Vimentin in cancer and its potential as a molecular target for cancer therapy (original) (raw)
Abstract
Vimentin, a major constituent of the intermediate filament family of proteins, is ubiquitously expressed in normal mesenchymal cells and is known to maintain cellular integrity and provide resistance against stress. Vimentin is overexpressed in various epithelial cancers, including prostate cancer, gastrointestinal tumors, tumors of the central nervous system, breast cancer, malignant melanoma, and lung cancer. Vimentin’s overexpression in cancer correlates well with accelerated tumor growth, invasion, and poor prognosis; however, the role of vimentin in cancer progression remains obscure. In recent years, vimentin has been recognized as a marker for epithelial–mesenchymal transition (EMT). Although EMT is associated with several tumorigenic events, vimentin’s role in the underlying events mediating these processes remains unknown. By virtue of its overexpression in cancer and its association with tumor growth and metastasis, vimentin serves as an attractive potential target for cancer therapy; however, more research would be crucial to evaluate its specific role in cancer. Our recent discovery of a vimentin-binding mini-peptide has generated further impetus for vimentin-targeted tumor-specific therapy. Furthermore, research directed toward elucidating the role of vimentin in various signaling pathways would reveal new approaches for the development of therapeutic agents. This review summarizes the expression and functions of vimentin in various types of cancer and suggests some directions toward future cancer therapy utilizing vimentin as a potential molecular target.
Keywords: Vimentin, Targeted therapy, Cancer, Epithelial mesenchymal transition
Introduction
The microfilaments, intermediate filaments (IFs), and microtubules constitute the three major groups of non–muscle cell cytoskeletal proteins. The IF family of proteins is encoded by a large gene family of close to 70 members in humans, mice, and other mammals [1]. There are six major classes of IFs, and they are believed to be restricted to certain cell types [2, 3]. These classes are types I and II, comprising acidic and basic keratins (found mainly in the epithelial cells); type III, e.g., vimentin (in mesenchymal cells) and desmin (in muscle cells); type IV, e.g., neurofilaments (in neurons); type V, lamins (in cell nuclei); and type VI, nestin (in embryonic neurons). In addition, there are the IF-associated proteins which organize IFs in bundles and networks; these proteins include plectin, ankyrin, desmoplakin, filaggrin, and others like them [4]. The IF-associated proteins are known to coordinate the interactions between IFs and other cytoskeletal elements and organelles. Together, IFs and IF-associated proteins serve as organizers of cytoplasmic space within cells and of cells that make up the tissue architecture, thereby stabilizing and strengthening various organs [4].
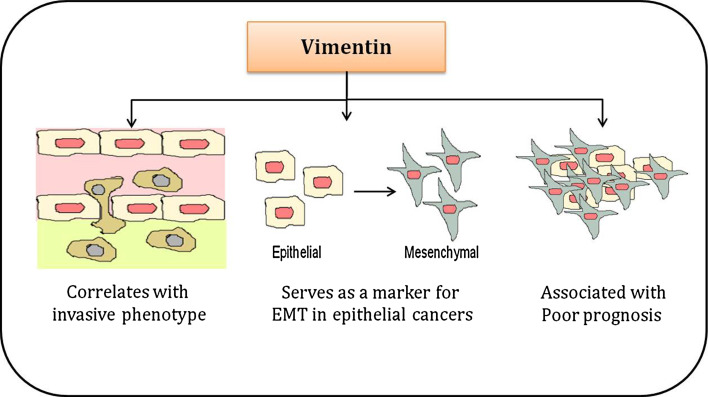
Vimentin, a 57-kDa protein, is one of the most widely expressed and highly conserved proteins of the type III IF protein family. During murine development, vimentin expression commences on embryonic day 8.5 (E8.5) and becomes predominant in the primitive streak stage [5], while in adult mice, vimentin expression was reported to be limited to connective tissue mesenchymal cells in the central nervous system and muscle [6]. Vimentin’s expression is also reported in a wide range of other cell types including pancreatic precursor cells, Sertoli cells, neuronal precursor cells, trophoblast giant cells, fibroblasts, endothelial cells lining blood vessels, renal tubular cells, macrophages, neutrophils, mesangial cells, leukocytes, and renal stromal cells [7–12]. Vimentin is now regarded as a canonical marker of epithelial–mesenchymal transition (EMT) (reviewed in [13]), a cellular reprogramming process in which epithelial cells acquire a mesenchymal phenotype that causes them to dramatically alter their shape and exhibit increased motility (Fig. 1). EMT is characterized by the expression of vimentin IFs in epithelial cells, which normally express only keratin IFs. Accordingly, during the reverse process of EMT, known as mesenchymal–epithelial transition, the cells start to exhibit an epithelial phenotype and show decreased vimentin expression with lower motility rates [14]. Increased vimentin expression has been reported in various tumor cell lines and tissues, including prostate cancer, breast cancer, endometrial cancer, tumors of the central nervous system, malignant melanoma, and gastrointestinal tract tumors that include pancreatic, colorectal, and hepatic cancers; further details are discussed in later sections of this review.
Fig. 1.
Vimentin’s role in cancer. Overexpression of vimentin is frequently associated with increased migratory/invasive capacity of cancer cells. Vimentin is used mainly as a marker for epithelial–mesenchymal transition in association with other known markers. The majority of cancers overexpress vimentin, and it is used as an indicator of poor prognosis
Although vimentin is believed to maintain the structural processes of the cell and mediate many other functions in vitro (reviewed in [15]), knockout mice lacking vimentin had virtually normal phenotypes and no apparent defects [16]. This observation suggests that vimentin is not critical for the survival of mice under normal physiological conditions. However, later studies showed that vimentin (−/−) mice exhibited impaired wound healing in both embryonic and adult stages because their fibroblasts were weak and severely disabled in their capacity to migrate [17, 18]. Furthermore, vimentin (−/−) mice died because of end-stage renal failure in a pathological situation involving the reduction of renal mass [19]; and showed decreased flow-induced dilation during arterial remodeling, suggesting that vimentin modulates arterial structural responses to altered blood flow [20]. Finally, vimentin-deficient lymphocytes showed a decreased capacity for homing to lymph nodes and spleen [21]. Interestingly, vimentin (−/−) embryonic stem cell-derived tumors showed a pattern of composition similar to that of teratocarcinomas induced by wild-type embryonic stem cells, suggesting that vimentin is not essential for efficient tumor growth and differentiation in vivo [22]; conversely, several in vitro results have suggested that vimentin may play a tumor promoter role in cancer [15]. The discrepancy between these results may be attributable to the fact that vimentin is differentially expressed in different cell types and might have tissue-specific functions; alternatively, there might be redundancy in the functions of the various IF proteins. Thus, the use of vimentin (−/−) mice can provide valuable information on the roles of vimentin and also contribute towards bridging the gap between in vitro results and clinical data. Furthermore, understanding the expression patterns and functions of this protein in different cancerous tissues may provide new diagnostic, prognostic, and therapeutic approaches to tackle this deadly disease.
Structure and regulation of vimentin
Vimentin is a polypeptide comprising 466 amino acids with a highly conserved α-helical "rod" domain that is flanked by non-α-helical N- and C-terminal end domains the "head" domain (77 residues) and "tail" domain (61 residues), respectively [23]. Together, these molecules associate in parallel and in register to form a coiled coil, which forms the basic structural building block for the entire IF family of proteins [24]. Vimentin is also known to form homopolymers and heteropolymers (i.e., it associates with other type III and with type IV IFs), a common feature among the members of the IF family; this feature is attributed to the presence of a coiled-coil α-helical domain that contributes to the formation of highly stable polymers, the stability of which in turn is controlled by the phosphorylation status of the integral proteins [25]. The head domains of a vimentin dimer are known to form a symmetric structure, and three sites have been identified which indicate that the distance between the head regions increases upon phosphorylation, a post-translational modification that regulates vimentin assembly/disassembly [26–28].
Vimentin serves as an excellent substrate for a number of kinases in vitro, and multiple phosphorylation sites on vimentin have been identified [29–33]. Phosphorylation of vimentin is associated with functional consequences, including the regulation of IF structure and of several signaling pathways [27]. For example, vimentin is phosphorylated by protein kinase A on the sites S38 and S72 that leads to decreased filament formation in vivo; however, site-directed mutagenesis of these sites showed no significant effect on filament assembly, indicating that phosphorylation primarily regulates disassembly of vimentin IFs [27]. p21-activated kinase was shown to phosphorylate vimentin at several sites and to be involved in the regulation of vimentin structural reorganization [34]. Aurora B kinase, a key regulator involved in the mitotic processes, was shown to phosphorylate vimentin and regulate vimentin filament segregation during the cytokinetic process [35]. Furthermore, protein phosphatase 2A was shown to associate with vimentin and prevent its phosphorylation, suggesting that protein phosphatase 2A plays a key role in the regulation of interphase IF dynamics [36].
Vimentin was also shown to be a target for other types of post-translational modifications, including citrullination, sumoylation, and _O_-GlcNAc modification. Citrullination is a post-translational modification in which the arginine residues are enzymatically deiminated to citrulline by peptidylarginine deiminase [37]. Vimentin was shown to be citrullinated in macrophages undergoing apoptosis, and antibodies to this citrullinated vimentin were shown to be generated in the event of improper disposal of apoptotic material [38]. _O_-GlcNAcylation of glial vimentin has been suggested to prevent hyperphosphorylation of vimentin, thus allowing vimentin to retain its ability to maintain a rigid structure and provide a scaffold for neuronal migration [39]. Vimentin was sumoylated at site 354 in the nucleus upon stimulation with protein inhibitor of activated STAT3 (PIAS3), and this modification was suggested to regulate the structure and motility of glioblastoma multiforme cells [40]. Taken together, these studies suggest an important role for post-translational modifications in regulating vimentin’s function. Furthermore, these modifications are cell- and tissue-specific. However, additional post-translational modifications of vimentin may be identified in the future, and thereby improve our understanding of this IF’s functions both in vitro and in vivo.
Vimentin is encoded by a single-copy gene located on chromosome 10p13. Initially, vimentin promoter was shown to be composed of three elements that regulate vimentin’s expression [41]. Later, several _cis_-elements and associated factors were identified within the human vimentin promoter, suggesting that the vimentin gene is subjected to complex control. These additional elements included a TATA box, eight putative GC boxes [41], the NF-κB-binding site [42], AP-1-binding sites [43], the PEA3-binding site [44], the Sp/XKLF-binding site [45], and the ZBP-89-binding site [45, 46]. Furthermore, vimentin expression was shown to be transactivated by β-catenin/TCF that binds to the putative site 468 bp upstream of the transcription initiation site of the vimentin promoter and thus increases tumor cell’s invasive potential [47]. It has been shown that NF-κB, a key protein regulating the immune and inflammatory process, also plays an important role in regulating the EMT process [48] and that its inhibition in mesenchymal cells can reverse the EMT process [49], suggesting that NF-κB is important in both activation and maintenance of EMT. Since vimentin is overexpressed during the EMT process and NF-κB is one of the transcription factors binding to the vimentin promoter, it is tempting to speculate that this overexpression of vimentin results from the activation of NF-κB in cancer cells. Also, the TGFβ1 response element was found within the activated protein complex-1 region of the vimentin promoter and was shown to be involved in the regulation of vimentin expression in myoblasts and myotubes [50].
The vimentin gene was also shown to be a target of epigenetic modifications. It is frequently methylated in advanced colorectal carcinomas and was suggested to serve as a diagnostic marker in the detection and monitoring of colorectal carcinoma using serum and stool samples [51]. Furthermore, upon treatment with 5-aza-deoxycytidine, a methylation inhibitor, vimentin mRNA expression was increased several-fold in different colon cancer cell lines [52], indicating that DNA demethylation is sufficient to activate vimentin transcription in colon cancer cells. It has also been shown that transcription factor ZBP-89 recruits histone deacetylase 1 (HDAC1) to the vimentin promoter, which leads to a decrease in vimentin expression [53]. This effect was abrogated in the presence of trichostatin A, a HDAC1 inhibitor, indicating that the vimentin gene is vulnerable to HDACs and contributes to one of the possible mechanisms of vimentin silencing. These studies demonstrate the multitude of endogenous transcriptional activators or repressors that can regulate vimentin expression in a given cell type and clearly show that these factors regulate the EMT-associated events that take place during cell migration. A rational drug design to inhibit vimentin expression will require deeper insights into vimentin gene regulation; therefore, further investigation may be promising in identifying newer drug targets.
Subcellular distribution and export of vimentin to the cell surface
Vimentin, being a cytoskeletal structural protein, is expected to be restricted to the cytosolic portion of cells; however, vimentin has been shown to be a nuclear and extracellular protein, as well. Traub et al. [54] predicted the possibility that IF proteins not only participate in the cytosolic functions but also mediate certain DNA- and RNA-mediated events in the nucleus; however, this latter function of IFs was questioned because they lack nuclear localization sequences, which would allow them to enter the nucleus. Later, a novel mechanism of vimentin entry into the nucleus by single-stranded DNA via a piggyback mechanism (that is, "piggybacking" on single-stranded DNA) was suggested [55]. At the nuclear envelope, the 6.6-kDa tail region of vimentin was shown to interact with lamin B; this interaction was suggested to provide a continuous set of contacts between the plasma membrane skeleton and the karyoskeleton of eukaryotic cells [56]. Recently, it was shown that vimentin localizes in the nucleus of neuroblastoma cells and regulates p21Waf1 expression; however, the principal events involved in this regulation remain unclear [57]. That report also shed light on vimentin’s ability to act as a regulator of transcription, suggesting that other proteins under the control of nuclear vimentin will be identified. At present, little is known about vimentin’s role in the nucleus, and much more investigation is required in this area.
Although vimentin is also an extracellular protein, it lacks a signal sequence for secretion; however, Perides et al. [58] suggested that the positively charged amino terminal of vimentin, rich in hydroxylated and hydrophobic amino acid residues, can assist in directing the protein toward membranes and binding it to the lipid bilayer. Although the mechanism of this transport remains unclear, there have been several other reports of vimentin transport to the cell surface. For instance, vimentin on the surface of cardiomyocytes and vascular smooth muscle cells, respectively, accounted for almost 8 and 14% of the total biotinylated cell surface proteins [59]. Moreover, extracellular staining showed a punctate appearance, confirming that only a fraction of the vimentin was expressed on the cell surface. However, our recent analysis of mouse organs confirmed that vimentin levels are nearly undetectable in most tissues [60]. In another study, it was shown that vimentin interacts with β3 integrins and plectin, which together regulate the organization and distribution of vimentin in several cell types [61]. The authors also suggested that a possible function of β3 integrin–mediated recruitment of vimentin to the cell surface is to regulate the strength of cell binding to the substrate. Vimentin was shown to promote cell motility in a protein kinase Cε (PKCε)-dependent manner and its phosphorylation by PKCε in integrin containing intracellular vesicles regulated its exit to the plasma membrane [62], suggesting a possible mechanism of its translocation to the plasma membrane.
Interestingly, neutrophils undergoing spontaneous apoptosis were shown to express vimentin and lamin B1 on the cell surface [63], which lends credence to the aforementioned possibility that apoptotic cells participate in the development of corresponding autoantibodies in the serum, a condition frequently associated with inflammatory disorders [64]. Mor-Vaknin et al. [65] have shown that vimentin undergoes phosphorylation in activated monocyte-derived macrophages, which leads to its expression on the cell surface. The authors reported an increased association of vimentin with the endoplasmic reticulum and the Golgi apparatus, suggesting that vimentin is secreted into the extracellular milieu. This secretion of vimentin was blocked in the presence of the Golgi blocker monensin and the glycosylation blocker tunicamycin, both of which disrupt early to intermediate events involved in the conventional secretory pathway that includes vesicular trafficking through the endoplasmic reticulum–Golgi compartments, leading to budding of secretory vesicles from the _trans_-Golgi network and exocytosis. Further, the anti-inflammatory cytokine interleukin-10 blocked the secretion of vimentin through protein kinase C inhibition, whereas the pro-inflammatory cytokine tumor necrosis factor-α triggered secretion of vimentin [65]. In another study, mass spectrometry analysis determined that vimentin secreted from the surface of _Mycobacterium tuberculosis_-infected monocytes acted as a putative ligand for NKp46 receptor on natural killer cells [66]. This binding contributes to the recognition of infected monocytes by natural killer cells. Furthermore, it was suggested that this binding was highly specific to the secreted vimentin and did not occur with intracellular vimentin. Neutralizing the surface vimentin resulted in only a 50% inhibition of the natural killer cytolytic activity, indicating that vimentin may act as a cross-linking protein between the natural ligand and NKp46 receptor [66]. Although NKp46 receptor expression is highly specific to natural killer cells, its expression has been reported in several other cell types, including cancer cells [67], suggesting that vimentin binding to the surface receptors of specific cells elicits specific functions, which are yet to be investigated.
Huet et al. [68] have reported SC5, a monoclonal antibody that specifically detects the secreted vimentin at the surface of viable Sézary cells or activated normal T lymphocytes. The authors suggested that anti-vimentin autoantibodies may be present in the serum of patients with Sézary syndrome; if so, these antibodies may serve as an excellent marker for diagnosis or prognosis. Recently, proteomic analysis of serum samples from patients with hepatocellular carcinoma (HCC) showed that vimentin is heavily secreted by small HCCs [69]. Although few of the cells did not express endogenous vimentin, all secreted the IF. However, neither the mechanism of secretion nor the function of secreted vimentin is known. It is possible that secreted vimentin neutralizes the activity of natural killer cells that harbor the NKp46 receptors [70, 71], thereby creating a tumor immune escape environment that enhances tumor progression. Taken together, these results imply that vimentin transport to the cell surface is tightly controlled during development and that several factors, including cytokines, can modulate vimentin’s extracellular transport. It is interesting to note that although most epithelial cancers express vimentin during EMT, the extracellular location of vimentin remains to be investigated; once determined, this location could serve as a possible target for therapeutic intervention.
Vimentin in cell signaling
Vimentin IFs are found in the cytoplasm of mesenchymal cells, where it maintains the cytoarchitecture and tissue integrity [5]. Vimentin is known to interact with a large number of proteins (Table 1 shows a few) and to participate in various cellular functions.
Table 1.
Molecules interacting with vimentin and their possible functions
| Interacting protein | Function | References |
|---|---|---|
| TSGA10 | Influences the function of antigen presenting cells (APC) | [72] |
| AptA (A. phagocytophilum toxin A) | Activates mammalian Erk1/2 mitogen-activated protein kinase | [73] |
| Scrib | Cell migration and aggregation | [74] |
| Rab9 | Intracellular lipid transport | [75] |
| Caveolin-1 | Anterior polarization of caveolin-1 in transmigrating cells | [76] |
| Plectin | Integration of cytoplasm | [77] |
| IFAP-300 | Lens cell differentiation | [78] |
| hsc70 | Regulation of heat-shock genes | [79] |
| Filamin | Formation of cell extensions | [80] |
| Alpha-crystallin | Enhanced vimentin aggregation | [81] |
| cGMP kinase | Vimentin phosphorylation | [82] |
| Yes kinase | Molecular support for Yes kinase | [83] |
| Desmoplakin | Links IFs with desmosomes | [84] |
| Periplakin | Mediates cellular localization | [85] |
| Actin-containing structures | Vimentin filament organization | [86] |
| hnRNP | Viral replication | [87] |
| Uridine phosphorylase | Function unknown | [88] |
| Formiminotransferase cyclodeaminase | Integrates Golgi complex with IF cytoskeleton | [89] |
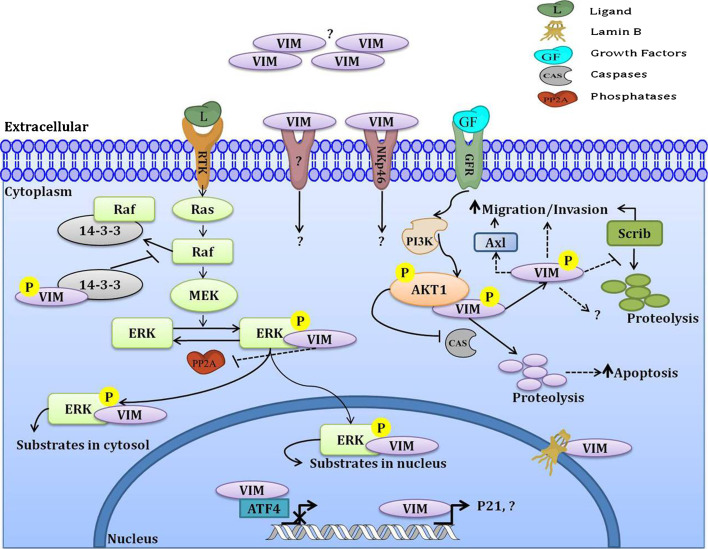
Vimentin is also involved in other processes that involve the formation of complexes with cell signaling molecules and other adaptor proteins (Fig. 2). For example, vimentin was shown to interact with phosphorylated Erk, a mitogen-activated protein kinase, and protect it from dephosphorylation [90]. Through biochemical and molecular modeling approaches, it was observed that phosphorylated Erk binding was localized to the second coiled-coil domain of vimentin and that this binding was calcium dependent. From these observations, it was suggested that vimentin stabilizes phosphorylated Erk by protecting it from dephosphorylation by calcium-dependent steric hindrance, thereby enabling long-distance transport of phosphorylated Erk within the cell [90]. AKT1 kinase was shown to bind to phosphorylated vimentin and protect it from caspase-induced proteolysis, thus leading to increased motility and invasiveness of soft-tissue sarcoma cells [91]. Phosphorylated vimentin was shown to interact with 14-3-3 proteins, which participate in a multitude of cell signaling and cell cycle processes. This interaction prevents the assembly of Raf-14-3-3 and similar complexes, suggesting that vimentin regulates 14-3-3 complexes and controls various intracellular signaling and cell cycle control pathways by modifying 14-3-3 availability [92]. Scrib, a protein involved in cell migration, is protected from proteasomal degradation upon interaction with vimentin, suggesting that vimentin upregulation during EMT leads to stabilization of Scrib, thereby promoting directed cell migration and increasing the invasive capacity of cells [74]. Vimentin acts as a brake on differentiation in immature osteoblasts by interacting with activating transcription factor 4 [93]. Vimentin was shown to function as a regulator of the receptor tyrosine kinase Axl and was shown to enhance cell migration by inducing Axl expression [94]. Further, Slug- and Ras-induced EMT changes were shown to be dependent on the upregulation of vimentin [94]. Vimentin has been reported to be a downstream target of PI3Kγ signaling, the activation of which results in increased vimentin phosphorylation that is required for efficient leukocyte transendothelial migration [95]. From these studies, it is evident that vimentin not only acts as a scaffolding protein but also mediates several signaling pathways and cellular processes. Also, it would be interesting to discover other functions of vimentin in the nucleus with possible roles in mediating cell cycle processes. Furthermore, extracellular vimentin may also mediate several signaling processes by binding to specific receptors that are yet to be investigated. Elucidating the molecular mechanisms involved in the EMT process may provide deeper insights into vimentin’s role in perturbing different signaling pathways by targeting specific proteins.
Fig. 2.
Vimentin’s role in cell signaling. In cytosol, vimentin was shown to interact with phosphorylated Erk and protect its phosphorylation by inhibiting phosphatases, which allows it to travel long distances within the cell. Also, vimentin’s interaction with 14-3-3 proteins prevents the formation of the 14-3-3-Raf complex and thereby regulates several cell processes by depleting the availability of free 14-3-3. In addition, AKT1 was shown to phosphorylate vimentin and protect it from caspase-induced proteolysis; therefore, the freely available vimentin participates in processes that lead to increased migratory and invasive capacity of the cells. In the nucleus, vimentin was shown to regulate p21 expression, while the complex formed between ATF4 and vimentin prevents active transcription by ATF4. Extracellularly, vimentin-specific receptors have not yet been identified; however, vimentin was shown to act as a specific ligand to one possible receptor, NKp46, on natural killer cells. Although reports indicate that vimentin is also secreted, neither the function of the secreted protein nor the mechanism of secretion is clear
Vimentin in cancer
Evaluating the expression pattern of vimentin in normal and cancerous tissues can be of great value in tumor diagnosis and prognosis. In this section, we summarize the observations of vimentin’s expression profile and functions in several cancers.
Prostate cancer
In prostate cancer, vimentin was detected mainly in poorly differentiated cancers and bone metastases and was nearly undetectable in well- or moderately differentiated tumors [96, 97]. Furthermore, vimentin expression was associated with motile prostate cancer cell lines [97], and its downregulation in PC-3 cells led to a significant decrease in tumor cell motility and invasive activity [96]. Similarly, vimentin was found to be overexpressed in prostate cancer cell line CL1, which was derived from an aggressive tumor; after experimental abrogation of vimentin expression, the cells showed a significant decrease in invasiveness [98]. Surprisingly, in the same study, there was no change in the invasiveness of LNCaP cells after vimentin expression was induced. The authors speculated that vimentin’s expression contributes to the development of an invasive phenotype in conjunction with other undiscovered proteins. Inhibition of vimentin significantly reduced the tumor growth of a highly tumorigenic and metastatic M12 subline (derived from prostate epithelial p69 cell line) [99]. Further, three-dimensional cultures showed that M12 cells containing vimentin did not form acini when compared to P69 cells, however, upon blocking vimentin, M12 cells showed the growth of acini-like structures, suggesting vimentin plays a key role in homeostasis of the normal acinus in prostate. In another study, vimentin was shown to be overexpressed in highly metastatic, human prostate epithelial cancer cell line PC-3M-1E8, and its role in modulating these cell’s invasiveness was attributed to its ability to regulate the E-cadherin/β-catenin complex via c-Src regulation [100]. Since PKCε is known to phosphorylate vimentin, which imparts increased cell motility [62], and recently PKCε ablation was shown to inhibit prostate cancer development and metastasis [101] suggests a possibility that PKCε could be a target to prevent vimentin induced tumorigenic events in prostate cancer cells. Results from several other studies also support the view that vimentin is overexpressed in prostate cancer and contributes to its invasive and metastatic potential [102, 103].
Gastrointestinal tract cancers
Gastrointestinal tract cancers include cancers of the stomach, small intestine, colon, rectum, liver, and pancreas. In gastric cancers, vimentin expression has been most often associated with the invasive phenotype of gastric carcinoma and was suggested to play an important role in the metastasis of gastric carcinomas and serve as a prognostic marker for gastric cancers [104, 105]. Analysis of esophageal squamous cell carcinoma samples from patients showed that vimentin expression was associated with a significantly higher incidence of lymph node metastasis than a lack of expression was [106]. In HCC, vimentin expression has been mainly associated with metastatic HCC [107] and in small HCC’s, vimentin was detectable in serum samples [69]. This finding seems to be inconsistent with another group’s observation that overexpression of vimentin in HCC cells decreased their proliferative and invasive capabilities in vitro [108]; however, the mechanisms underlying the latter effects remain unclear.
Vimentin gene methylation has gained much attention in colorectal cancer and is suggested to occur frequently in advanced colorectal cancers [51]. This phenomenon has the potential to be harnessed and used in high-sensitivity techniques for detecting advanced cancer by analysis of clinical samples, including serum and stool [52, 109, 110]. Similarly, vimentin gene methylation may serve as a biomarker of early or recurrent colorectal cancer. Vimentin overexpression in colorectal cancers is mainly associated with the stromal component and is restricted to stromal fibroblasts, endothelial cells lining the microvessels, and tumor-infiltrating lymphocytes [111, 112]. However, overexpression of vimentin has also been detected in colorectal cancer cells, with the level of expression correlating well with increases in migratory and invasive potentials, which decreased upon vimentin knockdown using vimentin-specific small interfering RNA (siRNA) [113]. It has also been suggested that 70% knockdown of vimentin is sufficient to impair the invasive and migratory capacities of carcinoma cells. Proteomic expression analysis of colorectal cancer samples from patients by two-dimensional gel electrophoresis showed a differential regulation of vimentin expression between the cancerous and surrounding normal tissues [114].
Vimentin expression in pancreatic duct-like cells starts from E12.5 in rodent embryos and reaches peak levels soon after birth, most likely due to simultaneous upregulation of TGF-β protein, which upregulates vimentin [50, 115]. Pancreatic cancers had a threefold higher vimentin expression than other tumors; however, a more specific antigenic isoform of vimentin was found at five- to tenfold higher levels, and autoantibodies generated against this isoform were suggested to have possible utility in the early diagnosis of pancreatic cancer [116]. Transient knockdown of methyl-CpG-binding domain protein 1, a suppressor of gene transcription, led to drastic downregulation of vimentin mRNA levels, suggesting that methyl-CpG-binding domain protein 1 mediates the expression of vimentin in pancreatic cancer [117]. Also, TGF-β was shown to be overexpressed in pancreatic cancers and to regulate the expression of vimentin in Panc-1 cells [118]. Further, vimentin was shown to increase the invasive potential of pancreatic cancer cells, which was reduced in the presence of vimentin-specific siRNA [119].
Breast cancer
Vimentin expression was shown to be elevated in several aggressive breast cancer cell lines [47], and this overexpression was very well correlated with increased migration and invasion of breast cancer cells [47, 120]. Furthermore, the noninvasive MCF-7 cells exhibited increased motility and invasiveness upon vimentin overexpression, and these characteristics were downregulated by vimentin antisense oligonucleotides in MDA-MB-231 cells, which constitutively express vimentin [120]. It is interesting to note that vimentin was overexpressed in MCF-10A cells at the edge of the wound in a wound healing assay and provided the cells with enhanced cell migratory potential, which was dependent on the level/presence of epidermal growth factor [121]. Histological examination of human breast carcinoma samples found that vimentin expression is predominantly found in high-grade ductal carcinomas with low estrogen receptor levels [122]. Several other studies have reported overexpression of vimentin in breast cancer cell lines and tissues (reviewed by Kokkinos et al. [123]). Recent studies have reported that vimentin plays a major role in the EMT process of breast cancers, and its knockdown resulted in a decrease in genes linked with breast cancer invasion and the basal-like phenotype, including Axl, ITGB4, and PLAU, with a subsequent increase in the genes abundant in normal mammary epithelium, including RAB25 and EHF [94]. Furthermore, vimentin was shown to play a key role in the regulation of Axl and in the Slug- and Ras-induced migration of breast cancer cells [94].
Malignant melanoma
Recently, utilizing proteomic analysis on a wide array of melanoma samples, Li et al. [124] showed that overexpression of vimentin in primary tumors not only serves as a diagnostic marker but also acts as a predictor of hematogenous metastasis. The authors also suggested the possibility of using vimentin expression as a predictor of clinical outcome, thereby providing individual treatment strategies for melanoma patients. Several other reports have also addressed the relationship between the overexpression of vimentin and its association with metastases and increased invasive potential of melanoma cells [125–128].
Central nervous system tumors
In the normal human brain, moderate to strong vimentin expression has been found mainly in ependymal cells, the choroid plexus, meningeal cells, and some subpial cells, while weak expression has been found in endothelial cells [129]. Vimentin expression in glioma cells was shown to be dependent on the cellular density and chemotherapy/radiation and was predominantly expressed in low-density cell cultures [130]. Further, in glioblastoma cells, galectin-1 was shown to regulate protein kinase C epsilon- and vimentin-mediated integrin trafficking that is essential in promoting glioma malignancy [131]. In meningiomas, expression of a specific phosphorylated form of vimentin was associated with noninfiltrative tumors [132], and this form of vimentin was suggested to determine the migratory potential of the meningiomas and to differentiate between types of meningiomas. Vimentin expression was also detected in schwannomas and neurofibromas [133].
Lung cancer
Vimentin is expressed in the bronchial epithelium of the fetus and decreases with increasing age, whereas in the adult bronchial epithelium, its expression is restricted mainly to the basal and the columnar cells [134]. In lung cancers, vimentin has been detected in moderately and well-differentiated adenocarcinomas and in giant cell carcinomas [135]. In non-small-cell lung cancer, vimentin overexpression was found to be an independent predictor of poor survival in patients with resected non-small-cell lung cancer [136]. In another study, it was observed that glycosylated vimentin was downregulated in human lung adenocarcinomas; glycosylated vimentin thus was suggested to represent a new functional biomarker for the diagnosis and treatment response of lung cancers [137]. Furthermore, vimentin was shown to be differentially expressed in lung cancer cell lines, and poly(ADP-ribose) polymerase 1 was shown to bind the vimentin promoter region and induce its expression in lung cancer cells [138].
Other cancers
Vimentin is also overexpressed in other types of cancers, including cervical cancer [139], clear-cell renal cell carcinoma [140], certain types of lymphomas [141], papillary thyroid carcinoma [142], and endometrial carcinomas [143].
Taken together, the evidence indicates that vimentin not only serves as a potential diagnostic tool in the detection of cancer but also plays a key role in the development and progression of cancer. Although its expression is best characterized in the EMT process, it is equally possible that tumorigenic events, including tumor cell migration and invasion, are a consequence of vimentin overexpression in cancer cells, as most studies have indicated a positive association between the invasive phenotype and vimentin overexpression and have shown a decrease in these characteristics upon knockdown of vimentin in vitro. Furthermore, vimentin’s overexpression during metastasis [100, 106, 107, 124, 127] suggests it has a role as a metastasis promoter. However, it is unclear whether tumorigenic events are a result of fine tuning occurring in the cancer cells, wherein vimentin might be acting as a scaffolding protein during signal transduction and promoting tumorigenic events in association with other tumor-promoting oncogenes.
Drug targets of vimentin
Vimentin overexpression in different cancer cell lines and tissues and its association with increased cancer cell growth, invasion, and migration suggests that vimentin is in fact participating in the promotion of these tumorigenic events and may serve as an excellent target for cancer therapy. Also, because of its overexpression in different cancers, vimentin can be used as a target to deliver therapeutic agents to the tumor site. Although most reports in the literature have shown that certain agents decrease vimentin levels, this effect is always considered a secondary and indirect effect on vimentin expression resulting from the intended effect, reduction in EMT. Very few reports have shown direct inhibition of vimentin expression and its consequences.
Withaferin-A, a bioactive compound isolated from Withania somnifera, was shown to bind tetrameric vimentin at a unique binding pocket site between the pair of head-to-tail α-helical dimers [144]. Recently, it was shown that withaferin-A-induced apoptosis is significantly more pronounced in vimentin-expressing cells than in other cells and that vimentin knockdown abrogated the apoptotic effect [145]. The authors suggested a mechanism involving the degradation of vimentin upon binding to withaferin-A, which then results in increased apoptosis of cancer cells. Silibinin, the major active constituent of silymarin isolated from milk thistle (Silybum marianum) and a flavonolignan, which has shown strong chemopreventive and anticancer activities, was recently shown to inhibit the invasion, motility, and migration of ARCaPM prostate cancer cells via downregulation of vimentin and matrix metalloproteinase-2 [146]. These results are similar to those reported by Singh et al. [147], which revealed antimetastatic and anti-invasive activities of silibinin in TRAMP mice via the agent’s ability to induce cells to re-express E-cadherin, with a concomitant strong decrease in the level of vimentin, thereby inhibiting EMT. Salinomycin, an antibiotic, resulted in a drastic reduction in the level of vimentin and a concomitant increase in E-cadherin levels in CD133+ colorectal cancer cells [148]. Although salinomycin exhibited anticancer properties by downregulating cell growth, migration, and invasion, the vimentin-related mechanism underlying these events remains elusive. Zhang et al. [149] have reported that vimentin is the target of microRNA-17-3p, which functions as a tumor suppressor in prostate cancer. Re-expression of this microRNA results in decreased vimentin expression along with altered cellular functions, suggesting its importance as an alternative target for gene therapy. Recently, microRNAs miR-200 and miR-30 family member were shown to significantly reduce vimentin expression in anaplastic thyroid carcinomas [150]. Pools of microRNA induced mesenchymal-epithelial transition in anaplastic thyroid carcinomas, which was accompanied by an increase in E-cadherin and a decrease in vimentin protein and mRNA levels. However, it is still unclear how these microRNAs inhibit the expression of vimentin and induce an epithelial phenotype that has decreased invasive potential in cancer cells.
Our lab recently identified a novel linear peptide called comprehensive carcinoma homing peptide (CHP, sequence VNTANST), which when administered in the form of CHP-IL-12 fusion gene construct (that is, with the DNA fragment encoding the VNTANST sequence inserted directly before the stop codon of the p40 subunit in an interleukin-12 plasmid DNA), resulted in an increased accumulation of interleukin-12 in the tumor microenvironment and showed promising results in different cancer models [60]. Those results provide evidence that CHP possesses excellent targeting and homing properties, which make it a potentially useful tool in cancer treatment. From mass spectrometry analysis, it was observed that one of the binding partners for CHP is vimentin. These observations indicate a distinct possibility that vimentin is expressed on the surface of cancer cells and is internalized upon contact with specific ligands. Also, we found that normal lung tissue expressed vimentin but CHP binding did not occur; while in tumors, CHP binding was decreased when vimentin antibodies were supplemented, suggesting that CHP specifically binds to the surface-bound vimentin on cancer cells. It is equally possible that upon internalization, CHP interacts with intracellular vimentin and interferes with various signaling pathways, thereby affecting different cellular functions; however, this possibility is yet to be tested. That report thus identified vimentin as an attractive target to direct therapeutic agents to tumor sites.
In recent years, aptamers have gained a lot of attention in the field of cancer therapeutics [151]. Identification of vimentin-specific aptamers would be of great value, as aptamers are known to exhibit high binding specificity and can be chemically modified to improve their therapeutic properties. In addition, vimentin-specific antibodies can be used to deliver anticancer agents to tumor sites. Therefore, it is of utmost importance to check the profile of vimentin expression in different cancer types with respect to its localization and isoforms, which may then guide the development of novel treatment options.
The reader should note that the majority of the targets discussed here have been tested in vivo and that the therapies did not exhibit any significant toxicity, indicating that these modalities are specific for the vimentin expressed in cancer cells, not that in normal mesenchymal cells. A possible explanation for such an outcome could be the lower expression levels of vimentin in normal cells than in EMT-transformed cells, differences in subcellular localization patterns of vimentin in these cells, or the expression of vimentin variants in cancer cells. Thus, by utilizing vimentin as an anticancer target, there will be a plethora of therapeutically pertinent opportunities to overcome the current predicaments in cancer therapy.
Concluding remarks
From the above studies, it is evident that vimentin is a multifunctional protein, and its ability to interact with a large number of proteins makes it a potential regulator of several physiological functions; however, the true function of vimentin, apart from maintaining the structural integrity of cells, is yet to be unraveled. In the majority of cancers, vimentin is overexpressed, and in some tumors, vimentin was found to be expressed on tumor cell membranes. Several studies have linked the overexpression of vimentin to the aggressiveness of cancer. Importantly, vimentin expression has been strongly associated with the metastatic phenotype and poor prognosis. Interestingly, cancer-related studies have mostly addressed the functions of intracellular vimentin, while the role of extracellular/cell surface-associated vimentin remains unclear. Understanding the mechanism of vimentin gene regulation and the role of extracellular/cell surface vimentin may contribute to a better understanding of cancer and to better means of controlling the invasiveness of cancer cells. Although all findings have indicated that vimentin may someday serve as a clinically relevant biomarker for different malignancies, more research will be necessary to assess the major functions of vimentin in tumorigenesis. Moreover, by use of vimentin knockout mice, valuable information on the role of vimentin in cancer can be established, which can also be used to bridge the gap between in vitro results and clinical data. In view of the available data, vimentin expression in cancer is likely to become an attractive and promising therapeutic target and has great potential for providing novel clinical prognostic and diagnostic tools. Further, the use of vimentin-specific chemical inhibitors as well as novel therapeutic agents, including antibodies, peptides, aptamers, and siRNA directed against vimentin in combination with other anticancer agents, must be encouraged.
Acknowledgments
We apologize to the authors of many other relevant studies that are not cited because of space limitations. Work in the authors’ laboratory was supported by Grants from the National Institutes of Health to Dr. Shulin Li (NIH RO1CA120895).
Conflict of interest
We declare that none of the authors has a financial interest related to this work.
References
- 1.Hesse M, Magin TM, Weber K. Genes for intermediate filament proteins and the draft sequence of the human genome: novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J Cell Sci. 2001;114:2569–2575. doi: 10.1242/jcs.114.14.2569. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 3.Niki T, Pekny M, Hellemans K, Bleser PD, Berg KV, Vaeyens F, Quartier E, et al. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999;29:520–527. doi: 10.1002/hep.510290232. [DOI] [PubMed] [Google Scholar]
- 4.Green KJ, Bohringer M, Gocken T, Jones JC. Intermediate filament associated proteins. Adv Protein Chem. 2005;70:143–202. doi: 10.1016/S0065-3233(05)70006-1. [DOI] [PubMed] [Google Scholar]
- 5.Franke WW, Grund C, Kuhn C, Jackson BW, Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. III. Primary mesenchymal cells and the first appearance of vimentin filaments. Differentiation. 1982;23:43–59. doi: 10.1111/j.1432-0436.1982.tb01266.x. [DOI] [PubMed] [Google Scholar]
- 6.Larsson A, Wilhelmsson U, Pekna M, Pekny M. Increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of old GFAP(−/−)Vim(−/−) mice. Neurochem Res. 2004;29:2069–2073. doi: 10.1007/s11064-004-6880-2. [DOI] [PubMed] [Google Scholar]
- 7.Cochard P, Paulin D. Initial expression of neurofilaments and vimentin in the central and peripheral nervous system of the mouse embryo in vivo. J Neurosci. 1984;4:2080–2094. doi: 10.1523/JNEUROSCI.04-08-02080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Souza PC, Katz SG. Coexpression of cytokeratin and vimentin in mice trophoblastic giant cells. Tissue Cell. 2001;33:40–45. doi: 10.1054/tice.2000.0148. [DOI] [PubMed] [Google Scholar]
- 9.Ko SH, Suh SH, Kim BJ, Ahn YB, Song KH, Yoo SJ, Son HS, et al. Expression of the intermediate filament vimentin in proliferating duct cells as a marker of pancreatic precursor cells. Pancreas. 2004;28:121–128. doi: 10.1097/00006676-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Mahrle G, Bolling R, Osborn M, Weber K. Intermediate filaments of the vimentin and prekeratin type in human epidermis. J Invest Dermatol. 1983;81:46–48. doi: 10.1111/1523-1747.ep12538403. [DOI] [PubMed] [Google Scholar]
- 11.Carter V, Shenton BK, Jaques B, Turner D, Talbot D, Gupta A, Chapman CE, et al. Vimentin antibodies: a non-HLA antibody as a potential risk factor in renal transplantation. Transplant Proc. 2005;37:654–657. doi: 10.1016/j.transproceed.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 12.Evans RM. Vimentin: the conundrum of the intermediate filament gene family. Bioessays. 1998;20:79–86. doi: 10.1002/(SICI)1521-1878(199801)20:1<79::AID-BIES11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 14.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 15.Ivaska J, Pallari HM, Nevo J, Eriksson JE. Novel functions of vimentin in cell adhesion, migration, and signaling. Exp Cell Res. 2007;313:2050–2062. doi: 10.1016/j.yexcr.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 16.Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 17.Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci. 2000;113(Pt 13):2455–2462. doi: 10.1242/jcs.113.13.2455. [DOI] [PubMed] [Google Scholar]
- 18.Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, et al. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci. 1998;111(Pt 13):1897–1907. doi: 10.1242/jcs.111.13.1897. [DOI] [PubMed] [Google Scholar]
- 19.Terzi F, Henrion D, Colucci-Guyon E, Federici P, Babinet C, Levy BI, Briand P, et al. Reduction of renal mass is lethal in mice lacking vimentin. Role of endothelin-nitric oxide imbalance. J Clin Invest. 1997;100:1520–1528. doi: 10.1172/JCI119675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffers PM, Henrion D, Boulanger CM, Colucci-Guyon E, Langa-Vuves F, van Essen H, Fazzi GE, et al. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:611–616. doi: 10.1161/01.ATV.20.3.611. [DOI] [PubMed] [Google Scholar]
- 21.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 22.Langa F, Kress C, Colucci-Guyon E, Khun H, Vandormael-Pournin S, Huerre M, Babinet C. Teratocarcinomas induced by embryonic stem (ES) cells lacking vimentin: an approach to study the role of vimentin in tumorigenesis. J Cell Sci. 2000;113(Pt 19):3463–3472. doi: 10.1242/jcs.113.19.3463. [DOI] [PubMed] [Google Scholar]
- 23.Goldie KN, Wedig T, Mitra AK, Aebi U, Herrmann H, Hoenger A. Dissecting the 3D structure of vimentin intermediate filaments by cryo-electron tomography. J Struct Biol. 2007;158:378–385. doi: 10.1016/j.jsb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs E, Hanukoglu I. Unraveling the structure of the intermediate filaments. Cell. 1983;34:332–334. doi: 10.1016/0092-8674(83)90367-7. [DOI] [PubMed] [Google Scholar]
- 25.Ku NO, Liao J, Chou CF, Omary MB. Implications of intermediate filament protein phosphorylation. Cancer Metastasis Rev. 1996;15:429–444. doi: 10.1007/BF00054011. [DOI] [PubMed] [Google Scholar]
- 26.Aziz A, Hess JF, Budamagunta MS, Voss JC, Fitzgerald PG Site-directed spin labeling and electron paramagnetic resonance determination of vimentin head domain structure. J Biol Chem 285:15278–15285 [DOI] [PMC free article] [PubMed]
- 27.Eriksson JE, He T, Trejo-Skalli AV, Harmala-Brasken AS, Hellman J, Chou YH, Goldman RD. Specific in vivo phosphorylation sites determine the assembly dynamics of vimentin intermediate filaments. J Cell Sci. 2004;117:919–932. doi: 10.1242/jcs.00906. [DOI] [PubMed] [Google Scholar]
- 28.Evans RM. Phosphorylation of vimentin in mitotically selected cells. In vitro cyclic AMP-independent kinase and calcium-stimulated phosphatase activities. J Cell Biol. 1989;108:67–78. doi: 10.1083/jcb.108.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang TJ, Lee TT, Lee WC, Lai YK, Yu JS, Yang SD. Autophosphorylation-dependent protein kinase phosphorylates Ser25, Ser38, Ser65, Ser71, and Ser411 in vimentin and thereby inhibits cytoskeletal intermediate filament assembly. J Protein Chem. 1994;13:517–525. doi: 10.1007/BF01901533. [DOI] [PubMed] [Google Scholar]
- 30.Ando S, Tanabe K, Gonda Y, Sato C, Inagaki M. Domain- and sequence-specific phosphorylation of vimentin induces disassembly of the filament structure. Biochemistry. 1989;28:2974–2979. doi: 10.1021/bi00433a035. [DOI] [PubMed] [Google Scholar]
- 31.Ando S, Tokui T, Yamauchi T, Sugiura H, Tanabe K, Inagaki M. Evidence that Ser-82 is a unique phosphorylation site on vimentin for Ca2(+)-calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1991;175:955–962. doi: 10.1016/0006-291X(91)91658-Y. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura Y, Hashimoto R, Amano M, Nagata K, Matsumoto N, Goto H, Fukusho E, et al. Localized phosphorylation of vimentin by rho-kinase in neuroblastoma N2a cells. Genes Cells. 2000;5:823–837. doi: 10.1046/j.1365-2443.2000.00372.x. [DOI] [PubMed] [Google Scholar]
- 33.Cheng TJ, Tseng YF, Chang WM, Chang MD, Lai YK. Retaining of the assembly capability of vimentin phosphorylated by mitogen-activated protein kinase-activated protein kinase-2. J Cell Biochem. 2003;89:589–602. doi: 10.1002/jcb.10511. [DOI] [PubMed] [Google Scholar]
- 34.Goto H, Tanabe K, Manser E, Lim L, Yasui Y, Inagaki M. Phosphorylation and reorganization of vimentin by p21-activated kinase (PAK) Genes Cells. 2002;7:91–97. doi: 10.1046/j.1356-9597.2001.00504.x. [DOI] [PubMed] [Google Scholar]
- 35.Goto H, Yasui Y, Kawajiri A, Nigg EA, Terada Y, Tatsuka M, Nagata K, et al. Aurora-B regulates the cleavage furrow-specific vimentin phosphorylation in the cytokinetic process. J Biol Chem. 2003;278:8526–8530. doi: 10.1074/jbc.M210892200. [DOI] [PubMed] [Google Scholar]
- 36.Turowski P, Myles T, Hemmings BA, Fernandez A, Lamb NJ. Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol Biol Cell. 1999;10:1997–2015. doi: 10.1091/mbc.10.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Venrooij WJ, Pruijn GJ. Citrullination: a small change for a protein with great consequences for rheumatoid arthritis. Arthritis Res. 2000;2:249–251. doi: 10.1186/ar95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vossenaar ER, Radstake TR, van der Heijden A, van Mansum MA, Dieteren C, de Rooij DJ, Barrera P, et al. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63:373–381. doi: 10.1136/ard.2003.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farach AM, Galileo DS. O-GlcNAc modification of radial glial vimentin filaments in the developing chick brain. Brain Cell Biol. 2008;36:191–202. doi: 10.1007/s11068-008-9036-5. [DOI] [PubMed] [Google Scholar]
- 40.Liming Wang JZ, Banerjee Sipra, Barnes Laura, Sajja Venkateswara, Liu Yiding, Guo Baochuan, Yuping Du, Agarwal MukeshK, Wald DavidN, Wang Qin, Yang Jinbo. Sumoylation of vimentin354 is associated with PIAS3 inhibition of Glioma cell migration. Oncotarget. 2010;1:620–627. doi: 10.18632/oncotarget.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rittling SR, Baserga R. Functional analysis and growth factor regulation of the human vimentin promoter. Mol Cell Biol. 1987;7:3908–3915. doi: 10.1128/mcb.7.11.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lilienbaum A, Paulin D. Activation of the human vimentin gene by the Tax human T-cell leukemia virus. I. Mechanisms of regulation by the NF-kappa B transcription factor. J Biol Chem. 1993;268:2180–2188. [PubMed] [Google Scholar]
- 43.Rittling SR, Coutinho L, Amram T, Kolbe M. AP-1/jun binding sites mediate serum inducibility of the human vimentin promoter. Nucleic Acids Res. 1989;17:1619–1633. doi: 10.1093/nar/17.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen JH, Vercamer C, Li Z, Paulin D, Vandenbunder B, Stehelin D. PEA3 transactivates vimentin promoter in mammary epithelial and tumor cells. Oncogene. 1996;13:1667–1675. [PubMed] [Google Scholar]
- 45.Zhang X, Diab IH, Zehner ZE. ZBP-89 represses vimentin gene transcription by interacting with the transcriptional activator, Sp1. Nucleic Acids Res. 2003;31:2900–2914. doi: 10.1093/nar/gkg380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wieczorek E, Lin Z, Perkins EB, Law DJ, Merchant JL, Zehner ZE. The zinc finger repressor, ZBP-89, binds to the silencer element of the human vimentin gene and complexes with the transcriptional activator, Sp1. J Biol Chem. 2000;275:12879–12888. doi: 10.1074/jbc.275.17.12879. [DOI] [PubMed] [Google Scholar]
- 47.Gilles C, Polette M, Mestdagt M, Nawrocki-Raby B, Ruggeri P, Birembaut P, Foidart JM. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res. 2003;63:2658–2664. [PubMed] [Google Scholar]
- 48.Min C, Eddy SF, Sherr DH, Sonenshein GE. NF-kappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–744. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 49.Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, Kraut N, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004;114:569–581. doi: 10.1172/JCI21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Y, Zhang X, Salmon M, Lin X, Zehner ZE. TGFbeta1 regulation of vimentin gene expression during differentiation of the C2C12 skeletal myogenic cell line requires Smads, AP-1 and Sp1 family members. Biochim Biophys Acta. 2007;1773:427–439. doi: 10.1016/j.bbamcr.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shirahata A, Sakata M, Sakuraba K, Goto T, Mizukami H, Saito M, Ishibashi K, et al. Vimentin methylation as a marker for advanced colorectal carcinoma. Anticancer Res. 2009;29:279–281. [PubMed] [Google Scholar]
- 52.Zou H, Harrington JJ, Shire AM, Rego RL, Wang L, Campbell ME, Oberg AL, et al. Highly methylated genes in colorectal neoplasia: implications for screening. Cancer Epidemiol Biomarkers Prev. 2007;16:2686–2696. doi: 10.1158/1055-9965.EPI-07-0518. [DOI] [PubMed] [Google Scholar]
- 53.Wu Y, Zhang X, Salmon M, Zehner ZE. The zinc finger repressor, ZBP-89, recruits histone deacetylase 1 to repress vimentin gene expression. Genes Cells. 2007;12:905–918. doi: 10.1111/j.1365-2443.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 54.Traub P, Shoeman RL. Intermediate filament proteins: cytoskeletal elements with gene-regulatory function? Int Rev Cytol. 1994;154:1–103. doi: 10.1016/S0074-7696(08)62198-1. [DOI] [PubMed] [Google Scholar]
- 55.Hartig R, Shoeman RL, Janetzko A, Tolstonog G, Traub P. DNA-mediated transport of the intermediate filament protein vimentin into the nucleus of cultured cells. J Cell Sci. 1998;111(Pt 24):3573–3584. doi: 10.1242/jcs.111.24.3573. [DOI] [PubMed] [Google Scholar]
- 56.Georgatos SD, Blobel G. Lamin B constitutes an intermediate filament attachment site at the nuclear envelope. J Cell Biol. 1987;105:117–125. doi: 10.1083/jcb.105.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mergui X, Puiffe ML, Valteau-Couanet D, Lipinski M, Benard J, Amor-Gueret M p21Waf1 expression is regulated by nuclear intermediate filament vimentin in neuroblastoma. BMC Cancer 10:473 [DOI] [PMC free article] [PubMed]
- 58.Perides G, Harter C, Traub P. Electrostatic and hydrophobic interactions of the intermediate filament protein vimentin and its amino terminus with lipid bilayers. J Biol Chem. 1987;262:13742–13749. [PubMed] [Google Scholar]
- 59.Ise H, Kobayashi S, Goto M, Sato T, Kawakubo M, Takahashi M, Ikeda U, et al. Vimentin and desmin possess GlcNAc-binding lectin-like properties on cell surfaces. Glycobiology. 2010;20:843–864. doi: 10.1093/glycob/cwq039. [DOI] [PubMed] [Google Scholar]
- 60.Cutrera J, Dibra D, Xia X, Hasan A, Reed S, Li S Discovery of a Linear Peptide for Improving Tumor Targeting of Gene Products and Treatment of Distal Tumors by IL-12 Gene Therapy. Mol Ther [DOI] [PMC free article] [PubMed]
- 61.Bhattacharya R, Gonzalez AM, Debiase PJ, Trejo HE, Goldman RD, Flitney FW, Jones JC. Recruitment of vimentin to the cell surface by beta3 integrin and plectin mediates adhesion strength. J Cell Sci. 2009;122:1390–1400. doi: 10.1242/jcs.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ivaska J, Vuoriluoto K, Huovinen T, Izawa I, Inagaki M, Parker PJ. PKCepsilon-mediated phosphorylation of vimentin controls integrin recycling and motility. EMBO J. 2005;24:3834–3845. doi: 10.1038/sj.emboj.7600847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moisan E, Girard D. Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis. J Leukoc Biol. 2006;79:489–498. doi: 10.1189/jlb.0405190. [DOI] [PubMed] [Google Scholar]
- 64.Kurki P, Virtanen I. The detection of human antibodies against cytoskeletal components. J Immunol Methods. 1984;67:209–223. doi: 10.1016/0022-1759(84)90462-9. [DOI] [PubMed] [Google Scholar]
- 65.Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 66.Garg A, Barnes PF, Porgador A, Roy S, Wu S, Nanda JS, Griffith DE, et al. Vimentin expressed on Mycobacterium tuberculosis-infected human monocytes is involved in binding to the NKp46 receptor. J Immunol. 2006;177:6192–6198. doi: 10.4049/jimmunol.177.9.6192. [DOI] [PubMed] [Google Scholar]
- 67.Srivastava BI, Srivastava MD. Expression of natural cytotoxicity receptors NKp30, NKp44, and NKp46 mRNAs and proteins by human hematopoietic and non-hematopoietic cells. Leuk Res. 2006;30:37–46. doi: 10.1016/j.leukres.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 68.Huet D, Bagot M, Loyaux D, Capdevielle J, Conraux L, Ferrara P, Bensussan A, et al. SC5 mAb represents a unique tool for the detection of extracellular vimentin as a specific marker of Sezary cells. J Immunol. 2006;176:652–659. doi: 10.4049/jimmunol.176.1.652. [DOI] [PubMed] [Google Scholar]
- 69.Sun S, Poon RT, Lee NP, Yeung C, Chan KL, Ng IO, Day PJ, et al. Proteomics of hepatocellular carcinoma: serum vimentin as a surrogate marker for small tumors (< or = 2 cm) J Proteome Res. 2010;9:1923–1930. doi: 10.1021/pr901085z. [DOI] [PubMed] [Google Scholar]
- 70.Uchida A, Colot M, Micksche M. Suppression of natural killer cell activity by adherent effusion cells of cancer patients. Suppression of motility, binding capacity and lethal hit of NK cells. Br J Cancer. 1984;49:17–23. doi: 10.1038/bjc.1984.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, Reyburn HT. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70:481–489. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roghanian A, Jones DC, Pattisapu JV, Wolfe J, Young NT, Behnam B. Filament-associated TSGA10 protein is expressed in professional antigen presenting cells and interacts with vimentin. Cell Immunol. 2010;265:120–126. doi: 10.1016/j.cellimm.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 73.Sukumaran B, Mastronunzio JE, Narasimhan S, Fankhauser S, Uchil PD, Levy R, Graham M, et al. Anaplasma phagocytophilum AptA modulates Erk1/2 signalling. Cell Microbiol. 2011;13:47–61. doi: 10.1111/j.1462-5822.2010.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phua DC, Humbert PO, Hunziker W. Vimentin regulates scribble activity by protecting it from proteasomal degradation. Mol Biol Cell. 2009;20:2841–2855. doi: 10.1091/mbc.E08-02-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walter M, Chen FW, Tamari F, Wang R, Ioannou YA. Endosomal lipid accumulation in NPC1 leads to inhibition of PKC, hypophosphorylation of vimentin and Rab9 entrapment. Biol Cell. 2009;101:141–152. doi: 10.1042/BC20070171. [DOI] [PubMed] [Google Scholar]
- 76.Santilman V, Baran J, Anand-Apte B, Evans RM, Parat MO. Caveolin-1 polarization in transmigrating endothelial cells requires binding to intermediate filaments. Angiogenesis. 2007;10:297–305. doi: 10.1007/s10456-007-9083-z. [DOI] [PubMed] [Google Scholar]
- 77.Svitkina TM, Verkhovsky AB, Borisy GG. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J Cell Biol. 1996;135:991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lieska N, Shao D, Kriho V, Yang HY. Expression and distribution of cytoskeletal IFAP-300 kD as an index of lens cell differentiation. Curr Eye Res. 1991;10:1165–1174. doi: 10.3109/02713689109024134. [DOI] [PubMed] [Google Scholar]
- 79.Cheng TJ, Lai YK. Transient increase in vimentin phosphorylation and vimentin-HSC70 association in 9L rat brain tumor cells experiencing heat-shock. J Cell Biochem. 1994;54:100–109. doi: 10.1002/jcb.240540111. [DOI] [PubMed] [Google Scholar]
- 80.Brown KD, Binder LI. Identification of the intermediate filament-associated protein gyronemin as filamin. Implications for a novel mechanism of cytoskeletal interaction. J Cell Sci. 1992;102(Pt 1):19–30. doi: 10.1242/jcs.102.1.19. [DOI] [PubMed] [Google Scholar]
- 81.Song S, Hanson MJ, Liu BF, Chylack LT, Liang JJ. Protein–protein interactions between lens vimentin and alphaB-crystallin using FRET acceptor photobleaching. Mol Vis. 2008;14:1282–1287. [PMC free article] [PubMed] [Google Scholar]
- 82.MacMillan-Crow LA, Lincoln TM. High-affinity binding and localization of the cyclic GMP-dependent protein kinase with the intermediate filament protein vimentin. Biochemistry. 1994;33:8035–8043. doi: 10.1021/bi00192a007. [DOI] [PubMed] [Google Scholar]
- 83.Ciesielski-Treska J, Ulrich G, Chasserot-Golaz S, Aunis D. Immunocytochemical localization of protein kinases Yes and Src in amoeboid microglia in culture: association of Yes kinase with vimentin intermediate filaments. Eur J Cell Biol. 1995;68:369–376. [PubMed] [Google Scholar]
- 84.Stappenbeck TS, Bornslaeger EA, Corcoran CM, Luu HH, Virata ML, Green KJ. Functional analysis of desmoplakin domains: specification of the interaction with keratin versus vimentin intermediate filament networks. J Cell Biol. 1993;123:691–705. doi: 10.1083/jcb.123.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van den Heuvel AP, de Vries-Smits AM, van Weeren PC, Dijkers PF, de Bruyn KM, Riedl JA, Burgering BM. Binding of protein kinase B to the plakin family member periplakin. J Cell Sci. 2002;115:3957–3966. doi: 10.1242/jcs.00069. [DOI] [PubMed] [Google Scholar]
- 86.Cary RB, Klymkowsky MW, Evans RM, Domingo A, Dent JA, Backhus LE. Vimentin’s tail interacts with actin-containing structures in vivo. J Cell Sci. 1994;107(Pt 6):1609–1622. doi: 10.1242/jcs.107.6.1609. [DOI] [PubMed] [Google Scholar]
- 87.Kanlaya R, Pattanakitsakul SN, Sinchaikul S, Chen ST, Thongboonkerd V. Vimentin interacts with heterogeneous nuclear ribonucleoproteins and dengue nonstructural protein 1 and is important for viral replication and release. Mol Biosyst. 2010;6:795–806. doi: 10.1039/b923864f. [DOI] [PubMed] [Google Scholar]
- 88.Russell RL, Cao D, Zhang D, Handschumacher RE, Pizzorno G. Uridine phosphorylase association with vimentin. Intracellular distribution and localization. J Biol Chem. 2001;276:13302–13307. doi: 10.1074/jbc.M008512200. [DOI] [PubMed] [Google Scholar]
- 89.Gao Y, Sztul E. A novel interaction of the Golgi complex with the vimentin intermediate filament cytoskeleton. J Cell Biol. 2001;152:877–894. doi: 10.1083/jcb.152.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Perlson E, Michaelevski I, Kowalsman N, Ben-Yaakov K, Shaked M, Seger R, Eisenstein M, et al. Vimentin binding to phosphorylated Erk sterically hinders enzymatic dephosphorylation of the kinase. J Mol Biol. 2006;364:938–944. doi: 10.1016/j.jmb.2006.09.056. [DOI] [PubMed] [Google Scholar]
- 91.Zhu QS, Rosenblatt K, Huang KL, Lahat G, Brobey R, Bolshakov S, Nguyen T, et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene. 2011;30:457–470. doi: 10.1038/onc.2010.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tzivion G, Luo ZJ, Avruch J. Calyculin A-induced vimentin phosphorylation sequesters 14-3-3 and displaces other 14-3-3 partners in vivo. J Biol Chem. 2000;275:29772–29778. doi: 10.1074/jbc.M001207200. [DOI] [PubMed] [Google Scholar]
- 93.Lian N, Wang W, Li L, Elefteriou F, Yang X. Vimentin inhibits ATF4-mediated osteocalcin transcription and osteoblast differentiation. J Biol Chem. 2009;284:30518–30525. doi: 10.1074/jbc.M109.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, Tiron C, et al. Vimentin regulates EMT induction by Slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30(12):1436–1448. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
- 95.Barberis L, Pasquali C, Bertschy-Meier D, Cuccurullo A, Costa C, Ambrogio C, Vilbois F, et al. Leukocyte transmigration is modulated by chemokine-mediated PI3Kgamma-dependent phosphorylation of vimentin. Eur J Immunol. 2009;39:1136–1146. doi: 10.1002/eji.200838884. [DOI] [PubMed] [Google Scholar]
- 96.Zhao Y, Yan Q, Long X, Chen X, Wang Y. Vimentin affects the mobility and invasiveness of prostate cancer cells. Cell Biochem Funct. 2008;26:571–577. doi: 10.1002/cbf.1478. [DOI] [PubMed] [Google Scholar]
- 97.Lang SH, Hyde C, Reid IN, Hitchcock IS, Hart CA, Bryden AA, Villette JM, et al. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. Prostate. 2002;52:253–263. doi: 10.1002/pros.10088. [DOI] [PubMed] [Google Scholar]
- 98.Singh S, Sadacharan S, Su S, Belldegrun A, Persad S, Singh G. Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 2003;63:2306–2311. [PubMed] [Google Scholar]
- 99.Zhang X, Fournier MV, Ware JL, Bissell MJ, Yacoub A, Zehner ZE. Inhibition of vimentin or beta1 integrin reverts morphology of prostate tumor cells grown in laminin-rich extracellular matrix gels and reduces tumor growth in vivo. Mol Cancer Ther. 2009;8:499–508. doi: 10.1158/1535-7163.MCT-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wei J, Xu G, Wu M, Zhang Y, Li Q, Liu P, Zhu T, et al. Overexpression of vimentin contributes to prostate cancer invasion and metastasis via src regulation. Anticancer Res. 2008;28:327–334. [PubMed] [Google Scholar]
- 101.Hafeez BB, Zhong W, Weichert J, Dreckschmidt NE, Jamal MS, Verma AK. Genetic ablation of PKC epsilon inhibits prostate cancer development and metastasis in transgenic mouse model of prostate adenocarcinoma. Cancer Res. 2011;71:2318–2327. doi: 10.1158/0008-5472.CAN-10-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu M, Bai X, Xu G, Wei J, Zhu T, Zhang Y, Li Q, et al. Proteome analysis of human androgen-independent prostate cancer cell lines: variable metastatic potentials correlated with vimentin expression. Proteomics. 2007;7:1973–1983. doi: 10.1002/pmic.200600643. [DOI] [PubMed] [Google Scholar]
- 103.Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial–mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res. 2010;3:90–99. [PMC free article] [PubMed] [Google Scholar]
- 104.Fuyuhiro Y, Yashiro M, Noda S, Kashiwagi S, Matsuoka J, Doi Y, Kato Y, et al. Clinical significance of vimentin-positive gastric cancer cells. Anticancer Res. 2010;30:5239–5243. [PubMed] [Google Scholar]
- 105.Takemura K, Hirayama R, Hirokawa K, Inagaki M, Tsujimura K, Esaki Y, Mishima Y. Expression of vimentin in gastric cancer: a possible indicator for prognosis. Pathobiology. 1994;62:149–154. doi: 10.1159/000163895. [DOI] [PubMed] [Google Scholar]
- 106.Jin H, Morohashi S, Sato F, Kudo Y, Akasaka H, Tsutsumi S, Ogasawara H, et al. Vimentin expression of esophageal squamous cell carcinoma and its aggressive potential for lymph node metastasis. Biomed Res. 2010;31:105–112. doi: 10.2220/biomedres.31.105. [DOI] [PubMed] [Google Scholar]
- 107.Hu L, Lau SH, Tzang CH, Wen JM, Wang W, Xie D, Huang M, et al. Association of Vimentin overexpression and hepatocellular carcinoma metastasis. Oncogene. 2004;23:298–302. doi: 10.1038/sj.onc.1207150. [DOI] [PubMed] [Google Scholar]
- 108.Li ZM, Wen YJ, Yang HB, Qin G, Tian L, Deng HX, Wen B. Enhanced expression of human vimentin intermediate filaments in hepatocellular carcinoma cells decreases their proliferative and invasive abilities in vitro. Zhonghua Zhong Liu Za Zhi. 2008;30:408–412. [PubMed] [Google Scholar]
- 109.Zou H, Harrington J, Rego RL, Ahlquist DA. A novel method to capture methylated human DNA from stool: implications for colorectal cancer screening. Clin Chem. 2007;53:1646–1651. doi: 10.1373/clinchem.2007.086223. [DOI] [PubMed] [Google Scholar]
- 110.Chen WD, Han ZJ, Skoletsky J, Olson J, Sah J, Myeroff L, Platzer P, et al. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst. 2005;97:1124–1132. doi: 10.1093/jnci/dji204. [DOI] [PubMed] [Google Scholar]
- 111.von Bassewitz DB, Roessner A, Grundmann E. Intermediate-sized filaments in cells of normal human colon mucosa, adenomas and carcinomas. Pathol Res Pract. 1982;175:238–255. doi: 10.1016/S0344-0338(82)80111-8. [DOI] [PubMed] [Google Scholar]
- 112.Ngan CY, Yamamoto H, Seshimo I, Tsujino T, Man-i M, Ikeda JI, Konishi K, et al. Quantitative evaluation of vimentin expression in tumour stroma of colorectal cancer. Br J Cancer. 2007;96:986–992. doi: 10.1038/sj.bjc.6603651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McInroy L, Maatta A. Down-regulation of vimentin expression inhibits carcinoma cell migration and adhesion. Biochem Biophys Res Commun. 2007;360:109–114. doi: 10.1016/j.bbrc.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 114.Alfonso P, Nunez A, Madoz-Gurpide J, Lombardia L, Sanchez L, Casal JI. Proteomic expression analysis of colorectal cancer by two-dimensional differential gel electrophoresis. Proteomics. 2005;5:2602–2611. doi: 10.1002/pmic.200401196. [DOI] [PubMed] [Google Scholar]
- 115.Di Bella A, Regoli M, Nicoletti C, Ermini L, Fonzi L, Bertelli E. An appraisal of intermediate filament expression in adult and developing pancreas: vimentin is expressed in alpha cells of rat and mouse embryos. J Histochem Cytochem. 2009;57:577–586. doi: 10.1369/jhc.2009.952861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hong SH, Misek DE, Wang H, Puravs E, Hinderer R, Giordano TJ, Greenson JK, et al. Identification of a specific vimentin isoform that induces an antibody response in pancreatic cancer. Biomark Insights. 2006;1:175–183. [PMC free article] [PubMed] [Google Scholar]
- 117.Liu C, Chen Y, Yu X, Jin C, Xu J, Long J, Ni Q, et al. Proteomic analysis of differential proteins in pancreatic carcinomas: effects of MBD1 knock-down by stable RNA interference. BMC Cancer. 2008;8:121. doi: 10.1186/1471-2407-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yin T, Wang C, Liu T, Zhao G, Zhou F. Implication of EMT induced by TGF-beta1 in pancreatic cancer. J Huazhong Univ Sci Technolog Med Sci. 2006;26:700–702. doi: 10.1007/s11596-006-0619-z. [DOI] [PubMed] [Google Scholar]
- 119.Walsh N, O’Donovan N, Kennedy S, Henry M, Meleady P, Clynes M, Dowling P. Identification of pancreatic cancer invasion-related proteins by proteomic analysis. Proteome Sci. 2009;7:3. doi: 10.1186/1477-5956-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Korsching E, Packeisen J, Liedtke C, Hungermann D, Wulfing P, van Diest PJ, Brandt B, et al. The origin of vimentin expression in invasive breast cancer: epithelial–mesenchymal transition, myoepithelial histogenesis or histogenesis from progenitor cells with bilinear differentiation potential? J Pathol. 2005;206:451–457. doi: 10.1002/path.1797. [DOI] [PubMed] [Google Scholar]
- 121.Gilles C, Polette M, Zahm JM, Tournier JM, Volders L, Foidart JM, Birembaut P. Vimentin contributes to human mammary epithelial cell migration. J Cell Sci. 1999;112(Pt 24):4615–4625. doi: 10.1242/jcs.112.24.4615. [DOI] [PubMed] [Google Scholar]
- 122.Domagala W, Lasota J, Bartkowiak J, Weber K, Osborn M. Vimentin is preferentially expressed in human breast carcinomas with low estrogen receptor and high Ki-67 growth fraction. Am J Pathol. 1990;136:219–227. [PMC free article] [PubMed] [Google Scholar]
- 123.Kokkinos MI, Wafai R, Wong MK, Newgreen DF, Thompson EW, Waltham M. Vimentin and epithelial-mesenchymal transition in human breast cancer—observations in vitro and in vivo. Cells Tissues Organs. 2007;185:191–203. doi: 10.1159/000101320. [DOI] [PubMed] [Google Scholar]
- 124.Li M, Zhang B, Sun B, Wang X, Ban X, Sun T, Liu Z, et al. A novel function for vimentin: the potential biomarker for predicting melanoma hematogenous metastasis. J Exp Clin Cancer Res. 2010;29:109. doi: 10.1186/1756-9966-29-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chu YW, Seftor EA, Romer LH, Hendrix MJ. Experimental coexpression of vimentin and keratin intermediate filaments in human melanoma cells augments motility. Am J Pathol. 1996;148:63–69. [PMC free article] [PubMed] [Google Scholar]
- 126.Hendrix MJ, Seftor EA, Chu YW, Seftor RE, Nagle RB, McDaniel KM, Leong SP, et al. Coexpression of vimentin and keratins by human melanoma tumor cells: correlation with invasive and metastatic potential. J Natl Cancer Inst. 1992;84:165–174. doi: 10.1093/jnci/84.3.165. [DOI] [PubMed] [Google Scholar]
- 127.Ben-Ze’ev A, Raz A. Relationship between the organization and synthesis of vimentin and the metastatic capability of B16 melanoma cells. Cancer Res. 1985;45:2632–2641. [PubMed] [Google Scholar]
- 128.Caselitz J, Janner M, Breitbart E, Weber K, Osborn M. Malignant melanomas contain only the vimentin type of intermediate filaments. Virchows Arch A Pathol Anat Histopathol. 1983;400:43–51. doi: 10.1007/BF00627007. [DOI] [PubMed] [Google Scholar]
- 129.Yamada T, Kawamata T, Walker DG, McGeer PL. Vimentin immunoreactivity in normal and pathological human brain tissue. Acta Neuropathol. 1992;84:157–162. doi: 10.1007/BF00311389. [DOI] [PubMed] [Google Scholar]
- 130.Trog D, Yeghiazaryan K, Schild HH, Golubnitschaja O. Up-regulation of vimentin expression in low-density malignant glioma cells as immediate and late effects under irradiation and temozolomide treatment. Amino Acids. 2008;34:539–545. doi: 10.1007/s00726-007-0007-4. [DOI] [PubMed] [Google Scholar]
- 131.Fortin S, Le Mercier M, Camby I, Spiegl-Kreinecker S, Berger W, Lefranc F, Kiss R. Galectin-1 is implicated in the protein kinase C epsilon/vimentin-controlled trafficking of integrin-beta1 in glioblastoma cells. Brain Pathol. 2010;20:39–49. doi: 10.1111/j.1750-3639.2008.00227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bouamrani A, Ramus C, Gay E, Pelletier L, Cubizolles M, Brugiere S, Wion D, et al. Increased phosphorylation of vimentin in noninfiltrative meningiomas. PLoS One. 2010;5:e9238. doi: 10.1371/journal.pone.0009238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kawahara E, Oda Y, Ooi A, Katsuda S, Nakanishi I, Umeda S. Expression of glial fibrillary acidic protein (GFAP) in peripheral nerve sheath tumors. A comparative study of immunoreactivity of GFAP, vimentin, S-100 protein, and neurofilament in 38 schwannomas and 18 neurofibromas. Am J Surg Pathol. 1988;12:115–120. doi: 10.1097/00000478-198802000-00004. [DOI] [PubMed] [Google Scholar]
- 134.Broers JL, de Leij L, Rot MK, ter Haar A, Lane EB, Leigh IM, Wagenaar SS, et al. Expression of intermediate filament proteins in fetal and adult human lung tissues. Differentiation. 1989;40:119–128. doi: 10.1111/j.1432-0436.1989.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 135.Upton MP, Hirohashi S, Tome Y, Miyazawa N, Suemasu K, Shimosato Y. Expression of vimentin in surgically resected adenocarcinomas and large cell carcinomas of lung. Am J Surg Pathol. 1986;10:560–567. doi: 10.1097/00000478-198608000-00006. [DOI] [PubMed] [Google Scholar]
- 136.Al-Saad S, Al-Shibli K, Donnem T, Persson M, Bremnes RM, Busund LT. The prognostic impact of NF-kappaB p105, vimentin, E-cadherin and Par6 expression in epithelial and stromal compartment in non-small-cell lung cancer. Br J Cancer. 2008;99:1476–1483. doi: 10.1038/sj.bjc.6604713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rho JH, Roehrl MH, Wang JY. Glycoproteomic analysis of human lung adenocarcinomas using glycoarrays and tandem mass spectrometry: differential expression and glycosylation patterns of vimentin and fetuin A isoforms. Protein J. 2009;28:148–160. doi: 10.1007/s10930-009-9177-0. [DOI] [PubMed] [Google Scholar]
- 138.Chu S, Xu H, Ferro TJ, Rivera PX. Poly(ADP-ribose) polymerase-1 regulates vimentin expression in lung cancer cells. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1127–L1134. doi: 10.1152/ajplung.00197.2007. [DOI] [PubMed] [Google Scholar]
- 139.Gilles C, Polette M, Piette J, Delvigne AC, Thompson EW, Foidart JM, Birembaut P. Vimentin expression in cervical carcinomas: association with invasive and migratory potential. J Pathol. 1996;180:175–180. doi: 10.1002/(SICI)1096-9896(199610)180:2<175::AID-PATH630>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 140.Williams AA, Higgins JP, Zhao H, Ljunberg B, Brooks JD. CD 9 and vimentin distinguish clear cell from chromophobe renal cell carcinoma. BMC Clin Pathol. 2009;9:9. doi: 10.1186/1472-6890-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gustmann C, Altmannsberger M, Osborn M, Griesser H, Feller AC. Cytokeratin expression and vimentin content in large cell anaplastic lymphomas and other non-Hodgkin’s lymphomas. Am J Pathol. 1991;138:1413–1422. [PMC free article] [PubMed] [Google Scholar]
- 142.Yamamoto Y, Izumi K, Otsuka H. An immunohistochemical study of epithelial membrane antigen, cytokeratin, and vimentin in papillary thyroid carcinoma. Recognition of lethal and favorable prognostic types. Cancer. 1992;70:2326–2333. doi: 10.1002/1097-0142(19921101)70:9<2326::AID-CNCR2820700919>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 143.Coppola D, Fu L, Nicosia SV, Kounelis S, Jones M. Prognostic significance of p53, bcl-2, vimentin, and S100 protein-positive Langerhans cells in endometrial carcinoma. Hum Pathol. 1998;29:455–462. doi: 10.1016/S0046-8177(98)90060-0. [DOI] [PubMed] [Google Scholar]
- 144.Bargagna-Mohan P, Hamza A, Kim YE, Khuan Abby Ho Y, Mor-Vaknin N, Wendschlag N, Liu J, et al. The tumor inhibitor and antiangiogenic agent withaferin A targets the intermediate filament protein vimentin. Chem Biol. 2007;14:623–634. doi: 10.1016/j.chembiol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lahat G, Zhu QS, Huang KL, Wang S, Bolshakov S, Liu J, Torres K, et al. Vimentin is a novel anti-cancer therapeutic target; insights from in vitro and in vivo mice xenograft studies. PLoS One. 2010;5:e10105. doi: 10.1371/journal.pone.0010105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 146.Wu KJ, Zeng J, Zhu GD, Zhang LL, Zhang D, Li L, Fan JH, et al. Silibinin inhibits prostate cancer invasion, motility and migration by suppressing vimentin and MMP-2 expression. Acta Pharmacol Sin. 2009;30:1162–1168. doi: 10.1038/aps.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Singh RP, Raina K, Sharma G, Agarwal R. Silibinin inhibits established prostate tumor growth, progression, invasion, and metastasis and suppresses tumor angiogenesis and epithelial-mesenchymal transition in transgenic adenocarcinoma of the mouse prostate model mice. Clin Cancer Res. 2008;14:7773–7780. doi: 10.1158/1078-0432.CCR-08-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dong TT, Zhou HM, Wang LL, Feng B, Lv B, Zheng MH. Salinomycin selectively targets ‘CD133+’ cell subpopulations and decreases malignant traits in colorectal cancer lines. Ann Surg Oncol. 2011;18(6):1797–1804. doi: 10.1245/s10434-011-1561-2. [DOI] [PubMed] [Google Scholar]
- 149.Zhang X, Ladd A, Dragoescu E, Budd WT, Ware JL, Zehner ZE. MicroRNA-17-3p is a prostate tumor suppressor in vitro and in vivo, and is decreased in high grade prostate tumors analyzed by laser capture microdissection. Clin Exp Metastasis. 2009;26:965–979. doi: 10.1007/s10585-009-9287-2. [DOI] [PubMed] [Google Scholar]
- 150.Braun J, Hoang-Vu C, Dralle H, Huttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 151.Bouchard PR, Hutabarat RM, Thompson KM. Discovery and development of therapeutic aptamers. Annu Rev Pharmacol Toxicol. 2010;50:237–257. doi: 10.1146/annurev.pharmtox.010909.105547. [DOI] [PubMed] [Google Scholar]