Long-Term Cytokine Production from Engineered Primary Human Stromal Cells Influences Human Hematopoiesis in an In Vivo Xenograft Model (original) (raw)
. Author manuscript; available in PMC: 2011 Feb 27.
Published in final edited form as: Stem Cells. 1997;15(6):443–454. doi: 10.1002/stem.150443
Abstract
Human hematopoiesis can be supported in beige/nude/XID (bnx) mice by coinjection of human bone marrow stromal cells engineered to secrete human interleukin 3 (HuIL-3). The major limitation is a total absence of human B cell development in the mice, which could be due to supraphysiological levels of HuIL-3 in the circulation. In an effort to obtain human B lymphoid, as well as T lymphoid and myeloid cell development in the mice, CD34+ cells were coinjected with human marrow stromal cells engineered to secrete human IL-2, IL-7, stem cell factor or FLT3 ligand, ± IL-3. No single factor other than IL-3 supported sustained human hematopoiesis in the mice, although cytokines were expressed for four to six months post-transplantation. Production of both HuIL-3 and IL-7 in the mice supported extrathymic development of human T lymphocytes, but no B cells, myeloid cells, or clonogenic progenitors were detected. Human B cells were not produced from CD34+ cells in the bnx mice under any condition tested. Another limitation to the bnx/Hu system is a lack of maturation of human red blood cells, although BFU-E are maintained. Stromal cells secreting human erythropoietin and IL-3 were cotransplanted into mice with HuCD34+ cells and an increase in hematocrit from 40%–45% to 80%–85% resulted, with production of human and murine red blood cells. Unfortunately, all mice (n = 9) suffered strokes, displayed paralysis and died within three weeks. The bnx/Hu cotransplantation model provides an interesting system in which to study human hematopoietic cell differentiation under the influence of various cytokines.
Keywords: Differentiation, Retroviral-mediated transduction, Marrow stroma immune deficient mice, Stem cells, Xenotransplantation
INTRODUCTION
A major limitation to models of hematopoiesis and gene therapy based on in vitro cultures and colony-forming assays is that development of multilineage progeny from individual pluripotent human stem cells is not measured. Stem cell biology is best measured by transplantation of cytoablated recipients, with subsequent analysis of the progeny developing from the infused cells in the new host. The transplantation of immune deficient mice with human bone marrow (BM) provides a suitable in vivo model of human hematopoiesis.
The beige/nude/xid (bnx) strain is a triple recessive mutant which is suitable for engraftment of human marrow [1]. The bnx mouse has three homozygous recessive mutations which render it immune deficient. The nude mutation, on chromosome 11, causes dysgenesis of the thymic epithelium [2]. Few mature T lymphocytes develop in nude mice due to the lack of an environment for positive selection, although some αβT cells develop by extrathymic mechanisms in older animals [3]. The beige mutation, on chromosome 13, causes a defect in serotonin storage in the dense granules of basophils, platelets and natural killer (NK) cells [4]. The NK cell activity in the beige mouse is therefore severely impaired [5]. The xid, or x-linked immunodeficiency allele, causes a blockage in early B lymphocyte development [6]. The B cells do not mature functionally past the early B220+ stage. Immunoglobulin gene rearrangement at the VD to J recombination step does not occur normally and mature B cells are seldom produced [7]. The few mature B cells that are able to develop cannot mount an immune response against a xenograft, due to the lack of T cells to provide necessary costimuli.
We established a successful xenograft system using bnx mice as recipients of human CD34+ (HuCD34+) hematopoietic progenitor cells that have been marked by retroviral vectors. Long-term hematopoiesis (up to one year post-transplantation) from the human stem and progenitor cells is sustained by cotransplantation of human BM stromal cells engineered to produce human interleukin 3 (HuIL-3). Production of IL-3, which is species specific, in mice has no discernible effect on murine hematopoiesis [8]. Reciprocally, human hematopoietic cells do not undergo long-term hematopoiesis in bnx mice in the absence of HuIL-3. The bnx/Hu xenograft model allows investigation of the impact of different ex vivo transduction protocols on both the efficiency of gene transfer into stem cells and the maintenance of pluripotentiality.
Human cells of all myeloid lineages and T lymphocytes develop in the BM of bnx mice cotransplanted with human marrow-derived hematopoietic progenitor cells and IL-3-producing stroma [8, 9]. Human colony-forming progenitors (colony-forming units [CFU]-granulocyte/macrophage, CFU-mixture and BFU-E) can be easily recovered, but B lymphocytes and mature glycophorin A+ RBCs are not observed. The major limitation of this system is the absolute lack of development of the human B lymphoid lineage in the mice, possibly due to the supraphysiological levels of HuIL-3 in the circulation. IL-3 has been shown to interfere with the differentiation of stem cells into B lymphocytes in the murine system [10, 11]. Providing the appropriate human cytokines via engineered stromal cells in the mice might lead to human B lymphoid, as well as T lymphoid and myeloid cell development from cotransplanted human stem cells, and would allow examination of the development of the full spectrum of hematopoietic lineages from individual, marked, human hematopoietic stem cells.
In the current studies, we transplanted HuCD34+ cells into immune deficient mice along with human marrow stromal cells engineered to produce human IL-2, IL-7, FLT3 ligand (FL), or stem cell factor (SCF) with and without IL-3, to analyze the extent of engraftment of human hematopoietic cells and the types of mature cells produced, specifically seeking the production of B lymphoid cells in addition to T lymphoid and myeloid cells. Stroma engineered to express each factor was used singly or in combination with IL-3 to yield maximal multilineage cell production. Transplantation of human stromal cells engineered to produce human erythropoietin (HuEPO) was also examined to study human RBC maturation in the mice and to potentially provide a model in which to study gene therapy for globinopathies. Our goals for these studies were to provide an environment that would allow long-term expansion of particular human hematopoietic lineages in the mice and to study methods of gene therapy to treat disorders affecting specific cell types, such as X-linked severe combined immune deficiency (B cells), thalassemia and sickle cell anemia (RBCs).
MATERIALS AND METHODS
Vectors and Packaging Cells
Retroviral vectors designed to express human cytokines were constructed in the LXSN backbone [12], with the exception of the JZENhIL-7/tkneo vector, which was a gift from Dr. James Econoumou, UCLA School of Medicine (Los Angeles, CA). Construction of the L-IL-3-SN vector has been described [8]. The L-HuEPO-SN vector was constructed by Karen Pepper, Children’s Hospital of Los Angeles (Los Angeles, CA). The SCF cDNA was obtained in the pGEM3Z plasmid from Dr. Van Parker at Amgen (Thousand Oaks, CA). This cDNA encodes a variably spliced SCF mRNA, which will generate both membrane-bound and soluble forms of the cytokine. The plasmid was cut with HindIII and KPN (GIBCO BRL; Gaithersburg, MD) to release an 820 bp fragment containing the complete SCF cDNA. The fragment was inserted into complementary sites in the pGEM7ZF plasmid multicloning region. An 800 bp fragment containing the SCF cDNA was then removed with BamH1 and moved into the complementary site in the multicloning region of the vector LXSN. The IL-2 cDNA was obtained from R&D Systems (Minneapolis, MN) in the pUC18 plasmid. The plasmid was cut with HindIII and EcoR1, then cloned into complementary sites in pGEM7. The 400 bp cDNA was removed from the pGEM7 plasmid with BamH1, and moved into the LXSN BamH1 site using T4 ligase (GIBCO BRL). The FLT3 cDNA was obtained as the T110 insert in the PME185 vector from Charles Hannum at DNAX (Palo Alto, CA) [13]. The plasmid was cut with EcoR1 and Xho1 and inserted into the complementary site in the multicloning region of LXSN. Vector plasmids were transfected into GP + E ecotropic packaging cells (ATCC) using Dotap transfection reagent (Boerhinger Mannheim; Gmbh, Germany). The 48-h transient supernatants were collected, filtered through a 0.45 µm pore size syringe filter (Uniflo-25 with calcium acetate membrane; Schleicher & Schuell; Keene, NH) and used to transduce PA317 amphotropic packaging cells (ATCC). High titer clones were derived as published [8], and expanded to generate supernatant for transduction of primary human stromal cells. The LN vector, which carries the neo gene [14], but no cytokine cDNA, was used as the negative control in all experiments. The titers of the clones used for transduction of stromal cells were between two and five million infectious particles/ml, and were determined to be free of recombinant helper virus as described [15]. All supernatants were collected after 48 h production from 80% confluent flasks of vector-producing fibroblasts that had been incubated at 32°C with 5% CO2 [16]. Supernatants were passed through 0.45 µm filters and stored at −70°C in 15 ml polypropylene tubes (Becton-Dickinson; San Jose, CA).
Transduction of Primary Human Stromal Cells
Screens used to filter marrow during harvest of normal human donors were the source of stromal cells for all experiments. The use of these samples, otherwise discarded as waste, was approved by the Children’s Hospital of Los Angeles Committee on Clinical Investigation. Many small bony spicules packed with stroma had lodged in the screens during filtration of marrow, and were removed by immersion of the screens in Iscove’s modified Dulbecco’s medium (IMDM) with 20% fetal calf serum (FCS) followed by vigorous agitation. Spicules from unseparated BM were then collected by gravity sedimentation and plated in T-75 flasks (Costar ventcap) in stromal medium, which is IMDM with 15% FCS, 15% horse serum, 10−4 M 2-mercaptoethanol (Sigma; St. Louis, MO), 10−6 M hydrocortisone (Sigma), 50 U/ml penicillin G, 50 µg/ml streptomycin sulfate, and 2 mM L-glutamine (Irvine Scientific; Irvine, CA). The medium was removed, and adherent layers flushed well, then refed the same medium 12–18 h after plating. This step removes many of the contaminating hematopoietic cells. Stromal cells were then grown for four to seven days, until flasks were approximately 50% confluent. Subconfluent layers of primary stromal cells were split by trypsinization (trypsin-EDTA, Irvine Scientific), and distributed to fresh flasks to a confluency of less than 50% for transduction. Human hematopoietic cells differentiated to erythroid and macrophage lineages very rapidly in the stromal medium and were lost with the first rounds of trypsinization. Erythroid cells were removed by flushing, and the macrophages were left behind during brief trypsinizations of subconfluent flasks. By the third passage, hematopoietic cells had been eradicated, except for mature macrophages which comprised less than 1% of the culture, as demonstrated by fluorescence-activated cell sorter (FACS) analysis and immunohistochemical staining for the panleukocyte antigen CD45 using the monoclonal antibody HLE-1 (Becton-Dickinson) and the macrophage-specific anti-CD14 antibody (Becton-Dickinson).
To transduce subconfluent stromal monolayers, equal volumes of rapidly thawed retroviral supernatant and Dulbecco’s modified Eagle’s medium with high glucose and 10% heat-inactivated FCS were added to each T-75 flask. Protamine sulfate (Lyphomed; Deerfield, IL) was added to a concentration of 4 ug/ml. Twelve h after the initial supernatant addition, 100% of the spent transduction media was removed and replaced with fresh supernatant, media and protamine sulfate in the same ratios. This procedure was done three times, at 12 h intervals, for a total of four additions of retroviral supernatant. The day after the final transduction, stromal cells were trypsinized and split between two fresh T-75 flasks in stromal medium. The selective agent G418 (Geneticin, GIBCO BRL) was added to a concentration of 0.75 mg/ml active drug, and selection was allowed to proceed for seven days. Nontransduced stroma from the same donor was used as a control, to ensure complete killing. Following the selection period, monolayers were trypsinized, and the transduced, selected stromal cells were cryopreserved for future cotransplantation experiments. A portion of each sample was expanded and used to generate supernatant for testing by enzyme-linked immunosorbent assay (ELISA) and bioassay.
ELISA and Bioassay to Quantitate Cytokine Production from Transduced Stromal Monolayers
The quantitation of HuIL-3 levels produced by stromal cells and detected in the serum of cotransplanted bnx mice was performed by bioassay and ELISA as described [8]. The production of HuIL-7 from bnx serum and engineered stromal cells was measured by ELISA (R&D Systems). Production of HuSCF and EPO were measured by bioassay systems, using HuCD34+ progenitors isolated from normal HuBM by immunomagnetic selection as target cells. Five hundred CD34+ cells per dish were plated in 1 ml basal methylcellulose medium [8] in quadruplicate. The supernatant or serum to be tested was then added in a range of dilutions, in a volume of 10–100 µls, to each plate. A standard curve was generated by addition of a range of dilutions of the recombinant cytokine being tested. BFU-E only develop fully if SCF or IL-3 are present, in addition to EPO. The smaller CFU-erythroid, containing single clusters of 50–100 erythroid cells, will develop in response to EPO alone, but enter apoptosis within three days in the absence of EPO. Therefore, to assay for IL-3 and SCF, the recombinant cytokines and supernatants to be tested were added on day 1 of culture, then EPO was added on day 4 to all CFU dishes. Development of large erythroid and mixed lineage colonies was observed only in plates that contained IL-3 or SCF using this technique. The number of erythroid colonies obtained from each sample was plotted on the line generated by the standard curve from colonies that had formed in response to the recombinant cytokine dilutions.
To assay for EPO levels, 500 CD34+ cells were first plated in methylcellulose medium containing recombinant HuSCF (50 ng/ml; R&D systems). Diluted samples of supernatant and serum to be tested were added on day 4 of culture, in comparison to a range of dilutions of recombinant EPO (Epoietin alpha; Amgen). Again, erythroid colonies were obtained only in dishes containing both SCF and EPO. IL-2 levels were assessed by bioassay using the CTLL2 line (ATCC) as target cells. Cells were maintained in RPMI media (GIBCO) with 10% FCS and 100 units/ml recombinant HuIL-2 (Biosource International; Camarillo, CA). For bioassay, cells were washed three times in Hank’s buffered saline (GIBCO) and plated at 1 × 104 cells per well in a 96-well flat-bottomed plate in RPMI with 10% FCS. Diluted supernatant, serum or recombinant IL-2 in several concentrations were added to the wells in a volume of 100 µls. Plates were pulsed with tritiated thymidine after 20 h, then harvested and counted six h later. The concentration of IL-2 in each sample was calculated from the standard curve generated by the incorporation of thymidine in cells treated with the recombinant cytokine.
Immunodeficient Mice and Cotransplantation
Six-week-old bnx homozygous mice (bg.bg/nu.nu/xid.xid) bred at Children’s Hospital Los Angeles (Los Angeles, CA) were used for all studies. Cotransplantation of transduced human progenitors and stroma-producing IL-3 was done as described [8]. Mice were sacrificed by 75% CO2/25% O2 narcosis two to eight months after transplantation with human cells. BM was flushed from the tibiae and femurs of each mouse into 1×phosphate-buffered saline, dispersed with a fine needle, and used for FACS analysis and human-specific CFU plating.
Antibody Staining and FACS Analysis
Single cell suspensions from the marrow and spleen of cotransplanted mice were preincubated for 15 min on ice with unconjugated mouse immunoglobulin (MsIgG; Coulter; Hialeah, FL). Directly conjugated murine monoclonal antibodies used to identify human-specific cell surface antigens were: HLE-1 (anti-CD45; Becton-Dickinson), My9-RD1 (anti-CD33; Coulter), Leu-12 (anti-CD19; Becton-Dickinson), Leu-3a (anti-CD4; Becton-Dickinson), Leu-2a (anti-CD8; Becton-Dickinson), and anti-glycophorin A-fluorescein isothiocyanate (FITC; Becton-Dickinson). The rat-anti-mouse CD45-R-PE antibody (Pharmingen; San Diego, CA) was used to identify murine leukocytes, and the TER119-PE antibody (Pharmingen) was used to quantitate murine cells of the erythroid lineage. FITC-conjugated monoclonal antibodies directed against the different T cell receptor Vβ regions were obtained from Immunotech (Marseilles, France). Following a 15 min antibody binding period on ice, cells were depleted of RBCs by resuspension in ortho lysis buffer (Becton-Dickinson), washed, and fixed in 1% paraformaldehyde. Tubes stained with the anti-erythroid lineage markers were not subjected to the lysis step, and were washed in 1×phosphatebuffered saline (GIBCO, BRL). Samples were acquired on a Becton-Dickinson FACScan and analyzed using the CellQuest software package (Becton-Dickinson). Ten thousand events were acquired for each sample. In all experiments, parallel staining and FACS analyses were done on normal human and nontransplanted bnx mouse BM controls to confirm that the human-specific antibodies did not cross-react with murine cells.
Human-Specific CFU Assay
Marrow cells harvested from each mouse were plated in a human-specific colony-forming assay to determine the number of human hematopoietic progenitor cells engrafted within the murine BM as described [8]. Screened lots of FCS from Biowhittaker, Inc. (Walkersville, MD) were used, and HuIL-3 was the only recombinant cytokine added to the basal methylcellulose medium. HuEPO was added on day 4 of culture, without disruption of developing colonies. If added on day 1 of culture, small bursts of murine erythroid cells develop, but by day 4 the CFU-erythroid progenitors have entered apoptosis, and EPO addition will stimulate erythroid development from the more primitive human cells, supported by the IL-3 in the medium. Colonies were counted on day 14–21, and the number of human BFU-E, CFU-mixture, and CFU-granulocyte/macrophage were assessed as described [8].
Statistical Analyses
The significance of each set of values was assessed using the two-tailed _t_-test assuming equal variance, with the Excel 5.0 software (Microsoft Corporation; Seattle, WA). Average values are listed with standard deviations.
RESULTS AND DISCUSSION
Following transduction by retroviral vectors carrying the neo gene and the cDNA for IL-2, IL-3, IL-7, SCF, FL and EPO, stromal cells were selected in 0.75 mg/ml (active) G418, subjected to ELISA or bioassay to determine the level of cytokine production, and cryopreserved or used directly in cotransplantation experiments. The first series of experiments examined the effects of each human cytokine expressed from stromal cells cotransplanted into bnx mice with HuCD34+ cells. Stromal cells expressing IL-3 were used as the positive control, and stroma transduced by the LN vector [14] were used as the negative control in all experiments. We had previously demonstrated that LN-transduced stroma supported little detectable long-term engraftment of human cells in the mice in comparison to stromal cells producing HuIL-3 [8]. For side-by-side comparison of the human cell engraftment potential in each experiment, 1 × 106 stromal cells from a normal human donor, transduced by each vector, were cotransplanted into sublethally irradiated bnx mice with 1 × 106 CD34+ human hematopoietic progenitors enriched from BM of a second (allogeneic) donor. The effects of production of each cytokine on murine hematopoiesis and on human hematopoietic cell engraftment and subsequent lineage development were assessed.
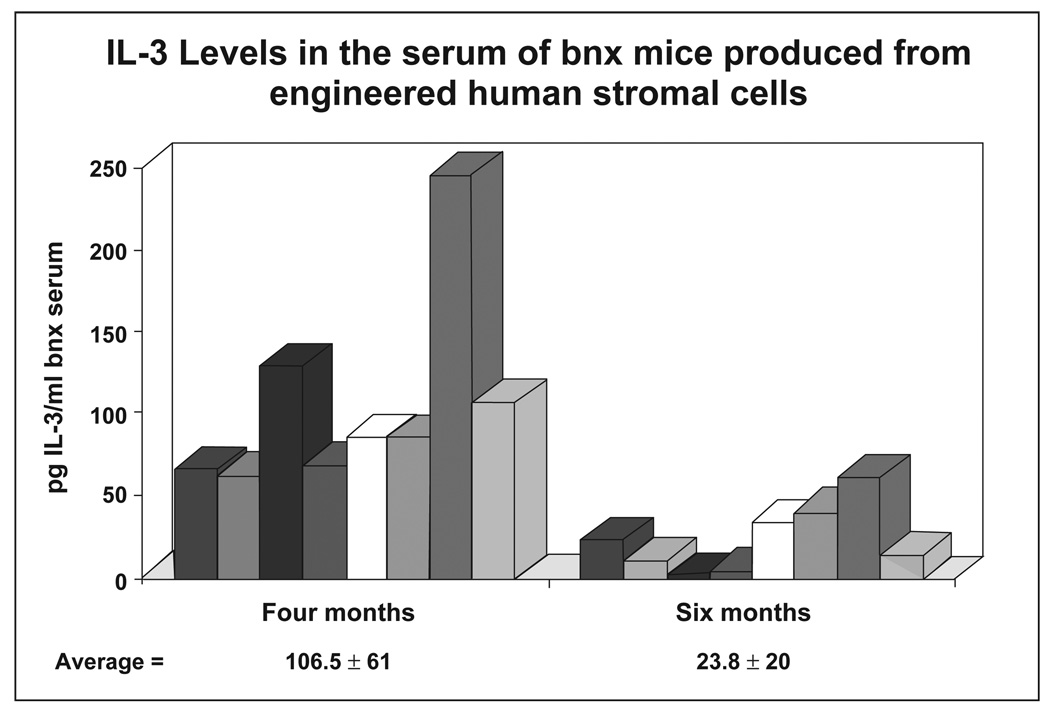
The levels of HuIL-3 in the serum of one set of eight mice cotransplanted with HuCD34+ progenitors and human stromal cells engineered to secrete HuIL-3 were assessed at four months post-transplantation, then again at six months (Fig. 1). The average level was 106.5 ± 61.0 pg HuIL-3/ml serum at four months, and 23.8 ± 20.4 pg/ml at six months. In previous studies we had detected levels as high as 350 pg/ml in the serum of cotransplanted mice at four months post-transplantation [8]. FL and IL-2 could not be measured above background levels from bnx serum at the time of sacrifice, two to eight months post-transplantation, possibly due to the insensitivity of the bioassays used for their detection. HuIL-7, SCF and EPO secreted from the stromal cells were maintained at supraphysiological levels in the serum for the duration of the experiments, as measured by bioassay and confirmed by ELISA when possible (discussed individually below). Human stromal cells expressing the cytokine products of the retroviral vector with which they were transduced have been recovered for up to six months from the spleens of cotransplanted bnx mice, and occasionally liver and lung, but never from the BM, in over 80 mice tested. Following several passages in vitro to achieve transduction by retroviral vectors and selection in G418, the stromal cells have a large, well-differentiated appearance. There may be a physical size barrier which precludes their entry into the murine marrow upon transplantation. Alternatively, ex vivo culture may downregulate an adhesion molecule required for stromal cell homing. Future studies tracking human hematopoietic progenitor and stromal cell homing to bnx tissues may give insight into this phenomenon.
Figure 1. IL-3 levels in the serum of bnx mice four and six months after cotransplantation with engineered human stromal cells.
Stromal cell monolayers were developed from normal HuBM and depleted of hematopoietic cells. Transduction by the retroviral vector L-IL-3-SN, carrying the neo gene and the cDNA for HuIL-3, was achieved by four rounds of supernatant addition. Cells were selected in 0.75 mg/ml G418, re-expanded, and cotransplanted into sublethally irradiated bnx mice together with 1 × 106 HuCD34+ cells that had been subjected to transduction by the LN retroviral vector for marking and subsequent tracking of progeny. At four and six months post-transplantation, blood was collected from the tail vein of the mice. The level of HuIL-3 in each sample was assessed by bioassay using HuCD34+ progenitor targets as described [8]. Serum levels were calculated by linear regression analysis in comparison to a standard curve generated from dilutions of recombinant HuIL-3 and the same CD34+ target cells.
Since the human cytokines IL-2, IL-7 and FL are not species specific, we analyzed the effects of systemic expression on murine hematopoiesis. We had previously determined that expression of HuIL-3 in the mice had no discernible effect on murine hematopoiesis [8] but feared adverse effects resulting from expression of the speciescross-reactive cytokines. HuIL-3 and SCF are both species specific, and no adverse effect was observed from their expression in the mice. Production of FL had no observed effect on murine hematopoiesis in the immunodeficient host. Average spleen weights, hematocrit and cells developing in the pro-B cell, NK, granulocyte and monocyte/macrophage lineages in mice transplanted with IL-3, SCF, or FL-producing stroma were not significantly different from nontransplanted control littermates or mice that had been transplanted with stroma transduced by the LN vector. Cotransplantation of IL-7-producing stromal cells caused an increase in the number of murine B220+ cells in all recipients, but did not adversely affect health or significantly change the hematocrit, spleen weight or development of other murine lineages.
Results of the initial experiments to study the effects from single cytokine production on human hematopoiesis in bnx mice are shown in Table 1. No significant engraftment of human cells, defined as greater than 1% of the bnx BM WBCs replaced with HuCD45+ cells, was obtained in sibling bnx mice cotransplanted with stroma expressing HuIL-2, IL-7, FL, or SCF. IL-3-producing stroma cotransplanted with CD34+ progenitor cells from the same donors supported normal xenograft levels, ranging from 2.8% HuCD45+ cells in the bnx BM to 7.4% (Table 1). This demonstrates that the HuCD34+ cells were healthy and capable of sustaining hematopoiesis in the mice for at least six months, but that only the IL-3-producing stroma was able to support their long-term survival. Loss of cytokine expression was not likely to be a reason for the lack of engraftment because stromal cells secreting the human cytokines could be recovered from the spleens of the mice for up to six months. Extensive silencing of retroviral vectors in transduced, cotransplanted stromal cells has not been observed. It is possible that vector silencing, by promoter methylation or other mechanisms, has occurred in some cells, and those that have retained expression of neomycin phosphotransferase (the product of the neo gene) have been isolated by selective pressure in the neomycin analog G418 upon recovery from the mice. To examine this possibility, we will correlate the extents of vector marking in the splenic DNA with the amount of expression obtained from the same tissue. The problem of vector silencing might not be as profound in human cells as in murine hematopoietic cells that have evolved mechanisms to combat integration of multiple murine retroviruses. An attractive alternative could be that the human stromal cell may be more permissive for long-term expression from the Moloney murine leukemia virus long-terminal repeat than the murine stem cell, for reasons not yet ascertained.
Table 1.
Engraftment of human marrow CD34+ progenitors in bnx mice cotransplanted with primary human stroma engineered to express cytokines
| Mouse # | MonthsPost-BM Transplantation | Cytokine Expressedby Stromal Cells | % Engraftmentawith Human Cells |
|---|---|---|---|
| 1 | 4.0 | LN | <1% |
| 2 | 6.0 | LN | <1% |
| 3 | 4.5 | LN | <1% |
| 4 | 6.0 | LN | <1% |
| 5 | 4.0 | SCF | <1% |
| 6 | 5.5 | SCF | <1% |
| 7 | 6.0 | SCF | <1% |
| 8 | 6.0 | SCF | <1% |
| 9 | 4.0 | IL-3 | 5.3% |
| 10 | 5.5 | IL-3 | 2.8% |
| 11 | 6.0 | IL-3 | 6.2% |
| 12 | 6.0 | IL-3 | 5.1% |
| 13 | 3.0 | IL-3 | 7.4% |
| 14 | 4.0 | IL-3 | 3.6% |
| 15 | 4.0 | IL-7 | <1% |
| 16 | 5.5 | IL-7 | <1% |
| 17 | 6.0 | IL-7 | <1% |
| 18 | 4.0 | IL-7 | <1% |
| 19 | 4.0 | IL-7 | <1% |
| 20 | 2.0 | IL-2 | <1%b |
| 21 | 2.5 | IL-2 | <1%b |
| 22 | 4.0 | IL-2 | <1% |
| 23 | 4.0 | IL-2 | <1% |
| 24 | 4.0 | IL-2 | <1% |
| 25 | 3.0 | IL-2 | <1% |
| 26 | 4.0 | FL | <1% |
| 27 | 4.0 | FL | <1% |
| 28 | 4.0 | FL | <1% |
| 29 | 5.0 | FL | <1% |
| 30 | 5.0 | FL | <1% |
Mice cotransplanted with human hematopoietic progenitors and stromal cells engineered to secrete HuIL-2 developed severe skin problems including eczema and small sores. Two of the six mice transplanted with the IL-2-producing stroma developed a pro-T cell lymphoma of murine origin, perhaps caused by overstimulation of inappropriate divisions in that cell population. Possibly, murine pre-T cells were driven into an unnaturally proliferative state in the nude mice, and had aberrant T cell receptor rearrangements that became transforming. Samples of the tissues from both mice have been saved for future analyses. There was no extended engraftment of human hematopoietic cells supported by the IL-2-producing stroma and no human B cell production, therefore use of the L-IL-2-SN vector has been discontinued due to adverse effects on murine hematopoiesis.
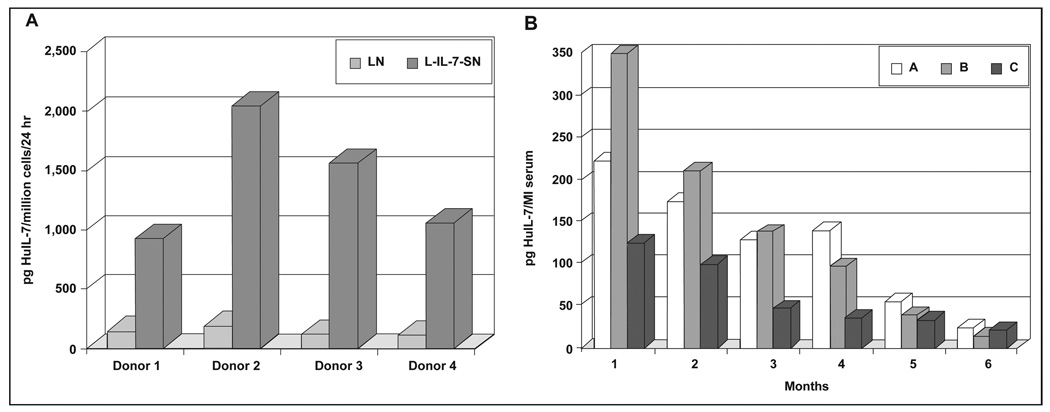
Next, effects from production of IL-7 from engineered stromal cells on cotransplanted CD34+ cells were examined. IL-7 had been previously reported to stimulate growth of lymphoid progenitors [17, 18], and so was hoped to aid in development of human B lymphocytes in bnx mice. Figure 2A shows production of HuIL-7 from engineered stromal cells, assessed by ELISA. The average was 1–2 nanograms per million cells in 24 h, in comparison to the LN-transduced stroma, which was making very low levels. When IL-7-producing stromal cells were transplanted into the mice, no long-term engraftment of cotransplanted HuCD34+ progenitors resulted (Table 1), and no development of human B cells occurred, although HuIL-7 could be detected in the serum of the mice for at least six months (Fig. 2B).
Figure 2. HuIL-7 production by transduced primary human stromal cells.
A) Production in vitro. Supernatants were collected from 1 × 106 stromal cells stably transduced by the L-IL-7-SN or LN retroviral vectors, following 24 h incubation. Each sample was subjected to ELISA (R&D Systems) to determine the level of IL-7 produced. Stromal cells transduced by the LN vector were assayed as a control. B) Measurement of HuIL-7 levels in bnx serum. Samples of blood were obtained from the tail vein of mice A, B and C at monthly intervals after transplantation of stromal cells from Donor 2, transduced by the L-IL-7-SN vector. Cells were removed by centrifugation, and the serum was subjected to ELISA for HuIL-7. Mice that had received stroma transduced by the LN vector were used as controls and had undetectable levels of HuIL-7.
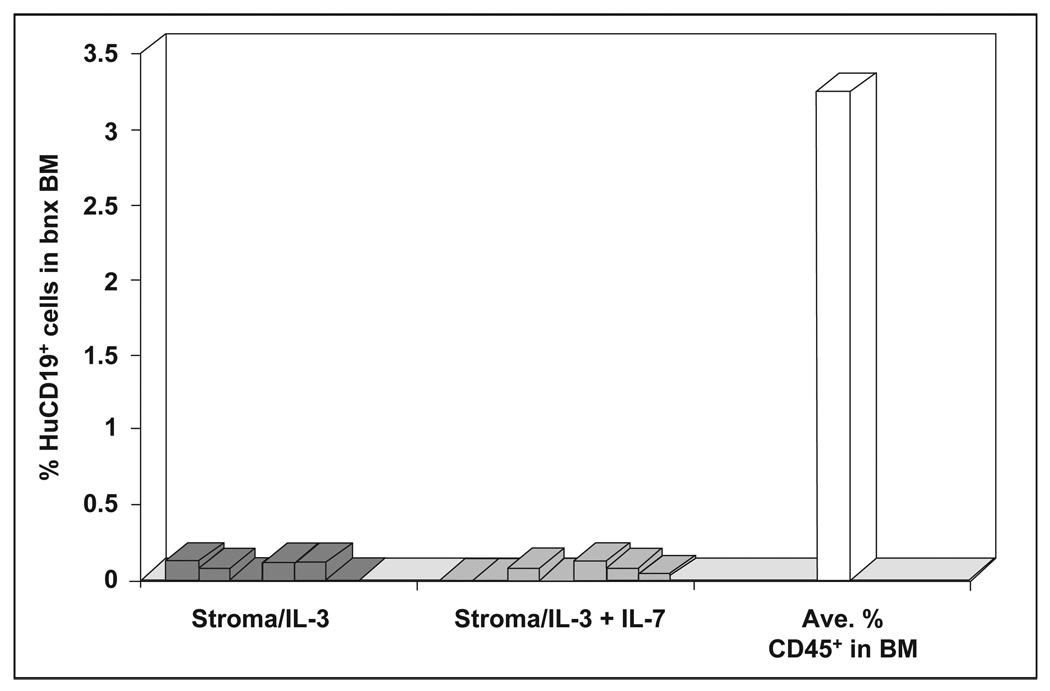
Since expression of the cytokines IL-2, IL-7, SCF and FL from stromal cells did not result in significant levels of human hematopoiesis from cotransplanted HuCD34+ progenitors, the engineered stromal cells were next transplanted in addition to IL-3-producing stroma. To minimize the potential negative effect of HuIL-3 on B cell production, 1 × 105 IL-3-producing stromal cells, tenfold lower than the usual dose, were transplanted with 9 × 105 stromal cells producing the second cytokine. We hoped that a lower level of IL-3 production in the mice would be adequate to allow human cell engraftment and subsequent hematopoiesis, without providing an inhibitory effect on B lymphopoiesis. The combination of IL-3 and IL-7 production in bnx mice was assessed first. A total of 1 × 105 IL-3-producing cells and 9 × 105 IL-7-producing cells were transplanted into one cohort of mice, and one million IL-3-producing stromal cells from the same donor were transplanted into the second group. One million CD34+ progenitors enriched from the marrow of a second donor were coinjected with the stromal cells into each mouse. No significant development of HuCD45+/CD19+ B lymphocytes from the transplanted CD34+ cells was obtained in either group of mice. Figure 3 shows the HuCD19+ B cell levels that resulted, essentially baseline, in comparison to the average extent of human cell engraftment obtained in this experiment.
Figure 3. HuCD19+ cell engraftment in bnx mice cotransplanted with CD34+ cells and stroma producing IL-3 + IL-7 or IL-3 alone.
The percentage of HuCD19+ B cells obtained in mice cotransplanted with stroma producing IL-3 + IL-7 or IL-3 is shown in comparison to the average extent of human hematopoietic cell engraftment obtained in these experiments. Mice were sacrificed six to eight months after transplantation.
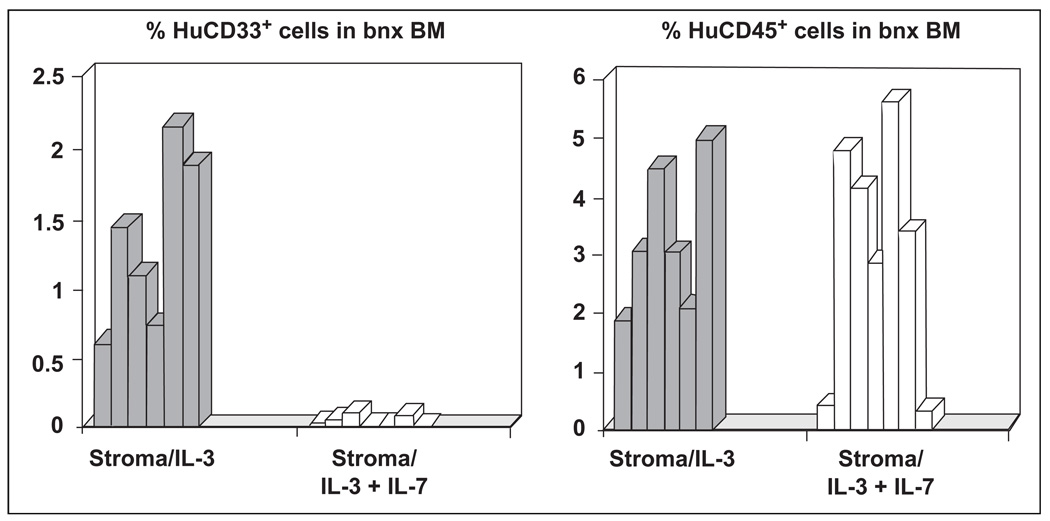
The combination of IL-3- and IL-7-producing stroma sustained engraftment of HuCD45+ cells at normal xenograft levels, although a log reduction in IL-3-producing stromal cells had been infused. In the mice transplanted with IL-3- and IL-7-producing stroma there was a complete absence of HuCD33+ cells (Fig. 4), and the engrafted human cells were primarily T lymphocytes (Table 2). In contrast, mice that had received HuCD34+ cells from the same donors, cotransplanted with one million IL-3-producing stromal cells, and no IL-7-producing stroma, had the typical pattern of human hematopoietic cell phenotypes normally obtained in the bnx/Hu model, including CD33+ human myeloid progenitors (Fig. 4 and Table 2). Statistical analysis of the percentages of human cells of each lineage that had developed in the groups of mice transplanted with stroma-producing IL-3 plus a second cytokine showed that only the CD33+ cells were significantly lower in mice that had received IL-3- and IL-7-producing stromal cells. There were no significant differences in the development of cells of any other lineage shown in Table 2.
Figure 4. HuCD33+ versus CD45+ cell engraftment in bnx mice cotransplanted with stroma expressing IL-3 alone or in combination with IL-7.
One group of mice was transplanted with 1 × 105 IL-3-producing stromal cells and 9 × 105 IL-7-producing stromal cells. The second group received 1 × 106 IL-3-producing stromal cells. Mice were sacrificed six to eight months after transplantation and the percentage of HuCD45+ versus CD33+ cells in the marrow was determined by FACS.
Table 2.
Development of human hematopoietic lineages in bnx mice cotransplanted with CD34+ progenitors and human stroma expressing dual cytokines
| Mouse # | Hu StromaProducing: | %HuCD45 | %HuCD4 | %HuCD8 | %HuCD33 | %HuCD19 | %Hu 45 | |
|---|---|---|---|---|---|---|---|---|
| SPLN | BLD | |||||||
| 31 | IL-3 + IL-7 | 0.4 | 0.3 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 |
| 32 | IL-3 + IL-7 | 4.8 | 0.7 | 3.1 | 0.1 | 0.0 | 0.0 | 0.0 |
| 33 | IL-3 + IL-7 | 4.1 | 1.4 | 2.3 | 0.1 | 0.0 | 0.0 | 0.0 |
| 34 | IL-3 + IL-7 | 2.9 | 1.0 | 1.6 | 0.0 | 0.1 | 0.0 | 0.0 |
| 35 | IL-3 + IL-7 | 5.6 | 2.7 | 2.7 | 0.0 | 0.2 | 0.0 | 3.6 |
| 36 | IL-3 + IL-7 | 3.4 | 2.0 | 1.8 | 0.1 | 0.3 | 0.0 | 0.0 |
| 37 | IL-3 + IL-7 | 0.3 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| Ave. | 3.1 | 1.2 | 1.7 | 0.0 | 0.0 | |||
| 38 | IL-3 + SCF | 10.7 | 2.8 | 3.6 | 2.0 | 0.0 | 1.2 | 4.9 |
| 39 | IL-3 + SCF | 5.3 | 2.7 | 1.2 | 1.4 | 0.0 | 2.4 | 3.3 |
| 40 | IL-3 + SCF | 3.0 | 0.9 | 1.5 | 1.1 | 0.0 | 0.9 | 0.0 |
| 41 | IL-3 + SCF | 0.6 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 |
| 42 | IL-3 + SCF | 2.2 | 0.8 | 0.7 | 0.9 | 0.0 | 0.0 | 0.0 |
| Ave. | 4.4 | 1.4 | 1.4 | 1.2 | 0.0 | |||
| 43 | IL-3 + FL | 3.4 | 1.2 | 1.0 | 1.5 | 0.0 | 0.0 | 0.0 |
| 44 | IL-3 + FL | 0.7 | 0.2 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 |
| 45 | IL-3 + FL | 4.8 | 1.4 | 2.1 | 1.5 | 0.1 | 1.6 | 0.8 |
| 46 | IL-3 + FL | 2.6 | 0.7 | 0.4 | 1.8 | 0.0 | 0.0 | 0.0 |
| Ave. | 2.7 | 0.9 | 0.9 | 1.4 | 0.0 | |||
| 47 | IL-3 | 1.9 | 0.0 | 1.5 | 0.6 | 0.0 | 0.0 | 0.0 |
| 48 | IL-3 | 3.1 | 0.1 | 2.0 | 1.4 | 0.0 | 0.0 | 0.0 |
| 49 | IL-3 | 4.5 | 1.2 | 2.4 | 1.1 | 0.0 | 0.0 | 0.0 |
| 50 | IL-3 | 3.0 | 1.0 | 0.9 | 0.7 | 0.0 | 1.5 | 0.0 |
| 51 | IL-3 | 2.2 | 0.2 | 0.4 | 1.9 | 0.0 | 2.7 | 1.4 |
| 52 | IL-3 | 4.9 | 1.5 | 0.8 | 2.2 | 0.1 | 0.0 | 0.9 |
| Ave. | 3.4 | 0.7 | 1.3 | 1.3 | 0.0 |
The colony-forming assays done on marrow recovered from the mice revealed that those transplanted with normal HuCD34+ progenitors and stromal cells producing IL-3 and IL-7 harbored no human myeloid or erythroid colony-forming progenitors. In contrast, mice transplanted with HuCD34+ progenitors from the same donor, and IL-3-producing stroma alone, had normal levels of human CFU of all lineages. Stimulation of T lymphopoiesis at the expense of myeloid development by coproduction of HuIL-7 and IL-3 in the mice was unexpected. Although IL-7 is known to be important in early lymphopoiesis [17, 18], it has been reported to also have stimulatory effects on human myelopoiesis in vitro [19]. Thus, our results might be attributed to the interaction of IL-3 and IL-7 together with cross-reactive cytokines from the murine microenvironment.
Mice transplanted with IL-3 and SCF or FL-producing stroma, in the 1:10 ratios described in the previous paragraph had no discernible alterations in the human hematopoietic cell phenotypes that had developed from the coinjected CD34+ progenitors, in comparison to mice that had received IL-3-producing stroma alone (Table 2). It is possible that expression of the murine-to-human cross-reactive cytokines SCF and FL did not augment effects from the levels already present in the murine microenvironment. An alternative explanation might be that these cytokines are less effective when they reach the human cells systemically, and are most active in membrane bound form. Since, in our model, the engineered stromal cells do not home to the murine BM where the human progenitors are located, effects from the secreted cytokines must be achieved through the circulation. Membrane-bound SCF has been reported to be more active on stimulation of early hematopoietic progenitors than the soluble form [20, 21], and FL could function in a similar manner.
Human T Lymphocyte Development in bnx Mice
Development of all human myeloid lineages, T cells, and colony-forming progenitors occurs from cotransplanted CD34+ progenitors in the bnx/Hu xenograft system. The development of human T lymphocytes in bnx mice is fascinating because the mice are athymic due to the nude mutation, so maturation must occur through extrathymic mechanisms. Athymic differentiation of murine αβT cells is known to occur in older nude mice [3], and, indeed, populations of (primarily CD8+) murine T cells are routinely seen in bnx mice over six months of age (Dao and Nolta, unpublished data). Human T lymphocytes recovered from bnx mice are CD4+, CD8+, or double positive. All express T cell receptor for antigen (TCR)αβ, and significant populations of human CD3+ cells bearing TCRγδ have not been found in the marrow, spleen or blood of any xenografted bnx mouse in our system.
Evidence that T lymphocytes mature from the transplanted CD34+ cells, rather than expanding from mature T cells that have contaminated the graft, are threefold. First, the CD34+ populations obtained by our method of immunomagnetic selection are 95%–99% pure, as assessed by removal of the magnetic beads with chymopapain, counterstaining, and FACS analysis. The contaminating cells are monocytes that have nonspecifically bound the primary anti-CD34 antibody by the Fc receptor. Mature T cells cannot be identified in the sorted population, although CD34+/CD2+ lymphoid progenitors are present. In addition, for retroviral transduction the CD34+ cell population is cultured for 72 h ex vivo in media containing hydrocortisone, which is toxic to T lymphocytes, prior to transplantation. Second, CD34+ cell populations isolated from T cell-depleted HuBM that lacked mature T cells and CD2+ progenitors gave rise to the same T cell phenotypes as CD34+ populations isolated from whole marrow from the same donors, following long-term engraftment in bnx mice [8]. Third, several common stem cells have been marked by retroviral vectors ex vivo, then generated both T lymphoid and myeloid progeny in the mice with identical retroviral integration sites, as confirmed by clonal analysis by inverse polymerase chain reaction. Sequencing of the DNA flanking the integrated provirus in individual human T cell and granulocyte-macrophage clones recovered from the same mouse was done to confirm that the two disparate lineages had arisen from the same marked stem cell [9].
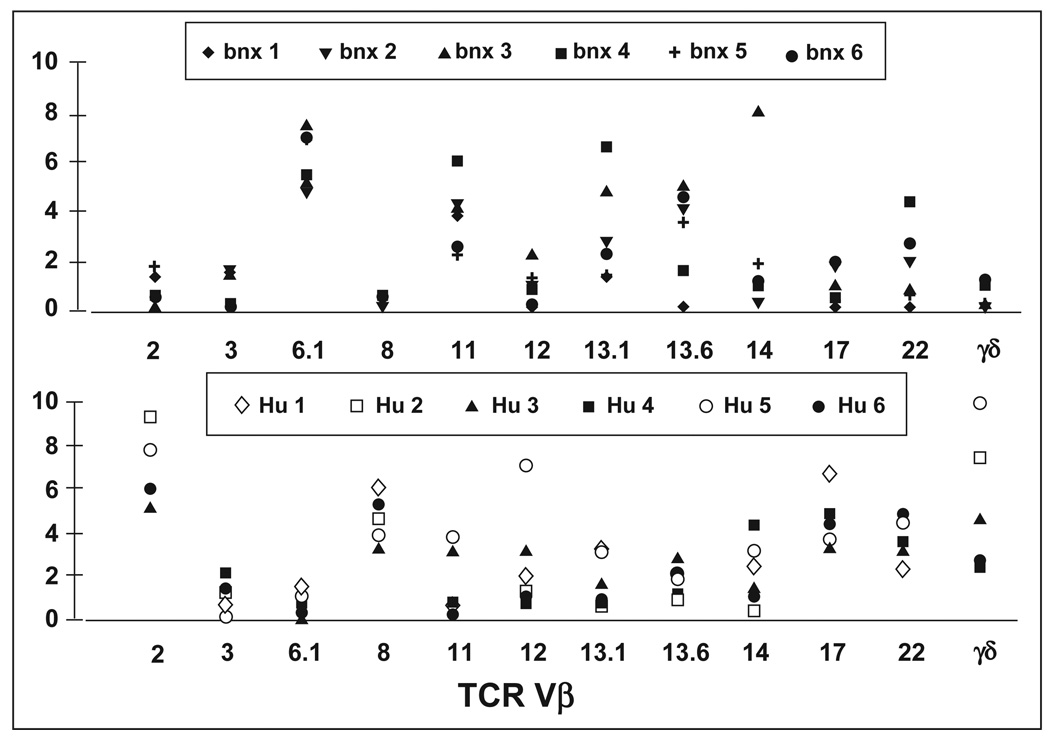
To begin to analyze T cell development in the bnx/Hu xenograft system, our first goal was to determine the pattern of TCR Vβ usage in human cells maturing extrathymically in the mice, as compared to human T cells that had developed by normal thymic selection in human controls. The pattern of TCR Vβ expression in HuCD45+/CD3+ cells recovered from bnx marrow after eight months engraftment was compared to the patterns observed in T cells from normal human peripheral blood samples. Cells were labeled with phycoerythrin-conjugated anti-CD3 and FITC-conjugated monoclonal antibodies directed against the most commonly expressed human Vβ regions. From Figure 5, which shows the TCR Vβ usage in six well-engrafted bnx/Hu mice and six normal donors, it can be seen that the Vβ usage is not equivalent in human cells that have developed extrathymically in bnx mice, and in human cells that have developed via normal routes in humans. Vβ genes 6.1, 11, 13.1, 13.6 and 22 were most commonly expressed in the bnx/HuT cells, whereas 2, 8, and 17 were most frequently represented in human samples. In future studies, we will couple Vβ analysis with clonal integration analysis by inverse polymerase chain reaction [9] to determine whether marked human T cells with limited Vβ are expanded in the mice from a few or many different human T cell progenitors. It is possible that the T cells that develop in the bnx mice represent progenitors that fortuitously rearranged a T cell receptor with weak reactivity to the murine major histocompatibility complex, and then expanded over time in an unchecked xenogeneic response. However, we have seen no signs of graft-versus-host disease (GVHD) in bnx mice with levels of human T lymphocytes in their marrow ranging from 1% to 4%. The human T cells may be in an anergic state in the mice, or they might be unable to mount a full GVH response without the presence of human B cells. In NOD/LtSz-severe combined immunodeficient mice, GVH was observed only when cells from human donors previously conditioned to murine antigens were transplanted [22]. Future studies will examine the functional status of the bnx/Hu T cells, and will determine whether they arise from few or multiple human lymphoid progenitors.
Figure 5. TCR usage by human T lymphocytes recovered from long-term engrafted bnx mice versus normal human donors.
The percentage of CD3+ T cells expressing the most commonly used TCR Vβ genes was determined by FACS. The pattern of TCR usage in marrow from six well-engrafted bnx mice is shown in the top panel. The usage in peripheral blood samples from normal human donors is shown in the lower panel.
EPO Production from Engineered Stromal Cells in bnx Mice
The effects of HuEPO expression in the mice were also assessed. It was hoped that systemic production of HuEPO might allow a higher percentage of human RBCs to mature to the glycophorin A+ stage to facilitate in vivo studies of gene therapy for human globinopathies. In the standard bnx/Hu xenograft model, the human cells mature to the BFU-E stage only, then there is no further development. It was possible that murine EPO was not cross-reactive to human cells at physiological levels. Human and murine EPO are 80% identical at the amino acid level. However, the HuEPO molecule has a site of O-linked glycosylation at serine 126. In murine EPO, this serine is replaced by a proline [23]. Since N- and O- linked glycosylation are required for full activity, this difference leads to reduced stimulation of human cells by murine EPO.
We, therefore, cotransplanted HuCD34+ cells and stromal cells engineered to produce HuEPO into bnx mice. Production of HuEPO from engineered stromal cells is shown in Figure 6. EPO produced by the primary human stromal cells was equivalent to two units per million cells in a 24-h period. Stromal cells transduced by the L-EPO-SN vector were cotransplanted with HuCD34+ progenitors into two cohorts of mice (n = 8), and a transient burst of mature human RBCs, as well as an increase in murine RBCs and hematocrit was obtained. However, the production of human RBCs was transient, and after the first three weeks, human RBCs and BFU-E could no longer be recovered from the peripheral blood of either group of mice. No human cells were detected in the marrow at the time of sacrifice, two months post-transplantation.
Figure 6. HuEPO production by transduced primary human stromal cells.
Supernatants were collected from 1 × 106 stromal cells stably transduced by the L-HuEPO-SN or LN retroviral vectors, following 24-h incubation. Each sample was subjected to bioassay using freshly isolated HuCD34+ progenitors as target cells in a methylcellulose- based system to determine the level of EPO produced. Stromal cells transduced by the LN vector were used as a control.
To obtain more sustained human erythropoiesis in bnx mice, we next tried cotransplantation of IL-3-producing and EPO-producing stromal cells with the purified HuCD34+ progenitors. Inclusion of stromal cells producing IL-3 resulted in an increase in hematocrit to 65%–85% RBCs (normal values are 40%–45% for bnx mice), with increased production of mature murine and human RBCs. Spleen sizes were increased dramatically in all mice. Unfortunately, all of the mice (9 total) suffered strokes and died within three weeks post-transplantation. We will refine this system by reducing the level of HuEPO expression, or by introducing a vector which will express transiently, during the period when human RBC production from BFU-E is needed.
In summary, cotransplantation of HuCD34+ progenitors and primary human stromal cells engineered to produce cytokines that might lead to enhanced development of human B lymphocytes in bnx mice was examined. The cytokines IL-2, IL-7, FL and SCF were studied in direct comparison to IL-3, which we predicted, from a historic database of over 325 mice, would not promote human B cell development from cotransplanted human hematopoietic progenitors from bone marrow. Although the human cytokines were secreted at supraphysiological levels into the murine bloodstream for at least six months, no single factor supported detectable human hematopoiesis in the mice, without concomitant IL-3 production. Although some interesting results were obtained in the described studies, human B cell production from human hematopoietic progenitors was not achieved by cotransplantation of stromal cells producing any of the cytokines tested, with or without concurrent IL-3 production. We are currently evaluating the ex vivo culture conditions used to mark the cells with the LN retroviral vector to determine whether the potential for the cells to develop into B lymphocytes has been ablated prior to transplantation, as has been reported in the murine hematopoietic system [10, 11]. The bnx/Hu cotransplantation system provides an interesting tool for studying human lineage differentiation under the influence of different cytokines. By marking the input human stem cells with retroviral vectors, differentiation of individual cells into the various lineages can be followed by analysis of proviral integration site [9]. Cotransplantation of engineered stromal cells allows analysis of the impact of defined human cytokines on human hematopoietic cells in an in vivo system.
ACKNOWLEDGMENTS
Thank you to Don Kohn, Ken Weinberg, Gay Crooks, Sharyn Walker and Robertson Parkman, as always, for useful discussion. Dr. Paul Pattengale, at Children’s Hospital of Los Angeles, Department of Pathology (Los Angeles, CA), sectioned and analyzed tissues from the mice that had received the L-IL-2-SN vector, and had developed a pro-T cell lymphoma. Ellen Bolotin performed some of the ELISA assays to detect HuIL-7 expression. This work was made possible by Sally Worttman, who heads our animal facility, with Frederick Young and Renee Workman-Traub, who have maintained the bnx mouse colony since this work began in 1990.
This work was supported by the John Connell Gene Therapy Foundation and a grant from the NIH NHLBI (SCOR #1-P50-HL54850).
REFERENCES
- 1.Andriole GL, Mule JJ, Hansen CT, et al. Evidence that lymphokine-activated killer cells and natural killer cells are distinct based on an analysis of congenitally immunodeficient mice. J Immunol. 1985;135:2911–2920. [PubMed] [Google Scholar]
- 2.Pantelouris EM. Absence of thymus in a mouse mutant. Nature. 1968;217:370–373. doi: 10.1038/217370a0. [DOI] [PubMed] [Google Scholar]
- 3.Speiser DE, Stubi U, Zinkernagel R. Extrathymic positive selection of αβT-cell precursors in nude mice. Nature. 1990;355:170–173. doi: 10.1038/355170a0. [DOI] [PubMed] [Google Scholar]
- 4.Holland JM. Serotonin deficiency and prolonged bleeding in beige mice. Proc Soc Exp Biol Med. 1976;151:32–38. doi: 10.3181/00379727-151-39137. [DOI] [PubMed] [Google Scholar]
- 5.Roder J, Duwe A. The beige mutation in the mouse selectively impairs natural killer cell function. Nature. 1979;278:451–454. doi: 10.1038/278451a0. [DOI] [PubMed] [Google Scholar]
- 6.Smith HR, Yaffe LJ, Kastner DL, et al. Evidence that Lyb-5 is a differentiation antigen in normal and xid mice. J Immunol. 1986;136:1194–1201. [PubMed] [Google Scholar]
- 7.Karagogeos D, Rosenberg N, Wortis HH. Early arrest of B cell development in nude, X-linked immunodeficient mice. Eur J Immunol. 1986;16:1125–1132. doi: 10.1002/eji.1830160916. [DOI] [PubMed] [Google Scholar]
- 8.Nolta JA, Hanley MB, Kohn DB. Sustained human hematopoiesis in immunodeficient mice by co-transplantation of marrow stroma expressing human IL-3: analysis of gene transduction of long-lived progenitors. Blood. 1994;83:3041–3049. [PubMed] [Google Scholar]
- 9.Nolta JA, Dao MA, Wells S, et al. Transduction of pluripotent human hematopoietic stem cells demonstrated by clonal analysis after engraftment in immune-deficient mice. Proc Natl Acad Sci USA. 1996;93:2414–2419. doi: 10.1073/pnas.93.6.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball TC, Hirayama F, Ogawa M. Modulation of early B lymphopoiesis by interleukin-3. Exp Hematol. 1996;24:1225–1231. [PubMed] [Google Scholar]
- 11.Yonemura Y, Ku H, Hirayama F, et al. Interleukin 3 or interleukin 1 abrogates the reconstituting ability of hematopoietic stem cells. Proc Natl Acad Sci USA. 1996;93:4040–4047. doi: 10.1073/pnas.93.9.4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–988. [PMC free article] [PubMed] [Google Scholar]
- 13.Hannum C, Culpepper J, Campbell D, et al. Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of hematopoietic stem cells and is encoded by variant RNAs. Nature. 1994;368:643–647. doi: 10.1038/368643a0. [DOI] [PubMed] [Google Scholar]
- 14.Miller AD, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2905. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan RA, Cornetta K, Anderson WF. Applications of the polymerase chain reaction in retroviral mediated gene transfer and the analysis of gene marked human TIL cells. Hum Gene Ther. 1990;1:257–264. doi: 10.1089/hum.1990.1.2-135. [DOI] [PubMed] [Google Scholar]
- 16.Bauer GB, Valdez P, Kearns K, et al. Inhibition of human immunodeficiency virus-1 (HIV-1) replication after transduction of granulocyte colony-stimulating factor mobilized CD34+ cells from HIV-1 infected donors using retroviral vectors containing anti-HIV-1 genes. Blood. 1997;89:7–13. [PubMed] [Google Scholar]
- 17.Suda T, Zlotnik A. IL-7 maintains the T-cell precursor potential of CD3−CD4−CD8− thymocytes. J Immunol. 1991;146:3068–3077. [PubMed] [Google Scholar]
- 18.Godfrey DI, Zlotnik A. Control points in early T-cell development. Immunol Today. 1993;14:547–553. doi: 10.1016/0167-5699(93)90186-O. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen FW, Veiby OP, Skjonsberg C, et al. Novel role of interleukin 7 in myelopoiesis: stimulation of primitive hematopoietic progenitor cells. J Exp Med. 1993;178:1777–1782. doi: 10.1084/jem.178.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson DM, Lyman SD, Baird A, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in membrane bound and soluble forms. Cell. 1990;63:235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 21.Caruana G, Ashman LK, Fujita J, et al. Responses of the murine myeloid cell line FDC-P1 to soluble and membrane-bound forms of steel factor (SLF) Exp Hematol. 1993;21:761–768. [PubMed] [Google Scholar]
- 22.Greiner DL, Shultz LD, Yates J, et al. Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with C.B-17-scid/scid mice. Am J Pathol. 1995;146:888–902. [PMC free article] [PubMed] [Google Scholar]
- 23.Dexter TM, Garland JM, Testa NG, editors. Colony Stimulating Factors: Immunology Series. Volume 49. New York: Marcel Dekker, Inc.; 1990. pp. 177–203. [PubMed] [Google Scholar]