Apurinic/apyrimidinic endonuclease 1 inhibits protein kinase C-mediated p66shc phosphorylation and vasoconstriction (original) (raw)
Abstract
Aims
Phosphorylation of the adaptor protein p66shc is essential for p66shc-mediated oxidative stress. We investigated the role of the reducing protein/DNA repair enzyme apurinic/apyrimidinic endonuclease1 (APE1) in modulating protein kinase CβII (PKCβII)-mediated p66shc phosphorylation in cultured endothelial cells and PKC-mediated vasoconstriction of arteries.
Methods and results
Oxidized low-density lipoprotein (oxLDL)induced p66shc phosphorylation at serine 36 residue and PKCβII phosphorylation in mouse endothelial cells. Adenoviral overexpression of APE1 resulted in reduction of oxLDL-induced p66shc and PKCβII phosphorylation. Phorbol 12-myristate 13-acetate (PMA), which stimulates PKCs, induced p66shc phosphorylation and this was inhibited by a selective PKCβII inhibitor. Adenoviral overexpression of PKCβII also increased p66shc phosphorylation. Overexpression of APE1 suppressed PMA-induced p66shc phosphorylation. Moreover, PMA-induced p66shc phosphorylation was augmented in cells in which APE1 was knocked down. PMA increased cytoplasmic APE1 expression, compared with the basal condition, suggesting the role of cytoplasmic APE1 against p66shc phosphorylation. Finally, vasoconstriction induced by phorbol-12,13, dibutylrate, another PKC agonist, was partially inhibited by transduction of Tat-APE1 into arteries.
Conclusion
APE1 suppresses oxLDL-induced p66shc activation in endothelial cells by inhibiting PKCβII-mediated serine phosphorylation of p66shc, and mitigates vasoconstriction induced by activation of PKC.
Keywords: p66shc, Apurinic/apyrimidinic endonuclease1, Oxidized LDL, Protein kinase C, Endothelial cells
1. Introduction
Oxidative stress negatively impacts vascular homoeostasis and controls a number of signalling pathways relevant to vascular disease. P66shc belongs to the ShcA family of adaptor proteins and mediates oxidative stress in many cell types and tissues. Mice lacking the adaptor protein p66shc have increased resistance to oxidative stress and a 30% increase in life span.1 In the vasculature, p66shc plays an important role in endothelial dysfunction associated with pathophysiological conditions such as diabetes and hyperlipidaemia.2 ShcA proteins are phosphorylated at tyrosine residues in response to stimulation by a variety of growth factors and cytokines.3–5 However, p66Shc is functionally different from the p46 and p52 isoforms in that it also undergoes phosphorylation mainly at Ser36 after exposure to oxidative stress such as ultraviolet or H2O2.1,6–8 Phosphorylation at Ser36 is required for conferring increased susceptibility to oxidative stress and is critical for the cell death response elicited by oxidative damage.9 Therefore, prevention of this phosphorylation may have a therapeutic impact on diseases that are associated with oxidative damage. Activation of endothelial cells by oxidized low-density lipoprotein (oxLDL) is an early key event in hyperlipidaemia-induced endothelial dysfunction and atherosclerosis.10 OxLDL signals, in part, through p66shc. Apoptosis induced by oxLDL is associated with up-regulation of p66shc expression in intestinal cells.11 Moreover, p66shc phosphorylation is induced by protein kinase Cβ (PKCβ) which is activated by oxidative conditions in atherosclerosis.12,13
Among PKC isoenzymes, PKCβII was focused on the pathogenesis in cardiovascular disorder. Previously, reports showed that oxLDL-induced PKCβII membrane translocation and other PKC isoforms including PKCβI revealed no change in membrane translocation by oxLDL.12 Also, other studies suggested that oxidative stress and hyperglycaemia are able to trigger the activation of PKCβII.14,15 Diacylglycerol activates one or more PKC isoenzymes, PKCβII is preferentially activated in vascular smooth muscle and endothelial cells.16,17
Apurinic/apyrimidinic endonuclease1/redox factor-1 (APE1) is a multi-functional protein involved in base excision DNA repair and in transcriptional regulation of gene expression, and has a pleiotropic role in controlling cellular response to oxidative stress.18,19 APE1 reduces intracellular reactive oxygen species (ROS) production.20–22 Overexpression of APE1 suppresses tumour necrosis factor-alpha-induced monocyte adhesion to endothelial cells23 and inhibits hypoxia-induced endothelial cell apoptosis.24 Moreover, APE1 inhibits balloon injury-induced neointimal formation in rats,25 suggesting that it has an anti-inflammatory function in the vascular endothelium. However, whether APE1 affects p66shc, and in what context is unknown. Hypothesizing that APE1 suppresses cellular oxidative stress in part by inhibiting p66shc, we investigated the role and mechanism of APE1 in p66shc phosphorylation in endothelial cells.
2. Methods
2.1. Cell culture and reagent
Mouse MS-1 endothelial cells were purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were grown in Dulbecco's modified Eagle medium with 10% foetal bovine serum, 10 U/mL penicillin, and 10 µg/mL streptomycin. Antibodies to PKCβII (SC-210), APE1 (SC-17774), β-actin (SC-1616), GAPDH (SC-25778), and β-galactosidase (SC-65670) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies to phospho-ser36-p66shc (CN566807, Calbiochem, La Jolla, CA, USA), phospho-PKCβII (AB5785, Abcam, Cambridge, MA, USA), SHC (C#610082, BD Biosciences, Franklin Lakes, NJ, USA), and p84/N5 (Gene Tex, San Antonio, TX, USA) were used. Native LDL was purchased from Kalen Biomedical (Montgomery Village, MD, USA). Anilino-monoindolylmaleimide (3-(1-(3-imidazol-1-ylpropyl)-1H-indol-3-yl)-4-anilino-1H-pyrrole-2,5-dione, a PKCβ-specific inhibitor),26 phorbol 12-myristate 13-acetate (PMA), and phorbol-12,13, dibutylrate (PDBu) were purchased from Calbiochem. Go6976 was purchased from Tocris Bioscience (Bristol, UK). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated.
2.2. Adenoviral vector construction
Adenoviruses encoding β-galactosidase (Adβgal) and full-length APE1(AdAPE1) were generated by homologous recombination in human embryonic kidney 293 cells, and have been described previously.27 Adβgal was used as an adenoviral control. Overexpression of human PKCβII in endothelial cells was accomplished with a replication-incompetent adenovirus created by the Viralpower adenovirus expression system (Invitrogen, Carlsbad, CA, USA). Briefly, human PKCβII was isolated from PKCβ cDNA in pBluscriptR (C#MHS1010–7294004, Open Biosystems, Huntsville, AL, USA), which was matched with Genbank accession number BC036472 using the following primers with the restriction enzyme linker; sense primer: 5′- CGC GGA TCC ATG GCT GAC CCG GCT GCG-3′ (containing a _Bam_HI restriction site), anti-sense primer: 5′-CCG CTC GAG TTA GCT CTT GAC TTC GGG-3′ (containing a _Xho_I restriction site). After _Bam_HI and _Xho_I digestion, full-length hPKCβII constructs were cloned into the pENTR-CMV-FLAG vector, which has attL sites for site-specific recombination with a Gateway destination vector and an entry vector (Invitrogen). The site-specific recombination between hPKCβII cDNA in the pENTR-CMV-FLAG and the adenovirus vector pAd/PL-DEST was achieved with LR clonase II. Cells were grown until an 80% cytopathic effect was seen, and then harvested to prepare the stock recombination adenovirus. The adenovirus was amplified in 293A cells and purified using the CsCl2 gradient technique, as described previously.27 Cells were infected with 200 multiplicity of infection (MOI; particle forming units per cell) of adenovirus for 18 h. The virus was removed, and the cells were incubated for another 24 h
2.3. Oxidized LDL
To produce oxLDL, 1 mL of human native LDL (density 1.019–1.063g/mL, 5 mg/mL) was diluted to 1.5 mL in EDTA-free PBS and incubated with 20 μM CuSO4 for 18 h at 37°C. At the end of the incubation, 0.1 mM EDTA was added to prevent further oxidation, and the oxLDL was concentrated to 1.4 mg/mL. The extent of LDL oxidation was assessed in a TBARS assay.
2.4. Small interfering RNA for APE1
Mouse small interfering RNA (siRNA) and scrambled siRNA were purchased from Bioneer Co. (Deajeon, South Korea). The cells were transfected with 20 nM chemically synthesized siRNA targeting mouse APE1 (5′-GUC UGG UAA GAC UGG AGU ACC-3′), and scrambled siRNA was used as a control. siRNA and lipofectamine 2000 were separately diluted in OptiMEM medium, incubated for 5 min at room temperature, combined, and incubated for an additional 20 min followed by adding it to the cells for 48 h.
2.5. Recombinant Tat-APE1 protein expression and purification
Full-length human APE1 cDNA was cloned into the pTAT bacterial expression vector (pTAT-2.1, kindly provided by Steven Dowdy), which contains a six-histidine tag, for easy purification.28 pTAT-APE1 plasmids were then transformed into the BL21(DE3) strain of Escherichia coli. Following 4 h of IPTG induction, the cells were sonicated in buffer Z (8 M urea, 100 mM NaCl, and 20 mM HEPES), and recombinant proteins were purified on an Ni-NTA agarose column (Qiagen, Valencia, CA, USA). After washing, Tat-APE1 was eluted using 250 mM imidazole-containing buffer Z followed by desalting on a PD-10 column (Amersham Pharmacia Biotech, Piscataway, NJ, USA) in PBS. The eluate was frozen in 10% glycerol at −80°C.
2.6. Western blot analysis
MS-1 cells were harvested in 100 μL of lysis buffer containing 20 mM Tris-Cl (pH 7.5), 100 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1 mM Na3VO3, 1 mM β-glycerophosphate, 4 mM Na pyrophosphate, 5 mM NaF, 1% Triton X-100, and a protease inhibitor cocktail. The lysate was centrifuged at 12 000 rpm for 20 min, and the supernatant was collected. Protein (20–40 μg) was separated on 10% SDS–PAGE and was electrotransferred onto nitrocellulose membranes. After blocking with 5% skimmed milk for 2 h at room temperature, blots were incubated overnight at 4°C with specific primary antibody (1:1000) and subsequently with horseradish peroxidase-conjugated secondary antibody. Blots were developed for visualization using an enhanced chemiluminescence detection kit (Pierce Biotechnology, Rockford, IL, USA). Cells were serum starved for 18 h in some experiments to reduce basal phosphorylation of specific proteins. Nuclear and cytoplasmic extracts were prepared using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce). Protein concentration was measured with a Coomassie Protein assay kit (Pierce) using bovine serum albumin as the standard.
2.7. Immunofluorescent staining
For transfection and immnofluorescent staining, 5 × 104 cells of MS-1 were grown on glass coverslips, and then transiently transfected with 1 μg of pEGFP-APE1 by using lipofectamine 2000, as recommended by the manufacturer (Invitrogen). pEGFP-APE1 was generated by insertion of full length of human APE1 cDNA23 into pEGFP-N1 (Clonetech, Mountain View, CA, USA). Twenty-four hours after transfection, the cells were exposed to 100 nM PMA for 30 min and then fixed with ice-cold acetone. Coverslips were mounted on microscope slides, and fluorescence signals were visualized with a Zeiss fluorescent microscope.
2.8. Measurement of vasoreactivity
Male Sprague-Dawley rats 5–6 weeks old (150∼200g) were anaesthetized with a mixture of ketamine (80 mg/kg) and xylazine (12 mg/kg) intraperitoneally. Aortas were isolated, cleaned, and cut into rings (2–3 mm in width). Each ring was connected to an isometric force transducer (MultiMyograph 610M, Danish Myo Technology, Aarhus, Denmark), suspended in an organ chamber filled with 7.5 mL of Krebs buffer solution (NaCl, 99 mM; KCl, 4.7 mM; CaCl2, 1.9 mM; MgSO4, 1.2 mM; K2HPO4, 1.03 mM; NaHCO2, 25 mM; Glucose 11.1 mM; pH 7.4) and aerated with 95% O2/5% CO2. Isometric tension was recorded continuously, as previously described.29 After a 1 h equilibration period, the rings were pre-contracted with PDBu (100 nM) and then isometric tension was observed with Tat-APE1 or Tat-GFP (100 nM). Relaxation was expressed as a percentage of the pre-contracted tension obtained with PDBu (100 nM). All in vivo procedures were in accordance with US National Institutes of Health guidelines and were approved by the institutional animal care and use committee of Chungnam National University (South Korea).
2.9. Statistical analysis
Values are expressed as means ± SEM. The statistical evaluation was conducted with a one-way analysis of variance followed by a Tukey's post hoc test, and P < 0.05 was considered statistically significant.
3. Results
3.1. Oxidized LDL-induced p66shc and PKCβII phosphorylation
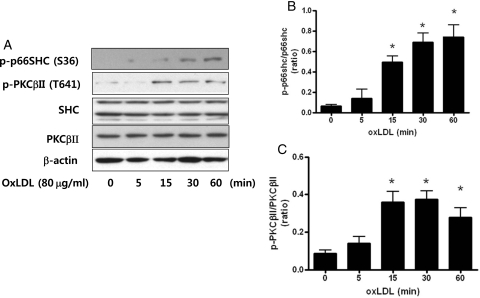
To investigate whether oxLDL activated p66shc and PKCβII phosphorylation, we treated endothelial cells with oxLDL (80 μg/mL) for various times. As shown in Figure 1, oxLDL induced p66shc phosphorylation (Ser 36) and PKCβII phosphorylation (T641). p66shc and PKCβII phosphorylation were induced by oxLDL within 15 min, which remained for 60 min. However, total Shc and PKCβII were not changed by oxLDL in endothelial cells.
Figure 1.
Oxidized LDL (oxLDL) increases p66shc and PKCβII phosphorylation in the MS-1 endothelial cell line. Cells were harvested after treatment with oxidized LDL (80 μg/mL) for the indicated times. Expression of p66shc phosphorylation and PKCβII phosphorylation were measured by western blotting. (A) Typical representative data. (B and C). Summarized data. Each bar represents the ratio of endogenous p66shc or PKCβII. Each bar shows the mean ± SEM (n = 4); *P < 0.05 vs. control.
3.2. APE1 suppressed oxLDL-induced p66shc phosphorylation and PKCβII phosphorylation
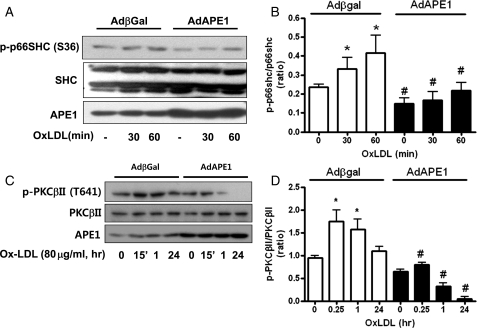
We investigated the effect of APE1 on oxLDL-induced p66shc phosphorylation. In Adβgal-infected endothelial cells, oxLDL (80 μg/mL) also increased p66shc phosphorylation within 30–60 min; however, APE1 overexpression using adenoviral APE1 gene transfer significantly reduced oxLDL-induced p66shc phosphorylation in endothelial cells (Figure 2A and B). Next, we investigated the effect of APE1 overexpression on oxLDL-induced PKCβII phosphorylation. OxLDL (80 μg/mL) increased PKCβII phosphorylation within 15 min, which returned to basal levels within 24 h. However, APE1 overexpression significantly reduced oxLDL-induced PKCβII phosphorylation (Figure 2C and D).
Figure 2.
APE1 overexpression inhibits oxLDL-induced p66shc phosphorylation (A) and PKCβII phosphorylation (C) in the MS-1 endothelial cell line. (A) and (C) Typical representative data. P66shc phosphorylation at serine 36 and PKCβII phosphorylation at threonine 641 were analysed with western blot after exposure to oxidized LDL (80 μg/mL). (B and D) Densitometry analysis of p66shc phosphorylation (B) and PKCβII phosphorylation (D). Each bar shows the mean ± SEM (n = 3); *P < 0.05 vs. basal, #P < 0.05 vs. Adβgal.
3.3. PMA or oxLDL-induced p66shc phosphorylation: involvement of PKCβII
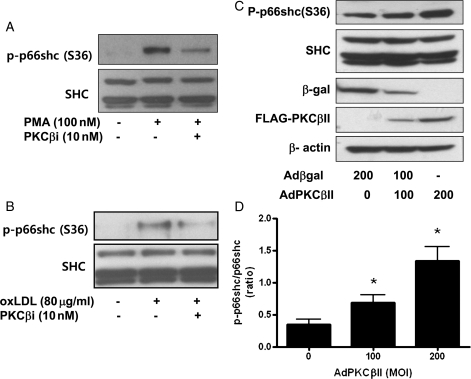
To investigate whether p66shc phosphorylation was activated by PKCβII, we examined the effect of a PKC antagonist on PMA or oxLDL-induced p66shc phosphorylation. The exposure of PMA (100 nM) or oxLDL (80 μg/mL) for 30 min markedly increased p66shc phosphorylation, which was inhibited significantly by 10 nM PKCβi, a specific inhibitor of PKCβII (PKCβi, aniline-monoindolylmaleimide inhibitor) (Figure 3A and B). To confirm whether p66shc phosphorylation was related to PKCβII activation, we examined the effect of PKCβII overexpression using an adenoviral flag-tagged PKCβII gene transfer on p66shc phosphorylation. Adenoviral flag-tagged PKCβII gene transfer in a range of 100–200 MOI successfully induced PKCβII expression, as accessed by western blot for FLAG. AdPKCβII-infected endothelial cells showed an increase in p66shc phosphorylation compared with Adβgal-infected endothelial cells, suggesting PKCβII-induced p66shc phosphorylation (Figure 3C). Therefore, our data suggested that PMA-induced p66shc phosphorylation can be mediated by PKCβII activation in endothelial cells.
Figure 3.
PKCβII activates p66shc phosphorylation in the MS-1 endothelial cell line. (A) Effect of PKCβi on PMA (100 nM)-induced p66shc phosphorylation. PKCβi (10 nM), a specific PKCβII inhibitor (PKCβi, aniline-monoindolylmaleimide inhibitor). (B) Effect of PKCβi on oxLDL (80 μg/mL)-induced p66shc phosphorylation. PKCβi was pretreated for 10 min before exposure to PMA or oxLDL. (C) Effect of PKCβII overexpression on p66shc phosphorylation. PKCβII overexpression with AdPKCβII was confirmed by western blot for FLAG-PKCβII. Total adenoviral gene transfection was balanced with 200 MOI Adβgal as a control virus. Each bar shows the mean ± SEM (n = 3); *P < 0.05 vs. control.
3.4. APE1 suppressed PMA-induced p66shc phosphorylation
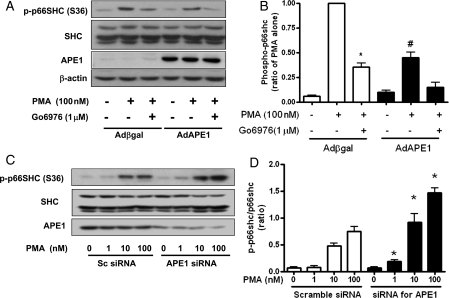
As we established that PMA induced p66shc phosphorylation, we next examined the effect of APE1 overexpression on p66shc phosphorylation in endothelial cells. PMA (100 nM, 30 min) markedly induced p66shc phosphorylation (S36), which was significantly inhibited by Go6976 (1 μM), an inhibitor of the PKCα/β isoenzyme. However, APE1 overexpression using AdAPE1 significantly suppressed PMA-induced p66shc phosphorylation, as shown in Figure 4A. A densitometric analysis is plotted in Figure 4B.
Figure 4.
APE1 controls PMA-induced p66shc phosphorylation in the MS-1 endothelial cell line. (A) APE1 overexpression significantly suppressed PMA-induced p66shc phosphorylation. Go6976 (1 μM) was pretreated for 10 min before exposure to PMA. (B) Densitometric analysis of p66shc phosphorylation. Each bar shows the mean ± SEM (n = 4); *P < 0.05 vs. PMA control; #P < 0.05 vs. Adβgal. (C) Gene silencing of APE1 with siRNA increased PMA-induced p66shc phosphorylation. (D) Densitometric analysis of p66shc phosphorylation. Cells were transfected with scrambled siRNA (sc siRNA) or APE1 siRNA (20 nM) using lipofectamine 2000 for 48 h. Each bar shows the mean ± SEM (n = 4); *P < 0.05 vs. control siRNA.
To investigate whether basal APE1 influences PMA-induced p66shc phosphorylation, we evaluated the effect of APE1 siRNA on PMA-induced p66shc phosphorylation. Following the treatment of endothelial cells with 20 nM APE1-specific siRNA for 48 h, endogenous APE1 expression was efficiently reduced compared with scrambled siRNA-transfected cells. PMA (1–100 nM) increased p66shc phosphorylation in a dose-dependent manner in scrambled siRNA-transfected cells. Even basal p66shc phosphorylation was not increased by the treatment with APE1 siRNA, but PMA-induced p66shc phosphorylation was increased in endothelial cells transfected with APE1 siRNA (Figure 4C). These data suggested that gene APE1 silencing increased PMA-induced p66shc phosphorylation in endothelial cells.
3.5. PKC activations induced cytoplasmic translocation of APE1
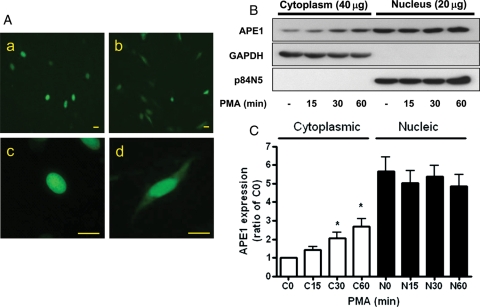
Under basal conditions, APE1 is localized mostly in the nucleus, whereas p66shc is mainly localized in the cytoplasm and/or mitochondria. We next focused on how APE1 inhibited PKC-induced p66shc phosphorylation. In the pEGFP-APE1-transfected MS-1, we investigated the effect of PMA on APE1 translocation. Twenty-four hours after transfection, the cells were exposed to 100 nM of PMA for 30 min and then visualized with fluorescent microscopy. As shown in Figure 5A, in the basal state, APE1 was mainly localized to the nucleus of MS-1 cells (a.c). However, cytoplasmic APE1 expression was detected in the cells that were exposed to PMA for 30 min (bd). Under a non-stimulated condition, APE1 was detected mainly in the nuclear fraction, as assessed by western blotting. However, cells treated with PMA for 15–60 min showed increased cytoplasmic APE1 expression, suggesting APE1 cytoplasmic translocation (Figure 5B and C).
Figure 5.
PMA increase cytoplasmic APE1 expression in the MS-1 endothelial cell line. (A) Immunofluorescence images of pEGFP-APE1 in basal and PMA-exposed MS-1 cells. The cells were transfected with 1 μg of pEGFP-APE1 and then were exposed to 100 nM PMA for 30 min and then fixed with ice-cold acetone. a, c; basal state, b, d; PMA (100 nM), a, b; ×100, c, d; ×400. Scale bars, 20 μm. (B) Effect of PMA (100 nM) on APE1 expression in the cytoplasmic and nuclear fraction of MS-1 cells. Cells were treated with PMA (100 nM) for the indicated times. Each bar shows the mean ± SEM (n = 3); *P < 0.05. GAPDH and p84N5 were used as cytoplasmic and nuclear markers, respectively. Note: 40 μg of cytoplasmic homogenate proteins and 20 μg of nucleic homogenate proteins were used for western blotting.
3.6. Cell-permeable APE1 (Tat-APE1) inhibited PKC-induced vasocontraction
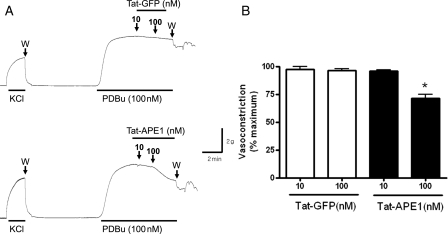
Tat-APE1 is a cell-permeable APE1 that inhibits monocyte adhesion in endothelial cells.28 Finally, we investigated whether Tat-APE1 affected PKC agonist-induced vasocontraction in rat aorta. To activate PKC in the rat aortas, we used PDBu to activate PKC in rat aorta.30 PDBu (100 nM)-induced tonic contraction in aortic rings, as shown in Figure 6A. However, Tat-APE1 (10–100 nM) exposure inhibited PDBu-induced contraction compared with Tat-GFP used as a control (Figure 6B).
Figure 6.
Cell-permeable APE1 (Tat-APE1) suppresses PDBu-induced vasocontraction in the rat aorta. When PDBu (100 nM)-induced contraction reached the maximum, 10–100 nM Tat-APE1 or Tat-GFP was added cumulatively. Tat-GFP was used as a control for Tat-APE1. After a 60 mM K+-induced contraction stabilized, the contraction was evoked by PDBu (100 nM). W, washing. (B) Summarized contractile response data. Each bar shows the mean ± SEM (n = 3); * P < 0.05 vs. Tat-GFP.
4. Discussion
It is widely accepted that the p66shc adaptor protein controls oxidative stress and life span in mammals and genetic deletion of the p66shc adaptor protein prevents endothelial dysfunction and oxidative stress.31–34 As p66shc is activated by oxidative stress, the endogenous biomolecules for preventing p66shc phosphorylation may have a therapeutic impact on oxidative stress diseases. APE1 is a multi-functional protein involved in base excision DNA repair and transcriptional regulation of gene expression.18,19 The multifunctional nature of APE1 is being uncovered and has been extensively studied in the cellular response against oxidative stress.18
In the present study, we demonstrated that APE1 inhibited oxLDL or PKC-induced p66shc phosphorylation in endothelial cells. The inhibitory action of APE1 on oxLDL-induced p66shc phosphorylation was mediated by inhibiting PKCβII phosphorylation. Furthermore, cell-permeable APE1 inhibited PKC-induced vasoconstriction, suggesting that APE1 plays a crucial inhibitory role in p66shc phosphorylation.
Oxidative stress, such as hydrogen peroxide, is able to phosphorylate p66shc via PKCβ activation in MEF cell lines. Hispidin, a specific blocker of a PKCβ isoform, inhibits p66shc phosphorylation.13 However, it is unknown which type of PKCβ isoforms affect p66shc phosphorylation in endothelial cells. In the present study, our data showed that transfection of PKCβII increased p66shc phosphorylation, but PKCβI did not increase it (Supplementary material online, Figure S1). Also, we verified that inhibiting PKCβII reduced PKC agonist-induced p66shc phosphorylation. The PKCβ inhibitor, an anilino-monoindolylmaleimide compound, acts as a potent, cell-permeable, reversible, and ATP-competitive inhibitor of PKCβ isozymes (IC50 = 5 nM and 21 nM for human PKCβII and βI, respectively) and displays greater selectivity over PKCα, ρ, and ɛ (IC50 = 331 nM, >1 µM, and 2.8 µM, respectively).26 We used 10 nM of a PKCβ inhibitor to specifically inhibit PKCβII. Treatment with 10 nM of a PKCβ inhibitor suppressed PKC agonist-induced p66shc phosphorylation, suggesting the involvement of PKCβII. We also confirmed that adenoviral overexpression of human-specific PKCβII cDNA increased p66shc phosphorylation (Figure 3). Therefore, our data strongly suggested that PKCβII is upstream of p66shc phosphorylation in endothelial cells.
OxLDL is an important cardiovascular disease biomarker. Hypercholestrolaemia increases ROS production, resulting in lipid and protein oxidation and peroxidation. p66shc(–/–) mice are more resistant to high-fat diet-induced atherogenesis and reduced expression of oxidation markers.34,35 Among the PKCs, PKCβ is a classic PKC, which is activated by calcium, diacylglycerol, and phorbol esters.36 Activation of PKCβII isoforms causes vascular dysfunction.37–39 Recent data have shown that a reduction of PKCβII attenuates neointimal formation in response to acute arterial injury in mice.40 In the present study, we confirmed that oxLDL exposure increased PKCβII phosphorylation and p66shc phosphorylation in endothelial cells. Moreover, oxLDL-induced PKCβII phosphorylation was suppressed by APE1 overexpression. These data suggest that APE1 plays an inhibitory role against oxLDL-induced p66shc phosphorylation by inhibition of PKCβII phosphorylation in endothelial cells. In rat aorta, we also demonstrated that cellular transduction of APE1 using Tat-APE1 inhibited PKC-induced vasoconstriction. This finding indicates that the cellular transduction of APE1 may be useful to reduce PKC-induced vascular dysfunction (Figure 6). Endothelial dysfunction is mediated by multiple mechanisms in the endothelium. Increase in p66 phosphorylation due to PKC activation could lead to endothelial dysfunction through several mechanisms including increase in endothelial production of ROS, and decrease in endothelial nitric oxide. Our in vitro data in endothelial cells, and ex vivo data in vascular rings, taken together, combined with the known role of endothelial p66shc in mediating endothelial dysfunction, suggests that APE1 mitigates endothelial dysfunction induced by PKC.
Some evidence suggests that APE1 expression and subcellular localization are finely tuned. The subcellular distribution of APE1 may be regulated by both nuclear import and export.41 APE1 is a ubiquitous protein, but its expression pattern differs based on the cell type. APE1 subcellular localization is mainly nuclear, but cytoplasmic staining has also been reported, the latter being associated with mitochondria and/or the endoplasmic reticulum.42 Subcellular localization of APE1 is crucial for its activity. In the present study, we investigated how APE1 is involved in the regulation of p66shc activation. Basal cytoplasmic APE1 was ∼20% of nuclear APE1 in endothelial cells. However, exposure to PKC agonists, such as PMA, increased cytoplasmic APE1, suggesting the cytoplasmic translocation of APE1 in endothelial cells. As acute exposure of PMA for 1 h has limited ability to induce de novo APE1 protein expression, increased cytoplasmic APE1 by PMA would be due to cytoplasmic translocation of APE1 in endothelial cells. Recently, Yuk et al.43 showed that forced cytoplasmic APE1 overexpression profoundly attenuates up-regulation of HMGB1-mediated ROS, cytokine secretion, and cyclooxygenase-2 expression in primary monocytes and macrophage-like THP-1 cell lines. Therefore, it is reasonable that cytoplasmic APE1 has been proposed as a potential therapeutic modality for inflammatory diseases.
In the present study, there was some limitation. PKCβ inhibitors, such as PKCβi and Go6976, did not completely suppress PMA-induced p66shc phosphorylation. Go6976 further suppressed PMA-induced p66shc phosphorylation in APE1-overexpressed endothelial cells, indicating that APE1 inhibition of PMA-induced p66shc phosphorylation may also involve a PKC-independent pathway in addition to PKC pathway. However, the role of APE1 on PKC-independent pathway needs further investigation.
Because APE1 is affected by p66shc phosphorylation in response to oxLDL or PKC, the present finding will reasonably improve the understanding of the crosstalk between APE1 and p66shc and potentially produce therapeutic modes against oxidative vascular diseases.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the Korea Science and Engineering Foundation through the Infection Signaling Network Research Center (R13–2007–020–01000–0 to B.H.J.) and NIH grants (HL070929 and HL094959 to K.I.)
Supplementary Material
Supplementary Data
References
- 1.Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 2.Camici GG, Cosentino F, Tanner FC, Luscher TF. The role of p66Shc deletion in age-associated arterial dysfunction and disease states. J Appl Physiol. 2008;105:1628–1631. doi: 10.1152/japplphysiol.90579.2008. [DOI] [PubMed] [Google Scholar]
- 3.Cutler RL, Liu L, Damen JE, Krystal G. Multiple cytokines induce the tyrosine phosphorylation of Shc and its association with Grb2 in hemopoietic cells. J Biol Chem. 1993;268:21463–21465. [PubMed] [Google Scholar]
- 4.Pelicci G, Lanfrancone L, Grignani F, McGlade J, Cavallo F, Forni G, et al. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992;70:93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- 5.Rozakis-Adcock M, McGlade J, Mbamalu G, Pelicci G, Daly R, Li W, et al. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992;360:689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- 6.Kao AW, Waters SB, Okada S, Pessin JE. Insulin stimulates the phosphorylation of the 66- and 52-kilodalton Shc isoforms by distinct pathways. Endocrinology. 1997;138:2474–2480. doi: 10.1210/endo.138.6.5203. [DOI] [PubMed] [Google Scholar]
- 7.Yang CP, Horwitz SB. Taxol mediates serine phosphorylation of the 66-kDa Shc isoform. Cancer Res. 2000;60:5171–5178. [PubMed] [Google Scholar]
- 8.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 9.Skulachev VP. The p66shc protein: a mediator of the programmed death of an organism? IUBMB Life. 2000;49:177–180. doi: 10.1080/713803613. [DOI] [PubMed] [Google Scholar]
- 10.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Giovannini C, Scazzocchio B, Matarrese P, Vari R, D'Archivio M, Di Benedetto R, et al. Apoptosis induced by oxidized lipids is associated with up-regulation of p66Shc in intestinal Caco-2 cells: protective effects of phenolic compounds. J Nutr Biochem. 2008;19:118–128. doi: 10.1016/j.jnutbio.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Harja E, Chang JS, Lu Y, Leitges M, Zou YS, Schmidt AM, et al. Mice deficient in PKCbeta and apolipoprotein E display decreased atherosclerosis. FASEB J. 2009;23:1081–1091. doi: 10.1096/fj.08-120345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, et al. Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- 14.Ceolotto G, Gallo A, Miola M, Sartori M, Trevisan R, Del Prato S, et al. Protein kinase C activity is acutely regulated by plasma glucose concentration in human monocytes in vivo. Diabetes. 1999;48:1316–1322. doi: 10.2337/diabetes.48.6.1316. [DOI] [PubMed] [Google Scholar]
- 15.Scivittaro V, Ganz MB, Weiss MF. AGEs induce oxidative stress and activate protein kinase C-beta(II) in neonatal mesangial cells. Am J Physiol Renal Physiol. 2000;278:F676–F683. doi: 10.1152/ajprenal.2000.278.4.F676. [DOI] [PubMed] [Google Scholar]
- 16.Braiman L, Sheffi-Friedman L, Bak A, Tennenbaum T, Sampson SR. Tyrosine phosphorylation of specific protein kinase C isoenzymes participates in insulin stimulation of glucose transport in primary cultures of rat skeletal muscle. Diabetes. 1999;48:1922–1929. doi: 10.2337/diabetes.48.10.1922. [DOI] [PubMed] [Google Scholar]
- 17.Bandyopadhyay G, Standaert ML, Galloway L, Moscat J, Farese RV. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721–4731. doi: 10.1210/endo.138.11.5473. [DOI] [PubMed] [Google Scholar]
- 18.Jeon BH, Irani K. APE1/Ref-1: versatility in progress. Antioxid Redox Signal. 2009;11:571–574. doi: 10.1089/ars.2008.2223. [DOI] [PubMed] [Google Scholar]
- 19.Tell G, Quadrifoglio F, Tiribelli C, Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal. 2009;11:601–620. doi: 10.1089/ars.2008.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angkeow P, Deshpande SS, Qi B, Liu YX, Park YC, Jeon BH, et al. Redox factor-1: an extra-nuclear role in the regulation of endothelial oxidative stress and apoptosis. Cell Death Differ. 2002;9:717–725. doi: 10.1038/sj.cdd.4401025. [DOI] [PubMed] [Google Scholar]
- 21.Ozaki M, Suzuki S, Irani K. Redox factor-1/APE suppresses oxidative stress by inhibiting the rac1 GTPase. FASEB J. 2002;16:889–890. doi: 10.1096/fj.01-0664fje. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Chen J, Zhao T, Fan Z. Granzyme K degrades the redox/DNA repair enzyme Ape1 to trigger oxidative stress of target cells leading to cytotoxicity. Mol Immunol. 2008;45:2225–2235. doi: 10.1016/j.molimm.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Kim CS, Son SJ, Kim EK, Kim SN, Yoo DG, Kim HS, et al. Apurinic/apyrimidinic endonuclease1/redox factor-1 inhibits monocyte adhesion in endothelial cells. Cardiovasc Res. 2006;69:520–526. doi: 10.1016/j.cardiores.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Hall JL, Wang X, Van A, Zhao Y, Gibbons GH. Overexpression of Ref-1 inhibits hypoxia and tumor necrosis factor-induced endothelial cell apoptosis through nuclear factor-kappab-independent and -dependent pathways. Circ Res. 2001;88:1247–1253. doi: 10.1161/hh1201.091796. [DOI] [PubMed] [Google Scholar]
- 25.Lee HM, Jeon BH, Won KJ, Lee CK, Park TK, Choi WS, et al. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Circ Res. 2009;104:219–227. doi: 10.1161/CIRCRESAHA.108.178699. 215p following 227. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka M, Sagawa S, Hoshi J, Shimoma F, Matsuda I, Sakoda K, et al. Synthesis of anilino-monoindolylmaleimides as potent and selective PKCbeta inhibitors. Bioorg Med Chem Lett. 2004;14:5171–5174. doi: 10.1016/j.bmcl.2004.07.061. [DOI] [PubMed] [Google Scholar]
- 27.Jeon BH, Gupta G, Park YC, Qi B, Haile A, Khanday FA, et al. Apurinic/apyrmidinic endonuclease 1 regulates endothelial NO production and vascular tone. Circ Res. 2004;95:902–910. doi: 10.1161/01.RES.0000146947.84294.4c. [DOI] [PubMed] [Google Scholar]
- 28.Song YJ, Lee JY, Joo HK, Kim HS, Lee SK, Lee KH, et al. Tat-APE1/ref-1 protein inhibits TNF-alpha-induced endothelial cell activation. Biochem Biophys Res Commun. 2008;368:68–73. doi: 10.1016/j.bbrc.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 29.Lee SK, Kim HS, Song YJ, Joo HK, Lee JY, Lee KH, et al. Alteration of p66shc is associated with endothelial dysfunction in the abdominal aortic coarctation of rats. FEBS Lett. 2008;582:2561–2566. doi: 10.1016/j.febslet.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Bilder GE, Kasiewski CJ, Perrone MH. Phorbol-12,13-dibutyrate-induced vasoconstriction in vivo: characterization of response in genetic hypertension. J Pharmacol Exp Ther. 1990;252:526–530. [PubMed] [Google Scholar]
- 31.Camici GG, Schiavoni M, Francia P, Bachschmid M, Martin-Padura I, Hersberger M, et al. Genetic deletion of p66(Shc) adaptor protein prevents hyperglycemia-induced endothelial dysfunction and oxidative stress. Proc Natl Acad Sci USA. 2007;104:5217–5222. doi: 10.1073/pnas.0609656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tomilov AA, Bicocca V, Schoenfeld RA, Giorgio M, Migliaccio E, Ramsey JJ, et al. Decreased superoxide production in macrophages of long-lived p66Shc knock-out mice. J Biol Chem. 2010;285:1153–1165. doi: 10.1074/jbc.M109.017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graiani G, Lagrasta C, Migliaccio E, Spillmann F, Meloni M, Madeddu P, et al. Genetic deletion of the p66Shc adaptor protein protects from angiotensin II-induced myocardial damage. Hypertension. 2005;46:433–440. doi: 10.1161/01.HYP.0000174986.73346.ba. [DOI] [PubMed] [Google Scholar]
- 34.Napoli C, Martin-Padura I, de Nigris F, Giorgio M, Mansueto G, Somma P, et al. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc Natl Acad Sci USA. 2003;100:2112–2116. doi: 10.1073/pnas.0336359100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin-Padura I, de Nigris F, Migliaccio E, Mansueto G, Minardi S, Rienzo M, et al. p66Shc deletion confers vascular protection in advanced atherosclerosis in hypercholesterolemic apolipoprotein E knockout mice. Endothelium. 2008;15:276–287. doi: 10.1080/10623320802487791. [DOI] [PubMed] [Google Scholar]
- 36.Edwards AS, Faux MC, Scott JD, Newton AC. Carboxyl-terminal phosphorylation regulates the function and subcellular localization of protein kinase C betaII. J Biol Chem. 1999;274:6461–6468. doi: 10.1074/jbc.274.10.6461. [DOI] [PubMed] [Google Scholar]
- 37.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckman JA, Goldfine AB, Gordon MB, Garrett LA, Creager MA. Inhibition of protein kinase Cbeta prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002;90:107–111. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]
- 39.Kouroedov A, Eto M, Joch H, Volpe M, Luscher TF, Cosentino F. Selective inhibition of protein kinase Cbeta2 prevents acute effects of high glucose on vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 2004;110:91–96. doi: 10.1161/01.CIR.0000133384.38551.A8. [DOI] [PubMed] [Google Scholar]
- 40.Huang C, Chang JS, Xu Y, Li Q, Zou YS, Yan SF. Reduction of PKCbetaII activity in smooth muscle cells attenuates acute arterial injury. Atherosclerosis. 2010;212:123–130. doi: 10.1016/j.atherosclerosis.2010.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson EB, Theriot CA, Chattopadhyay R, Mitra S, Izumi T. Analysis of nuclear transport signals in the human apurinic/apyrimidinic endonuclease (APE1/Ref1) Nucleic Acids Res. 2005;33:3303–3312. doi: 10.1093/nar/gki641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7:367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 43.Yuk JM, Yang CS, Shin DM, Kim KK, Lee SK, Song YJ, et al. A dual regulatory role of apurinic/apyrimidinic endonuclease 1/redox factor-1 in HMGB1-induced inflammatory responses. Antioxid Redox Signal. 2009;11:575–588. doi: 10.1089/ars.2008.2196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data