The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression (original) (raw)
Abstract
Rel and RelA, individually dispensable for lymphopoiesis, serve unique functions in activated B and T cells. Here their combined roles in lymphocyte development were examined in chimeric mice repopulated with c-_rel_–/– _rela_–/– fetal liver hemopoietic stem cells. Mice engrafted with double-mutant cells lacked mature IgMloIgDhi B cells, and numbers of peripheral CD4+ and CD8+ T cells were markedly reduced. The absence of mature B cells was associated with impaired survival that coincided with reduced expression of bcl-2 and A1. bcl-2 transgene expression not only prevented apoptosis and increased peripheral B-cell numbers, but also induced further maturation to an IgMloIgDhi phenotype. In contrast, the survival of double-mutant T cells was normal and the bcl-2 transgene could not rectify the peripheral T-cell deficit. These findings indicate that Rel and RelA serve essential, albeit redundant, functions during the later antigen-independent stages of B- and T-cell maturation, with these transcription factors promoting the survival of peripheral B cells in part by upregulating Bcl-2.
Keywords: apoptosis/Bcl-2/lymphopoiesis/NF-κB/transgenics
Introduction
During lymphopoiesis, maintenance of cell numbers in the various developmental compartments is tightly controlled by a dynamic balance between proliferation, differentiation and apoptosis (Strasser, 1995). B- and T-cell precursors with non-functional antigen receptors that arise during the error-prone assembly of immunoglobulin (Ig) or T-cell receptor (TCR) genes are eliminated by programmed cell death. In the bone marrow, productive Ig gene rearrangements in pre-B cells are thought to transduce a survival signal through a pre-B-cell receptor (pre-BCR) composed of the Ig heavy chain, the invariant λ5 chain and Vpre-B (Strasser, 1995). Upon productive Ig light chain gene rearrangement, immature B cells exit the bone marrow, and undergo differentiation and selection steps in the spleen that ultimately result in only 5% of these cells being recruited into the long-lived mature B-cell pool (Osmond, 1993). The most immature peripheral B cells are characterized by an sIgMhiIgDlo phenotype, cells of intermediate maturity express high levels of IgM and IgD (sIgMhiIgDhi), whereas mature B cells downregulate surface IgM (sIgMloIgDhi). In the thymus, immature CD3+CD4+CD8+ T cells are subject to positive and negative selection (von Boehmer, 1990). Approximately 95% of thymocytes have non-functional or autoreactive antigen receptors and are eliminated by apoptosis (von Boehmer, 1990; Strasser, 1995). The remaining thymocytes undergo positive selection and differentiate into long-lived mature T cells that migrate to peripheral lymphoid tissues (von Boehmer, 1990). Thymocytes bearing TCRα/β molecules that recognize class I major histocompatibility complex (MHC) proteins develop into CD3+CD4–CD8+ T cells, whereas those expressing class II MHC-binding receptors differentiate into CD3+CD4+CD8– T cells (von Boehmer, 1990).
While signaling through antigen receptors promotes differentiation and survival throughout B- and T-cell development (von Boehmer, 1990; Rajewsky, 1996), little is known about the gene regulatory networks that control these events. In particular, those transcription factors and downstream genes that regulate the later stages of B- and T-cell differentiation and selection remain largely elusive. One group of transcription factors important in the antigen-dependent activation, proliferation and survival of lymphocytes are members of the Rel/NF-κB family. These proteins comprise dimers of related subunits that control transcription by binding decameric sequences (κB elements) in the regulatory regions of target genes (Baldwin, 1996). The five subunits (Rel, RelA, RelB, NF-κB1 and NF-κB2), each encoded by distinct loci, share a conserved N-terminal domain (Rel homology domain) that encompasses sequences essential for DNA binding, dimerization and nuclear import (Baldwin, 1996). Rel, RelA (p65) and RelB possess C-terminal transcriptional transactivation domains, whereas NF-κB1 (p50) and NF-κB2 (p52) lack intrinsic transactivating properties, and instead function as homodimeric transcriptional repressors or modulators of transactivating dimer partners. In most cells, the major proportion of Rel/NF-κB proteins are retained in the cytoplasm in an inactive state through association with inhibitory (IκB) proteins (Whiteside and Israel, 1997). Cytokines, mitogens and stress stimuli promote the nuclear translocation of Rel/NF-κB by activating an IκB kinase complex (Karin, 1999) that phosphorylates IκB proteins, targeting them for ubiquitin-dependent proteosome- mediated degradation (Whiteside and Israel, 1997).
Analysis of mutant mice lacking individual Rel/NF-κB subunits has begun to reveal the essential roles of each transcription factor. _rela_–/– fetuses die at approximately embryonic day 15 (E15; E0 corresponds to the morning the vaginal plugs are identified) due to hepatocyte apoptosis caused by deregulated tumor necrosis factor (TNF)/TNF receptor 1 signaling (Beg and Baltimore, 1996; Doi et al., 1999). Hemopoietic repopulation studies using E13 _rela_–/– fetal liver cells established that RelA is not essential for lymphopoiesis, but is involved in lymphocyte activation (Doi et al., 1997; Horwitz et al., 1999). Rel, RelB, NF-κB1 or NF-κB2 are individually dispensable for embryogenesis and hemopoiesis, but serve essential roles during the activation of mature hemopoietic cells (Gerondakis et al., 1999). However, structural conservation of Rel/NF-κB proteins, coupled with overlapping expression patterns, suggests that functional redundancy could mask certain phenotypes in single-mutant mice due to compensation by other Rel/NF-κB members. Indeed, mice that lack different combinations of two Rel/NF-κB proteins exhibit novel phenotypes or more severe manifestations of those abnormalities seen in the single mutants (Gerondakis et al., 1999).
Since Rel and RelA, the transactivating Rel/NF-κB family members most widely expressed in hemopoietic cells (Gerondakis et al., 1998), display similar transcriptional activities (Baldwin, 1996), a likelihood of overlap in Rel and RelA function necessitated an examination of their combined roles in hemopoiesis. Although preliminary studies using E12 c-_rel_–/–_rela_–/– fetal liver cells to repopulate the hemopoietic compartment of lethally irradiated mice showed the loss of both transcription factors, impaired erythropoiesis and caused deregulated expansion of granulocytes (Grossmann et al., 1999), their combined roles in lymphopoiesis were unclear. While c-_rel_–/–_rela_–/– lymphocytes were virtually absent in reconstituted recipients, these findings were difficult to interpret in light of the rapid death of these mice from the anemia and granulocytosis. It was noted in the course of these experiments that wild-type hemopoietic cells suppress the c-_rel_–/–_rela_–/– granulocytosis. Since _rag-1_–/– mice lack lymphocytes, but otherwise have normal hemopoiesis and only require low dose irradiation to promote repopulation of the lymphoid system by donor stem cells (Chen et al., 1994), these mice were used as recipients of c-_rel_–/–_rela_–/– fetal liver hemopoietic stem cells to study the role of Rel and RelA in lymphopoiesis.
Here we show that the combined loss of Rel and RelA impairs the normal development of mature B and T cells. Mature (IgMloIgDhi) B cells were absent and apoptosis was dramatically increased in the immature (IgMhiIgDlo, IgMhiIgDhi) B-cell populations. This enhanced death of c-_rel_–/–_rela_–/– B cells coincided with reduced levels of Bcl-2. Consistent with a functional association between B-cell development and Bcl-2 expression, enforced expres sion of a bcl-2 transgene blocked the elevated apoptosis and promoted further differentiation of c-_rel_–/–_rela_–/– B cells. In contrast, the deficit of peripheral c-_rel_–/–_rela_–/– T cells did not appear to result from increased cell death. Collectively, these findings establish that Rel and RelA are important in regulating the survival of peripheral B cells during antigen-independent maturation, whereas the control of peripheral T-cell numbers by these transcription factors involves mechanisms independent of cell survival.
Results
The combined absence of Rel and RelA leads to a block in B-cell development
Sublethally irradiated _rag-1_–/– mice were engrafted with equivalent numbers of E12 control (wild-type, c-_rel_–/– or _rela_–/–) or c-_rel_–/–_rela_–/– fetal liver cell suspensions. Between 2 and 6 months later, B- and T-cell development was assessed by flow cytometric analysis of cell suspensions from bone marrow, thymus, spleen and lymph node that had been stained with fluorochrome-labeled surface marker-specific monoclonal antibodies. In all figures, unless stated otherwise, control data indicate that essentially equivalent results were obtained using mice engrafted with wild-type, c-_rel_–/– or _rela_–/– hemopoietic progenitors.
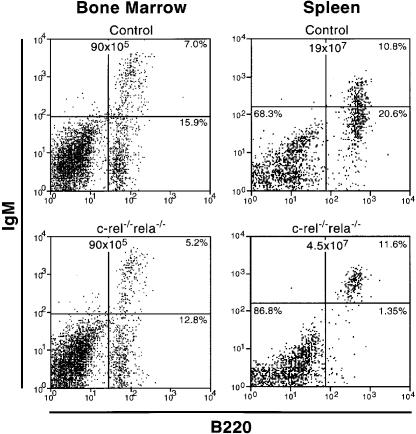
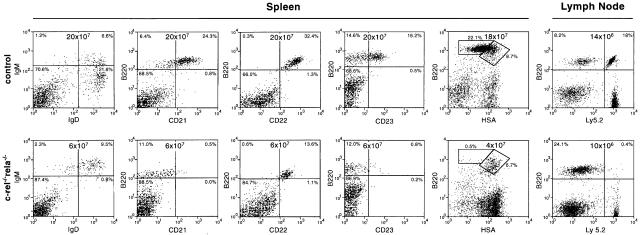
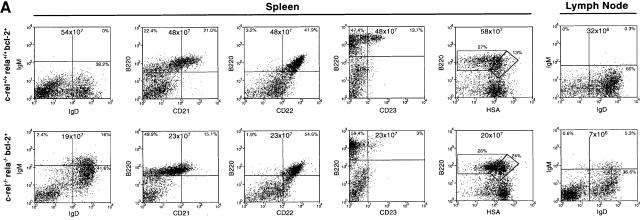
Typical data for c-_rel_–/–_rela_–/– B-cell development are presented in Figures 1 and 2. While the number of c-_rel_–/–_rela_–/– B220+IgM+ B cells in the bone marrow was relatively normal, 10-fold fewer c-_rel_–/–_rela_–/– B220+sIg+ B cells were present in the spleen (Figure 1). This result was consistent with the lower spleen weight in these mice (wild-type, 170 ± 8 mg; c-_rel_–/–, 160 ± 25 mg; _rela_–/–, 190 ± 33 mg; c-_rel_–/–_rela_–/–, 56 ± 5 mg). The reduced numbers of splenic c-_rel_–/–_rela_–/– B cells predominantly reflected the absence of the most mature, long-lived IgMloIgDhi population, which comprises the major splenic B-cell population in mice reconstituted with control fetal liver cells (Figure 2). The total numbers of immature IgMhiIgDlo and IgMhiIgDhi c-_rel_–/–_rela_–/– B cells were also reduced ∼3-fold. Mature recirculating B cells are normally characterized by an upregulation of CD21, CD22 and CD23, and low levels of heat-stable antigen (HSA) (Loder et al., 1999). Consistent with the absence of mature B cells in the spleen (Figure 2) and bone marrow (results not shown) of c-_rel_–/–_rela_–/– reconstituted mice, virtually none of the B220+sIg+ cells expressed CD21 or CD23, and only low levels of CD22 and high levels of HSA were observed on these cells (Figure 2). In addition to a deficit of mature splenic B cells, B220+ cells were almost completely absent in the lymph nodes of c-_rel_–/–_rela_–/– reconstituted mice (Figure 2). Collectively, these findings demonstrate that during B-cell differentiation, Rel and RelA together are essential for establishing and/or maintaining the long-lived mature B-cell pool.
Fig. 1. Peripheral B-cell numbers are reduced in _rag-1_–/– mice engrafted with c-_rel_–/–_rela_–/– fetal liver cells. Cell suspensions from the bone marrow and spleens of _rag-1_–/– mice that had been engrafted 2–6 months earlier with either E12 control (wild-type, c-_rel_–/– or _rela_–/–) or c-_rel_–/–_rela_–/– fetal liver cells were stained with monoclonal antibodies specific for IgM and B220, and examined by flow cytometry. Representative dot blots from one of four experiments are shown. Total cell numbers from each organ are presented, with the percentage of cells in the relevant quadrants indicated.
Fig. 2. Mature B cells are absent in _rag-1_–/– mice engrafted with c-_rel_–/–_rela_–/– fetal liver cells. Cell suspensions from spleens of _rag-1_–/– mice engrafted with control or c-_rel_–/–_rela_–/– fetal liver cells were stained with monoclonal antibodies specific for IgM, IgD, B220, CD21, CD22 and CD23, and analyzed by flow cytometry. Owing to the virtual absence of lymph nodes (LN) in _rag-1_–/– mice that only receive c-_rel_–/–_rela_–/– fetal liver cells, LN stains (B220 versus Ly5.2) were performed on _rag-1_–/– mice co-reconstituted with wild-type Ly5.1+ and Ly5.2+ control or c-_rel_–/–_rela_–/– fetal liver cells. The dot blots are representative of results obtained from three separate experiments using four or more animals from each genotype. Organ cellularity and the distribution of the relevant B-cell populations are indicated.
Rel and RelA are required for the production of normal numbers of mature T cells
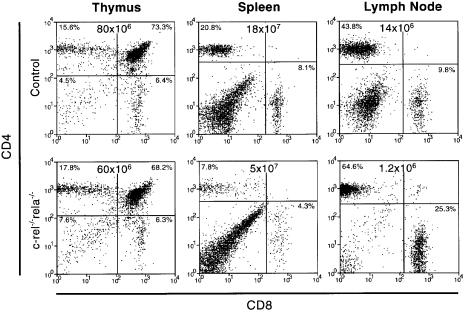
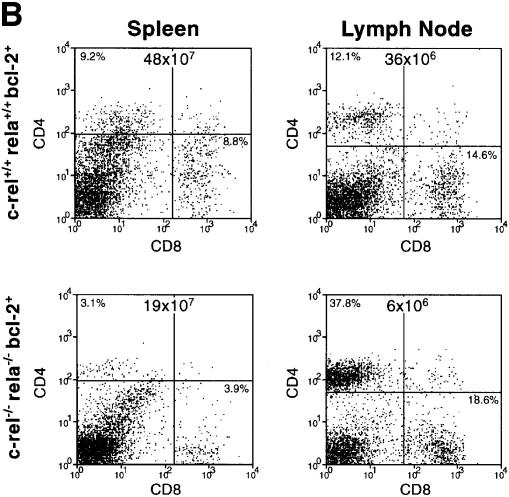
In _rag-1_–/– mice reconstituted with _rela_–/– or c-_rel_–/–_rela_–/– fetal liver cells, overall thymocyte cellularity was slightly reduced compared with those reconstituted with wild-type or c-_rel_–/– cells. The relative frequencies of the four major thymocyte subsets (CD4–CD8–, CD4+CD8+, CD4+CD8– and CD4–CD8+) were, however, normal (Figure 3). In the periphery, the total numbers of CD4+ and CD8+ c-_rel_–/–_rela_–/– T cells were significantly diminished compared with all control mice (Figure 3). In the spleen, there were between 5- and 10-fold fewer CD4+ and CD8+ c-_rel_–/–_rela_–/– T cells. Although absolute numbers of c-_rel_–/–_rela_–/– T cells in lymph nodes were 6-fold lower than normal, the relative proportion of CD4+ and CD8+ T cells was actually higher than in normal or single-mutant mice due to the absence of c-_rel_–/–_rela_–/– B cells. These findings indicate that Rel and RelA together are required for establishing and/or maintaining normal numbers of peripheral CD4+ and CD8+ T cells.
Fig. 3. The combined absence of Rel and RelA reduces peripheral T-cell numbers. Immunofluorescence staining with antibodies against CD4 and CD8 was performed on cell suspensions of thymus, spleen and lymph node of _rag-1_–/– mice reconstituted with fetal liver cells from control or c-_rel_–/–_rela_–/– embryos. Dot blots are representative of four separate experiments, with organ cellularity and the proportion of the different thymocyte and peripheral T-cell populations indicated.
c-rel–/–rela–/– B cells undergo accelerated apoptosis in culture and increased turnover in vivo
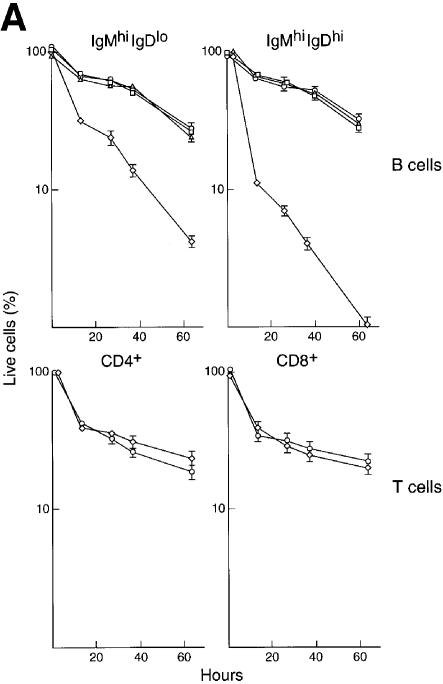
While Rel/NF-κB family members are essential for the survival of mature B and T cells during mitogenic activation (Barkett and Gilmore, 1999; Gerondakis et al., 1999), it was unknown whether these transcription factors also serve a survival role during earlier stages of lymphocyte development. Since Rel/NF-κB activity normally increases during the phase of B-cell maturation affected by the combined loss of Rel and RelA, we considered whether diminished survival could in part account for the defect in c-_rel_–/–_rela_–/– B-cell development. This was assessed by measuring c-_rel_–/–_rela_–/– splenic B-cell survival in tissue culture (Figure 4A). The loss of Rel or RelA did not alter the survival of IgMhiIgDlo or IgMhiIgDhi B cells, whereas both of these c-_rel_–/–_rela_–/– B-cell populations died more rapidly in culture than control cells. Of particular interest, control immature IgMhiIgDlo and IgMhiIgDhi B cells died at an equivalent rate, while IgMhiIgDhi c-_rel_–/–_rela_–/– B cells underwent apoptosis at a faster rate than the less mature IgMhiIgDlo population. Given this finding, the viability of quiescent c-_rel_–/–_rela_–/– splenic CD4+ and CD8+ T cells in culture was also examined. In contrast to the c-_rel_–/–_rela_–/– B cells, the Rel/RelA deficiency had no impact on in vitro survival of CD4+ and CD8+ T cells (Figure 4A).
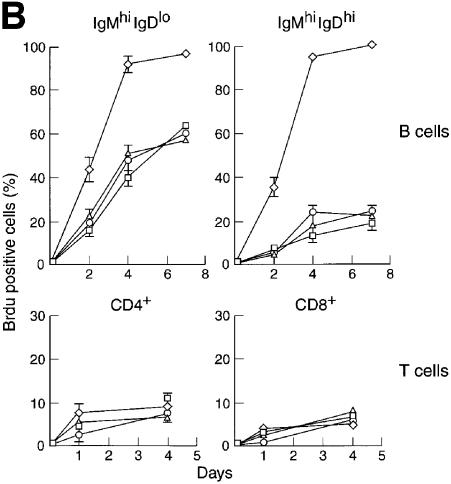
Fig. 4. (A) c-_rel_–/–_rela_–/– B cells undergo accelerated apoptosis in culture. Resting wild-type (open circles), _rela_–/– (open squares), c-_rel_–/– (open triangles) and c-_rel_–/–_rela_–/– (open diamonds) splenic sIgMhiIgDlo and sIgMhiIgDhi B cells or CD4+ and CD8+ T cells were cultured in DMEM/10% FCS/50 µM β-mercaptoethanol (no mitogens or cytokines) for a period of 60 h. At the indicated times, the frequency of live cells was determined by trypan blue exclusion and flow cytometric analysis of cells stained with propidium iodide. All cells were purified by negative sorting and, at the start of the experiments, >99% of cells of each genotype were viable. The data represent the mean ± SD of four experiments. (B) Abnormally rapid turnover of c-_rel_–/–_rela_–/– B cells in vivo. The turnover of sIgMhiIgDlo and sIgMhiIgDhi B cells, plus CD4+ and CD8+ T cells was determined by BrdU incorporation. Splenic cells isolated from mice with wild-type, c-_rel_–/–, _rela_–/– or c-_rel_–/–_rela_–/– immune systems that were fed BrdU for 2, 4 or 7 days were subjected to three-color immunofluorescence staining, and flow cytometric analysis was used to determine the fraction of B and T cells that had incorporated BrdU. The panels shown summarize the kinetics of BrdU incorporation for normal (open circles), _rela_–/– (open squares), c-_rel_–/– (open triangles) and c-_rel_–/–_rela_–/– (open diamonds) splenic sIgMhiIgDlo and sIgMhiIgDhi B cells, plus CD4+ and CD8+ T cells over a 4 or 7 day time course. The values are arithmetic means ± SD from the analysis of three mice of each genotype at the given time points.
To ascertain whether the increased death of double-mutant B cells in vitro was reflected in a greater turnover of these cells in vivo, we compared the extent of 5-bromo-2′-deoxyuridine (BrdU) incorporation into the IgMhiIgDlo and IgMhiIgDhi splenic populations from _rag-1_–/– mice engrafted with progenitors of the various genotypes (Figure 4B). In control mice, a kinetic analysis revealed that less BrdU was incorporated by IgMhiIgDhi cells compared with the IgMhiIgDlo population, a finding consistent with the known increase of cell survival as B cells mature (Osmond, 1993). In contrast, BrdU incorporation into c-_rel_–/–_rela_–/– B cells was markedly elevated. This increase was particularly pronounced for IgMhiIgDhi cells, the most mature B-cell population observed in recipients of c-_rel_–/–_rela_–/– fetal liver cells (Figure 4B). By day 4, virtually all IgMhiIgDlo and IgMhiIgDhi c-_rel_–/–_rela_–/– splenic B cells were BrdU labeled. By comparison, ∼50 and <30% of the respective control B-cell populations were labeled during that period. Consistent with the findings on T-cell survival in culture, BrdU labeling of CD4+ and CD8+ c-_rel_–/–_rela_–/– splenic T cells was not significantly higher than that of wild-type or single-mutant T cells (Figure 4B). These data indicate that together Rel and RelA promote survival during later stages of B-cell maturation, but do not appear to serve a survival role in mature quiescent T cells.
Immature c-rel–/–rela–/– B cells express abnormally low levels of Bcl-2
The prosurvival proteins A1, Bcl-xL and Bcl-2 are differentially expressed during the late stages of B-cell development (Chao and Korsmeyer, 1998). In resting peripheral B cells, Bcl-xL levels are downregulated (Grillot et al., 1996), while A1 and Bcl-2 expression increases (Chao and Korsmeyer, 1998; Tomayko and Cancro, 1998). Since the survival of B-lineage cells was reduced in mice lacking Bcl-2 (Veis et al., 1993; Nakayama et al., 1994) or Bcl-xL (Motoyama et al., 1995), and A1 promotes the survival of mature mitogen-activated B cells in culture (Grumont et al., 1999), bcl-2, _bcl-x_L and A1 expression was compared in purified wild-type, c-_rel_–/–, _rela_–/– and c-_rel_–/–_rela_–/– IgMhiIgDlo, IgMhiIgDhi and IgMloIgDhi splenic B cells using semi-quantitative RT–PCR (Figure 5A). _bcl-x_L expression was low and invariant in all populations, irrespective of genotype (lanes 1–11). Consistent with previous findings (Tomayko and Cancro, 1998), in wild-type B cells, A1 mRNA levels were higher in B cells of greater maturity (lanes 1, 5 and 9). While A1 expression in _rela_–/– B cells appeared to be normal (lanes 3, 7 and 11), it was not upregulated in more mature c-_rel_–/– (IgMhiIgDhi and IgDhiIgMlo) or c-_rel_–/–_rela_–/– (IgMhiIgDhi) B cells (lanes 6, 8 and 10). bcl-2 mRNA levels were similar in wild-type, c-_rel_–/– and _rela_–/– splenic B cells of varying maturity (lanes 1–3, 5–7 and 9–11), but were reduced ∼6-fold in both the IgMhiIgDlo and IgMhiIgDhi c-_rel_–/–_rela_–/– populations (lanes 4 and 8).
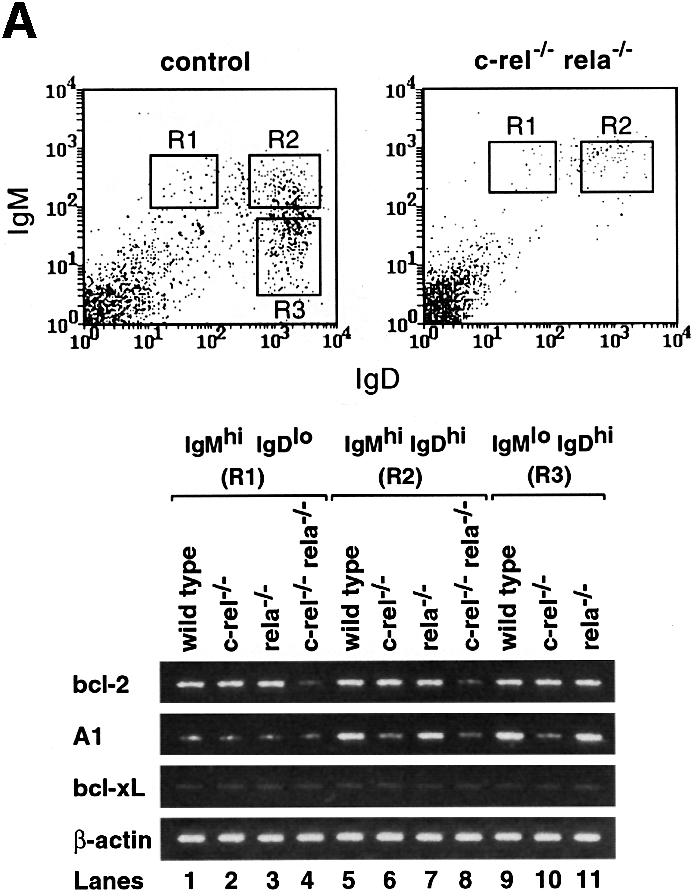
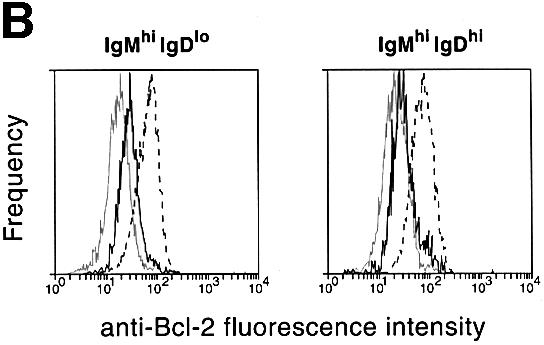
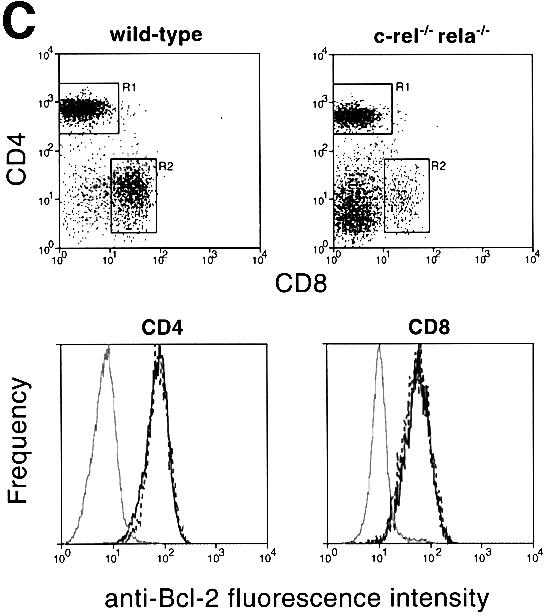
Fig. 5. (A) RT–PCR analysis of A1, _bcl-x_L and bcl-2 expression in control and c-_rel_–/–_rela_–/– splenic B cells. Cell suspensions from spleens of _rag-1_–/– mice engrafted with control (wild-type, c-_rel_–/– or _rela_–/–) or c-_rel_–/–_rela_–/– fetal liver cells were stained with antibodies specific for IgM and IgD. Typical staining profiles for control (wild-type, c-_rel_–/– or _rela_–/–) and c-_rel_–/–_rela_–/– B-cell populations are depicted as FACS dot blots. Boxed regions correspond to the sIgMhiIgDlo (R1), IgMhiIgDhi (R2) and IgMloIgDhi (R3; control only) B-cell populations purified by flow cytometry. Total RNA isolated from FACS-purified wild-type (lanes 1, 5 and 9), c-_rel_–/– (lanes 2, 6 and 10), _rela_–/– (lanes 3, 7 and 11) and c-_rel_–/–_rela_–/– (lanes 4 and 8) IgMhiIgDlo (lanes 1–4), IgMhiIgDhi (lanes 5–8) and IgMloIgDhi (lanes 9–11) splenic B cells was subjected to semi-quantitative RT–PCR using bcl-2, A1, _bcl-x_L and β-actin-specific primers. PCR products were fractionated on 1% agarose gels. These data are representative of three independent experiments. (B) Bcl-2 levels are lower than normal in c-_rel_–/–_rela_–/– sIgM+ B cells. Four-color immunofluorescence staining was used to identify sIgMhiIgDlo and sIgMhiIgDhi B cells, and to determine the intracellular levels of Bcl-2. Fixed, permeabilized wild-type or c-_rel_–/–_rela_–/– splenic B cells were stained with Cy5-labeled anti-B220, Texas Red-labeled anti-IgD and biotinylated anti-IgM antibodies, and revealed with R-phycoerythrin–streptavidin. Histograms depict the labeling intensity of wild-type B cells stained with an isotype-matched control antibody (gray line), or anti-Bcl-2 (3F11) FITC antibody stains of wild-type (broken line) and c-_rel_–/–_rela_–/– (solid line) B cells. (C) Mature c-_rel_–/–_rela_–/– T cells express normal levels of Bcl-2. Four-color immunofluorescence staining was performed to determine intracellular levels of Bcl-2 in wild-type and c-_rel_–/–_rela_–/– CD4+ and CD8+ T cells. Fixed, permeabilized splenic T cells (identified by staining with Cy-5-labeled anti-Thy-1) are shown as FACS dot-blot profiles (left panels, _x_-axis, staining with Texas Red-labeled anti-CD8+ antibodies; _y_-axis, staining with biotinylated anti-CD4+ antibodies plus R-phycoerythrin–streptavidin). The boxed regions R1 and R2 represent the gates for the CD4+ and CD8+ cells, respectively. The panels represent fluorescence intensity histograms of cells stained with control antibody (gray line) or anti-Bcl-2 antibody staining of wild-type (broken line) or c-_rel_–/–_rela_–/– (solid line) cells.
The findings for bcl-2 mRNA expression were supported by intracellular staining with Bcl-2-specific antibodies (Figure 5B). Bcl-2 levels in IgMhiIgDlo and IgMhiIgDhi c-_rel_–/–_rela_–/– B cells were 5-fold lower than in the corresponding wild-type cells. Consistent with the mRNA expression patterns, the reduction in Bcl-2 was observed in the c-_rel_–/–_rela_–/– but not in the c-_rel_–/– or _rela_–/– B-cell populations (results not shown). In peripheral T cells, where survival was unaffected by the combined loss of Rel and RelA, Bcl-2 levels were equivalent in wild-type and c-_rel_–/–_rela_–/– CD4+ and CD8+ T cells (Figure 5C). These findings indicate that Bcl-2 expression during peripheral B-cell maturation is dependent upon Rel and RelA.
Enforced Bcl-2 expression promotes survival and maturation of c-rel–/–rela–/– B cells
To determine whether the reduced survival and impaired maturation of c-_rel_–/–_rela_–/– B cells was in part a consequence of low Bcl-2 levels, we tested whether these defects could be overcome by enforced expression of a bcl-2 transgene. _rag-1_–/– mice were engrafted with wild-type or c-_rel_–/–_rela_–/– fetal liver cells derived from embryos that carry a bcl-2 transgene under the transcriptional control of the hemopoietic-restricted vav promoter (Ogilvy et al., 1999). Enforced bcl-2 expression, which inhibited the death of c-_rel_–/–_rela_–/– B cells in culture (Figure 6), not only increased double-mutant B-cell numbers in the spleen 25-fold, but also promoted their differentiation (Figure 7). The majority of c-_rel_–/–_rela_–/– splenic B cells expressing the bcl-2 transgene had a mature IgMloIgDhi phenotype, and CD21 as well as CD22 were upregulated in most of these splenic B cells (Figure 7A). Furthermore, the B220hiHSAlo B-cell population was restored in the spleen (Figure 7A) and mature B cells were now abundant in lymph nodes (Figure 7A). Yet, despite bcl-2 transgene expression promoting the development of a peripheral c-_rel_–/–_rela_–/– B-cell population of greater maturity, the process of differentiation was not completely normal. Total numbers of the IgMhiIgDhi and IgMloIgDhi cells in these mice were still somewhat lower than those observed in wild-type or control bcl-2 transgenic mice (compare Figures 2 and 7), and CD23 was not upregulated in these cells (Figure 7A).
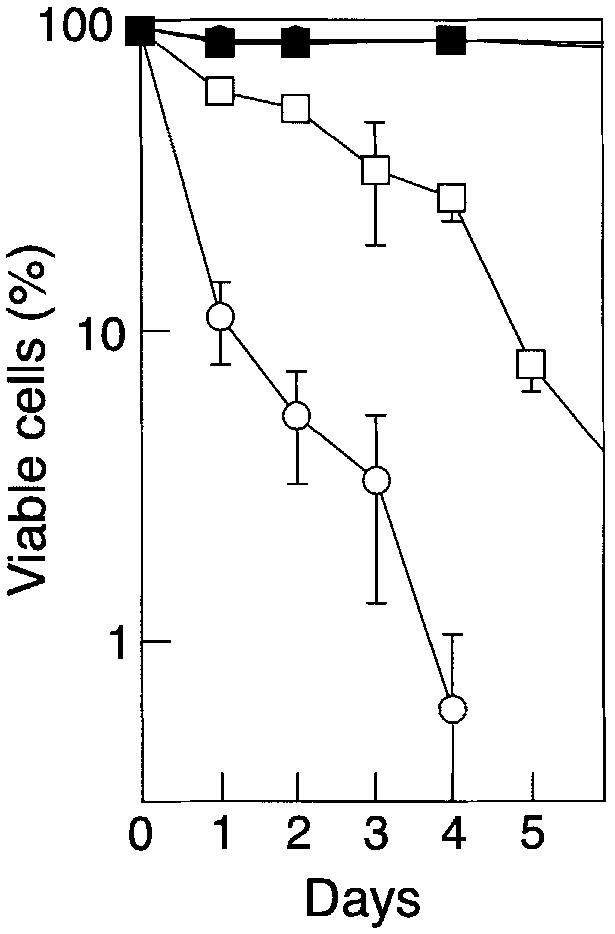
Fig. 6. bcl-2 transgene expression inhibits apoptosis of c-_rel_–/–_rela_–/– B cells. Resting wild-type (squares) and c-_rel_–/–_rela_–/– (circles) non-transgenic (open symbols) or bcl-2 transgenic (closed symbols) splenic sIgM+B220+ B cells were cultured in DMEM/10% FCS/50 µM β-mercaptoethanol for periods of up to 5 days. At various intervals, the frequency of live cells was determined by Trypan Blue exclusion or flow cytometric analysis of cells stained with propidium iodide. All cells were purified by negative sorting and, at the start of the experiments, >99% of cells of each genotype were viable. The data represent the mean ± SEM of four experiments.
Fig. 7. (A) bcl-2 transgene expression promotes c-_rel_–/–_rela_–/– B-cell maturation. Cell suspensions from the spleens and lymph nodes of _rag-1_–/– mice engrafted with bcl-2 transgenic control or c-_rel_–/–_rela_–/– fetal liver cells were stained with monoclonal antibodies specific for IgM, IgD, B220, CD21, CD22 and CD23, then analyzed by flow cytometry. The dot blots are representative of results obtained from three separate experiments using four or more animals from each genotype. Organ cellularity and the distribution of the relevant B-cell populations are indicated. (B) Enforced Bcl-2 expression does not rescue the deficit of peripheral c-_rel_–/–_rela_–/– T cells. Single-cell suspensions from the spleens and lymph nodes of _rag-1_–/– mice that had been reconstituted with bcl-2 transgenic wild-type or c-_rel_–/–_rela_–/– fetal liver cells were stained with antibodies specific for CD4 and CD8. The dot blots are representative of data obtained from three independent experiments.
Although the bcl-2 transgene inhibits apoptosis as effectively in T cells as it does in B cells (Ogilvy et al., 1999), it could not overcome the T-cell deficit provoked by the combined lack of Rel and RelA (Figure 7B). c-_rel_–/–_rela_–/– CD4+ and CD8+ T-cell numbers were elevated only 3-fold by the presence of the transgene and remained 6- to 10-fold lower than in _rag-1_–/– mice engrafted with control bcl-2 transgenic cells. This finding, coupled with the indistinguishable survival and turnover of control and c-_rel_–/–_rela_–/– splenic T cells, indicates that Rel and RelA are not critical for the survival of quiescent peripheral T cells.
Mitogen-induced proliferation and Ig secretion are defective in c-rel–/–rela–/– B cells and cannot be rescued by Bcl-2
The semi-redundant function of Rel and RelA during the latter phase of antigen-independent B-cell differentiation prompted us to examine whether Rel and RelA also had overlapping roles in the response to mitogens and the regulation of Ig secretion. Consistent with previous findings, c-_rel_–/– and _rela_–/– B cells displayed proliferative defects of varying severity when stimulated with single mitogens such as lipopolysaccharide (LPS), antibodies to the BCR or the Toll-like protein RP-105 (Kontgen et al., 1995; Doi et al., 1997). The proliferative defects exhibited by these mutant B cells to individual mitogens could, however, be overcome by certain combinations of these same stimuli (Grumont et al., 1998) (Table I). In contrast to the c-_rel_–/– or _rela_–/– B cells, c-_rel_–/–_rela_–/– B cells failed to proliferate when treated with any of the stimulus combinations (Table I), even though these cells express the surface receptors that engage these mitogens (results not shown). Antibody production by c-_rel_–/–_rela_–/– B cells was assessed by examining serum Igs in naive _rag-1_–/– mice reconstituted 3–6 months earlier with mutant E12 fetal liver cells. While different serum Igs were reduced but not abolished in _rag-1_–/– mice with c-_rel_–/– or _rela_–/– immune systems (Table II), in accordance with previously published findings (Kontgen et al., 1995; Doi et al., 1997), absolutely no serum Igs were detected in mice reconstituted with c-_rel_–/–_rela_–/– fetal liver cells (Table II).
Table I. Failure of mitogens to promote c-_rel_–/–_rela_–/– B-cell proliferation.
| Genotype | No stimulus | α-IgM | α-RP | LPS | α-IgM/α-RP | α-IgM/LPS | α-RP/LPS |
|---|---|---|---|---|---|---|---|
| c-rel+/+rela+/+ | 1150 ± 295 | 33 900 ± 11 200 | 54 000 ± 20 200 | 111 900 ± 1780 | 94 200 ± 7400 | 186 200 ± 14 3000 | 258 000 ± 16 500 |
| c-rel_–/–_rela+/+ | 410 ± 150 | 140 ± 29 | 500 ± 80 | 6100 ± 80 | 1400 ± 200 | 85 400 ± 12 000 | 125 000 ± 33 000 |
| c-rel+/+_rela_–/– | 500 ± 100 | 18 600 ± 8600 | 3200 ± 1300 | 31 300 ± 6100 | 92 700 ± 4900 | 106 000 ± 17 400 | 217 000 ± 46 200 |
| c-_rel_–/–_rela_–/– | 65 ± 7 | 94 ± 34 | 170 ± 37 | 310 ± 55 | 230 ± 51 | 450 ± 43 | 590 ± 115 |
| c-rel+/+rela+/+_bcl2_T | 760 ± 290 | 27 600 ± 2000 | 46 500 ± 1800 | 133 700 ± 2100 | 95 800 ± 4300 | 203 000 ± 38 700 | 333 900 ± 48 700 |
| c-_rel_–/–_rela_–/–_bcl2_T | 130 ± 30 | 200 ± 50 | 110 ± 40 | 220 ± 35 | 340 ± 140 | 320 ± 33 | 600 ± 100 |
Table II. c-_rel_–/–_rela_–/– B cells fail to secrete Igs.
| Genotype (lymphoid cells) | IgM | IgG1 | IgG2a | IgG2b | IgG3 | IgA |
|---|---|---|---|---|---|---|
| _rag-1_–/– control | <1 | <1 | <1 | <1 | <1 | <1 |
| c-rel_–/–_rela+/+ | 387 ± 85 | 491 ± 47 | 547 ± 190 | 1010 ± 143 | 31 ± 6 | 627 ± 93 |
| c-rel_–/–_rela+/+ | 104 ± 14 | 3.3 ± 1.4 | 27.5 ± 17 | 174 ± 50 | 12 ± 4 | 345 ± 131 |
| c-rel+/+_rela_–/– | 147 ± 143 | 60 ± 21 | 120 ± 29 | 685 ± 190 | 24 ± 12 | 20 ± 6 |
| c-_rel_–/–_rela_–/– | <1 | <1 | <1 | <1 | <1 | <1 |
| c-rel+/+rela+/+_bcl2_T | 2280 ± 740 | 297 ± 29 | 1940 ± 630 | 1700 ± 310 | 11.5 ± 6 | 1800 ± 150 |
| c-_rel_–/–_rela_–/–_bcl2_T | <1 | <1 | <1 | <1 | <1 | <1 |
Since c-_rel_–/–_rela_–/– B cells exhibit an immature phenotype, proliferative responses and Ig production were also examined in mice reconstituted with wild-type or double-mutant fetal liver hemopoietic progenitors expressing the bcl-2 transgene. Results from these experiments, summarized in Tables I and II, clearly show that these B lymphocytes were still unable to proliferate in culture when mitogen activated, and did not secrete any Igs. These findings indicate that in addition to regulating B-cell maturation and survival, Rel and RelA also serve overlapping roles in controlling antibody secretion and the response of mature B cells to mitogenic activation.
Discussion
The combined absence of Rel and RelA did not perturb the development of B-cell progenitors in the bone marrow, but proved essential for later stages of B-cell maturation. Immature c-_rel_–/–_rela_–/– B cells exit the bone marrow, yet fail to be recruited into the long-lived mature B-cell pool, having been arrested at the HSAhiB220loIgMhiIgDhi stage of development. This indicates that in addition to the distinct functions that Rel and RelA perform in mature activated B lymphocytes (Gerondakis et al., 1999), these transcription factors also serve redundant roles during B-cell maturation. Impaired development of mature B cells has also been described recently in mice expressing a B-lineage-restricted IκBα transgene (Bendall et al., 1999). However, unlike the c-_rel_–/–_rela_–/– mutation, there was only a modest reduction of the IgMloIgDhi B-cell population in these transgenic animals, a finding that may reflect incomplete inhibition of Rel and RelA dimers by IκBα. The c-_rel_–/–_rela_–/– B-cell maturation block also differs from the B-lineage defects that arise from other combinations of Rel/NF-κB null mutations. The combined loss of NF-κB1 and RelA leads to a progenitor defect that is reflected in an absence of B220+ cells (Horwitz et al., 1997); _nfkb1_–/–_nfkb2_–/– B-cell differentiation is effectively blocked at the immature IgMhiIgDlo stage (Franzoso et al., 1997) whereas, in _nfkb1_–/–_relb_–/– mice, overall numbers of both immature and mature B cells are reduced without a distinct developmental block (Weih et al., 1997). Collectively, these findings indicate that specific Rel/NF-κB dimers serve essential roles during distinct phases of B-cell development. This is consistent with earlier biochemical studies showing that the Rel/NF-κB subunit composition in B-lineage cells changes during the course of their development (Gerondakis et al., 1998).
The marked reduction in the survival of IgMhiIgDlo and IgMhiIgDhi c-_rel_–/–_rela_–/– B cells establishes that these transcription factors serve a key anti-apoptotic role during the later stages of B-cell maturation. A combined survival function for Rel and RelA during B-cell differentiation differs from previous findings on the anti-apoptotic function of Rel/NF-κB proteins in B cells. RelA had not been shown previously to have a survival activity in B cells (Gerondakis et al., 1999), while NF-κB1 and Rel have distinct anti-apoptotic roles in mature quiescent and activated B cells, respectively (Grumont et al., 1998).
Here we show that constitutive A1 and bcl-2 expression is reduced in peripheral B cells lacking Rel and RelA. Although the absence of Rel prevents the increased A1 expression associated with B-cell maturation, the differentiation of c-_rel_–/– B cells is not impaired (Grumont et al., 1998), a finding consistent with normal B-cell development in _A1_–/– mice (Hamasaki et al., 1998). Likewise, a mature IgMloIgDhi population still develops in _bcl-2_–/– mice even though Bcl-2 is critical for maintaining normal numbers of peripheral B cells (Matsuzaki et al., 1997). The heightened severity of the B-cell survival defects in the absence of Rel and RelA compared with the loss of Bcl-2 or A1 is most readily reconciled by a model in which Rel and RelA function in a semi-redundant manner to promote survival during B-cell maturation by upregulating both prosurvival proteins. Presumably, the reduction in A1 and Bcl-2 that accompanies the combined loss of Rel and RelA represents a level of anti-apoptotic protein expression that falls below a threshold necessary to maintain the survival of mature B cells. The need for Rel/NF-κB to activate multiple genes that encode prosurvival proteins may reflect different functions served by these Bcl-2 family members in protecting mature B cells from apoptosis. Alternatively, co-expression of two Bcl-2-like proteins with similar or equivalent activity could represent a fail-safe mechanism to ensure that a precautionary reserve of anti-apoptotic activity is available.
It remains to be determined whether the expression of Bcl-2 in peripheral B cells is regulated directly or indirectly by Rel/NF-κB. Although Bcl-2 is also crucial for the maintenance of the peripheral T-cell population (Chao and Korsmeyer, 1998), it is not reduced in splenic CD4+ or CD8+ c-_rel_–/–_rela_–/– T cells, indicating that Rel/NF-κB control of Bcl-2 expression in peripheral lymphocytes appears to be restricted to the B lineage. The findings described here, coupled with the importance of Rel/NF-κB-induced A1 and _bcl-x_L expression in the survival of mature activated B cells, indicate that Rel/NF-κB proteins regulate the survival of B-lineage cells during various stages of maturity by differentially controlling the expression of distinct _bcl-2_-like prosurvival genes.
In addition to inhibiting the increased apoptosis of IgMhiIgDlo and IgMhiIgDhi c-_rel_–/–_rela_–/– B cells, a bcl-2 transgene was found to promote further development to the IgMloIgDhi stage of maturation. This indicates that impaired c-_rel_–/–_rela_–/– B-cell differentiation arises in part from an inability to receive survival signals. This is similar to the observation that Bcl-2 can promote survival and differentiation of B-cell precursors in scid mutant mice (Strasser et al., 1994). It is noteworthy that even in wild-type splenic B cells, the bcl-2 transgene increases the proportion of mature cells, with immature B cells being virtually absent. This previously reported observation (Strasser et al., 1990) appears to result from the transgene being expressed at a higher level than the endogenous gene, and further supports the notion that the level of Bcl2 expression can influence B-cell maturation. Impaired survival, however, cannot account for all of the defects afflicting c-_rel_–/–_rela_–/– B cells. The proportion of bcl-2 transgenic c-_rel_–/–_rela_–/– IgMloIgDhi B cells expressing the maturation markers CD21 and CD23 was still lower than that observed for the bcl-2 transgenic controls. Further more, bcl-2 transgene expression did not overcome the failure of c-_rel_–/–_rela_–/– B cells to develop into antibody-forming cells, or rescue the inability of c-_rel_–/–_rela_–/– B cells to proliferate in response to mitogens. Such findings confirm that Rel and RelA regulate B-cell functions that are independent of cell survival.
Defects in peripheral B-cell maturation are also seen in mice that lack Btk (Hardy et al., 1983), phosphoinositide 3-kinase (Fruman et al., 1999) or Syk (Turner et al., 1997). These proteins, like Rel/NF-κB, constitute components of the BCR signaling pathway. Since continuous BCR signaling is critical for the survival of mature B cells (Lam et al., 1997), it is possible that Rel and RelA transmit BCR signals necessary to maintain B-cell survival and development. Our results also indicate that BCR signaling early in B-cell ontogeny does not require Rel and RelA, since, in contrast to c-_rel_–/–_rela_–/– B cells, those that lack Ig heavy or light chains are blocked at the pro-B or pre-B cell stage of development (Strasser, 1995).
While the absence of single Rel/NF-κB transcription factors did not impair T-cell production (Gerondakis et al., 1999), combined loss of Rel and RelA led to a significant reduction in peripheral CD4+ and CD8+ T cells. Since c-_rel_–/–_rela_–/– thymocyte numbers were only marginally smaller than normal, the reduction in mature T cells appears to be due to a defect in peripheral T cells. A similar defect has been described in transgenic mice expressing a mutant form of IκBα (IκBΔN) that cannot be phosphorylated (Boothby et al., 1997; Attar et al., 1998; Hettmann et al., 1999), although the reduction in peripheral T-cell numbers due to the combined loss of Rel and RelA was more pronounced. Despite IκBΔN effectively repressing nuclear Rel and RelA expression in thymocytes and T cells (Boothby et al., 1997), only peripheral CD8+ T cells were reduced in these transgenic mice. One plausible explanation for this difference may be that IκBΔN transgene expression was absent or insufficient to inhibit Rel/NF-κB in T-cell progenitors at a developmental stage crucial for the subsequent maturation of CD4+ T cells.
The reduced number of peripheral CD4+ and CD8+ T cells in c-_rel_–/–_rela_–/– mice did not appear to result from increased apoptosis. The survival of mature, resting, c-_rel_–/–_rela_–/– T cells in culture was normal, as was the turnover of peripheral T-cell populations in vivo. Moreover, enforced bcl-2 transgene expression did not rectify the c-_rel_–/–_rela_–/– T-cell deficit, a finding consistent with Bcl-2 levels being normal in these T cells. Possible mechanisms that could account for the reduction in peripheral c-_rel_–/–_rela_–/– T cells that are currently under investigation include impaired thymic emigration, defective homing to peripheral lymphoid organs or the ineffective post-thymic proliferative expansion of mature T cells.
Materials and methods
Fetal liver cell engraftment
The c-_rel_–/– (Kontgen et al., 1995) and _rela_–/– mice (Beg et al., 1995) were backcrossed for more than nine generations with C57BL/6 mice. Lymphopoiesis was examined by injecting fetal liver cells from E12 embryos into sub-lethally irradiated (300 rads) C57BL/6 _rag-1_–/– mice. Fetal liver cells from wild-type, c-_rel_–/–, _rela_–/– and c-_rel_–/–_rela_–/– embryos, which were genotyped by PCR, were obtained from c-rel+/+rela+/– × c-rel+/+rela+/– and c-rel_–/–_rela+/– × c-rel_–/–_rela+/– matings. Single-cell suspensions were obtained from fetal liver by repeated passage through a 21-gauge needle, and 3 × 106 viable nucleated cells were injected into the tail vein of 8- to 10-week-old _rag1_–/– mice. Co-reconstitutions using Ly5.1+ control and Ly5.2+ c-_rel_–/–_rela_–/– fetal liver cells were carried out as described (Grossmann et al., 1999). C57BL/6 transgenic mice expressing a human bcl-2 cDNA under the transcriptional control of the hemopoiesis-specific vav promoter have been described (Ogilvy et al., 1999). Fetal liver cells from E12 wild-type and c-_rel_–/–_rela_–/– bcl-2 transgenic (_bcl-2_T) embryos were used for reconstituting _rag-1_–/– mice as described above.
Immunofluorescence staining and flow cytometric analysis
Single-cell suspensions from the bone marrow, spleen, thymus and lymph nodes of reconstituted mice were surface stained as described (Grumont et al., 1998) using various combinations of monoclonal antibodies conjugated to biotin, fluorescein isothiocyanate (FITC), R-phycoerythrin, Cy5 or Texas Red. Staining with biotinylated antibodies was revealed by R-phycoerythrin–streptavidin or tricolor–streptavidin (Caltag, San Francisco, CA). Between 5000 and 10 000 viable cells were analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, CA). The following antibodies were used: anti-Ly5.2 (clone 5.430-15.2), anti-B220 (RA3-6B2), anti-Thy-1.2 (30H12), anti-CD4 (YTS-197.7.7.3), anti-CD8 (145-2C11), anti-IgM (5.1 anti-µ heavy chain), anti-IgD (11-26C), anti-CD21 (7G6), anti-CD22 (2D6), anti-CD23 (B3B4) and anti-HSA (MI69). Cytoplasmic staining of intracellular proteins was performed as described previously (Strasser et al., 1995). Spleen cells from reconstituted _rag-1_–/– mice were first surface stained with anti-B220, anti-IgM and anti-IgD for B cells, or with anti-Thy1, anti-CD4 and anti-CD8 for T cells. Stained cells were then fixed for 15 min at 4°C in phosphate-buffered saline (PBS) containing 1% paraformaldehyde, followed by incubation with hamster anti-mouse Bcl-2 monoclonal antibodies (3F11; PharMingen, San Diego, CA) in PBS containing 0.3% saponin (Sigma) and 1% fetal calf serum. Staining with anti-Bcl-2 antibodies or isotype-matched control antibodies was revealed by FITC-conjugated mouse anti-hamster IgG antibodies (PharMingen).
Lymphocyte purification
Immature (IgM+IgD–) and transitional (IgM+IgD+) splenic B cells stained with various combinations of anti-IgM and anti-IgD antibodies were purified from reconstituted _rag1_–/– mice by positive or negative cell sorting on a FACSII or FACStar Plus cell sorter (Becton Dickinson, San Jose, CA) as described previously (Grumont et al., 1998). B220+IgM+ B cells from bone marrow were sorted by staining with anti-B220 and anti-IgM, while CD4+CD8+, CD4+CD8– and CD4–CD8+ T-cell populations from the thymus and spleen of reconstituted _rag1_–/– mice were obtained after staining with anti-CD4 and anti-CD8 antibodies. The purity of B- and T-cell populations enriched by sorting was ≥95%.
Cell turnover analysis
Cell turnover in animals was determined by labeling proliferating cells with the thymidine analog BrdU (Sigma, St Louis, MO), provided continuously for 2, 4 or 7 days in the drinking water (1 mg/ml plus 2% glucose to overcome taste aversion). BrdU incorporation into cellular DNA was detected by immunofluorescence staining with an FITC-labeled monoclonal antibody (BU-1; Becton Dickinson) and flow cytometric analysis was carried out as described previously (Grumont et al., 1998). Combined analysis of incorporated BrdU and cell surface antigen expression by staining with surface marker-specific antibodies was used to identify B (IgM+IgD– and IgM+IgD+) and T (CD4+, CD8+) cells in the spleen. As a negative control for staining with the FITC-BU-1 anti-BrdU antibody, we included cells from a mouse that had not received BrdU in the drinking water. Routinely, <1% of cells from such control mice stained above background.
Lymphocyte survival in tissue culture
To assess cell survival, B and T cells sorted from the spleen of reconstituted _rag1_–/– mice were cultured at an initial concentration of 5 × 105 cells/ml in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 4.5 g/l glucose, 13 µM folic acid, 250 µM l-asparagine, 50 µM β-mercaptoethanol and 10% fetal bovine serum. At various time points, cell viability was determined by staining with trypan blue or by flow cytometric analysis of cells stained with propidium iodide.
Enzyme-linked immunosorbent assays
The levels of Igs of various isotypes in the sera of _rag-1_–/– mice reconstituted with E12 fetal liver cells from wild-type, c-_rel_–/–, _rela_–/– or c-_rel_–/–_rela_–/– embryos (with or without a bcl-2 transgene) were determined as described (Kontgen et al., 1995). The relative levels of each Ig isotype were determined by comparing the serum samples with a hyperimmune serum standard.
B-lymphocyte activation in culture
B lymphocytes were cultured in 96-well flat-bottom microtiter plates at an initial concentration of 3 × 105/ml in 100 µl of the high glucose version of DMEM supplemented with 13 µM folic acid, 250 µM l-asparagine, 50 µM β-mercaptoethanol and 10% fetal bovine serum. Cells were stimulated with LPS (Difco, Detroit, MI) at a concentration of 20 µg/ml, affinity-purified goat anti-mouse IgM (Fab′)2 fragments (Jackson ImmunoResearch, Bar Harbor, ME) at 20 µg/ml or rat anti-mouse RP monoclonal antibody at 1 µg/ml, individually or in combination.
Cellular proliferation was measured at various times by adding 0.5 µCi of [3H]thymidine for 6 h, after which cells were harvested onto glass fiber filters. Incorporated radioactivity was quantitated by scintillation counting.
Semi-quantitative RT–PCR
Total RNA was isolated from FACS-sorted IgMhiIgDlo, IgMhiIgDhi and IgMloIgDhi wild-type, c-_rel_–/–, _rela_–/– and c-_rel_–/–_rela_–/– splenic B cells (105 cells per population) using RNAgents (Promega). Using equivalent amounts of RNA, cDNA synthesis followed by semi-quantitative PCR amplification for 25 cycles was performed as described previously (Grumont and Gerondakis, 2000). Samples were then fractionated on a 1% agarose gel. The sequences of the oligonucleotides used for the amplification of murine bcl-2, _bcl-x_L, A1 and β-actin mRNA were: bcl-2 sense, 5′-TCGCTACCGTCGTGACTTC-3′; antisense, 5′-AAACAGAGGTCGCATGCTG-3′ according to Tomayko and Cancro (1998); A1 sense, 5′-CAAATCTGGCTGGCTGACTTTTC-3′; antisense, 5′-CAAGTGCTGATAACCATTCTCGTC-3′ according to Tomayko and Cancro (1998); _bcl-x_L sense, 5′-GTTGTACCTGCTTGCTGTCGCCGG-3′; antisense 5′-AGCTTGTAGGAGAGAAAGTCGACC-3′ according to Grillot et al. (1997); β-actin sense, 5′-GCATTGCTGACAGGATGCAG-3′; and antisense, 5′-CCTGCTTGCTGATCCACATC-3′ according to Tomayko and Cancro (1998). The bcl-2, A1, _bcl-x_L and β-actin PCR products are 315, 123, 195 and 156 bp, respectively.
Acknowledgments
Acknowledgements
We thank Drs Amer Beg and David Baltimore for making the _rela_–/– mice available, and Yukio Nakamura, Raelene Grumont and David Tarlinton for useful discussions, technical advice and reagents. This work is supported by grants from the National Health and Medical Research Council (Australia), the Anti-Cancer Council of Victoria, the Commonwealth Aids Research Council (grant no. 971274) and the International Association for Cancer Research (St Andrews, UK).
References
- Attar R.M., Macdonald-Bravo,H., Raventos-Suarez,C., Durham,S.K. and Bravo,R. (1998) Expression of constitutively active IκBβ in T cells of transgenic mice: persistent NF-κB activity is required for T-cell immune responses. Mol. Cell. Biol., 18, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A.S.,Jr (1996) The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol., 14, 649–683. [DOI] [PubMed] [Google Scholar]
- Barkett M. and Gilmore,T.D. (1999) Control of apoptosis by Rel/NF-κB transcription factors. Oncogene, 18, 6910–6924. [DOI] [PubMed] [Google Scholar]
- Beg A.A. and Baltimore,D. (1996) An essential role for NF-κB in preventing TNF-α-induced cell death. Science, 274, 782–784. [DOI] [PubMed] [Google Scholar]
- Beg A.A., Sha,W.C., Bronson,R.T., Ghosh,S. and Baltimore,D. (1995) Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature, 376, 167–170. [DOI] [PubMed] [Google Scholar]
- Bendall H.H., Sikes,M.L., Ballard,D.W. and Oltz, EM. (1999) An intact NF-κB signaling pathway is required for maintenance of mature B cell subsets. Mol. Immunol., 36, 187–195. [DOI] [PubMed] [Google Scholar]
- Boothby M.R., Mora,A.L., Scherer,D.C., Brockman,J.A. and Ballard,D.W. (1997) Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-κB. J. Exp. Med., 185, 1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao D.T. and Korsmeyer,S.J. (1998) BCL-2 family: regulators of cell death. Annu. Rev. Immunol., 16, 395–419. [DOI] [PubMed] [Google Scholar]
- Chen J., Shinkai,Y., Young,F. and Alt,F.W. (1994) Probing immune functions in RAG-deficient mice. Curr. Opin. Immunol., 6, 313–319. [DOI] [PubMed] [Google Scholar]
- Doi T.S., Takahashi,T., Taguchi,O., Azuma,T. and Obata,Y. (1997) NF-κB RelA-deficient lymphocytes: normal development of T cells and B cells, impaired production of IgA and IgG1 and reduced proliferative responses. J. Exp. Med., 185, 953–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T.S., Marino,M.W., Takahashi,T., Yoshida,T., Sakakura,T., Old,L.J. and Obata,Y. (1999) Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc. Natl Acad. Sci. USA, 96, 2994–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G. et al. (1997) Requirement for NF-κB in osteoclast and B-cell development. Genes Dev., 11, 3482–3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman D.A., Snapper,S.B., Yballe,C.M., Davidson,L., Yu,J.Y., Alt,F.W. and Cantley,L.C. (1999) Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science, 283, 393–397. [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Grumont,R., Rourke,I. and Grossmann,M. (1998) The regulation and roles of Rel/NF-κB transcription factors during lymphocyte activation. Curr. Opin. Immunol., 10, 353–359. [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Grossmann,M., Nakamura,Y., Pohl,T. and Grumont,R. (1999) Genetic approaches in mice to understand Rel/NF-κB and IκB function: transgenics and knockouts. Oncogene, 18, 6888–6895. [DOI] [PubMed] [Google Scholar]
- Grillot D.A., Merino,R., Pena,J.C., Fanslow,W.C., Finkelman,F.D., Thompson,C.B. and Nuñez,G. (1996) bcl-x exhibits regulated expression during B cell development and activation and modulates lymphocyte survival in transgenic mice. J. Exp. Med., 183, 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillot D.A., Gonzalez-Garcia,M., Ekhterae,D., Duan,L. Inohara,N., Ohta,S., Seldin,M.F. and Nuñez,G. (1997) Genomic organization, promoter region analysis and chromosome localization of the mouse bcl-x gene. J. Immunol., 158, 4750–4757. [PubMed] [Google Scholar]
- Grossmann M., Metcalf,D., Merryfull,J., Beg,A., Baltimore,D. and Gerondakis,S. (1999) The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc. Natl Acad. Sci. USA, 96, 11848–11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumont R.J., Rourke,I.J., O’Reilly,L.A., Strasser,A., Miyake,K., Sha,W. and Gerondakis,S. (1998) B lymphocytes differentially use the Rel and nuclear factor κB1 (NF-κB1) transcription factors to regulate cell cycle progression and apoptosis in quiescent and mitogen-activated cells. J. Exp. Med., 187, 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumont R.J., Rourke,I.J. and Gerondakis,S. (1999) Rel-dependent induction of A1 transcription is required to protect B cells from antigen receptor ligation-induced apoptosis. Genes Dev., 13, 400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumont R.J. and Gerondakis,S. (2000) Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor κB. J. Exp. Med., 191, 1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki A., Sendo,F., Nakayama,K., Ishida,N., Negishi,I., Nakayama,K. and Hatakeyama,S. (1998) Accelerated neutrophil apoptosis in mice lacking A1-a, a subtype of the _bcl-2_-related A1 gene. J. Exp. Med., 188, 1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R.R., Hayakawa,K., Parks,D.R. and Herzenberg,L.A. (1983) Demonstration of B-cell maturation in X-linked immunodeficient mice by simultaneous three-colour immunofluorescence. Nature, 306, 270–272. [DOI] [PubMed] [Google Scholar]
- Hettmann T., DiDonato,J., Karin,M. and Leiden,J.M. (1999) An essential role for nuclear factor κB in promoting double positive thymocyte apoptosis. J. Exp. Med., 189, 145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B.H., Scott,M.L., Cherry,S.R., Bronson,R.T. and Baltimore,D. (1997) Failure of lymphopoiesis after adoptive transfer of NF-κB-deficient fetal liver cells. Immunity, 6, 765–772. [DOI] [PubMed] [Google Scholar]
- Horwitz B.H., Zelazowski,P., Shen,Y., Wolcott,K.M., Scott,M.L., Baltimore,D. and Snapper,C.M. (1999) The p65 subunit of NF-κB is redundant with p50 during B cell proliferative responses and is required for germline CH transcription and class switching to IgG3. J. Immunol., 162, 1941–1946. [PubMed] [Google Scholar]
- Karin M. (1999) How NF-κB is activated: the role of the IκB kinase (IKK) complex. Oncogene, 18, 6867–6874. [DOI] [PubMed] [Google Scholar]
- Kontgen F., Grumont,R.J., Strasser,A., Metcalf,D., Li,R., Tarlinton,D. and Gerondakis,S. (1995) Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity and interleukin-2 expression. Genes Dev., 9, 1965–1977. [DOI] [PubMed] [Google Scholar]
- Lam K.P., Kuhn,R. and Rajewsky,K. (1997) In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell, 90, 1073–1083. [DOI] [PubMed] [Google Scholar]
- Loder F., Mutschler,B., Ray,R.J., Paige,C.J., Sideras,P., Torres,R., Lamers,M.C. and Carsetti,R. (1999) B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. J. Exp. Med., 190, 75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y., Nakayama,K., Tomita,T., Isoda,M., Loh,D.Y. and Nakauchi,H. (1997) Role of bcl-2 in the development of lymphoid cells from the hematopoietic stem cell. Blood, 89, 853–862. [PubMed] [Google Scholar]
- Motoyama N. et al. (1995) Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science, 267, 1506–1510. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Negishi,I., Kuida,K., Sawa,H. and Loh,D.Y. (1994) Targeted disruption of Bcl-2αβ in mice: occurrence of gray hair, polycystic kidney disease and lymphocytopenia. Proc. Natl Acad. Sci. USA, 91, 3700–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvy S., Metcalf,D., Print,C.G., Bath,M.L., Harris,A.W. and Adams,J.M. (1999) Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc. Natl Acad. Sci. USA, 96, 14943–14948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond D.G. (1993) The turnover of B-cell populations. Immunol. Today, 14, 34–37. [DOI] [PubMed] [Google Scholar]
- Rajewsky K. (1996) Clonal selection and learning in the antibody system. Nature, 381, 751–758. [DOI] [PubMed] [Google Scholar]
- Strasser A. (1995) Life and death during lymphocyte development and function: evidence for two distinct killing mechanisms. Curr. Opin. Immunol., 7, 228–234. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris,A.W., Vaux,D.L., Webb,E., Bath,M.L., Adams,J.M. and Cory,S. (1990) Abnormalities of the immune system induced by dysregulated bcl-2 expression in transgenic mice. Curr. Top. Microbiol. Immunol., 166, 175–181. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris,A.W., Corcoran,L.M. and Cory,S. (1994) Bcl-2 expression promotes B- but not T-lymphoid development in scid mice. Nature, 368, 457–460. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris,A.W., Huang,D.C., Krammer,P.H. and Cory,S. (1995) Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J., 14, 6136–6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomayko M.M. and Cancro,M.P. (1998) Long-lived B cells are distinguished by elevated expression of A1. J. Immunol., 160, 107–111. [PubMed] [Google Scholar]
- Turner M., Gulbranson-Judge,A., Quinn,M.E., Walters,A.E., MacLennan,I.C. and Tybulewicz,V.L. (1997) Syk tyrosine kinase is required for the positive selection of immature B cells into the recirculating B cell pool. J. Exp. Med., 186, 2013–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veis D.J., Sorenson,C.M., Shutter,J.R. and Korsmeyer,S.J. (1993) Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys and hypopigmented hair. Cell, 75, 229–240. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. (1990) Developmental biology of T cells in T cell-receptor transgenic mice. Annu. Rev. Immunol., 8, 531–556. [DOI] [PubMed] [Google Scholar]
- Weih F., Durham,S.K., Barton,D.S., Sha,W.C., Baltimore,D. and Bravo,R. (1997) p50–NF-κB complexes partially compensate for the absence of RelB: severely increased pathology in p50(–/–)relB(–/–) double-knockout mice. J. Exp. Med., 185, 1359–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside S.T. and Israel,A. (1997) IκB proteins: structure, function and regulation. Semin. Cancer Biol., 8, 75–82. [DOI] [PubMed] [Google Scholar]