Heterodimerization of Mdm2 and Mdm4 is critical for regulating p53 activity during embryogenesis but dispensable for p53 and Mdm2 stability (original) (raw)
Abstract
Mdm2 and Mdm4 are homologous RING domain-containing proteins that negatively regulate the tumor suppressor p53 under physiological and stress conditions. The RING domain of Mdm2 encodes an E3-ubiquitin ligase that promotes p53 degradation. In addition, Mdm2 and Mdm4 interact through their respective RING domains. The in vivo significance of Mdm2-Mdm4 heterodimerization in regulation of p53 function is unknown. In this study, we generated an Mdm4 conditional allele lacking the RING domain to investigate its role in Mdm2 and p53 regulation. Our results demonstrate that homozygous deletion of the Mdm4 RING domain results in prenatal lethality. Mechanistically, Mdm2-Mdm4 heterodimerization is critical for inhibiting lethal p53 activation during early embryogenesis. However, Mdm2-Mdm4 interaction is dispensable for regulating p53 activity as well as the stability of Mdm2 and p53 at later stages of development. We propose that Mdm4 is a key cofactor of Mdm2 that inhibits p53 activity primarily during early embryogenesis but is dispensable for regulating p53 and Mdm2 stability in the adult mouse.
Keywords: Mdmx, mouse models, p53 stability, ubiquitination
Murine double minute 2 (Mdm2) and its homolog Mdm4 (also known as Mdmx) are two major negative regulators of p53 (1–3). Genetic ablation of either in mice is embryonic lethal and rescued by concomitant deletion of p53 (4–8). Both proteins share considerable homology in their N-terminal p53 binding and C-terminal really interesting new gene (RING) domains (3). Although both proteins inhibit p53 activity by binding and masking its transactivation domain, the RING domain of Mdm2 also functions as an E3-ubiquitin ligase that promotes p53 degradation (9–12). However, similar analysis of the Mdm4 RING domain has remained inconclusive. Importantly, these homologous proteins also interact with each other through their respective RING domains (13). In vitro studies have suggested that Mdm2-Mdm4 heterodimerization is essential for stabilizing Mdm2 and directing its ubiquitin ligase activity toward p53 (14–16). However, the in vivo significance of this interaction in p53 regulation has not been investigated. Here we generated and characterized a conditional Mdm4flxRING knockin allele to specifically address these questions in vivo.
Results and Discussion
To generate a conditional allele of Mdm4 without the RING domain, we inserted a mutant copy of exon 11 lacking the RING domain between the endogenous exon 11 and 3′ UTR of the Mdm4 gene. The mutant exon 11 carried an in-frame deletion of the 49 RING-encoding amino acids. Before _cre_-mediated deletion, the targeted allele expressed the full-length wild-type Mdm4 (Mdm4flxRING). After _cre-loxP_–mediated recombination the endogenous RING domain containing exon 11 could be precisely replaced with the mutant version to generate Mdm4ΔRING/+ mice (Fig. 1_A_).
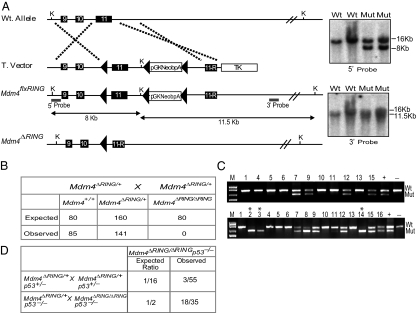
Fig. 1.
Generation of Mdm4ΔRING mice. (A) Targeting and screening strategy for generation of Mdm4ΔRING mice. Southern blots with 5′ and 3′ external probes were performed to confirm homologous recombination. Wt, wild type; T.Vector, targeting vector; Mut, mutant. (B) Table shows the expected and observed frequencies of homozygous mutant mice obtained. (C) Upper: PCR genotyping for the embryos recovered at E9.5. Lower: Corresponding PCR genotyping of all deciduas, including ones with the homozygous Mdm4ΔRING/ΔRING genotype. Asterisks (*) indicate empty deciduas homozygous for the Mdm4ΔRING/ΔRING genotype. (D) Table shows the expected and observed frequencies of viable homozygous mutant mice obtained on a _p53_-null background.
To examine the physiological consequences of deletion of the Mdm4 RING domain, we intercrossed Mdm4ΔRING/+ mice to generate Mdm4ΔRING/ΔRING mice. Surprisingly, we did not observe any homozygous pups from multiple crosses (Fig. 1_B_). The time point of this embryonic lethality was very similar to Mdm4 null lethality (6, 17). PCR genotyping did not reveal any homozygous embryos at embryonic day (E) 9.5. Genotyping analysis of the corresponding deciduas, however, revealed homozygosity of Mdm4ΔRING in the unresorped deciduas, suggesting recent demise of the conceptuses (Fig. 1_C_). Concomitant deletion of p53 rescued the embryo lethal phenotype, implicating unrestrained p53 activation in homozygous Mdm4ΔRING/ΔRING knockin mice (Fig. 1_D_). In addition, rescue of the Mdm4ΔRING/ΔRING lethality was also accomplished by crossing these mice onto a homozygous p53515A/515A allele, which encodes the transcriptionally null mutant p53R172H protein (18).
To confirm correct RNA splicing at the intron–exon junctions surrounding the RING deletion, we isolated RNA from Mdm4ΔRING/ΔRINGp53−/− mouse spleen and carried out RT-PCR sequencing of Mdm4 (Fig. S1). Mdm4ΔRING transcribed a truncated mRNA lacking the sequence corresponding to the 49 RING-encoding amino acids. We further confirmed presence of the smaller protein encoded by the Mdm4ΔRING allele by immunoprecipitating and Western blotting Mdm4 from p53−/− and Mdm4ΔRING/ΔRINGp53−/− mouse embryonic fibroblasts (MEFs) (Fig. 2_A_). We next examined the interaction of endogenously expressed Mdm4ΔRING protein with its two major binding partners, p53 and Mdm2. Mdm4 interacts with p53 via its N-terminal domain, whereas it uses the C-terminal RING domain to dimerize with Mdm2 (3). We performed this analysis in the mutant Mdm4ΔRING/ΔRINGp53515A/515A background because p53R172H, although transcriptionally inactive, retains its interaction with and regulation by Mdm2 (1, 19). As expected, coimmunoprecipitation analysis of cell lysates from p53515A/515A and Mdm4ΔRING/ΔRINGp53515A/515A MEFs with a p53 antibody confirmed an intact albeit weaker interaction between mutant Mdm4ΔRING and p53R172H under both normal and DNA-damaging conditions (Fig. 2_B_). Similar analysis with an Mdm2 antibody revealed binding between Mdm2 and Mdm4 but loss of the interaction between Mdm2 and Mdm4ΔRING. A slight decrease in Mdm4 levels after immunoprecipitation with an Mdm2 antibody is likely due to masking of the Mdm2 epitope or due to increased degradation of the interacting proteins after ionizing radiation (IR) (20–22).
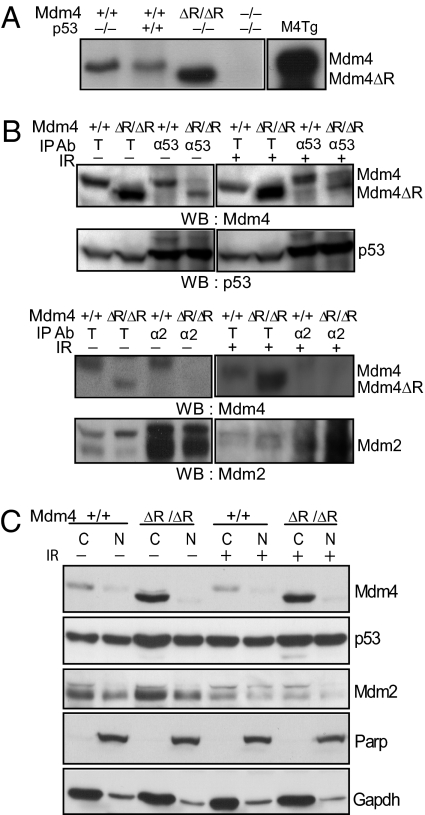
Fig. 2.
Characterization of Mdm4ΔRING protein. (A) Immunoprecipitation (IP) results from MEFs of different genotypes expressing the Mdm4 and Mdm4ΔRING protein. Protein extract from an Mdm4 transgenic mouse spleen (M4Tg) is used as positive control. ΔR, ΔRING. (B) Coimmunoprecipitation of Mdm4 from p53515A/515A (wild type for Mdm4) and Mdm4ΔRING/ΔRINGp53515A/515A (mutant for Mdm4) MEFs, treated with or without IR. Upper: Coimmunoprecipitation with p53 antibody (α53). Lower: Coimmunoprecipitation with Mdm2 antibody (α2). T, 10% of input. (C) Subcellular localization of Mdm2, Mdm4, and p53 in protein lysates from p53515A/515A and Mdm4ΔRING/ΔRINGp53515A/515A MEFs treated with or without IR. Parp and Gapdh were used as nuclear (N) and cytoplasmic (C) fraction markers, respectively.
The prevailing view suggests that subcellular localization of p53, Mdm2, and Mdm4 is due to the interactions between them (7, 23). We therefore tested whether a defect in Mdm2–Mdm4 interaction could alter the subcellular localization of these p53 pathway proteins. We also examined whether stress-induced modifications alter the subcellular distribution of these proteins. To that end, we carried out nuclear–cytoplasmic fractionation of p53515A/515A and Mdm4ΔRING/ΔRINGp53515A/515A MEF cell lysates under both normal and DNA damage conditions. We confirmed the purity of the nuclear and cytoplasmic fractions by immunoblotting with Parp and Gapdh antibodies, respectively. Interestingly, no differences in subcellular localization of p53R172H, Mdm2, Mdm4, or Mdm4ΔRING under both normal and DNA damage conditions were observed (Fig. 2_C_). Whereas p53 and Mdm2 remained distributed in the nucleus and cytoplasm, Mdm4 and Mdm4ΔRING proteins maintained primarily cytoplasmic localization. Of note, the slightly higher levels of p53R172H observed in the cytoplasm could be either due to the p53 mutation itself affecting localization or due to increased cytoplasmic retention of p53R172H by Mdm4ΔRING.
Mdm2-Mdm4 heterodimerization is perceived to stabilize Mdm2 by inhibiting its autoubiquitination and in turn promoting p53 ubiquitination (16, 24, 25). However, because p53R172H is easily stabilized under tissue culture conditions it could not be used for these experiments (19). To examine the effects of Mdm4ΔRING on wild-type p53 stability and activity, we therefore used a conditional hypomorphic p53Neo/Neo allele recently developed in our laboratory (26). In this model, normal wild-type p53 expression can be restored by either tamoxifen injection in conjunction with a Cre-ER transgene in mice or by adenovirus-encoded cre recombinase (adeno-cre) infection in cells. p53Neo/Neo mice express only ≈15% of normal wild-type p53 levels and could rescue lethality of Mdm4ΔRING mice. We generated MEFs from p53Neo/Neo (wild type for Mdm4) and Mdm4ΔRING/ΔRINGp53Neo/Neo for this analysis. A functional p53 allele (>90% recombination) was restored by overnight adeno-cre infection in these cells (Fig. 3_A_). Surprisingly, no difference in p53 and Mdm2 half-lives was observed in cycloheximide-treated MEFs of both genotypes (Fig. 3_B_). Both proteins maintained ≈20-min half-lives in either genotype. However, the truncated Mdm4ΔRING protein was not degraded in comparison with the full-length wild-type Mdm4 (Fig. 3_B_). These data suggest that the interaction between Mdm4 and Mdm2 is necessary for Mdm4 degradation but not Mdm2 stability or p53 degradation under homeostatic conditions. We next analyzed the role of the Mdm4 RING domain on DNA damage-induced degradation of p53 and Mdm2. We treated p53Neo/Neo and Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs with 10 Gy IR and after 4 h measured the protein stability of the accumulated proteins. Again, no differences in degradation kinetics were observed (Fig. 3_C_). Whereas p53 and Mdm4ΔRING remained stable during the course of the experiment, the half-life of Mdm2 was shortened to ≈10 min in MEFs of both genotypes after IR. Notably, Mdm4 was undetectable owing to its degradation after IR (21, 22). These data further corroborate that Mdm2-Mdm4 heterodimerization is also dispensable for Mdm2 and p53 degradation under genotoxic conditions.
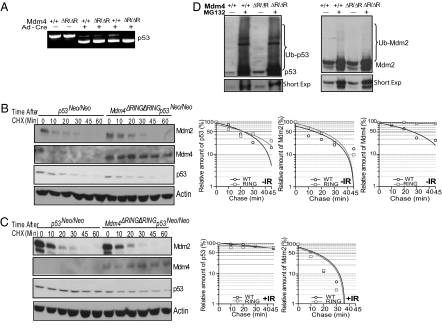
Fig. 3.
Mdm2-Mdm4 heterodimerization is dispensable for p53 and Mdm2 degradation. (A) PCR genotyping shows recombination efficiency in p53Neo/Neo and Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs after adeno-cre (Ad-Cre) virus infection. ΔR, ΔRING (B) Adeno-cre–infected p53Neo/Neo and Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs were treated with cycloheximide ((CHX) 20 μg/mL) and harvested at different time points. WT, wild type; RING, Mdm4ΔRING. (C). Adeno-cre–infected p53Neo/Neo and Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs were treated with 10 Gy IR. Proteins were allowed to accumulate for 4 h, followed by cycloheximide treatment (20 μg/mL) and cells harvested at different time points. (D) p53Neo/Neo and Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs were transfected with HA-ub, followed by infection with adeno-cre virus. Protein lysate was immunoprecipitated with HA-conjugated beads and immunoblotted with p53 and Mdm2 antibodies.
We next analyzed p53 and Mdm2 ubiquitination in the Mdm4ΔRING background. We transfected a hemagglutinin-tagged ubiquitin (HA-ub)–expressing plasmid in p53Neo/Neo and Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs and analyzed p53 and Mdm2 ubiquitination status after adeno-cre–mediated p53 restoration. In agreement with the above observations, we found no differences in p53 and Mdm2 ubiquitination between the two genotypes. Similar high molecular weight smears, characteristic of ubiquitination, were observed in the proteasome inhibitor MG132-treated MEFs of both genotypes (Fig. 3_D_).
Although Mdm2 and p53 stability was not altered in the Mdm4ΔRING background, the embryonic lethality at E9.5 suggested enhanced p53 activity during early development. We therefore next investigated whether lack of the Mdm4 RING domain also led to similar augmentation of p53 activity in MEFs generated from E13.5 Mdm4ΔRING/ΔRINGp53Neo/Neo embryos. We induced the expression of wild-type p53 in p53Neo/Neo and Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs by infecting with adeno-cre and performed RT–quantitative PCR (qPCR) for p53 targets. Indeed, Mdm2, p21, and Puma transcripts were up-regulated approximately twofold in Mdm4ΔRING/ΔRINGp53Neo/Neo cells. Nonetheless, the fold induction was similar between the genotypes, suggesting that the enhancement observed was due to increased basal p53 activity in Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs under tissue culture conditions (Fig. 4_A_). Corresponding Western blot analyses also revealed relatively higher levels of phospho-p53 and the p53 targets p21 and Puma in adeno-cre–treated Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs (Fig. 4_B_). We subsequently generated viable homozygous Mdm4ΔRING/ΔRINGp53Neo/Neo pups. This rescue is in contrast to the observation that p53Neo/Neo does not similarly rescue the Mdm2 null lethality (26). This suggests that deletion of the Mdm4 RING domain results in a relatively weaker phenotype compared with _Mdm2_-null and does not lead to the analogous lethal effects of p53 activation.
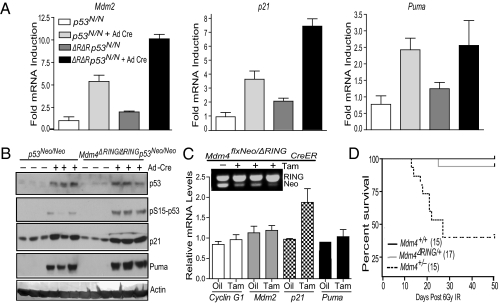
Fig. 4.
RING domain deletion does not promote lethal p53 activation in adult mouse tissues. (A) Real-time PCR for p53 targets in p53Neo/Neo (p53N/N) and Mdm4ΔRING/ΔRINGp53Neo/Neo (ΔRΔR_p53N/N_) MEFs after adeno-cre (Ad-Cre) infection. Data were normalized to expression in untreated p53Neo/Neo controls and represent a mean of three independent experiments ± SEM. (B) Western blot analysis from adeno-cre–treated p53Neo/Neo and Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs. (C) Five-week-old Mdm4flxRING/ΔRING:CreER mice were injected with either corn oil or tamoxifen. RNA from spleen was used for RT-qPCR analyses for p53 targets. Data were normalized to expression levels in oil-injected controls, n = 3, ±SEM. Inset: Homologous recombination after tamoxifen (Tam) injection. (D) Kaplan-Meyer survival curve of Mdm4+/+, Mdm4+/−, and Mdm4ΔRING/+ mice after 6 Gy irradiation.
We next examined whether the Mdm4 RING deletion elicited a similar p53 response in adult mice. We injected adult conditional Mdm4flxRING/ΔRINGCreER mice with tamoxifen to generate recombined Mdm4ΔRING/ΔRING mice. Recombination (89%) was analyzed by PCR amplification of genomic DNA from spleen (Fig. 4_C_). Notably, mice with recombined Mdm4ΔRING/ΔRING allele did not appear sick and were viable. RT-qPCR analysis on RNA from spleen of the recombined mice revealed slight up-regulation of p21 but no significant changes to other canonical p53 targets (Fig. 4_C_). These data established that Mdm2-Mdm4 heterodimerization is dispensable for p53 inhibition in adult mouse tissues.
Because Mdm4ΔRING remained stable after IR, whereas p53 and Mdm2 degraded with similar kinetics in MEFs, we further evaluated the long-term effects of Mdm4ΔRING expression on p53 regulation and survival after IR exposure in vivo. Mdm4+/− mice are radiosensitive and die after exposure to 6 Gy IR (27). We similarly irradiated Mdm4ΔRING/+ mice with 6 Gy IR and monitored them for 50 d (Fig. 4_D_). Whereas 65% of Mdm4+/− mice died after IR, only 1 of 17 Mdm4ΔRING/+ mice succumbed to radiation. This result may be attributed to a partial function of the Mdm4ΔRING because it retains the p53 interaction domain and is refractory to post-IR degradation. We also repeated this analysis in Mdm4flxRING/ΔRINGCreER mice, which express wild-type p53. We injected Mdm4flxRING/ΔRINGCreER mice once per week for 3 wk with tamoxifen to initiate recombination of the Mdm4 conditional allele. Forty-eight hours after the final injection, mice were irradiated with 6 Gy IR. Again, no mortality was observed during the subsequent 50-d observation period. Because sublethal doses of IR primarily cause death by hematopoietic failure in Mdm4+/− mice, these results suggest that the Mdm4ΔRING protein inhibits the lethal activation of p53 in the radiosensitive hematopoietic compartment under stress conditions.
Previous data suggest that an Mdm2 RING mutant that lacks the E3-ubiquitin ligase activity and the ability to heterodimerize with Mdm4 leads to p53 stabilization (28). Our results here demonstrate that in the absence of Mdm2-Mdm4 heterodimerization, p53 and Mdm2 degradation rates are not altered. Taken together, these data imply that p53 degradation by Mdm2 ubiquitin ligase activity is independent of its association with Mdm4. Additionally, Mdm2-Mdm4 heterodimerization is necessary for Mdm4 but not Mdm2 degradation.
Interestingly, Mdm4ΔRING protein was stable under DNA-damaging conditions. This phenomenon is similar to that observed with the Mdm4-3SA phosphorylation mutant protein, which resists degradation after radiation damage (29). This could explain the lack of radiosensitivity of Mdm4ΔRING/+ and tamoxifen-injected Mdm4flxRING/ΔRINGCreER mice after 6 Gy IR exposure. A nondegradable, RING-deficient Mdm4 protein could remain bound to p53 and effectively inhibit its transcriptional activity.
A short form of Mdm4 (Mdmx-S) has been reported in human normal and cancer cells (30). This short form lacks the RING domain and thus resembles Mdm4ΔRING. Mdmx-S is amplified in human cancers such as glioblastoma and soft tissue sarcoma and correlates with poor prognosis (31, 32). The consequences of increased levels of Mdmx-S in these patients after DNA-damaging chemo/radiotherapeutic agents may include inhibition of p53 activity and/or p53-independent activities and warrant further investigation.
Our results clearly show that RING domain-mediated Mdm2-Mdm4 heterodimerization is critical for regulating p53 activity during early embryogenesis. However, during later developmental stages and adult life this interaction becomes dispensable or redundant. In stark contrast to Mdm2 deletion, the relatively minor nonlethal phenotypes observed with deletion of Mdm4 in adult animal tissues also support this notion (33). In contrast to loss of Mdm4 function as studied in these animal models, amplification of Mdm4 occurs in multiple human tumors, and many of these retain wild-type p53 (27, 34–36). These data support the importance of Mdm4 in regulating p53 under tumor and nonphysiological stress conditions.
Collectively, these results suggest that Mdm4 primarily acts as a cofactor with Mdm2 to inhibit p53 during embryogenesis but is dispensable for regulating p53 and Mdm2 stability in the adult under homeostatic conditions.
Materials and Methods
Generation of Mdm4ΔRING/+ Mice.
A loxP flanked neomycin cassette and an additional copy of exon 11 lacking the RING domain was inserted between the endogenous exon 11 and the 3′ UTR of the Mdm4 gene. Additionally, a loxP sequence was placed upstream of the endogenous Mdm4. Hsv-Tk1 cassette was included for negative selection (Fig. 1_A_). The targeting construct was sequenced completely and electroporated into TC1 mouse embryonic stem cells. G418 resistant clones were analyzed for correct homologous recombination by Southern blotting using 5′ and 3′ external probes (Fig. 1_A_). Two independent correctly targeted clones were expanded and injected into C57BL/6 blastocysts to generate Mdm4ΔRING/+ chimeras. Male chimeras were backcrossed to C57BL/6 mice to obtain germline transmission of the mutant allele.
Mouse Breeding, Maintenance, and Genotyping.
All mice were maintained in >90% C57BL/6 background. All mouse studies were conducted in compliance with Institutional Animal Care and Use Committee protocols. Before neomycin cassette deletion, mouse genotyping was done by PCR amplification using primer sets 5′-cta gtg aga cgt gct act tc-3′ and 5′-gga gag atg tac acc tgt gt-3′. The neomycin selection cassette was deleted by crossing germline transmitted F1 generation mice with Zp3-Cre deleter mice (37). Subsequently, genotyping was carried out by PCR amplification with primer sets 5′-ggc aac tcc aga taa cta cc-3′ and 5′-cag tac ctc ttg ctt gga g-3′ and resolved on an agarose gel.
MEF Preparation and Cell Culture.
Embryonic day 13.5 embryos were used to generate MEFs, which were maintained in DMEM (Invitrogen) supplemented with 10% FBS, penicillin (100 IU/mL), and streptomycin (100 μg/mL). Early-passage MEFs (P2–P3) were used for analysis.
Protein Analysis.
MEFs were lysed in Nonidet P-40 buffer. Protein estimation was carried out with Bicinchoninic Acid (BCA Protein Assay Kit; Pierce). Approximately 100 μg of lysate was resolved on 8% SDS/PAGE and Western blotted with antibodies against p53 (CM5; Vector Laboratories, 1:1,000), Mdm2 (2A10; Calbiochem, 1:500), Mdm4 (MX82; Sigma, 1:500), S15-p53 (9284; Cell Signaling Technology, 1:1,000), vinculin (V9131; Sigma, 1:1,000), and β-actin (AC15; Sigma, 1:5,000). Two milligrams of protein was used for immunoprecipitation with the same antibodies. Subcellular fractionation was carried out using a nuclear extract kit (Active Motif, #40010) according to the manufacturer's protocol.
Ubiquitination Assay.
p53Neo/Neo and Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs were transfected with HA-ub plasmid (kind gift from Dr. M. H. Lee, M.D. Anderson Cancer Center, Houston, TX). After 48 h cells were infected with adeno-cre virus [100 multiplicity of infection (MOI)] and harvested 24 h later. Protein was immunoprecipitated with anti-hemagglutinin antibody-tagged protein-A agarose beads (Sigma, #E6779), resolved on 8% SDS/PAGE, and immunoblotted with p53 or Mdm2 antibody.
Protein Stability Assay.
p53Neo/Neo and Mdm4ΔRING/ΔRINGp53Neo/Neo MEFs were infected with adeno-cre for 24 h before treatment with cycloheximide (20 μg/mL). Cells were harvested at different time points and protein analyzed by Western blotting.
Conditional Recombination of Allele.
Conditional recombination of the mouse allele was carried out by immunoprecipitation. Tamoxifen injection was as previously described (26). Recombination in MEFs was achieved by overnight adeno-cre virus infection (100 MOI) to the cells. The rate of recombination was determined by PCR amplification of genomic DNA and subsequent densitometric ratio analysis of bands resolved on agarose gels.
Quantitative RT-PCR.
RNA was isolated from tissues/MEFs using TRIzol (Invitrogen). One microgram of RNA was reverse-transcribed to cDNA (First Strand Synthesis Kit; GE Healthcare). First-strand reaction was diluted 10-fold, and 2 μL of the diluted reaction was used in qPCR reaction for p53 targets in an ABI 7900HT real-time PCR machine. Primer sequences have been previously described (38).
IR Studies.
Mice were irradiated at 6 Gy in a cesium-137 irradiator and monitored for 50 d. MEFs were cultured in a 100-mm tissue culture dish, irradiated with 10 Gy IR, and incubated at 37 °C before harvesting at different time points for experimental analyses.
Supplementary Material
Supporting Information
Acknowledgments
We thank Drs. Sean Post and James Jackson for critical reading of the manuscript. Mice were made by the Genetically Engineered Mouse Facility at The M. D. Anderson Cancer Center. Studies were supported by National Institutes of Health Grant CA47296 (to G.L.). V.P. has been supported in part by Molecular Genetics of Cancer Training Grant CA009299 and is a recipient of fellowships from the Dodie P. Hawn and Lupe C. Garcia Foundations.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.H.V. is a guest editor invited by the Editorial Board.
References
- 1.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- 2.Shvarts A, et al. MDMX: A novel p53-binding protein with some functional properties of MDM2. EMBO J. 1996;15:5349–5357. [PMC free article] [PubMed] [Google Scholar]
- 3.Marine JC, Dyer MA, Jochemsen AG. MDMX: From bench to bedside. J Cell Sci. 2007;120:371–378. doi: 10.1242/jcs.03362. [DOI] [PubMed] [Google Scholar]
- 4.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 5.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 6.Parant J, et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet. 2001;29:92–95. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- 7.Migliorini D, et al. Mdm4 (Mdmx) regulates p53-induced growth arrest and neuronal cell death during early embryonic mouse development. Mol Cell Biol. 2002;22:5527–5538. doi: 10.1128/MCB.22.15.5527-5538.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finch RA, et al. mdmx is a negative regulator of p53 activity in vivo. Cancer Res. 2002;62:3221–3225. [PubMed] [Google Scholar]
- 9.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 10.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 11.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 12.Haines DS, Landers JE, Engle LJ, George DL. Physical and functional interaction between wild-type p53 and mdm2 proteins. Mol Cell Biol. 1994;14:1171–1178. doi: 10.1128/mcb.14.2.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanimura S, et al. MDM2 interacts with MDMX through their RING finger domains. FEBS Lett. 1999;447:5–9. doi: 10.1016/s0014-5793(99)00254-9. [DOI] [PubMed] [Google Scholar]
- 14.Pan Y, Chen J. MDM2 promotes ubiquitination and degradation of MDMX. Mol Cell Biol. 2003;23:5113–5121. doi: 10.1128/MCB.23.15.5113-5121.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawai H, Lopez-Pajares V, Kim MM, Wiederschain D, Yuan ZM. RING domain-mediated interaction is a requirement for MDM2’s E3 ligase activity. Cancer Res. 2007;67:6026–6030. doi: 10.1158/0008-5472.CAN-07-1313. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto K, Taya Y, Nakagama H. Mdmx enhances p53 ubiquitination by altering the substrate preference of the Mdm2 ubiquitin ligase. FEBS Lett. 2009;583:2710–2714. doi: 10.1016/j.febslet.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Grier JD, Xiong S, Elizondo-Fraire AC, Parant JM, Lozano G. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol Cell Biol. 2006;26:192–198. doi: 10.1128/MCB.26.1.192-198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang GA, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. doi: 10.1016/j.cell.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Terzian T, et al. The inherent instability of mutant p53 is alleviated by Mdm2 or p16INK4a loss. Genes Dev. 2008;22:1337–1344. doi: 10.1101/gad.1662908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Q, Chen J. The phenotype of MDM2 auto-degradation after DNA damage is due to epitope masking by phosphorylation. Cell Cycle. 2011;10:1162–1166. doi: 10.4161/cc.10.7.15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Gilkes DM, Pan Y, Lane WS, Chen J. ATM and Chk2-dependent phosphorylation of MDMX contribute to p53 activation after DNA damage. EMBO J. 2005;24:3411–3422. doi: 10.1038/sj.emboj.7600812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawai H, et al. DNA damage-induced MDMX degradation is mediated by MDM2. J Biol Chem. 2003;278:45946–45953. doi: 10.1074/jbc.M308295200. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Chen L, Chen J. DNA damage induces MDMX nuclear translocation by p53-dependent and -independent mechanisms. Mol Cell Biol. 2002;22:7562–7571. doi: 10.1128/MCB.22.21.7562-7571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson MW, Berberich SJ. MdmX protects p53 from Mdm2-mediated degradation. Mol Cell Biol. 2000;20:1001–1007. doi: 10.1128/mcb.20.3.1001-1007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu J, et al. Mutual dependence of MDM2 and MDMX in their functional inactivation of p53. J Biol Chem. 2002;277:19251–19254. doi: 10.1074/jbc.C200150200. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, et al. Restoring expression of wild-type p53 suppresses tumor growth but does not cause tumor regression in mice with a p53 missense mutation. J Clin Invest. 2011;121:893–904. doi: 10.1172/JCI44504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terzian T, et al. Haploinsufficiency of Mdm2 and Mdm4 in tumorigenesis and development. Mol Cell Biol. 2007;27:5479–5485. doi: 10.1128/MCB.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itahana K, et al. Targeted inactivation of Mdm2 RING finger E3 ubiquitin ligase activity in the mouse reveals mechanistic insights into p53 regulation. Cancer Cell. 2007;12:355–366. doi: 10.1016/j.ccr.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Wang YV, Leblanc M, Wade M, Jochemsen AG, Wahl GM. Increased radioresistance and accelerated B cell lymphomas in mice with Mdmx mutations that prevent modifications by DNA-damage-activated kinases. Cancer Cell. 2009;16:33–43. doi: 10.1016/j.ccr.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rallapalli R, Strachan G, Cho B, Mercer WE, Hall DJ. A novel MDMX transcript expressed in a variety of transformed cell lines encodes a truncated protein with potent p53 repressive activity. J Biol Chem. 1999;274:8299–8308. doi: 10.1074/jbc.274.12.8299. [DOI] [PubMed] [Google Scholar]
- 31.Riemenschneider MJ, et al. Amplification and overexpression of the MDM4 (MDMX) gene from 1q32 in a subset of malignant gliomas without TP53 mutation or MDM2 amplification. Cancer Res. 1999;59:6091–6096. [PubMed] [Google Scholar]
- 32.Bartel F, et al. Significance of HDMX-S (or MDM4) mRNA splice variant overexpression and HDMX gene amplification on primary soft tissue sarcoma prognosis. Int J Cancer. 2005;117:469–475. doi: 10.1002/ijc.21206. [DOI] [PubMed] [Google Scholar]
- 33.Xiong S, Van Pelt CS, Elizondo-Fraire AC, Fernandez-Garcia B, Lozano G. Loss of Mdm4 results in p53-dependent dilated cardiomyopathy. Circulation. 2007;115:2925–2930. doi: 10.1161/CIRCULATIONAHA.107.689901. [DOI] [PubMed] [Google Scholar]
- 34.Valentin-Vega YA, Barboza JA, Chau GP, El-Naggar AK, Lozano G. High levels of the p53 inhibitor MDM4 in head and neck squamous carcinomas. Hum Pathol. 2007;38:1553–1562. doi: 10.1016/j.humpath.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riemenschneider MJ, Knobbe CB, Reifenberger G. Refined mapping of 1q32 amplicons in malignant gliomas confirms MDM4 as the main amplification target. Int J Cancer. 2003;104:752–757. doi: 10.1002/ijc.11023. [DOI] [PubMed] [Google Scholar]
- 36.Laurie NA, et al. Inactivation of the p53 pathway in retinoblastoma. Nature. 2006;444:61–66. doi: 10.1038/nature05194. [DOI] [PubMed] [Google Scholar]
- 37.Lewandoski M, Wassarman KM, Martin GR. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol. 1997;7:148–151. doi: 10.1016/s0960-9822(06)00059-5. [DOI] [PubMed] [Google Scholar]
- 38.Post SM, et al. A high-frequency regulatory polymorphism in the p53 pathway accelerates tumor development. Cancer Cell. 2010;18:220–230. doi: 10.1016/j.ccr.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information