WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse (original) (raw)
Abstract
WNT4, a member of the Wnt family of ligands, is critical for the development of the female reproductive tract. Analysis of Wnt4 expression in the adult uterus during pregnancy indicates that it may play a role in the regulation of endometrial stromal cell proliferation, survival, and differentiation, which is required to support the developing embryo. To investigate the role of Wnt4 in adult uterine physiology, conditional ablation of Wnt4 using the PRcre mouse model was accomplished. Ablation of Wnt4 rendered female mice subfertile due to a defect in embryo implantation and subsequent defects in endometrial stromal cell survival, differentiation, and responsiveness to progesterone signaling. In addition to altered stromal cell function, the uteri of PRcre/+Wnt4f/f (Wnt4d/d) mice displayed altered epithelial differentiation characterized by a reduction in the number of uterine glands and the emergence of a p63-positive basal cell layer beneath the columnar luminal epithelial cells. The altered epithelial cell phenotype was further escalated by chronic estrogen treatment, which caused squamous cell metaplasia of the uterine epithelium in the Wnt4d/d mice. Thus, WNT4 is a critical regulator not only of proper postnatal uterine development, but also embryo implantation and decidualization.—Franco, H. L., Dai, D., Lee, K. Y., Rubel, C. S., Roop, D., Boerboom, D., Jeong, J.-W., Lydon, J.-P., Bagchi, I. C., Bagchi, M. K., DeMayo, F. J. WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse.
Keywords: p63, estrogen, Bmp2, Foxo1
Embryo implantation is a complex process consisting of a series of coordinated events, including embryo attachment to the uterine luminal epithelium, its subsequent invasion through the luminal epithelium, and then the proliferation and differentiation of the uterine stromal cells in a process known as decidualization (reviewed in ref. 1). These events require not only extensive crosstalk between the embryo and the uterus, but also the coordinate actions of the ovarian steroid hormones estrogen (E2) and progesterone (P4) to ensure that the uterine environment is receptive to the incoming embryo. Much work has been done in recent years to begin to elucidate the mechanisms by which these hormones regulate embryo implantation, largely through the use of animal models (reviewed in refs. 1–3). From these studies, a complex signaling network has emerged, demonstrating that embryo implantation is regulated by multiple signaling pathways with intricate positive and negative feedback loops (3). One signaling molecule, identified as critical for regulating the process of endometrial stromal cell decidualization, is bone morphogenetic protein 2 (BMP2). Conditional ablation of Bmp2 in the murine uterus, as well as in vitro studies in isolated primary mouse and human stromal cells, showed that this molecule is required for decidualization (4, 5). Mechanistic analysis of the pathways regulated by BMP2 during this process identified multiple targets, including wingless-related MMTV integration site 4 (Wnt4).
Wnt4 is one of 19 Wnt ligands related to wingless in Drosophila (6). These ligands can signal in either a canonical or noncanonical fashion. The canonical signaling pathway involves the binding of the Wnt ligand to its Frizzled receptor, which inhibits the degradation of β-catenin, resulting in its nuclear translocation and activation of target genes. Noncanonical Wnt signaling encompasses various signaling pathways that differ from the canonical pathway at multiple levels, including the Wnt receptors (i.e., ROR2, RYK) and the mediators of Wnt signaling (i.e., JNK, CAMKII) (reviewed in ref. 6). Wnt/β-catenin signaling has been implicated in the regulation of embryo implantation, as its inhibition greatly hindered this process (7–10). Specific members of the Wnt family have been shown to play a role in proper postnatal uterine differentiation, as its loss (such as total ablation of Wnt7a or Wnt5a or conditional ablation of β-catenin) results in altered postnatal uterine development (10–12). Wnt4 has been shown to be critical for the development of the female reproductive tract (13). Although the role of WNT4 in adult uterine physiology is not known, Wnt4 is expressed at specific times in the pregnant mouse uterus and is under E2 regulation (14–17). Wnt4 mRNA is expressed weakly in the luminal epithelium on pregnancy day 0.5 (vaginal plug=d0.5), at which point its expression is reduced until d4.5, when it localizes to stromal cells surrounding the implanting embryo and expands and persists in the decidua (16, 17). Analysis of the in vivo role of WNT4 in adult uterine function has been limited by the perinatal lethality and female to male sex reversal of _Wnt4_−/− mice (13, 18).
Here, we conditionally ablated Wnt4 (19) in the uterus using the PRcre mouse model (20). Female PRcre/+Wnt4f/f (Wnt4d/d) mice displayed severe subfertility, exhibiting a defect in embryo implantation and subsequent decidualization. In addition, these mice displayed altered postnatal uterine differentiation, characterized by stratification of the luminal epithelium and a reduction in the number of uterine glands. While the glands may contribute to the implantation defect, we demonstrate that WNT4 acts in the stroma to mediate P4-driven stromal cell survival. Thus, WNT4 is a critical mediator not only of proper postnatal uterine differentiation, but also of embryo implantation.
MATERIALS AND METHODS
Animals and hormone treatments
Mice were maintained in the designated animal care facility at Baylor College of Medicine according to the Institutional Animal Care and Use Committee guidelines for the care and use of laboratory animals. Fertility was assessed by mating 8-wk-old female mice with wild-type male mice for 6 mo and determining the number and size of litters delivered. Superovulation was induced in 3-wk-old female mice by administering 5 IU of pregnant mares' serum gonadotropin intraperitoneally (i.p.; VWR Scientific, West Chester, PA, USA), followed by 5 IU human chorionic gonadotropin i.p. (Pregnyl, Organon International, Oss, Netherlands) 48 h later, and they were placed with males. The mice were sacrificed 24 h later, and oocytes were flushed from the oviducts and counted. For the chronic E2 treatment, mice were ovariectomized at 6 wk of age. After 2 wk rest, a vehicle (beeswax) or E2 (20 μg/pellet) pellet was implanted subcutaneously. The mice were sacrificed either 2 wk or 3 mo later. At the time of dissection, uterine tissues were placed in 4% PFA (vol/vol) or flash-frozen and stored at −80°C.
Embryo implantation was assessed by mating 8-wk-old female mice with wild-type male mice. The presence of the postcoital vaginal plug was designated d0.5. Embryo transfer experiments were carried out by mating 8-wk-old female mice with vasectomized male mice. On d0.5, 20 blastocysts were transferred into the oviduct (10/horn) as detailed by Nagy (21). The mice were sacrificed on the designated day, and the uteri were fixed in 4% paraformaldehyde (PFA; v/v) overnight. Blood was collected, and serum was isolated by centrifugation using serum separator tubes (BD, Franklin Lakes, NJ, USA). The serum was sent to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, VA, USA) for analysis of P4 by radioimmunoassay.
The artificial induction of decidualization has been previously described (22). Briefly, 6-wk-old female mice were ovariectomized and treated with 3 daily injections of 100 ng E2/mouse. After 2 d of rest, mice were then treated with daily subcutaneous injections of 1 mg P4 + 6.7 ng E2/mouse for 3 d. At 6 h after the last injection, one uterine horn was traumatized by the injection of 50 μl of sesame oil. Mice were given daily subcutaneous injections of 1 mg P4 + 6.7 ng E2/mouse following the trauma. Mice were sacrificed on the designated days after the trauma by cervical dislocation while under anesthetic, 2,2-tribromoethyl alcohol (Avertin; Sigma-Aldrich, St. Louis, MO, USA). At the time of dissection, uterine tissues were placed in 4% PFA (v/v) or flash-frozen and stored at −80°C. For the day −1 time point, mice were sacrificed 6 h after the second P4 plus E2 injection, which corresponds to 1 day before the administration of the decidual trauma.
The rescue of decidualization using recombinant LIF has been previously described (23). Briefly, the artificial induction of decidualization was followed as above. However, on day −1, each uterine horn was injected with either 10 μl of 1% BSA or 10 μl of recombinant LIF (100 ng/ml in 1% BSA; Millipore, Billerica, MA, USA). The next day, the uterus was traumatized by a scratch with a 25-gauge needle. Mice were sacrificed 2 d after the decidual trauma and processed as above. As a positive control, LIF was used instead of E2 in the artificial decidual response as described previously (24). Briefly, 6-wk-old female mice were ovariectomized and administered daily subcutaneous injections of 100 ng E2 for 3 d. After 2 d of rest, mice were given daily injections of 1 mg P4. At 6 h after the second P4 injection, mice were injected intraluminally with either 10 μl of 1% BSA or 10 μl of recombinant LIF (100 ng/ml in 1% BSA). Then, 6 h after the third P4 injection, one of the horns received a decidual trauma by a scratch with a 25-gauge needle, while the other horn was left unstimulated as a control. Mice were sacrificed 2 d later and processed as above.
Quantitative real-time PCR
Total RNA was isolated from frozen uterine tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). One microgram of the RNA was reverse transcribed into cDNA with M-MLV (Invitrogen). Expression levels of mRNA were measured by quantitative RT-PCR TaqMan analysis using the ABI Prism 7700 Sequence Detector System (PE Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions. Real-time probes and primers were purchased from Applied Biosystems (Table 1). Standard curves were generated by serial dilution of a preparation of total cDNA. All real-time PCR was done using independent RNA sets. All mRNA quantities were normalized against 18S RNA using ABI rRNA control reagents (Applied Biosystems). Statistical analyses were performed using 1-way ANOVA followed by Tukey's post hoc multiple-range test with the Instat package from GraphPad (San Diego, CA, USA).
Table 1.
Applied Biosystems assay identification for quantitative RT-PCR analysis
| Gene | Assay identification |
|---|---|
| Bmp2 | Mm01340178_m1 |
| Fkbp4 | Mm00487391_m1 |
| Fkbp5 | Mm00487401_m1 |
| Foxa2 | Mm00839704_m1 |
| Foxo1 | Mm00490672_m1 |
| Fst | Mm00514982_m1 |
| Frap1 | Mm00444968_m1 |
| Id1 | Mm00775963_m1 |
| Lif | Mm00434761_m1 |
| p63 | Mm00495788_m1 |
| PR | Mm00435625_m1 |
| Ptgs2 | Mm00478384_m1 |
| Wnt4 | Mm00437341_m1 |
| Wnt6 | Mm00437351_m1 |
Immunohistochemistry
Uteri were fixed overnight in 4% PFA (v/v), followed by thorough washing in 70% ethanol, and tissues were processed, embedded in paraffin, and sectioned. Uterine sections were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series. Sections were preincubated with 10% normal goat serum in PBS (pH 7.5) or the M.O.M. kit (Vector Laboratories, Burlingame, CA, USA), according to manufacturer's instructions, and then incubated with anti-p63 (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-cytokeratin 5 (1:100; from D.R.), anti-phospho-histone H3 (1:2000; Upstate, Billerica, MA, USA), or anti-PR (1:200; DAKO, Carpinteria, CA, USA) in 10% normal serum in PBS (pH 7.5) or the M.O.M. Kit (Vector Laboratories). On the following day, sections were washed in PBS and incubated with biotinylated secondary antibody (5 μl/ml; Vector Laboratories) for 1 h at room temperature. Immunoreactivity was detected using the DAB Substrate kit (Vector Laboratories); the immunoreactivity was visualized as brown staining.
Immunofluorescence
Uteri were fixed overnight in 4% PFA (v/v), followed by thorough washing in 70% ethanol, and tissues were processed, embedded in paraffin, and sectioned. Uterine sections were cut at 5 μm and mounted on silane-coated slides, deparaffinized, and rehydrated in a graded alcohol series. Sections were preincubated with 10% normal goat serum in PBS (pH 7.5; Vector Laboratories) and then incubated with anti-FOXO1 (1:100; Millipore) in 10% normal serum in PBS (pH 7.5; Vector Laboratories). On the following day, sections were washed in PBS and incubated with biotinylated secondary antibody (5 μl/ml; Vector Laboratories) for 1 h at room temperature. Immunoreactivity was detected using the TSA kit (Invitrogen); the immunoreactivity was visualized as green fluorescence. The TUNEL assay was performed using the Roche in situ cell death detection kit, fluorescein (Roche, Boulder, CO, USA), according to manufacturer's instructions.
Alkaline phosphatase activity assay
Uteri were fixed in 4% PFA (v/v) overnight, followed by sucrose gradients in PBS (15 and 30%), and tissues were embedded in OCT. Tissue sections were cut at 16 μm. Slides were postfixed in 0.2% glutaraldehyde, washed in PBS, and incubated with a 100 mM Tris buffer (pH 9.5) containing chromogenic substrates for alkaline phosphatase (168.5 μl of 100 mg/ml nitro blue tetrazolium salt in dimethylformamide and 175 μl of 50 mg/ml 5-bromo-4-chloro-3-indoyl phosphate/toluidinium salt in dimethylformamide added to 50 ml of the Tris buffer; Roche). The development of a purple color is indicative of alkaline phosphatase activity.
RESULTS
Generation of mice with conditional ablation of Wnt4 in the uterus
Previously, WNT4 has been implicated in the regulation of endometrial stromal cell decidualization (4, 5). Because female _Wnt4_−/− mice exhibit perinatal lethality and sex reversal, conditional ablation of WnT4 was necessary in order to study its in vivo role in adult uterine function (13, 18). Therefore, Wnt4f/f mice (19) were crossed to the PRcre mouse model (20), which recombines alleles in all PR-expressing cells, which includes all compartments of the uterus (PRcre/+Wnt4f/f; Wnt4d/d). To assess the efficiency of ablation, the expression of Wnt4 in the uterus was examined 2 d after the administration of the decidual trauma in the artificial decidual response. Wnt4f/f mice exhibited strong expression of Wnt4, whereas this expression was significantly attenuated in the Wnt4d/d mice, as shown by quantitative RT-PCR analysis (Supplemental Fig. S1). Therefore, Wnt4 was efficiently ablated by PRcre in the mouse uterus.
Wnt4 regulates postnatal uterine development
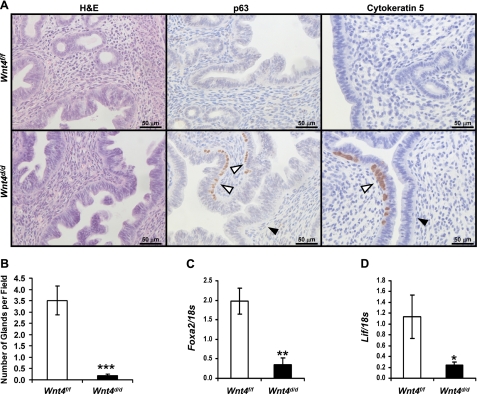
Because WNT4 affects prenatal uterine development (13), we investigated the effect of Wnt4 ablation on postnatal uterine development. Histological examination of the uteri of 6-wk-old Wnt4f/f mice showed normal uterine histology; however, the Wnt4d/d uteri exhibited an abnormal appearance (Fig. 1A). In lieu of the simple columnar luminal epithelial layer observed in the control uteri, the Wnt4d/d uteri displayed a second cell layer in addition to the columnar layer, which stained positive for the basal cell marker p63, as well as the marker of stratified epithelium cytokeratin 5 (25). These stratified regions were localized focally in the luminal epithelium. In addition, the Wnt4d/d uterus exhibited a reduction in the number of uterine glands compared to the Wnt4f/f uteri. The number of glands was quantified by taking the number of glands per uterine field, which confirmed the histological results (Fig. 1B). Furthermore, the expression of Foxa2, which is expressed specifically in the uterine glands and is essential for their development (23, 26), was assessed on day −1 in the artificial decidual response. An abbreviated artificial decidual response was used instead of natural pregnancy to avoid any possible gene changes due to the presence of the blastocyst (27) or variations in the staging of d3.5. Its expression was reduced in the Wnt4d/d uteri compared to controls, providing additional verification of the reduction in uterine glands (Fig. 1C). As secretions from uterine glands are critical for the viability of the embryo prior to placentation, we assessed the expression of one of these secretions, leukemia inhibitory factor (Lif), on day −1 of the artificial decidual response (28). Lif expression was significantly reduced in the Wnt4d/d uteri compared to controls, as expected, due to the reduction in uterine glands (Fig. 1D). These data demonstrate a critical role for WNT4 in proper postnatal uterine development.
Figure 1.
Wnt4d/d mice exhibit altered postnatal uterine development. A) Analysis of 6-wk-old Wnt4f/f and Wnt4d/d mice. Left panel: hematoxylin and eosin staining. Middle panel: immunohistochemical analysis of p63. Right panel: immunohistochemical analysis of cytokeratin 5. Counterstaining is hematoxylin. Scale bars = 50 μm. White arrowheads mark p63+ cells. Black arrowheads mark p63-negative cells. B) Quantification of number of uterine glands per field in Wnt4f/f and Wnt4d/d uteri at 6 wk of age. C) Quantitative RT-PCR analysis of the gland marker Foxa2 on day −1 in the artificial decidual response. D) Quantitative RT-PCR analysis of Lif on day −1 in the artificial decidual response. Results represent means ± se; n = 5 mice/genotype. *P < 0.05; **P < 0.01; ***P < 0.001.
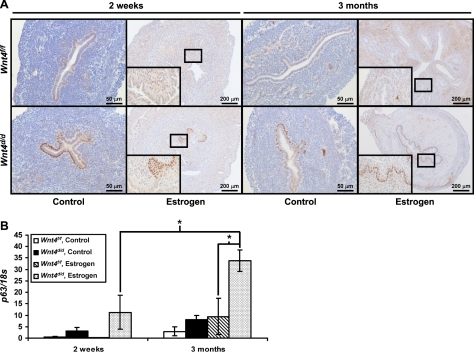
Previously, the presence of basal cells in the uterine luminal epithelium has been associated with in utero exposure to the synthetic estrogen diethylstilbestrol (DES; refs. 29, 30). Therefore, we wanted to determine whether chronic E2 treatment would expand the basal cell population in the Wnt4d/d mice. After 2 wk of E2 treatment, the Wnt4d/d mice developed focal regions of multiple p63+ cell layers (Fig. 2A). When the treatment was extended to 3 mo, the entire luminal epithelium was stratified and contained p63+ basal cells in the Wnt4d/d mice (Fig. 2A). Therefore, there is a progression from focal basal cells to focal squamous cell metaplasia to squamous cell metaplasia throughout the entire luminal epithelium. In the Wnt4f/f mice, rare p63+ basal cells were observed in the 3-mo E2-treated mice compared to the control Wnt4f/f mice, indicating that chronic E2 treatment can promote this phenotype (Fig. 2A, inset). In the control-treated mice, no basal cells were observed in the Wnt4f/f mice, but focal basal cells were observed in the Wnt4d/d mice, although they never progressed any further. Therefore, the presence of the p63+ basal cells occurs in the Wnt4d/d mice in the absence of hormones, indicating that loss of Wnt4 is sufficient to promote the formation of the basal cells. To quantitate these changes, we performed quantitative real-time PCR analysis for p63, as it is only expressed in this cell population in the mouse uterus and, therefore, serves as a marker of basal cells (Fig. 2B). These data confirmed the immunohistochemistry results. Thus, the appearance of p63+ basal cells occurs not only with loss of Wnt4, but also upon chronic E2 treatment, and, when the uterus is exposed to both loss of Wnt4 and chronic E2 treatment, this basal cell population is expanded and the epithelium undergoes squamous cell metaplasia.
Figure 2.
Wnt4 regulates epithelial differentiation in response to E2. A) Immunohistochemical analysis of p63 in Wnt4f/f and Wnt4d/d mice treated with vehicle or E2 for 2 wk or 3 mo. Scale bars = 50 (left panels; 200 μm (right panels). Insets: ×40 view. B) Quantitative RT-PCR analysis of p63 in Wnt4f/f and Wnt4d/d mice treated with vehicle or E2 for 2 wk or 3 mo. Results represent means ± se; n = 5 mice/group. *P < 0.05.
Wnt4 is required for female fertility
To assess the potential effect of Wnt4 ablation on female fertility, 8-wk-old Wnt4f/f and Wnt4d/d mice were mated with wild-type male mice for 6 mo. While the Wnt4f/f mice exhibited normal fecundity, the Wnt4d/d mice were severely subfertile (Table 2). Of the 10 mice examined, only 3 delivered litters, resulting in 4 litters with a total of 5 pups. Thus, WNT4 is critical for female fertility.
Table 2.
Female Wnt4d/d mice are severely subfertile
| Genotype | Females (n) | Pups (n) | Litters (n) | Average pups/litter | Average litters/female |
|---|---|---|---|---|---|
| Wnt4f/f | 10 | 330 | 55 | 6.00 ± 0.27 | 5.50 ± 0.17 |
| Wnt4d/d | 10 | 5 | 4 | 1.25 ± 0.25 | 0.40 ± 0.22 |
Because the PRcre mouse model also edits alleles in the pituitary gland and corpus luteum of the ovary, we examined the effect of Wnt4 ablation on the estrous cycle, ovulation, fertilization, and serum P4 levels (20). The estrous cycle was normal in the Wnt4d/d mice (data not shown). In response to superovulatory hormones, the Wnt4d/d mice ovulated comparably to control mice, and these ova were able to be fertilized in a similar manner to ova from control mice (Supplemental Table S1). Furthermore, serum P4 levels on d4.5 were comparable between the Wnt4f/f and Wnt4d/d mice (Supplemental Table S1). Thus, Wnt4 ablation does not affect the hypothalamo-pituitary-ovarian axis, suggesting that the cause of the reduced fertility of the Wnt4d/d mice is due to a uterine defect.
Embryo implantation is impaired in Wnt4d/d mice
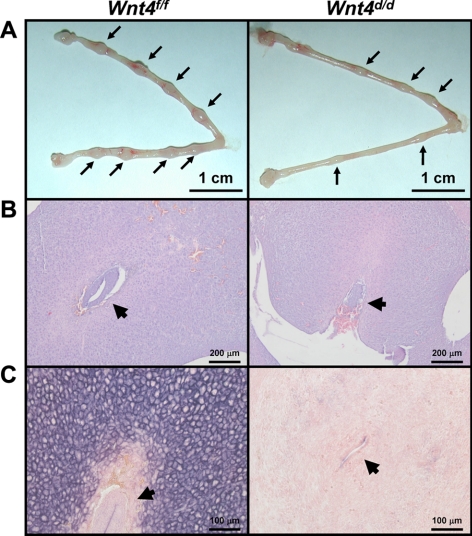
The attachment of the embryo to the uterine luminal epithelium and invasion through to the uterine stroma is a critical event in early pregnancy (1). To determine whether WNT4 plays a role in this process, female Wnt4f/f and Wnt4d/d mice were mated with vasectomized male mice. On d0.5, wild-type blastocysts were transferred into the oviducts of female mice, and the presence of visible implantation sites was assessed on d5.5. All of the Wnt4f/f mice displayed implantation sites, whereas only 25% of the Wnt4d/d mice displayed implantation sites. Wnt4f/f mice displayed 56 visible implantation sites, resulting from 120 embryos transferred (46.67%) whereas Wnt4d/d mice displayed 7 implantation sites from 180 embryos transferred (3.89%). In addition to having a reduced number of implantation sites, the size of the implantation sites was also smaller in the Wnt4d/d uteri compared to controls (1.52±0.06 vs. 2.44±0.14 mm; Fig. 3A).
Figure 3.
Embryo implantation is impaired in Wnt4d/d mice. A) Gross histology of Wnt4f/f and Wnt4d/d uteri on d5.5. Arrows mark implantation sites. B) Hematoxylin-and-eosin staining of d5.5 embryo implantation sites in Wnt4f/f and Wnt4d/d uteri. Arrowheads mark the embryo. C) Alkaline phosphatase staining of Wnt4f/f and Wnt4d/d implantation sites on d5.5. Positive staining is visualized as purple; counterstaining is nuclear fast red. Arrowheads mark the embryo. Scale bars = 1 cm (A); 200 μm (B); 100 μm (C).
Examination of the histology of these embryos revealed successful attachment and invasion into the uterine stroma of the embryos in the Wnt4f/f uterus (Fig. 3B). However, in the Wnt4d/d uterus, while embryos were able to attach to the uterine luminal epithelium, they failed to successfully invade into the uterine stroma. Many embryos failed to initiate any invasion, whereas some partially invaded through the luminal epithelium. Furthermore, the stromal cells of the Wnt4d/d uterus failed to decidualize compared to controls, as seen both histologically and by alkaline phosphatase activity, a marker of stromal cell differentiation during decidualization (Fig. 3C). Thus, WNT4 has a critical role in embryo implantation and may also be necessary for proper decidualization.
Wnt4 is a critical mediator of uterine stromal cell decidualization
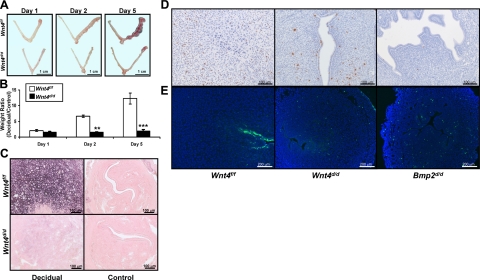
To further examine the role of WNT4 in decidualization, female Wnt4f/f and Wnt4d/d mice were assessed for their ability to undergo the artificially induced decidual response. The Wnt4f/f mice exhibited a robust response, as seen grossly by the increased size of the decidual (right) horn compared to the control (left) horn throughout the decidual time course, whereas the Wnt4d/d uteri failed to show an increase in size of the decidual horn (Fig. 4A). The extent of decidualization can be quantified by taking the weight ratio of the decidual horn to the control horn. This quantification confirms the observations of the gross anatomy, with the Wnt4d/d mice displaying a significantly reduced decidual response as early as 2 d after the administration of the decidual trauma (Fig. 4B).
Figure 4.
Wnt4d/d mice exhibit a decidualization defect. A) Gross histology of Wnt4f/f and Wnt4d/d uteri at 1, 2, and 5 d after administration of decidual trauma in the artificial decidual response. Decidual horn is at right; control horn is at left. B) Quantification of decidual response in Wnt4f/f and Wnt4d/d mice at 1, 2, and 5 d after administration of decidual trauma in the artificial decidual response by taking the weight ratio of the decidual vs. control horn. C) Alkaline phosphatase staining of Wnt4f/f and Wnt4d/d decidual and control horns on day 2. Positive staining is visualized as purple; counterstaining is nuclear fast red. D) Immunohistochemical analysis of phospho-histone H3 in Wnt4f/f, Wnt4d/d, and Bmp2d/d uteri on day 2. Counterstaining is hematoxylin. E) TUNEL analysis of Wnt4f/f, Wnt4d/d, and Bmp2d/d uteri on day 2. Nuclei are counterstained with DAPI. Scale bars = 1 cm (A); 100 μm (C, D); 200 μm (E). Results represent means ± se; n = 5 mice/group. **P < 0.01; ***P < 0.001.
Stromal cell decidualization is characterized by the proliferation and differentiation of the stromal cells in preparation for placentation (1). Stromal cell differentiation was assessed by staining for alkaline phosphatase activity, a known marker of stromal cell differentiation. Robust alkaline phosphatase staining was observed in the Wnt4f/f decidua, whereas it was markedly reduced in the Wnt4d/d decidua (Fig. 4C). The control horns were used as a negative control. Proliferation was assessed by immunohistochemical analysis of phospho-histone H3. This analysis revealed no difference in proliferation between Wnt4f/f and Wnt4d/d stromal cells (Fig. 4D). Because the reduced number of differentiating cells was not due to changes in cellular proliferation, we wanted to determine whether it may be due to changes in cellular apoptosis, which we assessed by the TUNEL assay, in which apoptotic cells are stained green. While there is little stromal cell apoptosis in the Wnt4f/f decidua, there are significant numbers of apoptotic stromal cells in the Wnt4d/d uteri (Fig. 4E). When comparing the defect in decidualization with Wnt4 ablation to that of Bmp2, ablation of Bmp2 also results in an increase in stromal cell apoptosis (Fig. 4E) and a lack of stroma cell differentiation, but ablation of Bmp2 also resulted in a decrease in stromal cell proliferation (4). Therefore, Wnt4 regulates stromal cell differentiation and survival during decidualization, but acts differently from Bmp2 with regard to stromal cell proliferation.
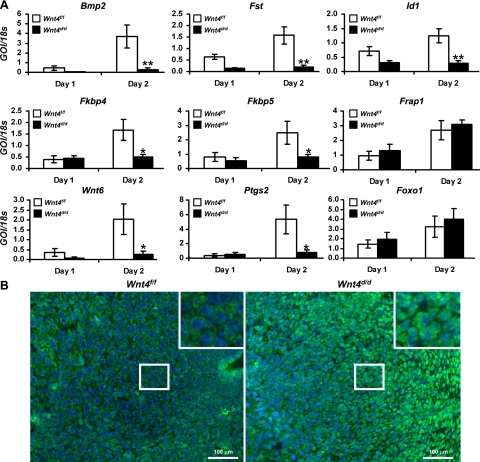
To identify the pathways regulated by WNT4 during decidualization, molecular analysis of genes known to be involved in decidualization was conducted. This analysis revealed a reduction in the decidual marker genes, bone morphogenetic protein 2 (Bmp2) and its downstream target genes, follistatin (Fst) and inhibitor of DNA binding 1 (Id1) (Fig. 5A). As Wnt4 expression was also decreased upon ablation of Bmp2 (4), these data suggest that a complex signaling network exists between the pathways regulated by WNT4 and by BMP2 during decidualization. Microarray analysis was conducted during decidualization in the Bmp2d/d uteri, which identified numerous genes regulated by BMP2 (4). To determine whether these genes are also regulated by WNT4, we analyzed the expression of a subset of these genes in the Wnt4d/d decidua. Similarly to the Bmp2d/d uteri, we observed a reduction in the expression of prostaglandin synthase 2 (Ptgs2); Wingless-type MMTV integration site family, member 6 (Wnt6); and FK506 binding proteins 4 and 5 (Fkbp4 and Fkbp5) (Fig. 6A). Interestingly, we saw no change in the expression of FK506 binding protein 12-rapamycin associated protein 1 [Frap1; otherwise known as a mammalian target of rapamycin (mTOR)] upon Wnt4 ablation, which differs from the Bmp2d/d uteri (Fig. 5A). These data suggest that some pathways are regulated by both WNT4 and BMP2 during decidualization, but that other pathways are regulated by either WNT4 or BMP2. Many of the deregulated genes are associated with P4 signaling (1). Therefore, we assessed PR levels on day 1 and 2 of the artificial decidual response. Analysis of PR levels demonstrated no difference at either the mRNA or protein level between the Wnt4f/f and Wnt4d/d mice, similar to the Bmp2d/d mice (Supplemental Fig. S2 and ref. 4). Thus, WNT4 acts to regulates P4 action, likely by regulating PR activity and not the level of the receptor itself.
Figure 5.
WNT4 regulates P4 action during decidualization. A) Quantitative RT-PCR analysis of decidual marker genes Bmp2, Fst, Id1, Fkbp4, Fkbp5, Frap1, Ptgs2, Wnt6, and Foxo1 in Wnt4f/f and Wnt4d/d uteri on days 1 and 2. B) Immunofluorescence analysis of FOXO1 in Wnt4f/f and Wnt4d/d uteri on day 2. Positive staining is visualized as green fluorescence; counterstaining is DAPI (blue). GOI, gene of interest. Scale bars = 100 μm. Results represent means ± se; n = 5 mice/group. *P < 0.05; **P < 0.01.
Figure 6.
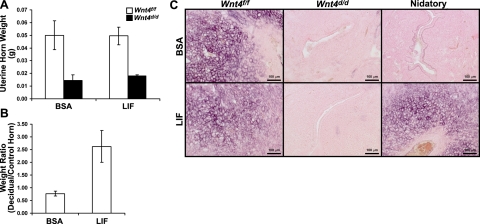
Administration of recombinant LIF is unable to rescue the Wnt4d/d decidual defect. A) Weight of uterine horns of Wnt4f/f and Wnt4d/d mice at 2 d after administration of decidual trauma in the artificial decidual response. Mice received intrauterine uterine injections of either vehicle (BSA) or LIF 1 d prior to decidual trauma; n = 5/group. B) Weight ratio of decidual vs. control horns in Wnt4f/f mice that received an injection of LIF instead of nidatory E2 at 2 d after decidual trauma in the artificial decidual response; n = 3/group. C) Alkaline phosphatase staining in Wnt4f/f and Wnt4d/d mice that received intrauterine vehicle (BSA) or LIF injections during decidualization (Wnt4f/f, Wnt4d/d) or instead of nidatory E2 (nidatory). Positive staining is visualized as purple; counterstaining is nuclear fast red. Scale bars = 100 μm. Results represent means ± se.
Since cell survival during decidualization appears to be regulated by WNT4, we wanted to determine the mechanism by which WNT4 regulates apoptosis. During decidualization, the localization of FOXO1 is regulated by P4, which promotes its cytoplasmic shuttling (31, 32). This shuttling prevents FOXO1 from regulating the transcription of its target genes, including numerous proapoptotic genes (33). To determine whether altered localization of FOXO1 may account for the increased apoptosis, we determined whether the localization of FOXO1 was altered on Wnt4 ablation. The mRNA expression of Foxo1 was unaltered in the Wnt4d/d mice (Fig. 5A). However, immunohistochemical analysis revealed a change in its localization pattern. While FOXO1 was primarily cytoplasmic in the decidual cells of the Wnt4f/f mice, it remained nuclear in the stromal cell of the Wnt4d/d mice (Fig. 5B). Therefore, these data suggest that WNT4 regulates decidualization by mediating P4-driven FOXO1 cytoplasmic shuttling, thereby promoting stromal cell survival.
The decidual defect of the Wnt4d/d mice is not due to the glandular developmental defect
Secretions from uterine glands, such as LIF, are essential for embryo implantation and decidualization (28, 34). Recently, it was demonstrated that the administration of recombinant LIF was able to partially rescue the decidual defect in a mouse model that lacked uterine glands (23). Therefore, we used this protocol to ascertain whether the decidual defect of the Wnt4d/d mice is due to the reduction in uterine glands or to a role for WNT4 in the uterine stroma. The administration of recombinant LIF was unable to rescue the decidual defect of the Wnt4d/d mice without affecting decidualization in the Wnt4f/f mice, as seen both by the weight of the uterine horns and by alkaline phosphatase activity (Fig. 6A, C). To ensure that the recombinant LIF was functional, we substituted LIF for nidatory E2, as described previously (24). In the Wnt4f/f mice, an injection of LIF instead of nidatory E2 was able to induce decidualization compared to an injection of vehicle, as assayed both by the ratio of the weights of the decidual vs. control horn and by alkaline phosphatase activity (Fig. 6B, C). Thus, the observed decidual defect of the Wnt4d/d mice is due to the loss of Wnt4 in the stroma and not due to the reduction in uterine glands.
DISCUSSION
WNT4 has been suggested to play a role in the regulation of stromal cell decidualization during embryo implantation (4, 5). To address this potential role, we examined the role of WNT4 in vivo. Because of the perinatal lethality and sex reversal of female _Wnt4_−/− mice (13, 18), Wnt4 (19) was conditionally ablated using the PRcre mouse model (20). After validating the successful ablation of Wnt4 in the uterus (Supplemental Fig. S1), we assessed the effect of Wnt4 ablation on uterine development and function.
Previous work has implicated WNT4 in the regulation of prenatal uterine development (13). In addition, the Wnt signaling pathway has been demonstrated to play roles in the regulation of postnatal uterine development as key regulators of uterine gland formation and epithelial differentiation (10–12). Examination of the Wnt4d/d uteri revealed a reduction in the number of uterine glands (Fig. 1A–C). This result is similar to those found upon ablation of Wnt7a or Wnt5a or conditional ablation of β-catenin (10–12). In addition to the gland formation defect, we also observed altered epithelial differentiation in the Wnt4d/d uteri, characterized by the presence of p63+ basal cells (Fig. 1A). This phenotype is similar to that observed upon ablation of Wnt7a or conditional ablation of β-catenin using the PRcre mouse model (10, 12). However, conditional ablation of β-catenin using the Amhr2cre mouse model resulted in uteri that contained glands (35). These data suggest that Wnt signaling in the epithelium is necessary for uterine gland formation; however, future studies are needed to confirm this observation, as the Amhr2cre mouse model exhibits weak Cre activity in the uterine stroma. Interestingly, Wnt4 expression was deregulated on ablation of Wnt7a, suggesting that it may act downstream of WNT7A in this process (12). The stratification observed in the cycling Wnt4d/d uteri was not as severe as in the other mouse models, suggesting that there may be some compensation from the other Wnt ligands in maintaining the integrity of the epithelium. Interestingly, p63 has been shown to regulate Wnt4 expression, suggesting the possible existence of a regulatory network between these two proteins (36). Squamous cell carcinoma accounts for a small portion of endometrial cancers and is thought to arise from the cervix (37). However, our studies suggest that these lesions may arise from the uterus itself. Patients with this type of cancer survive an average of 40 mo, even after treatment, demonstrating a need to better understand this disease (37). Previous studies have demonstrated a reduction in Wnt4 expression in basal cell carcinomas, supporting a role for this signaling pathway in the expansion of this cell population (38). Therefore, by using this mouse model and our understanding of basal cell carcinomas, we may be able to gain insight into uterine squamous cell carcinoma.
Stratification of the luminal epithelium has been linked to in utero exposure to the synthetic hormone DES (29, 30). This response was shown to be dependent on estrogen receptor α (ERα; ref. 39). Wnt4 is E2-regulated in the uterus, suggesting it may play a role in the uterine estrogenic response (14, 15). Therefore, we treated Wnt4d/d mice with E2 for an extended period of time. These mice developed squamous cell metaplasia, as marked by stratification of the luminal epithelium and the expression of the basal cell marker p63 (Fig. 2). Further, the control mice treated with E2 for 3 mo also demonstrated increased p63 expression. Interestingly, uterine gland formation has also been linked to in utero exposure to DES (29, 30). Thus, these data suggest that a complex relationship exists between Wnt and E2 signaling in the regulation of postnatal epithelial differentiation and WNT4 may represent a means by which the uterine epithelium is protected from stratification in response to endogenous or environmental E2s.
Previously, Wnt signaling was shown to be critical for female fertility, as female mice with ablation of β-catenin in the uterus displayed infertility (10). Interestingly, levels of Wnt signaling appear to be critical, as constitutive activation of β-catenin in the uterus also rendered female mice infertile (10). Female Wnt4d/d mice display severe subfertility (Table 2). PRcre recombines alleles in the pituitary gland and corpus luteum of the ovary, in addition to the uterus; however, examination of the pituitary gland and ovary demonstrated no observable phenotype (Supplemental Table S1). Previously, using the Amhr2cre mouse model to ablate Wnt4, it was shown that WNT4 is critical for follicle development, likely by regulating steroidigenic genes in the granulosa cells (19). The difference in the phenotypes of these two mouse models can likely be explained by the differences in Cre-expression, as the PRcre activity occurs late in follicular development. Thus, the likely reason for the reduced fertility of the Wnt4d/d mice is a uterine defect.
The uterus must be receptive in order for the embryo to attach to the luminal epithelium, invade through it, and establish a decidual response (1). The implantation defect observed in the Wnt4d/d mice may be due indirectly to the loss of uterine glands or due to direct action of WNT4 in stromal cells. Uterine glands are critical for implantation in the mouse due to the production of LIF in the preimplantation period (28). Ablation of Lif or loss of endometrial glands in the mouse uterus results in implantation and decidual defects (23, 28). However, in both cases, these defects may be rescued by administering recombinant LIF (23, 24). As expected because of the reduction in uterine glands, Lif expression is reduced in the Wnt4d/d mice (Fig. 1D), implicating the reduction in uterine glands as contributing to the implantation defect. However, the administration of recombinant LIF was unable to rescue the decidual defect of these mice, suggesting that WNT4 exerts a role in the stroma during decidualization (Fig. 6). In addition, the _Lif_−/− mice display reduced stromal cell proliferation during decidualization, which differs from the Wnt4d/d decidua, further supporting a role for WNT4 in the stroma (Fig. 4D and ref. 40). The Wnt4d/d mice did display initial invasion and decidualization. The reason for this may be because of the presence of a few uterine glands or the expression of Wnt4 in PR-negative cells, which may provide enough signaling to give these effects. However, it is evident that the ablation of Wnt4 affects embryo implantation, in part, through its regulation of gland formation.
Examination of the artificial decidual response confirmed that decidualization was reduced upon ablation of Wnt4 (Fig. 4). Unlike Bmp2 ablation, which caused a decrease in proliferation (4) and an increase in apoptosis (Fig. 4E), the Wnt4d/d decidua only showed an increase in apoptosis with no alteration in proliferation. Molecular analysis demonstrated decreased expression of the decidual marker genes Bmp2, Fst, and Id1 (Fig. 6A). These data suggest that a complex relationship between WNT4 and BMP2 exists during decidualization, and so we wanted to determine the pathways that may be regulated by these two signaling molecules. As microarray analysis was previously conducted to determine the BMP2-regulated genes, we analyzed a subset of these genes in the Wnt4d/d decidua. We observed reduced expression of Wnt6, Fkbp4, Fkbp5, and Ptgs2 during decidualization (Fig. 6A), which is consistent with what was previously observed on conditional ablation of Bmp2 in the uterus (4). Female _Ptgs2_−/− mice displayed a defect in embryo implantation and decidualization (41, 42). Ablation of Fkbp4 demonstrated its critical role in female fertility as a regulator of uterine P4 signaling (43, 44). Thus, WNT4 acts to regulate P4 signaling during decidualization. However, both the Bmp2d/d and Wnt4d/d mice failed to show changes in overall PR levels during decidualization (Supplemental Fig. S2 and ref. 4). Thus, these results suggest that WNT4 regulates decidualization by mediating P4 signaling, but not by regulating the level of the PR. However, not all genes that were deregulated on Bmp2 ablation were affected by Wnt4 ablation, as exemplified by Frap1/mTOR expression (Fig. 6A). mTOR has been implicated in the regulation of embryo implantation, potentially as a mediator of stromal cell proliferation (45). Therefore, the difference in stromal cell proliferation between the Bmp2d/d and Wnt4d/d decidua may potentially be explained by the difference in the expression of Frap1/mTOR.
Uterine stromal cell apoptosis in the Wntd/d mice may be linked to a deficit in PR signaling. The administration of the antiprogestin RU486 to mice that have undergone decidualization leads to stromal cell apoptosis and loss of the decidua, which mimics menstruation (46, 47). The ability of P4 to regulate apoptosis in human endometrial stromal cells has been linked to the cellular localization of FOXO1 (31, 32). When FOXO1 is nuclear, it regulates the transcription of its target genes by binding to its response elements, located in the promoters of its target genes, which include multiple proapoptotic genes (33). To prevent this transcription, FOXO1 is shuttled to the cytoplasm, which occurs in uterine stromal cells in response to P4 treatment (31, 32). In the Wnt4d/d uteri, FOXO1 is mislocalized in that it remains in the nucleus during decidualization when it should be cytoplasmic, as seen in the controls (Fig. 5B). These data implicate the mislocalization of FOXO1 as being a potential reason for the increased stromal cell apoptosis observed in the Wnt4d/d uteri. Interestingly, FOXO1 has been shown to be essential for human endometrial stromal cell decidualization, indicating that both the level and localization of FOXO1 need to be regulated for a successful decidual response (31, 48). Therefore, WNT4 regulates decidualization by promoting stromal cell differentiation and survival by mediating P4 action.
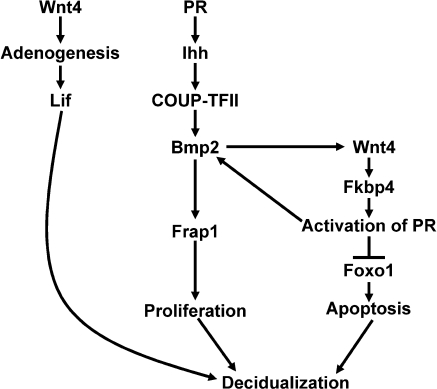
As summarized in Fig. 7, ablation of Wnt4 in the uterus using the PRcre mouse model rendered female mice subfertile due to defects at multiple steps in the implantation process. Ablation of Wnt4 results in a reduction in uterine glands and a stratification of the luminal epithelium, which was exacerbated by E2 treatment. During early pregnancy, a defect in embryo implantation and decidualization was observed in the Wnt4d/d mice due to an attenuation of P4 signaling, which led to a defect in stromal cell survival. Thus, these findings demonstrate a critical role for WNT4 in proper postnatal uterine development as well as normal uterine function. Further examination into these signaling pathways will provide insight not only into the complex signaling networks that regulate embryo implantation, but also into the pathways that are deregulated during endometrial diseases, such as endometrial cancer.
Figure 7.
Model of WNT4 action during embryo implantation.
Supplementary Material
Supplemental Data
Acknowledgments
The authors thank Joanne S. Richards for assistance with the Wnt4f/f mice; Sungnam Cho, Jie Yang, and Bryan Ngo for technical assistance; and Sophia Y. Tsai and Janet DeMayo for manuscript preparation.
This work was supported by U.S. National Institutes of Health (NIH) grants R01HD042311 (to F.J.D.), R01HD057873 (to J.W.J.), and R01CA77530 (to J.P.L.); an operating grant from the Canadian Institutes of Health Research (to D.B.); Reproductive Biology Training Grant 5T32HD07165 and a scholarship from Baylor Research Advocates for Student Scientists, supported in part by the Winterman Foundation and the Houston Livestock Show and Rodeo Scholarship Fund (to H.L.F.); and Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH grants U54HD0077495 (to F.J.D.), U54HD055787 (to I.C.B., M.K.B., and F.J.D.), and U54HD28934 (to the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core) through a cooperative agreement as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Footnotes
REFERENCES
- 1.Franco H. L., Jeong J. W., Tsai S. Y., Lydon J. P., DeMayo F. J. (2008) In vivo analysis of progesterone receptor action in the uterus during embryo implantation. Semin. Cell Dev. Biol. 19, 178–186 [DOI] [PubMed] [Google Scholar]
- 2.Lee K. Y., DeMayo F. J. (2004) Animal models of implantation. Reproduction 128, 679–695 [DOI] [PubMed] [Google Scholar]
- 3.Wang H., Dey S. K. (2006) Roadmap to embryo implantation: clues from mouse models. Nat. Rev. 7, 185–199 [DOI] [PubMed] [Google Scholar]
- 4.Lee K. Y., Jeong J. W., Wang J., Ma L., Martin J. F., Tsai S. Y., Lydon J. P., DeMayo F. J. (2007) Bmp2 is critical for the murine uterine decidual response. Mol. Cell. Biol. 27, 5468–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q., Kannan A., Wang W., Demayo F. J., Taylor R. N., Bagchi M. K., Bagchi I. C. (2007) Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J. Biol. Chem. 282, 31725–31732 [DOI] [PubMed] [Google Scholar]
- 6.Angers S., Moon R. T. (2009) Proximal events in Wnt signal transduction. Nat. Rev. Mol. Cell Biol. 10, 468–477 [DOI] [PubMed] [Google Scholar]
- 7.Li J., Zhang J. V., Cao Y. J., Zhou J. X., Liu W. M., Fan X. J., Duan E. K. (2005) Inhibition of the beta-catenin signaling pathway in blastocyst and uterus during the window of implantation in mice. Biol. Reprod. 72, 700–706 [DOI] [PubMed] [Google Scholar]
- 8.Mohamed O. A., Jonnaert M., Labelle-Dumais C., Kuroda K., Clarke H. J., Dufort D. (2005) Uterine Wnt/beta-catenin signaling is required for implantation. Proc. Natl. Acad. Sci. U. S. A. 102, 8579–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie H., Tranguch S., Jia X., Zhang H., Das S. K., Dey S. K., Kuo C. J., Wang H. (2008) Inactivation of nuclear Wnt-beta-catenin signaling limits blastocyst competency for implantation. Development 135, 717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong J. W., Lee H. S., Franco H. L., Broaddus R. R., Taketo M. M., Tsai S. Y., Lydon J. P., DeMayo F. J. (2009) Beta-catenin mediates glandular formation and dysregulation of beta-catenin induces hyperplasia formation in the murine uterus. Oncogene 28, 31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mericskay M., Kitajewski J., Sassoon D. (2004) Wnt5a is required for proper epithelial-mesenchymal interactions in the uterus. Development 131, 2061–2072 [DOI] [PubMed] [Google Scholar]
- 12.Miller C., Sassoon D. A. (1998) Wnt-7a maintains appropriate uterine patterning during the development of the mouse female reproductive tract. Development 125, 3201–3211 [DOI] [PubMed] [Google Scholar]
- 13.Vainio S., Heikkila M., Kispert A., Chin N., McMahon A. P. (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397, 405–409 [DOI] [PubMed] [Google Scholar]
- 14.Hou X., Tan Y., Li M., Dey S. K., Das S. K. (2004) Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol. Endocrinol. 18, 3035–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katayama S., Ashizawa K., Fukuhara T., Hiroyasu M., Tsuzuki Y., Tatemoto H., Nakada T., Nagai K. (2006) Differential expression patterns of Wnt and beta-catenin/TCF target genes in the uterus of immature female rats exposed to 17alpha-ethynyl estradiol. Toxicol. Sci. 91, 419–430 [DOI] [PubMed] [Google Scholar]
- 16.Daikoku T., Song H., Guo Y., Riesewijk A., Mosselman S., Das S. K., Dey S. K. (2004) Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol. Endocrinol. 18, 1238–1250 [DOI] [PubMed] [Google Scholar]
- 17.Hayashi K., Erikson D. W., Tilford S. A., Bany B. M., Maclean J. A., 2nd, Rucker E. B., 3rd, Johnson G. A., Spencer T. E. (2009) Wnt genes in the mouse uterus: potential regulation of implantation. Biol. Reprod. 80, 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stark K., Vainio S., Vassileva G., McMahon A. P. (1994) Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679–683 [DOI] [PubMed] [Google Scholar]
- 19.Boyer A., Lapointe E., Zheng X., Cowan R. G., Li H., Quirk S. M., Demayo F. J., Richards J. S., Boerboom D. (2010) WNT4 is required for normal ovarian follicle development and female fertility. FASEB J. 24, 4627–4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soyal S. M., Mukherjee A., Lee K. Y., Li J., Li H., DeMayo F. J., Lydon J. P. (2005) Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 41, 58–66 [DOI] [PubMed] [Google Scholar]
- 21.Nagy A. (2003) Manipulating the Mouse Embryo: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA [Google Scholar]
- 22.Finn C. A., Martin L. (1972) Endocrine control of the timing of endometrial sensitivity to a decidual stimulus. Biol. Reprod. 7, 82–86 [DOI] [PubMed] [Google Scholar]
- 23.Jeong J. W., Kwak I., Lee K. Y., Kim T. H., Large M. J., Stewart C. L., Kaestner K. H., Lydon J. P., Demayo F. J. Foxa2 is essential for mouse endometrial gland development and fertility. Biol. Reprod 83, 396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J. R., Cheng J. G., Shatzer T., Sewell L., Hernandez L., Stewart C. L. (2000) Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology 141, 4365–4372 [DOI] [PubMed] [Google Scholar]
- 25.Koster M. I., Roop D. R. (2007) Mechanisms regulating epithelial stratification. Annu. Rev. Cell Dev. Biol. 23, 93–113 [DOI] [PubMed] [Google Scholar]
- 26.Besnard V., Wert S. E., Hull W. M., Whitsett J. A. (2004) Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr. Patterns 5, 193–208 [DOI] [PubMed] [Google Scholar]
- 27.Hamatani T., Daikoku T., Wang H., Matsumoto H., Carter M. G., Ko M. S., Dey S. K. (2004) Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc. Natl. Acad. Sci. U. S. A. 101, 10326–10331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart C. L., Kaspar P., Brunet L. J., Bhatt H., Gadi I., Kontgen F., Abbondanzo S. J. (1992) Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79 [DOI] [PubMed] [Google Scholar]
- 29.Goldberg J. M., Falcone T. (1999) Effect of diethylstilbestrol on reproductive function. Fertil. Steril. 72, 1–7 [DOI] [PubMed] [Google Scholar]
- 30.Sassoon D. (1999) Wnt genes and endocrine disruption of the female reproductive tract: a genetic approach. Mol. Cell. Endocrinol. 158, 1–5 [DOI] [PubMed] [Google Scholar]
- 31.Brosens J. J., Gellersen B. (2006) Death or survival–progesterone-dependent cell fate decisions in the human endometrial stroma. J. Mol. Endocrinol. 36, 389–398 [DOI] [PubMed] [Google Scholar]
- 32.Labied S., Kajihara T., Madureira P. A., Fusi L., Jones M. C., Higham J. M., Varshochi R., Francis J. M., Zoumpoulidou G., Essafi A., Fernandez de Mattos S., Lam E. W., Brosens J. J. (2006) Progestins regulate the expression and activity of the forkhead transcription factor FOXO1 in differentiating human endometrium. Mol. Endocrinol. 20, 35–44 [DOI] [PubMed] [Google Scholar]
- 33.Huang H., Tindall D. J. (2007) Dynamic FoxO transcription factors. J. Cell Sci. 120, 2479–2487 [DOI] [PubMed] [Google Scholar]
- 34.Gray C. A., Burghardt R. C., Johnson G. A., Bazer F. W., Spencer T. E. (2002) Evidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongation. Reproduction 124, 289–300 [PubMed] [Google Scholar]
- 35.Arango N. A., Szotek P. P., Manganaro T. F., Oliva E., Donahoe P. K., Teixeira J. (2005) Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev. Biol. 288, 276–283 [DOI] [PubMed] [Google Scholar]
- 36.Osada M., Park H. L., Nagakawa Y., Begum S., Yamashita K., Wu G., Kim M. S., Trink B., Sidransky D. (2006) A novel response element confers p63- and p73-specific activation of the WNT4 promoter. Biochem. Biophys. Res. Commun. 339, 1120–1128 [DOI] [PubMed] [Google Scholar]
- 37.Mendivil A., Schuler K. M., Gehrig P. A. (2009) Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes. Cancer Control 16, 46–52 [DOI] [PubMed] [Google Scholar]
- 38.Bonifas J. M., Pennypacker S., Chuang P. T., McMahon A. P., Williams M., Rosenthal A., De Sauvage F. J., Epstein E. H., Jr. (2001) Activation of expression of hedgehog target genes in basal cell carcinomas. J. Invest. Dermatol. 116, 739–742 [DOI] [PubMed] [Google Scholar]
- 39.Couse J. F., Dixon D., Yates M., Moore A. B., Ma L., Maas R., Korach K. S. (2001) Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev. Biol. 238, 224–238 [DOI] [PubMed] [Google Scholar]
- 40.Kimber S. J. (2005) Leukaemia inhibitory factor in implantation and uterine biology. Reproduction 130, 131–145 [DOI] [PubMed] [Google Scholar]
- 41.Cheng J. G., Stewart C. L. (2003) Loss of cyclooxygenase-2 retards decidual growth but does not inhibit embryo implantation or development to term. Biol. Reprod. 68, 401–404 [DOI] [PubMed] [Google Scholar]
- 42.Lim H., Paria B. C., Das S. K., Dinchuk J. E., Langenbach R., Trzaskos J. M., Dey S. K. (1997) Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 91, 197–208 [DOI] [PubMed] [Google Scholar]
- 43.Yang Z., Wolf I. M., Chen H., Periyasamy S., Chen Z., Yong W., Shi S., Zhao W., Xu J., Srivastava A., Sanchez E. R., Shou W. (2006) FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol. Endocrinol. 20, 2682–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tranguch S., Cheung-Flynn J., Daikoku T., Prapapanich V., Cox M. B., Xie H., Wang H., Das S. K., Smith D. F., Dey S. K. (2005) Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc. Natl. Acad. Sci. U. S. A. 102, 14326–14331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen X., He J., Ding Y., Zeng L., Gao R., Cheng S., Liu X., Wang Y. (2009) The role of MTOR in mouse uterus during embryo implantation. Reproduction 138, 351–356 [DOI] [PubMed] [Google Scholar]
- 46.Brasted M., White C. A., Kennedy T. G., Salamonsen L. A. (2003) Mimicking the events of menstruation in the murine uterus. Biol. Reprod. 69, 1273–1280 [DOI] [PubMed] [Google Scholar]
- 47.Xu X. B., He B., Wang J. D. (2007) Menstrual-like changes in mice are provoked through the pharmacologic withdrawal of progesterone using mifepristone following induction of decidualization. Hum. Reprod. 22, 3184–3191 [DOI] [PubMed] [Google Scholar]
- 48.Grinius L., Kessler C., Schroeder J., Handwerger S. (2006) Forkhead transcription factor FOXO1A is critical for induction of human decidualization. J. Endocrinol. 189, 179–187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data