Signaling in thymic selection (original) (raw)
. Author manuscript; available in PMC: 2012 Apr 1.
Published in final edited form as: Curr Opin Immunol. 2011 Jan 15;23(2):207–212. doi: 10.1016/j.coi.2010.12.017
Summary
T cell receptor signaling allows the developing thymocyte to undergo positive or negative selection, which are required for formation of a useful mature T cell repertoire. Recent developments include the finding that much of the Lck kinase (required to initiate T cell signaling) is already in an active configuration before signaling. The analog strength of antigen binding to the T cell receptor binding may be translated into a digital signal by the amount of time the TCR is paired with a co-receptor carrying Lck. Downstream, the cellular localization of MAP kinase signaling is determined by the strength of the signal and in turn predicts positive or negative selection. A novel protein, Themis, is important in crossing the positive selection developmental checkpoint, but its mode of action is still uncertain. Commitment to the CD4 or CD8 lineage is influenced by the amount of ZAP-70 signaling and also by closely regulated responsiveness to intrathymic cytokines such as IL7.
Introduction
The development of a functional T cell repertoire requires selection of thymocytes based on specific T cell receptor (TCR) signals. There are two major developmental checkpoints that are dependent on TCR signaling, but the receptor is different at each. The TCR β-chain is rearranged during the early CD4−8− or “double negative” stage of thymocyte development, specifically at the DN3 stage. The β-chain associates with a surrogate α-chain or pre-Tα, to form the pre-TCR. Interactions at the cell-surface between pre-TCR molecules [1] result in signaling that leads to massive expansion of cell numbers and differentiation to the CD4+8+ (double positive, DP) stage. At this point, the V and J regions of the TCR α-chain rearrange to each other. This rearrangement continues sequentially and on both chromosomes, until a TCR α-chain is formed that can associate with the already-formed β-chain to make a complete mature TCR and recognize self MHC-peptide well enough to transduce a signal. This is the “positive selection” checkpoint. Cells that do not receive this positive selection signal eventually die through lack of stimulation, whereas those cells whose TCR binds too strongly to self pMHC undergo activation induced apoptosis–referred to as negative selection [2, 3].
Distinguishing positive from negative selection
The distinction between positive and negative selection during T cell development in the thymus is crucial to self-tolerance and therefore to the avoidance of autoimmunity [2, 3]. When self antigens are not presented to developing thymocytes to induce negative selection, for example due to a mutant Aire gene, then autoimmunity will develop [4].
a) Signal strength and kinetics
The strength of interaction between the TCR and thymus-expressed MHC-peptide complexes is a crucial parameter of selection; ligands that strongly activate the thymocytes cause negative selection by activation-induced cell death, whereas weaker ligands cause partial activation leading to positive selection [5, 6]. Experiments with TCR transgenic mice showed that strongly activating, negative-selecting ligands tend to be strong agonists for mature T cells. Positive selecting ligands are often antagonists or weak agonists [5, 7]. The agonist MHC-peptide complexes have relatively high affinity and/or a longer half-life of binding compared to the antagonists [8–14]. Weak agonists can induce a different type of positive selection, where the TCR signaling is attenuated by downregulation of the coreceptor. This is most clearly shown in MHC class I-restricted development, where the CD8β gene is downregulated in the DP cells, resulting in the development of CD8αα-expressing SP cells [15–17]. This agonist type of thymic selection can alternatively be considered as lineage deviation induced by a negatively selecting ligand.
The TCR is extremely discriminating in its responses to MHC-peptide ligands–a clear threshold between positive and negative selection has been defined, where the affinity difference between the weakest ligand that induces negative selection is barely higher than one that induces positive selection [18, 19]. The strength and kinetics of both Ca2+ [20–22] and Erk [18, 23–25] signaling is extremely important in positive versus negative selection. However, the mechanism by which these signals are regulated by TCR signal strength are not understood. One recent finding is that negative-selecting ligands induce Erk phosphorylation at the plasma membrane at the site of TCR stimulation [18, 26], whereas positive-selecting ligands induce p-Erk in a cytoplasmic location, possibly at the Golgi [18].
b) Proximal signaling
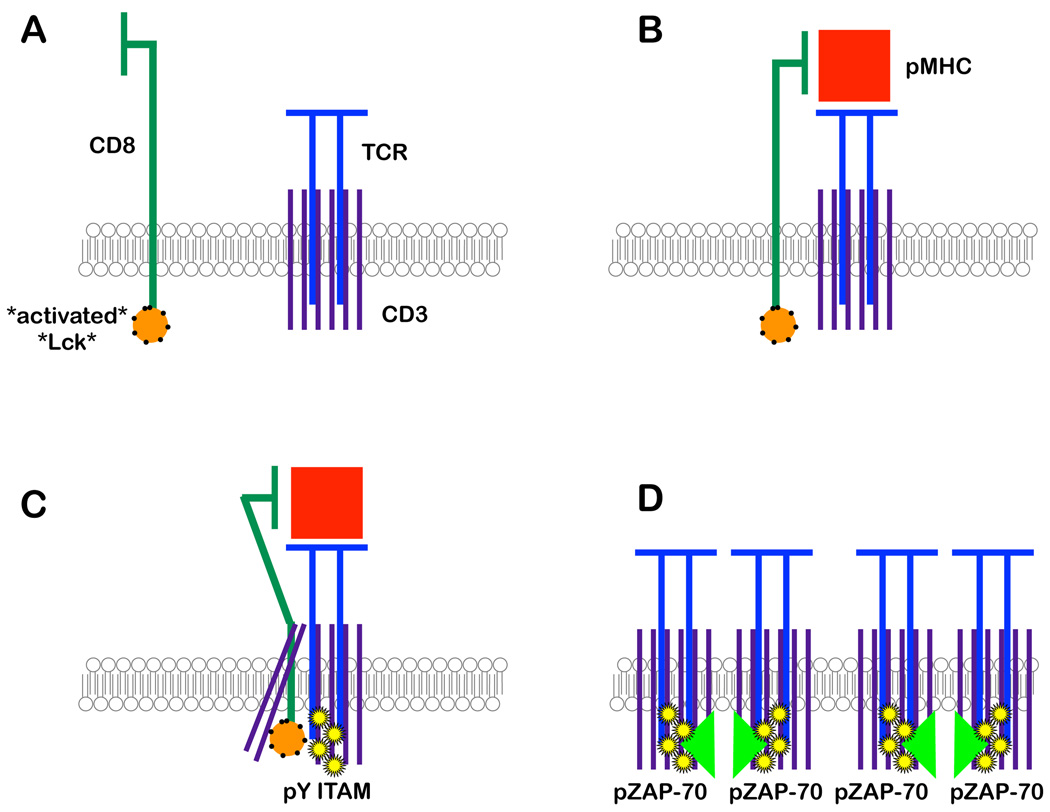
Recent work from O. Acuto’s lab has shown that up to 40% of the Lck in resting T cells and thymocytes is constitutively activated [27]. The sustained presence of the kinase in its active form requires trans-phosphorylation to maintain a high level of phosphorylated Y394 and binding to HSP90-CDC37 to prevent Lck’s degradation. The presence of a large pool of active Lck allows T cells and thymocytes to exist in “standby mode”. The authors suggest that TCR engagement by MHCp may induce changes in the conformation of the CD3 subunits or in the lipid environment around the TCR to allow the Lck carried by a co-engaged CD8 molecule access to the ITAMs of the CD3 chains. The presence of a large pre-existing pool of active Lck ensures the rapid phosphorylation and activation of an antigen bound TCR [27] (Fig. 1).
Figure 1.
Prior to antigen encounter, the CD8 co-receptor carries a pre-activated Lck molecule, placing the thymocyte in “stand-by” mode (A), ready to initiate a signal once cognate antigen co-engages CD8 and the TCR (B). If the pMHC co-engages TCR and CD8 for a sufficiently long time, a “zippered” structure is formed between the membrane proximal domains of CD8β and the TCR’s α chain, which brings the pre-activated Lck into the CD3 complex, leading to high level phosphorylation of the ζ chain ITAMs (C). Once enough ζ-phosphorylated TCRs are formed, they are able to bind ZAP-70. A second round of Lck phosphorylation is responsible for phosphorylating ZAP-70 at Y319, but the phosphorylation at Y493 is brought about by ZAP-70 trans-phosphorylation (D).
A digital signaling response is reflected not only in signaling through the Ras/Map kinase pathway, but also upstream at more TCR-proximal signaling events in response to the linear changes of TCR-MHCp binding strength. For example, when the authors’ labs looked at the induction of a close interaction between the TCR and the CD8 co-receptor using Foerster resonance energy transfer (FRET) microscopy, we found that negative selecting ligands induced a FRET signal that accumulated faster, but was more short-lived, than the signal induced by positive selecting ligands [26, 28]. Lck, the kinase associated with CD8, is now known to be in its active configuration in thymocytes [27]. Therefore, the digital kinetics of the TCR-CD8 interaction results in differential kinetics of phosphorylation of TCR by Lck in response to positive- or negative-selecting ligands. Based on one of our (EP) lab’s evidence that several different TCRs use the same TCR-pMHC affinity threshold to initiate negative selection [19], EP and D. Naeher [29] propose a mechanism for how the TCR and co-receptor read ligand affinity. They argue that for a self-antigen to induce negative selection, it must co-ligate TCR and CD8 for a minimum time. During this time, TCR and CD8 align through a zipper mechanism, which relies on the membrane proximal domains of the TCR α chain and CD8β (Fig. 1). This allows the Lck attached to the CD8α chain access to CD3’s ITAMs, enabling full ζ chain phosphorylation. Self-antigens, which reside for less than the minimum dwell time cannot “close” the CD8/TCR zipper, resulting in incomplete ζ chain phosphorylation and positive selection.
Recent data from EP’s group show that the amount of phosphorylated ZAP-70 recruited by phosphorylated TCR is responsible for the distinction between positive and negative selection [30]. While phosphorylation of ZAP-70’s Y319 is mediated by Lck, Y493 phosphorylation is clearly dependent on ZAP-70 to ZAP-70 trans-phosphorylation. The ability of negative selecting ligands to recruit three times more ZAP-70 to the synapse may promote the trans-phosphorylation of Y493, which is essential for enhanced ZAP-70 enzymatic activity.
c) Endogenous non-stimulatory peptides in recognition of antigen and negative selection stimuli
When a T cell recognizes antigenic MHCp in vivo, it does so under conditions where the antigenic MHCp is a tiny minority of the MHCp complexes offered by the APC. Typically a single antigenic MHCp complex is not enough to stimulate a T cell, but in the presence of other–not overtly stimulatory–endogenous MHCp, activation can occur [31, 32]. The exact mechanism by which this works is still somewhat obscure, but it may be because of a weak interaction between the TCR and the “co-agonist” endogenous MHCp [31], or because of the non-cognate interaction between coreceptor and endogenous MHC [32, 33], or a combination (reviewed in [34]). It is notable that work indicating the dominance of the TCR interaction with the endogenous MHCp came from MHC-class II-restricted T cells (i.e. requiring CD4 as the coreceptor) [31], In contrast, initial experiments from CD8-dependent MHC class I-restricted cells did not identify any TCR specificity for endogenous MHCp, leading to the suggestion that the CD8-class I interaction was more important [32, 33]. In this regard, the affinity of CD8 for class I is significantly higher than that of CD4 for class II, and whilst CD8 can stabilize a TCR-MHCp interaction, there is no evidence that CD4 can do the same. Indeed, recent work indicates that the role of the coreceptors has much more to do with bringing Lck to the proximity of the TCR than it is to stabilizing the TCR-MHCp interaction, even for CD8 [35].
Recent data from M. Davis’ lab shows that class I MHC restricted TCRs can utilize endogenous MHCp to aid recognition of negative selecting ligands by class I-restricted cells. Using dimers of MHC class I molecules, they demonstrated that very low affinity interactions occur between TCR and most endogenous MHC class I-peptide complexes; at this very low affinity, there was effectively no discrimination between different endogenous MHCp for their ability to act as co-agonists [36]. Thus, a response to negative selecting peptide could be induced in thymocytes provided that some endogenous MHC class I-peptide was available [33, 36]. These results support the idea that TCRs can be functionally cross-linked–resulting in signaling–when recognition of a single antigenic MHCp is accompanied by even very weak interactions between TCR and endogenous MHCp. However, positive selection signaling requires cross-linking of TCRs by intermediate-affinity ligands. Therefore, positive selection may be more stringent in its requirements for the TCR-MHCp interaction than is negative selection. These studies did not address the role of the coreceptor in recognition of endogenous MHCp [36]. Recently, NG’s group has used single-chain class I-peptide molecules to distinguish between [non-cognate CD8-endogenous MHCp] and [TCR-endogenous MHCp] interactions during antigen recognition. While an interaction between TCR and non-stimulatory class I MHCp was observed, its impact was small compared to the influence of the non-cognate CD8-MHC interaction (J.A. Hoerter, J. Brzostek, NRJG, in prep.).
Themis
_TH_ymocyte _E_xpressed _M_olecule _I_nvolved in _S_election (Themis) was recently described as a novel gene and protein that is required for passage through the positive selection checkpoint [37–41]. Themis is expressed in a tightly regulated manner during T cell development, in late DN and especially in DP thymocytes. It is down-regulated after positive selection, but is also expressed at low amounts in mature T cells. Themis is tyrosine phosphorylated within seconds after TCR stimulation in both mouse [37, 39] and human T cells [37, 42], suggesting that it may play a role early in signal transduction through the TCR. It is a member of a small gene family of unknown function, and it is highly conserved among vertebrates [37–39]. It has no classical conserved domains other than a polyproline region and a potential bipartite nuclear localization sequence. However, a pair of novel “CABIT” domains–of unknown function–were identified using bioinformatics [38]. CABIT domains are present in invertebrates; the Drosophila ortholog being Serrano [38, 43]. Studies with knockouts or chemically induced point mutant mice showed that Themis plays an important T-cell-intrinsic role in regulating thymocyte development through the positive selection checkpoint [37–41].
Other aspects of Themis’ biology are more contentious. There is some evidence for a slight defect in negative selection in Themis-deficient mice [37, 39], but this was not found in all of the studies [38]. Although any effect on negative selection is certainly much less striking than the effect on positive selection, there is no agreement how Themis’ exerts its’ effect on positive selection. Most investigators have so far failed to identify a defect in TCR signaling in Themis-deficient thymocytes [38–40]. However, studies from one of our labs (NG) found a subtle change in the strength and kinetics of Ca2+ influx and phosphorylation of Erk [37]. Recently, O. Acuto’s lab have confirmed this p-Erk difference in _THEMIS_-knockdown Jurkat cells [42]. As described above, small differences in p-Erk [18, 23–25] and Ca2+ signaling [20–22] in pre-selection thymocytes can distinguish positive from negative selecting TCR signals. Thus, these signaling differences between Themis sufficient and deficient pre-selection thymocytes suggest that Themis likely contributes to setting the signaling threshold for thymocyte responses to self-antigens [37].
Themis interactions with molecules in the TCR signaling cascade include interactions with the adaptor Grb2 [38–40], the tyrosine kinase Itk [37], and phospholipase C (PLCγ1) [37, 42]. Recent data also indicate that Themis interacts with LAT, likely through Grb2 [42]. There is some evidence that interactions with Grb2 and Itk are increased by TCR activation [37, 39], but at this stage there is no clear idea of where and how Themis fits into the signaling pathways downstream of TCR–except that it is crucial to positive selection signaling. A recent study using a conditional mutant of Grb2, where deletion was induced from the thymic DN3 stage on by the Lck-Cre transgene, showed a strong influence of Grb2 on positive selection [44]. Lack of Grb2 resulted in much weaker than normal phosphorylation of TCR-proximal signaling molecules, including CD3ζ, LAT and PLCγ1. These data suggest that both Themis and Grb2 are involved in TCR-proximal signaling events, so that their interaction is likely important in determining the cell fate decisions occurring during thymic selection.
Lineage commitment
To examine CD4/CD8 lineage commitment, B. Seddon’s group used at tet-inducible ZAP-70 transgene to induce the selection of SP thymocytes in ZAP-70 knockout mice [45]. While CD4 SP cells appeared in 30 hrs, the development of CD8 SPs was delayed until 4 days. This was likely related to the requirement for higher levels of ZAP-70 to promote the positive selection of cells into the CD8 lineage. This was supported by studies of DP thymocytes in unmanipulated mice, which showed that CD8 SP thymocytes are descended from DPs with higher levels of ZAP-70. Precisely why DPs destined for the CD8 lineage require more ZAP-70 to support their positive selection is not yet clear.
An interesting finding has recently come from A. Singer’s group, whose work over the last several years has supported a kinetic signaling model [46] to explain CD4/CD8 lineage commitment. Once a DP thymocyte receives a selecting signal, it downregulates CD8 but not CD4. If the thymocyte’s TCR recognizes class II MHC, then signaling persists, while if it recognizes class I MHC then signaling through the TCR is terminated. This difference in the length of signaling is a key parameter in determining differentiation into the correct T cell lineage. An open question concerning the selection of CD8 SP thymocytes is what signals take over once TCR signaling is terminated following CD8 downregulation. Singer’s group has shown that DP thymocytes genetically engineered to become IL-7 responsive develop into Runx positive, CD8 SP cells, even when TCR signaling is blocked. Their data support the idea that a short period of TCR signaling induces cytokine responsiveness in DP thymocytes and that subsequent signaling by intrathymic cytokines (IL-7) specifies CD8 lineage choice [47].
Conclusions
Signaling in thymocytes continues to be an active area of research. Despite the recent progress, many aspects of the multiple selection steps required to generate an MHC restricted and self tolerant T cell repertoire are not yet fully explained. The fact that a completely new molecule (Themis) with an important influence on positive selection could be discovered at this apparently mature stage of the field is itself remarkable. As Themis’ role is still unclear, it leaves open the possibility that new biochemical pathways important in thymocyte development are still to be uncovered.
Acknowledgement
Supported by NIH grants AI074074, AI073870 and GM065230 and grants from the Swiss National Science Foundation, the European Research Council (TerraIncognita) and EU FP7 (Sybilla). This is manuscript #21038 from TSRI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pang SS, Berry R, Chen Z, Kjer-Nielsen L, Perugini MA, King GF, Wang C, Chew SH, La Gruta NL, Williams NK, et al. The structural basis for autonomous dimerization of the pre-T-cell antigen receptor. Nature. 2010;467:844–888. doi: 10.1038/nature09448. [DOI] [PubMed] [Google Scholar]
- 2.Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- 3.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 4.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 5.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 6.Jameson SC, Hogquist KA, Bevan MJ. Positive selection of thymocytes. Annu. Rev. Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- 7.Sebzda E, Kündig TM, Thomson CT, Aoki K, Mak SY, Mayer JP, Zamborelli T, Nathenson SG, Ohashi PS. Mature T cell reactivity altered by peptide agonist that induces positive selection. J. Exp. Med. 1996;183:1093–1104. doi: 10.1084/jem.183.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam SM, Travers PJ, Wung JL, Nasholds W, Redpath S, Jameson SC, Gascoigne NRJ. T cell receptor affinity and thymocyte positive selection. Nature. 1996;381:616–620. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 9.Alam SM, Davies GM, Lin CM, Zal T, Nasholds W, Jameson SC, Hogquist KA, Gascoigne NRJ, Travers PJ. Qualitative and quantitative differences in T cell receptor binding of agonist and antagonist ligands. Immunity. 1999;10:227–237. doi: 10.1016/s1074-7613(00)80023-0. [DOI] [PubMed] [Google Scholar]
- 10.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by αβ T cell receptors. Annu. Rev. Immunol. 1998;16:523–534. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 11.Rosette C, Werlen G, Daniels MA, Holman PO, Alam SM, Travers PJ, Gascoigne NRJ, Palmer E, Jameson SC. The impact of duration versus extent of TCR occupancy on T cell activation: a revision of the kinetic proofreading model. Immunity. 2001;15:59–70. doi: 10.1016/s1074-7613(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 12.Gascoigne NRJ, Zal T, Alam SM. T-cell receptor binding kinetics in T-cell development and activation. Exp. Rev. Mol. Med. 2001 Feb 12;2001:1–17. doi: 10.1017/S1462399401002502. ( http://www.expertreviews.org/01002502h.htm). [DOI] [PubMed] [Google Scholar]
- 13.Holmberg K, Mariathasan S, Ohteki T, Ohashi PS, Gascoigne NRJ. TCR binding kinetics measured with MHC class I tetramers reveal a positive selecting peptide with relatively high affinity for TCR. J. Immunol. 2003;171:2427–2434. doi: 10.4049/jimmunol.171.5.2427. [DOI] [PubMed] [Google Scholar]
- 14.Gronski MA, Boulter JM, Moskophidis D, Nguyen LT, Holmberg K, Elford AR, Deenick EK, Kim HO, Penninger JM, Odermatt B, et al. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10:1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 15.Hogquist KA, Jameson SC, Bevan MJ. Strong agonist ligands for the T cell receptor do not mediate positive selection of functional CD8+ T cells. Immunity. 1995;3:79–86. doi: 10.1016/1074-7613(95)90160-4. [DOI] [PubMed] [Google Scholar]
- 16.Leishman AJ, Gapin L, Capone M, Palmer E, MacDonald HR, Kronenberg M, Cheroutre H. Precursors of functional MHC class I- or class II-restricted CD8αα+ T cells are positively selected in the thymus by agonist self-peptides. Immunity. 2002;16:355–364. doi: 10.1016/s1074-7613(02)00284-4. [DOI] [PubMed] [Google Scholar]
- 17.Yamagata T, Mathis D, Benoist C. Self-reactivity in thymic double-positive cells commits cells to a CD8αα lineage with characteristics of innate immune cells. Nat Immunol. 2004;5:597–605. doi: 10.1038/ni1070. [DOI] [PubMed] [Google Scholar]
- 18.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NRJ, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 19.Naeher D, Daniels MA, Hausmann B, Guillaume P, Luescher I, Palmer E. A constant affinity threshold for T cell tolerance. J Exp Med. 2007;204:2553–2559. doi: 10.1084/jem.20070254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kane LP, Hedrick SM. A role for calcium influx in setting the threshold for CD4+CD8+ thymocyte negative selection. J. Immunol. 1996;156:4594–4601. [PubMed] [Google Scholar]
- 21.Freedman BD, Liu QH, Somersan S, Kotlikoff MI, Punt JA. Receptor avidity and costimulation specify the intracellular Ca2+ signaling pattern in CD4(+)CD8(+) thymocytes. J Exp Med. 1999;190:943–952. doi: 10.1084/jem.190.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davey GM, Schober SL, Endrizzi BT, A.K. D, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J.Exp.Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mariathasan S, Zakarian A, Bouchard D, Michie AM, Zuniga-Pflucker JC, Ohashi PS. Duration and strength of extracellular signal-regulated kinase signals are altered during positive versus negative thymocyte selection. J Immunol. 2001;167:4966–4973. doi: 10.4049/jimmunol.167.9.4966. [DOI] [PubMed] [Google Scholar]
- 24.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 25.McNeil LK, Starr TK, Hogquist KA. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci U S A. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yachi PP, Ampudia J, Zal T, Gascoigne NRJ. Altered peptide ligands induce delayed and reduced CD8-TCR interaction - a role for CD8 in distinguishing antigen quality. Immunity. 2006;25:203–211. doi: 10.1016/j.immuni.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011.. This paper shows that a large proportion of Lck in resting T cells and thymocytes is pre-activated. This was surprising and counter to the received wisdom of the time.
- 28.Mallaun M, Naeher D, Daniels MA, Yachi PP, Hausmann B, Luescher IF, Gascoigne NRJ, Palmer E. The T cell receptor's α-chain connecting peptide motif promotes close approximation of the CD8 coreceptor allowing efficient signal initiation. J Immunol. 2008;180:8211–8221. doi: 10.4049/jimmunol.180.12.8211.. This paper showed that the recruitment of CD8 coreceptor to the TCR, as measured by FRET microscopy, is kinetically different for recognition of positive and negative selecting ligands. The positive selectors showed a slower induction of the interaction, compared to the negative selectors.
- 29.Palmer E, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat Rev Immunol. 2009;9:207–213. doi: 10.1038/nri2469.. This paper draws together many observations to formulate a mechanism how the TCR measures pMHC affinity and initiates negative selection.
- 30.Mallaun M, Zenke G, Palmer E. A discrete affinity-driven elevation of ZAP-70 kinase activity initiates negative selection. J Recept Signal Transduct Res. 2010 doi: 10.3109/10799893.2010.518151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 32.Yachi PP, Ampudia J, Gascoigne NRJ, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat Immunol. 2005;6:785–792. doi: 10.1038/ni1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yachi PP, Lotz C, Ampudia J, Gascoigne NRJ. T cell activation enhancement by endogenous pMHC acts for both weak and strong agonists but varies with differentiation state. J Exp Med. 2007;204:2747–2757. doi: 10.1084/jem.20062610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gascoigne NRJ. Do T cells need endogenous peptides for activation? Nat Rev Immunol. 2008;8:895–900. doi: 10.1038/nri2431. [DOI] [PubMed] [Google Scholar]
- 35.Artyomov MN, Lis M, Devadas S, Davis MM, Chakraborty AK. CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1010568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juang J, Ebert PJ, Feng D, Garcia KC, Krogsgaard M, Davis MM. Peptide-MHC heterodimers show that thymic positive selection requires a more restricted set of self-peptides than negative selection. J Exp Med. 2010;207:1223–1234. doi: 10.1084/jem.20092170.. This paper demonstrates that virtually any endogenous MHC class I-peptide can have a sufficiently strong interaction with TCR to allow it to act as a co-agonist for a negative-selecting peptide. This was in marked contrast to previous data with class II-restricted T cells. Positive selecting peptides, presumably because they are already lower affinity than negative selectors, could not make use of these very low affinity non-stimualtory peptides.
- 37.Fu G, Vallee S, Rybakin V, McGuire MV, Ampudia J, Brockmeyer C, Salek M, Fallen PR, Hoerter JAH, Munshi A, et al. Themis controls thymocyte selection through regulation of T cell antigen receptor-mediated signaling. Nat Immunol. 2009;10:848–856. doi: 10.1038/ni.1766.. Along with refs 38–41, his paper identified Themis as an important actor in positive selection.
- 38.Johnson AL, Aravind L, Shulzhenko N, Morgun A, Choi SY, Crockford TL, Lambe T, Domaschenz H, Kucharska EM, Zheng L, et al. Themis is a member of a new metazoan gene family and is required for the completion of thymocyte positive selection. Nat Immunol. 2009;10:831–839. doi: 10.1038/ni.1769..
- 39.Lesourne R, Uehara S, Lee J, Song KD, Li L, Pinkhasov J, Zhang Y, Weng NP, Wildt KF, Wang L, et al. Themis, a T cell-specific protein important for late thymocyte development. Nat Immunol. 2009;10:840–847. doi: 10.1038/ni.1768..
- 40.Patrick MS, Oda H, Hayakawa K, Sato Y, Eshima K, Kirikae T, Iemura S, Shirai M, Abe T, Natsume T, et al. Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. Proc Natl Acad Sci U S A. 2009;106:16345–16350. doi: 10.1073/pnas.0908593106..
- 41.Kakugawa K, Yasuda T, Miura I, Kobayashi A, Fukiage H, Satoh R, Matsuda M, Koseki H, Wakana S, Kawamoto H, et al. A novel gene essential for the development of single positive thymocytes. Mol Cell Biol. 2009;29:5128–5135. doi: 10.1128/MCB.00793-09..
- 42.Brockmeyer C, Paster W, Pepper D, Tan CP, Trudgian DC, McGowan S, Fu G, Gascoigne NRJ, Acuto O, Salek M. Quantitative kinetics of TCR-induced tyrosine phosphorylation networks identifies THEMIS as a new component of the TCR signalosome. J Biol Chem. 2010 doi: 10.1074/jbc.M110.201236. In press. This work identified a large number of proteins that are tyrosine-phosphorylated after TCR stimulation, and grouped them by their different kinetic profiles. One of the early PTK targets is Themis. They further show that it is part of the TCR signalosome.
- 43.Chung S, Vining MS, Bradley PL, Chan CC, Wharton KA, Jr, Andrew DJ. Serrano (sano) functions with the planar cell polarity genes to control tracheal tube length. PLoS Genet. 2009;5:e1000746. doi: 10.1371/journal.pgen.1000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jang IK, Zhang J, Chiang YJ, Kole HK, Cronshaw DG, Zou Y, Gu H. Grb2 functions at the top of the T-cell antigen receptor-induced tyrosine kinase cascade to control thymic selection. Proc Natl Acad Sci U S A. 2010;107:10620–10625. doi: 10.1073/pnas.0905039107.. In contrast to previous work, which showed that Grb-2 was involved in negative but not positive selection, this paper shows that Grb-2 is important in both types of thymic selection.
- 45.Saini M, Sinclair C, Marshall D, Tolaini M, Sakaguchi S, Seddon B. Regulation of Zap70 expression during thymocyte development enables temporal separation of CD4 and CD8 repertoire selection at different signaling thresholds. Sci Signal. 2010;3:ra23. doi: 10.1126/scisignal.2000702.. This paper shows that positive selection of CD8 lineage thymocytes requires DP thymocytes expressing a higher amount of ZAP-70.
- 46.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park J-H, Adoro S, Guinter T, Erman B, Alag A, Catalfamo M, Kimura MY, Cui Y, Lucas PJ, Gress RE, et al. Signaling by intrathymic cytokines, not T cell receptors, specifies CD8-lineage choice and promotes differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010 doi: 10.1038/ni.1840.. This work shows that a short period of TCR signaling induces cytokine responsiveness in DP thymocytes and that subsequent signaling by intrathymic cytokines (IL-7) specifies CD8 lineage choice