Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells (original) (raw)
. Author manuscript; available in PMC: 2011 Jul 20.
Summary
The CD4+Foxp3+ lineage of regulatory T (Treg) cells plays a key role in controlling immune and autoimmune responses. Treg cells originate primarily during T cell differentiation in the thymus, but conversion of mature T lymphocytes to Foxp3-positivity can be elicited by several means, including activation in the presence of transforming growth factor (TGF)β in vitro. Retinoic Acid (RA), the ubiquitous morphogen that exerts a particular shepherding effect on the gut immune system, increases TGFβ–induced expression of Foxp3, an effect shown here to be mediated through RA receptor (RAR)α. Part of RA’s influence may be due to it’s ability to down-modulate the receptor for IL-6, a cytokine that inhibits Foxp3 expression, but this effect appeared to be of relatively minor importance. Rather, RA negatively affected a population of CD4+ cells with a CD44hi phenotype, akin to that of memory or effector cells, which inhibited the TGFβ-induced conversion of naïve CD4+ T cells. This “contra-conversion” activity was mediated, at least in part, through the synthesis of a set of cytokines (IL-4, IL-21, IFNγ), which in combination had a potent dampening effect on Foxp3 induction. RA, via RARα, elicited a coordinated shut-down of the whole program of cytokine expression in CD44hi cells. The in vivo relevance of this observation was established by transferring RA-sensitive OT-II T cells, which showed less effective conversion to Foxp3+ in RARα-deficient hosts. Thus, CD44hi T cells can actively restrain the induction of Foxp3, and this balance can be shifted or fine tuned by RA.
Introduction
Foxp3+CD4+ regulatory T (Treg) cells are central to the maintenance of immunological homeostasis and tolerance in the T lymphocyte compartment (Sakaguchi et al., 2006). This role is exemplified by the devastating lymphoproliferation and multi-organ autoimmunity that occur in mice or humans deficient in this population, whether carrying spontaneous mutations (scurfy mice, IPEX patients (Ziegler, 2006)) or after experimental lineage ablation (Kim et al., 2007). A distinct gene-expression signature characterizes Foxp3+ Treg cells (Fontenot et al., 2005; Huehn et al., 2004; Herman et al., 2004; Hill et al., 2007). Foxp3 plays an important role in determining this signature, but is not the master regulator it was once thought to be, as it is neither sufficient to elicit the full Treg genomic profile nor strictly necessary for generation of the lineage (Gavin et al., 2007; Lin et al., 2007; Hill et al., 2007).
Most of the Treg cells present in lymphoid organs of normal mice are generated in the thymus (Hsieh et al., 2006; Pacholczyk et al., 2006; Wong et al., 2007a), and the specific TCR repertoire that distinguishes them from conventional CD4+ T cells (Tconv) can be tracked from the thymus to peripheral lymphoid organs (Hsieh et al., 2006; Pacholczyk et al., 2006; Wong et al., 2007b). In addition, mature CD4+ T cells from peripheral lymphoid organs can be converted to Foxp3-positivity in a variety of conditions: chronic suboptimal stimulation by agonist peptide (Kretschmer et al., 2005; Apostolou and von Boehmer, 2004), exposure to agonist administered orally (Mucida et al., 2005; Coombes et al., 2007), or during lymphopenia-driven homeostatic expansion (Sun et al., 2007). Finally, activation in the presence of IL-2 and TGFβ in vitro can induce Foxp3 expression in naïve Tconv cells, which then acquire some characteristics of Treg cells, including suppressive properties in some contexts (Chen et al., 2003; Fantini et al., 2004; Wan and Flavell, 2005). On the other hand, Foxp3 expression in TGFβ-induced Treg cells is unstable (Floess et al., 2007), these cells are not suppressive in all assays, and converted cells acquire only a partial segment of the genomic signature typical of Treg cells (Hill et al., 2007).
Most interesting in this context was the observation that dendritic cells from gut origin, in particular a CD103+ population from the lamina propria (LP), can significantly enhance TGFβ-induced conversion of CD4+ T cells to the Foxp3+ phenotype in vitro, and that this effect can be ascribed to all-trans retinoic acid (RA), which also represses differentiation to an IL17-secreting phenotype (Mucida et al., 2007; Sun et al., 2007; Coombes et al., 2007; Benson et al., 2007; Elias et al., 2008). RA, the key metabolite of Vitamin A, is an important morphogen that impacts the development and maintenance of a wide variety of tissues, as exemplified by the pleiotrophic abnormalities that appear in Vitamin-A-deficient embryos or adults (reviewed in (Mark et al., 2006)). Concerning hematopoietic cells, RA can have general stimulatory effects on lymphocyte responses, possibly by inhibiting apoptotic pathways (Iwata et al., 2004), and affects natural killer (NK) activity by modulating interferons and NK cell ligands (Abb et al., 1982a; Abb et al., 1982b; Cerwenka et al., 2000). In addition, RA seems to play a predominant role in the homeostasis and homing of lymphoid populations of the gut-associated lymphoid tissue (GALT). It is synthesized in abundance by gut dendritic cells (Iwata et al., 2004; Coombes et al., 2007), induces the specific gut-homing molecules CCR9 and α4β7 integrin on T cells, and also promotes GALT-related functions in B cells (Iwata et al., 2004; Mora et al., 2006). RA’s important role in controlling Foxp3 expression mediated by TGFβ also suggests that the GALT has evolved a specific system to maintain a balanced symbiosis between the gut flora and the immune system (Iwata et al., 2004; Mora et al., 2006; Mucida et al., 2007; Sun et al., 2007; Coombes et al., 2007; von Boehmer, 2007),
Retinoic acid receptors (RARs) belong to the family of nuclear hormone receptors, and act as ligand-dependent transcriptional regulators. There are three subtypes of RARs (RARα, RARβ and RARγ) (Chambon, 1994), all of which bind all-trans RA at high affinity, but each has distinct developmental effects and genomic footprints. RARα and RARγ are the predominant forms expressed in immune cells (Purton et al., 2006). More recently, it has been realized that RA can also serve as an activating ligand for the PPARβ/δ receptor, with different effects on cell growth and apoptosis (Schug et al., 2007).
In this context, it was clearly of interest to investigate the molecular mechanisms by which RA promotes Foxp3 expression mediated by TGFβ. Beyond their direct effects as transactivators, RARs also influence transcription by transrepression of AP1 activity (Nicholson et al., 1990; Salbert et al., 1993; Chen et al., 1995; Altucci and Gronemeyer, 2001). This could conceivably impact Foxp3 expression in many ways; by altering signals induced by T cell costimulation (Wu et al., 2006): or by disturbing the competition between AP1 and Foxp3 for NFAT binding (von Boehmer, 2007), and therefore any down-stream events. Alternatively, liganded RARs could potentiate TGFβ signaling. The explorations reported here started from these premises, but ended up with a rather different conclusion, unveiling a multi-cell interplay underlying RA’s action.
RESULTS
Treg conversion and homeostasis in RAR deficient mice
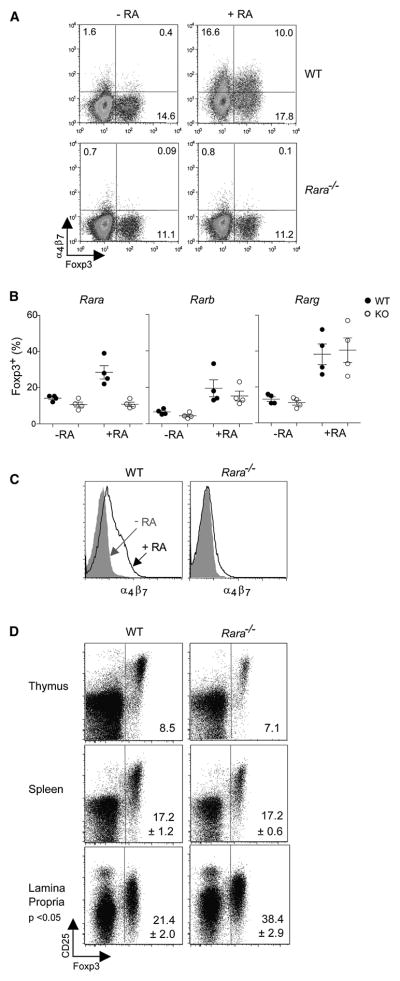
Three main nuclear receptors for RA have been described. Recent studies, using pharmacological inhibitors, have implicated RARα in RA-boosted Foxp3 induction (Schambach et al., 2007; Kang et al., 2007; Elias et al., 2008), but we felt it worthwhile to exploit genetically deficient mice to evaluate the individual contributions of the three receptors, and to asses the impact of the RA pathway on Treg populations in vivo. Thus, knockout mice with loss-of-function mutations in RARα, RARβ or RARγ (Chapellier et al., 2002c; Chapellier et al., 2002a; Chapellier et al., 2002b) were bred and analyzed. Purified CD4+Foxp3− T cells from each of the knockout lines (or their control littermates) were stimulated in vitro by anti-CD3 monoclonal antibodies together with splenic DCs and TGFβ, with or without RA. As expected, the proportion of Foxp3+ cells was boosted by RA in cultures from control littermates and from RARβ- and RARγ-deficient mice, but the effect was completely abrogated by the RARα-knockout mutation (Fig. 1A, B). Similarly, the induction of α4β7 integrin by RA was dependent on RARα (Fig. 1C).
Fig. 1. RARα is responsible for the enhanced conversion mediated by retinoic acid.
CD4+CD25− T cells were sorted and cultured in vitro with CD11c+ dendritic cells (ratio 10:1 T cell to DC) in the presence of anti-CD3 antibody (1μg/ml) and TGFβ (10 ng/ml), with or without 10nM RA for 5 days. A) Foxp3 and α4β7 expression is reduced in CD4+ T cells when cells are obtained from RARα KO mice compared to WT littermate controls (representative FACS plot from 2 or more independent experiments). B) Summary of TGFβ mediated conversion in cultures from WT or RARα, RARβ or RARγ KO mice treated with or without RA (p<0.05, students t-test). C) RARα also controls the expression of α4β7 expression in cultures treated with TGFβ and RA (representative FACS plot from 2 or more independent experiments). D) RARα deletion in mice alters the proportion of CD4+Foxp3+ T cells in the lamina propria, but not the thymus or spleen. Organs were processed from RARα +/+ or −/− mice and Foxp3 and CD25 expression on CD4+ T cells was determined by FACS. Numbers in the gates represent the mean (+/− SD where applicable) for Foxp3 expression (thymus, n=2, spleen, n=5, and lamina propria, n=8), and p-value was determined by t-test.
To investigate the impact of this blockade of RA signaling on the selection and steady-state levels of Treg populations in vivo, we analyzed CD4+ lymphocytes from the thymus, secondary lymphoid organs and GALT for expression of Foxp3 and CD25 (Fig. 1D). Positive selection of Treg cells in the thymus appeared unaffected, with the usual proportion of Foxp3+ cells among CD4+CD3hi thymocytes. Steady-state levels of Treg cells were not decreased in the lymphoid organs of RARα-KO mice, and if anything showed an increase in the LP. Thus, a deficiency in signaling through the RARα did not decrease the overall frequency of Treg cells, whose steady-state levels appear to be set by other factors. Correspondingly, RARα-deficient mice do not present with obvious autoimmune manifestations.
Impact of RA on the Treg signature
As reported previously, Foxp3-positive cells induced by TGFβ in the presence of IL2 and TCR activation cannot be equated to bone fide Treg cells, as they are missing important elements of the Treg gene-expression signature (Hill et al., 2007); TGFβ-induced Foxp3 expression is unstable and can be rapidly lost upon removal of the cytokine, consistent with a different chromatin state at the Foxp3 locus (Floess et al., 2007). One possible explanation for the RARα-mediated effect of RA was that it allowed converted cells to acquire the “missing segment” of the Treg signature, thus resulting in a more complete and stable phenotype. (In preliminary experiments, cells converted in the presence of LP DCs did appear to better maintain their Foxp3+ phenotype upon in vivo transfer than did those generated in the presence of spleen (Sp) DCs; see also (Benson et al., 2007)) Thus, we compared the gene-expression profiles of Foxp3+ cells, generated by 5 days of TGFβ treatment from sorted CD4+Foxp3− cells, in the presence of LP or Sp DCs, the latter with or without 10 nM RA. The gene-expression profiles were obtained from purified T cell populations, using Foxp3-eGFP reporter mice as donors. Replicates were generated, hybridized to Affymetrix M430v2 microarrays, and RMA-normalized expression values were used for analysis (all datasets deposited at NCBI/GEO under accession # XXX). We focused particularly on the transcripts that constitute the robust “Treg signature”, derived from multiple datasets in a previous study (Hill et al., 2007).
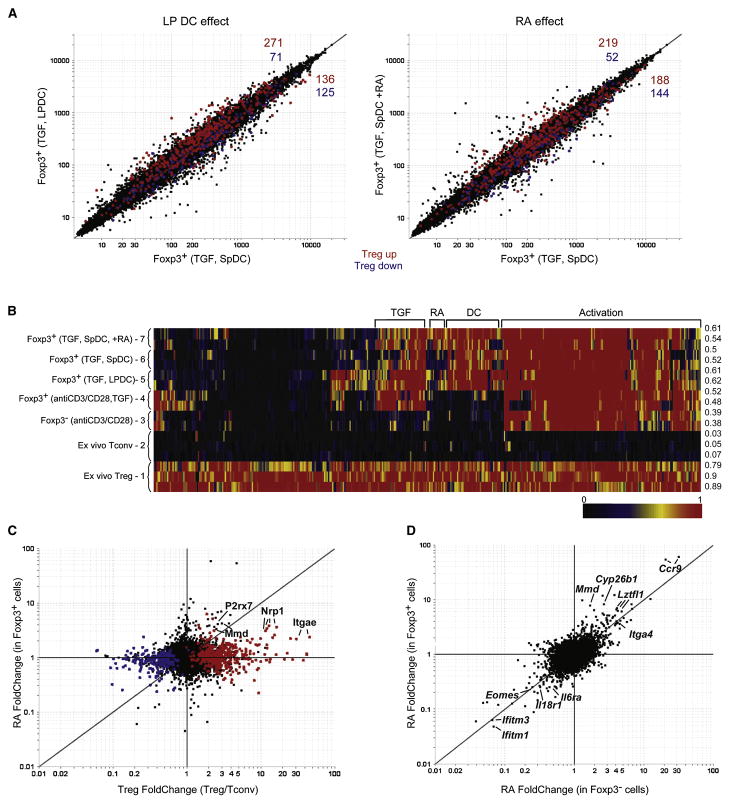
In the graphs of Fig. 2A, the expression profiles of Foxp3+ cells generated with TGFβ and LP DCs (left) versus RA supplementation (right) are compared in parallel to those from Foxp3+ cells generated simply with TGFβ and splenic DCs, a condition that leads to less conversion (Sun et al., 2007). Although there was no general displacement, a subtle but distinct off-diagonal shift of the Treg signature transcripts was observed, those normally over-represented in Treg cells being as a group slightly more expressed in samples from the LP DC co-cultures (red dots, 271 over- vs 125 under-expressed, χ2 p= 1.5 10−11). Accordingly, transcripts under-represented in Treg cells were also under-expressed in Foxp3+ cells from LP DC co-cultures (blue dots, 125 vs 71, p= 1.2 10−4). These biases were also observed with Treg signature transcripts in Foxp3+ cells from RA-supplemented cultures (219 vs 188, 52 vs 144, χ2 p= 4.9 10−11). A direct comparison of transcripts affected by retinoic acid or LP DCs showed a clear parallel, supporting the notion that the ability of LP DCs to enhance Foxp3 expression is due to the production of RA (Figure S1). Thus, the presence of RA did appear to reinforce slightly the Treg gene-expression signature. But did it promote complementation of the “holes”, i.e. those transcripts normally over-expressed in Tregs that are not induced in TGFβ-elicited Foxp3+ cells (Hill et al., 2007). This question was addressed by the SignatureMatch analysis of Fig. 2B. This algorithm is designed to test how well a signature is achieved in test populations. It uses normalized expression values, that are then standardized relative to two reference populations that define the expression minima and maxima for each transcript of the signature (here ex vivo Treg and Foxp3- Tconv cells, pops. 1 and 2), In agreement with our previous report, in vitro activation in the presence of IL-2 (pop. 3) induces a significant fraction of the Treg signature, and the addition of TGFβ (pop. 4) only brings forth a minor subset of Treg signature transcripts (mainly in the TGF bracket). Although there was an additional impact of LP DCs in the cultures (pop. 5), partially mimicked by Sp DCs + RA (pop. 7), much of the Treg signature transcripts remained at the basal level of Tconv cells. Thus, the “holes” in the signature elicited by activation in the presence of TGFβ largely persist in spite of RA. The FoldChange plots of Fig. S2 confirm this impression, and there are only substantial changes in a small number of Treg signature genes. This inability of RA to complement the partial effect of TGFβ on the Treg signature is also evident from the representation of Fig. 2C, which compares the effect of RA (in the presence of TGFβ) to the Treg profile: only a few genes of the Treg signature were directly affected in any sizeable manner by RA (e.g. Nrp1, P2rx7).
Fig. 2. Retinoic acid influences the expression of a discrete group of genes that are largely independent of the canonical Treg cell genomic signature.
A) Comparison of probe expression values in Foxp3+ T cells after culture with anti-CD3, TGFβ and: lamina propria DCs (y-axis) or spleen DCs (x-axis), left pannel; or spleen DCs with (y-axis) or without (x-axis) retinoic acid (100 nM), right pannel. Probes highlighted in red and blue correspond to genes either up or down-regulated (respectively) in the canonical Treg cell genomic signature. B) “Signature Match” Heat map analysis of the Treg cell genomic signature in ex vivo Treg cells or TGFβ converted cells (Foxp3+, anti-CD3/CD28, TGF, and Foxp3-, anti-CD3/CD28 are from (Hill et al., 2007)). Raw expression values were normalized to 1 or zero for ex vivo Treg cells or Tcov cells respectively. The expression values for genes from the TGFβ converted cells were normalized within this range (for a detailed description of the algorithm see supplemental experimental procedures) and displayed as a heat map where red represents the expression of a gene at the same or a greater level than what is found in an ex vivo Treg cell, while black is the expression of a gene that is at the same or lower level than an ex vivo Tconv cell. Numbers to the right of the diagram show the average score for each group across the entire signature. C) FcFc plot comparing the effects of retinoic acid in Foxp3+ TGFβ converted cells (y-axis) with ex vivo Treg cells (x-axis). D) FcFc plot comparing the effects of retinoic acid in Foxp3+ (y-axis) and Foxp3-(x-axis) TGFβ treated cells.
Most of the changes elicited by RA were independent of the Treg signature, a conclusion bolstered by the plot of Fig. 2D, which compares the effects of RA in Foxp3+ cells versus in the cells remaining Foxp3-negative in those cultures: RA-responsive transcripts were very similar in both cell types. The RA signature (listed in Supplementary Table S1 and S2) included some of the transcripts one expects to be induced, such as Ccr9 and Itga4 (α4 integrin), but also some repressed transcripts. It did not show any significant overlap with the “TGFβ signature”, i.e. the transcripts affected by TGFβ treatment independently of Treg conversion (Hill et al., 2007), indicating that RA does not enhance conversion by increasing the intensity of TGFβ signaling. On the other hand, the observation that the IL-6 receptor was down-regulated by 3.7-fold was quite suggestive, since IL-6, in the presence of TGFβ, promotes the expression of IL-17, while inhibiting that of Foxp3 (Veldhoen et al., 2006; Bettelli et al., 2006; Mangan et al., 2006; Stockinger and Veldhoen, 2007).
IL-6: only a minor player in RA’s action
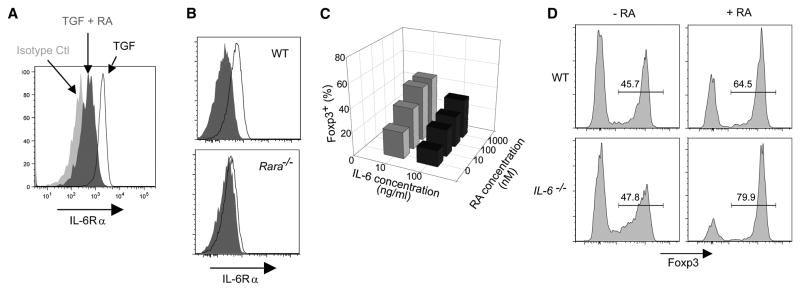
The repression of IL-6R by RA observed in the gene expression profiles suggested a mechanism for RA’s action: since IL-6 inhibits the induction of Foxp3, RA might simply be reducing the sensitivity of naïve T cells to inhibition by IL-6. We first confirmed by flow cytometry that the alpha chain of IL-6R was indeed repressed by RA (Fig. 3A; over 6 experiments, the mean fluorescence intensity dropped from 526 +/− 116 to 255 +/− 81, p<0.001). This regulation of IL-6R expression was also lost when testing cells from RARαKO mice (Fig. 3B). Under our culture conditions, addition of recombinant IL-6 did have the expected inhibitory effect on Foxp3 expression, and this influence was partially reversed by RA, consistent with the notion that RA might help to relieve the inhibition by IL-6 (Fig. 3C). On the other hand, RA was still very effective in cultures of cells from IL-6-deficient mice; if anything, its influence was even stronger than with cultures from WT mice (Fig. 3D). Thus, an inhibition of IL-6 action, while it may partially contribute to RA’s impact on Foxp3 expression, particularly in IL-6-rich environments, cannot be the main mechanism through which RA promotes TGFβ mediated Foxp3 expression.
Fig. 3. Retinoic acid down-regulates the IL-6Rα but enhancement of Foxp3 expression by RA occurs in the absence of IL-6.
A) Expression of IL-6Rα is down-regulated in CD4+ T cells by retinoic acid. Splenocytes (1×105) were activated with anti-CD3/CD28 beads in the absence (TGF, open histogram) or presence of RA (TGF +RA, dark grey histogram). Representative FACS plots are shown (n=6 independent experiments). B) CD4+ T cells from retinoic acid receptor alpha deficient (RARα KO) mice do not down regulate the IL-6R after culture with RA. CD4+ T cells (0.5 × 105) from wild-type (WT) or RARαKO mice were activated with anti-CD3/CD28 beads and TGFβ (10 ng/ml) in the absence (open histogram) or presence (filled histogram) of retinoic acid (100nM). Representative FACS plots are shown (n=3 independent experiments). C) Retinoic acid prevents the inhibition of Foxp3 expression mediated by IL-6 in CD4+ T cells activated with TGFβ. CD4+ T cells (0.5 × 105) were activated with anti-CD3/CD28 beads and various concentrations of RA. After 24hrs TGFβ (10 ng/ml) and IL-6 were added and cells were cultured for an additional 4 days. Representative FACS data is shown for Foxp3 expression in CD4+ T cells (n=3 independent experiments). D) Retinoic acid enhances Foxp3 expression in CD4+ T cells in the absence of IL-6. Splenocytes from wild-type or IL-6KO mice were stimulated (as in A) and analyzed 4 days later for Foxp3 expression in CD4+ T cells. Representative FACS plots are shown (n=3 independent experiments).
RA acts indirectly on Treg conversion
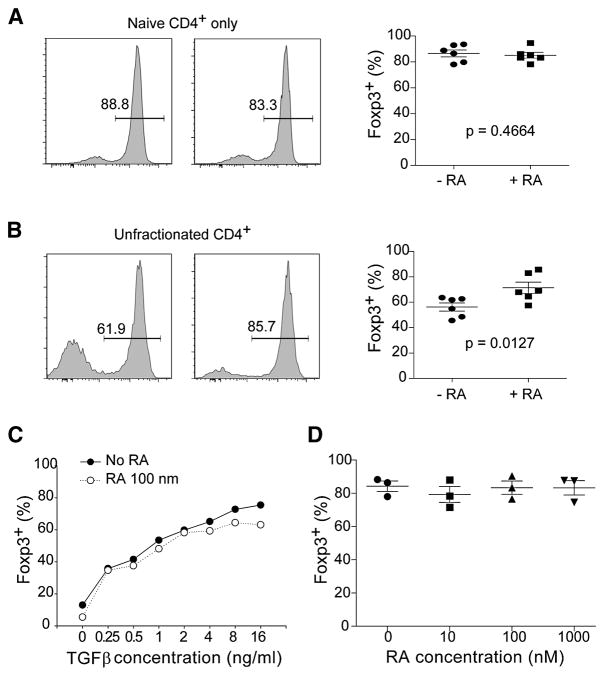
The rather limited impact of RA on transcriptional profiles of converted Treg cells raised the possibility of an indirect effect, acting on something other than the responding cells. This hypothesis emerged to the forefront by a serendipitous observation, reported in Fig. 4. In experiments designed to test the effect of RA on isolated CD4+ T cell subsets, we observed that the enhancing effect of RA was lost when the fully naïve fraction of CD4+ cells (purified as CD44loCD62Lhi) was used as responders (Fig. 4A) rather than CD4+ T cells from unfractionated splenocytes (Fig. 4B). Also apparent in these experiments was that purified naïve CD4+ cells gave rise to a higher proportion of Foxp3+ convertants when cultured alone than when cultured as part of unfractionated splenocytes (compare the left panels of Fig. 4A and 4B). The insensitivity of naïve CD4+ cells to RA was not due to a saturating response to TGFβ, as it was also observed through suboptimal doses of TGFβ (Fig. 4C). Similarly, the conversion of purified naïve cells could not be boosted through a range of RA doses (Fig. 4D). This lack of response did not mean that purified naïve CD4+ T cells were refractory to RA, as CCR9 and the α4β7 integrin were induced effectively in these cells by RA exposure (not shown).
Fig. 4. Retinoic acid acts indirectly on Foxp3 expression.
A) Retinoic acid does not enhance TGFβ mediated Foxp3 expression in purified naïve CD4+ T cells. Naïve CD25−CD44loCD62Lhi CD4+ T cells (0.5 × 105) were activated with anti-CD3/CD28 beads and TGFβ (10 ng/ml) in the absence (left panel) or presence of RA (100 nM) (middle panel) for 4 days. Representative FACS data is shown for Foxp3 expression with the results of multiple experiments plotted in the right panel (n=6, and p-value for student’s t-test). B) Retinoic acid does enhance TGFβ mediated Foxp3 expression in CD4+ T cells from unfractionated splenocytes. Splenocytes (0.5 × 105) were activated in vitro (as in A) in the absence (left panel) or presence of RA (100 nM) (middle panel) for 4 days. Representative FACS data is shown for Foxp3 expression in CD4+ T cells with the results of multiple experiments plotted in the right panel (n=6, and p value for student’s t-test). C) Retinoic acid does not enhance Foxp3 expression in purified naïve CD4+ T cells over a range of TGFβ concentrations. Naïve CD4+ T cells (purified as in A) were activated with anti-CD3/CD28 beads in the presence of 100nM RA and the indicated doses of TGFβ, then analyzed at day 4 for Foxp3 expression by FACS. Representative data is shown for 3 independent experiments. D) Foxp3 expression is not enhanced in naïve CD4+ T cells over a range of retinoic acid doses. Naïve CD4+ T cells were activated (as in A) in the presence of the indicated concentrations of RA, then analyzed for Foxp3 expression by FACS. Summarized data from 3 independent experiments is shown.
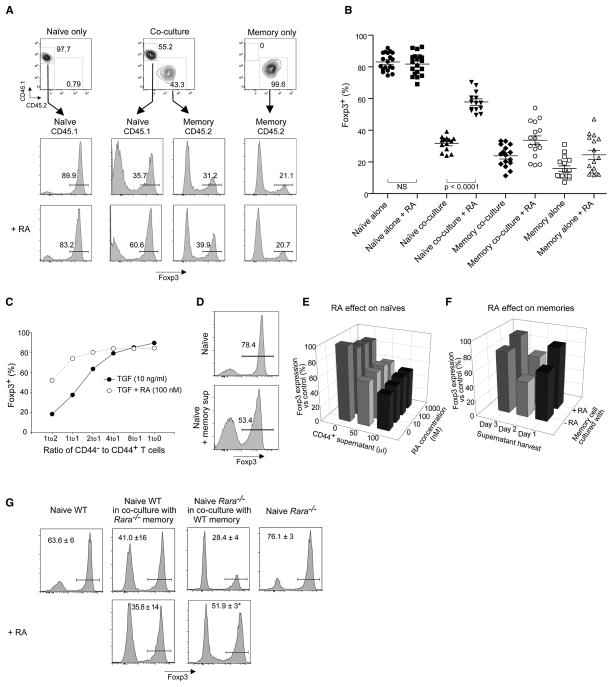
These data suggested that the increased generation of Foxp3+ cells in response to RA in these cultures of unseparated splenocytes might represent the lifting of an inhibition imparted by other cells, a dampening lifted equally by RA or by purification of the naïve responder cells. It seemed likely that the inhibitory cell might also be a CD4+ T cell, as it was clearly possible to obtain strong responses to RA when whole CD4+ populations were used as responders to TGFβ (as in the experiments of Figs 1 and 2, and as in all the initial descriptions of RA’s effect (Mucida et al., 2007; Lufkin et al., 1993; Coombes et al., 2007; Benson et al., 2007; Elias et al., 2008)). This inhibitory population would then be predicted to have a CD44hi memory phenotype, and would thus be lost during the purification of naïve responder cells. We directly tested this hypothesis by the reconstitution experiments depicted in Fig. 5, in which CD4+ cells were purified as CD44-negative or positive (each identifiable through CD45 allotypes so as to track their fate after culture), and admixed in stimulation cultures supplemented or not with RA. The induction of Foxp3 in naïve cells was clearly inhibited by coculture with an equal number of CD44hi cells, an inhibition reversed by the addition of RA (Fig. 5A; several experiments tabulated in Fig. 5B). This inhibitory effect of CD44hi cells (hereafter referred to as “contra-conversion” for short), and its reversal by RA, titrated through a range of cell ratios (Fig. 5C). This dose-response range is compatible with the results obtained with whole CD4+ cells (e.g. Fig. 4), where the 44−/44+ ratio was approximately 4.
Fig. 5. CD44hiMemory CD4 T cells restrain TGFβ mediated Foxp3 expression in naïve CD4 T cells.
A) Naïve CD25−CD44loCD62Lhi CD4+ T cells (0.5 × 105) were activated with anti-CD3/CD28 beads and TGFβ (10 ng/ml) in the absence or presence of RA (100 nM). These cells were either cultured alone, or in combination with CD25−CD44hiD62Llo memory CD4+ T cells (0.5 × 105 memory cells to 0.25 × 105 naïve cells) for 4 days. For comparison, CD44hi memory T cells were also cultured alone (under identical conditions as naïve cells cultured alone). Individual populations were tracked using CD45.1 or CD45.2 congenic markers. Foxp3 expression in congenically marked populations was determined by FACS and a representative experiment is shown. B) FACS analysis for Foxp3 expression in multiple experiments as described in A (n=14–19). Statistically significant differences, with p-values < 0.05 determined by student’s t-test, were found between all of the groups except for ▲ vs ○, and ◆ vs △. C) The contra-conversion effect of CD44hi memory T cells is dose dependent and parallels the responsiveness to RA. Co-culture experiments with congenically marked CD44hi memory or naïve T cells were performed in the absence or presence of RA and using different ratios of CD44hi memory T cells to naïve T cells (0.5 × 105). Cells were activated (as in A) and analyzed for Foxp3 expression 4 days later. Data presented is representative of 3 independent experiments. D) Supernatant from memory T cells activated with anti-CD3/CD28 beads for 48hrs can inhibit Foxp3 expression in naïve T cells. E) Retinoic acid does not act on the responsiveness of naïve T cells to the soluble inhibitory factor produced by memory T cells. Naïve T cells were activated with anti-CD3/CD28 beads and the indicated concentrations of retinoic acid for 24 hrs, then cultured in the presence of TGFβ (10 ng/ml) and the indicated concentration of supernatant from memory T cells for 4 days. Representative FACS data from one of 3 experiments is shown. Data is presented as percent Foxp3 expression in the test condition relative to that found in naïve T cells alone (control). F) Retinoic acid inhibits the production of the soluble factor produced by memory T cells. Memory T cells were activated and cultured in the presence or absence of RA. Supernatant were harvested at different time points and added to naïve T cells in the conversion assay. Representative FACS data from one of 3 experiments is shown. Data is presented as percent Foxp3 expression in the test condition relative to that found in naïve T cells alone (control). G) Retinoic acid receptor alpha mediated signaling in CD44hi memory T cells is necessary to enhance Foxp3 expression in naïve cells. Criss-cross experiments with RARα KO memory (left panel) or naïve (right panel) CD4+ T cells in co-culture with congenically marked WT naïve (left panel) or memory (right panel) CD4+ T cells were conducted as described in A). No enhancement of Foxp3 expression was seen when RARα KO memory CD4+ T cells were cultured with WT naïve CD4+ T cells in the presence of RA. A significant enhancement (p<0.01) of Foxp3 expression was seen when RARα KO naïve CD4+ T cells were cultured with WT memory CD4+ T cells in the presence of RA. Representative FACS plots are shown with the mean and SD from 3 independent experiments.
We then asked how CD44hi memory T cells influence the TGFβ-induced conversion of naive T cells, the production of a soluble factor being the most likely candidate. Culture supernatants from CD44hi cells grown under the same conditions as the coculture of Fig. 5A (anti-CD3/28, TGFβ) were indeed able to mediate the inhibition of Foxp3 induction (although this dampening by supernatants was never quite as effective as when cells were added directly – compare Figs. 5C and 5D). Preliminary time-course analyses showed that production of the inhibitory factor(s) required stimulation of the CD44hi population, peaking at 48 hrs of culture (not shown). Thus, the impact of CD44hi cells can be explained, at least in part, by the production of soluble inhibitors.
This observation prompted us to ask how RA affects contra-conversion activity. Conceptually, RA might prevent the production of inhibitory factors by CD44hi cells, or it might decrease the sensitivity of responder naïve cells. These two alternatives were tested in the experiments illustrated in Fig. 5E–F. Treatment of the responder naive cells with RA had no impact on their susceptibility to inhibitory supernatant (Fig. 5E). On the other hand, treatment of the CD44hi population did reduce the production of inhibitory factor(s) in the supernatants (Fig. 5F).
As an independent confirmation that RA signaled through the CD44hi population, rather than through the naïve converting cells themselves, we exploited the identification of RARα, as the primary receptor in this context, and conducted mixed cell experiments using naïve or memory populations from RARαKO mice or congenically marked WT mice (Fig. 5G). Under these conditions, expression of Foxp3 in naïve cells from RARαKO mice could still be increased by RA.. On the other hand, RA was without effect when memory cells from RARαKO mice were co-cultured with WT naïve cells. As expected, the genotype of the naïve T cells was immaterial (Fig. 5G) Thus, the primary mode of action of RA in enhancing the conversion to a Foxp3+ phenotype appears to be through the reduction of inhibitory factors produced by CD44hi cells.
Contra-conversion results from synergistic cytokine action
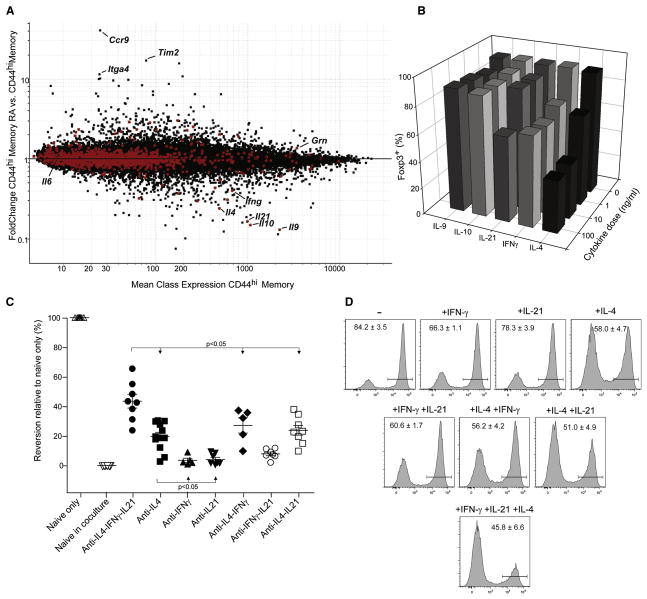
We then sought to identify the soluble factor(s) mediating contra-conversion. Such a factor should have two characteristics: be produced by CD44hi CD4+ cells, and be reduced by RA treatment. Triplicate microarray profiles were generated on CD45 allotype marked CD44hi cells in stimulated cocultures prepared exactly as in Fig. 5, and these were compared with or without RA treatment. The results are displayed on the expression vs FC plot of Fig. 6A, where GeneOntology identifiers were used to identify cytokines and proteins released in the extracellular space (shown as red dots). Several factors immediately stood out in this analysis, matching both criteria of transcript abundance and repression by RA: IL-4, -9, -10, -21 and γ-Interferon (IFNγ). This analysis also confirmed the RA responsiveness of these cells, as RA-responsive transcripts identified in Fig. 2 were also found differentially expressed in CD44hi cells (ie Itga4 and Ccr9). In contrast, the vast majority of secreted proteins were unaffected by RA, or were even induced (e.g. granulin). Contra-conversion was not likely to be mediated by IL-6 since the CD44hiCD4+ cells produced virtually no IL-6 transcript.
Fig. 6. Contra-conversion is mediated by cytokines and alleviated by blocking IL-4, IFNγ and IL-21.
Congenically marked CD44hi memory CD4+ T cells were isolated from the co-culture assay at 48 hrs and processed for microarray analysis. A) Mean expression (CD44hi memory T cells, x-axis) vs FoldChange (CD44hi memory RA/CD44hi memory T cells, y-axis) plot of expression data from memory T cells treated with or without RA in the co-culture assay. Secreted extra-cellular factors identified from gene ontology (GO) analysis are highlighted in red. A) Recombinant cytokines were added individually to cultures of naïve CD4+ T cells activated with anti-CD3/CD28 beads and TGFβ. Cells were harvested at day 4 to test for Foxp3 expression by FACS. Representative data is shown for one of 3 or more experiments. B) Inhibition of cytokine signaling in co-culture experiments using blocking antibodies. Naïve and memory co-culture experiments were performed in the absence or presence of cytokine blocking antibodies (5 μg/ml). Cells were harvested at day 4 to test for Foxp3 expression in congenically marked cells by FACS. Statistically significant differences, with p-values < 0.05 determined by student’s t-test, were found between all of the groups except for ■ vs ◆ or □, and ▲ vs ▽ or ○. C) Recombinant IFNγ (1 ng/ml), IL-4 (1 ng/ml), and IL-21 (10 ng/ml) was added individually, in pairs or all together to cultures of naïve CD4+ T cells activated with anti-CD3/CD28 beads and TGFβ. Cells were harvested at day 4 and tested for Foxp3 expression by FACS. Representative FACS data with average values +/− SEM for 6 individual experiments.
In order to confirm the regulation of expression of some of these cytokines by RA, we performed intracellular staining of the memory cells (identified in the cocultures as CD45.2+) after activation for 48hrs. Clear reductions in IL-10 and IL-4 production were evident in memory T cells treated with retinoic acid (Supplemental Figure S3). Somewhat surprisingly we did not see the same trend for IFNγ, possibly due to a more complex post-transcriptional regulation. Notably, none of these cytokines were detected in naïve CD44− T cells cultured under the same conditions (not shown).
We then tested the effect of these candidate cytokines on TGFβ-induced conversion of naïve CD4+ cells (Fig. 6B). IL-9 and IL-10 had no effect. IL-21 was inhibitory as expected, but only at relatively high doses. IFNγ had a modest effect, but IL-4 led to a marked decrease of Foxp3 expression, consistent with recent studies (Wei et al., 2007; Mantel et al., 2007). Antibody blockade experiments were performed to determine which of these cytokines played the most important part in contra-conversion in coculture experiments (Fig. 6C). Blocking IL-21 or IFNγ individually had little or no effect, but anti-IL-4 antibodies significantly impacted on contra-conversion activity (the importance of IL-4 was also apparent from using CD44hi cells from IL-4-KO mice, Figure S4). Pairwise combinations of antibodies had little more effect than each alone, but blockade of all three cytokines was more effective at eliminating contra-conversion activity, albeit still incompletely (Fig. 6C). It should also be noted that these blocking antibodies did not affect Foxp3 expression in cultures with naïve T cells only, paralleling the insensitivity seen to RA (data not shown).
This result prompted us to further explore whether these cytokines act synergistically to inhibit Foxp3 expression by adding them back at sub-optimal doses to conversion cultures of purified naïve T cells (Fig. 6D). At the doses used, IFNγ and IL-21 had little effect on their own, and IL-4 lead to only a partial reduction in Foxp3 expression. Limited synergy was seen when cytokines were added in pairs, for example IL-21 enhanced the inhibitory effects of IFNγ and IL-4. The largest reduction of Foxp3 expression was seen when all three cytokines were added at once, confirming their 3-way synergy.
Thus, CD44hi memory T cells release of a set of cytokines that inhibit TGFβ-driven Foxp3 expression. Their effect seems to be elicited by their combined and synergistic action, rather than by any one alone, and RA inhibits the transcription of the whole panel.
RA acts indirectly on antigen specific conversion in GALT
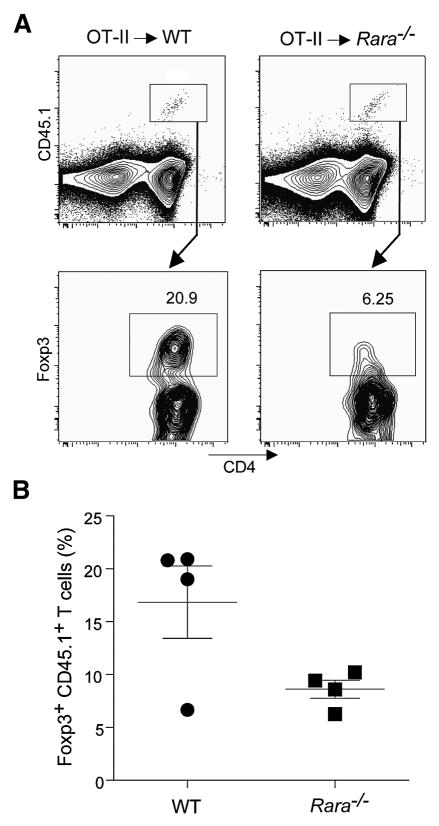
To verify the in vivo relevance of the indirect mode of RA action demonstrated in vitro, we exploited the known role of RARα in the process, asking how the same WT CD4+ T cells would convert when transferred into WT or RARα-deficient hosts. We used as a model Foxp3-negative OT-II T cells transferred into hosts also fed with oral ovalbumin. This model of in vivo antigen specific conversion is restricted to the GALT, as conversion did not occur in subcutaneous lymph nodes or in spleen (Sun et al., 2007). Consistent with previous findings, transferring OT-II RAG T cells (identifiable with a CD45.1 allotype, and devoid of any detectable Foxp3+ cells) to either WT or RARαKO hosts led to detectable conversion in both the lamina propria and mesenteric lymph nodes of ova-fed mice (Fig. 7A; as expected, no conversion was observed in the spleen or other lymph nodes), OT-II cells in WT recipients showied a 2-fold higher proportion of Foxp3-positive cells compared to RARαKO hosts (Fig. 7B). These data provide direct evidence that RA signaling via RARα affects Foxp3 induction in vivo in an indirect manner, through cells other than the naïve CD4+ cells.
Fig. 7. RARα signaling can indirectly alter Foxp3 conversion in vivo.
A) Foxp3− OT-II RAG-1−/− CD45.1 CD4+ T cells were sorted then injected IV into RARα KO mice or WT littermates (0.5–1 × 106 cells/mouse). Mice were given water supplemented with ovalbumin (1.5%) for 7 days after which point they were sacrificed and donor OT-II CD45.1 T cells from various lymphoid organs were analyzed for Foxp3 expression by FACS. Representative FACS plots showing Foxp3 expression in CD45.1+ CD4+ T cells from the mesenteric lymph nodes of either a WT or RARα KO host. B) Summary of Foxp3 expression in donor CD45.1+ OT-II T cells harvested from lamina propria or mesenteric lymph nodes of RARα KO mice or WT littermate controls from multiple experiments.
DISCUSSION
This study set out to elucidate the molecular mechanisms by which RA promotes the TGFβ-induced conversion of naive CD4+ T cells to the Foxp3+ phenotype. Perhaps surprisingly, we did not observe major effects of RA on the responding cells. There was no enhancement of TGFβ signalling, no complementation of the Treg transcriptional signature, and the consequences of RA treatment on gene-expression were relatively modest and largely shared between Foxp3-positive and -negative cells in the same cultures. Instead, the data point to a rather different mode of action, where RA counteracts the dampening effects of inhibitory populations whose existence was revealed by cell fractionation and complementation analyses. RA’s activity was entirely mediated by RARα, which provided a useful experimental handle to confirm this indirect effect through criss-cross experiments, in vitro and in vivo.
The use of purified CD4+ cells in the assays allowed us to discern the indirect effect of RA, and the impact of contra-conversion mediated by CD44hi cells. On the other hand, the ability of RA to repress IL-6R in naïve T cells, which would reduce their susceptibility to inhibition by IL-6, also suggests the possible existence of direct effects. While this direct effect appeared of secondary importance in our culture conditions, it may be more prominent in some in vivo situations, where IL-6 made by non-T cells may be more important. RA would thus safeguard TGFβ-induced conversion in several settings.
An indirect effect of RA is compatible with the observation made by most groups that the compound has no effect whatsoever in the absence of TGFβ, which is understandable if it serves to relieve a repression rather than to directly promote conversion. That RA would interact with inhibitory cells is also compatible with the fact that there was significant variability in the baseline efficacy of conversion (as noted by (Benson et al., 2007)): most likely, the actual proportion of CD44hi cells in the input population varied between donor pools. The conditions used for these cultures (engagement of CD3 and/or CD28, IL2 supplementation, DCs) were conducive to cytokine production, and one can conceive of how small experimental variation might affect contra-conversion. In fact, the nature of the activating stimulus may modulate the efficacy of RA in enhancing TGFβ-driven Foxp3 expression.
It is currently unclear whether contra-conversion may also affect other modes of T cell differentiation elicited by TGFβ. In these co-cultures, even with high concentrations of T-cell-derived IL-21, we found no evidence for IL-17 production at the protein or mRNA level. Thus, there is clearly more than a dichotomous set of possible outcomes (Foxp3 or IL-17 expression) for naïve T cells stimulated with TGFβ.
The ability of CD4+ Tconv cells to convert to a Foxp3+ phenotype when stimulated in a TGFβ-rich environment has been proposed as a means to achieve a balanced and regulated response, particularly in mucosal areas, such as the GALT, where peaceful co-existence (if not actual tolerance) must be achieved with the intestinal commensal flora. The ability to generate Treg cells when intestinal homeostasis is threatened is thus desirable, but it is also important that the system limit the potential for a “runaway” conversion to Treg phenotypes, which would overly suppress needed responses. The existence of a contra-conversion pathway would be a logical means to control this potential: a balance between a TGFβ-fueled drive to conversion and contra-conversion activity. In this context, RA would serve as an external modulator of this balance. It is provided by a third-party cell, and one can conceive of the DCs acting as a sensor and integrator of local microbial challenge and inflammatory responses, influencing the conversion/contra-conversion balance by releasing variable amounts of RA. The particular ability of gut DCs to produce RA (Iwata et al., 2004; Coombes et al., 2007) is clearly in line with such a notion.
On the other hand, RA has little to no influence on the selection of Treg cells in the thymus, not on their overall proportions in secondary lymphoid organs, as evidenced by the normal Treg populations in RARα-deficient mice. This is compatible with a view where RA-influenced conversion of conventional CD4+ T cells to a Foxp3+ phenotype represents a focused adaptation, only involving particular reactivities or locations, but that overall homeostatic control of Treg populations falls under a different control, such as the supply of trophic cytokines.
The dampening of Foxp3 induction by cytokines showed clear synergistic effects. IL-4 was the most active in this respect, but IL-21 and IFNγ clearly enhanced this ability, and complemented each other in doing so. This synergy is unusual for IL-4 and IFNγ, which are usually antagonistic. It will be important to elucidate the signaling pathways involved, but one can imagine complex interactions wherein one cytokine induces the receptor or the signaling cascade downstream of the other.
It should also be pointed out that, while contra-conversion can be mediated by cytokines in this synergistic fashion, soluble factors do not account for the whole effect of CD44hi cells. Supernatants from CD44hi cells were never quite as effective as the cells themselves, and inhibition of contra-conversion with antibody combinations only incompletely reversed the inhibitory effect. While these elements could be explained by focused release and action of cytokines, or by additional soluble mediators, they also suggest that cell-cell interactions may be involved in contra-conversion.
What cells mediate contra-conversion? The results indicate that the function resides, at least in part, in CD44hi CD4+ T cells. It does not involve NK-T cells, a priori attractive candidates because of their ability to produce IL-4 and IFNγ: sorted NK1.1-positive cells within the CD44hi fraction had no contra-conversion ability (not shown). Similarly, Treg cells themselves did not show activity (a priori an appealing hypothesis in which Treg cells would negatively feed back on their own generation), as purified Foxp3-GFP+ cells added to the cultures did not influence conversion (not shown). In contrast, contra-conversion ability was found in the CD44hi subset of CD8+ T cells, suggesting that the activity resides in populations of antigen-experienced Tconv cells with markers consistent with effector/memory cells.
In fairness, while the concept of contra-conversion is logical and has operational value, we do not know whether a dedicated “contra-convertor” population exists. Such a label does carry the teleological connotation of a distinct and identifiable population whose role (or one of whose roles) is to maintain the conversion/contra-conversion balance. An alternative scenario is one in which there is no such uniquely identifiable population, but rather a set of differentiated states which produce a set of cytokines that dampen TGFβ-mediated Foxp3 induction. This effect could be instilled in an antigen- or location-specific manner and perpetuate the dominance of effective T cell responses, by inhibiting the neo-generation of Treg cells, even in locations of strong TGFβ exposure. Whether one or several cell types affect contra-conversion, it is striking that RA represses the entire inhibitory program.
Experimental Procedures
Mice
C57BL/6J (CD45.1 congenic), C57BL/6J, C57BL/6-H2g7, C57BL/6-IL6KO, and C57BL/6-IL4KO mice were bred in the SPF Joslin facility or purchased from Jackson Laboratories. RAR-deficient mice (Chapellier et al., 2002c; Chapellier et al., 2002a; Chapellier et al., 2002b) were bred in the SPF facility at IGBMC while Foxp3-eGFP reporter mice (Bettelli et al., 2006) and OT-II RAG-1−/−(CD45.1 congenic) were bred in the SPF facility at NIAID.
Cells
Cells used for in vitro activation and in vivo transfer were obtained from spleen and lymph nodes or lamina propria of 6–8 week old mice. Unless otherwise noted, cells were sorted to obtain individual T cell or dendritic cell sub-populations.
Cell sorting and flow cytometry
For Figures 1 and 2, CD4+CD25− T cells or CD4+Foxp3/eGFP− T cells, lamina propria dendritic cells (LP DC) or spleen dendritic cells (Sp DC) were isolated as described previously (Sun et al., 2007). For experiments subsetting CD4+ cells types, naïve cells were sorted as B220−,CD8−,CD11c−,CD25−,CD4+,CD44−CD62Lhi, while the “memory” pool was sorted as B220−,CD8−,CD11c−,CD25−,CD4+,CD44hiCD62Llo. OT-II RAG-1−/− CD45.1 CD4+ T cells were sorted as a naïve population (as described above but without staining for CD62L). Post-activation analysis assessed CD4, CD45.1, CD45.2, α4β7, CCR9, IL6Rα, Foxp3, IL-10, IL-4, or IFNγ expression by cell surface and intracellular antibody staining.
In vitro activation
For conversion assays, T cells were activated with anti-CD3/CD28 coated beads (Dynal) at a concentration of one bead/cell in the presence of 20 U/ml recombinant human IL-2 (Proleukin®, Chiron) and 10 ng/ml recombinant TGFβ (Peprotech), for 4 days in a 96-well plate. Naïve CD4+ T cell were cultured at a concentration of 0.5×105/well or 0.25×105/well when used in co-culture assays. Memory CD4+ T cells were seeded at a 2:1 (memory:naïve) cell ratio in co-culture experiments (unless otherwise noted). Some cultures were also treated all-trans RA (100nM unless otherwise noted, Sigma), recombinant mouse IL-4, IL-6, IL-9, IL-10, IL-21, or IFNγ (at the indicated concentrations, Peprotech), or with blocking antibodies specific for IL-6Rα, IL-4, IL-21 or IFNγ (5 μg/ml, R&D systems). For the assays of Figs 1 and 2, 105 CD4+CD25− or CD4+eGFP− T cells were cultured with 0.1×105 purified LP or Sp DCs in the presence of anti-CD3 (1 μg/ml), TGFβ (3 ng/ml), IL-2 (5 ng/ml), and retinoic acid (10 or 100 nM) as described previously (Sun et al., 2007).
In vivo conversion assay
OT-II RAG-1−/− CD45.1 CD4+ T cells (0.5–1×106/mouse) were transferred IV into either RARαKO or WT littermate controls as described previously (Sun et al., 2007). The drinking water of recipient mice was supplemented with 1.5% ovalbumin and changed every 2 days. Mice were sacrificed 7 days after T cell transfer and Foxp3 expression in CD45.1+ donor T cells were detected in skin draining lymph nodes, spleen, mesenteric lymph node, and the lamina propria.
Microarrays
RNA was prepared from sorted cell populations as described (Trizol; (Yamagata et al., 2004)). RNA was amplified for two rounds (MessageAmp aRNA, Ambion), biotin-labeled (BioArray High Yield RNA Transcription Labeling, Enzo), and purified using the RNeasy Mini Kit (Qiagen). The resulting cRNAs were hybridized to M430 2.0 chips (Affymetrix). All cell populations analyzed were generated in duplicate or triplicate. Raw data were normalized using the RMA algorithm implemented in the “Expression File Creator” module from the GenePattern software package (Reich et al., 2006). Data were visualized using the “Multiplot” modules from GenePattern.
Supplementary Material
1
2
3
4
Acknowledgments
We thank S. Vitolo, K. Hattori for assistance with mice, J. LaVecchio and G. Buruzala for help with cytometry, J. Perez and K. Leatherbee for microarrays, and C. Laplace for graphics. This work was supported by grants from the JDRF (4-2004-368) and the NIH (1R01AI51530-5), by Young Chair funds to DM and CB, and by Joslin’s NIDDK-funded DERC core facilities. J.A. Hill, was supported by a post-doctoral fellowship from the Canadian Institutes of Health Research, CMS and J.A. Hall by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
Treg
regulatory T cell
Tconv
conventional T cell
RA
retinoic acid
FC
fold-change
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abb J, Abb H, Deinhardt F. Effect of retinoic acid on the spontaneous and interferon-induced activity of human natural killer cells. Int J Cancer. 1982a;30:307–310. doi: 10.1002/ijc.2910300309. [DOI] [PubMed] [Google Scholar]
- Abb J, Abb H, Deinhardt F. Retinoic acid suppression of human leukocyte interferon production. Immunopharmacology. 1982b;4:303–310. doi: 10.1016/0162-3109(82)90051-0. [DOI] [PubMed] [Google Scholar]
- Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer. 2001;1:181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Cerwenka A, Bakker AB, McClanahan T, Wagner J, Wu J, Phillips JH, Lanier LL. Retinoic acid early inducible genes define a ligand family for the activating NKG2D receptor in mice. Immunity. 2000;12:721–727. doi: 10.1016/s1074-7613(00)80222-8. [DOI] [PubMed] [Google Scholar]
- Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- Chapellier B, Mark M, Bastien J, Dierich A, Lemeur M, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor beta (RARbeta) gene. Genesis. 2002a;32:91–94. doi: 10.1002/gene.10073. [DOI] [PubMed] [Google Scholar]
- Chapellier B, Mark M, Garnier JM, Dierich A, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor gamma (RARgamma) gene. Genesis. 2002b;32:95–98. doi: 10.1002/gene.10072. [DOI] [PubMed] [Google Scholar]
- Chapellier B, Mark M, Garnier JM, Lemeur M, Chambon P, Ghyselinck NB. A conditional floxed (loxP-flanked) allele for the retinoic acid receptor alpha (RARalpha) gene. Genesis. 2002c;32:87–90. doi: 10.1002/gene.10071. [DOI] [PubMed] [Google Scholar]
- Chen JY, Penco S, Ostrowski J, Balaguer P, Pons M, Starrett JE, Reczek P, Chambon P, Gronemeyer H. RAR-specific agonist/antagonists which dissociate transactivation and AP1 transrepression inhibit anchorage-independent cell proliferation. EMBO J. 1995;14:1187–1197. doi: 10.1002/j.1460-2075.1995.tb07102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, Klein-Hessling S, Serfling E, Hamann A, Huehn J. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3-dependent and independent regulation of the Treg transcriptional signature. Immunity. 2007;25:693–695. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–410. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK, Niesner U, de la RM, Schmidt CA, Brauer R, Buer J, Scheffold A, Hamann A. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, Lemeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci U S A. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, Karagiannidis C, Lambrecht BN, Hendriks RW, Crameri R, Akdis CA, Blaser K, Schmidt-Weber CB. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark M, Ghyselinck NB, Chambon P. Function of retinoid nuclear receptors: lessons from genetic and pharmacological dissections of the retinoic acid signaling pathway during mouse embryogenesis. Annu Rev Pharmacol Toxicol. 2006;46:451–480. doi: 10.1146/annurev.pharmtox.46.120604.141156. [DOI] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest. 2005;115:1923–1933. doi: 10.1172/JCI24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal Th-17 and Regulatory T Cell Differentiation Mediated by Retinoic Acid. Science. 2007 doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Nicholson RC, Mader S, Nagpal S, Leid M, Rochette-Egly C, Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990;9:4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–259. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Purton LE, Dworkin S, Olsen GH, Walkley CR, Fabb SA, Collins SJ, Chambon P. RARgamma is critical for maintaining a balance between hematopoietic stem cell self-renewal and differentiation. J Exp Med. 2006;203:1283–1293. doi: 10.1084/jem.20052105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. GenePattern 2.0. Nat Genet. 2006;38:500–501. doi: 10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- Salbert G, Fanjul A, Piedrafita FJ, Lu XP, Kim SJ, Tran P, Pfahl M. Retinoic acid receptors and retinoid X receptor-alpha down-regulate the transforming growth factor-beta 1 promoter by antagonizing AP-1 activity. Mol Endocrinol. 1993;7:1347–1356. doi: 10.1210/mend.7.10.8264664. [DOI] [PubMed] [Google Scholar]
- Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. Eur J Immunol. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19:281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Oral tolerance: is it all retinoic acid? J Exp Med. 2007;204:1737–1739. doi: 10.1084/jem.20071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–18174. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Mathis D, Benoist C. TCR-based lineage tracing: no evidence for conversion of conventional into regulatory T cells in response to a natural self-antigen in pancreatic islets. J Exp Med. 2007a doi: 10.1084/jem.20070822. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007b;178:7032–7041. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, Mathis D, Benoist C, Chen L, Rao A. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Yamagata T, Mathis D, Benoist C. Self-reactivity in thymic double-positive cells commits cells to a CD8 alpha alpha lineage with characteristics of innate immune cells. Nat Immunol. 2004;5:597–605. doi: 10.1038/ni1070. [DOI] [PubMed] [Google Scholar]
- Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
2
3
4