A small-molecule IAP inhibitor overcomes resistance to cytotoxic therapies in malignant gliomas in vitro and in vivo (original) (raw)
Abstract
We tested the use of the small-molecule Inhibitor of Apoptosis Protein (IAP) inhibitor LBW242 in combination with the standard-of-care therapies of irradiation and temozolomide for malignant gliomas. In vitro assays demonstrated that LBW242 enhanced the cytotoxic activity of radiotherapy, and clonogenic assays showed that the combination therapy led to a synergistic anti-glioma effect in multiple cell lines. Neurosphere assays revealed that the combination of radiation and LBW242 led to a pro-apoptotic effect in these glioma–initiating cell-enriched assays, with a corresponding inhibition of primary tumor cell growth. Athymic mice bearing established human malignant glioma tumor xenografts treated with LBW242 plus radiation and temozolomide demonstrated a synergistic suppression of tumor growth. Taken together, these experiments show that the pro-apoptotic and anti-glioma effects of radiotherapy and chemotherapy can be enhanced by the addition of a small-molecule IAP inhibitor. These results are readily translatable to clinical trial and offer the potential for improved treatment outcomes for patients with glioma.
Keywords: apoptosis, glioma, Inhibitor of Apoptosis Protein, radiotherapy
Novel strategies for treating malignant gliomas are urgently needed because malignant gliomas remain inherently resistant to standard treatment modalities and, despite intensive treatment with surgery and radiotherapy, are almost always incurable.1 The addition of temozolomide to multi-modality therapy has been shown to improve outcomes in adult patients; however, despite this treatment, the disease ultimately progresses, resulting in dismal overall survival rates.2 One approach to improve patient outcomes has been to escalate the dose intensity of therapy to increase tumor cell killing. Although this has resulted in some short-term responses, it has not made any impact on patient survival.3 Another approach has been to develop therapies that specifically target the oncogenic pathways that are activated in gliomas, such as the epidermal growth factor receptor (EGFR) pathway. However, despite the availability of therapies that effectively inhibit these targets, clinical trials of novel targeted agents have, to date, not demonstrated any significant improvement in outcomes for patients with newly diagnosed glioma.
To improve survival rates in patients with malignant gliomas, it is critical to understand the reasons that current treatment strategies have failed. There is now considerable evidence that resistance to chemo- and radiotherapy in gliomas often results from the activation of anti-apoptotic mechanisms. The apoptotic pathway has been shown to be heavily dysregulated in high-grade gliomas.4 Perturbation of this pathway occurs at multiple levels through a variety of mechanisms. For example, PTEN is one of the most common mutations in malignant gliomas, resulting in activation of the PI3K/AKT pathway and a strong anti-apoptotic signal.5,6 Similarly, multiple receptor tyrosine kinases, such as EGFR and platelet-derived growth factor receptor (PDGFR), are known to be abnormally active, resulting in potent and persistent anti-apoptotic signalling.6–13 Multiple members of the BCL-2 family have also been shown to be dysregulated and to contribute to gliomagenesis.14,15 The Inhibitor of Apoptosis Proteins (IAPs) represent the final molecular blockade preventing apoptosis by inhibiting the activity of caspases 3, 7, and 9. A number of different IAPs have been shown to be highly expressed in malignant gliomas, with an adverse correlation with patient outcomes.16–18 More importantly, peptides that inhibit IAPs have been shown to synergize with tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) and to enhance apoptosis in glioma cells both in vitro and in vivo_._19–21 However, the extraordinarily high concentrations of peptides required to inhibit their target, plus the potential toxicity of TRAIL, have limited these studies to preclinical proof of principle only.
There are now a number of therapeutic agents entering clinical development that specifically target the apoptotic pathway and offer a new avenue for the development of anti-glioma treatment strategies.22 Several Smac mimetics have been developed that are able to inhibit the activity of IAPs; furthermore, unlike their predecessors, they are orally bioavailable and able to penetrate effectively into malignant cells at pharmacologic concentrations.23–25 We have previously shown that a novel, small-molecule IAP inhibitor can be successfully combined with growth factor receptor inhibitors to stimulate apoptosis, resulting in an additive or synergistic anti-glioma effect.26 We hypothesized that targeting the apoptotic pathway in conjunction with conventional cytotoxic therapies in malignant gliomas would overcome treatment resistance, increase levels of apoptosis, and significantly enhance anti-tumor activity.
We show here that the pro-apoptotic and anti-glioma effects of radiotherapy and chemotherapy can be dramatically enhanced by the addition of a small-molecule IAP inhibitor. These results are potentially translatable to clinical trials and offer the potential for improved treatment outcomes for patients with glioma.
Materials and Methods
Cell Culture
The human glioma cell lines U87, U87VIII, LN827, and Gli36 were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, penicillin (100 U/mL), and streptomycin (100 µg/mL). Cells were cultured in a humidified 10% CO2 atmosphere at 37°C and maintained in a logarithmic growth phase for all experiments. Transformed human brain microvasculature endothelial cells (THBMVECs) were cultured in Cambrex EBM-2 media with an EGM-2MV bullet kit (Cambrex Corporation).
Drugs
Temozolomide (Temodar; Schering Plough) was obtained from the pharmacy and resuspended in 1% carboxy-methylcelulose for in vivo administration. LBW242 was generously provided by Novartis Pharma. Stock solutions of LBW242 were dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich), stored at −20°C, and diluted in fresh medium immediately prior to use.
Antibodies and Western Blotting
Cells were grown to 70% confluence; washed twice in ice-cold phosphate buffered saline (PBS); lysed with Radio Immuno Precipitation (RIPA) buffer (Boston Bioproducts) containing 50 mM Tris-Cl (pH 7.4), 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 0.1% sodium dodecyl sulfate with the addition of 1 mM of EDTA, 1 mM of sodium orthovanadate, 50 mM of NaF, and Complete Protease Inhibitor cocktail (Roche Applied Science, Mannheim, Germany); vortexed for 5 s; and centrifuged at 17 530_g_ for 10 min at 4°C. Equal amounts of protein were loaded onto 4%–12% Bis-Tris-polyacrylamide gel (Invitrogen), separated by electrophoresis, and transferred to nitrocellulose membranes (Invitrogen). Western blots were probed with β-actin (Sigma-Aldrich), cIAP-1 (Cell Signaling), cIAP-2 (abcam), and XIAP (Cell Signaling) antibodies as per manufacturer instructions. Blots were then labeled with anti-rabbit or anti-mouse IgG-HRP antibody (Vector Laboratories) and were visualized using chemiluminescence (ChemiDoc XRS System; Bio Rad).
Clonogenic Assay
A total of 500–1000 cells were plated into 100-mm dishes (Falcon) using DMEM plus 10% fetal bovine serum. After 8 h, LBW242 or vehicle was added, and cells were cultured overnight. After 16 h of incubation, cells were exposed to radiation as indicated and were then returned to the incubator. After 10–14 days of additional incubation, colonies were fixed with 100% cold ethanol, then stained with 0.5% crystal violet. Plating efficiency in control plates was calculated as the number of colonies counted/number of cells seeded × 100. The surviving fraction in treatment groups was calculated as the number of colonies/plating efficiency. The combinatorial indices for radiation (400 cGy) and LBW242 (10 μM) were expressed as the ratio of observed/expected proportion of cells surviving,27 where the expected result was calculated as the proportion of surviving cells after treatment with LBW242 alone multiplied by the proportion of surviving cells after treatment with radiation alone. With use of this method, values <1 are considered to indicate synergy, values close to 1.0 are considered to represent an additive effect, and values >1 represent an antagonistic effect.27
Caspase 8 and 9 Activity Assays
Caspase 8 and Caspase 9 activity was measured with the Caspase-Glo 8 and Caspase-Glo 9 assays (Promega) in accordance with the manufacturer's protocol. In brief, 3 × 104 U87VIII cells/mL were irradiated at the indicated doses and cells plated in 100 microliters of medium in 96 well microtiter plates and incubated for 24 h. The indicated concentration of inhibitors was added, and the cells were incubated for an additional 72 h. One hundred microliters of labeling reagent were added to each well, and luminescence was measured after 3 h of incubation on a plate-reading luminometer. Luminesence was normalized to viable cell number using a resazurin assay (Sigma-Aldrich).
Assessment of Apoptosis by Annexin V Staining
1 × 105 U87VIII cells were plated in a 25-cm flask of medium, cultured for 24 h, and treated with the indicated concentration of inhibitors. Sixteen hours later, cells were irradiated at the indicated dose, incubated for an additional 6 days, collected for analysis, washed twice in cold PBS, and resuspended in 100 μL of annexin-binding buffer containing 2.5 μL of Annexin V-FITC and 2 μL propidium iodide (BD Biosciences), in accordance with the manufacturer's instructions. Before FACS analysis, an additional 400 μL of binding buffer was added to the cell suspension. FACS analysis was performed on CellQuest and gated to exclude cellular debris; 10, 000 events were collected for each sample.
Endothelial Cell Assay
A total of 3 × 104 THMBVECs per well were plated in 24-well plates and incubated overnight. On day 2, medium was removed and replaced with medium containing LBW242 (10 µM) or DMSO. After 1 h, all plates were removed from the incubator and treated with or without 6 Gy irradiation, as indicated, and were then incubated for an additional 48 h. Plates were then washed with PBS and trypsinized, and the cell count was determined with a coulter counter.
Glioma Neurosphere Assay
Collection and use of fresh and discarded human tumor tissue was approved by the Brigham and Women's Hospital Institutional Review Board (Boston, MA). After frozen section diagnosis of malignant glioma by the attending neuropathologist, tumor material was grossly dissected from the tissue sample. Portions of the tumors were collected in chilled media for the studies described here, and other portions were allocated for paraffin embedding for histological diagnosis and for genotyping. Expansion of tumor material and propagation was accomplished by subcutaneous implantation in lcr SCID mice (cells were never grown on plastic). When tumors reached ∼1 cm, tumors were disaggregated, and cells were counted and then grown in serum-free media with EGF, FGF, and LIF, as described previously,28,29 to form tumorspheres. LBW242 (10 µM) was added 24 h after plating, and tumor neurospheres were counted 10 days after plating. For assessment of apoptosis, tumor neurospheres were pre-established for 7 days, treated with LBW242 and/or radiation therapy, and stained with propidium iodide after 10 days to assess for live or dead cells by fluorescence microscopy.
Tumor Cell Line Xenografts
Tumor cell lines were harvested in mid-logarithmic growth phase and resuspended in PBS. Homozygous NCr nude mice (Charles River Laboratories) were anesthetized with ketamine hydrochloride at 150 mg/kg and xylazine at 12 mg/kg (Phoenix Pharmaceuticals) IP. A small surgical incision along the midline was made to expose the calvarium. Mice were restrained in a stereotactic frame (Stoeltling), and a small burr-hole (size 34; Roboz) was created at 2 mm lateral and 2 mm posterior to the bregma. Fifty thousand U87-LucNeo cells in 10 µL PBS were injected through a 27-gauge needle over 3 min (3.3 µL/min) at 3 mm below the dura. The incision was closed with wound clips (Becton Dickenson) that were removed 5–7 days after surgery.
In Vivo Treatment
Mice were imaged at least twice after implantation of cells to identify those in which tumor burden increased over time. Two weeks after implantation of U87-LucNeo cells, cohorts of 40 mice per experiment with approximately equivalent tumor bioluminescence were divided into equal control and treatment groups. Mice treated with LBW242 alone were given 50 mg/kg intraperitoneally once daily for 14 days. The standard-of-care arm consisted of temozolomide, 5 mg/kg orally, followed 1.5 h later by 400 cGy irradiation administered daily on 2 consecutive days. For irradiation, anesthetized mice were placed in a custom lead shielding apparatus that limited radiation exposure to the cranium only, and treatment was delivered using a Gammacell 40 irradiator (MDS Nordion).
In Vivo Imaging
Mice were anesthetized, injected with D-luciferin at 50 mg/mL intraperitoneally (Promega), and imaged with the IVIS Imaging System (Caliper Life Sciences) for 10–120 s, bin size 2. To quantify bioluminescence, identical circular regions of interest were drawn to encircle the entire head of each animal, and the integrated flux of photons (photons per second) in each region of interest was determined by using the Living Images software package (Caliper Life Sciences). Data were normalized to the bioluminescence at the initiation of treatment for each animal.
All animal studies were performed under protocols approved by the Dana-Farber Cancer Institute Animal Care and Use Committee.
Statistical Analysis
To assess the statistical significance of the differences observed between the relative change in caspase activity, cellular proliferation (clonogenic and angiogenic assays), and Annexin-V staining between different treatment groups, the Student t test was used and calculated with Microsoft Excel. All tests were 2-tailed, and P values <.05 were considered statistically significant. For analysis of the effect of LBW242 combined with radiation therapy and temozolomide in the in vivo orthograft, the difference in luminescence between the 4 treatment groups was assessed using the 1-way Anova Kruskal Wallis test and the Dunn Multiple Comparison test (Graphpad Prism software, version 4). Survival was assessed by using a bioluminscence score >1.0 × 109 as an end point, with significance calculated with the log-rank (Mantel-Cox) test (Graphpad Prism software, version 4).
Results
IAP Inhibition Sensitises Glioma Cells to Radiation Therapy
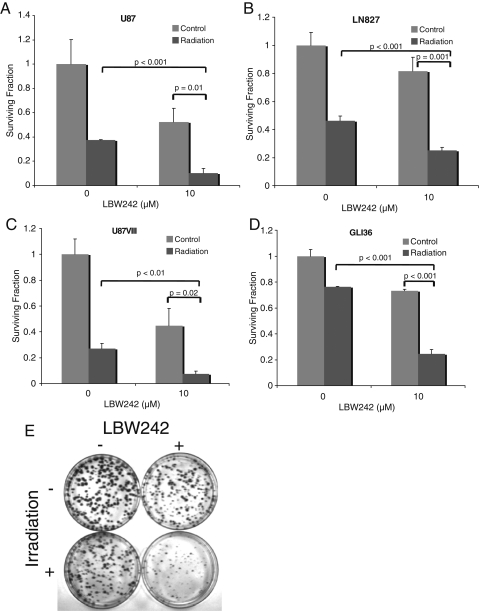
LBW242 is an orally bioavailable small-molecule IAP inhibitor designed to mimic the structure and function of the endogenous peptide Smac.23,24 We and others have previously shown that LBW242 binds tightly to the BIR3 domain of members of the IAP family of proteins, preventing their binding to caspases, and thereby facilitating caspase activation. Our previous work has further shown that LBW242 penetrates the blood-brain barrier, and in glioma cell lines, LBW242 specifically prevents the binding of XIAP to caspase 9, thus facilitating the induction of apoptosis when the intrinsic pathway is activated by targeted therapies.26 Because radiation therapy is known to activate the intrinsic apoptotic pathway and because radiation therapy is a first-line treatment for malignant gliomas, we therefore questioned whether LBW242 could enhance the effect of radiation therapy and act as a radiation sensitiser. To address this, we assessed the effect of pretreating glioma cells with LBW242 prior to their exposure to radiation therapy (Fig. 1A–E). Both LBW242 and radiation as single agents each reduced the number of colonies formed in a number of different glioma cell lines. The combination of both LBW242 and radiation therapy led to a synergistic reduction in colony formation in all adherent cell lines tested (Table 1).
Fig. 1.
Inhibitor of apoptosis protein (IAP) inhibition enhances the efficacy of radiation therapy in multiple glioma cell lines. (A–D) Clonogenic assays in multiple glioma cell lines show enhancement of the effect of radiation therapy by the addition of 10 μM of LBW242. Each experiment was performed in triplicate; data points represent the mean, and error bars represent standard deviations. P values were calculated using the Student t test (2-tailed). (E) A representative image showing results of a typical clonogenic assay in Gli36 cells.
Table 1.
Combinatorial indices for cells treated with radiation plus LBW242
| Cell line | Expected proportion surviving | Observed proportion surviving | Combinatorial indexa |
|---|---|---|---|
| U87 | 0.05 | 0.02 | 0.46 |
| LN827 | 0.37 | 0.25 | 0.67 |
| U87VIII | 0.12 | 0.07 | 0.60 |
| Gli36 | 0.56 | 0.24 | 0.44 |
| BT74 | 0.51 | 0.40 | 0.79 |
| BT79 | 0.07 | 0.08 | 1.14 |
Because apoptosis is thought to depend on the relative balance of pro- versus anti-apoptotic cellular signals, it is possible that the presence of an anti-apoptotic signal, such as EGFRvIII, which is known to be common in malignant gliomas and to induce radiation resistance, could potentially protect cells from this synergistic effect. Therefore, 2 cell lines that overexpress EGFRVIII were assessed: a U87 cell line that had been transformed to overexpress EGFRVIII (U87VIII), and the Gli36 cell line that is known to overexpress EGFRVIII. In both cases, the same synergy between LBW242 and radiation therapy was observed (Fig. 1 and Table 1).
IAP Inhibition Stimulates Apoptosis and Inhibits Angiogenesis
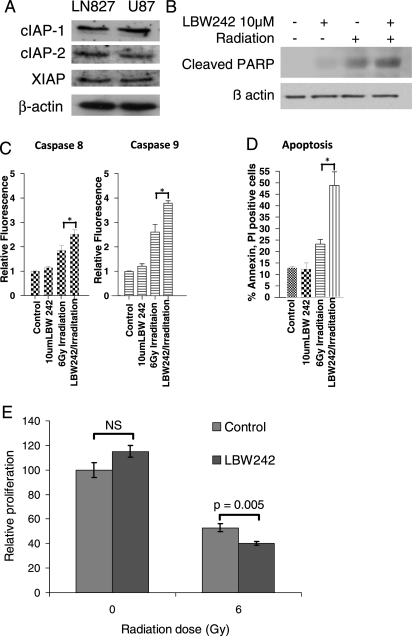
Because the BIR3 domain is common to multiple members of the family of IAP proteins and because LBW242 binds to the BIR3 domain, LBW242 is able to simultaneously inhibit the effects of multiple anti-apoptotic proteins. We assessed the expression of XIAP, cIAP1, and cIAP2 and confirmed that all 3 were expressed in the cell lines used in our experiments (Fig. 2A).
Fig. 2.
Mechanism of action of LBW242 in malignant gliomas. (A) Western blot for 3 members of the IAP family of proteins in malignant glioma cell lines. (B) Western blot for cleaved Poly ADP-ribose polymerase (PARP) protein in U87 glioma cells treated with or without irradiation (400 cGy) ± 10 μM of LBW242. (C) U87VIII cells were treated with LBW242 and/or irradiation for 72 h. Caspase 8 and 9 activity is expressed relative to controls as mean ± standard error of the mean of triplicates (*P < .05, by 2-tailed Student t test). (D) Apoptosis was measured as the proportion of cells staining positive for annexin V and propidium iodide 96 h after treatment with or without LBW242 and 6 Gy irradiation (* P < .05, by 2-tailed Student t test). (E) THMBVEC, human brain endothelial cells were treated with radiation (600 cGy) ± 10 μM of LBW242. Data are represented as mean ± standard deviation of triplicate samples (P value calculated by 2-tailed Student t test).
A direct result of caspase activation is the cleavage of the protein Poly ADP-ribose polymerase (PARP), which is used as a marker of apoptosis. To determine whether the combination of LBW242 and radiation resulted in activation of apoptosis, we measured PARP cleavage and annexin V staining in treated glioma cells. Although cells treated with LBW242 alone showed minimal PARP cleavage, the cells exposed to LBW242 prior to radiation therapy had increased abundance of the cleaved PARP protein, reflecting an enhancement in the rate of apoptosis (Fig. 2B). Similarly, administration of LBW242 alone did not result in any change in annexin V staining (Fig. 2D). There was a minor increase in annexin V staining after administration of radiation therapy; however, this was dramatically and significantly enhanced by the co-administration of both LBW242 and radiation therapy (Fig. 2D). To determine whether the increase in apoptosis was due to activation of the intrinsic or extrinsic apoptotic pathways, we next measured the effect of the therapy on caspase 8 and 9 activity. As seen in Fig. 2C, the combination of LBW242 and radiation therapy led to significantly enhanced activation of both the extrinsic (caspase 8) and intrinsic (caspase 9) pathways. Taken together, these results suggest that the addition of LBW242 to glioma cells directly inhibits the effect of IAPs and acts as a radiation sensitizer by directly enhancing the pro-apoptotic effect of radiation therapy.
Although these results would suggest that LBW242 may be an effective radio sensitizer, we considered recent evidence that radiotherapy may have a greater anti-tumor effect by inhibiting angiogenesis via the induction of apoptosis in endothelial cells rather than tumor cells.30 We therefore asked whether LBW242 could also enhance the effect of radiation in immortalized human brain endothelial cells. Although LBW242 alone had no single-agent effect on endothelial cell proliferation, it did lead to a modest—but statistically significant—enhancement of the effect of radiotherapy in endothelial cells (Fig. 2E). Thus, the ability of LBW242 to sensitize both glioma cells and endothelial cells to the effects of radiation suggest a potential therapeutic benefit.
IAP Inhibition Sensitizes Primary Glioma Cells to Radiotherapy
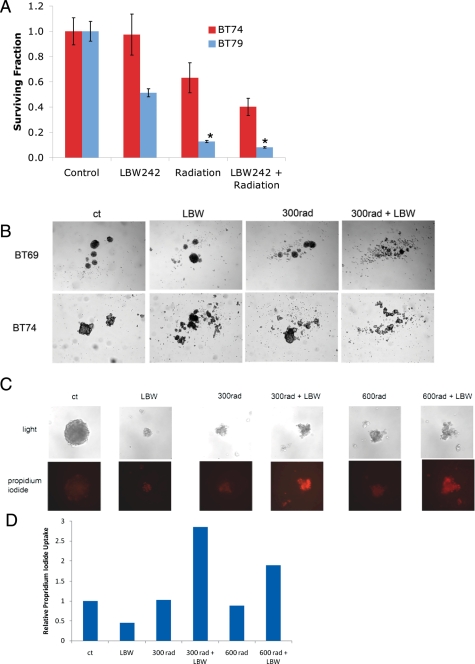
It has recently been suggested that the response of tumor-initiating cell–enriched glioma neurospheres to biologic agents may be a more specific measure of potential therapeutic efficacy than adherent glioma cell lines. Furthermore, CD133-positive glioma-intiating cells have been shown to be more resistant to radiation therapy and less likely to undergo apoptosis than CD133-negative cells.31 It has been postulated that this radiation resistance may be an important mechanism leading to disease progression in the majority of patients.31 We therefore assessed the effect of the combination therapy in glioma neurospheres derived from primary patient tumor specimens. When total neurosphere numbers were measured, LBW242 had variable levels of activity as a single agent (Fig. 3). However, even in tumor specimens in which LBW242 had no stand-alone activity, it still led to a synergistic reduction in the number of surviving neurospheres when combined with radiotherapy (Fig. 3A and Table 1). Similarly, in specimens in which single agent activity was seen, the combination therapy led to a statistically significant further reduction in neurosphere numbers (Fig. 3A), with a combinatorial index suggesting an additive effect (Table 1). Examination of the morphology of glioma neurospheres by light microscopy showed that cells treated with the combination of LBW242 and radiotherapy consistently had the appearance of dead aggregates (Fig. 3B). To assess whether this combinatorial effect observed in neurospheres was secondary to enhanced induction of apoptosis, we assessed propidium iodide uptake into treated neurospheres (Fig. 3C). No significant uptake of propidium iodide was seen in either control groups or in those treated with LBW242 or radiation therapy alone. However, the results for the combination treatment groups were markedly positive (Fig. 3D), indicating the induction of cell death in these cells. Thus, the addition of an IAP inhibitor is able to enhance the pro-apoptotic effect and anti-tumor efficacy of radiotherapy in highly resistant glioma neurospheres.
Fig. 3.
LBW242 enhances the effects of radiation and induces apoptosis in glioma neurospheres. Glioma neurospheres (tumorspheres) derived from different patients were treated with radiation ± 10 μM of LBW242. (A) Total neurosphere numbers were counted 10 days after plating, with data expressed as relative mean ± standard error of the mean of triplicates. *P = .007, by 2-tailed t test. (B) Representative photographs of glioma neurospheres after treatment with 10 μM of LBW242 and/or radiation. (C) Tumorspheres 72 h after treatment with radiation ± 10 μM of LBW242 were stained with propidium iodide and photographed, and (D) uptake measured by counting the relative number of red pixels in each photograph (ImageJ software; National Institutes of Health).
IAP Inhibition Combines with Radiation and Temozolomide in Glioma Xenografts
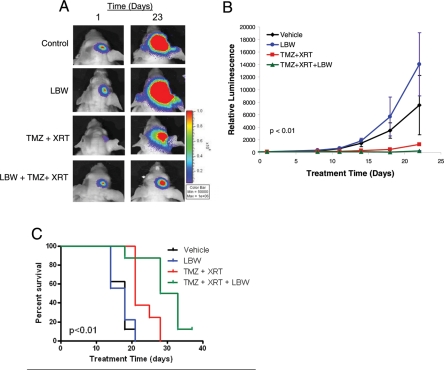
We next sought to extend these positive findings to an in vivo system using an orthotopic glioma model. To mimic the clinical setting, we sought to test the efficacy of LBW242 when added to standard-of-care treatment with radiation plus temozolomide. U87 cells were stereotactically implanted into the brains of nude mice. Tumor burden was serially assessed by bioluminescence imaging, which has previously been shown to be highly correlated with tumor volume.32,33 Animals with established tumors, which are characterized by logarithmically increasing tumor burden, were divided into treatment cohorts. One group was treated with 2 fractions of stereotactic radiation (400 cGy each) plus temozolomide (5 mg/kg) by oral gavage 1.5 h prior to radiation, 1 group was treated with LBW242 at 50 mg/kg twice per day by intraperitoneal injection for 14 days, 1 group was treated with vehicle, and the fourth group was treated with the combination of LBW242, temozolomide and radiation. Consistent with the in vitro studies, while monotherapy with either LBW242 alone or radiation plus temozolomide had some effect, the animals treated with radiation, temozolomide and LBW242 had complete cessation of tumor growth (Fig. 4A and B).
Fig. 4.
IAP inhibition enhances the effect of radiation and temozolomide in glioma xenografts. (A) Bioluminescent images from control and treated animals at the start and end of a treatment course of temozolomide, radiation, and/or LBW242, as indicated. (B) Growth curves were derived from serial measurements with 6 animals per treatment group. Data are mean values ± standard error of the mean. P value compares tumor size at final measurement (1-way Anova Kruskal Wallis test; Graphpad Prism software) (C) Survival curves from a repeated experiment showed a similar combinatorial effect with the addition of LBW242 to standard of care therapy (P < .01, by log-rank [Mantel-Cox] test; Graphpad Prism software).
To confirm these findings and to assess reproducibility, an additional in vivo experiment was performed using the same orthograft model and treatment groups described above. In this experiment, persistent tumor growth to a bioluminescence intensity >1.0 × 109 was used as a surrogate end point for animal survival. As shown in Fig. 4C, LBW242 again demonstrated a highly significant impact on final outcome when combined with radiation and temozolomide. These results establish that IAP inhibition enhances the anti-tumor efficacy of standard-of-care therapies in vivo in an orthotopic glioma model.
Discussion
Novel therapies for malignant gliomas are urgently needed. Surgery, radiation, and temozolomide are now accepted as standard-of-care therapies, however despite their well-documented activity in this disease, they do not result in long-term cures.2 Furthermore, despite the multitude of potential therapeutic targets that have been identified in malignant gliomas and validated in preclinical studies, previous attempts to inhibit those targets have met with limited clinical success.
The results shown here demonstrate a method of applying a targeted therapy to improve treatment responses. We have shown that the addition of a small-molecule IAP inhibitor sensitizes both glioma cell lines and primary patient neurospheres to the pro-apoptotic effects of radiation in vitro. In vivo studies confirm that this synergy results in a significant anti-glioma effect above and beyond that seen with the standard-of-care therapy of radiation plus temozolomide. These results are further supported by the in vitro studies performed by Fulda et al.,34 which showed radio sensitization of glioma cell lines to XIAP inhibition.
Two potential reasons for the previous failure of targeted therapies include the inter- and intra tumoral heterogeneity that is characteristic of malignant gliomas and, perhaps more importantly, the large number of different oncogenic signalling mechanisms that are dysregulated in these tumors. These 2 factors mean that no targeted therapy is likely to demonstrate single agent efficacy in clinical trials and that ultimately, improvements in outcome will only be seen with combination therapies. Currently, temozolomide and radiation are the most effective therapies available for patients with malignant gliomas.2 Use of a treatment combination that builds on and further improves the best available therapy maximizes the probability of achieving clinical success. An additional strength of this strategy is that the IAP inhibitors used are able to simultaneously inhibit multiple members of the same family of proteins, thus overcoming the potential for a tumor escape mechanism.
It is important to note that malignant gliomas contain multiple abnormalities of the apoptotic pathway upstream of the target inhibited in these studies. Despite the anti-apoptotic signals that result from these molecular aberrancies, the addition of an IAP inhibitor appeared to be sufficient to initiate apoptosis and suppress tumor growth when combined with cytotoxic therapy. The induction of apoptosis is thought to relate to the relative balance of pro- versus anti-apoptotic cellular signals, even when these signals occur at different points in the apoptotic pathway. The combination of radiation plus IAP inhibition led to sufficient pro-apoptotic signaling, such that the addition of other anti-apoptotic signals (such as via the EGFRVIII mutation) did not prevent the synergistic effect.
Considerable attention has been paid to the recent discovery that smac mimetics can lead to the degradation of cIAP1 in certain cell lines and thus sensitize some malignant cells to the effects of TNF-α via the extrinsic pathway.35 It remains unclear how the interaction between smac mimetics and TNF-α will impact the clinical use of these compounds. Thus, it is important to note that despite the important discovery of a novel mechanism of action for smac mimetics, the effect on caspase binding is still significant and potentially more important in terms of the development of rational treatment combinations.
Finally, a critically important feature of this therapeutic strategy is that it can be feasibly translated to the clinic. The IAPs have previously been identified as therapeutic targets in malignant gliomas, but the methods employed to inhibit their activity in other preclinical studies were not tractable for clinical translation. For example, direct intratumoral injection of full-length peptides was shown to have an anti-glioma effect,19,36,37 but this is not a strategy that can be readily employed in clinical trials. The IAP inhibitors used in this study are orally bioavailable and have a favorable pharmacokinetic profile, and we previously confirmed their ability to penetrate the blood-brain barrier.26 Several additional smac mimetics are entering clinical testing. The synergy demonstrated here with standard-of-care therapies provides a tractable path for clinical translation and suggests that IAP inhibitors could rationally be tested in patients with newly diagnosed high-grade glioma.
Acknowledgments
Funding provided by the DFCI-Novartis Drug Discovery Program (A.L.K.), the Royal Australasian College of Physicians Rowden White Fellowship (D.S.Z.), the Hagerty Fund Research Award (D.S.Z., A.L.K.), the Leonard Florence and Joanna Bruner Brain Tumor Research Funds (S.K.), the Australian-American Fulbright Commission (D.S.Z.), and the Balnaves Foundation (D.S.Z., J.K.). We thank Renee Wright for her assistance with the in vivo experiments.
References
- 1.Wen PY, Kesari S. Malignant Gliomas in Adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. doi:10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler DS, Cohn RJ, McCowage G, et al. Efficacy of vincristine and etoposide with escalating cyclophosphamide in poor-prognosis pediatric brain tumors. Neuro-oncology. 2006;8(1):53–59. doi: 10.1215/S1522851705000463. doi:10.1215/S1522851705000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler DS, Kung AL, Kieran MW. Anti-apoptosis mechanisms in malignant gliomas. J Clin Oncol. 2008;26(3):493–500. doi: 10.1200/JCO.2007.13.9717. doi:10.1200/JCO.2007.13.9717. [DOI] [PubMed] [Google Scholar]
- 5.Bogler O, Mikkelsen T. Angiogenesis and apoptosis in glioma: two arenas for promising new therapies. J Cell Biochem. 2005;96(1):16–24. doi: 10.1002/jcb.20475. doi:10.1002/jcb.20475. [DOI] [PubMed] [Google Scholar]
- 6.Broniscer A, Gajjar A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: two challenges for the pediatric oncologist. Oncologist. 2004;9(2):197–206. doi: 10.1634/theoncologist.9-2-197. doi:10.1634/theoncologist.9-2-197. [DOI] [PubMed] [Google Scholar]
- 7.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. doi: 10.1056/NEJMoa051918. doi:10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 8.Gammeltoft S, Ballotti R, Kowalski A, Westermark B, Van Obberghen E. Expression of two types of receptor for insulin-like growth factors in human malignant glioma. Cancer Res. 1988;48(5):1233–1237. [PubMed] [Google Scholar]
- 9.Kiaris H, Schally AV, Varga JL. Antagonists of growth hormone-releasing hormone inhibit the growth of U-87MG human glioblastoma in nude mice. Neoplasia. 2000;2(3):242–250. doi: 10.1038/sj.neo.7900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleming TP, Saxena A, Clark WC, et al. Amplification and/or overexpression of platelet-derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res. 1992;52(16):4550–4553. [PubMed] [Google Scholar]
- 11.Hermanson M, Funa K, Hartman M, et al. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52(11):3213–3219. [PubMed] [Google Scholar]
- 12.Guha A, Dashner K, Black PM, Wagner JA, Stiles CD. Expression of PDGF and PDGF receptors in human astrocytoma operation specimens supports the existence of an autocrine loop. Int J Cancer. 1995;60(2):168–173. doi: 10.1002/ijc.2910600206. doi:10.1002/ijc.2910600206. [DOI] [PubMed] [Google Scholar]
- 13.Kilic T, Alberta JA, Zdunek PR, et al. Intracranial inhibition of platelet-derived growth factor-mediated glioblastoma cell growth by an orally active kinase inhibitor of the 2-phenylaminopyrimidine class. Cancer Res. 2000;60(18):5143–5150. [PubMed] [Google Scholar]
- 14.Steinbach JP, Weller M. Apoptosis in gliomas: molecular mechanisms and therapeutic implications. J Neurooncol. 2004;70(2):247–256. doi: 10.1007/s11060-004-2753-4. [DOI] [PubMed] [Google Scholar]
- 15.Stegh AH, Kim H, Bachoo RM, et al. Bcl2L12 inhibits post-mitochondrial apoptosis signaling in glioblastoma. Genes & development. 2007;21(1):98–111. doi: 10.1101/gad.1480007. doi:10.1101/gad.1480007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagenknecht B, Glaser T, Naumann U, et al. Expression and biological activity of X-linked inhibitor of apoptosis (XIAP) in human malignant glioma. Cell Death Differ. 1999;6(4):370–376. doi: 10.1038/sj.cdd.4400503. doi:10.1038/sj.cdd.4400503. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Naito M, Hori S, Mashima T, Yamori T, Tsuruo T. A human IAP-family gene, apollon, expressed in human brain cancer cells. Biochem Biophys Res Commun. 1999;264(3):847–854. doi: 10.1006/bbrc.1999.1585. doi:10.1006/bbrc.1999.1585. [DOI] [PubMed] [Google Scholar]
- 18.Chakravarti A, Noll E, Black PM, et al. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20(4):1063–1068. doi: 10.1200/JCO.2002.20.4.1063. doi:10.1200/JCO.20.4.1063. [DOI] [PubMed] [Google Scholar]
- 19.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8(8):808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFα-mediated cell death. Science. 2004;305(5689):1471–1474. doi: 10.1126/science.1098231. doi:10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 21.Roa WH, Chen H, Fulton D, et al. X-linked inhibitor regulating TRAIL-induced apoptosis in chemoresistant human primary glioblastoma cells. Clin Invest Med. 2003;26(5):231–242. [PubMed] [Google Scholar]
- 22.Ziegler DS, Kung AL. Therapeutic targeting of apoptosis pathways in cancer. Curr Opin Oncol. 2008;20(1):97–103. doi: 10.1097/CCO.0b013e3282f310f6. doi:10.1097/CCO.0b013e3282f310f6. [DOI] [PubMed] [Google Scholar]
- 23.Chauhan D, Neri P, Velankar M, et al. Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM) Blood. 2007;109(3):1220–1227. doi: 10.1182/blood-2006-04-015149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaither A, Porter D, Yao Y, et al. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 2007;67(24):11493–11498. doi: 10.1158/0008-5472.CAN-07-5173. doi:10.1158/0008-5472.CAN-07-5173. [DOI] [PubMed] [Google Scholar]
- 25.Weisberg E, Kung AL, Wright RD, et al. Potentiation of antileukemic therapies by Smac mimetic, LBW242: effects on mutant FLT3-expressing cells. Molecular Cancer Therapeutics. 2007;6(7):1951–1961. doi: 10.1158/1535-7163.MCT-06-0810. doi:10.1158/1535-7163.MCT-06-0810. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler DS, Wright RD, Kesari S, et al. Resistance of human glioblastoma multiforme cells to growth factor inhibitors is overcome by blockade of inhibitor of apoptosis proteins. Journal of Clinical Investigation. 2008;118(9):3109–3122. doi: 10.1172/JCI34120. doi:10.1172/JCI34120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in Enzyme Regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. doi:10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 28.Ligon KL, Huillard E, Mehta S, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53(4):503–517. doi: 10.1016/j.neuron.2007.01.009. doi:10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. doi:10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300(5622):1155–1159. doi: 10.1126/science.1082504. doi:10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 31.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. doi:10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 32.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100(23):13513–13518. doi: 10.1073/pnas.2235846100. doi:10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szentirmai O, Baker CH, Lin N, et al. Noninvasive bioluminescence imaging of luciferase expressing intracranial U87 xenografts: correlation with magnetic resonance imaging determined tumor volume and longitudinal use in assessing tumor growth and antiangiogenic treatment effect. Neurosurgery. 2006;58(2):365–372. doi: 10.1227/01.NEU.0000195114.24819.4F. discussion -72 doi:10.1227/01.NEU.0000195114.24819.4F. [DOI] [PubMed] [Google Scholar]
- 34.Vellanki SH, Grabrucker A, Liebau S, et al. Small-molecule XIAP inhibitors enhance gamma-irradiation-induced apoptosis in glioblastoma. Neoplasia. 2009;11(8):743–752. doi: 10.1593/neo.09436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vince JE, Wong WW, Khan N, et al. IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis. Cell. 2007;131(4):682–693. doi: 10.1016/j.cell.2007.10.037. doi:10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 36.Giagkousiklidis S, Vogler M, Westhoff MA, Kasperczyk H, Debatin KM, Fulda S. Sensitization for gamma-irradiation-induced apoptosis by second mitochondria-derived activator of caspase. Cancer Res. 2005;65(22):10502–10513. doi: 10.1158/0008-5472.CAN-05-0866. doi:10.1158/0008-5472.CAN-05-0866. [DOI] [PubMed] [Google Scholar]
- 37.Mizukawa K, Kawamura A, Sasayama T, et al. Synthetic Smac peptide enhances the effect of etoposide-induced apoptosis in human glioblastoma cell lines. J Neurooncol. 2006;77(3):247–255. doi: 10.1007/s11060-005-9045-5. doi:10.1007/s11060-005-9045-5. [DOI] [PubMed] [Google Scholar]