The T helper type 2 response to cysteine proteases requires dendritic cell–basophil cooperation via ROS-mediated signaling (original) (raw)
. Author manuscript; available in PMC: 2011 Jul 28.
Published in final edited form as: Nat Immunol. 2010 May 23;11(7):608–617. doi: 10.1038/ni.1883
Abstract
The mechanisms that initiate T helper type 2 (TH2) responses are poorly understood. Here we demonstrate that cysteine protease–induced TH2 responses occur via ‘cooperation’ between migratory dermal dendritic cells (DCs) and basophils positive for interleukin 4 (IL-4). Subcutaneous immunization with papain plus antigen induced reactive oxygen species (ROS) in lymph node DCs and in dermal DCs and epithelial cells of the skin. ROS orchestrated TH2 responses by inducing oxidized lipids that triggered the induction of thymic stromal lymphopoietin (TSLP) by epithelial cells mediated by Toll-like receptor 4 (TLR4) and the adaptor protein TRIF; by suppressing production of the TH1-inducing molecules IL-12 and CD70 in lymph node DCs; and by inducing the DC-derived chemokine CCL7, which mediated recruitment of IL-4+ basophils to the lymph node. Thus, the TH2 response to cysteine proteases requires DC-basophil cooperation via ROS-mediated signaling.
Immune responses to T cell–dependent antigens show striking heterogeneity in terms of the cytokines made by helper T cells and the class of antibody secreted by B cells. In response to intracellular microbes, CD4+ helper T cells differentiate into T helper type 1 (TH1) cells, which produce interferon-γ (IFN-γ); in contrast, helminths induce the differentiation of TH2 cells, whose cytokines (principally interleukin 4 (IL-4), IL-5 and IL-13) induce immunoglobulin E (IgE) and eosinophil-mediated destruction of the pathogens1,2. Furthermore, TH17 cells (IL-17-producing helper T cells) mediate protection against fungal infections3. In addition to those subsets, other subsets have been identified, including TH9 cells (IL-9-producing helper T cells), TH22 cells (IL-22-producing helper T cells) and follicular helper T cells, located in the B cell–rich follicles of lymphoid organs2; but their physiological relevance and relationship to TH1, TH2 and TH17 cells are still being defined. Although much is known about the cytokines produced early in the response and the transcription factors that determine helper T cell polarization, the early ‘decision-making’ mechanisms that result in a given helper T cell response remain poorly understood. There is now ample evidence of a fundamental role for dendritic cells (DCs) in this process4–6. DCs comprise several functionally distinct subsets, which express a wide array of pathogen-recognition receptors (PRRs), including Toll-like receptors (TLRs); these enable them to ‘sense’ microbes7.
Despite the increasing knowledge about how the innate immune system shapes TH1 and TH17 responses, very little is known about its effect on TH2 responses. Basophils and mast cells promote TH2 responses by rapidly producing IL-4 after crosslinking of their Fc receptor for IgE (FcεRI) through preexisting antigen-IgE complexes8–13. Basophils can also prime TH2 responses to helminths and protein allergens14–16. Despite such advances, the potential importance of DC subsets and PRRs in sensing helminths or protein allergens and in ‘programming’ TH2 immunity remains largely unknown.
Although certain TLR ligands and ligands for the cytosolic PRR Nod1 induce TH2 responses17–21, the extent to which such receptors are involved in the initiation of TH2 responses to classic TH2 stimuli such as protease allergens or helminths is unknown. Furthermore, there is now a substantial body of data on the vital importance of DCs in modulating TH2 responses. Distinct subsets of DCs induce TH2 responses differently22,23, and specific microbial stimuli and allergens can ‘program’ DCs to prime TH2 responses24. Consistent with those findings, depletion of DCs abrogates asthma in mice25. Despite evidence of the involvement of DCs in TH2 responses, very little is understood about the nature of the DC subsets that induce TH2 responses in vivo, how DCs sense TH2-inducing stimuli, the nature of the intracellular signaling pathways that ‘program’ DCs to induce TH2 responses, and whether DCs act in concert with other cell types such as mast cells and basophils (which produce copious IL-4) to orchestrate TH2 responses. In addition, the role of DCs in initiating TH2 responses has been challenged by a published study suggesting that DCs are neither necessary nor sufficient for a TH2 response induced by papain15.
Here we demonstrate that migratory skin-derived dermal DCs were essential to the induction of a TH2 response to the cysteine protease papain. Subcutaneous immunization with papain plus antigen induced reactive oxygen species (ROS) in lymph node DCs and in dermal DCs and epithelial cells of the skin. ROS orchestrated TH2 responses by inducing oxidized lipids that triggered induction of thymic stromal lymphopoietin (TSLP) mediated by TLR4 and the adaptor TRIF in epithelial cells, by suppressing production of the TH1-inducing molecules IL-12 and CD70 by lymph node DCs, and by inducing the DC-derived chemokine CCL7, which mediated the recruitment of IL-4+ basophils to the lymph node.
RESULTS
DCs and TH2 differentiation in vivo
The cysteine protease papain, when injected together with ovalbumin protein (OVA), induced OVA-specific IgE and IgG1 antibodies and IL-4-producing CD4+ T cells (Fig. 1a), as described before15,26. In contrast, CpG DNA plus OVA stimulated IFN-γ-producing CD4+ T cells and OVA-specific IgG2b antibodies (Fig. 1a). Bromelain, a related cysteine protease, also induced TH2 responses (Supplementary Fig. 1). To determine whether DCs were required for induction of the TH2 response to OVA plus papain, we used the transgenic CD11c–diphtheria toxin receptor (CD11c-DTR) mouse model27. We selectively and transiently depleted CD11c-DTR mice of DCs by systemic administration of diphtheria toxin before immunizing the mice with OVA plus papain. Analysis by flow cytometry showed that intraperitoneal injection of diphtheria toxin into CD11c-DTR mice resulted in efficient depletion of DCs from lymph nodes and the dermis (Supplementary Fig. 2). We immunized CD11c-DTR and wild-type mice with OVA plus papain 24 h after injection of diphtheria toxin. After immunization, the production of IL-4 by CD4+ T cells was much lower in mice depleted of DCs (Fig. 1b). These results demonstrate that DCs are required for the induction of a TH2 response to papain. To further confirm the role of DCs in inducing antigen-specific TH2 responses, we transferred various numbers of CD4+ OT-II (ovalbumin-specific T cell antigen receptor) T cells into wild-type mice or CD11c-DTR mice (depleted of DCs by injection of diphtheria toxin) and then immunized the mice with OVA plus papain. We collected draining lymph node cells 4 d after immunization and restimulated the cells for 4 d ex vivo with OVA peptide (amino acids 323–339). After depletion of DCs, IL-4 production by CD4+ T cells was much lower (Fig. 1c). Together, these data demonstrate that DCs are required for the induction of antigen-specific TH2 responses in response to papain.
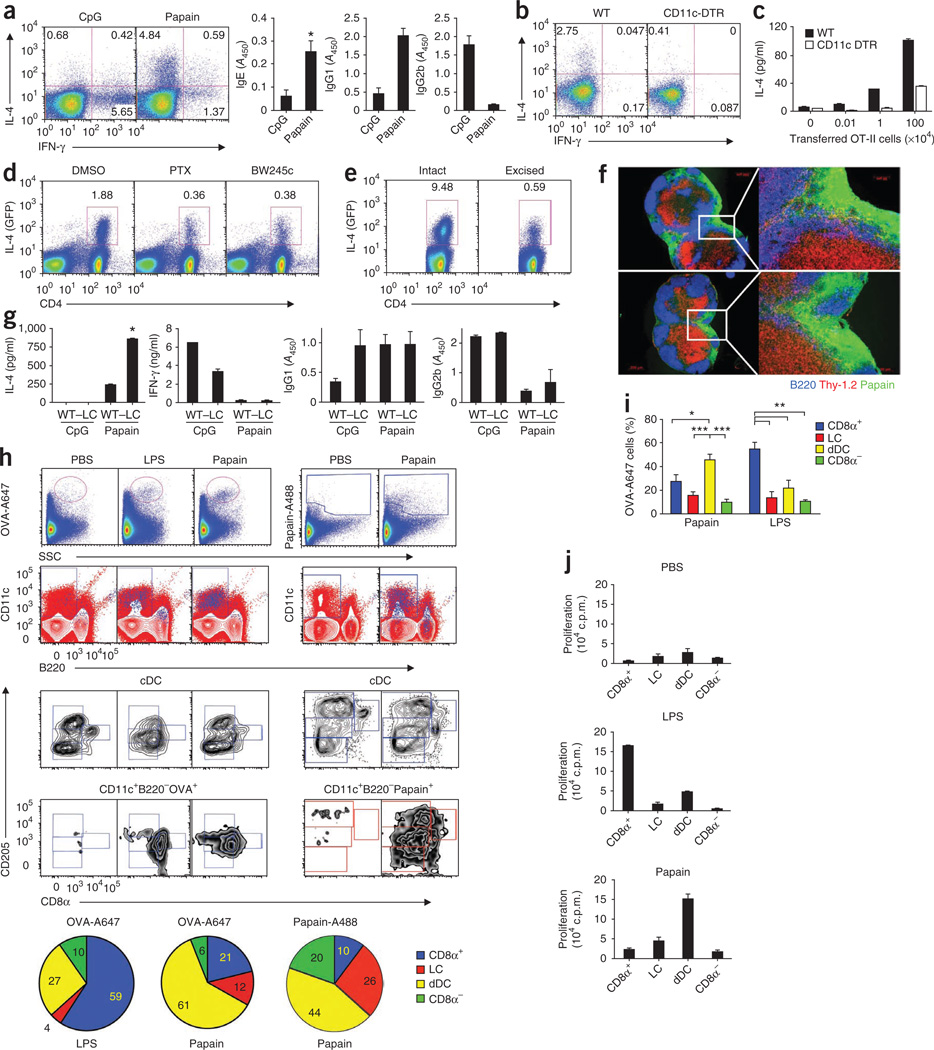
Figure 1.
Vital role of DCs in papain-induced TH2 responses. (a) Intracellular staining of IL-4 and IFN-γ in CD4+ T cells (left; day 21) and anti-OVA IgE, IgG1 and IgG2b in serum (right; day 21) from mice immunized on days 0, 7 and 14 with CpG or papain. _A_450, absorbance at 450 nm. (b) Intracellular staining of IFN-γ and IL-4 in CD4+ T cells from DC-depleted wild-type (WT) and CD11c-DTR mice immunized 4 d earlier with OVA plus papain. Numbers in quadrants (a,b) indicate percent cells in each. (c) ELISA of IL-4 in supernatants of draining lymph node cells from wild-type or CD11c-DTR mice given various numbers of CD4+ OT-II T cells 24 h before diphtheria toxin treatment, then immunized with OVA plus papain; 4 d later, cells were restimulated for 4 d ex vivo with OVA peptide (amino acids 323–339). (d) IL-4-producing CD4+ T cells from 4get mice given no pretreatment (dimethyl sulfoxide (DMSO)) or pretreated with pertussis toxin (PTX) or BW245c, then immunized with OVA plus papain; IL-4 was assessed 4 d later as green fluorescent protein (GFP). (e) IL-4-producing CD4+ T cells 4 d after immunization with OVA plus papain, with the site of immunization excised 6 h after immunization. Numbers above outlined areas (d,e) indicate percent IL-4+CD4+ T cells. (f) Immunofluorescence microscopy of frozen sections of draining lymph nodes (n = 2) from mice 2 h after injection of Alexa Fluor 488–labeled papain (green). Blue, B220 (B cell–associated marker); red, Thy-1.2 (CD90.2); right, enlargement of area outlined at left. Original magnification, ×5 (left) or ×20 (right). (g) Production of IL-4 and IFN-γ by CD4+ T cells (left) and anti-OVA IgG1 and IgG2b in serum (right; day 14) from wild-type mice and Langerhans cell–depleted langerin-DTR mice (–LC) after immunization with OVA plus papain, as described in a. *, P < 0.05 (_t_-test). (h) Uptake of OVA or papain by DC subsets (identified and defined as described in Results) in draining lymph nodes isolated from mice 24 h after subcutaneous immunization with Alexa Fluor 647–labeled OVA (OVA-A647) plus papain, or Alexa Fluor 488–labeled papain (Papain-A488) alone. Bottom, proportion of fluorescence-labeled cells in conventional DC (cDC) subsets. SSC, side scatter; LC, Langerhans cell; dDC, dermal DC. (i) Pooled data from h. (j) Immunostimulatory capacity of the four lymph node DC subsets sorted by flow cytometry from mice immunized 24 h earlier with OVA plus papain or OVA plus LPS, then cultured with OT-II CD4+ T cells; proliferation was assessed by thymidine labeling. *P < 0.05, **P < 0.01 and ***P < 0.001 (analysis of variance). Data are representative of three to five independent experiments (mean and s.e.m.).
Peripheral tissue–resident DCs take up antigen and migrate to draining lymph nodes to initiate adaptive immune responses4–6. Given that stimulation with papain effectively induced DC migration to and accumulation in the draining lymph node15,26, we hypothesized that skin-derived DCs have a critical role in the induction of TH2 responses to papain. To determine the role of skin-derived DCs, we blocked the migration of skin DCs in mice by injecting pertussis toxin or Bw245c (an agonist of the prostanoid receptor DP1), each of which can inhibit the migration of skin DCs28. To monitor TH2 responses in vivo, we used 4get mice, in which IL-4 production can be detected by flow cytometry analysis of the expression of green fluorescent protein29. We treated 4get mice with pertussis toxin or Bw245c before immunizing them with OVA plus papain and examined IL-4 secretion by CD4+ T cells in the draining lymph nodes. Treatment with either pertussis toxin or Bw245c resulted in much less IL-4 production by CD4+ T cells (Fig. 1d). This experiment suggested that the papain-induced TH2 response was dependent on the migration of skin-derived DCs to the draining lymph nodes. To further confirm that finding, we immunized 4get mice in the ear with OVA plus papain and then excised the injection site 6 h after immunization to physically block the migration of skin DCs28,30. IL-4 production by CD4+T cells from mice that underwent excision of the injection site was much lower than that of cells from mice with an intact site of immunization (Fig. 1e). To exclude the possibility that excision of the injection site could result in removal of the antigen depot, thus potentially diminishing presentation by any cell type, we determined whether we could visualize OVA or papain in the draining lymph node before excision of the site. Consistent with published reports30, at 2 h after immunization with labeled OVA or labeled papain, we detected a large amount of fluorescence in the subcapsular sinus and the underlying area between the B cell–rich follicles (Fig. 1f and Supplementary Fig. 3). Consistent with published studies30, it is very likely that soluble protein reached the lymph node via the lymphatic vessels. Therefore, excision of the injection site at 6 h does not preclude antigen availability in the lymph node. Together, these data (Fig. 1d–f) suggest that the skin-derived migratory DCs have a prominent role in the induction of TH2 responses after stimulation with papain.
The skin is populated by at least two subsets of DCs: epidermal Langerhans cells and resident dermal DCs. To investigate which skin DC subset was involved in the TH2 response to papain, we used a transgenic langerin-DTR mouse model in which Langerhans cells could be completely ablated within 24 h of the injection of diphtheria toxin31 and the epidermis remained largely devoid of Langerhans cells for at least 4 weeks after injection of diphtheria toxin (Supplementary Fig. 4). We immunized mice at day 14 after treatment with diphtheria toxin, a time at which other langerin-positive cells in the dermis would have returned31,32. There was no noticeable change in the induction of the TH2-dependent OVA-specific IgG1 antibody response after depletion of Langerhans cells (Fig. 1g). In fact, we observed significantly more IL-4 production by CD4+ T cells isolated from langerin-DTR mice treated with diphtheria toxin than by cells from wild-type mice (Fig. 1g). These data demonstrate that papain-induced TH2 responses were not promoted by Langerhans cells. We therefore sought to determine if the TH2 response was dependent on dermal DCs. We immunized C57BL/6 mice with Alexa Fluor 488–labeled papain or Alexa Fluor 647–labeled OVA plus papain, then analyzed the uptake of labeled papain or labeled OVA and their distribution in various DC subsets in the draining lymph node at 24 h after immunization (Fig. 1h,i). First, we identified CD11c+B220− ‘conventional’ DCs, then we used the expression of CD8α and the DC marker DEC-205 on this subset to resolve four main DC subsets in the lymph node as described before33. Here CD8α+DEC-205+ cells are CD8α+ DCs, CD8α−DEC-205hi cells are Langerhans cells, CD8α−DEC-205+ cells are dermal DCs, and CD8α−DEC-205− cells are CD8α− DCs33. We found that dermal DCs were the main population of cells that contained both papain and OVA. In contrast, immunization with OVA plus lipopolysaccharide (LPS) resulted in the ‘preferential’ uptake of antigen by CD8α+ DCs (Fig. 1h,i). A subset of DCs in the dermis has been shown to express CD103 (refs. 32,34,35). To determine if that subset was involved in antigen uptake, we stained draining lymph node cells from mice immunized with labeled papain and OVA by using a panel of flow cytometry antibodies as described36 (Supplementary Fig. 5) and did not find CD103+ DCs that efficiently took up antigen (Supplementary Fig. 6).
To further investigate the ability of each DC subset to present antigen to T cells, we sorted the four main conventional DC subsets by flow cytometry from the draining lymph node after immunization with OVA, OVA plus papain, or OVA plus LPS, and then cultured those cells together with naive OT-II T cells in vitro. We assessed the proliferation of OT-II T cells by incorporation of tritiated thymidine ([3H]thymidine). Dermal DCs, but not CD8α+ DCs, Langerhans cells or CD8α− DCs, isolated from mice immunized with papain plus OVA induced robust proliferation of OT-II T cells; this was consistent with uptake of antigen (Fig. 1h,j). However, in mice immunized with LPS plus OVA, the proliferation of OT-II cells was induced mainly by CD8α+ DCs, which was again consistent with uptake of antigen (Fig. 1h,j). In summary, dermal DCs, but not Langerhans cells, have an essential role in the uptake and presentation of papain and OVA that results in robust antigen-specific TH2 responses in mice.
Cooperation between DCs and basophils
To investigate whether DCs were sufficient to induce TH2 differentiation in response to papain in vivo, we subcutaneously mice immunized with OVA plus papain or OVA plus CpG. We collected draining lymph nodes 24 h after immunization, digested the nodes and isolated CD11c+ DCs by flow cytometry sorting. We cultured DCs for 72 h together with OVA-specific T cells from OT-II mice to examine the induction of T cell differentiation. DCs isolated from mice immunized with CpG plus OVA induced robust TH1 cytokine responses characterized by the production of IFN-γ without detectable IL-4 (Fig. 2a). Although, as shown above (Fig. 1j), DCs isolated from mice immunized with OVA plus papain were able to induce the proliferation of OT-II cells, they failed to induce IL-4 production. These experiments suggested the involvement of accessory cells in the induction of a TH2 response to papain. Studies have suggested that basophils are critically involved in the induction of TH2 in response to protease allergens and infection with helminths14–16,26. Basophils can be recruited to lymph nodes in response to challenge with papain15,26. To determine whether basophils and DCs have a shared role in TH2 immunity to papain, we isolated both cell subsets from lymph nodes of mice subcutaneously immunized with OVA plus papain. We purified DCs 22 h after immunization, as DC migration was first apparent at that time point15,26. Recruitment of basophils to the draining lymph nodes is known to peak at day 3 after immunization15,26. We found that basophils produced IL-4 (Fig. 2b). We isolated DCs and basophils from mice immunized with OVA plus papain and cultured naive OT-II helper T cells in vitro with DCs, basophils, or a combination of DCs and basophils. We collected cell culture supernatants at 5 d and analyzed IL-4 production. Consistent with our data above (Fig. 2a), we detected no IL-4 in the supernatants of T cells cultured with DCs (Fig. 2c). We observed moderate concentrations of IL-4 in the supernatants of T cells cultured with basophils and substantial enhancement of IL-4 production (about fivefold) for T cells cultured with both DCs and basophils. To confirm those findings and to establish the finding of production of IL-4 by OT-II CD4+ T cells, we did intracellular staining for IL-4. We detected very few IL-4-producing T cells when we cultured OT-II T cells together with DCs alone (Fig. 2d). This demonstrates that DCs are insufficient to polarize a TH2 response after stimulation with papain. Furthermore, there was no IL-4 production in T cells cultured with basophils alone, although we detected small amounts of IL-4 cytokine in the culture supernatants by enzyme-linked immunosorbent assay (ELISA). Notably, CD4+ T cells cultured with both DCs and basophils produced IL-4 (Fig. 2d). Together, these data demonstrate that DCs or basophils alone are unable to stimulate TH2 responses to papain; instead, they act in concert to promote antigen-specific TH2 differentiation.
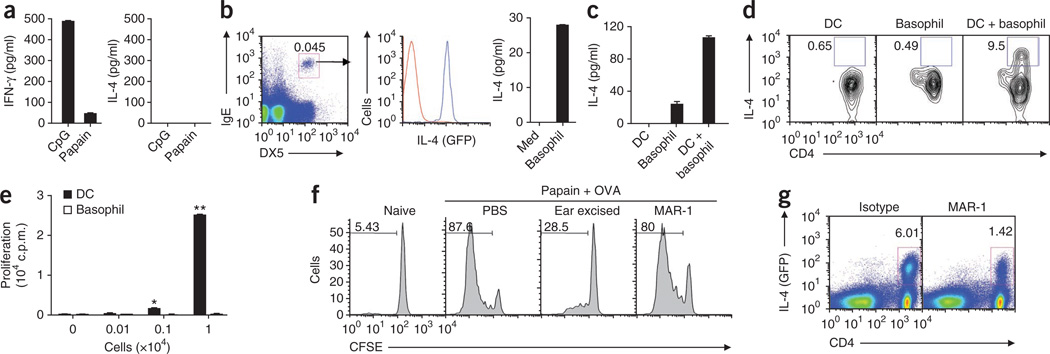
Figure 2.
DCs and basophils act in concert to drive TH2 responses. (a) Production of IFN-γ and IL-4 by OT-II CD4+ T cells after culture with CD11c+ lymph node DCs from mice immunized 24 h before with OVA plus papain or OVA plus CpG. (b) Flow cytometry analysis of IL-4 expression (middle) by IgE+DX5+ basophils (blue line) and nonbasophils (red line) sorted (left) from draining lymph nodes of 4get mice immunized subcutaneously 3 d earlier with papain, and ELISA of IL-4 production by flow cytometry–sorted basophils from mice immunized subcutaneously with papain plus OVA (right). Med, well with medium only. Number above outlined area (left) indicates percent IgE+DX5+ cells. (c) IL-4 production by cocultures of OT-II CD4+ T cells and CD11c+ DCs, basophils or a combination of DCs plus basophils isolated from mice immunized with OVA plus papain. (d) Intracellular flow cytometry analysis of IL-4-producing OT-II CD4+ T cells cultured as in c. Numbers adjacent to outlined areas indicate percent IL-4+CD4+ cells. (e) Proliferation of OT-II CD4+ T cells stimulated in vitro with various numbers of lymph node CD11c+ DCs or basophils isolated from mice immunized with OVA plus papain, with no exogenous OVA added, assessed by [3H]thymidine incorporation. (f) Proliferation of OT-II cells (labeled with the cytosolic dye CFSE) from unimmunized mice (Naive) or mice immunized with OVA plus papain and assessed with no further treatment (PBS), after ablation of skin-derived DCs by ear excision 6 h after immunization, or after depletion of basophils with MAR-1. Numbers above bracketed lines indicate percent CSFE+ (dividing) cells. (g) Flow cytometry analysis of IL-4 expression in CD4+ T cells in 4get mice, assessed (as green fluorescent protein) after basophil depletion and immunization as in f. Numbers above outlined areas indicate percent IL-4+CD4+ cells. *P < 0.05 and **P < 0.01 (_t_-test). Data are representative of three independent experiments (error bars (a–c,e), s.e.m.).
Basophils respond to papain by migrating to lymph nodes and producing TH2-inducing cytokines in vivo as described before15,26 and as shown here (Fig. 2b). Basophils express major histocompatibility complex class II molecules14–16 and costimulatory molecules14,15 and can endocytose soluble proteins in vitro15. Yet basophils were unable to promote a TH2 response in the absence of DCs in vivo. IL-4 expression in T cells is thought to be dependent on the cell cycle, with at least three cell divisions being required37. We hypothesized that basophils may not be able to present antigen to T cells or stimulate T cell proliferation in vivo. To establish the role of basophils in the ability to stimulate the proliferation of antigen-specific CD4+ T cells, we assayed [3H]thymidine incorporation in antigen-specific CD4+ T cells cultured with either basophils sorted by flow cytometry or DCs from draining lymph nodes. Basophils did not stimulate T cell proliferation, whereas DCs stimulated robust proliferation of CD4+ T cells (Fig. 2e). Even in the presence of exogenous OVA in the culture system, basophils showed much less antigen-presentation ability than did DCs (Supplementary Fig. 7). Therefore, the failure of basophils to prime a TH2 response after immunization with OVA plus papain was most probably due to their inability to stimulate T cell proliferation. Consistent with those findings, we observed no change in T cell proliferation after depletion of basophils in vivo with the MAR-1 antibody to FcεRIα (anti-FcεRIα)10 (Fig. 2f and Supplementary Fig. 8), although IL-4 production by CD4+ T cells was much lower (Fig. 2g), consistent with published reports26. T cells proliferated less when we blocked the migration of DCs by surgical excision of the site of injection (Fig. 2f). These data demonstrate that DCs or basophils alone are insufficient to polarize a papain-induced TH2 response. The ‘cooperation’ between these two cell types suggests a role for DCs in inducing T cell proliferation and a role for basophils in providing the IL-4 cytokine required for TH2 differentiation in response to papain.
ROS and TH2 responses to papain
To obtain insight into the molecular mechanism by which papain induced TH2 responses, we first analyzed the cytokine responses of lymph node DCs stimulated with papain. Notably, papain-stimulated DCs did not produce detectable amounts of several pro- and anti-inflammatory mediators, as measured by multiplex bead analysis (Supplementary Fig. 9). We then assessed by microarray analysis changes in gene expression in lymph node DCs cultured in vitro with papain or LPS. We found upregulation of 447 or 567 genes by stimulation with papain or LPS, respectively, relative to expression without stimulus (medium only). LPS-activated DCs had higher expression of several TH1-related genes, including Il12a, Ebi3 (encoding IL-27), Ifng, Cd70 and Tbx21 (encoding the transcription factor T-bet; Fig. 3a). Notably, a group of ROS-related genes were upregulated after papain stimulation (Fig. 3a), including Hmox1 (encoding HO-1) and Ncf4 (encoding p40phox). HO-1 is recognized as a sensitive and reliable indicator of cellular oxidative stress, and p40phox is a subunit of the NADPH complex38.
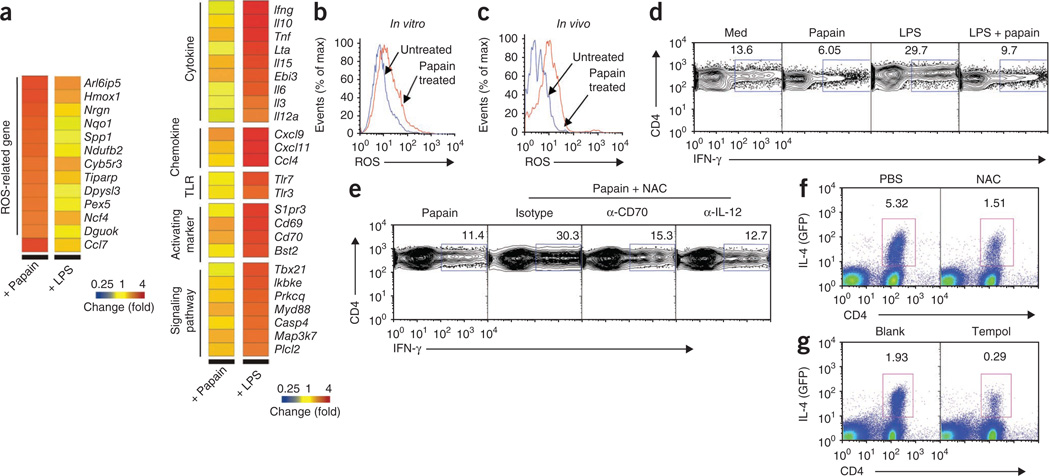
Figure 3.
ROS production by papain-activated DCs is critical for TH2 differentiation. (a) Microarray analysis of gene expression in lymph node DCs stimulated for 4 or 17 h in vitro with papain or LPS. (b,c) Flow cytometry analysis of ROS production by untreated and papain-stimulated DCs in vitro (b) and in vivo (c). (d) Flow cytometry analysis of intracellular IFN-γ production by OT-II T cells stimulated for 72 h with lymph node DCs pulsed with OVA peptide, amino acids 323–339, alone (Med) or together with papain, LPS, or LPS plus papain. (e) IFN-γ production assessed as in d but for cells pulsed with papain alone (far left) or with papain plus NAC in the presence of neutralizing anti-CD70 (α-CD70) or anti-IL-12 (α-IL-12) or isotype-matched control antibody (Isotype). (f,g) Flow cytometry analysis of IL-4 expression (as green fluorescent protein) in CD4+ T cells from draining lymph nodes of 4get mice immunized with papain, with no pretreatment (PBS (f) or Blank (g)) or after pretreatment with NAC (f) or microparticle-encapsulated tempol (g). Numbers above outlined areas indicate percent CD4+IFN-γ+ cells (d,e) or IL-4+CD4+ cells (f,g). Data are representative of one experiment (a) or two to five experiments (b–g).
To confirm the production of ROS by DCs, we obtained lymph node DCs activated in vitro with papain or DCs from lymph nodes of papain-immunized mice and stained cells with the fluorescent dye DCF. We detected the production of ROS, as indicated by an increase in DCF fluorescence (Fig. 3b,c). The presence of ROS is recognized as an endogenous signal for the induction of inflammation, acute lung injury and artherosclerosis39,40. Although the role of ROS in asthma has been well documented41, the involvement of ROS in induction of TH2 responses to cysteine proteases is unknown at present. However, the production of ROS by macrophages diminishes their capacity to stimulate TH1 responses42. We therefore determined whether ROS ‘programmed’ papain-induced DCs to stimulate TH2 responses in vitro. OVA-pulsed DCs induced the TH1 differentiation of OT-II cells in vitro (Fig. 3d). In contrast, the TH1 response was enhanced by stimulation of DCs with LPS, a TH1-polarizing stimulus. Notably, papain suppressed the ability of DCs to stimulate IFN-γ production. Furthermore, the increase in the TH1 response stimulated by LPS was decreased by culture of DCs with papain (Fig. 3d). We then determined whether ROS produced by papain-activated DCs were involved in the suppression of TH1 differentiation. Blocking ROS by _N_-acetyl-cysteine (NAC), a ROS-specific inhibitor, restored the IFN-γ production suppressed by papain (Fig. 3e). IL-12 is a key cytokine in directing the development of TH1 cells2–5,43. An IL-12-independent but CD70-dependent pathway of DC-mediated TH1 polarization has been described44. We thus determined whether blocking ROS with NAC enhanced the expression of CD70 and IL-12p70 by DCs and found that it did (Supplementary Fig. 10). To further confirm that the TH1 response restored by NAC was due to enhanced IL-12 or CD70, we added neutralizing antibody to IL-12 or CD70 to the in vitro cocultures. Neutralization of either CD70 or IL-12 resulted in a lower frequency of IFN-γ-producing T cells (Fig. 3e). These results suggest that the inhibition of TH1 responses in DCs treated with papain is mediated by the production of ROS, which in turn suppresses the expression of CD70 or IL-12.
To assess the involvement of ROS in papain-mediated TH2 responses in vivo, we injected 4get mice with PBS or NAC, then immunized the mice with OVA plus papain. At 4 d after immunization, we examined the production of IL-4 in CD4+ T cells by flow cytometry. IL-4 production was much lower in mice treated with NAC (Fig. 3f), which indicated that ROS is critical in the induction of TH2 responses by papain. To ‘preferentially’ target ROS inhibitors to phagocytic cells, including DCs45, we encapsulated the hydrophobic ROS inhibitor tempol in biodegradable poly(ketal)-based microparticles46 and treated mice with this before immunizing them with OVA plus papain. In mice injected with microparticle-encapsulated tempol before immunization, there was considerable inhibition of TH2 responses (Fig. 3g). Similarly, inhibition of ROS also impaired TH2 responses induced by the related cysteine protease bromelain (Supplementary Fig. 11). Together, these results demonstrate that ROS produced in cysteine protease–activated DCs is critical for the suppression of TH1 responses and enhancement of TH2 differentiation.
Papain-induced TSLP production
TSLP has a key role in the induction of TH2 responses47,48. To investigate the role of TSLP in papain-induced TH2 responses, we isolated RNA from the skin at the site of injection and from lymph node DCs at various time points (1, 2, 6 and 18 h) after immunization with papain. We first assessed TSLP mRNA by real-time PCR. We detected no TSLP mRNA in lymph node DCs (data not shown). However, in the skin, TSLP mRNA was induced by papain stimulation (Fig. 4a). Protein expression of TSLP, assessed by immunofluorescence staining of skin cryosections, was predominantly in the epidermis (Fig. 4b).
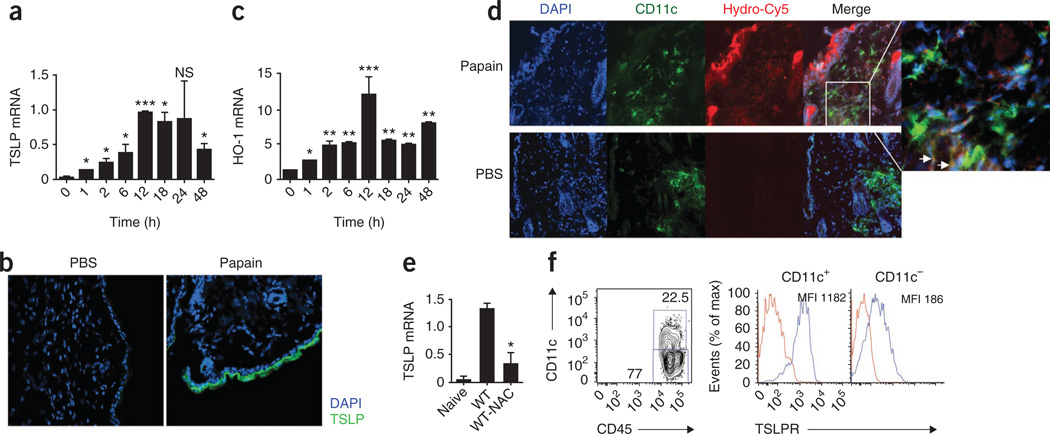
Figure 4.
TSLP production in skin in response to immunization with papain is dependent on ROS. (a) Quantitative RT-PCR analysis of TLSP mRNA expression in ears of mice injected with papain, presented relative to the expression of GAPDH mRNA (‘housekeeping’ gene encoding glyceraldehyde phosphate dehydrogenase). (b) Immunofluorescence confocal microscopy of frozen ear sections from mice immunized with OVA plus papain. Blue, DAPI (DNA-intercalating dye); green, TSLP. Original magnification, ×20. (c) Quantitative RT-PCR analysis of HO-1 mRNA expression in ears of mice injected with papain, presented relative to GAPDH mRNA expression. (d) Immunofluorescence confocal microscopy of the site of immunization with OVA plus papain, stained for DAPI (blue), hydro-Cy5 (red) and CD11c (green) to assess ROS activity. Far right, enlargement of area outlined at left; arrows indicate some hydro-Cy5 staining in DCs. Original magnification, ×20 (main images). (e) Quantitative RT-PCR analysis of TSLP mRNA expression in ears of mice injected with papain, with (WT-NAC) or without (WT) pretreatment with NAC, presented relative to GAPDH mRNA expression. (f) Flow cytometry analysis of expression of the TSLP receptor (TLSPR; blue lines) on CD11c+ and CD11c− dermal hematopoietic cells (sorted as shown at left), including dermal DCs. Red lines, isotype-matched control antibody. Numbers adjacent to outlined areas (left) indicate percent CD11c+CD45+ cells (top) or CD11c−CD45+ cells (bottom); MFI (right), mean fluorescent intensity. NS, not significant; *P < 0.05, **P < 0.01 and ***P < 0.001 (_t_-test). Data are representative of two to three independent experiments (error bars (a,c,e), s.e.m.).
ROS are produced by epithelial cells38. We thus analyzed the presence of ROS at the site of injection. Hmox1 expression has been used as a marker of intracellular oxidative stress38. We assessed Hmox1 expression in skin by quantitative real-time PCR. We observed robust induction of HO-1 mRNA in skin at the site of injection with papain (Fig. 4c). We detected HO-1 mRNA expression as early as 1 h after papain injection; it peaked at 12 h and lasted for at least 48 h. The hydrocyanine dye hydro-Cy5 is a membrane-permeable molecule that, after oxidation with ROS, is modified into a membrane-impermeable dye, which allows accumulation of the dye in cells producing ROS49. We treated mice with papain for 6 h and then injected them with hydro-Cy5 at the same injection site 1 h before excising skin for staining. We excised skin from the injection site and costained it for CD11c to delineate the presence of ROS in epithelial cells. We detected robust ROS production mainly in epithelial cells, with a weak signal in CD11c+ DCs in the dermis (Fig. 4d). Finally, we determined whether the expression of TSLP was ROS dependent. Real-time PCR data indicated that TSLP expression was significantly lower in NAC-treated mice (Fig. 4e). Also, we detected higher expression of TSLP receptor mRNA on DCs in the dermis (Fig. 4f) and CD4+ T cells in lymph node (Supplementary Fig. 12). These data indicate a role for ROS in the induction of TSLP in epithelial cells that might in turn induce signaling in dermal DCs via the TSLP receptor as well as in CD4+ T cells in draining lymph nodes, thereby promoting TH2 differentiation47,48.
The TLR4-TRIF signaling axis
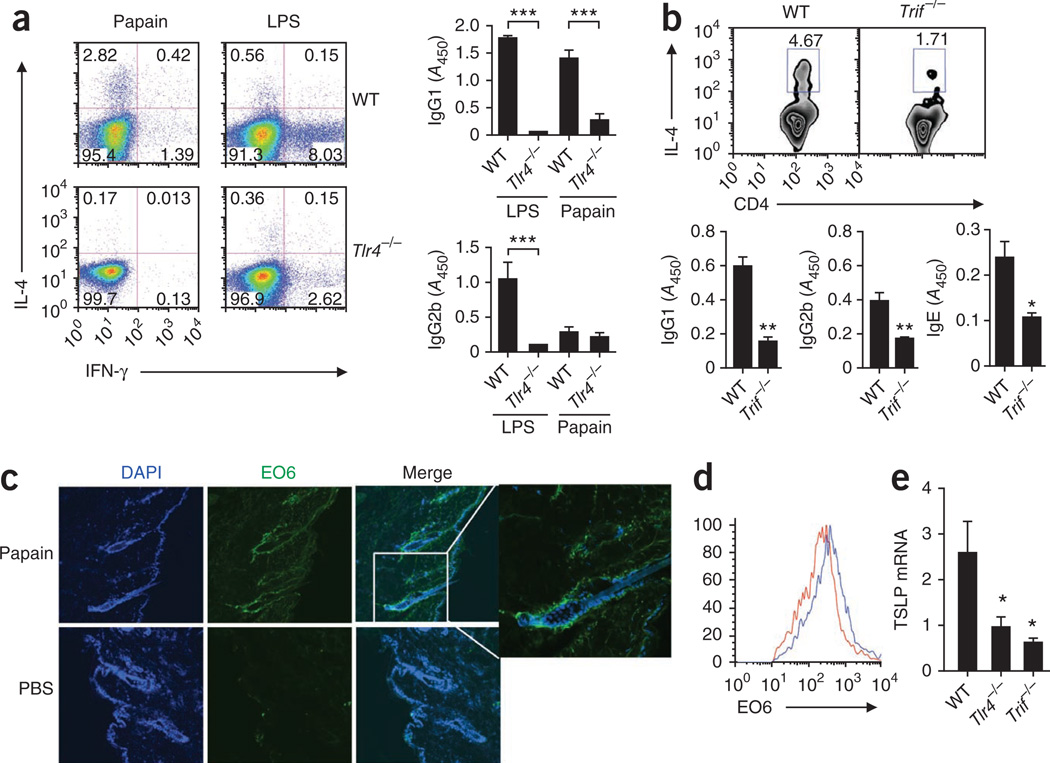
Very little is known about the role of PRRs in the induction of TH2 responses. We observed that papain-induced TH2 responses were independent of signaling via TLR2, TLR3, TLR6, TLR7 or TLR9 (Supplementary Fig. 13). In addition, neither the Nod-like receptors NALP3 and IPAF nor their downstream signaling adaptor proteins, such as ASC and caspase-1, were required for the induction of TH2 responses to papain (Supplementary Fig. 14). However, IL-4 production by CD4+ T cells, as well as the production of OVA-specific IgG1 antibodies, were significantly lower in _Tlr4_−/− mice after immunization with OVA plus papain (P < 0.05). In contrast, the induction of TH1 responses to OVA plus LPS was dependent on TLR4 (Fig. 5a). These data demonstrate that TH2 induction by papain is dependent on TLR4 signaling. To eliminate endotoxin contamination, we used endotoxin-free OVA in these experiments, and in addition, we used polymixin B to neutralize any endotoxin. Treatment with polymyxin B did not alter the IL-4 production by CD4+ T cells (Supplementary Fig. 15), which suggested that the TH2-inducing effect of papain was not caused by endotoxin contamination. Furthermore, IL-4 production by CD4+ T cells in 4get mice was much lower after stimulation with heat-inactivated papain (Supplementary Fig. 15), which indicated a role for the intrinsic enzymatic activity of papain in the induction of TH2 responses. TH2 responses were also significantly higher in mice deficient in the adaptor MyD88 (P < 0.05; Supplementary Fig. 16). In contrast, mice deficient in TRIF signaling showed much less production of IL-4 by CD4+ T cells, as well as less OVA-specific IgG1 and IgE (Fig. 5b). As both MyD88 and TRIF are necessary for endotoxin signaling, it is unlikely that the papain-induced TH2 responses were due to endotoxin contamination. Collectively, these data demonstrate that TLR4-TRIF signaling is involved in TH2 immunity induced by papain, but they raise questions about the nature of the ligand that initiates signaling via TLR4. One clue came from experiments demonstrating that induction of HO-1 was independent of TLR4 (data not shown). However, oxidized moieties, including oxidized phospholipids (OxPLs), can activate TLR4 on macrophages39,40,50,51, and ROS can induce the formation of OxPLs, which signal via the TLR4-TRIF-dependent pathway40. We thus hypothesized that the induction of ROS by papain could lead to the formation of OxPLs. The monoclonal antibody EO6 is specific to OxPLs and distinguishes them from nonoxidized phospholipids40. We observed considerable EO6-stained OxPLs in skin epithelial cells by immunofluorescence staining (Fig. 5c). In addition, flow cytometry analysis demonstrated the generation of EO6-detectable OxPLs in papain-activated DCs in draining lymph nodes (Fig. 5d). These data demonstrate that ROS generated by stimulation with papain triggers the oxidative-stress machinery and the production of OxPLs in skin and in draining lymph nodes. Furthermore, consistent with published studies of OxPLs39, we observed phosphorylation of the ubiquitin ligase TRAF6 in papain-treated DCs (data not shown). Together, our results indicate a link among ROS, OxPLs and TLR4- and TRIF-based signaling in the induction of TH2 responses after stimulation with papain. Furthermore, the induction of TSLP in skin was also tightly regulated by TLR4 and TRIF signaling (Fig. 5e).
Figure 5.
Papain-induced TH2 responses are dependent on TLR4-TRIF signaling. (a) Flow cytometry of intracellular staining for IL-4 and IFN-γ in CD4+ T cells from draining lymph nodes (left) and OVA-specific antibody titers (right) of wild-type or _Tlr4_−/− mice immunized with OVA plus papain or OVA plus LPS. Numbers in quadrants (left) indicate percent cells in each. (b) Flow cytometry of intracellular IL-4 staining in CD4+ T cells from draining lymph nodes (above) and OVA-specific antibody titers (below) of wild-type and _Trif_−/− mice immunized as in a. Numbers above outlined areas (top) indicate percent IL-4+CD4+ cells. (c) Immunofluorescence microscopy of frozen tissue sections of skin at the site of immunization, obtained from C57BL/6 mice injected with PBS or papain, fixed and stained with the EO6 antibody specific for OxPLs. Far right, enlargement of area outlined at left. Original magnification, ×20 (main images). (d) Flow cytometry analysis of the expression of OxPLs in draining lymph node CD11c+ DCs from mice injected with PBS (red line) or papain (blue line). (e) Quantitative RT-PCR analysis of TSLP mRNA expression in skin tissue derived from the site of immunization of papain-injected wild-type, TLR4-deficient or TRIF-deficient mice, presented relative to GAPDH mRNA expression. *P < 0.05, **P < 0.01 and ***P < 0.001 (_t_-test). Data are representative of two to three independent experiments (error bars (a,b,e), s.e.m.).
Recruitment of basophils to lymph nodes
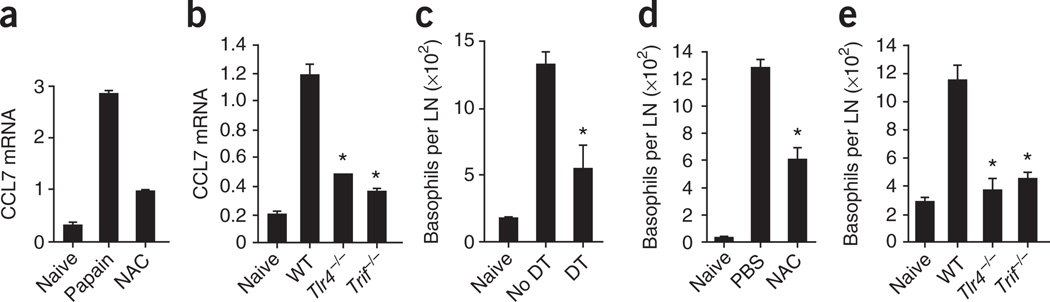
As described above (Fig. 2c–e), the recruitment of basophils to draining lymph nodes is critical in papain-induced TH2 responses. Microarray analysis showed that CCL7 (MCP-3), a basophil-attracting chemokine52, was selectively upregulated in papain-stimulated lymph node DCs but not in LPS-stimulated DCs (Fig. 3a). We further confirmed higher CCL7 mRNA expression by real-time PCR in lymph node–resident DCs activated by papain in vivo. Moreover, as described earlier for TSLP, the production of CCL7 by DCs was tightly regulated by the ROS, TLR4 and TRIF signaling pathways (Fig. 6a,b). We further hypothesized that activation of lymph node DCs by papain greatly increased secretion of CCL7, which subsequently recruited basophils to the draining lymph nodes to support TH2 responses. To test our hypothesis, we ablated DCs in CD11c-DTR mice by injection of diphtheria toxin and quantified the migration of basophils to the draining lymph nodes. The absolute number of basophils in draining lymph nodes was significantly lower after depletion of DCs (Fig. 6c), which suggests that DCs are critical in attracting basophils to lymph nodes. In contrast, we did not find significantly fewer basophils in draining lymph node after depletion of T cells through the use of anti-CD3 (P > 0.1; Supplementary Fig. 17), which indicated that T cells did not have a role in the recruitment of basophils. Next, we evaluated the migration of basophils after treatment with NAC (ROS blockade) and in _Tlr4_−/− and _Trif_−/− mice. The migration of basophils to draining lymph nodes was significantly less after treatment with NAC (Fig. 6d). Furthermore, we observed significantly fewer basophils in _Tlr4_−/− and _Trif_−/− mice than in wild-type mice in response to immunization with papain (Fig. 6e). Our data suggest that papain-activated DCs efficiently recruit basophils to draining lymph nodes and indicate a role for the DC-derived chemokine CCL7 in attracting basophils. Furthermore, ROS, TLR4 and TRIF signaling were critical in the induction of CCL7 in papain-stimulated DCs. Together, these results demonstrate that TH2 responses to papain are orchestrated by ROS-dependent TLR4-TRIF signaling, which mediates the concerted action of DCs and basophils (Supplementary Fig. 18).
Figure 6.
Regulation of basophil migration by ROS, TLR4 and TRIF signaling in DCs. (a) RT-PCR analysis of CCL7 mRNA expression by lymph node DCs isolated from unimmunized mice (Naive; left) or mice immunized subcutaneously with papain with (right) or without (middle) NAC pretreatment. (b) CCL7 mRNA expression by lymph node DCs from wild-type, TLR4-deficient or TRIF-deficient mice left unimmunized or immunized with papain. Results in a,b are presented relative to GAPDH mRNA expression. (c) Recruitment of basophils to the lymph nodes in CD11c-DTR mice left undepleted (no DT) or depleted of DCs (DT) and then immunized subcutaneously 1 d later with papain and evaluated 3 d later. LN, lymph node. (d) Recruitment of basophils to the lymph nodes in wild-type mice immunized with papain, with (NAC) or without (PBS) pretreatment with NAC. (e) Recruitment of basophils to the lymph nodes in unimmunized mice and wild-type, _Tlr4_−/− and _Trif_−/− mice immunized with papain. *P < 0.05 (_t_-test). Data are representative of two to three independent experiments.
Optimal TH2 induction
Papain induced IL-4 in basophils15 (Fig. 2a) and TSLP in epithelial cells15 (Fig. 4a,b) and also suppressed IL-12 production in DCs and directly inhibited their ability to stimulate TH1 responses (Fig. 3 and Supplementary Fig. 10). We determined the relative importance of IL-4, TSLP and suppression of IL-12 in TH2 induction by papain. First we did an experiment in vivo to neutralize both IL-4 and TSLP, as well as to supplement IL-12. We reconstituted _Il4_−/− mice on day −1 with OT-II CD4+ T cells and injected the mice on day 0 subcutaneously with anti–mouse TSLP and intraperitoneally with recombinant mouse IL-12, then immunized them 2 h later with OVA plus papain. We injected wild-type mice with isotype-matched control antibody at the same time and immunized them with OVA plus papain. On days +2 and +3, we injected mice again with anti-TSLP and also injected them with IL-12 on days +1, +2 and +3. On day +4, we isolated lymph node cells and restimulated them for 5 h in vitro on plates precoated with anti-CD3 and anti-CD28 in the presence of GolgiStop. We then analyzed IL-4 production by intracellular flow cytometry staining. We observed a lower frequency of IL-4+ CD4+ T cells (Supplementary Fig. 19a). To determine the relative contributions of IL-4 and TSLP to this result, we did an independent experiment in which we immunized wild-type and _Il4_−/− mice injected with anti-TSLP, as well as uninjected wild-type and _Il4_−/− mice, with OVA plus papain (Supplementary Fig. 19b). We then evaluated the antigen-specific CD4+ T cell response as described above. Blockade of either IL-4 or TSLP alone resulted in TH2 responses only modestly lower than those of mice that received isotype-matched control antibodies (Supplementary Fig. 19b); this result was consistent with published work15. In contrast, combined blockade of TSLP and IL-4 resulted in a more pronounced effect (Supplementary Fig. 19b). Together, these findings demonstrate that papain-mediated induction of IL-4 and TSLP, along with the suppression of IL-12, creates a permissive environment for the optimal induction of TH2 responses (Supplementary Fig. 18).
DISCUSSION
The role of DCs in the induction of TH2 responses has been addressed before22–25. However, the role of DCs in the induction of TH2 immunity to allergens has been challenged by a study reporting that DCs are neither sufficient nor essential for the induction of TH2 responses to papain15. The finding that DCs were not essential was demonstrated by ablation of DCs in lethally irradiated wild-type mice reconstituted with bone marrow derived from CD11c-DTR mice (chimeric mice)27 and subsequently immunized with papain plus antigen15. In contrast, our results have indicated that depletion of DCs in CD11c-DTR mice through the use of diphtheria toxin abrogated the induction of TH2 responses to papain plus antigen. Results obtained by excision of the site of injection, as well as blocking DC migration with inhibitors, supported the idea of a role for skin-derived migratory DCs in the induction of TH2 responses. In vitro analysis of sorted DC subsets indicated the involvement of dermal DCs in the induction of antigen-specific proliferation of CD4+ T helper cells in response to immunization with OVA plus papain. A possible explanation for the differences between our study here and the previously published study15 may be explained by earlier work demonstrating that a substantial proportion of CD11c+ cells in the dermis are resistant to depletion by irradiation53. Chimeric mice generated with CD11c-DTR bone marrow could potentially carry 25% residual dermal DCs derived from the host bone marrow (wild-type; CD11c-DTR−) that cannot be depleted by treatment with diphtheria toxin and hence potentially contribute to the adaptive response. In addition, incomplete depletion of donor-derived DCs by diphtheria toxin could result in substantial numbers of dermal DCs that promote TH2 responses. Furthermore, independent studies have demonstrated impairment of TH2 responses after depletion of DCs in CD11c-DTR mice25. The previously published study15 further demonstrated that DCs are not sufficient for TH2 responses to papain by using CD11c-Aβb mice54,55, in which major histocompatibility complex class II is selectively expressed on CD11c+ DCs; this is consistent with our results presented here.
The key DC-derived signals that mediate TH2 immunity to allergens are still unclear. IL-4 and TSLP can initiate TH2 responses, but our results have indicated that DCs do not produce such cytokines yet have a vital role in the induction of TH2 responses. Basophils can migrate to draining lymph nodes in response to allergens or helminths15,16,26 and secrete IL-4 and TSLP26. Furthermore, basophils express major histocompatibility complex class II as well as costimulatory molecules15,16 and take up soluble antigen in vitro and can present antigens to T cells15. However, how efficiently they present antigens relative to antigen presentation by DCs is unclear. Our data have demonstrated that basophils from mice immunized with OVA plus papain were unable to stimulate efficient proliferation of CD4+ T cells, even in the presence of exogenous OVA. Consistent with that finding, depletion of basophils in vivo by injection of MAR-1 (anti-FcεRIα) had no effect on T cell division, which demonstrates that basophils are not essential for the proliferation of antigen-specific CD4+ T cells in vivo. Together, these data indicate a critical role for the concerted action of basophils and DCs in driving TH2 immunity, with DCs providing antigen and basophils providing IL-4.
As for the molecular mechanisms by which cysteine proteases induce TH2 responses, our results have demonstrated a key role for ROS. ROS generated by macrophages can suppress TH1 responses42. Our results have shown that ROS suppress expression of IL-12 and CD70 in DCs, thereby favoring a TH2 bias. In vivo suppression of ROS production in DCs, by targeting of an ROS inhibitor to DCs in microparticles, resulted in lower TH2 responses. In addition to being generated by DCs, ROS were also generated by epithelial cells in response to papain immunization. TSLP, a cytokine known to be involved in TH2 differentiation, is regulated in airway epithelial cells by the production of ROS56. In our studies, we observed that TSLP production in epithelial cells in response to papain at the site of injection was significantly lower after treatment with NAC, which suggests a role for ROS in modulating TSLP expression in epithelial cells in response to papain.
Finally, it is still unclear how helminths and allergens are sensed by innate immune cells. Few studies have attempted to study the role of PRRs in the response to helminths and allergens. Data indicate that in both airway epithelial cells and keratinocytes, PAR2 is an important protease-mediated mediator of TSLP expression55,56. Our preliminary data suggest that PAR-2 deficiency has a modest effect on papain-induced TH2 responses (data not shown). In contrast, our results indicate that TLR4-mediated TRIF signaling is critical in papain-induced TH2 responses. Studies have demonstrated that a TLR4-dependent, MyD88-independent pathway is critical in oxidative stress–related diseases57. It is unlikely that the TLR activation was due to endotoxin, for the following reasons: we used endotoxin-free OVA; polymixin B treatment did not affect TH2 induction by OVA plus papain; TH2 induction was significantly lower after immunization with heat-inactivated papain; the TH2 response to papain was independent of MyD88, which is critical for endotoxin-mediated TLR4 triggering, and in fact, papain-induced TH2 responses were greater in _Myd88_−/− mice; and the induction of HO-1 by papain was independent of TLR4 and TRIF (data not shown). Therefore, which-ever TLR4 ligand was induced by papain must be downstream of HO-1. In this context, OxPLs can activate TLR4 (refs. 40,51), and our results indicated robust production of OxPLs in DCs and epithelial cells after stimulation with papain.
In summary, our data have demonstrated that TH2 responses to cysteine proteases require DC-basophil ‘cooperation’ via ROS signaling. Cysteine proteases stimulate ROS production in DCs and epithelial cells. ROS have a central role in orchestrating TH2 responses by inducing the formation of oxidized lipids that trigger TLR4-TRIF–mediated induction of TSLP by epithelial cells. In addition, ROS suppress production of the TH1-inducing molecules IL-12 and CD70 in lymph node DCs and induce the DC-derived chemokine CCL7, thus facilitating the recruitment of IL-4+ basophils to the lymph node.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureimmunology/.
Supplementary Material
Supplementary Materials
01
ACKNOWLEDGMENTS
We thank S.A. Mertens, L. Bronner and Y. Wang for assistance with cell sorting; Y. Wang, D. Levesque and D. Bonenberger for assistance with the maintenance of mice at the Emory Vaccine Center vivarium; S. Akira (Osaka University) for _Tlr2_−/−, _Tlr3_−/−, _Tlr4_−/−, _Tlr6_−/−, _Tlr7_−/−, _Tlr9_−/−, _Myd88_−/− and _Ticam1_lps−2/lps−2 mice; V. Dixit (Genentech) for _Nalp3_−/−, _Ipaf_−/− and _Asc_−/− mice; K.A. Hogquist (University of Minnesota) for Langerin-EGFP-DTR mice; and J. Witztum (University of California at San Diego) for EO6. Supported by the National Institutes of Health (U54AI057157, R37AI48638, R01DK057665, U19AI057266, HHSN266 200700006C, N01 AI50019, N01 AI50025) and the Bill & Melinda Gates Foundation.
Footnotes
Accession codes. GEO: microarray data, GSE21602.
Note: Supplementary information is available on the Nature Immunology website.
AUTHOR CONTRIBUTIONS
H.T. and B.P. designed experiments; H.T. did experiments; R.R. and W.C. assisted with experiments; S.P.K. K.K. and N.M. designed microparticles and assisted with ROS imaging; T.B.K. and H.L.N. assisted with data analyses.; B.M. provided mice; and H.T. and B.P. wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008;29:319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Heath WR, Carbone FR. Dendritic cell subsets in primary and secondary T cell responses at body surfaces. Nat. Immunol. 2009;10:1237–1244. doi: 10.1038/ni.1822. [DOI] [PubMed] [Google Scholar]
- 7.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 8.Urban JF, Jr, et al. The importance of Th2 cytokines in protective immunity to nematodes. Immunol. Rev. 1992;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 9.Khodoun MV, Orekhova T, Potter C, Morris S, Finkelman FD. Basophils initiate IL-4 production during a memory T-dependent response. J. Exp. Med. 2004;200:857–870. doi: 10.1084/jem.20040598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denzel A, et al. Basophils enhance immunological memory responses. Nat. Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 11.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat. Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 12.Min B, Paul WE. Basophils: in the spotlight at last. Nat. Immunol. 2008;9:223–225. doi: 10.1038/ni0308-223. [DOI] [PubMed] [Google Scholar]
- 13.Min B, et al. Basophils produce IL-4 and accumulate in tissues after infection with a Th2-inducing parasite. J. Exp. Med. 2004;200:507–517. doi: 10.1084/jem.20040590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimoto T, et al. Basophils contribute to TH2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat. Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 15.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat. Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrigoue JG, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat. Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon S, et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol. 2004;172:4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 18.Redecke V, et al. Cutting edge: activation of Toll-like receptor 2 induces a Th2 immune response and promotes experimental asthma. J. Immunol. 2004;172:2739–2743. doi: 10.4049/jimmunol.172.5.2739. [DOI] [PubMed] [Google Scholar]
- 19.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang D, et al. Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J. Exp. Med. 2008;205:79–90. doi: 10.1084/jem.20062027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fritz JH, et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26:445–459. doi: 10.1016/j.immuni.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Maldonado-Lopez R, et al. CD8α+ and CD8α− subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pulendran B, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat. Rev. Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 25.van Rijt LS, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J. Exp. Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung S, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allan RS, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 29.Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 2001;15:303–311. doi: 10.1016/s1074-7613(01)00186-8. [DOI] [PubMed] [Google Scholar]
- 30.Itano AA, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 31.Kissenpfennig A, et al. Dynamics and function of Langerhans cells in vivo: dermal dendritic cells colonize lymph node areas distinct from slower migrating Langerhans cells. Immunity. 2005;22:643–654. doi: 10.1016/j.immuni.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Bursch LS, et al. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 2007;204:3147–3156. doi: 10.1084/jem.20071966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henri S, et al. The dendritic cell populations of mouse lymph nodes. J. Immunol. 2001;167:741–748. doi: 10.4049/jimmunol.167.2.741. [DOI] [PubMed] [Google Scholar]
- 34.Poulin LF, et al. The dermis contains langerin+ dendritic cells that develop and function independently of epidermal Langerhans cells. J. Exp. Med. 2007;204:3119–3131. doi: 10.1084/jem.20071724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ginhoux F, et al. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J. Exp. Med. 2007;204:3133–3146. doi: 10.1084/jem.20071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 37.Bird JJ, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 38.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 39.Binder CJ, et al. Innate and acquired immunity in atherogenesis. Nat. Med. 2002;8:1218–1226. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 40.Imai Y, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riedl MA, Nel AE. Importance of oxidative stress in the pathogenesis and treatment of asthma. Curr. Opin. Allergy Clin. Immunol. 2008;8:49–56. doi: 10.1097/ACI.0b013e3282f3d913. [DOI] [PubMed] [Google Scholar]
- 42.Gelderman KA, et al. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J. Clin. Invest. 2007;117:3020–3028. doi: 10.1172/JCI31935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 44.Soares H, et al. A subset of dendritic cells induces CD4+ T cells to produce IFN-γ by an IL-12-independent but CD70-dependent mechanism in vivo. J. Exp. Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao W, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat. Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heffernan MJ, Kasturi SP, Yang SC, Pulendran B, Murthy N. The stimulation of CD8+ T cells by dendritic cells pulsed with polyketal microparticles containing ion-paired protein antigen and poly(inosinic acid)-poly(cytidylic acid) Biomaterials. 2009;30:910–918. doi: 10.1016/j.biomaterials.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu YJ, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 48.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat. Immunol. 2006;7:709–714. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 49.Kundu K, et al. Hydrocyanines: a class of fluorescent sensors that can image reactive oxygen species in cell culture, tissue, and in vivo. Angew. Chem. Int. Edn Engl. 2009;48:299–303. doi: 10.1002/anie.200804851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller YI, Chang MK, Binder CJ, Shaw PX, Witztum JL. Oxidized low density lipoprotein and innate immune receptors. Curr. Opin. Lipidol. 2003;14:437–445. doi: 10.1097/00041433-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Miller YI, et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 52.Dahinden CA, et al. Monocyte chemotactic protein 3 is a most effective basophil-and eosinophil-activating chemokine. J. Exp. Med. 1994;179:751–756. doi: 10.1084/jem.179.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bogunovic M, et al. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J. Exp. Med. 2006;203:2627–2638. doi: 10.1084/jem.20060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Niu N, Laufer T, Homer RJ, Cohn L. Cutting edge: limiting MHC class II expression to dendritic cells alters the ability to develop Th2-dependent allergic airway inflammation. J. Immunol. 2009;183:1523–1527. doi: 10.4049/jimmunol.0901349. [DOI] [PubMed] [Google Scholar]
- 55.Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity. 2008;29:795–806. doi: 10.1016/j.immuni.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura Y, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to TH2-type immune responses and airway inflammation. J. Allergy Clin. Immunol. 2008;122:1208–1214. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 57.Zhai Y, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J. Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials
01