Not(ch) just development: Notch signalling in the adult brain (original) (raw)
. Author manuscript; available in PMC: 2011 Nov 1.
Published in final edited form as: Nat Rev Neurosci. 2011 May;12(5):269–283. doi: 10.1038/nrn3024
Abstract
The Notch pathway is often regarded as a developmental pathway, but components of Notch signalling are expressed and active in the adult brain. With the advent of more sophisticated genetic manipulations, evidence has emerged that suggests both conserved and novel roles for Notch signalling in the adult brain. Not surprisingly, Notch is a key regulator of adult neural stem cells, but it is increasingly clear that Notch signalling also has roles in the regulation of migration, morphology, synaptic plasticity and survival of immature and mature neurons. Understanding the many functions of Notch signalling in the adult brain, and its dysfunction in neurodegenerative disease and malignancy, is crucial to the development of new therapeutics that are centred around this pathway.
Normal brain development requires exquisite timing of differentiation programs, and Notch signalling is well known as a master regulator of neural stem cells (NSCs) and neural development1,2. But Notch expression persists throughout the adult brain and in mature, differentiated cells in the CNS3,4. The importance of Notch signalling for normal human adult brain function is demonstrated by its implication in diseases as diverse as Allagile5,6, CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy)7 and Hajdu–Cheney8,9 syndromes, which have functional mutations in key Notch pathway elements, and Down’s syndrome10 and Alzheimer’s disease3,11, which have abnormal Notch expression levels. Furthermore, although they have distinct pathogeneses, each of these diseases has a cognitive impairment and/or a neurodegenerative component, which emphasizes the need to understand the functions of Notch in the adult brain.
Research into Notch function in fully differentiated cells and in the adult brain was initially hampered because of the embryonic lethality of Notch knockout mice2. With the development of Cre/loxP and viral gene transduction technologies (BOX 1), Notch expression can now be manipulated in mature animals, thus circumventing its developmental requirement. Not surprisingly, the topic has received much attention in the past few years, and the field has seen a surge in publications on Notch signalling in the adult brain.
Box 1. Methods to manipulate Notch in the adult rodent brain.
Mice with mutations in genes that encode elements of the Notch pathway frequently die before birth2. Thus, it has been essential to use alternative methods to analyse this signalling pathway. Owing to the exquisite dosage-dependence of Notch signalling, heterozygote mutant mice52 probably have developmental abnormalities, as may transgenic Notch antisense mice58. Perhaps the most frequently used method of manipulating Notch signalling is by inhibiting γ-secretase. Pharmacological inhibitors block the S3 cleavage of Notch by the γ-secretase complex, are easy to use and have robust effects on Notch signalling87. However, Notch blockade by γ-secretase inhibitors rapidly becomes toxic to the animals, owing to effects on intestinal precursor cells148. As an alternative — and more specific — means to target Notch, antibodies to the receptor and its ligands have been used, but delivery to the brain parenchyma of such antibodies is challenging owing to the difficulty in administration and limited diffusion. In vitro, and more rarely in vivo, soluble ligands and ligand domain peptides have been used as both blockers and stimulators of Notch signalling81,149. As can be expected from such discrepant effects (both inhibitory and stimulating), the mechanism of action of soluble ligands is somewhat controversial149. There has been speculation that high concentrations of soluble ligands can result in multimers that can activate Notch, whereas inhibitory monomers might predominate at lower concentrations150. Immobilized or clustered ligands are more commonly used but require proper titration149.
Conditional and inducible mutant mice exist for many of the key elements of the Notch pathway, including conditional Notch knockout (loss-of-function) and transgenic Notch intracellular domain (NICD) (gain-of-function) lines2. These lines make use of the Cre/loxP system, in which the gene of interest (or a portion of the gene) is flanked by loxP sites (‘floxed’). The floxed sequence can be removed using Cre recombinase, which recognizes the loxP sites. Tissue- and cell-specificity is achieved by driving Cre expression with an appropriate promoter, whereas inducibility is achieved by fusing Cre to a domain that sequesters it in the cytoplasm until administration of an agent, most commonly a tamoxifen-responsive mutated oestrogen receptor, promotes nuclear translocation. For neuron-specific manipulation, many Cre driver lines exist with which to conditionally manipulate the Notch pathway, though few studies have been performed using such mice51. The study of adult neural stem cells requires the use of inducible Cre lines, as knocking out Notch will always cause developmental abnormalities in neural stem cells when conditional drivers such as nestin–Cre and glial fibrillary acidic protein (GFAP)–Cre are employed2. However, inducible lines require extensive breeding, and given the need to induce nuclear translocation of inducible Cre, recombination rates are usually lower than traditional Cre lines. Frequently, recombination reporter genes, such as ROSA yellow fluorescent protein (YFP) or ROSA–lacZ, are used as a surrogate for recombination of the desired locus, but the recombination of reporter lines and floxed target genes are not linked processes, and demonstrating efficient recombination of both can be challenging151. Furthermore, many of the Notch receptors may have redundant roles152, necessitating recombination of more than one Notch receptor, or alternatively, common downstream targets. Conditional transgenic dominant-negative mastermind-like (Maml) mice, and conditional alleles of recombining binding protein suppressor of hairless (Rbpj) or Maml — all being part of the complex that forms with NICD to activate transcription — offer alternatives to block the function of all Notch receptors but may also have additional, Notch-independent effects153–155.
Notch signalling is used reiteratively in stages beyond stem and progenitor cells, including in the migratory and postmitotic stages of the neuronal lifecycle. That is to say, the core elements of the Notch pathway as used by precursors are also used to initiate several processes in neurons. Here, we review the evidence that Notch acts as master regulator of plasticity in the adult brain — from stem cells to mature neurons to degenerating neurons. We explore how Notch can affect each stage of the neuronal life cycle to generate an adaptive response, and we discuss the possible mechanisms and accessory pathways that are involved in these processes. Understanding how a master regulator like Notch functions in such diverse roles furthers our ability to develop feasible strategies to manipulate Notch in a clinically beneficial manner.
A brief history of Notch
The Notch signalling pathway is among the most well-conserved signalling pathways in animals. It arose with the evolution of multicellular organisms and the concomitant need for juxtacrine cell-to-cell communication to coordinate development12. As it is beyond the scope of this Review to explore the literature on Notch signalling in embryonic neurogenesis in detail1,2,12, we briefly highlight here our current understanding of the core elements of Notch signalling (FIG. 1).
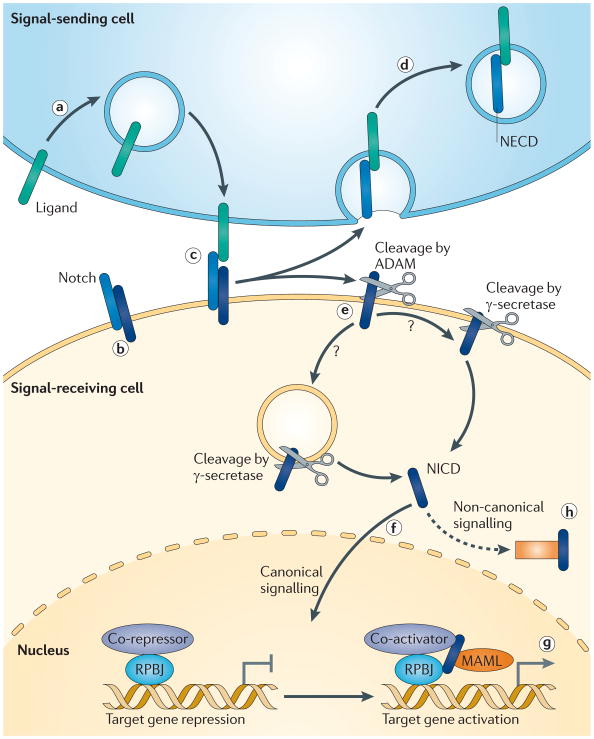
Figure 1. Notch signal transduction.
A model of canonical Notch signalling. Notch signalling is unidirectional, with a ‘signal-sending cell’ that presents the Notch ligand (the ‘signal‘) to the ‘signal-receiving cell’, which expresses the Notch receptor. a | Notch ligands, such as Delta-like protein 1 (DLL1; shown in green), are presented on the neuronal membrane and subsequently endocytosed. These ligands can be ‘activated’ by an as-yet-unknown mechanism and re-presented to the membrane. b | Notch is synthesized as a single peptide and then cleaved in the golgi compartment (not shown) to form a heterodimer (shown in dark and light blue) that is presented on the cell membrane. c | Upon being re-established on the membrane, the ligand can bind Notch. d | According to one model, the Notch heterodimer is pulled apart through the force of endocytosis in the signal-sending cell, thereby transendocytosing the Notch extracellular domain (NECD). e | The Notch domain that remains on the signal-receiving cell is cleaved by disintegrin and metalloproteinase domain-containing protein (ADAM) and subsequently by γ-secretase. The precise location of the γ-secretase cleavage is controversial, with some data indicating that it occurs in the endosome and other data indicating that it can happen both on the membrane and in the endosome, leading to different Notch intracellular domain (NICD) molecules. f | In either case, after cleavage NICD translocates to the nucleus. g | In the nucleus it dislodges the repressor complex from recombining binding protein suppressor of hairless (RBPJ), forming a complex that is stabilized by mastermind-like protein (MAMLs). NICD also recruits co-activators to initiate transcription of Notch target genes. h | More recently, it has been determined that Notch signalling can occur in the absence of transcriptional activation, through protein–protein interactions, or that it can activate non-RBPJ-dependent transcription (not shown), collectively referred to as ‘non-canonical’ signalling.
Notch functions as a receptor, and mammals have four Notch receptors (Notch1, Notch2, Notch3 and Notch4) and many ligands (TABLE 1), including jagged 1 (JAG1) and JAG2 (homologues of serrate), and delta-like proteins (reviewed in REFS 13,14). Notch and its ligands are single-pass transmembrane heterodimers (FIG. 1a,b). The extracellular domains of both Notch and its ligands consist of multiple epidermal growth factor (EGF) repeats, which can be modified by the addition of sugar moieties. Notch receptors also contain several domains that maintain the receptor in an inactivated state in the absence of a ligand13,15. Upon ligand binding, a series of cleavage events culminates in γ-secretase-mediated cleavage of the transmembrane domain of Notch, and release of the Notch intracellular domain (NICD) into the cytosol. The extracellular domain of Notch (NECD) is endocytosed, along with the ligand, into the ‘signal-sending cell’ — that is, the ligand-presenting cell (FIG. 1c–e; reviewed in REF. 13). Ligands are also subject to γ-secretase-mediated cleavage, but the function of ligand cleavage is not well understood16.
Table 1.
Notch receptor and selected ligand expression
| Notch pathway protein | Expression pattern in forebrain | Refs |
|---|---|---|
| Notch1 | Neuron, astrocyte, precursor, ependymal cell, endothelium | 4,24,26,144 |
| Notch2 | Precursor? Neuron? | 4,168 |
| Notch3 | Precursor? | 168 |
| Notch4 | Endothelium | 169 |
| DLL1 | Intermediate neural progenitor, postmitotic neuron? | 26,170,171 |
| DLL3 | Intermediate neural progenitor? | 170 |
| DLL4 | Endothelium | 172 |
| JAG1 | Precursor, intermediate neural progenitor, neuron | 24,26,62,170,173 |
| JAG2 | Neuron | 26,169 |
| DNER | Neuron | 174 |
In the canonical Notch signalling pathway, NICD translocates to the nucleus and, with mastermind-like protein 1 (MAML1), MAML2 or MAML3, converts the recombining binding protein suppressor of hairless (RBPJ) complex from a transcriptional inhibitor to an activator (FIG. 1f,g). The most studied Notch targets are the hairy and enhancer of split-related (HESR) genes, with many additional genes recently having been identified as Notch targets17. NICD can also signal through a non-canonical pathway, presumably through protein–protein interactions and RBPJ-independent gene activation18 (FIG. 1h).
Ligand binding permits disintegrin and metalloproteinase domain-containing protein (ADAM)-mediated S2 cleavage of the extracellular juxtamembrane region13. The γ-secretase cleavage of Notch is a proteolytic event that then occurs rapidly after ligand-mediated removal of the extracellular domain of Notch13. Cleavage by γ-secretase is a common target of pharmacological inhibition by molecules such as DAPT and DBZ (BOX 1). Such γ-secretase inhibitors are powerful blockers of Notch activity, with the important caveat that the γ-secretase complex cleaves at least 60 different proteins (and counting)19. Indeed, as shown in a recent study, γ-secretase-mediated effects frequently ascribed to Notch (for example, neural stem cell renewal) can be, and often may be, in fact Notch-independent20. Specifically, this study showed that Notch activation was sufficient to cause astrogliogenesis from neural stem cells but that the decreased stem cell self-renewal following γ-secretase inhibition was independent of Notch signalling, as NICD could not rescue these deficits20. Thus, although γ-secretase inhibitors may be a useful tool to screen for Notch pathway involvement, genetic approaches are necessary for more definitive conclusions regarding such involvement. Indeed, the lack of substrate specificity of γ-secretase inhibitors and the concomitant challenges in interpreting clinical data have hampered progress in the field of Alzheimer’s disease therapeutics21.
Notch in the adult brain
The neurogenic niches of the adult brain — the subventricular zone (SVZ) and the subgranular zone (SGZ) — represent a preservation of the embryonic germinal zones, and continue to generate cells throughout life to varying extents, depending on the species22. These niches are only a fraction of total adult brain tissue, yet Notch pathway components are expressed throughout the adult brain23–26. This suggests that Notch might have roles beyond regulating stem cell maintenance and differentiation. Here we review recent evidence for Notch signalling in the adult CNS, and explore its role in regulating both progenitors and mature cell types in the adult brain (FIG. 2).
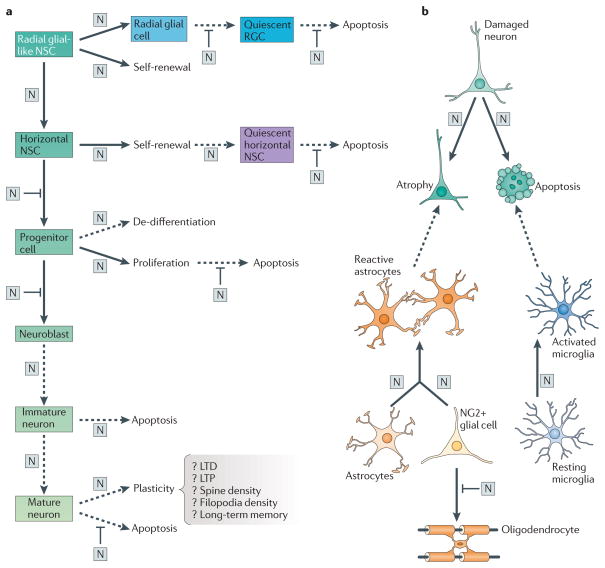
Figure 2. Pleiotropic roles for Notch signalling in the adult brain.
a | The generation of neurons in the adult brain is thought to proceed through several stages, beginning with radial glial-like neural stem cells (top left part) and ending in a mature neuron (bottom part). Early in the development of adult-born neurons, cells proceed through several divisions (shown by solid black arrows). Notch activation (N) occurs during, or immediately after, these divisions. Notch activation can also occur in postmitotic or quiescent cells in the absence of division (shown by dashed arrows). In radial glial-like stem cells (RGCs) Notch promotes a radial glial fate. In both RGCs and horizontal neural stem cells (NSCs), Notch activation promotes self-renewing divisions. Notch has also been implicated in regulating quiescence of NSCs. In progenitors, Notch activation is important for proliferation. Interestingly, in the subventricular zone, pigment epithelium-derived factor (PEDF) can sensitize progenitors such that low-level activation of Notch immediately following division can promote de-differentiation (that is, induce ‘stemness’). In its most well-known role, Notch inhibits neuronal differentiation of NSCs and progenitors. More recently, it has been appreciated that Notch regulates migration of neuroblasts and arborization of postmitotic newborn neurons. Notch has also been implicated in regulating synaptic plasticity of mature neurons (bottom right part) in the adult brain, regulating features of synaptic strengthening such as long-term potentiation (LTP) and long-term depression (LTD), and increases in the density of filopodia and spines. Finally, Notch is crucial for the survival of all stages of neurogenesis in both the developing and adult brain, either promoting or inhibiting apoptosis, depending on the stage. b | Following brain injury or degeneration, the developmental programs of Notch are reactivated to control a diverse set of responses to the insult, including promoting neuronal atrophy and death, increasing microglial activation, and potentially regulating reactive gliosis by initiating proliferation and astrogliogenesis from resident progenitors. However, the response is counter-productive and Notch activation limits the ability of oligodendrocytes to differentiate and remyelinate damaged neurons.
To cycle or not to cycle?
In stem and progenitor cells, proliferation is central to cell fate decision making because differentiation programs are tightly coupled to the process of cell cycle exit27,28. It is not surprising then, that Notch regulates the cell cycle to balance stem cell maintenance with daughter cell production, thereby preventing premature depletion of the niche. In zebrafish, which have a prominent adult telencephalic germinal zone, high levels of Notch activation in radial glia maintain the cells in a quiescent state, whereas a decrease in Notch activity leads to NSC proliferation and an increased net number of adult-born neurons29. Moreover, increased cell cycle exit of early precursor types24 and impaired expansion of the progenitor pool30 has been found in the SGZ of adult inducible Notch knockout mice, with eventual depletion of the stem population31,32. Consistent with the idea that Notch levels regulate the cell cycle, treating NSCs derived from human embryonic stem cells with the Notch inhibitor DAPT decreases levels of cyclin-dependent kinase 4 (CDK4) and S-phase kinase-associated protein 2 (SKP2), and increases levels of the cell cycle inhibitor cyclin-dependent kinase inhibitor 1B (p27KIP1). This prolongs the G1–S transition, and if levels of Notch signalling remain low and growth factors are withdrawn, the cell exits the cycle and differentiates into a neuron33. Interestingly, the relative levels of Notch signalling in neural stem cells (NSCs) can regulate the opposing states of quiescence versus proliferation in NSCs — with low levels leading to proliferation and high levels causing growth arrest34. However, neuronally committed, doublecortin-positive (DCX-positive) progenitors are less dependent on Notch and more responsive to environmental cues for the regulation of proliferation30, suggesting that the capacity of Notch to regulate proliferation in the adult brain is cell type-dependent, as discussed below.
Migration and early differentiation
Although the role of Notch in NSC maintenance is well described, Notch signalling in migrating and maturing cells in the perinatal and adult brain is less well characterized. For example, Notch signalling seems to perturb neuronal migration in the cerebral cortex by altering the morphology of migrating neurons. Increased Notch signalling leads to a bipolar morphology that favours migration, whereas decreased Notch signalling leads to a more multipolar morphology that stalls migration35. The mechanism by which Notch regulates migration involves reelin–DAB1 (disabled homolog 1) signalling, which inhibits NICD degradation35. Notably, the phenotype of reelin-lacking reeler mice — which are characterized by abnormal lamination of cortical neurons due to failed migration — could be rescued by overexpression of an NICD transgene35. Although these results were cell-autonomous to neurons (established by using neuron-specific promoters to drive transgene expression), it is notable that in the postnatal dentate gyrus a similar cooperative mechanism between NICD and reelin is essential for the formation and maintenance of the radial glial scaffold36. In DCX-positive immature neurons of the adult SGZ, NICD accumulates in the nucleus as the morphology changes to that of a migrating neuron24,37,38. More ramified cells show higher, more intense immunostaining for NICD, with mature cells exhibiting the highest levels. Interestingly, this pattern is consistent with the changes seen in the localization of neurogenin 3 (NGN3), as NGN3 becomes increasingly localized to the nucleus during neuronal differentiation39.
Another possible mechanism by which Notch can modulate neuronal migration and early differentiation came from studies in primary cortical neurons. Here, treatment with the Notch ligand JAG1 downregulated spastin, a protein involved in severing microtubules, and this resulted in increased microtubule stability. Immunostaining for acetylated α-tubulin revealed notable increases in microtuble branching and neurite thickness in these cortical neurons. This effect on microtubule stability could be blocked by γ-secretase inhibition40, indicating that it depended on NICD. Taken together, these findings demonstrate a role for Notch in regulating microtubule dynamics. Future studies will be needed to determine how direct this regulation is.
There is also evidence from studies in Drosophila that Notch may act non-canonically on axon guidance during development through tyrosine-protein kinase ABL signalling41–43. Specifically, it seems that Notch interacts with DAB and triple functional domain protein (TRIO)–ABL accessory proteins and directs axon guidance and dynamic patterning through modulation of the growth cone43. This is consistent with the interaction between DAB1 and Notch1 that occurs during neocortical neuron migration in cortical development35. An interaction between Notch and atonal has also been shown to cause dramatic alterations in axonal arborization in Drosophila44. In this study, high Notch activity diminished axonal branching, whereas low Notch activity had the opposite effect44. It remains to be seen whether Notch regulates axon dynamics in vertebrate neuronal development.
Structural plasticity
In developing cortical cells, Notch seems to modulate the complexity of the dendritic arbour. Transgenic NICD expression, ligand presentation and a high cell density — all leading to canonical Notch activation — cause a more complex, but often stunted, dendritic tree4,45,46. Conversely, loss-of-function experiments demonstrated that lack of Notch signalling reduced neurite numbers4,45,46. The modulation of dendritic arbour complexity by Notch seems to be dependent on canonical Notch signalling, as nuclear translocation of NICD is prominent and RBPJ-dependent Notch activity has been reported to occur in this process4,46. Similarly, levels of Notch signalling correlate with the complexity of the dendritic arbour of neurons in the postnatal and adult SGZ24. More recently, it was noted that adult inducible Notch1 knockout mice have granule cell neurons with smaller dendritic trees in the dentate gyrus30. NGN3 has been implicated as a possible player in the regulation of structural plasticity39,47, as have NUMB and numb-like (NUMBL) in neocortical cells4 and in sensory neurons48. In the case of NGN3, which promotes neurite outgrowth, Notch activation leads to expression of Hes genes that inhibit Ngn3 expression and neurite outgrowth47. Thus, a balance of NGN3 and Notch activity regulates dendritic complexity. NUMB and NUMBL, however, directly antagonize Notch signalling through endocytic control49, preventing surface localization and, presumably, activation of Notch.
Strikingly, in contrast to the effect of genetic manipulations of immature neurons, rodents with transgenic manipulations of Notch in mature, postmitotic cortical neurons seem to have normal gross dendritic morphology, suggesting that there may be a critical period for Notch signalling in the regulation of neuronal aborization, possibly coinciding with the critical period for integration of new neurons50. An elegant Cre/loxP system was used to express active Notch1 selectively in cortical pyramidal cells in adult mice51, and this manipulation resulted in reduced spine and filopodia densities without any gross changes in the dendritic arbour. These findings show that although Notch may not be able to reorganize a mature dendritic arbour during early adulthood, it can continue to fine-tune the dendritic spines.
Synaptic plasticity
A few studies have suggested that genetic manipulation of Notch influences plasticity and behaviour. An initial report described specific deficits in spatial learning and memory in adult Notch1 heterozygote mutant mice52. Adult Rbpj heterozygote mutant mice had similar but somewhat more severe disruptions in this form of memory52. However, the exquisite dosage-dependence of Notch makes it difficult to interpret these findings as these mice probably had developmental defects such as premature myelination53 and, potentially, changes in total neuron numbers54. Sidestepping such developmental confounds, a temperature-sensitive allele was used to examine the effect of Notch dysfunction in memory tasks in Drosophila. This study found that short-term memory was spared, whereas long-term memory was impaired55. Another study in Drosophila, using conditional Notch mutants or an induced dominant-negative Notch, found a similar deficit in long-term memory that could be rescued by overexpression of a wild-type Notch transgene56. Neuralized (NEUR), another molecule that modulates the Notch signalling pathway (among others) has also been implicated in long-term memory in Drosophila57. However, whether this role is dependent on Notch remains to be determined.
In the hippocampus of adult mice, reduced Notch levels (by expression of a transgenic antisense construct) led to impaired long-term potentiation (LTP) and enhanced long-term depression (LTD)58. Furthermore, treating hippocampal slices from these mice with an activating jagged peptide facilitated LTP58. However, much like Notch is transiently attenuated in the differentiation process of neurons, there is evidence that Notch signalling is interrupted — and that mRNA levels of the Notch target gene Hes1 are reduced — during the consolidation of long-term memory 10–12 hours after passive avoidance conditioning59. Indeed, administration of a Notch-specific antibody, which stimulates receptor activation, blocked consolidation. Perhaps surprisingly, this study found an NICD fragment of 66 kDa (visualized using two separate antibodies) in the nuclear fraction of lysed dentate gyrus neurons — a fragment well below the usual size59. Further study is needed to confirm these findings and to determine whether this is a breakdown product of NICD, a novel functional fragment or perhaps an imprecise cleavage event, as has been described in vitro60.
The role of Notch in synaptic plasticity is likely to be complex, as has been shown by studies in the Drosophila neuromuscular junction (NMJ). Here, Notch is necessary for appropriate NMJ complexity (measured in terms of axonal branches and presynaptic boutons), which can be blocked by expressing a dominant-negative Notch receptor61. However, the same study found that expression of higher levels of transgenic Notch or of constitutively active Notch blocks plasticity in the NMJ, suggesting that a moderate level of Notch might be ideal61 — and again demonstrating the dose-dependent nature of Notch signalling. Interestingly, expression of Notch in the NMJ correlates with neuronal activity61. The finding in this study61 that Notch can have opposing roles depending on the level of receptor activation could explain discrepant findings in different studies. For example, one study found that Notch activation decreases LTP51, whereas another demonstrated that it enhances LTP58. This paradoxical pattern is also seen in studies of Notch during neurodevelopment and — as discussed below — is probably due to the exquisite context- and dosage-dependence of Notch signalling.
Our understanding of the role of Notch in neuronal morphology and synaptic plasticity is still in its infancy and awaits further investigation. For example, the signal that stimulates Notch cleavage in neurons in vivo was unclear until recently published work greatly extended our understanding of the role of Notch signalling in neuronal plasticity in the mammalian62 and Drosophila63 brain. Gaiano and colleagues used transgenic Notch reporter mice and antibodies to Notch1 to confirm the expression and activity of Notch1 in neurons62, and demonstrated the localization of JAG1 to synapses primarily along the axon. They noted that Notch1 expression and cleavage increased in response to synaptic activity, with Notch1 cleavage being dependent on the neuronal activity-related gene Arc (activity-regulated cytoskeleton-associated protein; also known as Arg3.1). Fascinatingly, this dependence on Arc was only present in neurons and not during embryonic neural stem cell maintenance, when NICD is present in abundance. Notch1 knockout in mature neurons did not alter gross morphology but caused alterations in dendritic spines and disruption of LTP and LTD, leading to marked deficits in performance in several memory tasks62. Struhl and colleagues showed that in Drosophila, Notch was activated specifically in brain regions that are responsive to distinct odours63. Notably, Notch activity peaked at 24 hours after odour exposure and decreased in the following days. The authors speculated that this long decay period may be crucial for pairing of stimuli over extended periods of time63. Although Gaiano and colleagues noted that JAG1 was the likely ligand for Notch at the synapse in the rodent brain62, Struhl and colleagues showed that neuronal Notch cleavage in Drosophila was due to Delta stimulation63. As is evident from these studies, increasingly precise temporal and spatial manipulations of Notch will no doubt be invaluable in the elucidation of the precise function (or functions) of Notch in neuronal plasticity.
Ageing neurons
Some of the initial studies of Notch expression were performed in the context of age-related human disease, most notably Alzheimer’s disease, owing to the fact that -secretase cleavage is common to both Notch signalling and amyloid precursor protein (APP) processing3,64. In situ hybridization studies showed that mRNAs encoding Notch and presenilin — a protein that is part of the γ-secretase complex — are strongly expressed in normal brains in hippocampal and entorhinal neurons, with additional expression in cortical neurons and Purkinje cells3. Notably, Notch cleavage was dramatically increased in brains of patients with Alzheimer’s disease compared with controls3. In further support of the hypothesis that Notch processing may be abnormally high in animal models of Alzheimer’s disease, several studies have shown an age-dependent decrease of γ-secretase activity on Notch, as well as APP, in the normal brain65,66. Additionally, neuron cultures with presenilin mutations that are associated with familial forms of Alzheimer’s disease frequently display altered Notch signalling11,67, and several studies have reported interactions between Notch and APP10,68–72, and APP and NUMB73. More recent studies suggest that, although not causative, aberrant Notch signalling as a result of NUMB dysregulation, and alterations in presenilin and APP, may contribute to the progressive neurodegeneration characteristic of Alzheimer’s disease74. Unfortunately, thus far, γ-secretase inhibitors have not been very successful as potential treatments for Alzheimer’s disease.
An association between Notch and neuronal disease is also seen in prion disorders. NICD protein is elevated in the cortex of mice with prion disease, and this increases with rising levels of PrPSc (REF. 75). Notably, Notch1 mRNA abundance and NICD translocation correlate with dendritic atrophy of neurons75. Using small interfering RNA (siRNA) to knock down Notch1 in neuroblastoma (N2a) cells (an in vitro model for neurons), it was determined that activated Notch1 was primarily responsible for the decrease in neurites75. Moreover, treatment of mice with γ-secretase inhibitors (plus quinacrine to reduce PrPSc levels) was sufficient to prevent dendritic atrophy76. It is interesting to contrast these findings with those of Dahlhaus et al.51 and Alberi et al.62, who found no change in dendritic arborization in mature, postmitotic, cortical neurons following transgenic expression of NICD or conditional Notch1 ablation in much younger, disease-free rodents. This suggests that NICD can modulate dendritic complexity in an age-dependent manner during disease.
Ageing and pathology are often linked to shortened telomeres. A novel, mechanistic link between p53, Notch and telomere shortening in the process of neuritogenesis has recently been demonstrated77. Telomerase-deficient mice displayed reduced neurogenesis and had less complex neurite arbours, and this effect was dependent on p53. γ-secretase inhibition phenocopied p53 knockouts in terms of neuritic complexity, and this effect could be reversed by NICD overexpression. However, p53 did not directly regulate Notch1 or Notch3 levels77, indicating that p53 and Notch signalling act in parallel or cooperatively. Notch and p53 could converge on RHO-associated protein kinase 1 (ROCK1) and ROCK2 (REF. 77) — downstream effectors of RHOA — to regulate actin dynamics and thus, neurite extension. It will be interesting to explore these interactions in vivo and to ascertain whether neurons born from aged stem cells display intrinsic differences in Notch-dependent arborization — a fact that might impact the development of strategies for endogenous repair of the aged brain.
Notch in response to neurotrauma
Several studies in rodents have reported conflicting results regarding Notch signalling in ischaemic brain injury, with some studies reporting beneficial effects of Notch signalling on progenitor proliferation and survival78–81, and others reporting detrimental effects of Notch activation82,83. The caveat of many of these studies is that they examined gross changes in Notch signalling within a brain region. As we have discussed, changes in Notch signalling may differ between cell types. In addition to its roles in neurons and astroglia, Notch has profound roles in other cell types in the brain, including endothelial cells84 and oligodendrocytes85,86. It may therefore be inappropriate to draw conclusions regarding the potential effect of changes in whole-tissue expression levels of Notch protein or mRNA as there are probably pleiotropic roles for Notch in these different cell types. For example, Notch blockade improves functional outcome in a mouse model of stroke through diverse means83. First, Notch2 expression in neurons correlates with levels of post-stroke apoptosis82, and Notch activation in neurons predisposes them to apoptosis83, suggesting that Notch inactivation may protect against apoptosis cell-autonomously in neurons. Second, Notch increases inflammation through increased infiltration of immune cells into the infarct83, and Notch blockade may therefore decrease neuronal cell death in a non-cell-autonomous manner, by dampening the microglial (immune) response. Third, Notch may be involved in reactive gliosis following neurotrauma or disease25,87,88. This is consistent with findings seen following NICD expression in the intact brain, notably proliferation and maintenance of the glial fibrillary acidic protein (GFAP)-positive cell type in daughter cells, which are hallmarks of reactive gliosis24,89.
Notch signalling has also been implicated in the inability to recover from demyelinating injuries and diseases. Following a compression injury of the spinal cord in adult mice, Notch pathway components were upregulated in neurons, NUMB was upregulated in GFAP-positive astrocytes and sonic hedgehog (SHH) was expressed by myelin basic protein (MBP)-positive oligodendrocytes90. Although differentiation programs are activated in response to injury, integration of new neurons and remyelination of existing axons does not occur, perhaps owing to a gliogenic switch in adult NSCs. Notch activation in NSCs can inhibit the generation of neuronal progenitors and promote the generation of a gliogenic progenitor that can give rise to both oligodendrocytes and astrocytes. Further activation of Notch in the gliogenic progenitor inhibits oligodendrocyte differentiation and promotes astrocytic differentiation91. For example, in a mouse model of the demyelinating disease multiple sclerosis, Notch activation prevented oligodendrocyte differentiation and subsequent remyelination, leading to the generation of a glial scar92. However, another study found that Notch was needed to promote remyelination and differentiation of oligodendrocytes93, again highlighting the importance of the level of Notch activation. Future studies will need to use cell type-specific means of Notch manipulation (BOX 1) to sort out the role of Notch signalling during pathologies and subsequent recovery.
It’s all about the context
One of the greatest challenges in studying Notch signalling is the inability to predict the outcome of Notch activation, owing to its multiple roles (FIG. 3). The canonical Notch pathway is strikingly simple; there are no second messengers. How is it, then, that Notch signalling can have such pleiotropic functions? One mechanism is through extensive crosstalk with other pathways. It is generally accepted that Notch signalling integrates multiple pathways, thereby tuning downstream expression to fit the cell’s current needs and situation, that is, the cell’s context (reviewed in references REFS 94,95). This context is composed of several parts, such as the capacity to receive Notch activation, cell type, age of the animal, stimulus type and brain area. In addition, epigenetic factors96 and tissue-specific Notch cofactors97 can modulate the downstream targets receptive to the Notch ternary complex though changes in chromatin conformation, binding specificity or other nuclear modifications. The multiple functions of Notch signalling could be considered complementary rather than contradictory. In the following sections we will explore the factors that may determine which function of Notch signalling is relevant in a particular context. We propose that the varied and complex outcomes of Notch signalling (FIG. 3) are manifestations of a Notch signalling ‘network’ that controls the crucial choices that cells must make at particular stages in life12,94,98. When cells lose the ability to recognize their context and modulate Notch signalling accordingly, they can no longer make appropriate choices, as has been suggested in some types of brain tumour (BOX 2).
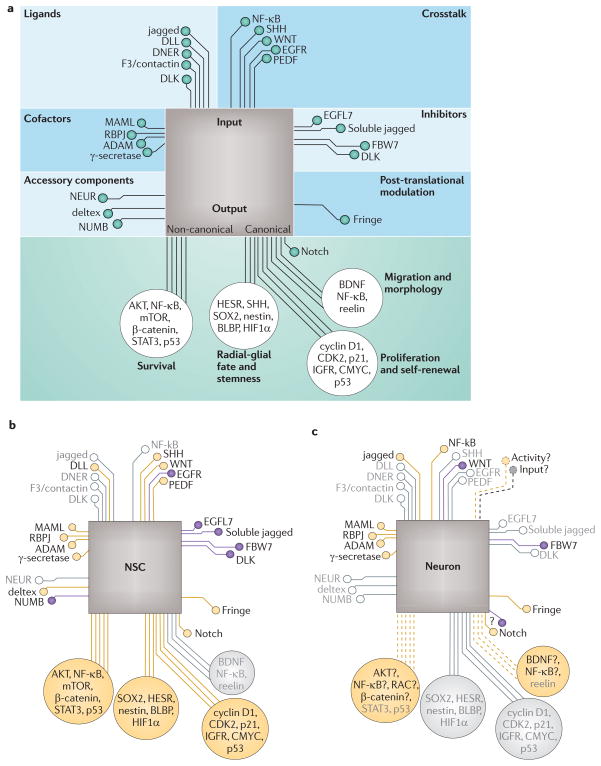
Figure 3. Notch integrates signals to coordinate a context-specific response.
It is generally accepted that Notch signalling integrates multiple pathways, thereby tuning downstream expression to match the cell’s current needs and situation. This could be considered to resemble a processor on a circuit board, integrating inputs from various computing processes and directing output to appropriate hardware to generate a coordinated response. a | A representative sample of the various ‘inputs’, which include Notch ligands and input through crosstalking pathways, is shown (top part). The ability of a cell to receive input is regulated by the availability of the Notch receptor (not shown) and ligands, and by post-translational modification of both the receptor and ligands by fringe family proteins — such modification results in some Notch ligands being activating and others inhibiting. Input capacity is further modulated by intracellular ‘accessory pathway’ components and cofactors, such as deltex, neuralized (NEUR) and mastermind-like proteins (MAMLs), and extracellular inhibitors, all of which can bias Notch activation in one cell of the signalling pair. The output of Notch signalling (a representative sample of the currently identified ‘outputs’ is shown; bottom part) results from both canonical Notch signalling (activation of genes whose expression is regulated by recombining binding protein suppressor of hairless (RBPJ)-dependent Notch signalling) and presumably non-canonical Notch signalling mechanisms. However, data on non-canonical signalling are limited. The output depends in part on the Notch pathways that are available in a given cell type and in part on the combination of inputs. b | In neural stem cells (NSCs), for example, Notch integrates growth factor (epidermal growth factor receptor (EGFR) and pigment epithelium-derived factor (PEDF)) signalling with input from key stem pathways, such as sonic hedgehog (SHH) and WNT, to promote expression of genes that maintain stemness and self-renewal. c | In mature neurons, in which SHH has a less prominent role, Notch may integrate WNT, neurotrophin and NF-κB signalling to modulate synaptic complexity and survival. Most of the input and key pathways that interact with Notch in mature neurons are unknown (shown by dashed lines), as are the downstream effectors. Probable candidates include neurotrophins (for example, brain-derived neurotrophic factor (BDNF)), activity-dependent modulators of synaptic and structural plasticity (for example, NF-κB), as well as effectors of cytoskeletal rearrangement (for example, RAS-related C3 botulinum toxin (RAC) proteins and β-catenin). Inputs that inhibit Notch signalling are shown in purple, and inputs that potentiate Notch signalling are shown in yellow. Inputs and outputs that play a less prominent part in a given stage are shown in grey with open circles. ADAM, a disintegrin and metalloproteinase domain-containing protein; AKT, serine/threonine-protein kinase AKT; BLBP, fatty acid-binding protein, brain; CDK2, cyclin-dependent kinase 2; DLL, Delta-like protein; DNER, Delta and Notch-like epidermal growth factor-related receptor; DLK, protein delta homologue 1; EGFL7, epidermal growth factor-like protein 7; FBW7, F-box/WD repeat-containing protein 7; HESR, hairy and enhancer of split-related genes; HIF1α, hypoxia-inducible factor 1A; IGFR, insulin-like growth factor receptor; mTOR, mammalian target of rapamycin; STAT3, signal transducer and activator of transcription 3.
Box 2. Pathological stem cell maintenance: Notch and glioma.
Gliomas are brain tumours that arise from glia cells, including radial glia. These are often aggressive, rapidly growing tumours with poor prognosis, mainly because of their resistance to chemotherapy and radiation, and their high incidence of recurrence. Gliomas are thought to arise from aberrant neural stem cells — in cancer contexts they are often referred to as cancer stem cells — and not surprisingly, Notch signalling is involved in the generation and/or progression of gliomas. For example, overexpression of the Notch intracellular domain (NICD) in a neural stem cell line is sufficient to transform it into a tumorigenic cell line156. Aberrant Notch signalling leads to aberrant epidermal growth factor receptor (EGFR) signalling157, a feature of the most malignant glioma, glioblastoma158. Likewise, disrupted regulation of Notch owing to mutations in NUMB or neuralized (NEUR) leads to tumour formation159,160.
Although not all gliomas arise due to disrupted Notch signalling, Notch signalling is involved in maintaining the cancer stem cells and in promoting their proliferation. It is activated in response to hypoxia and angiogenesis, two processes characteristic of gliomas, especially the aggressive glioblastoma161,162. Notch may be moderately expressed and inactive in low-grade tumours, but it is highly expressed and active in glioblastoma163. By maintaining the relatively quiescent cancer stem cells, Notch promotes radioresistance of gliomas, as these quiescent cells are not sensitive to the therapy and survive to repopulate the tumour164. However, Notch signalling can also limit the growth and severity of gliomas. In some medulloblastomas, Notch1 inhibits growth, whereas Notch2 promotes tumorigenesis165. Although current understanding of how Notch signalling contributes to the formation and progression of gliomas is limited, it has led to the development of more effective therapies. For example, inhibition of Notch using γ-secretase inhibitors decreases proliferation and increases radiosensitivity of glioblastoma cell lines164,166. Studies are underway to determine if treating patients with γ-secretase inhibitors may make gliomas more sensitive to current interventions (see the ClinicalTrials.gov website. Identifiers: NCT00572182, NCT01119599 and NCT01122901).
Directional signalling: sender or receiver?
If a cell is to use Notch signalling to make a choice, it must possess functional Notch receptors and be in contact with cells that express the ligand. It is generally thought that activation of the Notch pathway results in a mix of non-cell-autonomous (ligand-mediated) and cell-autonomous (receptor-mediated) effects. Indeed, disruption of Notch ligands seemingly leads to non-cell-autonomous impairment of neurogenesis99, whereas disruption of Notch receptors or RBPJ has been reported to lead to cell-autonomous effects31,32. This suggests that Notch signalling occurs in one direction, from the signal- sending (that is, ligand-presenting) cell to the signal-receiving (that is, Notch-presenting) cell12 (FIG. 1). The mechanisms that determine whether a cell will be a signal sender or a signal receiver remain largely unknown. Some studies indicate that this process may be stochastic100, whereas studies by Kageyama and colleagues, using elegant bioluminescence studies, attribute it to oscillation of the levels of pathway components, especially HES1 (REF. 101), or to post- translational modifications that affect receptor and ligand affinity and availability102. Intrinsic and extrinsic factors, as well as transcriptional feedback, regulate the levels of ligand and receptor, such that subtle initial differences in the ligand-to-receptor ratio are amplified and a signalling or receiving mode is established13,103. Cis ligand–receptor interactions inhibit Notch activation104, proteins such as deltex, NUMB and NEUR regulate the cellular distribution and surface availability of Notch, and non-canonical ligands modulate signalling strength — that is, the probability that a sufficient number of Notch receptors is activated to elicit a response14. The level of functional receptor that is available dictates the ability of the cell to receive a signal, especially given that each receptor can only be activated once owing to the irreversible cleavage events that are necessary for signalling12,13,34.
Molecular context
Many factors converge to determine the outcome of Notch activation, but perhaps the single most important factor is the type of cell that is receiving activation. This is because the repertoire of genes available for modulation (that is, the ‘molecular context’) varies greatly between cell types. There is extensive crosstalk between Notch and pathways implicated in different phases of adult SGZ and SVZ neurogenesis, and it is this crosstalk that allows signals to be integrated and consolidated (FIG. 3). Conditional knockout mice of the SHH pathway component protein patched homologue 1 (PTCH1)105 and of cilia proteins106 (which seem to mediate SHH signalling) display similar deficiencies as _Notch1_-inducible mutant mice — notably the depletion of primary, radial glial-like SGZ precursors81,107–109 — suggesting that both pathways are necessary for precursor maintenance. Likewise, WNT–β-catenin110 and Notch signalling seem to interact on several levels. WNT stimulates the expression of Notch ligands in progenitors111, thus provoking Notch signalling in adjacent NSCs, whereas Notch activation modulates the threshold of WNT signalling112. The two pathways also interact through glycogen synthase kinase-3β (GSK3β)113 and fibroblast growth factor (FGF)114 to link inhibition of differentiation with proliferation, and to cooperatively regulate the number of new neurons generated. Notch can also integrate signals from multiple growth factors in adult neural stem and progenitor cells. Pigment epithelium-derived factor (PEDF) increases the self-renewal capability of stem cells in the adult SVZ by upregulating the Notch target gene Egfr (epidermal growth factor receptor)23. However, another report indicates that in the adult SVZ, EGFR-expressing cells limit Notch signalling through a NUMB-dependent mechanism115 and suggests that Notch and EGFR cooperate to balance stem and progenitor cell numbers.
In contrast to its role in promoting stem cell maintenance and survival, Notch activation can also be pro-apoptotic in cycling cells. Putative disinhibition of Notch signalling using inducible Numb and Numbl knockout mice compromises progenitor survival116. However, the relationship between Notch and the cargo-specific adaptor protein NUMB and its closely related and partially- redundant NUMBL protein is quite complex. In Drosophila, NUMB acts as a cell-fate determinant by asymmetrically localizing during mitosis and by inhibiting Notch in one of the daughter cells117. However, although the role of Notch in maintaining neural stem in vertebrate cells exquisitely mirrors its role in the fruitfly, the role of NUMB is apparently not as well conserved117. For example, although Notch manipulations that result in gain-of-function consistently maintain the neural stem cell fate, manipulations of NUMB and NUMBL that result in loss of function, and that would be expected to result in an upregulation of Notch, do not show the anticipated outcome of massively decreased neurogene-sis118. However, morphogenesis is grossly disrupted. This is probably due to the role of NUMB in cadherin recycling116,118 and its interactions with other pathways and molecules such as p53 (REF. 119), SHH120, integrins and partitioning defective (PAR)-family proteins121. Indeed, overexpression of NUMB promoted the abnormal perdurance of the radial glial morphology — a phenotype that would be more commonly expected after Notch activation — owing to cadherin-based maintenance of the adherens junctions118. In the postnatal brain, a similar cadherin-based phenotype (that is, loss of ventricular zone integrity owing to loss of adherens junctions) was seen upon Numb and Numbl ablation116. However, Notch can be inhibited by overexpressing Numb in postmitotic neurons4. Futhermore, multiple groups have found interactions between Notch, NUMB and APP69,72,73, allowing for the possibility that changes in the dynamics122 of these interactions may be involved in the pathogenesis of Alzheimer’s disease. Taken together, owing to its effects on many diverse signalling pathways, the role of NUMB defies a simple categorization as a Notch inhibitor117.
Several Notch-interacting pathways that are beyond the scope of this Review have been identified in adult NSCs123,124. However, little is known about interacting pathways in postmitotic neurons. Notch signalling can promote the survival of postmitotic newborn neurons through α-synuclein in the adult hippocampus38. As discussed above, Notch signalling in neurons is dependent on activity-induced genes62 and cooperates with nerve growth factor (NGF) and its receptor p75 to regulate synaptic plasticity47. Hopefully, the growing body of knowledge about these signalling pathways and their context-dependent, cell type-specific interactions may one day allow us to fit together the molecular hierarchy to create a mechanistic model of the neurobiology of Notch signalling in the adult brain.
Physical context
The neurogenic niches are discrete areas of the brain, and it is only in these niches that neurogenesis normally occurs in adults22. It is well established that the niche itself provides extrinsic cues that Notch integrates to modulate cellular output accordingly125. It is not surprising, therefore, to find that neural stem cells isolated from the SVZ are distinct from stem cells in the SGZ. For example, SVZ stem cells generate inhibitory interneurons, whereas SGZ stem cells generate excitatory granule cell neurons123. Thus, caution should be taken when extrapolating findings from one niche to the other. Nevertheless, results from conditional and inducible Notch knockout mice suggest that Notch signalling maintains astroglial stem cells in both the SVZ and SGZ by preventing differentiation32.
Although much work is needed to fully understand the role of the niche environment in modulating the identity, potency and proliferation of neural stem cells, we have learned that both extracellular signals and neighbouring cells contribute to this modulation. Both vascular endothelial cells and astrocytes (which wrap the cerebral vasculature) stimulate neurogenesis from adult NSCs126,127, and this has led to the hypothesis that neurogenesis occurs preferentially in a ‘vascular niche’ created by this network of vessels and astrocytes in the SVZ and SGZ128,129. An intruiging study showed that endothelial cells in the adult SVZ induce Notch activation in NSCs indirectly through the candidate factor PEDF, thus biasing cell fate towards radial glia23. PEDF activates NF-κB in a non-canonical manner, and this induced nuclear export of both the p65 subunit of NF-κB and a nuclear receptor co-repressor (NCOR) protein from the Notch-responsive promoters of Hes1 and Egfr. By removing NCOR — and thereby disinhibiting the Notch target genes — stem cells are more likely to activate the Notch target genes responsible for self-renewing, asymmetric divisions.
Contrary to expectation, the vascular niche does not depend on high levels of oxygenation to support NSCs in the adult brain. Unlike mature neurons, NSCs are resistant to hypoxia, and even display more rapid expansion and enhanced self-renewal in 1% O2 compared with 20% O2 (REF. 130). In fact, evidence suggests that hypoxia — which is a physiological condition during embryonic development — contributes to a niche that supports stem cells and that is does so through Notch signalling131. The hypoxia-inducible factor 1α (HIF1α) is stabilized under hypoxic conditions and cooperates with Notch signalling to promote expression of Notch target genes and proliferation of NSCs and progenitors in the adult brain (such as in some tumours130,132 and perhaps after ischaemia), although no studies to date have examined the interaction of Notch and HIF1α after ischaemia in the adult brain. Intriguingly, under grossly normoxic conditions HIF1α is expressed and stabilized specifically in NSCs and progenitors in the adult brain133, suggesting that a physiological role for hypoxia may persist in the adult stem cell niche.
Neighbouring progenitor cells constitute a second important constituent of the neurogenic niche, providing both direct Notch ligands and a myriad of other cell–cell interactions. In the SVZ and SGZ, stem cells are surrounded by progenitors and mature cells134,135, and cell–cell interactions between NSC and progenitors, which provide the major Notch ligand input136, are required for neurogenesis — although few studies have formally examined the ability of adult progenitors to send a signal. Despite evidence that mature cells in these niches (for example, neurons or ependymal cells) express ligands, it has not been shown that either of these cell types can directly activate Notch signalling in adult brain. Further studies are needed to understand the implication of Notch signalling in different brain regions beyond the neurogenic niches and beyond stem and progenitor cells. For example, does Notch activation in neurons of the cortex have the same effect as in neurons of the cerebellum?
Neurogenic demand
Adult neurogenesis in the SVZ and SGZ is activity dependent137–139, but the mechanisms that underlie this responsiveness remain unknown. Given the integral part that Notch plays in regulating proliferation and quiescence of NSCs, it seems likely that Notch would be involved in activating stem cells in response to exercise, learning or injury (BOX 3). In this section we discuss the current evidence for this and speculate about the pathways that might interact with Notch to regulate neurogenesis in response to stimuli.
Box 3. A role for Notch in response to neurogenic demand?
Neural stem cells (NSCs) are quiescent but can be recruited in response to demand ranging from physiologic, such as learning or exercise, to pathologic, such as injury167. Notch has been implicated in regulating the transition from quiescence to activation, and levels of Notch signalling have been shown to grossly change in each of these situations. NSCs are not the only cells that can respond to stimuli. The effect of various stimuli on each stage of neurogenesis are shown in the table. Although Notch signalling has been implicated in regulating some or all of these processes in other contexts, we have limited data on its functional role in the adult brain (see the table, bottom row). However, it is clear that Notch remains a key player in cellular plasticity at all stages, and we must understand how it works to fine-tune potential Notch-based therapies.
| Cell type | Exercise | Learning | Stroke and injury |
|---|---|---|---|
| Radial glial-like NSC | Activated Increased proliferation but no increase in cell number140,141 Increased asymmetric divisions? | ? | Activated Increased proliferation158 |
| Horizontal NSC | • Quiescence | • Activated? | ? |
| •No change in proliferation or cell number140 | • Delayed proliferation?37 | ||
| Progenitors | • Increased proliferation and cell number137 | • Delayed proliferation?37 | • Increased proliferation and cell number79,80 |
| Neuroblasts | Increased proliferation and cell number137 Increased cell cycle exit37 | Delayed increase in apoptosis147 | Increased apoptosis158 |
| Newborn neurons | Increased cell number, owing to increased survival and more progenitors142 Increased structural complexity143 | Increased survival Increased structural complexity Increased synaptic plasticity147 | Increased apoptosis83 |
| Ependymal cells | – | – | • Conversion to neuroblast? |
| Associated changes in Notch signalling | • NICD increased in neuroblasts but not in NSC or progenitors37 | • Activated during learning but generates a novel 66-kDa NICD fragment59 | • Increased NICD and Hes1 in progenitors79 |
| •Inducible Notch ablation in NSC and progenitors blocks running-induced proliferation30 | • Sharply attenuated during consolidation59. | • Notch inhibition blocks ischaemia-induced progenitor proliferation80 and neuronal death83 but promotes neuroblast differentiation78,79 |
Exercise is one of the most potent stimulators of adult neurogenesis, particularly in the SGZ137, and several recent studies have shown that in mice, NSCs in the SGZ proliferate in response to running140,141. A study using _Hes5_–green fluorescence protein (GFP) reporter mice found that proliferation of Notch-responsive SGZ stem cells increased after running140, but it is unknown whether Notch is required for this response. In inducible Notch1 knockout mice, running was not sufficient to rescue the number of SGZ stem cells to wild-type levels30, but this study did not explore the proliferative status of NSCs. However, uncommitted SGZ progenitors did not proliferate in response to running, whereas the number of DCX-positive neuroblasts was increased, suggesting that there may be a stage-specific role for Notch in activity-dependent neurogenesis. Exercise increases survival of adult-born neurons in the murine SGZ through serine/threonine-protein kinase AKT signalling142,143 with a concomitant rise in levels of nuclear NICD in these newborn DCX-positive cells after running37, suggesting that Notch may have a role in promoting activity-dependent survival. However, survival of new neurons in the SGZ was not affected in inducible Notch1 knockout mice after running30. Thus, it remains to be determined if there is a causal link between Notch signalling and enhanced SGZ neurogenesis after exercise. It is likely that Notch plays an accessory part in other pathways, possibly through crosstalk with growth factor signalling and neurotrophic signalling, to permit responsiveness to neurogenic stimuli.
In a similar way to exercise, ischaemia induces proliferation of adult NSCs. However, Notch activation in differentiated ependymal cells after ischaemia has the opposite effect. Ependymal cells, which line the ventricles in the adult brain, do not proliferate under normal conditions and cannot self-renew. Yet, ependymal cells in forkhead box protein J1 (FOXJ1)-specific _RBPJ_-inducible knockout mice generate proliferating neuroblasts in response to ischaemic injury, unlike their wild-type littermates144. These results highlight a role for Notch signalling in postmitotic cells, preventing de-differentiation and cell-cycle entry. However, Kageyama and colleagues could not replicate these findings using similar nestin-specific inducible RBPJ knockout mice32. It was later found that the purportedly ependymal cell-specific FOXJ1 promoter employed in REF. 144 is also present in neurogenic astroglia and many other cells in that lineage145, indicating that there may be methodological confounds in the interpretation that FOXJ1-derived neurons exclusively descend from ependymal cells. Nevertheless, a recent study showed that there is an astonishing degree of plasticity of cell types in the SVZ, with ependymal cells and astrocytes transitioning back and forth between phenotypes146. This study also showed that Notch is upstream of ephrin-type B receptor 2 (EPHB2) forward signalling in maintaining ependymal cell differentiation. This raises the intriguing question of how Notch mechanistically mediates the maintenance of astrocytic neural stem cells23,115 and the differentiation of ependymal cells simultaneously when both have the potential to transform phenotypically into each other146. For example, do the same ligand(s) regulate both processes? And how is the equilibrium between cell fates regulated? Thus, further studies are needed to determine the precise role of Notch in regulating ependymal cell differentiation from the astroglial lineage and vice versa.
Although still incompletely understood, a role for Notch in modulating neurogenesis during memory and learning is emerging. Studies in adult rats and mice suggest that spatial learning correlates with increased neurogenesis in the SGZ — specifically, the survival and integration of newborn neurons147, indicating that the addition of new neurons is important for the formation of new memories. It is plausible that Notch may be involved in recruiting quiescent NSCs to generate the needed neurons, although no studies have examined this so far. As we have discussed above, Notch can promote survival of new postmitotic neurons38, and is specifically required for spatial memory52, suggesting that Notch signalling may modulate neurogenic demand at the level of the newborn neuron. It is possible that Notch could regulate the survival of newborn neurons directly or indirectly by modulating integration and synaptogenesis during learning — processes that are known to be crucial for the survival of new neurons. However, no studies have yet determined a causal or mechanistic link between Notch signalling and integration of adult-born neurons that are recruited during learning and memory formation.
Conclusions
The studies reviewed here suggest that Notch signalling in the adult brain displays a wealth of contextual diversity that we are only beginning to understand. Clearly, Notch signalling plays a prominent part in balancing stem cell maintenance with progenitor differentiation, but we do not understand how Notch signalling can fine-tune neurogenesis (from NSC proliferation to neuron integration) to suit the demand, under various conditions, in the life of an animal. In this Review we have suggested that this fine-tuning occurs in cooperation with crosstalking pathways that themselves might be regulated by the particular context or situation, whether it is running or recovering from ischaemia, learning or ageing. However, much of this is speculation and future studies will have to determine whether there is indeed any role for Notch in regulating neuronal output under demand conditions. It will not be enough to simply show that Notch is involved; rather, future studies must also focus on the underlying molecular mechanisms, so that we may gain a clearer understanding of the ‘Notch signalling network’. Notch may be a master regulator of cell choices, but it does not do this in isolation. Finally, as we have emphasized in this Review, the outcome of Notch activation is to a large extent dependent on the type of cell that receives the signal. We now have some of the tools that we need (BOX 1) to parse out the complex nature of Notch signalling in a cell-specific manner. For example, knowing that Notch regulates the structure and function of newly born neurons and that it potentially has crucial roles in synaptic plasticity and in the response to disease and trauma, using conditional and inducible mutant mice we can begin to isolate and study Notch signalling specifically in new neurons of the adult brain, both under normal conditions, in response to activity, and in disease models.
Understanding how Notch can modulate stem cell fate and the addition of neurons to the adult brain, as well as the survival and function of mature neurons, has broad implications for the development of therapies aimed at combating brain injury and neurodegeneration. First and foremost, in many cases of diffuse, widespread neurodegeneration, modulation of Notch may be sufficient to increase neuron survival, such as in the case of prion-related disorders — or, more speculatively, in Alzheimer’s disease. Alternative stem cell therapies are often proposed as potential treatments for neurodegenerative disorders, but we do not know the critical pathways involved in adding new neurons to existing circuitry. Notch signalling might be used to differentiate stem cells into a particular neuron type, or it might be important in regulating the survival of the new neurons once they have been implanted. Crosstalk with other pathways will need to be considered in addition to manipulating Notch itself. It is clear, however, that Notch is a key player in the adult brain and it will be the focus of many future studies towards understanding function and dysfunction.
Acknowledgments
J.L.A. would like to thank the Medical Science Training Program (MSTP) at both the University of Texas Southwestern Medical Centre, at Dallas, Texas, USA, and Mount Sinai School of Medicine, New York, New York, USA, as well as the US National Institute of Mental Health (NIMH) and National Institute on Drug Abuse (NIDA) for their support. J.J.B. was supported by the Connecticut Stem Cell Research Grant Program during the preparation of this manuscript and is currently supported by the Cedars-Sinai Regenerative Medicine Institute, Los Angeles, California, USA. A.J.E. is supported by grants from the US National Institutes of Health (NIH), including grants from NIDA (DA016765, DA016765-07S, DA023555) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK079328), the National Alliance for Research on Schizophrenia and Depression, the National Aeronautics and Space Administration, and the Norwegian Department of Public Health. P.R. is supported by grants from the US National Institute of Neurological Disorders and Stroke (NINDS), NIDA and the Kavli Institute of Neuroscience at Yale University School of Medicine, New Haven, Connecticut, USA.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nature Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- 2.Yoon K, Gaiano N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nature Neurosci. 2005;8:709–715. doi: 10.1038/nn1475. [DOI] [PubMed] [Google Scholar]
- 3.Berezovska O, Xia MQ, Hyman BT. Notch is expressed in adult brain, is coexpressed with presenilin-1, and is altered in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:738–745. doi: 10.1097/00005072-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Sestan N, Artavanis-Tsakonas S, Rakic P. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 1999;286:741–746. doi: 10.1126/science.286.5440.741. This paper is the first to show Notch function in postmitotic neurons in regulating density-dependent dendritic arborization. [DOI] [PubMed] [Google Scholar]
- 5.Li L, et al. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nature Genet. 1997;16:243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- 6.Oda T, et al. Mutations in the human Jagged1 gene are responsible for Alagille syndrome. Nature Genet. 1997;16:235–242. doi: 10.1038/ng0797-235. [DOI] [PubMed] [Google Scholar]
- 7.Joutel A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 8.Isidor B, et al. Truncating mutations in the last exon of NOTCH2 cause a rare skeletal disorder with osteoporosis. Nature Genet. 2011 Mar 6; doi: 10.1038/ng.778. [DOI] [PubMed] [Google Scholar]
- 9.Simpson MA, et al. Mutations in NOTCH2 cause Hajdu-Cheney syndrome, a disorder of severe and progressive bone loss. Nature Genet. 2011 Mar 6; doi: 10.1038/ng.779. [DOI] [PubMed] [Google Scholar]
- 10.Fischer DF, et al. Activation of the Notch pathway in Down syndrome: cross-talk of Notch and APP. FASEB J. 2005;19:1451–1458. doi: 10.1096/fj.04-3395.com. [DOI] [PubMed] [Google Scholar]
- 11.Veeraraghavalu K, Choi SH, Zhang X, Sisodia SS. Presenilin 1 mutants impair the self-renewal and differentiation of adult murine subventricular zone-neuronal progenitors via cell-autonomous mechanisms involving notch signaling. J Neurosci. 2010;30:6903–6915. doi: 10.1523/JNEUROSCI.0527-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 13.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovall RA, Blacklow SC. Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr Top Dev Biol. 2010;92:31–71. doi: 10.1016/S0070-2153(10)92002-4. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto S, Charng WL, Bellen HJ. Endocytosis and intracellular trafficking of Notch and its ligands. Curr Top Dev Biol. 2010;92:165–200. doi: 10.1016/S0070-2153(10)92005-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bray S, Bernard F. Notch targets and their regulation. Curr Top Dev Biol. 2010;92:253–275. doi: 10.1016/S0070-2153(10)92008-5. [DOI] [PubMed] [Google Scholar]
- 18.Sanalkumar R, Dhanesh SB, James J. Non-canonical activation of Notch signaling/target genes in vertebrates. Cell Mol Life Sci. 2010;67:2957–2968. doi: 10.1007/s00018-010-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Strooper B, Annaert W. Novel research horizons for presenilins and γ-secretases in cell biology and disease. Annu Rev Cell Dev Biol. 2010;26:235–260. doi: 10.1146/annurev-cellbio-100109-104117. [DOI] [PubMed] [Google Scholar]
- 20.Yang MS, et al. Among γ-secretase substrates Notch1 alone is sufficient to block neurogenesis but does not confer self-renewal properties to neural stem cells. Biochem Biophys Res Commun. 2011;404:133–138. doi: 10.1016/j.bbrc.2010.11.080. [DOI] [PubMed] [Google Scholar]
- 21.Extance A. Alzheimer’s failure raises questions about disease-modifying strategies. Nature Rev Drug Discov. 2010;9:749–751. doi: 10.1038/nrd3288. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 23.Andreu-Agullo C, Morante-Redolat JM, Delgado AC, Farinas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nature Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 24.Breunig JJ, Silbereis J, Vaccarino FM, Sestan N, Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc Natl Acad Sci USA. 2007;104:20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Givogri MI, et al. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- 26.Stump G, et al. Notch1 and its ligands Delta-like and Jagged are expressed and active in distinct cell populations in the postnatal mouse brain. Mech Dev. 2002;114:153–159. doi: 10.1016/s0925-4773(02)00043-6. [DOI] [PubMed] [Google Scholar]
- 27.Ostenfeld T, Svendsen CN. Requirement for neurogenesis to proceed through the division of neuronal progenitors following differentiation of epidermal growth factor and fibroblast growth factor-2-responsive human neural stem cells. Stem Cells. 2004;22:798–811. doi: 10.1634/stemcells.22-5-798. [DOI] [PubMed] [Google Scholar]
- 28.Rakic P, Ayoub AE, Breunig JJ, Dominguez MH. Decision by division: making cortical maps. Trends Neurosci. 2009;32:291–301. doi: 10.1016/j.tins.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapouton P, et al. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J Neurosci. 2010;30:7961–7974. doi: 10.1523/JNEUROSCI.6170-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ables JL, et al. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehm O, et al. RBPJκ-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci. 2010;30:13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borghese L, et al. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 2010;28:955–964. doi: 10.1002/stem.408. [DOI] [PubMed] [Google Scholar]
- 34.Guentchev M, McKay RD. Notch controls proliferation and differentiation of stem cells in a dose-dependent manner. Eur J Neurosci. 2006;23:2289–2296. doi: 10.1111/j.1460-9568.2006.04766.x. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto-Torii K, et al. Interaction between Reelin and Notch signaling regulates neuronal migration in the cerebral cortex. Neuron. 2008;60:273–284. doi: 10.1016/j.neuron.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sibbe M, Forster E, Basak O, Taylor V, Frotscher M. Reelin and Notch1 cooperate in the development of the dentate gyrus. J Neurosci. 2009;29:8578–8585. doi: 10.1523/JNEUROSCI.0958-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandt MD, Maass A, Kempermann G, Storch A. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neurogenesis. Eur J Neurosci. 2010;32:1256–1264. doi: 10.1111/j.1460-9568.2010.07410.x. [DOI] [PubMed] [Google Scholar]
- 38.Crews L, et al. α-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci. 2008;28:4250–4260. doi: 10.1523/JNEUROSCI.0066-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon-Areces J, Membrive G, Garcia-Fernandez C, Garcia-Segura LM, Arevalo MA. Neurogenin 3 cellular and subcellular localization in the developing and adult hippocampus. J Comp Neurol. 2010;518:1814–1824. doi: 10.1002/cne.22304. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari-Toninelli G, Bonini SA, Bettinsoli P, Uberti D, Memo M. Microtubule stabilizing effect of notch activation in primary cortical neurons. Neuroscience. 2008;154:946–952. doi: 10.1016/j.neuroscience.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 41.Giniger E. A role for Abl in Notch signaling. Neuron. 1998;20:667–681. doi: 10.1016/s0896-6273(00)81007-7. [DOI] [PubMed] [Google Scholar]
- 42.Crowner D, Le Gall M, Gates MA, Giniger E. Notch steers Drosophila ISNb motor axons by regulating the Abl signaling pathway. Curr Biol. 2003;13:967–972. doi: 10.1016/s0960-9822(03)00325-7. [DOI] [PubMed] [Google Scholar]
- 43.Le Gall M, De Mattei C, Giniger E. Molecular separation of two signaling pathways for the receptor, Notch. Dev Biol. 2008;313:556–567. doi: 10.1016/j.ydbio.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassan BA, et al. Natonal regulates neurite arborization but does not act as a proneural gene in the Drosophila brain. Neuron. 2000;25:549–561. doi: 10.1016/s0896-6273(00)81059-4. [DOI] [PubMed] [Google Scholar]
- 45.Berezovska O, et al. Notch1 inhibits neurite outgrowth in postmitotic primary neurons. Neuroscience. 1999;93:433–439. doi: 10.1016/s0306-4522(99)00157-8. [DOI] [PubMed] [Google Scholar]
- 46.Redmond L, Oh SR, Hicks C, Weinmaster G, Ghosh A. Nuclear Notch1 signaling and the regulation of dendritic development. Nature Neurosci. 2000;3:30–40. doi: 10.1038/71104. [DOI] [PubMed] [Google Scholar]
- 47.Salama-Cohen P, Arevalo MA, Grantyn R, Rodriguez-Tebar A. Notch and NGF/p75NTR control dendrite morphology and the balance of excitatory/ inhibitory synaptic input to hippocampal neurones through Neurogenin 3. J Neurochem. 2006;97:1269–1278. doi: 10.1111/j.1471-4159.2006.03783.x. [DOI] [PubMed] [Google Scholar]
- 48.Huang EJ, et al. Targeted deletion of numb and numblike in sensory neurons reveals their essential functions in axon arborization. Genes Dev. 2005;19:138–151. doi: 10.1101/gad.1246005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santolini E, et al. Numb is an endocytic protein. J Cell Biol. 2000;151:1345–1352. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 51.Dahlhaus M, et al. Notch1 signaling in pyramidal neurons regulates synaptic connectivity and experience-dependent modifications of acuity in the visual cortex. J Neurosci. 2008;28:10794–10802. doi: 10.1523/JNEUROSCI.1348-08.2008. This is one of the first studies to show activity-dependent Notch signalling in vivo. Using genetic approaches the authors show that Notch regulates structural and functional plasticity of pyramidal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costa RM, Honjo T, Silva AJ. Learning and memory deficits in Notch mutant mice. Curr Biol. 2003;13:1348–1354. doi: 10.1016/s0960-9822(03)00492-5. [DOI] [PubMed] [Google Scholar]
- 53.Givogri MI, et al. Central nervous system myelination in mice with deficient expression of Notch1 receptor. J Neurosci Res. 2002;67:309–320. doi: 10.1002/jnr.10128. [DOI] [PubMed] [Google Scholar]
- 54.Heitzler P, Simpson P. The choice of cell fate in the epidermis of Drosophila. Cell. 1991;64:1083–1092. doi: 10.1016/0092-8674(91)90263-x. [DOI] [PubMed] [Google Scholar]
- 55.Presente A, Boyles RS, Serway CN, de Belle JS, Andres AJ. Notch is required for long-term memory in Drosophila. Proc Natl Acad Sci USA. 2004;101:1764–1768. doi: 10.1073/pnas.0308259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ge X, et al. Notch signaling in Drosophila long-term memory formation. Proc Natl Acad Sci USA. 2004;101:10172–10176. doi: 10.1073/pnas.0403497101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pavlopoulos E, Anezaki M, Skoulakis EM. Neuralized is expressed in the α/β lobes of adult Drosophila mushroom bodies and facilitates olfactory long-term memory formation. Proc Natl Acad Sci USA. 2008;105:14674–14679. doi: 10.1073/pnas.0801605105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Y, et al. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci USA. 2004;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Conboy L, et al. Notch signalling becomes transiently attenuated during long-term memory consolidation in adult Wistar rats. Neurobiol Learn Mem. 2007;88:342–351. doi: 10.1016/j.nlm.2007.04.006. This study carefully examines the time course of Notch signalling during memory formation. Furthermore, it identifies a novel NICD fragment. [DOI] [PubMed] [Google Scholar]
- 60.Tagami S, et al. Regulation of Notch signaling by dynamic changes in the precision of S3 cleavage of Notch-1. Mol Cell Biol. 2008;28:165–176. doi: 10.1128/MCB.00863-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Bivort BL, Guo HF, Zhong Y. Notch signaling is required for activity-dependent synaptic plasticity at the Drosophila neuromuscular junction. J Neurogenet. 2009;23:395–404. doi: 10.3109/01677060902878481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alberi L, et al. Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron. 2011;69:437–444. doi: 10.1016/j.neuron.2011.01.004. This is the first study to demonstrate that Notch is present at the rodent synapse. They also elegantly show that Notch signalling is dependent on neuronal activity and regulates the structure and function of neurons in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lieber T, Kidd S, Struhl G. DSL-Notch signaling in the Drosophila brain in response to olfactory stimulation. Neuron. 2011;69:468–481. doi: 10.1016/j.neuron.2010.12.015. Using the power of Drosophila*** genetics, this study illuminates the dynamics of activity-related Notch signalling elicited by specific odorants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McGeer PL, Kawamata T, McGeer EG. Localization and possible functions of presenilins in brain. Rev Neurosci. 1998;9:1–15. doi: 10.1515/revneuro.1998.9.1.1. [DOI] [PubMed] [Google Scholar]
- 65.Franberg J, Karlstrom H, Winblad B, Tjernberg LO, Frykman S. γ-Secretase dependent production of intracellular domains is reduced in adult compared to embryonic rat brain membranes. PLoS ONE. 2010;5:e9772. doi: 10.1371/journal.pone.0009772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Placanica L, Zhu L, Li YM. Gender- and age-dependent γ-secretase activity in mouse brain and its implication in sporadic Alzheimer disease. PLoS ONE. 2009;4:e5088. doi: 10.1371/journal.pone.0005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song W, et al. Proteolytic release and nuclear translocation of Notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc Natl Acad Sci USA. 1999;96:6959–6963. doi: 10.1073/pnas.96.12.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen CD, Oh SY, Hinman JD, Abraham CR. Visualization of APP dimerization and APP-Notch2 heterodimerization in living cells using bimolecular fluorescence complementation. J Neurochem. 2006;97:30–43. doi: 10.1111/j.1471-4159.2006.03705.x. [DOI] [PubMed] [Google Scholar]
- 69.Fassa A, Mehta P, Efthimiopoulos S. Notch 1 interacts with the amyloid precursor protein in a Numb-independent manner. J Neurosci Res. 2005;82:214–224. doi: 10.1002/jnr.20642. [DOI] [PubMed] [Google Scholar]
- 70.Oh SY, Chen CD, Abraham CR. Cell-type dependent modulation of Notch signaling by the amyloid precursor protein. J Neurochem. 2010;113:262–274. doi: 10.1111/j.1471-4159.2010.06603.x. [DOI] [PubMed] [Google Scholar]
- 71.Oh SY, et al. Amyloid precursor protein interacts with notch receptors. J Neurosci Res. 2005;82:32–42. doi: 10.1002/jnr.20625. [DOI] [PubMed] [Google Scholar]
- 72.Roncarati R, et al. The γ-secretase-generated intracellular domain of β-amyloid precursor protein binds Numb and inhibits Notch signaling. Proc Natl Acad Sci USA. 2002;99:7102–7107. doi: 10.1073/pnas.102192599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kyriazis GA, et al. Numb endocytic adapter proteins regulate the transport and processing of the amyloid precursor protein in an isoform-dependent manner: implications for Alzheimer disease pathogenesis. J Biol Chem. 2008;283:25492–25502. doi: 10.1074/jbc.M802072200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kyriazis GA, et al. Stress-induced switch in Numb isoforms enhances Notch-dependent expression of subtype-specific transient receptor potential channel. J Biol Chem. 2010;285:6811–6825. doi: 10.1074/jbc.M109.074690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ishikura N, et al. Notch-1 activation and dendritic atrophy in prion disease. Proc Natl Acad Sci USA. 2005;102:886–891. doi: 10.1073/pnas.0408612101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spilman P, et al. A γ-secretase inhibitor and quinacrine reduce prions and prevent dendritic degeneration in murine brains. Proc Natl Acad Sci USA. 2008;105:10595–10600. doi: 10.1073/pnas.0803671105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferron SR, et al. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J Neurosci. 2009;29:14394–14407. doi: 10.1523/JNEUROSCI.3836-09.2009. This study details the fascinating finding that age-related telomere changes may fundamentally alter neural stem cell and daughter cell behavior through p53 and Notch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oya S, et al. Attenuation of Notch signaling promotes the differentiation of neural progenitors into neurons in the hippocampal CA1 region after ischemic injury. Neuroscience. 2009;158:683–692. doi: 10.1016/j.neuroscience.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 79.Wang L, et al. The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience. 2009;158:1356–1363. doi: 10.1016/j.neuroscience.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang X, Mao X, Xie L, Greenberg DA, Jin K. Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J Cereb Blood Flow Metab. 2009;29:1644–1654. doi: 10.1038/jcbfm.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Androutsellis-Theotokis A, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 82.Alberi L, et al. Neonatal stroke in mice causes long-term changes in neuronal Notch-2 expression that may contribute to prolonged injury. Stroke. 2010;41:S64–S71. doi: 10.1161/STROKEAHA.110.595298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arumugam TV, et al. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nature Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- 84.Hellstrom M, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 85.John GR, et al. Multiple sclerosis: re-expression of a developmental pathway that restricts oligodendrocyte maturation. Nature Med. 2002;8:1115–1121. doi: 10.1038/nm781. [DOI] [PubMed] [Google Scholar]
- 86.Wang S, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- 87.Magnus T, et al. Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes. J Neurosci Res. 2007;85:2126–2137. doi: 10.1002/jnr.21368. [DOI] [PubMed] [Google Scholar]
- 88.Morga E, et al. Jagged1 regulates the activation of astrocytes via modulation of NFκB and JAK/STAT/ SOCS pathways. Glia. 2009;57:1741–1753. doi: 10.1002/glia.20887. [DOI] [PubMed] [Google Scholar]
- 89.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J, Leong SY, Schachner M. Differential expression of cell fate determinants in neurons and glial cells of adult mouse spinal cord after compression injury. Eur J Neurosci. 2005;22:1895–1906. doi: 10.1111/j.1460-9568.2005.04348.x. [DOI] [PubMed] [Google Scholar]
- 91.Grandbarbe L, et al. Delta–Notch signaling controls the generation of neurons/glia from neural stem cells in a stepwise process. Development. 2003;130:1391–1402. doi: 10.1242/dev.00374. [DOI] [PubMed] [Google Scholar]
- 92.Jurynczyk M, Jurewicz A, Bielecki B, Raine CS, Selmaj K. Overcoming failure to repair demyelination in EAE: γ-secretase inhibition of Notch signaling. J Neurol Sci. 2008;265:5–11. doi: 10.1016/j.jns.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 93.Nakahara J, Kanekura K, Nawa M, Aiso S, Suzuki N. Abnormal expression of TIP30 and arrested nucleocytoplasmic transport within oligodendrocyte precursor cells in multiple sclerosis. J Clin Invest. 2009;119:169–181. doi: 10.1172/JCI35440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hurlbut GD, Kankel MW, Lake RJ, Artavanis-Tsakonas S. Crossing paths with Notch in the hyper-network. Curr Opin Cell Biol. 2007;19:166–175. doi: 10.1016/j.ceb.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 95.Poellinger L, Lendahl U. Modulating Notch signaling by pathway-intrinsic and pathway-extrinsic mechanisms. Curr Opin Genet Dev. 2008;18:449–454. doi: 10.1016/j.gde.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 96.Martinez AM, et al. Polyhomeotic has a tumor suppressor activity mediated by repression of Notch signaling. Nature Genet. 2009;41:1076–1082. doi: 10.1038/ng.414. [DOI] [PubMed] [Google Scholar]
- 97.Bernard F, Krejci A, Housden B, Adryan B, Bray SJ. Specificity of Notch pathway activation: twist controls the transcriptional output in adult muscle progenitors. Development. 2010;137:2633–2642. doi: 10.1242/dev.053181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bray SJ. Notch signalling: a simple pathway becomes complex. Nature Rev Mol Cell Biol. 2006;7:678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 99.Kawaguchi D, Yoshimatsu T, Hozumi K, Gotoh Y. Selection of differentiating cells by different levels of delta-like 1 among neural precursor cells in the developing mouse telencephalon. Development. 2008;135:3849–3858. doi: 10.1242/dev.024570. [DOI] [PubMed] [Google Scholar]
- 100.Johnston RJ, Jr, Desplan C. Stochastic neuronal cell fate choices. Curr Opin Neurobiol. 2008;18:20–27. doi: 10.1016/j.conb.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shimojo H, Ohtsuka T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron. 2008;58:52–64. doi: 10.1016/j.neuron.2008.02.014. This study elegantly demonstrates the oscillation of Hes1 and other Notch-related genes during development, providing a mechanism for cell fate choices during neurogenesis. [DOI] [PubMed] [Google Scholar]
- 102.D’Souza B, Miyamoto A, Weinmaster G. The many facets of Notch ligands. Oncogene. 2008;27:5148–5167. doi: 10.1038/onc.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sprinzak D, et al. Cis-interactions between Notch and Delta generate mutually exclusive signalling states. Nature. 2010;465:86–90. doi: 10.1038/nature08959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miller AC, Lyons EL, Herman T. G cis-Inhibition of Notch by endogenous Delta biases the outcome of lateral inhibition. Curr Biol. 2009;19:1378–1383. doi: 10.1016/j.cub.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shikata Y, et al. Ptch1-mediated dosage-dependent action of Shh signaling regulates neural progenitor development at late gestational stages. Dev Biol. 2011;349:147–159. doi: 10.1016/j.ydbio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 106.Breunig JJ, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci USA. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nature Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 108.Machold R, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 109.Palma V, et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–344. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 111.Estrach S, Ambler CA, Lo Celso C, Hozumi K, Watt FM. Jagged 1 is a β-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 2006;133:4427–4438. doi: 10.1242/dev.02644. [DOI] [PubMed] [Google Scholar]
- 112.Hayward P, et al. Notch modulates Wnt signalling by associating with Armadillo/β-catenin and regulating its transcriptional activity. Development. 2005;132:1819–1830. doi: 10.1242/dev.01724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Espinosa L, Ingles-Esteve J, Aguilera C, Bigas A. Phosphorylation by glycogen synthase kinase-3 β down-regulates Notch activity, a link for Notch and Wnt pathways. J Biol Chem. 2003;278:32227–32235. doi: 10.1074/jbc.M304001200. [DOI] [PubMed] [Google Scholar]
- 114.Shimizu T, et al. Stabilized β-catenin functions through TCF/LEF proteins and the Notch/RBP-Jκ complex to promote proliferation and suppress differentiation of neural precursor cells. Mol Cell Biol. 2008;28:7427–7441. doi: 10.1128/MCB.01962-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aguirre A, Rubio ME, Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467:323–327. doi: 10.1038/nature09347. This study demonstrates the interplay of Notch and EGFR and shows how the two pathways inhibit each other to establish a balance of progenitor production and stem cell maintenance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kuo CT, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pece S, Confalonieri S, Romano PR, Di Fiore PP. NUMB-ing down cancer by more than just a NOTCH. Biochim Biophys Acta. 2011;1815:26–43. doi: 10.1016/j.bbcan.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 118.Rasin MR, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nature Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- 119.Colaluca IN, et al. NUMB controls p53 tumour suppressor activity. Nature. 2008;451:76–80. doi: 10.1038/nature06412. [DOI] [PubMed] [Google Scholar]
- 120.Di Marcotullio L, et al. Numb is a suppressor of Hedgehog signalling and targets Gli1 for Itch-dependent ubiquitination. Nature Cell Biol. 2006;8:1415–1423. doi: 10.1038/ncb1510. [DOI] [PubMed] [Google Scholar]
- 121.Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 122.Chigurupati S, et al. Evidence for altered numb isoform levels in Alzheimer’s disease patients and a triple transgenic mouse model. J Alzheimers Dis. 2011 Jan 21; doi: 10.3233/JAD-2011-101797. [DOI] [PubMed] [Google Scholar]
- 123.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Johnson MA, Ables JL, Eisch AJ. Cell-intrinsic signals that regulate adult neurogenesis in vivo: insights from inducible approaches. BMB Rep. 2009;42:245–259. doi: 10.5483/bmbrep.2009.42.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13:543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 126.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 127.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 128.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 129.Tavazoie M, et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. 2010;177:1491–1502. doi: 10.2353/ajpath.2010.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gustafsson MV, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. This paper provided an intriguing mechanism for the observation that hypoxia maintains certain stem cell populations. Notably, HIF1α interacts with and stabilizes NICD, promoting receptor signalling. [DOI] [PubMed] [Google Scholar]
- 132.Pistollato F, et al. Interaction of hypoxia-inducible factor-1α and notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells. 2010;28:1918–1929. doi: 10.1002/stem.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roitbak T, Surviladze Z, Cunningham LA. Continuous expression of HIF-1α in neural stem/ progenitor cells. Cell Mol Neurobiol. 2011;31:119–133. doi: 10.1007/s10571-010-9561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci USA. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–378. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- 136.Nyfeler Y, et al. Jagged1 signals in the postnatal subventricular zone are required for neural stem cell self-renewal. EMBO J. 2005;24:3504–3515. doi: 10.1038/sj.emboj.7600816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fabel K, Kempermann G. Physical activity and the regulation of neurogenesis in the adult and aging brain. Neuromolecular Med. 2008;10:59–66. doi: 10.1007/s12017-008-8031-4. [DOI] [PubMed] [Google Scholar]
- 138.Li G, Pleasure SJ. Ongoing interplay between the neural network and neurogenesis in the adult hippocampus. Curr Opin Neurobiol. 2010;20:126–133. doi: 10.1016/j.conb.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma DK, Kim WR, Ming GL, Song H. Activity-dependent extrinsic regulation of adult olfactory bulb and hippocampal neurogenesis. Ann NY Acad Sci. 2009;1170:664–673. doi: 10.1111/j.1749-6632.2009.04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lugert S, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. In this study the authors identify radial and horizontal stem cells and find that different stem cell pools are recruited depending on the stimulus. Furthermore, they find that some stem cells cannot be activated. [DOI] [PubMed] [Google Scholar]
- 141.Suh H, et al. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bruel-Jungerman E, Veyrac A, Dufour F, Horwood J, Laroche S, et al. Inhibition of PI3K–Akt signalling blocks exercise-mediated enhancement of adult neurogenesis and synaptic plasticity in the dentate gyrus. PLoS ONE. 2009;4:e7901. doi: 10.1371/journal.pone.0007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Carlen M, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nature Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 145.Jacquet BV, et al. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development. 2009;136:4021–4031. doi: 10.1242/dev.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nomura T, Goritz C, Catchpole T, Henkemeyer M, Frisen J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell. 2010;7:730–743. doi: 10.1016/j.stem.2010.11.009. This study demonstrates an incredible phenotypic plasticity of adult neural stems and niche cell types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Milano J, et al. Modulation of notch processing by γ-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–358. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 149.Hicks C, et al. A secreted Delta1-Fc fusion protein functions both as an activator and inhibitor of Notch1 signaling. J Neurosci Res. 2002;68:655–667. doi: 10.1002/jnr.10263. [DOI] [PubMed] [Google Scholar]
- 150.Beckstead BL, et al. Methods to promote Notch signaling at the biomaterial interface and evaluation in a rafted organ culture model. J Biomed Mater Res A. 2009;91:436–446. doi: 10.1002/jbm.a.32214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Breunig JJ, Arellano JI, Macklis JD, Rakic P. Everything that glitters isn’t gold: a critical review of postnatal neural precursor analyses. Cell Stem Cell. 2007;1:612–627. doi: 10.1016/j.stem.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 152.Mason HA, Rakowiecki SM, Gridley T, Fishell G. Loss of notch activity in the developing central nervous system leads to increased cell death. Dev Neurosci. 2006;28:49–57. doi: 10.1159/000090752. [DOI] [PubMed] [Google Scholar]
- 153.Hori K, et al. A nonclassical bHLH Rbpj transcription factor complex is required for specification of GABAergic neurons independent of Notch signaling. Genes Dev. 2008;22:166–178. doi: 10.1101/gad.1628008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Saint Just Ribeiro M, Wallberg AE. Transcriptional mechanisms by the coregulator MAML1. Curr Protein Pept Sci. 2009;10:570–576. doi: 10.2174/138920309789630543. [DOI] [PubMed] [Google Scholar]
- 155.Zhang L, Gallagher PJ. Mind bomb 1 regulation of cFLIP interactions. Am J Physiol Cell Physiol. 2009;297:C1275–C1283. doi: 10.1152/ajpcell.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang XP, et al. Notch activation promotes cell proliferation and the formation of neural stem cell-like colonies in human glioma cells. Mol Cell Biochem. 2008;307:101–108. doi: 10.1007/s11010-007-9589-0. [DOI] [PubMed] [Google Scholar]
- 157.Purow BW, et al. Notch-1 regulates transcription of the epidermal growth factor receptor through p53. Carcinogenesis. 2008;29:918–925. doi: 10.1093/carcin/bgn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci Signal. 2009;2:re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- 159.Chen J, et al. Characterization of human LNX, a novel ligand of Numb protein X that is downregulated in human gliomas. Int J Biochem Cell Biol. 2005;37:2273–2283. doi: 10.1016/j.biocel.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 160.Nakamura H, et al. Identification of a human homolog of the Drosophila neuralized gene within the 10q25.1 malignant astrocytoma deletion region. Oncogene. 1998;16:1009–1019. doi: 10.1038/sj.onc.1201618. [DOI] [PubMed] [Google Scholar]
- 161.Rehman AO, Wang CY. Notch signaling in the regulation of tumor angiogenesis. Trends Cell Biol. 2006;16:293–300. doi: 10.1016/j.tcb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 162.Zheng X, et al. Interaction with factor inhibiting HIF-1 defines an additional mode of cross-coupling between the Notch and hypoxia signaling pathways. Proc Natl Acad Sci USA. 2008;105:3368–3373. doi: 10.1073/pnas.0711591105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stockhausen MT, Kristoffersen K, Poulsen HS. The functional role of Notch signaling in human gliomas. Neuro Oncol. 2010;12:199–211. doi: 10.1093/neuonc/nop022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wang J, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fan X, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:7787–7793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 166.Chen J, et al. Inhibition of Notch signaling blocks growth of glioblastoma cell lines and tumor neurospheres. Genes Cancer. 2010;1:822–835. doi: 10.1177/1947601910383564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus. 2008;18:793–806. doi: 10.1002/hipo.20439. [DOI] [PubMed] [Google Scholar]
- 168.Irvin DK, Zurcher SD, Nguyen T, Weinmaster G, Kornblum HI. Expression patterns of Notch1, Notch2, and Notch3 suggest multiple functional roles for the Notch-DSL signaling system during brain development. J Comp Neurol. 2001;436:167–181. [PubMed] [Google Scholar]
- 169.Murphy PA, et al. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc Natl Acad Sci USA. 2008;105:10901–10906. doi: 10.1073/pnas.0802743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Irvin DK, Nakano I, Paucar A, Kornblum HI. Patterns of Jagged1, Jagged2, Delta-like 1 and Delta-like 3 expression during late embryonic and postnatal brain development suggest multiple functional roles in progenitors and differentiated cells. J Neurosci Res. 2004;75:330–343. doi: 10.1002/jnr.10843. [DOI] [PubMed] [Google Scholar]
- 171.Yoon KJ, et al. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron. 2008;58:519–531. doi: 10.1016/j.neuron.2008.03.018. This fascinating study demonstrates that daughter progenitor cells can directly provide ligand stimulation to the Notch receptor present on neural stem cells. [DOI] [PubMed] [Google Scholar]
- 172.Shutter JR, et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- 173.Lavado A, Lagutin OV, Chow LM, Baker SJ, Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010;8:e1000460. doi: 10.1371/journal.pbio.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kurisu J, Fukuda T, Yokoyama S, Hirano T, Kengaku M. Polarized targeting of DNER into dendritic plasma membrane in hippocampal neurons depends on endocytosis. J Neurochem. 2010;113:1598–1610. doi: 10.1111/j.1471-4159.2010.06714.x. [DOI] [PubMed] [Google Scholar]