Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation (original) (raw)
Abstract
Neutrophils play a major role in the innate immune system and are normally considered to be short-lived effector cells that exert anti-microbial activity and sometimes immunopathology. Here, we show that these cells possess an additional function as professional antigen-presenting cells capable of priming a Th1- and Th17-acquired immune response. Using flow cytometry, fluorescence microscopy and western blotting, we show that mouse neutrophils express MHC class II and co-stimulatory molecules CD80 and CD86 after T-cell co-incubation. Neutrophils pulsed with ovalbumin (OVA) process and present peptide antigen to OVA-specific T cells in an MHC class II-dependent manner. Importantly, we demonstrate that neutrophils can prime antigen-specific Th1 and Th17 immune responses even without the addition of exogenous cytokines to cell cultures.
Keywords: antigen processing, interleukin 17, MHC class II molecule, polymorphonuclear leukocyte
Introduction
Neutrophils are well known to rapidly and in large number accumulate at sites of infection and inflammation. The classical view of these cells is that they function as kamikazi pilots, following chemokine gradients to target sites where they kill microbes, simultaneously causing collateral tissue damage through discharge of toxic mediators that also result in their own death. Subsequent phagocytosis of apoptotic neutrophils is thought to allow their immunologically silent removal. Thus, neutrophils are often dismissed as short-term effectors that have no lasting impact on the ensuing adaptive immune response to infection (1).
However, there is mounting evidence that polymorphonuclear leukocytes (PMN) may play more complex and nuanced roles in immunoregulation. Although the lifespan of neutrophils in the blood is only 8–12 h, proinflammatory mediators prevent onset of apoptosis, increasing their longevity greatly (2). It is also clear that neutrophils produce a large number of cytokines and chemokines during infection (3, 4). While on a per cell basis, the amount of any given neutrophil-derived cytokine or chemokine is often less than that produced by dendritic cells (DC) or macrophages, the sheer number of PMN (they are the most common leukocyte) suggests that on a population basis, neutrophils can contribute significantly to the cytokine response during infection. There is evidence that neutrophils differentiate into distinct subsets based upon Toll-like receptor expression and reciprocal secretion of IL-12 and IL-10 (5, 6). The ability of neutrophils to produce immunoregulatory cytokines and chemoattractants has led some investigators, including us, to propose that these cells play a role in recruitment and activation of DC during infection (7, 8).
Another immunoregulatory aspect of neutrophil function comes from evidence that these cells may be induced to express MHC class II molecules (9). While PMN from healthy donors do not express MHC class II glycoproteins, cytokines such as IFN-γ, granulocyte-monocyte colony-stimulatory factor (GM-CSF), and IL-3 can stimulate up-regulation (10–12). Interestingly, at least two of these mediators (IFN-γ and GM-CSF) also increase neutrophil survival, suggesting that prolonged contact between neutrophils and T cells is possible (13–15). It has also been reported that neutrophils can express co-stimulatory molecules CD80 and CD86, as well as the DC marker CD83. This has led some to propose that PMN are able to transdifferentiate into DC-like cells under appropriate stimulatory conditions (16–18). There is evidence that human neutrophils mediate proliferation of T cells in response to superantigen (11, 19), and it was recently shown that mouse neutrophils can stimulate ovalbumin (OVA)-specific T-cell proliferation (20). However, whether these responses truly reflect an ability to process and present antigen has been less clear.
Here, we show for the first time unequivocal evidence that neutrophils function as bona fide professional antigen-presenting cells (APCs), inducibly expressing MHC class II and co-stimulatory molecules and stimulating MHC class II-dependent proliferation of OVA-specific T cells after pulsing with antigenic peptide or intact OVA. Most importantly, we show that antigen-pulsed neutrophils are potent inducers of Th1 and Th17 differentiation in vitro independent of any exogenous cytokine addition. Together, our results demonstrate that neutrophils function as a new type of professional APC specializing in driving generation of proinflammatory T-cell effectors.
Methods
Mice
Female C57BL/6 and BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) or Taconic Farms (Germantown, NY, USA) and used at 6–8 weeks of age. MHC class II knockout mice on a C57BL/6 background (B6.129-H2-Ab1tm1Gru N12) were purchased from Taconic Farms. OT-II transgenic mice expressing an OVA-specific T-cell receptor on a Rag-1−/− C57BL/6 background [C57BL/6-Rag2tm1Fwa Tg(TcraTcrb)425Cbn] were obtained from Taconic Farms. All mice were maintained in the Transgenic Mouse Core Facility at the Cornell University College of Veterinary Medicine, which is accredited by the American Association for Accreditation of Laboratory Animal Care.
Percoll gradient purification of neutrophils
Mice were intraperitoneally (i.p.) injected with 0.5 ml of 10% thioglycollate (Becton Dickinson, Franklin Lakes, NJ, USA) and 18–20 h later, peritoneal exudate cells (PECs) were collected by washing the peritoneal cavity with ice-cold PBS (Cellgro, Manassas, VA, USA). After washing cells twice with PBS, neutrophils were purified by continuous Percoll gradient centrifugation as described elsewhere (21). Briefly, Percoll (GE Healthcare, Fairfield, CA, USA), adjusted to pH 7.4, was mixed at a ratio of 1: 9 with PEC re-suspended in PBS. The mixture was then transferred to a 10 ml polycarbonate centrifuge bottle and ultracentrifugation was performed at 60 000 × g for 65 min at 4°C using a 50 Ti rotor (Beckman Centrifuges, Brea, CA, USA). The layer enriched for neutrophils was collected using a gel-loading pipette tip. Neutrophils were subsequently washed twice with PBS and re-suspended in complete DMEM (cDMEM), consisting of 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, 30 mM HEPES (all purchased from Invitrogen Life Technologies, Carlsbad, CA, USA), 10% bovine growth serum (Hyclone, Logan, UT, USA) and 0.05 mM β-mercaptoethanol in DMEM. Neutrophil preparations were consistently >95% pure, as determined by flow cytometry and differential staining.
Bone marrow-derived DC
Bone marrow derived DC were prepared from flushed femur bone marrow cells. Briefly, bone marrow cells were suspended in RPMI (Cellgro, Manassas, VA, USA) supplemented with 10% FCS (Hyclone), 100 U ml−1 penicillin (Gibco, Carlsbad, CA, USA), 100 μg ml−1 streptomycin (Gibco) as well as GM-CSF (20 ng ml−1; Peprotech, Rocky Hill, NJ, USA). Cells were fed 3 and 6 days after culture initiation with fresh medium and cells were collected on day 9 for use in experiments.
Magnetic cell sorting of CD4 T cells
Splenocyte single-cell suspensions were obtained by gently mashing spleens and passing them through a 70-μm filter (BD Falcon, Franklin Lakes, NJ, USA) followed by erythrocyte lysis with red blood cell lysis buffer (Sigma–Aldrich, St Louis, MO, USA). Cells were centrifuged at 300 × g for 10 min at 4°C. The pellet was re-suspended in MACS buffer (PBS, 0.5% BSA and 2 nM EDTA) and MACS anti-CD4 magnetic beads (Miltenyi Biotec, Auburn, CA, USA) were added. The sample was mixed and incubated for 15 min at 4°C. Cells were washed using MACS buffer and centrifuged at 300 × g for 10 min. The cell pellet was re-suspended in MACS buffer and CD4-positive and -negative fractions were separated using an AutoMACS Separator.
Flow cytometry
Single-cell suspensions were incubated for 30 min at 4°C with FACS buffer (PBS, 1% bovine growth serum and 0.01% NaN3) containing 10% normal mouse serum to block Fc receptor binding. Samples were centrifuged and pellets were re-suspended in FACS buffer containing fluorochrome-conjugated antibody for 30 min at 4°C. The antibody used in this study were anti-Gr-1 FITC (BD Biosciences, San Jose, CA, USA); PE-conjugated anti-Ly6G (BD Biosciences), PE-conjugated anti-CD80, PE-conjugated anti-CD86 (BD Biosciences), PE-conjugated anti-MHC class II and anti-CD4 conjugated to allophycocyanin. Antibodies were purchased from eBioscience (San Diego, CA, USA) unless indicated otherwise. After washing, cells were re-suspended in FACS buffer and collected on a BD FACSCalibur flow cytometer. Data analysis was performed using FlowJo software (Tree Star, Ashland, OR, USA).
Intracellular cytokine staining was performed in neutrophil T-cell cultures following a previously described protocol (22). At day 3, after initiating cultures of OT-II T cells and OVA peptide-pulsed neutrophils, cells were pelleted and new medium supplemented with IL-2 (10 ng ml−1; Peprotech) was added. At day 6, cells were collected, washed and stimulated with phorbol myristate acetate (10 ng ml−1; EMD, Darmstadt, Germany) and ionomycin (1 μg ml−1; Calbiochem, Darmstadt, Germany) for 6 h in the presence of Brefeldin A. Cells were subsequently permeabilized and stained for intracellular cytokines and surface stained for membrane antigens.
Cell sorting
In some experiments, high purity neutrophils were obtained by staining thioglycollate-elicited cells with PE-conjugated antibody specific for Ly6G (1A8; BD Pharmingen, San Diego, CA, USA) for 30 min at 4°C. After extensive washing, cells were re-suspended in PBS with 1% bovine growth serum (Hyclone) and sorted based upon expression of Ly6G using a FACSAria cell sorter (BD Biosciences). In other experiments, CD4+ T cells were isolated by flow cytometric sorting after staining cells with anti-CD4 antibody conjugated to allophycocyanin. The purity of sorted populations was routinely >98%.
5-(and 6-)Carboxyfluorescein diacetate succinimidyl ester staining of CD4 T cells
CD4-positive T cells were stained with 5-(and 6-)carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Carlsbad, CA, USA) according to the manufacturer's instructions. Briefly, cells were re-suspended in PBS with 0.1% BSA and CFSE stock solution was used to yield a final concentration of 10 μM. Cells were incubated at 37°C for 10 min. The staining was quenched by adding five volumes of ice-cold cDMEM followed by incubation on ice for 5 min. CFSE-stained cells were further washed for a total of three washes using cDMEM.
Neutrophil and CD4 T cell co-cultures
Neutrophils, purified by Percoll gradient centrifugation or flow sorting, were either pre-incubated with medium alone, OVA peptide (amino acids 323–339; AnaSpec, Fremont, CA, USA) at a concentration of 2 μg ml−1 or whole OVA antigen (Thermo Scientific, Rockford, IL, USA) at a concentration of 20 μg ml−1 for 4 h at 37°C. After extensive washing in cDMEM, immunomagnetic bead-purified, or in some cases flow sorted, CFSE-labeled OT-ll CD4+ T cells were added to the neutrophils. After 4 days of culture, cells were collected and surface stained for CD4 as described above and CFSE peak dilutions were assayed using flow cytometry. Anti-MHC class II antibody blocking experiments were performed using Ab M5/114 (eBioscience) at a concentration of 5 μg ml−1. Transwell contact inhibition experiments were performed using a 24-well transwell plate (Costar, Lowell, MA, USA). Neutrophils and T cells were co-incubated in direct contact or separated by the transwell membrane with neutrophils in the lower chamber and T cells in the upper chamber. After incubation for 2 h at 37°C, neutrophils were collected and stained for MHC class II expression as described above.
Western blotting
Percoll gradient purified neutrophils, isolated by flow cytometric sorting of Ly6Ghigh cells after incubation with T cells, were lysed using SDS sample reducing buffer and stored at −80°C. Samples were subsequently resolved by SDS–PAGE, and immunoblotted using an anti-MHC class II Aβ-specific antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or stripped and re-probed for anti-GAPDH-specific antibody (Cell Signaling Technology, Danvers, MA, USA) as a loading control. Blots were visualized using an ECL-based detection system (Thermo Scientific).
Cytokine ELISA
IFN-γ in culture supernatants was measured using a commercial kit according to the manufacturer's recommendations (eBioscience). IL-17 was measured using a kit from R&D systems and IL-4 was measured using a kit from BD Biosciences (BDoptEIA).
Immunofluorescence microscopy
Neutrophils before or after T cell co-incubation were centrifuged on to glass cover slips for 5 min at 750 r.p.m. Cover slips were fixed with 3% paraformaldehyde (20 min, room temperature), then blocked with normal mouse serum and FCS-supplemented PBS for an hour at room temperature. Cover slips were incubated with anti-Gr-1 FITC (BD Biosciences); and PE-conjugated anti-MHC class II (eBioscience) for an hour at room temperature. After washing in PBS, cover slips were mounted with ProLong Antifade containing 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes). Images were collected with an Olympus BX51 fluorescence microscope equipped with a DP 70 camera using Olympus DP controller software and Olympus DP manager software.
Statistical analyses
Statistical analyses were performed using the Prism Software. Unpaired _t_-tests were performed to analyze data. P values <0.05 were considered significant.
Results
Isolation of mouse neutrophils
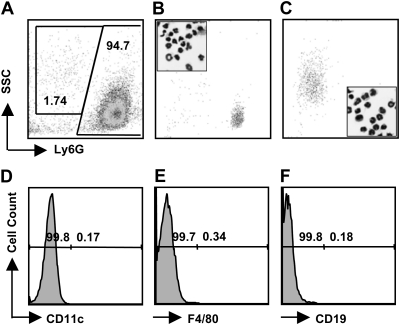
To obtain neutrophils, mice were i.p. injected with 10% thioglycollate and PEC were collected 18–22 h later. The cell population obtained was routinely 60–80% neutrophils with macrophages, mast cells, eosinophils and lymphocytes making up the remaining fraction of cells (data not shown). To purify neutrophils, PEC were centrifuged over a Percoll continuous density gradient consistently yielding a population of cells that were 96–98% positive for the neutrophil-associated marker Ly6G (Fig. 1A). There was routinely <0.5% CD11c+, F4/80+, and CD19+ cells in this population (Fig. 1D–F). The Ly6G+ population could be divided into Ly6Ghigh and Ly6Ginterm cells (94.7 and 1.7%, respectively; Fig. 1A). These two populations were isolated by flow cytometric sorting of Ly6Ghigh (Fig. 1B) and Ly6Ginterm (Fig. 1C) cells. Differential staining of these cells showed that essentially all were neutrophils, as determined by their characteristic multilobed nuclei (Fig. 1B and C, insets). We speculate that the Ly6Ginterm PMN are recent bone marrow emigrants recruited in response to thioglycollate injection because it is known that expression of this marker increases with neutrophil maturation (23).
Fig. 1.
Source of neutrophils and absence of APC populations in purified neutrophils. (A) Expression of Ly6G by thioglycollate-elicited neutrophils isolated by centrifugation over Percoll. The numbers indicate the relative percentage of cells falling within each indicated gate. PMN were sorted into Ly6G high (B) and intermediate (C) expressing populations. Insets in panels B and C show the morphological appearance of the flow-sorted populations. There was minimal cross contamination as determined by staining for DC (CD11c, panel D), macrophages (F4/80, panel E) and B cells (CD19, panel F). Data are representative of at least three independent experiments.
Expression of MHC and costimulatory molecules on mouse neutrophils
We previously reported that mouse neutrophils express the molecule CD80 that is associated with co-stimulation of T lymphocytes during MHC-restricted antigen presentation (Fig. 2F, inset) and (21). Therefore, we asked whether thioglycollate-elicited PMN also express CD86 and MHC class II molecules. We found that freshly isolated cells expressed neither MHC class II molecules (Fig. 2A and B) nor did they express CD86 (Fig. 2E, gray histogram). However, after 2-h co-incubation with purified CD4+ T cells, we detected up-regulation of MHC class II molecules (Fig. 2C and D) as well as increased expression of CD86 (Fig. 2E) on PMN. Levels of CD80 on neutrophils were also slightly up-regulated following T-cell co-incubation (Fig. 2F).
Fig. 2.
Co-incubation with T cells induces expression of MHC class II and co-stimulatory molecules on neutrophils. (A and C) Expression of MHC class II molecules on Ly6C/G (Gr-1)-gated neutrophils without (A) or with (C) 2-h T-cell co-incubation (red histograms). Gray shaded histograms show isotype antibody staining. (B and D) Neutrophils were imaged by fluorescence microscopy after staining with anti-Ly6C/G antibody (green), anti-MHC class II antibody (red). Nuclei were stained with DAPI (blue). MHC class II expression on neutrophils without (B) and with (D) T-cell co-incubation is shown. (E and F) Expression of CD86 (E) and CD80 (F) on Ly6C/G-gated neutrophils without T-cell co-incubation (shaded histogram) relative to expression after T-cell co-incubation (red histogram). The insets in panel E and F show isotype antibody staining (shaded blue histogram) compared with CD86 and CD80 expression on neutrophils with T-cell co-incubation respectively (red histogram). MHC class II and co-stimulatory molecule flow cytometry data are representative of at least four independent experiments. The microscopy data are representative of two independent experiments.
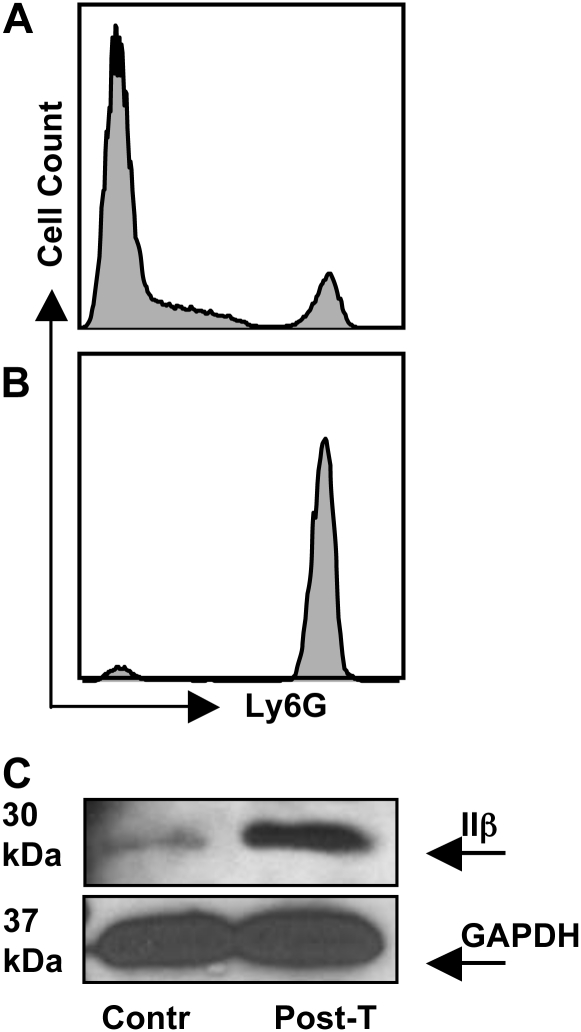
To further substantiate expression of MHC class II molecules on PMN, we co-incubated neutrophils with magnetic bead purified CD4+ T cells (Fig. 3A), then we re-isolated neutrophils 2 h later by cell sorting following labeling with Ly6G-specific antibody (Fig. 3B). Neutrophil cell lysates were subjected to western blot analysis using an MHC class II Aβ-specific antibody. As shown in Fig. 3(C), neutrophils lysed without pre-incubation with T cells (Contr) expressed minimal levels of Aβ chain, consistent with data shown in Fig. 2(A and B). However, supporting the flow cytometry results in Fig. 2, neutrophil lysates prepared after co-incubation with T cells (Post-T) clearly contained increased levels of the MHC class II Aβ molecule (Fig. 3C). This difference in expression is not due to unequal loading of samples as shown by blotting against GAPDH (Fig. 3C).
Fig. 3.
Biochemical evidence for MHC class II expression on neutrophils. (A) Neutrophils and T cells were co-cultured for 2 h at 37°C at a ratio of 1:10, then cells were labeled with Ly6G-specific antibody. Expression of Ly6G before (A, 10% positive) and after (B, 98% positive) cell sorting for neutrophils. (C) Western blot showing MHC class IIβ expression on neutrophils after T-cell co-incubation (Post-T). C also shows GAPDH loading control blotting from the same experiment. Contr, neutrophil lysate prepared without prior T-cell incubation. These data were repeated twice with the same result.
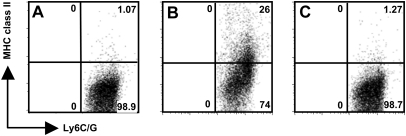
We sought to determine whether T-cell-driven expression of MHC class II on PMN was dependent upon release of soluble factors or direct cell-to-cell contact. To answer this question, neutrophils and T cells were incubated together or separated by a Transwell membrane. Freshly isolated neutrophils incubated alone did not express MHC class II molecules (Fig. 4A). Neutrophils incubated in direct contact with T cells up-regulated expression of MHC class II molecules (Fig. 4B). Strikingly, up-regulation of MHC class II molecules on neutrophils was completely abrogated when the cells were separated by a Transwell membrane (Fig. 4C). We conclude that cell contact is required for T lymphocyte-triggered up-regulation of MHC class II molecules on PMN.
Fig. 4.
Physical contact between neutrophils and T cells is required for up-regulation of MHC class II by neutrophils. (A) Background expression of cell surface MHC class II molecules on Ly6C/G-positive neutrophils prior to incubation with T cells. (B) Expression of MHC class II molecules on the surface of Ly6C/G-positive neutrophils after 2-h co-incubation with T cells. (C) Expression of MHC class II molecules by neutrophils when the T cells and neutrophils are separated by a Transwell membrane. The data are representative of three independent experiments.
Neutrophils process and present antigen to trigger T-cell activation
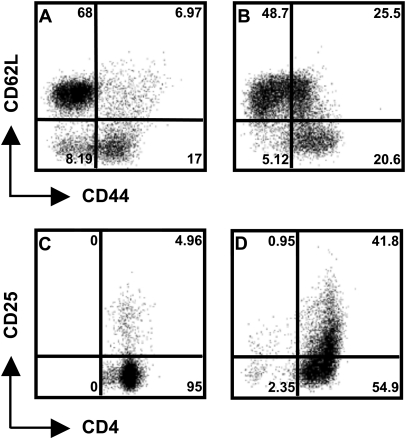
We next tested whether neutrophils expressing MHC class II molecules and co-stimulatory molecules were capable of triggering antigen-specific T-cell activation. To address this question, we used CD4+ T lymphocytes from OT-ll mice that recognize OVA323–339 peptide in the context of I-Ad. Percoll gradient-purified neutrophils were incubated with OVA antigen, washed and then added to CFSE-stained OT-ll CD4+ T cells and the cells were cultured for 4 days. PMN survived the 4-day co-culture with T cells, and the relative proportion at day 4 was very similar to the input proportion of T cells to neutrophils (Fig. 5A). In contrast, neutrophils cultured in isolation rapidly lost viability (data not shown). Cells were collected, stained for CD4 and analyzed using flow cytometry to determine CFSE peak dilutions as an indicator of T-cell proliferation. As expected, there was minimal proliferation when T cells were cultured with non-pulsed PMN (Fig. 5B). In striking contrast, neutrophils pre-incubated with native OVA stimulated T-cell proliferation (Fig. 5C). Similarly, pre-incubation of PMN with OVA323–339 peptide also triggered strong T-cell proliferation (Fig. 5D). We asked whether T-cell proliferation depended upon MHC class II expression, as would be predicted for bona fide antigen presentation. As shown in Fig. 5(E), inclusion of a blocking antibody to MHC class II completely abrogated the ability of antigen-pulsed neutrophils to trigger proliferation of OVA-specific T cells. Notably, that the activation status of isolated CD4+ T cells from mice prior to incubation with neutrophils was naive, as evidenced by the expression levels of CD44, CD62L and CD25 (Fig. 6A and C) and that these markers change expression upon activation after antigen presentation by neutrophils (Fig. 6B and D).
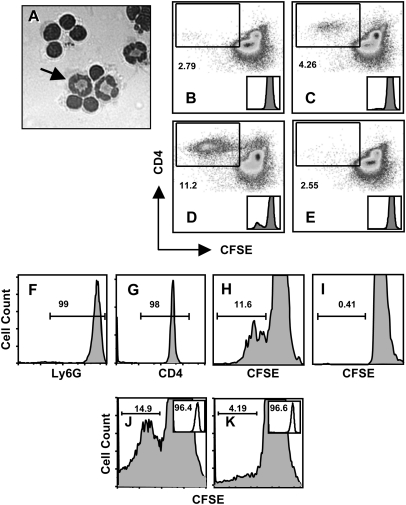
Fig. 5.
Neutrophils process and present antigen to stimulate T-cell proliferation. (A) Neutrophils were pre-incubated with OVA, then cells were added to OVA-specific OT-ll cells at a PMN to T cell ratio of 1: 10. Four days later, cells were examined under the microscope. The arrow points to one of three neutrophils in this image. In panels B–E, the ability of Percoll gradient-isolated neutrophils (97% purity) to stimulate immunomagnetic bead-isolated CD4+ T cell (98% purity) proliferation was examined. (B) Non-pulsed neutrophils induce low levels of OT-II T-cell proliferation in day 4 cultures as measured by CFSE dilution of labeled CD4+ T cells. (C) Proliferation of OVA-specific OT-II T cells after day 4 co-culture with neutrophils pre-incubated (4 h, 37°C) with whole OVA. (D) OT-II T cells proliferation after 4-day culture with OVA peptide-pulsed PMN. (E) Addition of anti-MHC class II blocking antibody (M5/114) prevents OT-II proliferation stimulated by OVA peptide-pulsed neutrophils. Insets in B through E show CFSE peak dilution histograms. Samples in B through E were incubated at a ratio of 10 T-cells for every one neutrophil. In panels F–I, the experiments were reiterated using flow sorted Ly6Ghigh neutrophils and CD4+ OT-II T cells. (F) Neutrophil purity after cell sorting (99% Ly6G positive). (G) Purity of OT-II T cells following cell sorting (98% CD4 positive). (H) Day 4 proliferation of sorted CFSE-labeled OT-II CD4+ T cells after incubation with sorted neutrophils pulsed with OVA peptide. (I) Proliferation of sorted OT-II T cells after incubation with non-pulsed sorted neutrophils. (J) Proliferation of OVA-specific OT-II T cells following incubation with OVA peptide-pulsed neutrophils from MHC class II expressing C57BL/6 mice. (K) Proliferation of CD4+ T cell following 4-day incubation with OVA peptide-pulsed neutrophils from MHC class II-deficient mice. Neutrophil purity, based on Ly6G expression, is shown in the inserts and was 96.4 and 96.6% in J and K, respectively. Samples in (F) through (K) were incubated at a ratio of five T-cells for every one neutrophil. Experiments in (A–D) were performed five times with the same result and the MHC blocking experiment (E) was performed three times with the same result. The cell sorting experiment (F–I) was performed three times and experiments with MHC class II KO cells were performed two times with the same result.
Fig. 6.
OTII CD4+ T-cells exhibit a naive phenotype prior to antigen presentation by neutrophils. Expression of CD44 and CD62L by CD4+ T-cells from a naive spleen of an OTII mouse (A) and expression of these markers after neutrophils antigen presentation in (B). Expression of CD25 on CD4+ T cells before and after neutrophils antigen presentation is shown in (C and D), respectively. The numbers indicate the relative percentage of cells falling within each indicated gate.
Although Percoll gradient-purified PMN and immunomagnetic bead-isolated CD4+ T lymphocytes were routinely >95% pure in repeated experiments, we could not completely exclude the possibility that residual macrophages or DC in the neutrophil preparations were responsible for antigen presentation, or that OVA antigen was acquired by residual APC in the T-cell preparations. Therefore, to further substantiate antigen presentation by neutrophils, we repeated the experiments using high-purity cells generated by flow cytometric cell sorting. The purity of Ly6G+ neutrophils and CD4+ T lymphocytes is shown in Fig. 5(F and G) and was 99 and 98% respectively. Pre-incubation of PMN with OVA323–339 triggered robust proliferation of CFSE-labeled T cells (Fig. 5H), while co-incubation with non-pulsed neutrophils did not induce a T cell response (Fig. 5I).
We further addressed the possibility that residual APC in the T-cell preparation were cross-presenting OVA antigen by determining the requirement for MHC class II molecules on the neutrophil population. As shown in Fig. 5(J), OVA-pulsed PMN generated from control mice stimulated proliferation of CFSE-labeled OVA-specific CD4+ T lymphocytes. However, when MHC class II-deficient PMN were used, levels of T-cell proliferation were reduced (Fig. 5K), with the insets showing purity of the neutrophil populations used as APC. We conclude from these results that neutrophils acquire antigen and stimulate T-cell proliferation in an MHC class II-dependent manner.
Neutrophils induce differentiation of Th1 and Th17 cells
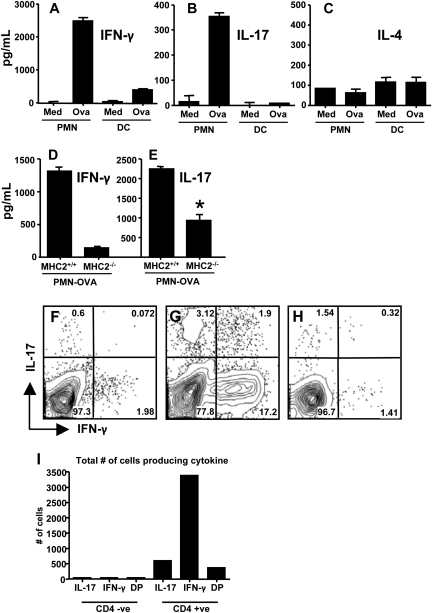
Given that OVA-pulsed PMN triggered antigen-specific T-cell proliferation, we were interested in the type of immune response generated. Therefore, we measured cytokine levels in supernatants from PMN and OVA-specific T cell co-cultures and compared these with responses in co-cultures of T cells and DC. Surprisingly, even without addition of exogenous cytokines, OVA-pulsed, but not control, neutrophils stimulated production of IFN-γ (Fig. 7A) and IL-17 (Fig. 7B). In contrast, parallel cultures of OVA-pulsed DC and T cells displayed minimal IFN-γ and no detectable IL-17. Neither PMN nor DC triggered production of IL-4 in T cell co-cultures (Fig. 7C). We then examined if cytokine production stimulated by OVA-pulsed neutrophils, like the T-cell proliferative response, required PMN expression of MHC class II molecules. As predicted, IFN-γ (Fig. 7D) responses were highly dependent upon neutrophil MHC class II molecule expression. For the case of IL-17 (Fig. 7E), there was a major reduction in cytokine production using MHC class II-negative neutrophils, but some IL-17 production clearly remained. The basis for this residual response is not clear. One possibility is that neutrophils, which are known to sometimes produce IL-17 (24, 25), release the cytokine in response to T cell co-culture. It is also possible that OVA-pulsed PMN produce factors that trigger MHC class II-independent T-cell IL-17 production.
Fig. 7.
OVA-pulsed neutrophils drive differentiation of Th1 and Th17 cells in vitro. (A–C) PMN and bone marrow-derived DC were pulsed with OVA peptide or medium alone (Med) then cultured with OVA-specific T cells. At day 4 after culture initiation, supernatants were collected and assayed for IFN-γ (A), IL-17 (B) and IL-4 (C). In (D and E) similar cultures were initiated using neutrophils from wild-type and MHC class II knockout mice. Day 4 supernatants were tested for IFN-γ (D) and IL-17 (E, where * indicates P <0.001). In panels (F and G), the co-cultures were subjected to intracellular cytokine staining following incubation with non-pulsed (F) and OVA peptide-pulsed (G) neutrophils. The results in F and G show intracellular IFN-γ and IL-17 after gating on CD4+ T cells. Panel (H) shows intracellular staining on CD4-negative gated population, while panel (I) shows the total number of cells staining positive for IL-17, IFN-γ or both (DP, double positive) in both the CD4 negative and positive populations. These experiments were performed twice with the same result.
We also performed intracellular cytokine staining on CD4+ T cells following incubation with OVA-pulsed neutrophils. As shown in Fig. 7(F), there was minimal IFN-γ and IL-17 expression in T cells after co-culture with non-pulsed neutrophils. However, as shown in Fig. 7(G), incubation with OVA-pulsed PMN stimulated generation of both Th1 and Th17 T cells as defined by production of IFN-γ and IL-17, respectively. Interestingly, we also detected a small population of T cells expressing both of these cytokines. Notably, production of these cytokines is largely restricted to CD4+ T-cells as gating on the CD4− population shows a minimal percentage of cytokine being produced (Fig. 7H), and when plotted as total number of cytokine producing cells, it is evident that the cytokines are produced by CD4+ T cells (Fig. 7I). The cytokines IL-6 and TGF-β together are known to drive Th17 differentiation. Therefore, we used blocking mAb to ask whether these cytokines were involved in neutrophil-induced generation of Th17 cells. Surprisingly, presence of these antibodies had no effect on the PMN-induced IL-17 response (Fig. 8A). In contrast, and as expected, the same antibodies were capable of blocking skewing toward IL-17 responses induced by bone marrow-derived DC cultured with IL-6 and TGF-β (Fig. 8B). Thus, antigen-pulsed neutrophils are capable of stimulating differentiation of both Th1 and Th17 T cells even without the addition of exogenous polarizing cytokines.
Fig. 8.
Th17 induction by neutrophils is both IL-6 and TGF-β independent. Purified neutrophils (A) and bone marrow-derived DC (B) were pulsed with OVA peptide and incubated with OVA-specific CD4+ T cells for 4 days in the presence of a Th17 skewing cocktail. In the indicated samples, an anti-skew mAb cocktail was included. On day 4, cultures were spun down and fresh medium supplemented with IL-2 added to co-cultures. Supernatant was collected 3 days after fresh media addition. Cytokine IL-17 levels were measured by ELISA. M, medium only; Skew, IL-17 skewing cocktail consisting of IL-6 (20 ng ml−1), TGF-β (1 ng ml−1), anti-IFN-γ (10 μg ml−1) and anti-IL-12 (10 μg ml−1); αSkew, anti-skewing cocktail consisting of anti-IL6 and anti- TGF-β mAb each at 10 μg ml−1.
Discussion
Neutrophils are conventionally viewed as short-lived cells that migrate rapidly and in large number to sites of infection and inflammation (1). Here, they phagocytose particulate antigen and release granule-associated microbicidal mediators, then rapidly undergo apoptotic death. The response is completed when tissue macrophages and DC phagocytose apoptotic PMN, thereby contributing to resolution of infection and inflammation (26, 27). Yet, it is known that neutrophils respond to inflammatory cytokines by producing immunoregulatory mediators and delaying their own apoptotic death, suggesting a more active role for PMN during infection (28–30). The results of the present study unequivocally demonstrate that neutrophils express MHC class II molecules that directly present antigenic peptide, induce T-cell proliferation and promote generation of Th17 effector cells. MHC class II molecules were not constitutively expressed by neutrophils, but instead up-regulation of these proteins required contact with T cells. Therefore, the present results reinforce an emerging view of PMN as active orchestrators of innate and adaptive immunity (1).
Our data show that OVA-pulsed neutrophils are programmed to induce Th17 differentiation even without addition of exogenous cytokines. This would appear to be an important, and possibly unique, property of PMN. Other APC, such as DC, typically require addition of recombinant cytokines to mediate optimal T-lymphocyte subset differentiation during cell culture. Th17 cells are now understood to be an independent T-cell lineage whose differentiation is controlled by TGF-β and IL-6 (31–33). Th17 cells are implicated in autoimmunity and inflammation associated with several diseases, including Crohn's disease in humans and experimental autoimmune encephalitis in mice (31). The cells are also important in host defense, insofar as they have been shown to play a protective role during infection with extracellular pathogens such as Klebsiella pneumoniae, Staphylococcus aureus and Candida albicans (34–36).
A key property of IL-17, the signature cytokine of Th17 cells, is its ability to promote neutrophil recruitment and granulopoiesis. This is mediated by chemokines such as macrophage inflammatory protein-2 and growth factors such as granulocyte colony-stimulating factor and stem cell factor (36). IL-17 is also known to potentiate neutrophil cytotoxic and phagocytic activity (37, 38). Our results reveal an amplification loop in which antigen-loaded PMN induce Th17 generation; production of IL-17, in turn, can be expected to promote increased neutrophil activity. Further evidence for cross talk between neutrophils and Th17 cells is provided by data showing mutual chemoattraction between these cell types mediated by reciprocal expression of chemokines and chemokine receptors (39).
While neutrophils can produce IL-12 that drives Th1 generation (3, 4), the neutrophil factors responsible for Th17 induction are not yet known. In this regard, it has been reported that apoptotic PMN favor Th17 generation. This is mediated through IL-6 and TGF-β elaboration by APC phagocytosing apoptotic neutrophils (40). Whether a similar pathway is involved in neutrophil-dependent Th17 generation described here is not known. However, we found that blocking antibodies specific for IL-6 and TGF-β failed to affect Th17 generation driven by OVA-pulsed PMN, arguing that these cytokines are not involved in neutrophil driven Th17 induction. We are currently pursuing the possibility that presently undefined neutrophil mediators induce differentiation of this T-cell subset independently of TGF-β or IL-6.
Neutrophils are not generally known for their ability to serve as professional APC. Nevertheless, some studies provide evidence that PMN can be induced to express MHC class II and co-stimulatory molecules. For example, both precursor and mature human PMN up-regulate MHC class II molecules following stimulation with either GM-CSF, IFN-γ or IL-3 (10, 12, 41). It has also been reported that cross-linking of neutrophil CD11b results in up-regulation of MHC class II molecules on PMN (42, 43). Unlike several cytokines secreted by neutrophils (44–46), expression of MHC class II molecules results, at least in part, from up-regulation of mRNA synthesis, rather than exocytosis of preformed MHC class II protein from intracellular granules (11). Neutrophil MHC class II expression has also been reported at the site of inflammation in rheumatoid arthritis, in Wegener's granulomatosis and in persistent Staphylococcus aureus infection (17, 47, 48). Similar to MHC class II molecules, the co-stimulatory molecules CD80 and CD86 have been shown to be up-regulated on neutrophils in response to inflammatory cytokines and during autoimmune pathology (16, 17, 42).
Despite this evidence, the functional consequences of inducible MHC class II and co-stimulatory CD80/CD86 on neutrophils has been unclear. Using human PMN, it has been shown that these cells stimulate T-cell proliferation in response to the superantigens Staphyloccocal enterotoxin A and E (11, 19). In related experiments, neutrophils have been found to process exogenous bacterial antigens for MHC class I-restricted presentation to CD8+ T lymphocytes (49). These cells could also secrete processed peptide that was subsequently acquired by neighboring macrophages or DC. More directly related to our studies, it was recently shown that mouse neutrophils loaded with antigenic OVA peptide stimulated proliferation and cytokine secretion by antigen-specific CD4+ T cells (20). Our results are significant insofar as they are the first to demonstrate a neutrophil capability to actively process and present peptide antigen to T cells and to simultaneously trigger Th17 differentiation.
In addition to their effects on T cells, there is clear evidence that PMN exert immunoregulatory effects on DC (8). Neutrophils release chemokines that attract both DC and T cells and they release cytokines that trigger DC co-stimulatory molecule expression as well as IL-12 and TNF-α secretion (50–53). This is mediated through physical contact mediated by LewisX carbohydrate moeities on PMN Mac-1 and DC-SIGN expressed by DC, combined with neutrophil release of TNF-α (54). Neutrophils also release several mediators during degranulation or apoptosis, and many of these serve as ‘alarmins’ that mobilize and activate APC (55). Among the alarmins produced by neutrophils are the human α-defensins HNP-1 and HNP-2 that chemoattract immature DC and naive T cells (53). Notably, these peptides function as adjuvants when administered to mice with OVA (56). Thus, neutrophils possess the ability to activate DC as well as directly trigger antigen-specific T cell immunity during infection.
The concept that a single APC can present antigen to T cells and provide polarizing signals that drive T-cell subset differentiation has received considerable attention. DC are well known to serve this role during Th1 induction. Recently, it was proposed that IL-4-producing basophils can function similarly in presenting antigen and stimulating Th2 differentiation (57–59). Our results suggest that neutrophils are a third type of APC that possesses a parallel ability to directly stimulate naive T cells and instruct differentiation to the Th1 and Th17 effector T cell subsets, a finding with broad implications with regard to disease pathogenesis and control of infection.
Funding
National Institutes of Health (AI47888 to E.Y.D).
Acknowledgments
We thank M. Hossain for expert technical assistance and M. Bynoe for critical review of the manuscript.
References
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Edwards SW, Moulding DA, Derouet M, Moots RJ. Regulation of neutrophil apoptosis. Chem. Immunol. Allergy. 2003;83:204. doi: 10.1159/000071562. [DOI] [PubMed] [Google Scholar]
- 3.Cassatella MA. Neutrophil-derived proteins: selling cytokines by the pound. Adv. Immunol. 1999;73:369. doi: 10.1016/s0065-2776(08)60791-9. [DOI] [PubMed] [Google Scholar]
- 4.Denkers EY, Del Rio L, Bennouna S. Neutrophil production of IL-12 and other cytokines during microbial infection. Chem. Immunol. Allergy. 2003;83:95. doi: 10.1159/000071557. [DOI] [PubMed] [Google Scholar]
- 5.Romani L, Mencacci A, Cenci E, et al. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J. Immunol. 1997;158:5349. [PubMed] [Google Scholar]
- 6.Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, Suzuki F. Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity. 2004;21:215. doi: 10.1016/j.immuni.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Denkers EY, Butcher BA, Del Rio L, Bennouna S. Neutrophils, dendritic cells and Toxoplasma. Int. J. Parasitol. 2004;34:411. doi: 10.1016/j.ijpara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. Close encounters of neutrophils and DCs. Trends Immunol. 2005;26:626. doi: 10.1016/j.it.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Hansch GM, Wagner C. Expression of MHC class II antigen and coreceptor molecules in polymorphonuclear neutrophils. Chem. Immunol. Allergy. 2003;83:45. doi: 10.1159/000071556. [DOI] [PubMed] [Google Scholar]
- 10.Gosselin EJ, Wardwell K, Rigby WF, Guyre PM. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J. Immunol. 1993;151:1482. [PubMed] [Google Scholar]
- 11.Radsak M, Iking-Konert C, Stagmaier S, Andrassy K, Hansch GM. Polymorphonuclear leukocytes as accessory cells for T-cell activation: MHC class II-restricted antigen-dependent induction of T-cell proliferation. Immunology. 2000;101:521. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith WB, Guida L, Sun Q, et al. Neutrophils activated by granulocyte-macrophage colony-stimulating factor express receptors for interleukin-3 which mediate class II expression. Blood. 1995;86:3938. [PubMed] [Google Scholar]
- 13.Bach EA, Aguet M, Schreiber RD. The IFN-g receptor: a paradigm for cytokine receptor signaling. Ann. Rev. Immunol. 1997;15:563. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 14.Brach MA, deVos S, Gruss HJ, Herrmann F. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood. 1992;80:2920. [PubMed] [Google Scholar]
- 15.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012. [PubMed] [Google Scholar]
- 16.Iking-Konert C, Cseko C, Wagner C, Stegmaier S, Andrassy K, Hansch GM. Transdifferentiation of polymorphonuclear neutrophils: acquisition of CD83 and other functional characteristics of dendritic cells. J. Mol. Med. 2001;79:4644. doi: 10.1007/s001090100237. [DOI] [PubMed] [Google Scholar]
- 17.Iking-Konert C, Vogt S, Radsak M, Wagner C, Hansch GM, Andrassy K. Polymorphonuclear neutrophils in Wegener's granulomatosis acquire characteristics of antigen presenting cells. Kidney Int. 2001;60:2247. doi: 10.1046/j.1523-1755.2001.00068.x. [DOI] [PubMed] [Google Scholar]
- 18.Iking-Konert C, Wagner C, Denefleh B, et al. Up-regulation of the dendritic cell marker CD83 on polymorphonuclear neutrophils (PMN): divergent expression in acute bacterial infections and chronic inflammatory disease. Clin. Exp. Immunol. 2002;130:501. doi: 10.1046/j.1365-2249.2002.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanger NA, Liu C, Guyre PM, et al. Activation of human T cells by major histocompatibility class II expressing neutrophils: proliferation in the presence of superantigen but not tetanus toxoid. Blood. 1997;89:4128. [PubMed] [Google Scholar]
- 20.Culshaw S, Millington OR, Brewer JM, McInnes IB. Murine neutrophils present Class II restricted antigen. Immunol. Lett. 2008;118:49. doi: 10.1016/j.imlet.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sukhumavasi W, Egan CE, Denkers EY. Mouse neutrophils require JNK2 MAPK for Toxoplasma gondii-induced IL-12p40 and CCL2/MCP-1 release. J. Immunol. 2007;179:3570. doi: 10.4049/jimmunol.179.6.3570. [DOI] [PubMed] [Google Scholar]
- 22.Jankovic D, Kullberg M, Caspar P, Sher A. Parasite-induced Th2 polarization is associated with down-regulated dendritic cell responsiveness to Th1 stimuli and a transient delay in T lymphocyte cycling. J. Immunol. 2004;173:419. doi: 10.4049/jimmunol.173.4.2419. [DOI] [PubMed] [Google Scholar]
- 23.Ueda Y, Kondo M, Kelsoe G. Inflammation and the reciprocal production of granulocytes and lymphocytes in bone marrow. J. Exp. Med. 2005;201:1771. doi: 10.1084/jem.20041419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: iL-15 as a possible trigger. J. Immunol. 2003;170:2106. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 25.Hoshino A, Nagao T, Nagi-Miura N, et al. MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. J. Autoimmun. 2008;31:79. doi: 10.1016/j.jaut.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Aleman M, de la Barrera S, Schierloh P, et al. Spontaneous or Mycobacterium tuberculosis-induced apoptotic neutrophils exert opposite effects on the dendritic cell-mediated immune response. Eur. J. Immunol. 2007;37:1524. doi: 10.1002/eji.200636771. [DOI] [PubMed] [Google Scholar]
- 27.Clayton AR, Prue RL, Harper L, Drayson MT, Savage CO. Dendritic cell uptake of human apoptotic and necrotic neutrophils inhibits CD40, CD80, and CD86 expression and reduces allogeneic T cell responses: relevance to systemic vasculitis. Arthritis Rheum. 2003;48:2362. doi: 10.1002/art.11130. [DOI] [PubMed] [Google Scholar]
- 28.Dunican A, Grutkoski P, Leuenroth S, Ayala A, Simms HH. Neutrophils regulate their own apoptosis via preservation of CXC receptors. J .Surg. Res. 2000;90:32. doi: 10.1006/jsre.2000.5829. [DOI] [PubMed] [Google Scholar]
- 29.Lee A, Whyte MKB, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 1993;54:283. [PubMed] [Google Scholar]
- 30.Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol. Today. 1992;13:169. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 31.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 32.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 33.Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 34.Conti HR, Gaffen SL. Host responses to Candida albicans: Th17 cells and mucosal candidiasis. Microbes Infect. 2010;12:518–527. doi: 10.1016/j.micinf.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin L, Ibrahim AS, Xu X, et al. Th1-Th17 cells mediate protective adaptive immunity against Staphylococcus aureus and Candida albicans infection in mice. PLoS Pathog. 2009;5:e1000703. doi: 10.1371/journal.ppat.1000703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoshino H, Laan M, Sjostrand M, Lotvall J, Skoogh BE, Linden A. Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J. Allergy Clin. Immunol. 2000;105:143. doi: 10.1016/s0091-6749(00)90189-1. [DOI] [PubMed] [Google Scholar]
- 38.Lu YJ, Gross J, Bogaert D, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelletier M, Maggi L, Micheletti A, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 40.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 41.Oehler L, Majdic O, Pickl WF, et al. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characterisitcs. J. Exp. Med. 1998;187:1019. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandilands GP, Ahmed Z, Perry N, Davison M, Lupton A, Young B. Cross-linking of neutrophil CD11b results in rapid cell surface expression of molecules required for antigen presentation and T-cell activation. Immunology. 2005;114:354. doi: 10.1111/j.1365-2567.2004.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandilands GP, McCrae J, Hill K, Perry M, Baxter D. Major histocompatibility complex class II (DR) antigen and costimulatory molecules on in vitro and in vivo activated human polymorphonuclear neutrophils. Immunology. 2006;119:562. doi: 10.1111/j.1365-2567.2006.02471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils with prestored IL-12 during microbial infection. J. Immunol. 2000;165:4515. doi: 10.4049/jimmunol.165.8.4515. [DOI] [PubMed] [Google Scholar]
- 45.Matzer SP, Baumann T, Lukacs NW, Rollinghoff M, Beuscher HU. Constitutive expression of macrophage-inflammatory protein 2 (MIP-2) mRNA in bone marrow gives rise to peripheral neutrophils with preformed MIP-2 protein. J. Immunol. 2001;167:4635. doi: 10.4049/jimmunol.167.8.4635. [DOI] [PubMed] [Google Scholar]
- 46.Terebuh PD, Otterness IG, Strieter RM, et al. Biologic and immunohistochemical analysis of interleukin-6 expression in vivo. Constitutive and induced expression in murine polymorphonuclear and mononuclear phagocytes. Am. J. Pathol. 1992;140:649. [PMC free article] [PubMed] [Google Scholar]
- 47.Iking-Konert C, Ostendorf B, Sander O, et al. Transdifferentiation of polymorphonuclear neutrophils to dendritic-like cells at the site of inflammation in rheumatoid arthritis: evidence for activation by T cells. Ann. Rheum. Dis. 2005;64:1436. doi: 10.1136/ard.2004.034132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner C, Iking-Konert C, Hug F, et al. Cellular inflammatory response to persistent localized Staphylococcus aureus infection: phenotypical and functional characterization of polymorphonuclear neutrophils (PMN) Clin. Exp. Immunol. 2006;143:70. doi: 10.1111/j.1365-2249.2005.02963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Potter NS, Harding CV. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J. Immunol. 2001;167:2538. doi: 10.4049/jimmunol.167.5.2538. [DOI] [PubMed] [Google Scholar]
- 50.Bennouna S, Bliss SK, Curiel TJ, Denkers EY. Cross-talk in the innate immune system: neutrophils instruct early recruitment and activation of dendritic cells during microbial infection. J. Immunol. 2003;171:6052. doi: 10.4049/jimmunol.171.11.6052. [DOI] [PubMed] [Google Scholar]
- 51.Bennouna S, Denkers EY. Microbial antigen triggers rapid mobilization of TNF-a to the surface of mouse neutrophils transforming them into inducers of high level dendritic cell TNF-a production. J. Immunol. 2005;174:4845. doi: 10.4049/jimmunol.174.8.4845. [DOI] [PubMed] [Google Scholar]
- 52.Megiovanni AM, Sanchez F, Robledo-Sarmiento M, Morel C, Gluckman JC, Boudaly S. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J. Leukoc. Biol. 2006;79:977. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- 53.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J. Leukoc. Biol. 2000;68:9. [PubMed] [Google Scholar]
- 54.van Gisbergen KP, Sanchez-Hernandez M, Geijteenbeek TB, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005;201:1281. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang D, de la Rosa G, Tewary P, Oppenheim JJ. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531. doi: 10.1016/j.it.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lillard JW, Jr., Boyaka PN, Chertov O, Oppenheim JJ, McGhee JR. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc. Natl Acad. Sci. USA. 1999;96:651. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrigoue JG, Saenz SA, Siracusa MC, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat. Immunol. 2009;10:697. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 2009;10:713. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshimoto T, Yasuda K, Tanaka H, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat. Immunol. 2009;10:706. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]