TGF-β inhibits p70 S6 kinase via protein phosphatase 2A to induce G1 arrest (original) (raw)
Abstract
On TGF-β binding, the TGF-β receptor directly phosphorylates and activates the transcription factors Smad2/3, leading to G1 arrest. Here, we present evidence for a second, parallel, TGF-β-dependent pathway for cell cycle arrest, achieved via inhibition of p70s6k. TGF-β induces association of its receptor with protein phosphatase-2A (PP2A)-Bα. Concomitantly, three PP2A-subunits, Bα, Aβ, and Cα, associate with p70s6k, leading to its dephosphorylation and inactivation. Although either pathway is sufficient to induce G1 arrest, abrogation of both, the inhibition of p70s6k, and transcription through Smad proteins is required for release of epithelial cells from TGF-β-induced G1 arrest. TGF-β thereby modulates the translational and posttranscriptional control of cell cycle progression.
Keywords: TGF-β p70s6k, PP2A, G1 arrest, cell cycle
TGF-β inhibits G1/S progression in a variety of eukaryotic cell types. Among these, untransformed epithelial cells are particularly sensitive to the growth inhibition by TGF-β. TGF-β binds the TGF-β receptor type I and type II (TβRI/TβRII). TβRI has been shown to transduce all known signals induced by TGF-β TβRI binds and phosphorylates the transcription factor Smad2 or, alternatively, its close homolog Smad3. Smad2 and 3 associate with Smad4 and modulate transcription of TGF-β responsive genes (Massague and Chen 2000; ten Dijke et al. 2000; Wrana and Attisano 2000).
It has been noted, however, that several other molecules interact with TβRI, indicating additional downstream effectors. FKBP12, the cellular target of the immunosuppressant Rapamycin, for example, binds the nonactive TβRI (Wang et al. 1994, 1996; Chen et al. 1997; Huse et al. 1999). In addition, the regulatory subunit Bα of protein phosphatase 2A (PP2A) has been shown to specifically interact with the activated TβRI (Griswold-Prenner et al. 1998). Among many other targets PP2A dephosphorylates and inactivates p70s6k (Ballou et al. 1988; Schonthal 1998; Goldberg 1999; Millward et al. 1999), a serine/threonine kinase that induces translation of mRNAs containing 5′TOP sequences (Jefferies et al. 1994; Pearson and Thomas 1995) and is essential for G1/S progression (Lane et al. 1993).
In this study we first observe that inhibition of Smad signaling is not sufficient to release epithelial cells from TGF-β-induced G1 arrest, indicating that there might be additional pathways for growth inhibition by TGF-β. We then show that PP2A and p70s6k are components of a TGF-β-induced signal transduction pathway to control protein translation and G1/S progression. Dependent on PP2A-Bα, TGF-β inhibits p70s6k by establishing or stabilizing complex formation of PP2A with p70s6k. Finally, we argue that repression of p70s6k functions as an alternative mechanism to Smad-mediated transcriptional control of the cell cycle. Once activated, either pathway is sufficient to induce TGF-β-dependent cell cycle arrest in G1.
Results
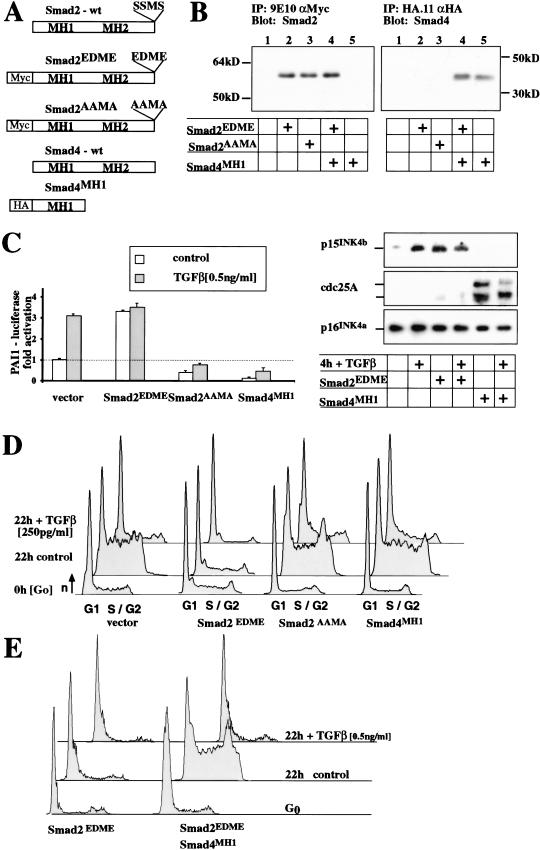
To test whether the Smad signal transduction pathway is necessary and sufficient for growth control by TGF-β, we generated dominant-negative and constitutively active mutants of Smad2 and Smad4. The transcriptional activity of these mutant proteins was assessed by their potential to regulate TGF-β-induced transcription in polarized mammary epithelial cells, EpH4 (Reichmann et al. 1992). Multiclonal populations were analyzed for the retroviral-expressed mutant Smad proteins (Fig. 1A,B), the activity of TGF-β reporter constructs, endogenous TGF-β target genes (Fig. 1C), and cell cycle progression by Fluorescence Activated Cell Scanning (FACS; Fig. 1D,E). TβRI activates Smad2 by multiple phosphorylation of its C-terminal motif, SSMS. Mutation of those serines in Smad2 to acidic residues (Smad2EDME) activated Smad2 to induce transcription (Fig. 1C; Macias-Silva et al. 1996; Liu et al. 1997; Souchelnytskyi et al. 1997). Mutation of the same serines to alanines (Smad2AAMA) or expression of the DNA-binding domain of Smad4 (Smad4MH1) inhibited TGF-β-induced transcription of a transient transfected PAI1 reporter or the endogenous p15ink4b gene (Fig. 1C). Both molecules thereby act as dominant-negative regulators of TGF-β-induced transcription.
Figure 1.
Smad proteins are sufficient and necessary for transcription but only sufficient and not necessary for G1 arrest induced by TGF-β. (A,B) Smad-induced transcription was modified in EpH4 mammary epithelial cells by expressing activated Smad2 (Smad2EDME) or dominant-negative Smad2 (Smad2AAMA) or the Mad Homology domain 1 of Smad4 (Smad4MH1) using high-titer retroviral vectors. (C) Transcription of transient TGF-β reporter normalized to β-actin control and Western blot for the endogenous p15 (TGF-β activated), cdc25A (TGF-β repressed), and p16 (control). (D) FACS-DNA profiles of EpH4 cells expressing the respective Smad protein. Cells were released from contact inhibition into cell cycle for 22 h, in the presence or absence of TGF-β. The activated Smad2EDME arrests cells in G1 under all conditions and activates transcription; dominant-negative Smad2AAMA or SMAD4MH1 were unable to prevent the TGF-β-induced G1 arrest in EpH4 cells despite their ability tototo antagonize TGF-β-induced transcriptional effects. (E) The expression of Smad4MH1 was sufficient to overcome Smad2EDME-mediated cell cycle arrest but not TGF-β-mediated arrest, arguing for an additional, not transcription-mediated, mechanism for induction of G1 arrest by TGF-β.
TGF-β induces G1 arrest (Fig. 1D) and Smad2EDME indeed mimicked this effect (Fig. 1D). Surprisingly, however, neither of the dominant-negative Smad proteins released from TGF-β-induced cell cycle arrest (Fig. 1D). Because receptor-activated Smads form a complex with Smad4, expression of Smad4MH1 should inhibit the TGF-β activated Smad2 and the Smad2EDME mutant and relieve Smad2EDME-mediated cell cycle arrest (Fig. 1D). Although expression of Smad4MH1 indeed reversed the cell cycle arrest induced by the dominant active Smad2 (Smad2EDME), it failed to release the same cells from TGF-β-induced G1 arrest (Fig. 1E), indicating that TGF-β induces additional pathways to induce G1 arrest, independent of Smad-mediated transcription.
Similar experiments performed previously in other cell systems had identified Smad-induced transcription as the only mediator of TGF-β-induced G1 arrest (Liu et al. 1997). In our experimental settings, Smad-induced transcription was likewise sufficient to induce G1 arrest in epithelial cells, but was clearly not the only mechanism. We attempted therefore to identify the additional mechanism mediating TGF-β-induced G1 arrest.
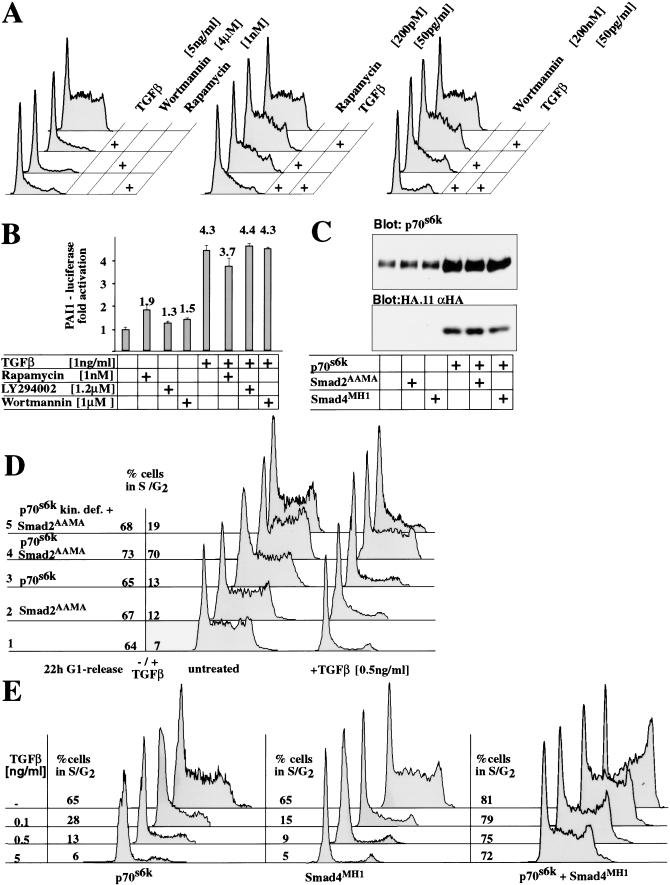
To identify signaling pathways essential for G1/S progression in epithelial cells, we applied known cell cycle inhibitors. We found that Rapamycin, an immunosuppressant and Wortmannin or LY294002, two PI3-kinase inhibitors, each induced G1 arrest in EpH4 cells (Fig. 2A). We asked whether Rapamycin or PI3-kinase inhibitors and TGF-β would synergize to induce G1 arrest and tested each drug for synergism with TGF-β. To pursue this approach, dose response curves for cell cycle entry in the presence of drugs alone or with TGF-β were determined (Fig. 2A; data not shown). Rapamycin, Wortmannin, and LY294002 synergized with TGF-β to induce G1 arrest when applied at nonsaturating conditions of either drug or factor alone (Fig. 2A), indicating that the drugs might modulate similar downstream targets as TGF-β to control cell cycle progression. The compounds, however, did not complement all aspects of TGF-β signaling, and neither drug significantly induced or synergized with Smad-mediated transcription (Fig. 2B).
Figure 2.
p70s6k pathway is rate limiting for TGF-β-induced G1 arrest. (A,B) Synergistic action of TGF-β with Wortmannin and Rapamycin on G1 arrest, but not transcriptional activation of a TGF-β responsive promoter. (A) FACS analysis of EpH4 cells 22 h after cell cycle release. Cells were treated with various drugs as indicated during the entire release period. High concentrations of TGF-β Wortmannin or Rapamycin alone induce G1 arrest in epithelial cells (left panel). Ineffective doses of Wortmannin and Rapamycin complement low, noninhibitory TGF-β concentrations to cause a complete G1 arrest (central and right panels). (B) Wortmannin, LY 294002, and Rapamycin did not affect TGF-β-induced PAI-1 reporter gene transcription. (C,D) Overexpression of p70s6k (C, Western blot) and FACS analysis determining proportion of cells in G1 after TGF-β induction in dependence of p70s6k and Smad2AAMA expression (D). Only coexpression of p70s6k and dominant-negative Smad2 protein release cells from TGF-β-induced G1 arrest. Neither p70s6k (D, lane 3) nor Smad2AAMA (D, lane 2) alone are capable to relieve the TGF-β-mediated cell cycle arrest, whereas both proteins expressed together effectively do so (lane 4). (E) FACS analysis of cells released from G1 arrest 22 h after TGF-β induction in dependence of p70s6k and Smad4MH1 expression. Activation of p70s6k and inhibition of Smad-induced transcription are sufficient to completely protect cells from TGF-β-induced cell cycle arrest.
All three compounds inhibit activation of p70s6k (Cheatham et al. 1994; Chung et al. 1994; Petritsch et al. 1995). Overexpression of p70s6k did not influence TGF-β-induced and Smad mediated transcription (data not shown). Next, we asked if regulation of p70s6k activity is a rate limiting step in the control of G1/S progression by TGF-β p70s6k was overexpressed in EpH4 epithelial cells or derivatives coexpressing either dominant-negative Smad2 or Smad4 (Fig. 2C) and cells released into G1/S in the absence or presence of TGF-β (Fig. 2D,E). In the absence of TGF-β cell cycle progression was not altered by expressing Smads and p70s6k (Fig. 2D). Expression of both p70s6k and the Smad2AAMA, however, released a high proportion of epithelial cells from G1 arrest into S-phase regardless of the presence of TGF-β (Fig. 2D). A kinase deficient p70s6k (p70s6k kin.def.) was not able to rescue from TGF-β-induced cell cycle arrest. Similar results were obtained when the dominant-negative Smad4 (Smad4MH1) and p70s6k were coexpressed (Fig. 2E; data not shown). Thus, p70s6k confers resistance to TGF-β-induced, but Smad-independent, G1 arrest.
To further test this model, we exposed cells expressing Smad4MH1 and p70s6k to increasing concentrations of TGF-β. Whereas 100pg/mL TGF-β-induced complete G1 arrest in EpH4 cells expressing either p70s6k or Smad4MH1 (Fig. 2E), cells coexpressing p70s6k and Smad4MH1 continued to undergo G1/S progression efficiently even at a TGF-β concentration of 5ng/mL. Thus, the cooperation of p70s6k and Smad4MH1 rendered the cells essentially insensitive to TGF-β-induced inhibition of cell cycle progression (Fig. 2E).
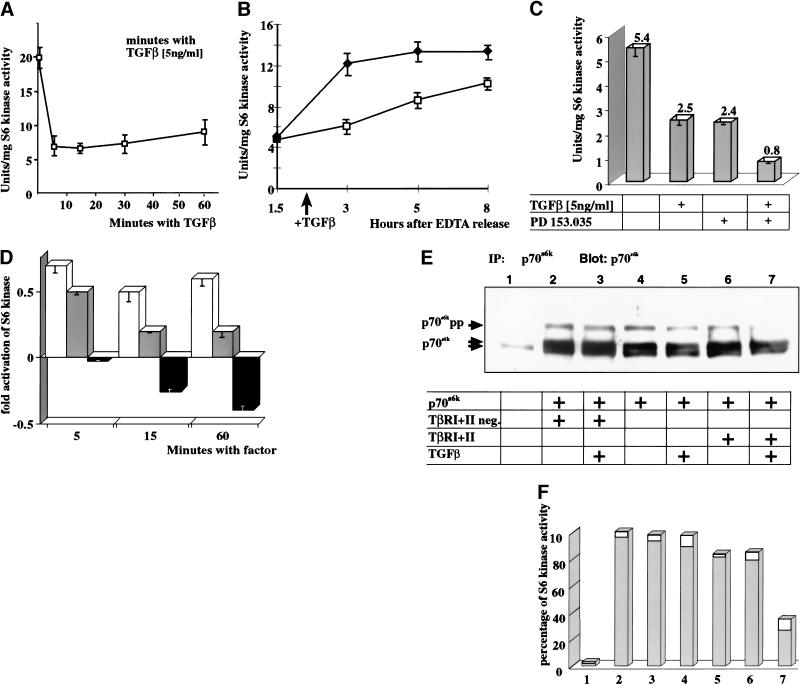
In mammalian cells, p70s6k becomes activated on G0/G1 or M/G1 transition, the activity peaks early in G1 and declines on progression into S-phase (Edelmann et al. 1996). We therefore were interested if TGF-β would suppress p70s6k activity in G0 and early G1 cells. EpH4 cells were cultured at confluency to induce early G1 arrest and subsequently treated for various times with TGF-β TGF-β addition lead to a decrease of p70s6k activity by 70% within 5 min compared with untreated lysates, and the inhibition was sustained for more than 1 h after factor addition (Fig. 3A,B). This rapid onset of inhibition indicates a direct posttranslational mechanism.
Figure 3.
TGF-β inhibits p70s6k and antagonizes EGFR signaling pathways at the level of p70s6k. (A) p70s6k kinase activity in EpH4 cells arrested in G1 and exposed to TGF-β for various times. Sixty-five percent inhibition of p70s6k activity by TGF-β was observed after 5 min, persisting for more than 1 h. (B) p70s6k kinase activity was determined in epithelial cells, arrested in G1 by contact inhibition, released into cell cycle, and treated with TGF-β or left untreated. TGF-β inhibits the increase of p70s6k activity in early G1. (♦) Control; (□) plus 5ng/mL TGF-β. (C) p70s6k activity in response to TGF-β and an EGFR kinase inhibitor in epithelial EpH4 cells after 20 min. The inhibitory effects of a combination of TGF-β and PD 153.035 are synergistic. (D) p70s6k activity in response to TGF-β and TGF-α in unsynchronized cycling cells. TGF-β inhibits the p70s6k; TGF-α activates p70s6k and partially neutralizes the inhibitory action of TGF-β on p70s6k activity. (Open bars) 5 ng/mL of TGF-α; (gray bars) TGF-α + TGF-β; (black bars) 5ng/mL of TGF-β. (E,F) Immunoprecipitation and Western blot of p70s6k transiently cotransfected in 293 cells with wild-type TβRI and II or inactive mutants (TβRI +II dn). (E) TGF-β inhibits the hyper-phosphorylation of p70s6k in dependence on an activatable TGF-β–TβR complex. Serum-induced hyperphosphorylated forms of p70s6k (p70s6k pp) disappear only after TGF-β stimulation of the coexpressed wild-type TβRI and TβRII (lanes 6,7), but not by stimulation of kinase inactivated TβRI/II (lane 2,3). (F) Corresponding p70s6k kinase activities determined in immunoprecipitates shown in E. The decrease of p70s6k pp (Fig. 4a, lane 7) is accompanied by a reduction of p70s6k kinase activity by more than 60%.
Having observed a TGF-β-induced inhibition of p70s6k in arrested cells we examined its effect on p70s6k activity in cells entering the cell cycle. Because overexpression of p70s6k and inhibition of Smad signaling released epithelial cells from TGF-β-induced G1 arrest (Fig. 2D,E), we anticipated that TGF-β would inhibit activation of p70s6k during G1/S progression. Indeed, the rapid increase of p70s6k kinase activity observed in the untreated population was delayed and strongly attenuated in cells treated with TGF-β (Fig. 3B).
We next looked for additive effects of inhibition of growth factor pathways and TGF-β on p70s6k activity. Activation of the epidermal growth factor receptor (EGFR) is essential for G1/S progression of epithelial cells (data not shown). TGF-β and an inhibitor of the EGFR tyrosine kinase, PD 153.035, both strongly reduced the activity of p70s6k. When TGF-β and PD 153.035 were added together, however, the inhibitory effect on p70s6k was synergistic (Fig. 3C). To further examine the interplay of growth signals, we asked if inhibition of p70s6k by TGF-β would antagonize activating signals from epithelial growth factors such as TGF-α that promote G1/S progression. Unsynchronized proliferating EpH4 cells (50% in G1 phase) were subjected to treatment with TGF-β TGF-α, or a combination of both. As expected, TGF-α stimulated p70s6k and TGF-β reduced the kinase activity of p70s6k with maximal inhibition after 60 min (Fig. 3D). When both factors were given at the same time, p70s6k activation was intermediate in value, indicating, again, that growth inhibition by TGF-β antagonizes growth promoting signals at the level of p70s6k activation.
We next analyzed if TGF-β-induced inhibition of p70s6k is mediated by the ligand-activated TβRI and TβRII. Accordingly, we immunoprecipitated overexpressed p70s6k from 293 cells, coexpressed with either wt TβRI and wt TβRII (Fig. 3E,F, lanes 6,7) or kinase-inactivated versions of TβRI and TβRII receptor (lanes 2,3) and stimulated the cells with TGF-β. The slower migrating form of p70s6k, corresponding to a highly phosphorylated and enzymatically active form of p70s6k (p70s6k pp; Fig. 3E) and p70s6k activity, was significantly reduced only in the presence of both wt TβR complex and TGF-β stimulation (Fig. 3E,F, lane 7). Thus, both the phosphorylation level and the kinase activity of p70s6k are significantly decreased on TGF-β receptor stimulation.
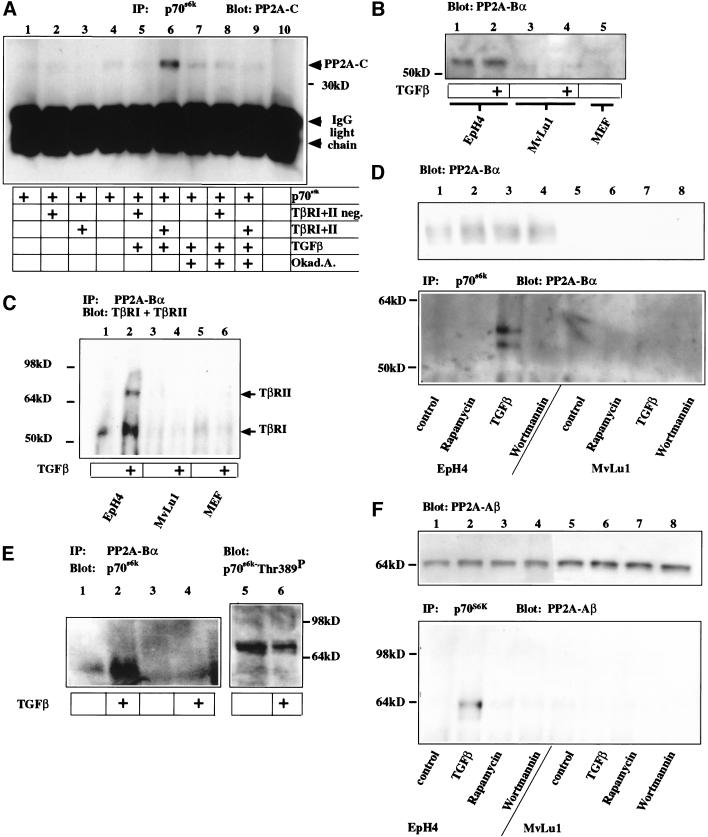
TGF-β-activated TβRI has been reported to interact directly with the regulatory subunit Bα of PP2A (Griswold-Prenner et al. 1998). PP2A consist of three subunits, a structural regulatory subunit A, a variable regulatory subunit B, and catalytic subunit C. PP2A-C has been shown to dephosphorylate and inactivate p70s6k (Ballou et al. 1988; Peterson et al. 1999). This indicated that TGF-β-induced inhibition of p70s6k might be mediated by PP2A. We therefore tested whether TGF-β might regulate the interaction of PP2A with p70s6k. HA-tagged p70s6k was subjected to anti-HA-mediated immunoprecipitation. The endogenous catalytic unit of PP2A (PP2A-C) formed a complex with p70s6k only in the presence of the active TGF-β receptor type I and II and only on addition of TGF-β (Fig. 4A, lane 6). No interaction was observed when kinase-dead versions of TβRI and TβRII were coexpressed with p70s6k and stimulated with TGF-β. Furthermore, inhibition of PP2A catalytic activity by addition of the phosphatase inhibitor okadaic acid at concentrations specific for PP2A disrupted formation of the TGF-β-induced PP2A-C/p70s6k complex. Taken together, these findings support the hypothesis that TGF-β inhibits p70s6k by inducing or stabilizing the interaction of PP2A-C with its substrate p70s6k. A similar mechanism has recently been postulated for the inhibition of p70s6k by Rapamycin (Peterson et al. 1999).
Figure 4.
TGF-β induces de-phosphorylation of p70s6k by stimulating association of PP2A subunits C, Aβ and Bα and p70s6k and association of PP2A-Bα and TβRI. (A) Western blot for PP2A-C after immunoprecipitation of p70s6k TβR expressing cells were treated with TGF-β and/or the PP2A inhibitor okadaic acid or left untreated. Interaction of PP2A with p70s6k is stimulated in cells expressing the TβRI/TβRII receptor complex on addition of TGF-β (lane 6), but inhibited by the presence of okadaic acid (Okad.A., lane 9). Coexpression of inactive TβRI/TβRII neg. fails to stimulate interaction (lanes 2,5,8) above basal binding activity (lanes 1,4,7). (B) Western blot for the expression of the endogenous regulatory subunit PP2A-Bα EpH4 epithelial cells express high levels of PP2A-Bα, and its expression is not altered by 1 h of TGF-β induction. Endogenous levels of PP2A-Bα in Mv1Lu cells and primary embryonic fibroblasts are below detection limits. (C) Immunoprecipitation for PP2A-Bα and Western blot for TβRI and TβRII. PP2A-Bα interacts with TβRI and TβRII on TGF-β stimulation in EpH4, but similar complexes are not detectable in Mv1Lu cells or primary embryonic mouse fibroblasts (MEF). (D) Western blot for PP2A-Bα after immunoprecipitation of p70s6k in EpH4 or Mv1Lu cells. PP2A-Bα interaction with p70s6k is observed specifically in response to TGF-β in EpH4 cells and not in response to other inhibitors of p70s6k or in Mv1Lu cells. (E) Western blot for p70s6k after immunoprecipitation of PP2A-Bα (lanes 1,2) or using a control antibody (lanes 3,4) and direct Western blot for Thr389 of p70s6k from cells stimulated with TGF-β in early G1. In response to TGF-β, PP2A-Bα is found in complexes with p70s6k and Thr 389 phosphorylation of p70s6k decreases. (F) Western blot for PP2A-Aβ before (top panel) and after (bottom panel) immunoprecipitation with p70s6k. In EpH4 cells, Aβ interacts with p70s6k specifically in response to TGF-β.
In certain cell lines such as mink lung epithelial cells (Mv1Lu), TGF-β does not inhibit p70s6k, and Smad signaling is both sufficient and necessary for TGF-β-mediated G1 arrest (Like and Massague 1986; Liu et al. 1997). Moreover, in contrast to wild-type murine embryonic fibroblasts (MEFs), MEFs lacking Smad3 do not respond to growth inhibition by TGF-β (Zhu et al. 1998; Datto et al. 1999; Yang et al. 1999). To understand this fundamental difference between primary embryonic fibroblasts and Mv1Lu cells on the one hand and epithelial cells on the other, we analysed the expression of the PP2A subunits and TGF-β receptors in these cell types. Both TβRI and TβRII were expressed at comparable levels in all three cell types (data not shown). We found, however, that the regulatory subunit Bα of PP2A is expressed in EpH4 cells (Fig. 4B), in primary small airway epithelial cells, and in a variety of epithelial tumor cell lines (data not shown), but not in Mv1Lu cells or primary MEFs (Fig. 4B). Therefore, it was not surprising that interaction of the endogenous PP2A-Bα subunit was detected with endogenous TβRI/TβRII in response to TGF-β in EpH4 cells, but not in Mv1Lu cells or primary MEFs (Fig. 4C). To examine the endogenous complexes formed by PP2A and p70s6k, EpH4 and Mv1Lu cells were treated in early G1 phase of the cell cycle with either Rapamycin, TGF-β, or Wortmannin. On p70s6k immunoprecipitation, complex formation with PP2A-Bα was only detected in EpH4 cells in response to TGF-β, indicating a TGF-β specific mechanism of p70s6k inhibition presumably absent in Mv1Lu cells (Fig. 4D). Moreover, if PP2A-Bα was immunoprecipitated from lysates of EpH4 cells in early G1, we were able to detect significantly increased amounts of p70s6k associated with PP2A-Bα in TGF-β treated cells (Fig. 4E). To better characterize p70s6k regulation, we analyzed the phosphorylation status of the Thr 389 in the linker domain of p70s6k (Fig. 4E). Phosphorylation of Thr 389 correlates with the kinase activity of p70s6k (Pullen et al. 1998; Weng et al. 1998). Thr 389 of p70s6k was found to be phosphorylated in EpH4 cells in early G1, but significantly reduced on TGF-β stimulation (Fig. 4E). These data further support our model that the inhibition of p70s6k by TGF-β is dependent on PP2A-Bα-mediated association of PP2A-C with p70s6k.
We then analyzed which A subunit of PP2A would interact with p70s6k in response to TGF-β The Aβ (PR65) regulatory subunit has recently been genetically linked to human lung and colon cancer (Wang et al. 1998). Aβ was expressed at comparable levels in both cells types, EpH4 and Mv1Lu (Fig. 4F). Interestingly, we detected Aβ associated with p70s6k only in EpH4 cells, but not in Mv1Lu cells, and only in response to TGF-β, but not in response to Rapamycin or Wortmannin (Fig. 4F).
Discussion
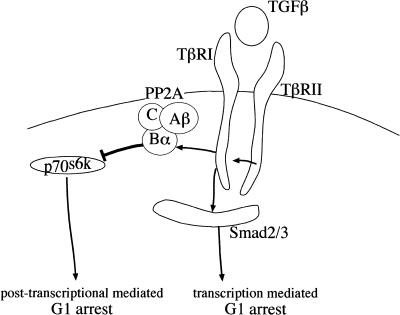
Taken together, our results show that TGF-β interferes with two independent pathways to induce G1 arrest in epithelial cells. The first is the pathway of transcriptional control mediated by Smad proteins (Eppert et al. 1996; Lagna et al. 1996; Macias-Silva et al. 1996; Zhang et al. 1996; Nakao et al. 1997). The second leads to the inhibition of p70s6k via PP2A (Fig. 5). This represents a novel observation with potential implication for our understanding of cell cycle regulation, cancer biology, and cancer development.
Figure 5.
Model for the two independent pathways initiated by TGF-β to induce G1-arrest. Both, inhibition of p70s6k and activation of Smad-induced transcription are independently sufficient to induce G1 arrest.
We show here that repression of p70s6k coincides with its dephosphorylation and association of p70s6k with three subunits of PP2A: PP2A-C and the two regulatory subunits PP2A-Aβ and PP2A-Bα. PP2A-Bα interacts with TβRI and thereby physically links TGF-β to the control of PP2A and to p70s6k. On receptor activation, PP2A-Bα specifically binds the activated TβRI and is catalytically activated by TGF-β (Griswold-Prenner et al. 1998; data not shown). PP2A-Bα then recruits PP2A-Aβ and PP2A-C to bind and dephosphorylate p70s6k. We were, however, not able to detect complexes containing at the same time PP2A, TβRI and p70s6k, indicating that on activation the phosphatase is released from the receptor to bind to the target molecule. Immunolocalization of the endogenous proteins supports this model (M. Oft, unpubl.). p70s6k activity controls the translational upregulation of proteins important for G1/S progression and is itself essential for cell cycle progression (Lane et al. 1993; Pearson and Thomas 1995). Most of the transcripts isolated to date represent ribosomal proteins and elongation factors of protein synthesis (Jefferies et al. 1994). TGF-β-induced inactivation of p70s6k leads to the translational regulation of a group of cell cycle regulators for G1 progression (M. Oft, unpubl.). It remains unclear, however, if the repression of those cell cycle regulators result from global repression of protein translation or represent a class of specifically translationally repressed mRNAs. It is conceivable that the regulation of crucial components of the cell cycle machinery is mediated at the transcriptional, translational, and posttranslational levels.
Expression of the regulatory subunit PP2A-Bα itself appears to be a prerequisite for the PP2A-mediated inhibition of p70s6k by TGF-β. Cells with nondetectable PP2A-Bα expression remain solely responsive to TGF-β-mediated transcriptional responses (Liu et al. 1997; Zhu et al. 1998; Datto et al. 1999; Yang et al. 1999). p70s6k is not inhibited by TGF-β in these cells (Like and Massague 1986; data not shown), which reflects the differential sensitivity of epithelial cells and mesenchymal cells to growth inhibitory effects of TGF-β. The chromosomal localization of PP2A-Bα has not been investigated; PP2A-Aβ, however, has been mapped to a human tumor suppressor locus on 11q22–24 and appears to be mutated in a subset of human lung tumors (Wang et al. 1998). It is tempting to speculate that mutations of the regulatory subunits of PP2A in human tumors abolish the regulation of p70s6k to TGF-β and confer a selective advantage to growing tumors.
Material and methods
Cell culture and cell cycle analysis
PAI1-promoter transcription assays were normalized to a internal β-actin–β-galactosidase control, and numbers represent the average of three independent experiments.
EpH4 cells were infected with supernatants of BOSC23 virus-producing cells (Pear et al. 1993), drug selected, tested for the transgene expression, and immediately analyzed as a mass culture to exclude clonal variations. For G1/S progression studies, the cells were seeded at a confluent density, arrested in G0/G1 by confluency for two days, EDTA released and replated as single cells. The majority of cells enter S-phase after 18 h. Samples for all G1/S profiles shown were collected 22 h after EDTA release. The results were, however, verified extensively throughout a 16-h to 30-h time window.
p70s6k kinase assays
Cells were washed twice with 10 mL cold Extraction Buffer (20 mM Tris at pH 7.5, 20 mM EDTA, 15 mM MgCl2, 40 mM 4-nitrophenyl phosphate [pNPP], 1 mM dithiothreitol [DTT], and 0.1 mM phenylmethylsulfonyl fluoride [PMSF]). For s6 kinase assays p70s6k was immunoprecipitated using polyclonal antibodies against p70s6k (Edelmann et al. 1996) or the HA-epitope and G protein–coupled sepharose beads. Precipitated beads were resuspended in S6 kinase assay buffer (50 mM Tris at pH 7.5, 0.1 mM EGTA, 5% ethylene glycol, 5 mM DTT, 10 mM MgCl2, 0.1% Triton X-100, and 0.25 mg/mL BSA) and assayed for S6 kinase activity using the ribosomal 40S subunit as a substrate as described previously (Petritsch et al. 1995). Immunoprecipitation of p90rsk and subsequent kinase assays revealed that s6 phosphorylation by p90rsk is negligible in EpH4 cells. All kinase assays were performed in duplicates and repeated twice.
Immunoprecipitations
TβRI, TβRII or their derivatives with inactivated kinase domains and HA-tagged p70s6k were transiently transfected into 293 cells. Cell lysates were collected 24 h after transfection and 20 min after stimulation with TGF-β or okadaic acid and subjected to anti-HA- or anti-p70s6k immunoprecipitation.
To precipitate endogenous TβRI and TβRII kinase-phosphatase complexes, lysates for immunoprecipitation were taken after 20 min of TGF-β treatment, normalized for protein content, and subjected to immunoprecipitation using the respective antibodies and Protein G sepharose beads.
Acknowledgments
We thank J. Massague and R. Wieser for sharing the expression vectors for various TβRI and TβRII and the 3TPlux construct, as well as Ali Fattay and especially Byron Hann for helpful discussion to improve the manuscript. C.P. was supported by a grant of the Sonderforschungsbereich.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL moft@cc.ucsf.edu; FAX (415) 502-6779.
References
- Ballou LM, Jeno P, Thomas G. Protein phosphatase 2A inactivates the mitogen-stimulated S6 kinase from Swiss mouse 3T3 cells. J Biol Chem. 1988;263:1188–1194. [PubMed] [Google Scholar]
- Cheatham B, Vlahos CJ, Cheatham L, Wang L, Blenis J, Kahn CR. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Liu F, Massague J. Mechanism of TGF-β receptor inhibition by FKBP12. EMBO J. 1997;16:3866–3876. doi: 10.1093/emboj/16.13.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370:71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor β-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelmann HM, Kuhne C, Petritsch C, Ballou LM. Cell cycle regulation of p70 S6 kinase and p42/p44 mitogen-activated protein kinases in Swiss mouse 3T3 fibroblasts. J Biol Chem. 1996;271:963–971. doi: 10.1074/jbc.271.2.963. [DOI] [PubMed] [Google Scholar]
- Eppert K, Scherer SW, Ozcelik H, Pirone R, Hoodless P, Kim H, Tsui LC, Bapat B, Gallinger S, Andrulis IL, et al. MADR2 maps to 18q21 and encodes a TGF-β-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell. 1996;86:543–552. doi: 10.1016/s0092-8674(00)80128-2. [DOI] [PubMed] [Google Scholar]
- Goldberg Y. Protein phosphatase 2A: Who shall regulate the regulator? Biochem Pharmacol. 1999;57:321–328. doi: 10.1016/s0006-2952(98)00245-7. [DOI] [PubMed] [Google Scholar]
- Griswold-Prenner I, Kamibayashi C, Maruoka EM, Mumby MC, Derynck R. Physical and functional interactions between type I transforming growth factor β receptors and bα, a WD-40 repeat subunit of phosphatase 2A. Mol Cell Biol. 1998;18:6595–6604. doi: 10.1128/mcb.18.11.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Chen YG, Massague J, Kuriyan J. Crystal structure of the cytoplasmic domain of the type I TGF β receptor in complex with FKBP12. Cell. 1999;96:425–436. doi: 10.1016/s0092-8674(00)80555-3. [DOI] [PubMed] [Google Scholar]
- Jefferies HB, Reinhard C, Kozma SC, Thomas G. Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc Natl Acad Sci. 1994;91:4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagna G, Hata A, Hemmati-Brivanlou A, Massague J. Partnership between DPC4 and SMAD proteins in TGF-β signalling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- Lane HA, Fernandez A, Lamb NJ, Thomas G. p70s6k function is essential for G1 progression. Nature. 1993;363:170–172. doi: 10.1038/363170a0. [DOI] [PubMed] [Google Scholar]
- Like B, Massague J. The antiproliferative effect of type β transforming growth factor occurs at a level distal from receptors for growth-activating factors. J Biol Chem. 1986;261:13426–13429. [PubMed] [Google Scholar]
- Liu X, Sun Y, Constantinescu SN, Karam E, Weinberg RA, Lodish HF. Transforming growth factor β-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci. 1997;94:10669–10674. doi: 10.1073/pnas.94.20.10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias-Silva M, Abdollah S, Hoodless PA, Pirone R, Attisano L, Wrana JL. MADR2 is a substrate of the TGF-β receptor and its phosphorylation is required for nuclear accumulation and signaling. Cell. 1996;87:1215–1224. doi: 10.1016/s0092-8674(00)81817-6. [DOI] [PubMed] [Google Scholar]
- Massague J, Chen TG. Controlling TGF-β signaling. Genes & Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- Nakao A, Roijer E, Imamura T, Souchelnytskyi S, Stenman G, Heldin CH, ten Dijke P. Identification of Smad2, a human Mad-related protein in the transforming growth factor β signaling pathway. J Biol Chem. 1997;272:2896–2900. doi: 10.1074/jbc.272.5.2896. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci. 1993;15:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RB, Thomas G. Regulation of p70s6k/p85s6k and its role in the cell cycle. Prog Cell Cycle Res. 1995;1:21–32. doi: 10.1007/978-1-4615-1809-9_3. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycinassociated protein. Proc Natl Acad Sci. 1999;96:4438–4442. doi: 10.1073/pnas.96.8.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petritsch C, Woscholski R, Edelmann HM, Ballou LM. Activation of p70 S6 kinase and erk-encoded mitogen-activated protein kinases is resistant to high cyclic nucleotide levels in Swiss 3T3 fibroblasts. J Biol Chem. 1995;270:26619–26625. doi: 10.1074/jbc.270.44.26619. [DOI] [PubMed] [Google Scholar]
- Pullen N, Dennis PB, Andjelkovic M, Dufner A, Kozma SC, Hemmings BA, Thomas G. Phosphorylation and activation of p70s6k by PDK1. Science. 1998;279:707–710. doi: 10.1126/science.279.5351.707. [DOI] [PubMed] [Google Scholar]
- Reichmann E, Schwarz H, Deiner EM, Leitner I, Eilers M, Berger J, Busslinger M, Beug H. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell. 1992;71:1103–1116. doi: 10.1016/s0092-8674(05)80060-1. [DOI] [PubMed] [Google Scholar]
- Schonthal AH. Role of PP2A in intracellular signal transduction pathways. Front Biosci. 1998;3:D1262–D1273. doi: 10.2741/A361. [DOI] [PubMed] [Google Scholar]
- Souchelnytskyi S, Tamaki K, Engstrom U, Wernstedt C, ten Dijke P, Heldin CH. Phosphorylation of Ser465 and Ser467 in the C terminus of Smad2 mediates interaction with Smad4 and is required for transforming growth factor-β signaling. J Biol Chem. 1997;272:28107–28115. doi: 10.1074/jbc.272.44.28107. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Miyazono K, Heldin CH. Signaling inputs converge on nuclear effectors in TGF-β signaling. Trends Biochem Sci. 2000;25:64–70. doi: 10.1016/s0968-0004(99)01519-4. [DOI] [PubMed] [Google Scholar]
- Wang SS, Esplin ED, Li JL, Huang L, Gazdar A, Minna J, Evans GA. Alterations of the PPP2R1B gene in human lung and colon cancer. Science. 1998;282:284–287. doi: 10.1126/science.282.5387.284. [DOI] [PubMed] [Google Scholar]
- Wang T, Donahoe PK, Zervos AS. Specific interaction of type I receptors of the TGF-β family with the immunophilin FKBP-12. Science. 1994;265:674–676. doi: 10.1126/science.7518616. [DOI] [PubMed] [Google Scholar]
- Wang T, Li BY, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. The immunophilin FKBP12 functions as a common inhibitor of the TGF β family type I receptors. Cell. 1996;86:435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]
- Weng QP, Kozlowski M, Belham C, Zhang A, Comb MJ, Avruch J. Regulation of the p70 S6 kinase by phosphorylation in vivo. Analysis using site-specific anti-phosphopeptide antibodies. J Biol Chem. 1998;273:16621–16629. doi: 10.1074/jbc.273.26.16621. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L. The Smad pathway. Cytokine Growth Factor Rev. 2000;11:5–13. doi: 10.1016/s1359-6101(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Yang X, Letterio JJ, Lechleider RJ, Chen L, Hayman R, Gu H, Roberts AB, Deng C. Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-β. EMBO J. 1999;18:1280–1291. doi: 10.1093/emboj/18.5.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng X, We R, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF-β response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]