. S. Shaw. 1998. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94**:**667-677. [DOI] [PubMed] [Google Scholar]
- 10.Gaide, O., B. Favier, D. F. Legler, D. Bonnet, B. Brissoni, S. Valitutti, C. Bron, J. Tschopp, and M. Thome. 2002. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nat. Immunol. 3**:**836-843. [DOI] [PubMed] [Google Scholar]
- 11.Gaide, O., F. Martinon, O. Micheau, D. Bonnet, M. Thome, and J. Tschopp. 2001. Carma1, a CARD-containing binding partner of Bcl10, induces Bcl10 phosphorylation and NF-κB activation. FEBS Lett. 496**:**121-127. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16**:**225-260. [DOI] [PubMed] [Google Scholar]
- 13.Kane, L. P., J. Lin, and A. Weiss. 2002. It's all Rel-ative: NF-κB and CD28 costimulation of T-cell activation. Trends Immunol. 23**:**413-420. [DOI] [PubMed] [Google Scholar]
- 14.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18**:**621-663. [DOI] [PubMed] [Google Scholar]
- 15.Khoshnan, A., D. Bae, C. A. Tindell, and A. E. Nel. 2000. The physical association of protein kinases C theta with a lipid raft-associated inhibitor of kappa B factor kinase (IKK) complex plays a role in the activation of the NF-κB cascade by TCR and CD28. J. Immunol. 165**:**6933-6940. [DOI] [PubMed] [Google Scholar]
- 16.Koseki, T., N. Inohara, S. Chen, R. Carrio, J. Merino, M. O. Hottiger, G. J. Nabel, and G. Nunez. 1999. CIPER, a novel NF-κB-activating protein containing a caspase recruitment domain with homology to herpesvirus-2 protein E10. J. Biol. Chem. 274**:**9955-9961. [DOI] [PubMed] [Google Scholar]
- 17.Lin, X., A. O'Mahony, Y. Mu, R. Geleziunas, and W. C. Greene. 2000. Protein kinase C-θ participates in NF-κB activation induced by CD3-CD28 costimulation through selective activation of IκB kinase β. Mol. Cell. Biol. 20**:**2933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monks, C. R., H. Kupfer, I. Tamir, A. Barlow, and A. Kupfer. 1997. Selective modulation of protein kinase C-θ during T-cell activation. Nature 385**:**83-86. [DOI] [PubMed] [Google Scholar]
- 19.Morgan, M. M., C. M. Labno, G. A. Van Seventer, M. F. Denny, D. B. Straus, and J. K. Burkhardt. 2001. Superantigen-induced T cell:B cell conjugation is mediated by LFA-1 and requires signaling through Lck, but not ZAP-70. J. Immunol. 167**:**5708-5718. [DOI] [PubMed] [Google Scholar]
- 20.Pomerantz, J. L., E. M. Denny, and D. Baltimore. 2002. CARD11 mediates factor-specific activation of NF-κB by the T cell receptor complex. EMBO J. 21**:**5184-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudd, C. E. 1999. Adaptors and molecular scaffolds in immune cell signaling. Cell 96**:**5-8. [DOI] [PubMed] [Google Scholar]
- 22.Ruland, J., G. S. Duncan, A. Elia, I. del Barco Barrantes, L. Nguyen, S. Plyte, D. G. Millar, D. Bouchard, A. Wakeham, P. S. Ohashi, and T. W. Mak. 2001. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell 104**:**33-42. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasula, S., M. Ahmad, J. Lin, J. Poyet, T. Fernandes-Alnemri, P. Tsichlis, and E. Alnemri. 1999. CLAP, a novel caspase recruitment domain-containing protein in the tumor necrosis factor receptor pathway, regulates NF-κB activation and apoptosis. J. Biol. Chem. 274**:**17946-17954. [DOI] [PubMed] [Google Scholar]
- 24.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-theta is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature 404**:**402-407. [DOI] [PubMed] [Google Scholar]
- 25.Thome, M., F. Martinon, K. Hofmann, V. Rubio, V. Steiner, P. Schneider, C. Mattmann, and J. Tschopp. 1999. Equine herpesvirus-2 E10 gene product, but not its cellular homologue, activates NF-κB transcription factor and c-Jun N-terminal kinase. J. Biol. Chem. 274**:**9962-9968. [DOI] [PubMed] [Google Scholar]
- 26.Viola, A., S. Schroeder, Y. Sakakibara, and A. Lanzavecchia. 1999. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science 283**:**680-682. [DOI] [PubMed] [Google Scholar]
- 27.Wang, D., Y. You, S. M. Case, L. M. McAllister-Lucas, L. Wang, P. S. DiStefano, G. Nunez, J. Bertin, and X. Lin. 2002. A requirement for CARMA1 in TCR-induced NF-κB activation. Nat. Immunol. 3**:**830-835. [DOI] [PubMed] [Google Scholar]
- 28.Weiss, A., and D. R. Littman. 1994. Signal transduction by lymphocyte antigen receptors. Cell 76**:**263-274. [DOI] [PubMed] [Google Scholar]
- 29.Willis, T. G., D. M. Jadayel, M. Q. Du, H. Peng, A. R. Perry, M. Abdul-Rauf, H. Price, L. Karran, O. Majekodunmi, I. Wlodarska, L. Pan, T. Crook, R. Hamoudi, P. G. Isaacson, and M. J. Dyer. 1999. Bcl10 is involved in t(1;14)(p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 96**:**35-45. [DOI] [PubMed] [Google Scholar]
- 30.Yan, M., J. Lee, S. Schilbach, A. Goddard, and V. Dixit. 1999. mE10, a novel caspase recruitment domain-containing proapoptotic molecule. J. Biol. Chem. 274**:**10287-10292. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, Q., R. Siebert, M. Yan, B. Hinzmann, X. Cui, L. Xue, K. M. Rakestraw, C. W. Naeve, G. Beckmann, D. D. Weisenburger, W. G. Sanger, H. Nowotny, M. Vesely, E. Callet-Bauchu, G. Salles, V. M. Dixit, A. Rosenthal, B. Schlegelberger, and S. W. Morris. 1999. Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14)(p22;q32). Nat. Genet. 22**:**63-68. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, W., and L. E. Samelson. 2000. The role of membrane-associated adaptors in T cell receptor signalling. Semin. Immunol. 12**:**35-41. [DOI] [PubMed] [Google Scholar]