Genetic control of neuronal activity in mice conditionally expressing TRPV1 (original) (raw)
. Author manuscript; available in PMC: 2011 Jun 30.
Published in final edited form as: Nat Methods. 2008 Mar 9;5(4):299–302. doi: 10.1038/nmeth.1190
Abstract
Here we describe a knock-in mouse model for Cre-_loxP_–based conditional expression of TRPV1 in central nervous system neurons. Expression of Cre recombinase using biolistics, lentivirus or genetic intercrosses triggered heterologous expression of TRPV1 in a cell-specific manner. Application of the TRPV1 ligand capsaicin induced strong inward currents, triggered action potentials and activated stereotyped behaviors, allowing cell type–specific chemical genetic control of neuronal activity in vitro and in vivo.
An important advance in mapping functional neural circuits has been the ability to simultaneously label and electrically activate targeted neurons1–5. Controlled expression of heterologous receptors has proven effective for such experimental manipulations1–4,6–8. Recent use of light-gated ion channels has provided optical control of neuronal activity on millisecond time scales2,3,5,8,9, but this approach requires direct optical access to brain tissue. Chemical genetic strategies for sensitizing neurons to exogenous ligands by engineered expression of heterologous receptors1,4 would allow an alternate approach for activation of deep brain structures, disperse neuronal populations, multiple brain regions or tissues otherwise inaccessible to light.
Here we describe a mouse model in which the rat vanilloid receptor TRPV1 (ref. 10) is conditionally expressed upon cell-specific recombination mediated by Cre recombinase. In recombined neurons, low concentrations of the TRPV1 ligand capsaicin elicited robust cation currents and action potential firing both in vitro and in vivo. This activation persisted for several seconds, could be repeatedly elicited in single neurons and was sufficient to drive behavioral responses.
To generate a conditional genetically encoded activator, we targeted the Gt(ROSA)26Sor (also known as ROSA26) locus11 for the insertion by homologous recombination. We inserted a _loxP_-flanked transcriptional termination cassette upstream of the rat Trpv1 cDNA, followed by an internal ribosomal entry site fused to enhanced cyan fluorescent protein (IRES-ECFP; Fig. 1a and Supplementary Fig. 1a online). We identified correctly targeted embryonic stem cell clones by Southern blot (Supplementary Fig. 1b and Supplementary Methods online) and used these clones to generate germline-targeted mice. To determine whether the conditional TRPV1-IRES-CFP was expressed upon removal of the stop cassette, we biolistically transfected plasmids encoding Cre recombinase into slices of main olfactory bulb from postnatal day 14 conditional TRPV1 mice. By 48 h, we observed overlapping expression of both TRPV1 and ECFP in neurons expressing Cre (Fig. 1b–d).
Figure 1.
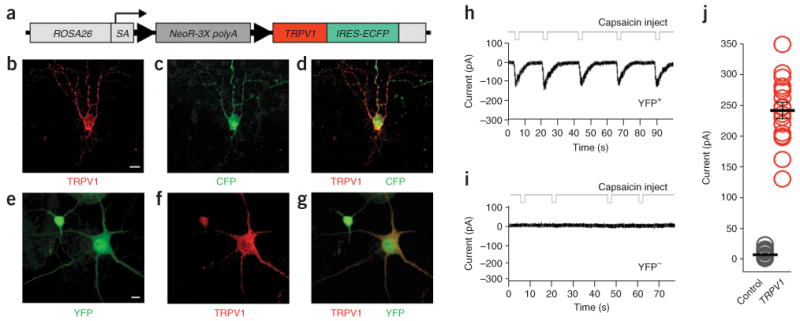
Conditional expression of TRPV1 by Cre recombinase. (a) Diagram of the conditional stopflox-TRPV1-IRES-ECFP allele targeted to the ROSA26 locus. SA, splice acceptor; arrowheads, loxP sites. (b–d) TRPV1-IRES-ECFP reporter expression in a brain slice from a ROSA-stopflox-TRPV1-IRES-ECFP +_/− mouse after introducing a Cre-expressing plasmid via biolistics, assayed by immunohistochemical detection of the conditionally expressed rat TRPV1 (b) and the CFP reporter (c). Colocalization of TRPV1 and CFP is shown in d. Scale bar, 20 μm. (e–g) Conditional expression of YFP (e) and TRPV1 (f) in cultured neurons from ROSA-stopflox-TRPV1-IRES-ECFP +/_− mouse after infection with a lentivirus expressing YFP-IRES-Cre. Colocalization of YFP and TRPV1 is shown in g. Scale bar, 10 μm. (h,i) Capsaicin-evoked currents recorded from a lentivirus-infected (YFP+) neuron expressing Cre (h) and a non-infected control cell (YFP−; i). Deflections in the top traces indicate capsaicin injections. (j) Currents measured after local application of capsaicin to single neurons expressing TRPV1 (YFP +) or control cells (YFP−) not expressing TRPV1. Black bar, mean peak current; error bars, s.e.m. (n = 15,17).
To test the function of the conditional Trpv1 allele, we performed whole-cell patch clamp recordings from cortical neurons derived from ROSA-stopflox-TRPV1-IRES-ECFP mice. To trigger TRPV1-IRES-CFP expression, we infected dissociated cortical neurons from postnatal day 1 conditional mice after 7 d in vitro with a lentiviral vector engineered to simultaneously express both yellow fluorescent protein (YFP) and Cre (Cre-IRES-YFP; Supplementary Methods). Positive immunoreactivity for TRPV1 in YFP-expressing cells verified Cre-mediated excision of the stop cassette (n = 100; Fig. 1e–g).
While blocking action potentials and all fast synaptic transmission (1 μM tetrodotoxin, 30 μM bicuculline, 25 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), 50 μM 2_R_-amino-5-phosphonovaleric acid (APV)), we locally applied small volumes of 1 μM capsaicin by a micropipette positioned near either virally infected Cre-expressing (YFP-positive) or uninfected (YFP-negative) cells, and recorded capsaicin-evoked currents. Local application of capsaicin elicited inward currents in all YFP-positive cells (mean peak current, 242 ± 13 pA; Fig. 1h). The average time to peak after local capsaicin application was 1.8 ± 0.1 s, and the time to baseline recovery was 9.4 ± 2.0 s (s.e.m.; n = 17; Fig. 1h). We never observed capsaicin-evoked currents in YFP-negative cells (n = 15; Fig. 1i,j). TRPV1-expressing neurons exhibited normal morphologies, resting membrane potentials and potassium currents to stepwise changes in membrane potential (Fig. 1e–g and Supplementary Fig. 2 online).
We next crossed ROSA-stopflox-TRPV1-IRES-ECFP conditional mice with mice harboring a transgenic nestin-Cre driver, allowing pan-neuronal TRPV1 expression12. All resulting offspring were morphologically and behaviorally indistinguishable from wild-type littermates. We identified _trans_-heterozygous mice by PCR genotyping (Supplementary Fig. 1c and data not shown), and using immunohistochemical analysis we verified Cre-mediated recombination by the widespread co-expression of TRPV1 and CFP throughout the brain (Fig. 2a–d). To test TRPV1 function, we monitored capsaicin-induced electrophysiological responses in acute neocortical slices from P21 trans_-heterozygous mice. We performed whole-cell current clamp recordings on layer 5 cortical pyramidal neurons during bath perfusion of capsaicin (250 nM) while blocking fast synaptic transmission (10 μM CNQX, 50 μM APV and 10 μM bicuculline). Application of capsaicin elicited action potentials in recombined cells (mean capsaicin-evoked firing rate above baseline, 7.9 ± 1.8 Hz, s.e.m.; n = 40; Fig. 2e), whereas recordings from cells in control slices derived from ROSA-stopflox-TRPV1-IRES-ECFP+/− mice not expressing Cre showed no response to capsaicin (n = 15; Supplementary Fig. 3a online). In response to capsaicin, neurons from nestin-Cre recombined ROSA-stopflox-TRPV1-IRES-ECFP +/− slices elicited dose-dependent depolarizing currents with an average half-maximal response at 350 nM (Fig. 2f). Acute application of capsaicin (1 μM) to individual cells using a pneumatic micropipette resulted in reproducible bursts of capsaicin-evoked action potentials with similar increases in firing rate (Fig. 2g). Recordings from activated cells showed normal resting membrane potentials (−65.6 ± 0.3 mV), spike shape and evoked firing patterns (Fig. 2e,g) as previously shown for this cell type13. However, the frequency of action potential firing was dependent on both the duration of application and capsaicin concentration. Changing these parameters elicited a range of responses from single capsaicin-evoked spikes to high-frequency firing and channel desensitization (Supplementary Fig. 4 online). In the absence of Cre, cells from ROSA-stopflox-TRPV1-IRES-ECFP+/_− mice did not respond to capsaicin (Supplementary Fig. 3a) but maintained glutamate responses (Supplementary Fig. 3b), indicating that the absence of capsaicin activation was not due to impaired excitability. Moreover, we did not observe any sensitivity to changes in bath pH over physiologically relevant ranges, a potential concern given the proton-gating nature of TRPV1 (ref. 10). Specifically, shifting pH from 7.4 to 6.0 produced no differences in pH-evoked currents between wild-type and TRPV1 mice (Supplementary Fig. 5a online; wild-type, −75 ± 35 pA; TRPV1, −72 ± 24 pA; P > 0.9) and had no measurable effects on excitability (Supplementary Fig. 5b).
Figure 2.
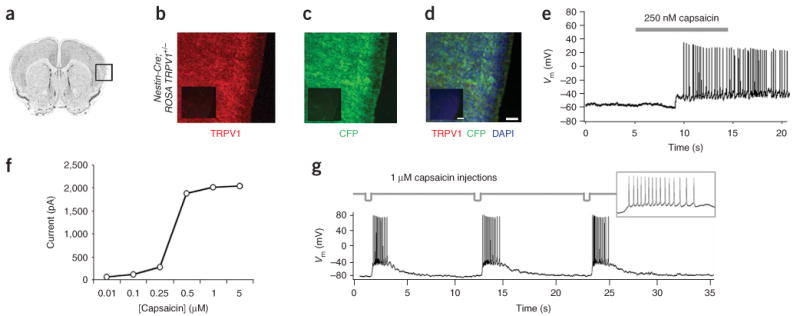
Conditional neuronal expression of TRPV1-IRES-ECFP in vivo by genetic intercross with mice harboring the nestin-Cre driver. (a) A Nissl-stained section of brain. (b–d) Immunohistochemical detection of TRPV1 (b) and CFP (c) and colocalization of the two markers (d) in the neocortex (region boxed in a) of P7 mice harboring both the ROSA-stopflox-TRPV1-IRES-ECFP (ROSA-TRPV1) and nestin-Cre alleles. DAPI staining indicates nuclei of both neuronal and non-neuronal cells. Insets, immunohistochemical detection of the same markers in ROSA-stopflox-TRPV1-IRES-ECFP mice not crossed with nestin-Cre. Scale bars, 50 μm (75 μm in inset). (e) Action potentials in TRPV1-expressing cortical neurons in acute brain slices from nestin-Cre∷ROSA-stopflox-TRPV1-IRES-ECFP mice upon bath application of capsaicin (indicated by the gray bar). (f) Dose-dependent current response to bath applied capsaicin in TRPV1-expressing cells. (g) Bursts of action potentials in TRPV1-expressing cortical neurons in acute brain slices with repeated local injections of capsaicin (deflections in top trace). Inset, magnified view of a capsaicin-evoked burst of action potentials.
We next targeted cortical pyramidal neurons in vivo for capsaicin stimulation in anesthetized conditional ROSA-stopflox-TRPV1-IRES-ECFP mice harboring the nestin-Cre driver. We placed an extracellular recording electrode fixed to a small injection pipette containing 1 μM capsaicin in the dorsal cortex of mice through a small craniotomy. We isolated single unit responses from individual neurons (n = 20, from 4 different mice) by stereotaxic targeting and monitoring of baseline neuronal activity (Supplementary Methods). In response to capsaicin application, nearly all (n = 18) cortical neurons fired high-frequency bursts of action potentials (Fig. 3a). Capsaicin-evoked spikes subsided after several seconds, could be repeatedly elicited with multiple capsaicin injections (Fig. 3b) and were dose-dependent (half-maximal effective concentration of 500 nM).
Figure 3.
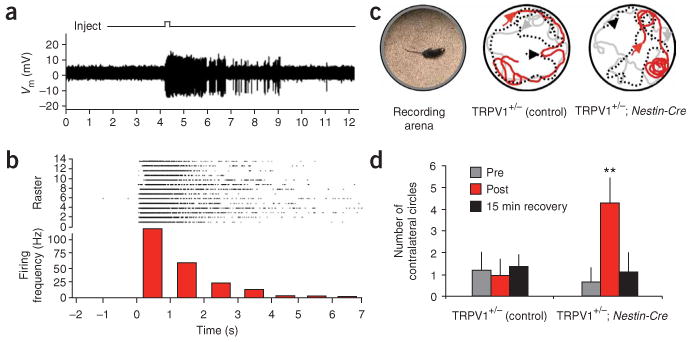
In vivo activation of neurons that conditionally express TRPV1. (a) Electrophysiological response from a cortical cell conditionally expressing TRPV1 upon local capsaicin injection (top trace deflection). (b) Peristimulus time histogram of firing rate binned at 1.0-s intervals, assembled from multiple trials of capsaicin-evoked stimulation of neurons conditionally expressing TRPV1. Top, raster sweeps of spikes from individual trials. (c) Capsaicin-induced circling behavior in awake nestin-Cre∷ROSA-stopflox-TRPV1-IRES-ECFP mice implanted with a ligand delivery cannula unilaterally in the dorsal left striatum. Image of recording arena (left). Tracked locomotor behavior from capsaicin-infused ROSA-stopflox-TRPV1-IRES-ECFP (control) mice lacking Cre expression (middle), and from capsaicin-infused nestin-Cre∷ROSA-stopflox-TRPV1-IRES-ECFP conditional expression mice (right). Gray traces, pre-infusion movement; red traces, post-infusion movement; black dotted traces, movement after a 15-min recovery period. Arrowheads indicate the direction of movement (see Supplementary Movies 1 and 2). (d) Number of contralateral circling events made by either TRPV1 conditional or control mice upon infusion with capsaicin. Data represent means ± s.e.m.; n = 6 mice each. **P < 0.001; _t_-test.
We next determined whether this technology could by used to activate genetically targeted populations of neurons in awake behaving mice. We unilaterally implanted cannulas into the striatum of either nestin-Cre∷ROSA-stopflox-TRPV1-IRES-ECFP mice or control ROSA-stopflox-TRPV1-IRES-ECFP mice lacking Cre and delivered 0.5 μM capsaicin by acute infusion. Exploratory and motor behavior in both nestin-Crer∷ROSA-stopflox-TRPV1-IRES-ECFP and control mice was unaffected after recovery from surgery or infusion of control saline solution (data not shown). In contrast, stereotyped contralateral circling behavior occurred within 5 min of capsaicin infusion in mice harboring the conditionally expressed TRPV1 receptor (n = 6). Control ROSA-stopflox-TRPV1-IRES-ECFP mice lacking Cre showed no apparent capsaicin-mediated behaviors (n = 6; Fig. 3c,d and Supplementary Movies 1 and 2 online).
To determine whether local infusion of capsaicin into striatal tissues resulted in cellular excitotoxicity, we performed cell death assays. At capsaicin concentrations used to elicit circling (500 nM), we observed no cell death, but infusion of much higher concentrations of capsaicin (5–10 μm) resulted in a dose-dependent excitotoxicity, likely resulting from excess calcium influx (Supplementary Fig. 6 online). Thus, in addition to ligand-induced neuronal activation, the conditional TRPV1 mice provide a new model system for focal excitotoxicity in genetically defined neuronal populations.
Finally, to test whether similar behaviors could be elicited in mice in which TRPV1 expression was induced focally in the brain, we expressed TRPV1 in small subsets of striatal neurons by stereotactic injection of YFP-IRES-Cre lentivirus in ROSA-stopflox-TRPV1-IRES-ECFP mice (Supplementary Fig. 7 online). One week after unilateral lentiviral Cre expression in the striatum, infusion of capsaicin recapitulated the stereotyped contralateral circling behavior (Supplementary Movie 3 online). In all experiments, circling behavior subsided within 15 min after capsaicin infusion (Fig. 3c,d and Supplementary Movies 1 and 3), and infusions did not cause any observable lasting behavioral effects. In no cases did we observe ipsilateral circling after capsaicin infusion into ROSA-stopflox-TRPV1-IRES-ECFP mice expressing Cre in striatal neurons, indicating that circling behaviors were not due to nonspecific motor disorientation. Thus, conditional expression of TRPV1 in conjunction with infusion of modest amounts of capsaicin allows for acute, transient and genetically targeted neuronal activation, and associated behavioral responses in vivo.
Here we described a mouse model designed for chemical genetic manipulation of neuronal activity both in vitro and in vivo. Targeting the ROSA26 locus for TRPV1 knock-in afforded the unique opportunity to express a potently activated ion channel in a controllable manner in vivo. Regulating the dose of capsaicin applied to TRPV1 expressing cells provided a range of control over neuronal activity from single action potentials to excitotoxic insult and neurodegeneration. Moreover, capsaicin was delivered to deep brain structures or disperse neuronal populations by standard methods of intracranial infusion.
A limitation of the system is the relatively coarse temporal resolution afforded by depolarizing neurons through TRPV1 channel activation. Unlike ChR2-mediated activation, which shows millisecond time scale activation in vivo2,3,5, capsaicin-induced firing is on the order of seconds. Nevertheless, the broad utility of this chemical genetic conditional system for in vivo applications, including activation of deep brain regions and disperse neuronal populations, provide a unique capability to reliably modulate neuronal activity in a manner complementary to optical methods3,14 and ligand-induced inhibition4. Photoreleasable forms of capsaicin might be used in a unique manner, possibly allowing finer temporal control, but still require light access similar to that for ChR2. Advantages of this system that complement existing mouse models include the genetic targetability via the Cre-loxP system, robust neuronal activation with low-level channel expression and the ease of in vivo manipulations afforded through standard cannula infusion techniques. Expanding the genetic tool kit targeting neuronal subtypes for ectopic stimulation will allow investigations into mammalian brain circuitry and circuit-driven behaviors.
All experimental procedures were conducted in accordance with guidelines and standards of the Duke University Institutional Animal Care and Use Committee.
Supplementary Material
supp figs 1-7
supp movie 1
supp movie 2
supp movie 3
Acknowledgments
We thank D. Julius (University of California, San Francisco) for the rat Trpv1X cDNA, F. Wang for sharing both space and embryonic stem cell culture reagents, J. Gross for technical assistance, and Y. Ben-Shaul, I. Davison, C. Hanus, T. Helton, J. Hernandez, M. Kennedy, R. Mooney and J. Yi for helpful input and critical review of the manuscript. This work was supported by grants from the US National Institutes of Health (to M.D.E.). M.D.E. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Note: Supplementary information is available on the Nature Methods website.
Author Contributions: B.R.A. designed experiments, generated and characterized knock-in mice, conducted electrophysiological and behavioral experiments, and wrote the paper. M.E.K. generated lentivirus. I.G.D. helped design and conduct electrophysiological experiments. L.C.K. participated in initial experimental design. M.D.E. designed experiments and wrote the manuscript.
References
- 1.Lima SQ, Miesenbock G. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Nagel G, et al. Curr Biol. 2005;15:2279–2284. doi: 10.1016/j.cub.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 3.Arenkiel BR, et al. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lerchner W, et al. Neuron. 2007;54:35–49. doi: 10.1016/j.neuron.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, et al. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 6.Zemelman BV, Lee GA, Ng M, Miesenbock G. Neuron. 2002;33:15–22. doi: 10.1016/s0896-6273(01)00574-8. [DOI] [PubMed] [Google Scholar]
- 7.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Proc Natl Acad Sci USA. 2003;100(Suppl. 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szobota S, et al. Neuron. 2007;54:535–545. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Han X, Boyden ES. PLoS ONE. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caterina MJ, et al. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 11.Soriano P. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 12.Betz UA, Vosshenrich CA, Rajewsky K, Muller W. Curr Biol. 1996;6:1307–1316. doi: 10.1016/s0960-9822(02)70717-3. [DOI] [PubMed] [Google Scholar]
- 13.Williams SR, Stuart GJ. J Physiol (Lond) 1999;521:467–482. doi: 10.1111/j.1469-7793.1999.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deisseroth K, et al. J Neurosci. 2006;26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supp figs 1-7
supp movie 1
supp movie 2
supp movie 3