Role of the angiotensin II AT2 receptor in inflammation and oxidative stress: opposing effects in lean and obese Zucker rats (original) (raw)
Abstract
Inflammation and oxidative stress are believed to contribute to hypertension in obesity/diabetes. Recently, we reported a role for the AT2 receptor in blood pressure control in obese Zucker rats. However, the role of AT2 receptors in inflammation and oxidative stress in obesity is not known. Therefore, in the present study, we tested the effects of the AT2 receptor agonist CGP-42112A on inflammation and oxidative stress in obese Zucker rats and compared them in their lean counterparts. Rats were systemically treated with either vehicle (control) or CGP-42112A (1 μg·kg−1·min−1; osmotic pump) for 2 wk. Markers of inflammation (CRP, MCP-1, TNF-α, and IL-6) and oxidative stress (HO-1, gp-91phox) as well as an antioxidant (SOD) were determined. Control obese rats had higher plasma levels of CRP, MCP-1, TNF-α, IL-6, and HO-1 compared with control lean rats. Conversely, plasma SOD activity was lower in control obese than in control lean rats. Furthermore, the protein levels of TNF-α and gp-91phox were higher in the kidney cortex of control obese rats. Interestingly, CGP-42112A treatment in obese rats reduced the plasma and kidney cortex inflammatory (TNF-α, IL-6) and oxidative stress (gp-91phox) markers and increased plasma SOD activity to the levels seen in lean control rats. However, CGP-42112A treatment in lean rats increased inflammatory (TNF-α, IL-6) and oxidative stress (gp-91phox) markers in the plasma and kidney cortex. Our present studies suggest anti-inflammatory and antioxidative functions of AT2 receptor in obese Zucker rats but proinflammatory and prooxidative functions in lean Zucker rats.
Keywords: CGP-42112A, superoxide dismutase, TNF-α, IL-6, NF-κB, nitric oxide
obesity is a major risk factor for development of hypertension, insulin resistance, atherosclerosis, dyslipidemia, and diabetes (7, 22). Early-stage obesity is associated with oxidative stress and inflammation, which may further contribute to the development of hypertension (11). Studies have reported increased oxidative stress markers [8-iso-prostane, heme oxygenase-1 (HO-1), SOD, and NAD(P)H oxidase activity and expression] and inflammatory markers [C-reactive protein (CRP), monocyte chemoattractant protein-1 (MCP-1), TNF-α, ILs, namely, IL-β and IL-6], in obese animals (12, 18, 21, 23, 26). However, the precise role of oxidative stress and inflammation in the development of obesity-related hypertension remains elusive.
ANG II is the main effector peptide of the renin-angiotensin system (RAS) (8). Studies have demonstrated that ANG II plays a significant role in the development of oxidative stress (32, 45). ANG II and oxidative stress, alone or together, can lead to the development of inflammation (20, 39). The “classic” effects of ANG II on blood pressure, electrolyte homeostasis, oxidative stress, and inflammation are attributed to the ANG II type 1 (AT1) receptors (13). The other subtype of ANG II receptors, type 2 (AT2) receptors, are less expressed during physiological conditions and mediate opposing effects to those of AT1 receptors (33). Recently, we have reported that renal AT2 receptor expression is increased, promotes natriuresis, and protects against blood pressure increase in obese animals (16, 17, 35). Since obesity is known to be associated with enhanced oxidative stress and inflammation (1, 14), a potential role of the AT2 receptor in inflammation and oxidative stress is not known.
In the present study, we determined the role of AT2 receptors in inflammation and oxidative stress in obese Zucker rats, a rat model of metabolic syndrome (4). Obese Zucker rats and their lean counterparts were chronically (2 wk) treated with the AT2 receptor-agonist CGP-42112A, and it was found that AT2 receptors had anti-inflammatory and antioxidative responses in obese Zucker rats. However, chronic activation of AT2 receptors in lean Zucker rats had proinflammatory and prooxidative responses.
MATERIALS AND METHODS
Animal model.
Age-matched (10–11 wk old) lean and obese Zucker rats were obtained from Harlan (Indianapolis, IN). Rats were housed in the University of Houston Animal Care Facility and were maintained on standard rat chow and water ad libitum. The Institutional Animal Care and Use Committee approved the experimental protocols. The lean and obese rats (n = 7) were divided into vehicle- and AT2 receptor agonist (CGP-42112A)-treated groups. Vehicle (saline) and CGP-42112A (1 μg·kg−1·min−1) separately were continuously infused for 2 wk by implanting of osmotic pumps (Alza, Palo Alto, CA) subcutaneously. The dose and length of CGP-42112A treatment were determined based on previous studies from our and other laboratories (5, 35, 44). Both in vitro and in vivo studies suggest that responses such as natriuresis and Na pump inhibition by CGP-42112A can be blocked by PD-123319, supporting the specificity of the agonist (5, 14, 19). After the treatment period, blood from the carotid artery and kidneys was collected under anesthesia. The blood was centrifuged at 1,500 g at 4°C to obtain plasma. The kidneys were decapsulated, rinsed with cold PBS to remove blood, sectioned sagittally with a razor blade, and cortices were separated. All samples were stored at −80°C until further analyses.
ELISA.
The level of CRP and MCP-1 was determined by using ELISA kits as per the manufacturer's instructions. Briefly, 100 μl of appropriate blank, standards, and samples (diluted 200- and 25-fold with assay buffer for CRP and MCP-1 determination, respectively) were added to appropriate wells in 96-well plates. Primary antibody (100 μl) was added to the MCP-1 plate. The plates were incubated for 1 h at room temperature for CRP and at 37°C for MCP-1. The wells were washed with wash buffer, and 100 μl of anti-rat CRP-HRP conjugate (secondary antibody) for CRP and chromogen for MCP-1 were added to each well. The plates were incubated for 30 min in the dark at room temperature. The horseradish peroxidase (HRP) substrate 3,3,5,5-tetramethylbenzidine was added to the CRP plate and incubated for 15 min in the dark. The reaction for CRP and MCP-1 was terminated with a stop solution (100 μl). The yellow color developed was read at 450 nm using a microplate reader.
SOD activity in plasma and kidney cortex.
SOD activity was determined using a kit-based assay (Cayman Chemical, Ann Arbor, MI). Briefly, 10 μl of standards and samples were added to appropriate wells along with 200 μl of diluted radical detector. To initiate the reaction, 20 μl of xanthine oxidase was added to each well. The plate was incubated for 20 min at room temperature. The absorbance was determined at 440–460 nm using a plate reader.
Western blotting.
The protein levels of cytokines (IL-6, TNF-α), superoxide radical-producing NADPH-gp91phox, HO-1, and antioxidant (Cu/Zn-SOD, Mn-SOD and Ec-SOD) enzymes were determined by standard Western blotting techniques. Briefly, kidney cortices were homogenized in a buffer containing (in mM) 50 Tris, 10 EDTA, and 1 PMSF, and a cocktail of protease inhibitors, and proteins were determined by the BCA method using a kit (Pierce, Rockford, IL). Equal amounts of protein (20 μg) from various rat groups were subjected to SDS-PAGE and were transferred onto nitrocellulose membranes (blot). The blots were blocked using 5% milk in PBS with 0.1% Tween 20 for 1 h at room temperature, followed by overnight incubation with primary antibodies for IL-6, TNF-α, HO-1, Cu/Zn-SOD, Mn-SOD, and Ec-SOD separately at 4°C. Appropriate HRP-conjugated anti-rabbit and anti-mouse secondary antibodies were used to detect protein bands using the enhanced chemiluminescence (ECL) system. The protein bands were analyzed by Fluorchem 8800 (Alpha Innotech Imaging System, San Leandro, CA) for the densitometric analysis. β-Actin was used as a protein loading control.
Chemicals.
CGP-42112A was custom synthesized (21st Century Biochemicals). ELISA kits for CRP and MCP-1 were purchased from Alpha Diagnostics (San Antonio, TX) and Immuno-Biological Laboratories, (Japan), respectively. The SOD activity kit was bought from Cayman Chemical. The HO-1 monoclonal antibody was from Assay Designs (Plymouth Meeting, PA), gp-91phox monoclonal antibody from BD Biosciences (San Jose, CA), TNF-α polyclonal antibody from Cell Signaling (Danvers, MA), rat IL-6 polyclonal antibody from Invitrogen (Carlsbad, CA), monoclonal antibody for β-Actin from Santa Cruz Biotechnology (Santa Cruz, CA), polyclonal Cu/Zn-SOD from Stressgen (Plymouth Meeting, PA), polyclonal Mn-SOD and polyclonal Ec-SOD from Chemicon (Temecula, CA), and ECL substrates were obtained from Alpha Diagnostics. All other chemicals were from Sigma (St. Louis, MO).
Statistical analysis.
Data are presented as means ± SE. To analyze variations between groups, Student's _t-_test was used. One-way ANOVA with post hoc (Newman-Keuls) tests was used to compare variations within groups. A value of P < 0.05 was considered statistically significant, with n = 5–7 rats in each group.
RESULTS
Effect of chronic CGP-42112A treatment on inflammatory markers in plasma and kidney cortex.
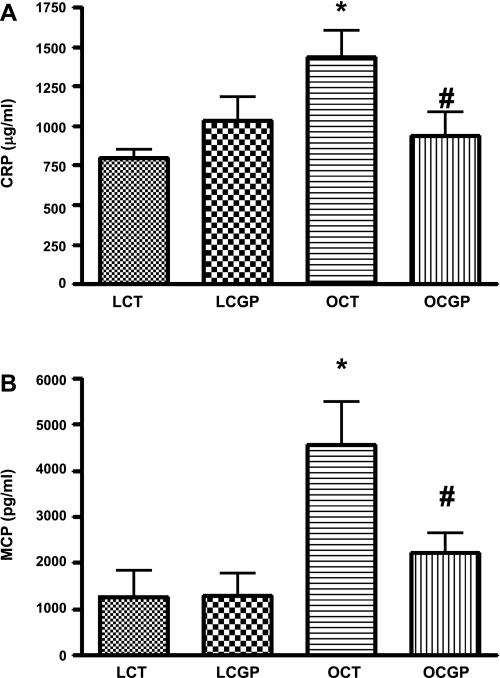
Obese control rats had significantly higher plasma CRP (1.2 ± .07 mg/ml) and MCP-1(4.9 ± 1.6 ng/ml) levels compared with the lean control rats (0.8 ± 0.2 and 0.3 ± 0.2 ng/ml). Chronic treatment with CGP-42112A for 2 wk significantly decreased the plasma CRP (0.6 ± 0.2 mg/ml) and MCP-1 (1.9 ± 0.8 ng/ml) levels in obese rats but had no significant effect in lean rats (Fig. 1). The trend of CRP level seen in lean CGP-42112A rats was subjected to power analysis, which suggests that a sample size of 12 for one-tailed analysis will show statistical significance.
Fig. 1.
Effect of 2-wk CGP-42112A treatment on plasma levels of CRP (A) and MCP-1 (B) in lean (L) and obese (O) Zucker rats. The infusion rate of CGP-42112A was 1 μg·kg−1·min−1 via subcutaneous osmotic pumps. Values are means ± SE. Student's _t_-test was used to compare the variations between groups, and 1-way ANOVA with the Newman-Keuls multiple comparison post hoc test was used to compare the data within a group. P < 0.05 was considered significant; n = 4–6. *Significantly different from lean vehicle-treated rats and/or lean CGP-42112A-treated rats. #Significantly different from obese vehicle-treated rats.
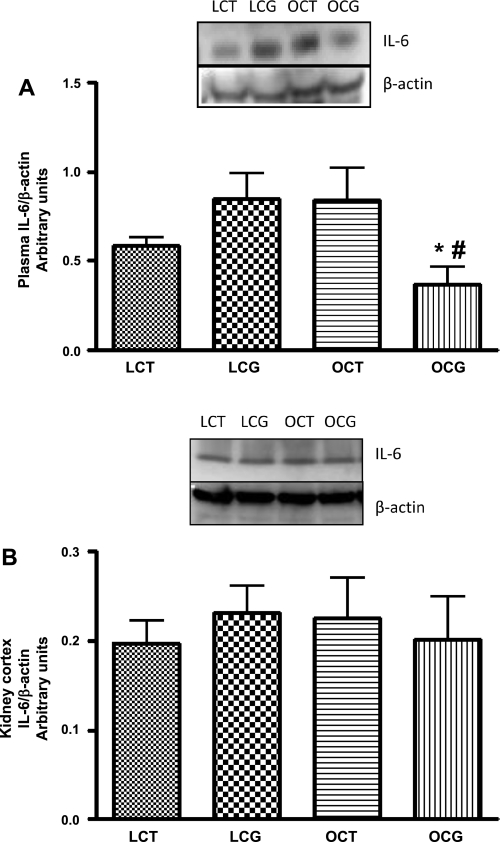
The plasma levels of IL-6 were higher in obese control than in lean control rats, which decreased with CGP-42112A treatment in obese rats (Fig. 2_A_). Conversely, CGP-42112A treatment in lean rats caused an insignificant increase in the plasma levels of IL-6 (Fig. 2_A_). Furthermore, the kidney cortical levels of IL-6 in control lean and obese Zucker rats were similar, which did not change with CGP-42112A treatment in these rats (Fig. 2_B_).
Fig. 2.
Effect of 2-wk CGP-42112A treatment on expression of IL-6 (24 kDa) in plasma (A) and kidney cortex (B) of lean and obese Zucker rats. Values are means ± SE. Student's _t_-test was used to compare the variations between groups, and 1-way ANOVA with the Newman-Keuls multiple comparison post hoc test was used to compare the data within a group. P < 0.05 was considered significant; n = 5–7. *Significantly different from lean CGP-42112A-treated rats. #Significantly different from obese vehicle-treated rats.
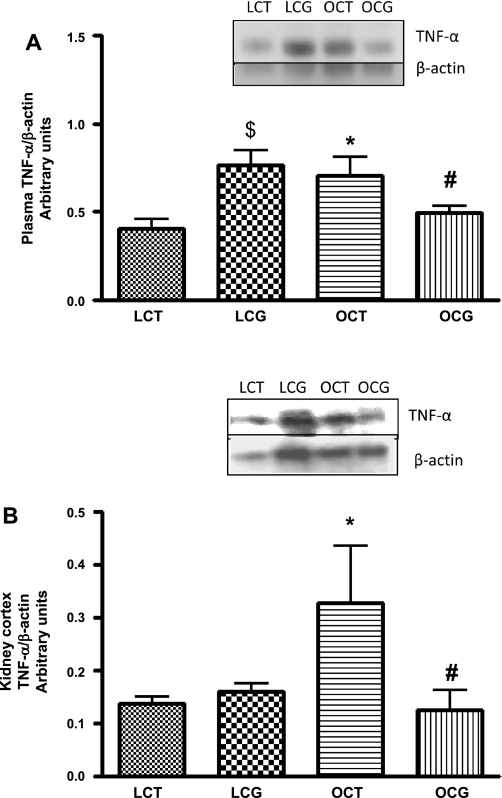
The plasma and kidney cortical levels of TNF-α were significantly higher in control obese compared with lean rats (Fig. 3, A and B), which decreased with CGP-42112A treatment in obese rats. However, CGP-42112A treatment in lean rats significantly increased the plasma levels of TNF-α (Fig. 3_A_).
Fig. 3.
Effect of 2-wk CGP-42112A treatment on expression of TNF-α (25 kDa) in plasma (A) and kidney cortex (B) of lean and obese Zucker rats. Values are means ± SE. Student's _t_-test was used to compare the variations between groups, and 1-way ANOVA with the Newman-Keuls multiple comparison post hoc test was used to compare the data within a group. P < 0.05 was considered significant; n = 5–7. $*Significantly different from lean vehicle-treated rats. #Significantly different from obese vehicle-treated rats and lean CGP-treated rats.
Effect of chronic CGP-42112A treatment on oxidative stress markers in plasma and kidney cortex.
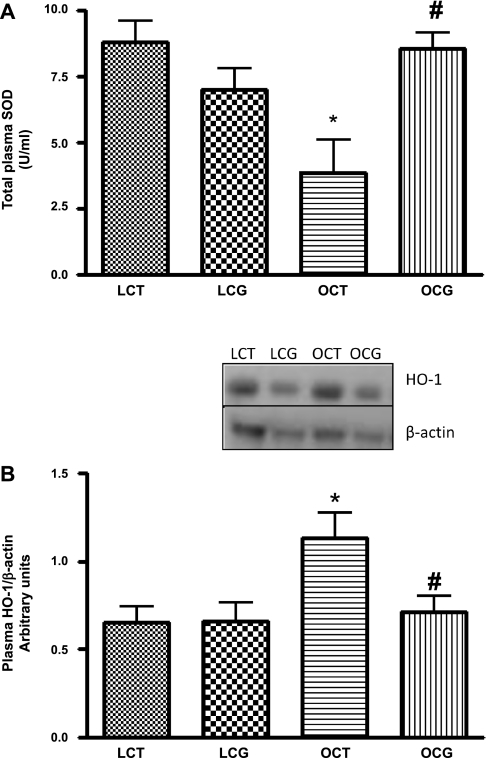
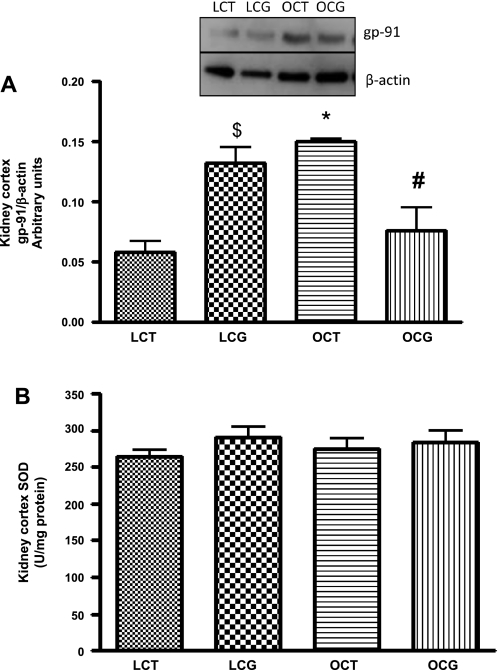
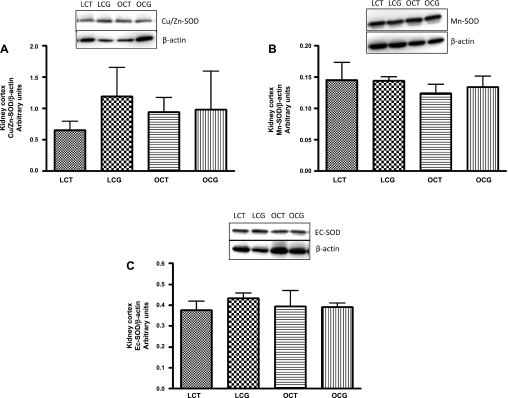
Plasma total SOD activity was significantly lower (Fig. 4_A_) in obese control (3.8 ± 1.2 U/ml) than in lean control rats (8.8 ± 0.7 U/ml), which with CGP-42112A treatment significantly increased in obese rats (8.5 ± 0.6 U/ml). CGP-42112A treatment caused no change in the plasma SOD activity in lean rats. In the kidney cortex, SOD activity (Fig. 5_B_) or expression (Fig. 6) of different SOD isoforms (Cu/Zn-SOD, Mn-SOD, and Ec-SOD) were not changed in either control or CGP-42112A-treated lean and obese rats.
Fig. 4.
Effect of 2-wk CGP-42112A treatment on total plasma SOD activity (A) and expression of 32-kDa plasma heme oxygenase (HO)-1 (B) in lean and obese Zucker rats. Values are means ± SE. Student's _t_-test was used to compare the variations between groups, and 1-way ANOVA with the Newman-Keuls multiple comparison post hoc test was used to compare the data within a group. P < 0.05 was considered significant; n = 5–8. *Significantly different from lean vehicle-treated and/or lean CGP-42112A-treated rats. #Significantly different from obese vehicle-treated rats.
Fig. 5.
Effect of 2-wk CGP-42112A treatment on expression of gp-91phox (A) and total SOD activity (B) in kidney cortex of lean and obese Zucker rats. Values are means ± SE. Student's _t_-test was used to compare the variations between groups, and 1-way ANOVA with the Newman-Keuls multiple comparison post hoc test was used to compare the data within a group. P < 0.05 was considered significant; n = 4–7. $*Significantly different from lean vehicle-treated rats. #Significantly different from obese vehicle-treated rats and lean CGP-42112A-treated rats.
Fig. 6.
Effect of 2-wk CGP-42112A treatment on expression of SOD isoforms Cu/Zn-SOD (A), Mn-SOD (B), and Ec-SOD (C) in kidney cortex of lean and obese Zucker rats. Values are means ± SE. Student's _t_-test was used to compare the variations between groups, and 1-way ANOVA with the Newman-Keuls multiple comparison post hoc test was used to compare the data within a group. P < 0.05 was considered significant; n = 4–5.
The plasma expression of HO-1 (32 kDa), an inducible enzyme, was significantly higher in obese compared with lean rats (Fig. 4_B_), which significantly decreased with CGP-42112A treatment for 2 wk in obese rats, and there was no change in lean rats.
The NAD(P)H oxidase (major superoxide-producing enzyme in the kidney) membranal subunit gp-91phox expression (58 kDa) was measured in kidney cortex. The expression of gp-91phox was found to be significantly higher in obese compared with lean control rats (Fig. 5_A_), which decreased with chronic CGP-42112A treatment in obese rats. However, CGP-42112A treatment in lean rats significantly increased the expression of gp-91phox.
Effect of chronic CGP-42112A treatment on mean arterial blood pressure.
Mean arterial blood pressure (MAP) measured under Inactin (100 mg/kg ip) anesthesia, as described earlier (16, 35), was significantly higher in obese control (131 ± 3 mmHg) than in lean control (95 ± 5 mmHg) rats. CGP-42112A treatment for 2 wk significantly reduced MAP by 19 mmHg in obese rats, and there was no detectable change in the lean rats.
DISCUSSION
In the present study, we clearly demonstrated that both inflammation and oxidative stress with chronic (2 wk) administration of the AT2 receptor agonist CGP-42112A decrease in obese but increase in lean rats, indicating its opposing effects in normal and pathophysiological situations.
Metabolic syndrome is a term given to a group of abnormalities such as obesity, hyperglycemia, hyperinsulinemia, dyslipidemia, and high blood pressure, which further increases the risk of overt diabetes mellitus and renal/cardiovascular diseases (7, 22). Obesity is one of the most common nutritional disorders and is associated with chronic low-grade inflammation and oxidative stress independent of hypertension (41). Studies suggest that increased inflammation and oxidative stress are tightly regulated by the elevated RAS activity in obese animals and hence can contribute to development or maintenance of hypertension (20, 32, 39). The main effector peptide of RAS is ANG II, which works via activation of AT1 and AT2 receptors (2). The proinflammatory and prooxidative actions of AT1 receptors are well established (37, 43); however, the reports about the role of AT2 receptor in inflammation and oxidative stress are sparse and quite ambiguous (29, 38). Therefore, the present study was designed to determine the effects of chronic AT2 receptor activation on inflammatory and oxidative stress markers in obese Zucker rats, a rodent model of metabolic syndrome (4), and compare these markers to their lean counterparts.
Obesity-related inflammation is associated with increase in circulating markers of inflammation such as CRP, cytokines (TNF-α, IL) and chemokines (MCP-1) (12, 20–22). Although they serve as independent markers of inflammation, on the molecular level they are intimately intertwined and regulate each other's expression and function (40). In the present study, the plasma levels of CRP, MCP-1, and TNF-α were significantly high in obese Zucker rats. There was a modest increase in the plasma level of IL-6 in obese rats. Previous studies have reported a significant increase in the IL-6 levels during inflammation (25, 42). This slight difference in observation can be attributed to the method of IL-6 determination used in the present study (Western blot vs. ELISA/RT-PCR). Chronic AT2 receptor agonist treatment significantly reduced the levels of all the markers of inflammation measured in this study in obese Zucker rats, suggesting an anti-inflammatory function of AT2 receptors in obese rats.
HO-1 is an inducible enzyme and is elevated during oxidative stress (23), and thus HO-1 measurement serves as an indirect measure of oxidative stress. We found that obese Zucker rats have significantly higher HO-1 expression in plasma, and chronic AT2 receptor activation significantly reduced HO-1 expression in obese rats. Lower plasma SOD activity in obese rat indicates that these rats have a poor anti-oxidant defense system, and chronic AT2 receptor activation is able to rescue this defect. Although there are other oxidative stress markers available, however, measurement of these two enzymes (HO-1, SOD) in the present study clearly demonstrates the anti-oxidant property of AT2 receptors in obese Zucker rats.
Numerous studies indicate that AT2 receptor expression is increased during pathological conditions such as hyperglycemia, cardiovascular disease, and renal failure (1, 16, 24). Recently, we have shown that in obese Zucker rats, renal AT2 receptor expression is increased and plays an important role in the regulation of Na excretion (15, 16). We sought to examine whether AT2 receptor activation in the kidney apart from Na excretion has any effect on inflammation and oxidative stress as seen in plasma. We measured inflammatory and oxidative stress markers in the renal cortex. TNF-α showed a similar trend as in plasma; however, the expression of IL-6 was not changed. The total SOD activity and expression of all the SOD isoforms in the kidney cortex were not significantly different in any of the treatment groups; however, the NAD(P)H oxidase membrane-bound subunit gp-91phox was significantly increased in obese rats. Although these results are difficult to explain, a plausible explanation would be that the renal damage at this age (11 wk) in obese rats was in the initial stages, where we found an increase in TNF-α and gp-91phox, but the inflammation was not at a stage where we could observe a plethora of inflammatory and oxidative stress markers. There are studies reporting increased inflammatory cytokines and oxidative stress markers in the kidneys of obese rats; however, these studies included older (18 wk) obese rats as opposed to the 11-wk-old animals used by us (6, 25, 42). One of the limitations of our study is that we have not measured the complete array of proinflammatory cytokines. There are studies which support the notion that not all proinflammatory cytokines are increased during inflammation (6). Apart from this, the other possible reasons could be the use of the renal cortex in our study rather than a whole kidney homogenate or the use of Western blotting techniques rather than ELISA or RT-PCR. It is also worth mentioning that increased circulating levels of plasma inflammatory markers can be an index of multiple organ inflammation like in adipocytes, liver, and spleen rather than just the kidney in obese rats.
One of the major and most unexpected findings of our study was increased expression/levels of some of the inflammatory and oxidative stress markers in AT2 agonist-treated lean rats. The expression of TNF-α in plasma and gp-91phox in the kidney cortex was significantly increased. There was a trend toward an increase, although not significantly, in plasma level/expression of CRP and IL-6. Total SOD activity in plasma was also less compared with the lean control rats. This unanticipated outcome of AT2 receptor activation in lean rats might be a clue to understanding why AT2 receptors are expressed less during normal physiological condition compared with AT1 receptors. However, the proinflammatory roles of AT2 receptors (10, 30) have been described in normal rats, and that is in agreement with our findings in lean rats. Although further comprehensive study is required to understand the underlying mechanism for the opposing role, a simple explanation can be attributed to the different signaling pathways of AT2 receptors in lean and obese Zucker rats. It is known that AT2 receptor activation leads to an increase in the levels of nitric oxide (NO)/cGMP as well as a decrease in the accumulation of cAMP (14, 31, 36). We recently reported that selective activation of AT2 receptors in the kidney increases NO/cGMP levels in obese but not in lean rats and causes a similar reduction in cAMP accumulation in both the groups (14, 31). The increase in cellular cAMP accumulation has been suggested to decrease oxidative stress (3) and suppresses the immune responses (9, 34). Hence it is plausible that AT2 receptor-mediated reduction in cAMP levels, without having any effect on NO/cGMP in lean rats (14), leads to an increase in oxidative and inflammatory stresses in lean rats. In obese rats AT2 receptors, by mediating NO/cGMP accumulation (14), produce antioxidant and anti-inflammatory responses and cancel out modest effects mediated via cAMP. Furthermore, we found that increases in oxidative and inflammatory stresses were independent of blood pressure change in lean rats. This suggests that early-stage oxidative stress and inflammation can exist independently of hypertension. Whether late-stage oxidative stress and inflammation produced by beyond a 2-wk CGP-42112A treatment in lean rats result in the hypertensive phenotype is not known.
The molecular mechanism(s) relating to the anti-inflammatory and/or antioxidative property of AT2 receptors in obese rats is not clear. However, from the present study it can be speculated that activation of AT2 receptors lowers oxidative stress by increasing SOD activity and decreasing the expression of the superoxide radical-producing enzyme NADPH oxidase (gp-91phox). The anti-inflammatory property, depending on very recent studies, can be attributed to an inhibitory role of AT2 receptors on nuclear translocation of NF-κB (28, 46), a well-known transcription factor regulating most of the proinflammatory cytokines (27).
To summarize, the present study underscores the anti-inflammatory and antioxidative properties of AT2 receptors in obese Zucker rats. Our findings suggest that CGP-42112A-induced activation of AT2 receptors has contradictory effects on inflammation and oxidative stress in normal and pathophysiological situations.
Perspective.
The present study supports the notion that selective activation of AT2 receptors can bring about beneficial effects on the management of disease conditions associated with inflammation and oxidative stress. However, considering the opposing effects in normal and pathophysiological condition, the use of AT2 receptor agonists in the treatment of inflammation and oxidative stress warrants serious consideration.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-61578.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Ali Q, Sabuhi R, Hussain T. High glucose up-regulates angiotensin II subtype 2 receptors via interferon regulatory factor-1 in proximal tubule epithelial cells. Mol Cell Biochem 344: 65– 71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bader M, Ganten D. Update on tissue renin-angiotensin systems. J Mol Med 86: 615– 621, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bokoch GM, Diebold B, Kim JS, Gianni D. Emerging evidence for the importance of phosphorylation in the regulation of NADPH oxidases. Antioxid Redox Signal 11: 2429– 2441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray GA. The Zucker-fatty rat: a review. Fed Proc 36: 148– 153, 1977 [PubMed] [Google Scholar]
- 5.Carey RM, Howell NL, Jin XH, Siragy HM. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension 38: 1272– 1277, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Ecelbarger CM, Rash A, Sinha RK, Tiwari S. The effect of chronic candesartan therapy on the metabolic profile and renal tissue cytokine levels in the obese Zucker rat. Mediators Inflamm 2010: 841343, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmarakby AA, Imig JD. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin Sci (Lond) 118: 291– 301, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiera F, Sharma AM. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol 35: 807– 825, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J Immunol 185: 1993– 1998, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteban V, Ruperez M, Vita JR, Lopez ES, Mezzano S, Plaza JJ, Egido J, Ruiz-Ortega M. Effect of simultaneous blockade of AT1 and AT2 receptors on the NFkappaB pathway and renal inflammatory response. Kidney Int Suppl: S33–S38, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Ferder L, Inserra F, Martinez-Maldonado M. Inflammation and the metabolic syndrome: role of angiotensin II and oxidative stress. Curr Hypertens Rep 8: 191– 198, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy 4: 281– 286, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Fyhrquist F, Saijonmaa O. Renin-angiotensin system revisited. J Intern Med 264: 224– 236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakam AC, Hussain T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am J Physiol Renal Physiol 290: F1430– F1436, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Hakam AC, Hussain T. Angiotensin II type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not in lean Zucker rats. Hypertension 47: 1117– 1124, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese Zucker rats. Hypertension 45: 270– 275, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol 290: F503– F508, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Inoguchi T, Nawata H. NAD(P)H oxidase activation: a potential target mechanism for diabetic vascular complications, progressive beta-cell dysfunction and metabolic syndrome. Curr Drug Targets 6: 495– 501, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Kambayashi Y, Takahashi K, Bardhan S, Inagami T. Molecular structure and function of angiotensin type 2 receptor. Kidney Int 46: 1502– 1504, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm 2010: 453892, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lind L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis 169: 203– 214, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clin Pharmacol Ther 87: 407– 416, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Ndisang JF. Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediators Inflamm 2010: 359732, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nio Y, Matsubara H, Murasawa S, Kanasaki M, Inada M. Regulation of gene transcription of angiotensin II receptor subtypes in myocardial infarction. J Clin Invest 95: 46– 54, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimatsu H, Suzuki E, Takeda R, Takahashi M, Oba S, Kimura K, Nagano T, Hirata Y. Blockade of endogenous proinflammatory cytokines ameliorates endothelial dysfunction in obese Zucker rats. Hypertens Res 31: 737– 743, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Ramos LF, Shintani A, Ikizler TA, Himmelfarb J. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol 19: 593– 599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razani B, Cheng G. NF-kappaB: much learned, much to learn. Sci Signal 3: pe29, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Rompe F, Artuc M, Hallberg A, Alterman M, Stroder K, Thone-Reineke C, Reichenbach A, Schacherl J, Dahlof B, Bader M, Alenina N, Schwaninger M, Zuberbier T, Funke-Kaiser H, Schmidt C, Schunck WH, Unger T, Steckelings UM. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension 55: 924– 931, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Rompe F, Unger T, Steckelings UM. The angiotensin AT2 receptor in inflammation. Drug News Perspect 23: 104– 111, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Ortega M, Lorenzo O, Ruperez M, Blanco J, Egido J. Systemic infusion of angiotensin II into normal rats activates nuclear factor-kappaB and AP-1 in the kidney: role of AT1 and AT2 receptors. Am J Pathol 158: 1743– 1756, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabuhi R, Asghar M, Hussain T. Inhibition of NAD(P)H oxidase potentiates AT2 receptor agonist-induced natriuresis in Sprague-Dawley rats. Am J Physiol Renal Physiol 299: F815– F820, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachse A, Wolf G. Angiotensin II-induced reactive oxygen species and the kidney. J Am Soc Nephrol 18: 2439– 2446, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Schulman IH, Raij L. The angiotensin II type 2 receptor: what is its clinical significance? Curr Hypertens Rep 10: 188– 193, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol 39: 127– 132, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siddiqui AH, Ali Q, Hussain T. Protective role of angiotensin II subtype 2 receptor in blood pressure increase in obese Zucker rats. Hypertension 53: 256– 261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siragy HM, Carey RM. The subtype 2 (AT2) angiotensin receptor mediates renal production of nitric oxide in conscious rats. J Clin Invest 100: 264– 269, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skultetyova D, Filipova S, Riecansky I, Skultety J. The role of angiotensin type 1 receptor in inflammation and endothelial dysfunction. Recent Pat Cardiovasc Drug Discov 2: 23– 27, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Steckelings UM, Rompe F, Kaschina E, Namsolleck P, Grzesiak A, Funke-Kaiser H, Bader M, Unger T. The past, present and future of angiotensin II type 2 receptor stimulation. J Renin Angiotensin Aldosterone Syst 11: 67– 73, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol 35: 881– 900, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Tzanavari T, Giannogonas P, Karalis KP. TNF-alpha and obesity. Curr Dir Autoimmun 11: 145– 156, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Van Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity (Silver Spring) 14: 2127– 2131, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Vaziri ND, Xu ZG, Shahkarami A, Huang KT, Rodriguez-Iturbe B, Natarajan R. Role of AT-1 receptor in regulation of vascular MCP-1, IL-6, PAI-1, MAP kinase, and matrix expressions in obesity. Kidney Int 68: 2787– 2793, 2005 [DOI] [PubMed] [Google Scholar]
- 43.Wassmann S, Nickenig G. Pathophysiological regulation of the AT1-receptor and implications for vascular disease. J Hypertens Suppl 24: S15– S21, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Whitebread SE, Taylor V, Bottari SP, Kamber B, de Gasparo M. Radioiodinated CGP 42112A: a novel high affinity and highly selective ligand for the characterization of angiotensin AT2 receptors. Biochem Biophys Res Commun 181: 1365– 1371, 1991 [DOI] [PubMed] [Google Scholar]
- 45.Wolf G. Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis. Antioxid Redox Signal 7: 1337– 1345, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Wu L, Iwai M, Li Z, Shiuchi T, Min LJ, Cui TX, Li JM, Okumura M, Nahmias C, Horiuchi M. Regulation of inhibitory protein-kappaB and monocyte chemoattractant protein-1 by angiotensin II type 2 receptor-activated Src homology protein tyrosine phosphatase-1 in fetal vascular smooth muscle cells. Mol Endocrinol 18: 666– 678, 2004 [DOI] [PubMed] [Google Scholar]