The emerging functions of septins in metazoans (original) (raw)
Abstract
Septins form a subfamily of highly related GTP-binding proteins conserved from eukaryotic protists to mammals. In most cases, septins function in close association with cell membranes and the actin and microtubule cytoskeleton to regulate a wide variety of key cellular processes. Further underscoring their importance, septin abnormalities are associated with several human diseases. Remarkably, septins have the ability to polymerize into assemblies of different sizes in vitro and in vivo. In cells, these structures act in the formation of diffusion barriers and scaffolds that maintain subcellular polarity. Here, we focus on the emerging roles of vertebrate septins in ciliogenesis, neurogenesis, tumorigenesis and host–pathogen interactions, and discuss whether unifying themes underlie the molecular function of septins in health and disease.
Keywords: septin, plasma membrane, actin, microtubule, diffusion barrier
See Glossary for abbreviations used in this article.
Glossary.
AFM atomic force microscopy
BBSome Bardet–Biedel syndrome proteins
CNS central nervous system
CRIK citron kinase
ERM ezrin–radixin–moesin domain
FcγR Fc gamma receptor
FRAP fluorescence recovery after photobleaching
IFT intraflagellar transport
Kar9 karyogamy protein 9
MAP microtubule-associated protein
MLC myosin light chain kinase
MLL mixed lineage leukaemia
NM2 type II non-muscle myosin
NHEFR Na+/H+ exchanger 3 regulatory factor
PCP planar cell polarity
PDZ PSD95–DlgA–ZO1 domain
RNAi RNA interference
ROCK Rho kinase
SEPT septin
Introduction
Cells contain a complex mixture of macromolecules. How order is established and maintained in cells remains a tantalizing question. A central strategy of eukaryotes seems to be the compartmentalization of cellular spaces—for example, through membrane encapsulation of organelles. Two additional layers of compartmentalization have become evident over the years. Scaffolding platforms, such as centrioles, provide a recruitment surface for the localized integration of signalling processes and anchorage of cytoskeletal structures. Lateral diffusion barriers, on the other hand, promote the compartmentalization of the plane of membranes into distinct domains. These barriers slow down the diffusion of membrane-associated molecules over boundaries and also act as scaffolding platforms. Increasing evidence suggests that such barriers are highly conserved among organisms, and are involved in the function of numerous cellular structures and appendages.
At the molecular level, a class of P-loop GTPase proteins—the septins—has been recurrently associated with lateral diffusion barriers. With the exception of land plants, septin genes are found in all eukaryotes. A group of related genes—the paraseptins—are also found in prokaryotes. The number of septin genes varies greatly between organisms: Homo sapiens has 13, the nematode Caenorhabditis elegans has only two and the budding yeast Saccharomyces cerevisiae has seven. Further complexity is added in many organisms by alternative splicing (Cao et al, 2007; Pan et al, 2007). Septins show a marked ability to oligomerize into hetero-complexes, which in turn polymerize into large assemblies composed of paired septin filaments (Bertin et al, 2008; DeMay et al, 2011; Sellin et al, 2011; Sirajuddin et al, 2007). On the basis of sequence homology, the 13 human septins can be divided into four subgroups (Table 1; Kinoshita, 2003). In cells, they form stable 6–8-subunit complexes containing members of at least three subgroups (II, VI and VII), with the 8-subunit complexes being marked by the presence of subgroup III septins (Table 1; Sellin et al, 2011). At least in yeast, the ability to from filaments is crucial for the function of septins (McMurray et al, 2011). Unlike actin filaments and microtubules, however, neither the septin complexes nor septin filaments are polar (Sirajuddin et al, 2007; John et al, 2007).
Table 1. Human septins, their classification, expression patterns and associations to human diseases.
| Septin | Aliases | Subgroup | Expression (*altered in cancer) | Disease association |
|---|---|---|---|---|
| SEPT1 | DIFF6, LARP, MGC20394, PNUTL3 | II | Lymphocytes, CNS* | Alzheimer disease, leukaemia, lymphoma, oral cancer |
| SEPT2 | DIFF6, KIAA0158, NEDD5 | II | Ubiquitous* | Alzheimer disease, bacterial infection, brain, liver and renal cancer, Von Hippel–Lindau syndrome |
| SEPT3 | MGC133218, bK250D10.3a | III | CNS* | Alzheimer disease, brain cancer, teratocarcinoma |
| SEPT4 | ARTS, BRADEION, Bradeion β, brain protein H5, CE5B3 | II | CNS, eye, testes* | Alzheimer disease, Parkinson disease, male infertility, skin, brain, breast, lung, liver, head and neck, urogenital and colon cancer, leukaemia, myeloma |
| SEPT5 | CDCREL, CDCREL-1, CDCREL1, CDCrel-1 | II | Ubiquitous* | Parkinson disease, schizophrenia, bipolar disorder, congenital hydrocephalus, leukaemia, pancreatic cancer |
| SEPT6 | KIAA0128, MGC16619, MGC20339 | VI | Ubiquitous* | Bipolar disorder, hepatitis C, leukaemia |
| SEPT7 | CDC10, CDC3, Nbla02942, SEPT7A | VII | Ubiquitous* | Male infertility, brain cancer |
| SEPT8 | KIAA0202 | VI | CNS, lymphocytes, intestinal track, placenta, eye* | Retinal degeneration |
| SEPT9 | AF17q25, FLJ75490, KIAA0991, MSF-A | III | Ubiquitous* | Hereditary neuralgic amyotrophy, bacterial infection, leukaemia, breast, ovarian, colorectal, liver and head and neck cancer, Hodgkin lymphoma |
| SEPT10 | FLJ11619 | VI | Ubiquitous* | |
| SEPT11 | FLJ10849 | VI | Ubiquitous* | Schizophrenia, bipolar disorder, bacterial infection, liver cancer, leukaemia |
| SEPT12 | FLJ25410 | III | Testes, lymphocytes* | Male infertility |
| SEPT14 | FLJ44060 | VI | Testes, CNS | – |
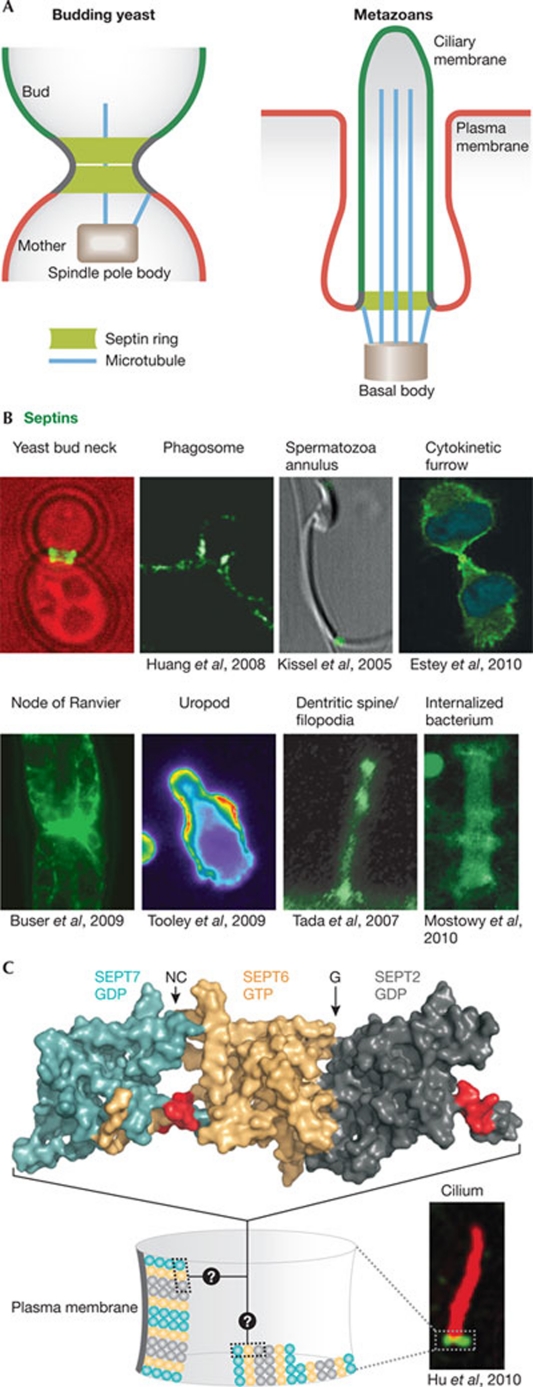
In cells, higher-order septin ring-like structures are found at the base of cilia, flagella, dendritic spines and yeast buds, where they associate tightly with the plasma membrane and recruit other molecules (the 'septin ring' paradigm, shown in Fig 1). Thereby septins mediate the formation of a lateral diffusion barrier in the membrane with which they have contact, and compartmentalize the membrane into two domains. The septin rings also act as cortical organizers for the actin and microtubule cytoskeleton. In yeast, the septin ring can exist in a 'frozen' state, in which subunits do not exchange, and in a 'fluid' state, in which subunits are rapidly exchanged (Dobbelaere et al 2003; Caviston et al, 2003). However, recent fluorescence intensity measurements suggested that septins are constantly, but slowly, recruited to the ring, until the ring splits (Chen et al, 2011). During bud growth and cytokinesis, the septin ring is in its frozen state, transiting through the fluid state only briefly during ring formation and splitting. During ring splitting, the filaments reorient 90° in a switch-like manner from parallel to perpendicular relative to the mother–bud axis (Vrabioiu & Mitchison, 2006; DeMay et al, 2011). However, the dynamics and orientation of septins in metazoan rings are not known (Fig 1C).
Figure 1.
The septin ring paradigm. (A) Septins assemble at the neck of budding yeast cells in a ring-like collar. This functions as a diffusion barrier to control the exchange of molecules between the mother and the bud, and as a scaffold to anchor other proteins, such as astral microtubules emanating from the spindle pole body (centriole equivalent in yeast). Cilia contain a structurally and functionally analogous septin structure at their base. (B) In metazoans, membrane-associated septin assemblies are found at the base of the indicated cellular structures and around intracellular pathogens (Shigella flexneri). (C) Structure of the SEPT2 (turquoise)–SEPT6 (gold)–SEPT7 (dark grey) complex (Protein Data Bank: 2QAG; Sirajuddin et al, 2007). The proposed membrane-binding interface (polybasic region; Zhang et al, 1999) is shown in red and the arrows denote the NC- and G-oligomerization interfaces. When assembled into rings, these oligomers probably form filaments, but the orientation of filaments in metazoan septin rings is unknown. Images in panels B and C are reproduced with permission from the relevant publishers. SEPT, septin.
This septin-ring-dependent mechanism of cellular compartmentalization was first described in yeast (Barral et al, 2000; Takizawa et al, 2000). The more recent finding that septin-dependent cellular compartmentalization has important roles in the structure and function of cilia, flagella and dendritic spines in metazoans might explain why septin abnormalities are linked to a variety of diseases, such as Alzheimer and Parkinson, schizophrenia, cancer, infertility and pathogen infection (Connolly et al, 2011b; Mostowy & Cossart, 2011; Toure et al, 2011; Peterson & Petty, 2010; Hall & Russell, 2004). Several recent reviews give a comprehensive view on septin biology and the structural and biochemical aspects of septin complexes (Cao et al, 2009; Caudron & Barral, 2009; Gilden & Krummel, 2010; Gladfelter, 2010; McMurray & Thorner, 2009; Spiliotis, 2010; Weirich et al, 2008). Here, we focus our attention on the emerging roles of animal septins and their adaptation to novel functions during the evolution of multicellularity.
Septins in the formation and maintenance of cilia
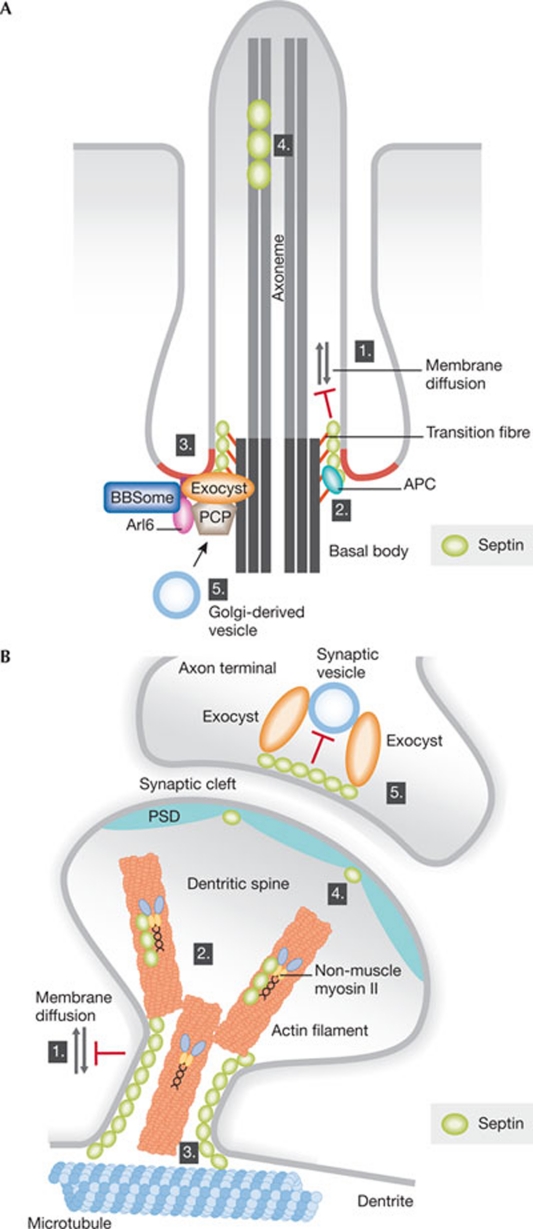
One of the best examples of how the septin ring paradigm (Fig 1) is conserved in higher eukaryotes comes from cilia, at the base of which septins form a ring-like structure. Cilia and flagella (hereafter referred to as 'cilia') are ancient organelles involved in generating movement and in sensing and transducing extracellular signals such as morphogens, odours, light and mechanical stress into the cell (Berbari et al, 2009). Their core structure, called an 'axoneme', is composed of nine cylindrically arranged microtubule pairs (9 + 0 in primary cilia), accompanied by one central microtubule pair in motile cilia (9 + 2). This axoneme arises from the distal end of the mother centriole, known as the 'basal body' (Figs 1A, 2A). During ciliogenesis, Golgi-derived vesicles are targeted to the distal pole of the basal body to form the ciliary membrane, which is anchored to the basal body by a set of microtubules called the transition fibres (Rohatgi & Snell, 2010; Sorokin, 1962). The elongation of cilia occurs through IFT-mediated delivery of structural subunits to the tip of the axoneme. This growth eventually leads to the fusion of the ciliary membrane with the plasma membrane and the emergence of the cilium from the surface of the cell (Rohatgi & Snell, 2010).
Figure 2.
Possible roles for septins in cilia and neuronal synapses. (A) Septins might contribute to ciliogenesis in different ways. (1) The septin ring acts as a lateral diffusion barrier at the base of the cilium and controls the transport of membrane proteins. (2) The septin ring could provide the attachment site for the transition fibres (orange), directly or through adaptor proteins such as APC. (3) The septin ring might function as a scaffold to recruit and/or organize the function of the exocyst, PCP proteins and the BBSome. (4) Septins are also present at the tip and shaft of the cilium and could influence microtubule dynamics and the IFT. (5) Septins could also function in vesicle transport to the basal body. The red colour at the base of the cilium indicates the ciliary necklace region, which displays a high degree of positive membrane curvature, possibly recognized by amphipathic helices (such as in the BBSome subunit Arl6). (B) (1) Septins localize to the base of the dendritic spines, where they might act as lateral diffusion barriers to restrict exchange between the dendritic shaft and the spine. Septins could also influence spine morphogenesis by regulating (2) actomyosin structures or (3) microtubules. Moreover, (4) septins are enriched in the PSD and (5) hinder synaptic vesicle release at the presynaptic compartment by acting as membrane-apposed barriers. APC, adenomatous polyposis coli; BBSome, Bardet–Biedel syndrome proteins; IFT, intraflagellar transport; PCP, planar cell polarity; PSD, postsynaptic density.
Although continuous with the plasma membrane, the ciliary membrane is insulated from it, with a distinct molecular composition adapted to its functions. Interestingly, septins assemble in a ring-like array at the base of both the motile cilia of frog embryos (Kim et al, 2010) and the primary cilia of mammalian cells (Hu et al, 2010). In both cases, reduction of septin expression causes a reduction in the number of cilia and their length, and defects in cilia-dependent sonic hedgehog signalling. In addition to its similarity with the septin ring of yeast cells, the localization of ciliary septins is reminiscent with that of SEPT4/12 at the annulus of the sperm tail (Fig 1B; Ihara et al, 2005; Kissel et al, 2005; Steels et al, 2007; Caudron & Barral, 2009).
How is the ciliary membrane insulated from the plasma membrane? FRAP assays of ciliary membrane proteins showed that although the proteins diffuse freely within the ciliary compartment, they do not display fluorescence recovery when the entire ciliary pool is bleached (Hu et al, 2010). Thus, their exchange with the rest of the plasma membrane is restricted at the base of the cilium. By contrast, non-membrane proteins exchange rapidly between the cilium and the cell. Remarkably, disruption of the septin ring at the base of the cilium by RNAi markedly increases the diffusional mobility of the ciliary membrane proteins between the cilium and the remainder of the plasma membrane (Hu et al, 2010). These data indicate that the septin ring at the base of cilia might mediate the formation of a lateral diffusion barrier that insulates the inner leaflet of the ciliary membrane from the plasma membrane. Notably, proteins mutated in nephronophthisis and Meckel syndrome localize to the base of cilia in a similar way to septins, where they link the axoneme to the ciliary membrane and might also have a barrier function (Williams et al, 2011). Furthermore, lipids are more condensed at the base of the cilium than in the apical part or in the surrounding plasma membrane (Vieira et al, 2006). Thus, accumulation of specific lipids and other proteins are likely to function together with septins in barrier formation.
Are the septin-dependent diffusion barriers impermeable, or do they only slow down the kinetics of molecule exchange between compartments? For the diffusion barriers in yeast, the latter is probably closer to reality (Valdez-Taubas & Pelham, 2003; Luedeke et al, 2005). If septin filament assembly is actually required for barrier formation in cilia as in yeast, the orientation and/or dynamics of these filaments are likely to determine the tightness of the barrier. How can the ciliary diffusion barrier be selective to allow some membrane proteins to enter whereas others are excluded? If the diffusion barrier allows some 'leaky' diffusion, this might promote proteins that are able to transiently interact with septins (or septin-associated molecules) to enter the cilium. This process might also be aided by the IFT machinery. By contrast, if septins form a tight barrier, import of membrane proteins would depend solely on vesicle delivery (Fig 2A). Some proteins containing a PDZ-binding motif—such as podocalyxin—might also be retained outside the cilium through interactions with an apical protein network composed of NHEFR, ERM proteins and actin (Francis et al, 2011). Thus, keeping some proteins in and excluding others from the cilium might in some cases be two distinct processes. Collectively, these data indicate that a lateral diffusion barrier formed by a scaffold of proteins (including septins) and possibly highly ordered lipids, controls the movement of membrane-bound material in and out of cilia. How the barrier works in terms of establishment, plasticity and selectivity remains to be determined.
Is septin function in ciliogenesis restricted to the formation of a diffusion barrier? The reduced number of cilia in SEPT2-depleted cells might be due to the dissolution of the cilia in the absence of a barrier, although this phenotype might also reflect the additional roles of septins during early ciliogenesis. Septins might direct the delivery and anchorage of vesicles to the basal body and/or inhibit their docking to the plasma membrane, as in neuronal synapses (Yang et al, 2010). It is unknown how vesicles initially reach the basal body and how subsequent vesicles are tethered to and fused with the ciliary membrane. SEPT2, which has a role in the transport of Golgi vesicles to the apical surface of epithelial cells (Spiliotis et al, 2008), might be involved in this process. Importantly, mammalian septins interact with and regulate the exocyst complex by directing it to the correct location and by regulating membrane fusion (Estey et al, 2010; Amin et al, 2008; Beites et al, 1999, 2005). Furthermore, the exocyst pathway contributes to the function of the PCP pathway during ciliogenesis, as the PCP protein Dishevelled mediates recruitment of Sec8-positive vesicles to the basal body (Park et al, 2008). These observations are particularly suggestive, because it was shown that SEPT2/7 depletion phenocopies the knockdown effect of the PCP component Fritz during Xenopus gastrulation and ciliogenesis (Kim et al, 2010). Remarkably, Fritz interacts with septin and Fritz controls the proper localization of septins during ciliogenesis. Thus, in addition to forming a diffusion barrier, septins might function in vesicle transport and the formation of the ciliary membrane, together with the exocyst and the PCP pathway (Fig 2A).
Septins are also good candidates to organize spatially the regulation of the BBSome, a protein complex linked to the Bardet–Biedl syndrome, which functions in the sorting and docking of vesicles to the base of the cilium, together with Rab8, Rab11 and Rabin8 (Knodler et al, 2010; Nachury et al, 2007). Localization of the BBSome to the membrane depends on BBS3, also known as the Arf GTPase Arl6 (Jin et al, 2010), which localizes to the base of the cilium in a ring-like array similar to septins (Wiens et al, 2010). Since mutations in Fritz are associated with BBS (Kim et al, 2010), it is tempting to speculate that a molecular pathway links PCP, septins and the BBSome in the regulation of ciliogenesis and ciliary transport.
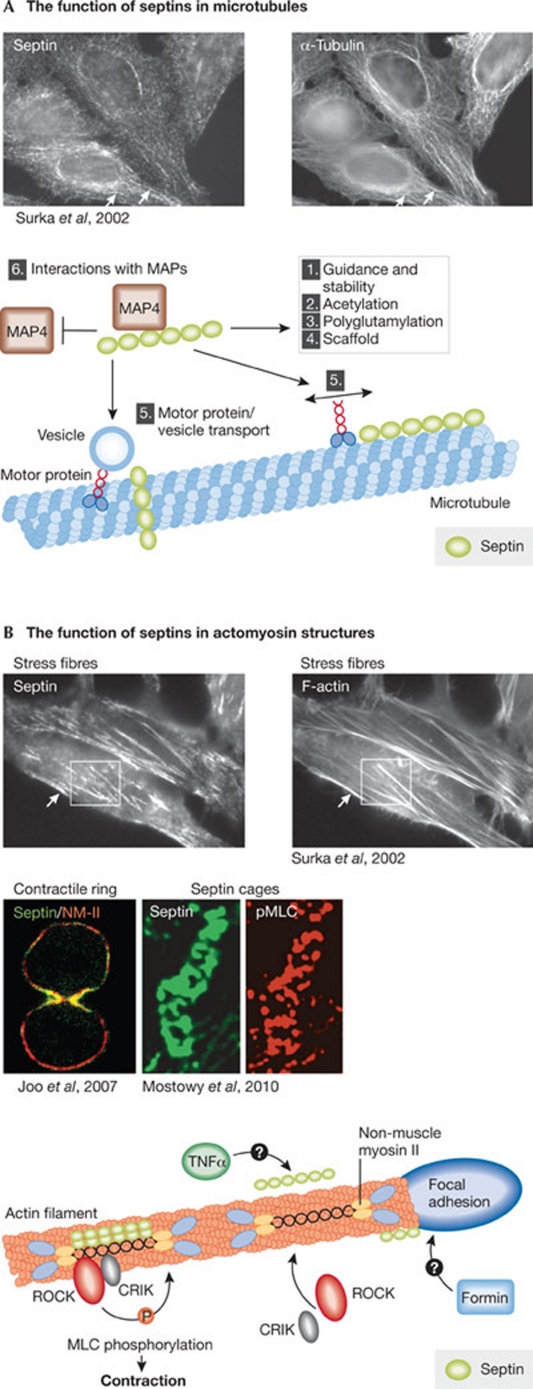
Finally, septins might contribute to the regulation of microtubule function during ciliogenesis. First, septins localize partly to the ciliary shaft (Hu et al, 2010; Kim et al, 2010), where they might regulate microtubule dynamics and vesicle transport. A recent study showed that, during epithelial polarization, septin scaffolds guide the growth and direction of microtubules by suppressing their disassembly (Bowen et al, 2011). Previous studies have also established that septins regulate microtubule stability through association with MAP4, microtubule acetylation and binding to polyglutamated microtubules (Fig 3A; Kremer et al, 2005; Nagata et al, 2003; Spiliotis et al, 2005, 2008; Spiliotis, 2010; Surka et al, 2002). Most intriguingly, however, the unique localization of septins to the base of the cilium makes them prime candidates for membrane attachment of the transition fibres. This possibility is attractive because budding yeast septins also contribute to the capture of astral microtubules to the plasma membrane (Kusch et al, 2002), and thereby to the proper localization of the centrosome and basal-body-equivalent to the bud neck (Figs 1A,2A). This interaction is mediated by MAP Kar9, which is at least functionally related to metazoan adenomatous polyposis coli (APC). Remarkably, deletion of APC in the renal epithelia of mice results in polycystic kidneys, a common consequence of cilia dysfunction (Qian et al, 2005). It will be interesting to test whether septins control microtubule organization in cilia.
Figure 3.
Interplay between septins and the actin and microtubule cytoskeletons. (A) Septins localize to microtubules and might influence them in different ways: (1) guide microtubule growth and prevent their disassembly; (2) influence microtubule acetylation; (3) bind to polyglutamated microtubules; (4) act as scaffolds to recruit other proteins to microtubules; (5) regulate the motility of microtubule-driven motors such as CENP-E and influence vesicle transport along microtubules; and (6) interact with MAPs to control the dynamics and identity of microtubules. (B) In mammalian cells, some septins localize to actomyosin structures, including stress fibres, contractile rings and around actomyosin-covered intracellular bacteria. Septins are recruited to stress fibres by binding to NM2, and this interaction might be enhanced by different factors such as TNFα. Septins recruit ROCK and CRIK to promote MLC phosphorylation and actomyosin contractility, and can possibly recruit formins to promote actomyosin assembly. Images in panels A and B are reproduced with permission from the relevant publishers. CENP-E, centromere-associated protein E; CRIK, citron kinase; MAP, microtubule-associated protein; MLC, myosin light chain kinase; NM2, type II non-muscle myosin; TNFα, tumour necrosis factor α; ROCK, Rho kinase.
Septins in the central nervous system
Another prime example in which a septin ring might function in cell compartmentalization comes from neurons. Many septins are abundantly expressed in the CNS where they display cell-type-specific expression patterns (Tada et al, 2007; Buser et al, 2009) and are associated with diverse neuropathological conditions (Table 1). Many septins localize in ring-like assemblies at the base of dendritic spines and to dendritic branch points of hippocampal neurons. Knockdown of SEPT7 or SEPT11 reduces dendritic branching and spine density, whereas SEPT7 overexpression has the opposite effect (Cho et al, 2011; Li et al, 2009; Tada et al, 2007; Xie et al, 2007). Dendritic spines are small protrusions on dendrites that function in processing and storing information. Although thousands of spines reside on the same dendrite, each of them functions independently from the others, indicating that each spine is functionally insulated. This insulation occurs partly at the spine neck, which restricts exchange of molecules between the spine head and the dendrite (Ashby et al, 2006; Bloodgood & Sabatini, 2005). Analogously to cilia, septins localize to the base of spines (Figs 1B,2B). However, whether septins affect the diffusion dynamics of membrane proteins in and out of spines is unknown. Individualization of spines contributes to memory formation and thereby septin defects might have an impact on this process. SEPT7 expression increases during formation of spatial memory in mice, which supports this idea (Engmann et al, 2011). Together, these data indicate an evolutionarily conserved function of the septin ring in individualizing subcellular structures.
As in the cilium and the yeast bud neck, the function of septins in the dendrites is probably not restricted to barrier formation, but might extend to the modulation of actin and microtubule organization. For example, the septin interaction partner myosin NM2 is required for proper morphogenesis of the spine neck and head (Ryu et al, 2006; Joo et al, 2007). Microtubules are also found in some spines (Dent et al, 2011). These events might be orchestrated by septins (Fig 2B). Similarly, interactions between septins, actomyosin and microtubules might underlie the role of septins in dendritic branching and neuronal migration (Shinoda et al, 2010).
Septins control several essential functions of neuronal cells in vitro, septin knockout mice show relatively mild phenotypes in CNS function. SEPT4 knockout mice have reduced dopaminergic transmission at the synapses of the striatonigral pathway and increased aggregation of α-synuclein, which are phenotypes associated with Parkinson disease (Ihara et al, 2007). Knockout of SEPT6, SEPT5 or SEPT3 alone or of SEPT5 and SEPT3 together does not lead to any gross abnormalities in CNS morphology in mice (Fujishima et al, 2007; Ono et al, 2005; Peng et al, 2002; Tsang et al, 2008). However, SEPT5 knockout mice behave abnormally (Suzuki et al, 2009). Together, these data indicate that the various septins have partly redundant roles in the CNS.
Septins and cell migration in cancer invasion
The septin ring paradigm might not always be well-suited to explain the reported functions of septins. Changes in the expression levels of septins have been found repeatedly in different types of cancer (reviewed in Connolly et al, 2011b; Peterson & Petty, 2010). There are many ways by which septins could contribute to tumorigenesis, ranging from their role in cell cycle progression (Spiliotis et al, 2005; Estey et al, 2010; Peterson & Petty, 2010), to the DNA damage response (Kremer et al, 2007) and being MLL fusion partners in leukaemia (Connolly et al, 2011b). Recently, considerable progress has been made in understanding the function of septins during cell motility and invasion—processes underlying cancer metastasis (Gonzalez et al, 2007; Shankar et al, 2010). Indeed, changes in septin expression alter single and collective modes of cell migration (Connolly et al, 2011a; Kim et al, 2010; Shankar et al, 2010; Tooley et al, 2009).
Orchestrated cellular movement depends on actin-polymerization-driven or bleb-expansion-driven protrusions at the front of the cell, followed by contractility-driven retraction of its rear. The requirements for adhesion, contraction and protrusion vary largely, depending on the mode of migration (Lämmermann & Sixt, 2009). T cells, which migrate in an amoeboid-like manner, express multiple septins—SEPT2/4/6/7/8/9/11—which form a 'corset' around the rear of these cells. Importantly, this rear, commonly known as the 'uropod', has a different molecular identity to the front of the cell. This asymmetry is partly driven by the enrichment of PI(4,5)P2, and PI(4,5)P2-binding proteins at the rear (Lokuta et al, 2007), which might enhance plasma-membrane–actin linkage and prevent unwanted protrusions. It is possible that the septin corset promotes this asymmetry by restricting lipid diffusion between the front and rear of the cell.
Interestingly, the polymerization of septin complexes into filaments and sheets is enhanced on the surface of lipid monolayers that contain PI(4,5)P2 (Bertin et al, 2010), and septins are also regulated by PI(4,5)P2 in vivo. Septins and PI(4,5)P2 localize together at the yeast bud neck (Garrenton et al, 2010) and the furrow of dividing mammalian cells (Field et al, 2005), and experimental conversion of PI(4,5)P2 into PI(3,4,5)P3 causes disassembly of the septin ring in yeast (Bertin et al, 2010; Rodriguez-Escudero et al, 2005). These data indicate that PI(4,5)P2 might have a major role in recruiting and orchestrating the assembly of septin complexes and higher-order assemblies in cells, but how this occurs is not yet known.
Remarkably, knockdown of the core structural unit of the T-cell corset, SEPT7, impairs cell migration but enhances cell invasion through narrow spaces. Additionally, it induces plasma membrane blebbing, a phenotype also seen after SEPT7 depletion during Xenopus gastrulation (Kim et al, 2010). Blebbing represents a protease-independent mode of 'amoeboid-type' cell motility that is used by leukocytes, invasive cancer cells and during developmental cell migration (Lämmermann & Sixt, 2009). The septin corset, which provides rigidity to the plasma membrane, might help cells to maintain direction during migration by preventing protrusions at the uropod. Consequently, loss of the septin corset might change the migratory behaviour of the cell by promoting blebbing, making the cell more elastic, and thus explaining why septin loss enhances the ability of cells to squeeze through narrow holes (Tooley et al, 2009). In favour of this model, SEPT2/11-depleted HeLa cells display reduced cortical elasticity as measured by AFM (Mostowy et al, 2011). This phenotype is mimicked by disruption of actin filaments, suggesting that submembranous septin assemblies impose similar rigidity to the plasma membrane as does the actin cytoskeleton. Furthermore, septins localize to submembranous actin filaments, and FRAP studies have shown that septin turnover is decreased after stabilization of actin filaments (Hagiwara et al, 2011). Thus, septins might function in membrane–actin linkage and/or promote plasma membrane rigidity by themselves. In either case, subtle disturbances in septin expression might predispose cells to blebbing and promote invasive behaviour of cancer cells.
In addition to maintaining plasma membrane rigidity, septins might contribute to the actomyosin contractility of motile cells. The abnormally long tail of septin-depleted migrating T cells could reflect this. Septins associate with the actin cytoskeleton both in yeast and mammals (Norden et al, 2004; Surka et al, 2002). In mammals, they associate with contractile actomyosin structures in non-muscle cells, both at the cleavage furrow and in stress fibres (Fig 3B; Joo et al, 2007; Kinoshita et al, 2002; Kremer et al, 2007; Mostowy et al, 2009; Surka et al, 2002) and also localize to focal adhesions where stress fibres are assembled (Bowen et al, 2011). The association with actin occurs indirectly, through binding to anillin and/or NM2 (Joo et al, 2007; Kinoshita et al, 2002). Anillin recruits septins to the actomyosin ring during cell division, but resides in the nucleus during interphase (Oegema et al, 2000). Therefore, during cell motility, septins probably associate with actomyosin by binding to NM2 (Joo et al, 2007). Although the function of septin–actomyosin interactions is incompletely understood, septins seem to act as a regulatory platform on contractile structures by recruiting myosin regulatory molecules such as ROCK and CRIK, to promote MLC phosphorylation and thereby contractility (Fig 3B; Joo et al, 2007). Notably, in yeast, septins also contribute to the recruitment of formins (Pruyne et al, 2004), although it is not clear how or whether this is also the case in metazoans. Finally, some septins—such as SEPT10/11—seem to be highly expressed in muscle tissue (http://biogps.gnf.org/). Thus, septins might have a more general role in actomyosin contractility than appreciated. All together, while the roles of septins in controlling cell motility are only starting to emerge, they are likely to involve the conserved functions in forming plasma-membrane-apposed barriers and scaffolds, and regulation of actomyosin contractility. Future studies will have to determine whether these functions are coupled to each other, and whether changes in septin expression indeed promote metastasis.
An army of septins fighting infectious pathogens
Microorganisms hijack host-cell machineries for different aspects of their life cycle. Septins have a general role in phagocytic processes, which can either act as an entry mechanism for microorganisms to promote infection, or as a host-cell defence mechanism leading to the elimination of the microbe. Phagocytosis is initiated when a 'professional' phagocyte—for example, a macrophage—encounters a microorganism. This leads to the sequential accumulation of phosphoinositide lipids, reorganization of submembranous actin and bending of the plasma membrane to form a cup-like protrusion around the microorganism. The phagocytic cup eventually seals and internalizes the target particle. The pathogen is then either degraded or escapes into the cytosol (Flannagan et al, 2009). Many septins (including SEPT2/6/10/11) are highly expressed in expert phagocytic cells, and SEPT2/11 transiently localize to Fcgγ-receptor (FcgγR)-dependent phagosomes of different cell types (Huang et al, 2008). The appearance of septins is concomitant with PI(4,5)P2 and F-actin accumulation at these sites, although septin inhibition reduces phagocytosis without any obvious effects on the actin cytoskeleton (Huang et al, 2008). Interestingly, SEPT2/9/11, also in 'non-professional' cells, concentrate beneath the attachment site of Listeria monocytogenes and Shigella flexneri bacteria, where they appear to form a ring-shaped collar and are important for bacterial entry (Mostowy et al, 2009). Despite the importance of septins for FcgγR-dependent phagocytosis and bacterial intake, their exact role remains unclear. A recent study in yeast showed that the septin ring assembled at the bud site maintains cell polarity by preventing Cdc42 diffusion from this site (Orlando et al, 2011). In a similar manner, during phagocytosis, septins might act to maintain local plasma membrane identity, possibly increasing membrane–cytoskeleton linkage and/or slowing down the dispersion of activated receptors and their targets to boost signalling and uptake.
Septins are also involved in later stages of Shigella and Listeria infection. Once internalized, Shigella and Listeria recruit the host-cell actin polymerization machinery to drive their intracellular and intercellular motility (Gouin et al, 2005). Surprisingly, intracellular bacteria with F-actin around them often display stable perpendicular filamentous rings, or 'cages', containing SEPT2/6/9/11 (Mostowy et al, 2010). These cages are not present around Shigella cells that lack actin staining, indicating that actin recruitment is a prerequisite for septin cage assembly. Surprisingly, NM2 and phosphorylated MLC also localize around the internalized bacteria and are required for septin cage formation (Mostowy et al, 2010). Importantly, septin cages suppress actin tail formation and the intercellular spread of bacteria (Mostowy et al, 2010). Moreover, they seem to represent an intermediate stage toward uptake and degradation of the bacterium by autophagy (Mostowy et al, 2010). Thus, septins function in determining the fate of internalized bacteria and as such are part of the host immune response. It is interesting to note that, by promoting autophagy, septins link cellular objects—such as actomyosin-surrounded bacteria—to membrane organization—such as formation of the phagophore—and compartmentalization of cellular space.
Conclusions and future perspectives
The recently identified roles for septins in higher eukaryotes have placed septin assemblies in structurally and functionally diverse subcellular structures; however, an increasing body of evidence suggests that the underlying molecular functions of septin assemblies are conserved across these processes from yeast to mammals. The assemblies act to spatially specify, recognize and individualize subcellular membranes and the structures associated with them. Furthermore, they form platforms that link membranes to actomyosin contractility and microtubule dynamics. The evolution to multicellularity seems to correlate with a more complex landscape of septins, septin complexes and the higher-order structures that they form. This plasticity has allowed septins to promote the formation and maintenance of a much wider panel of cellular appendages and ultrastructures, each with different dynamics. How septin function is modulated and adopted to the controlled formation of such a variety of structures, and how septins contribute to the function of these appendages, are important areas for future research (Sidebar A).
Sidebar A | In need of answers.
- What are the factors that dictate when and where septin assemblies are generated? Do different septin complexes differ in function and if so, how?
- Do certain lipids and/or membrane curvature prime septin filament assembly and can septin assemblies generate and/or stabilize membrane curvature in cells?
- What roles do the ring-like septin assemblies have in cells and how are septins assembled in these rings (Fig 1C)? How exactly do septin-dependent diffusion barriers function?
- What interfaces are used for septin–myosin and septin–tubulin interactions? Can septins crosslink the actin and/or microtubule cytoskeletons to membranes or are these interactions mutually exclusive?
Acknowledgments
This work was supported by a Federation of European Biochemical Societies (FEBS) Long Term Fellowship to J.S., and an advanced European Research Council (ERC) grant to Y.B. We thank Fabrice Caudron, Annina Denoth, Manuel Hotz and Alexander Rauch for comments on the manuscript and apologize to authors whose work we could not cite due to space limitations.
Footnotes
The authors declare that they have no conflict of interest.
References
- Amin ND, Zheng YL, Kesavapany S, Kanungo J, Guszczynski T, Sihag RK, Rudrabhatla P, Albers W, Grant P, Pant HC (2008) Cyclin-dependent kinase 5 phosphorylation of human septin SEPT5 (hCDCrel-1) modulates exocytosis. J Neurosci 28: 3631–3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby MC, Maier SR, Nishimune A, Henley JM (2006) Lateral diffusion drives constitutive exchange of AMPA receptors at dendritic spines and is regulated by spine morphology. J Neurosci 26: 7046–7055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral Y, Mermall V, Mooseker MS, Syder M (2000) Compartmentalization of the cell cortex by septins is required for the maintenance of cell polarity in yeast. Mol Cell 5: 841–851 [DOI] [PubMed] [Google Scholar]
- Beites CL, Xie H, Bowser R, Trimble WS (1999) The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat Neurosci 2: 434–439 [DOI] [PubMed] [Google Scholar]
- Beites CL, Campbell KA, Trimble WS (2005) The septin Sept5/CDCrel-1 competes with α-SNAP for binding to the SNARE complex. Biochem J 385: 347–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, O'Connor AK, Haycraft CJ, Yoder BK (2009) The primary cilium as a complex signaling center. Curr Biol 19: R526–R535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Grob P, Park SS, Garcia G 3rd, Patanwala I, Ng HL, Alber T, Thorner J, Nogales E (2008) Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci USA 105: 8274–8279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertin A, McMurray MA, Thai L, Garcia G 3rd, Votin V, Grob P, Allyn T, Thorner J, Nogales E (2010) Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization. J Mol Biol 404: 711–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL (2005) Neuronal activity regulates diffusion across the neck of dendritic spines. Science 310: 866–869 [DOI] [PubMed] [Google Scholar]
- Bowen JR, Hwang D, Bai X, Roy D, Spiliotis ET (2011) Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia. J Cell Biol 194: 187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser AM, Erne B, Werner HB, Nave KA, Schaeren-Wiemers N (2009) The septin cytoskeleton in myelinating glia. Mol Cell Neurosci 40: 156–166 [DOI] [PubMed] [Google Scholar]
- Cao L, Ding X, Yu W, Yang X, Shen S, Yu L (2007) Phylogenetic and evolutionary analysis of the septin protein family in metazoan. FEBS Lett 581: 5526–5532 [DOI] [PubMed] [Google Scholar]
- Cao L, Yu W, Wu Y, Yu L (2009) The evolution, complex structures and function of septin proteins. Cell Mol Life Sci 66: 3309–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caudron F, Barral Y (2009) Septins and the lateral compartmentalization of eukaryotic membranes. Dev Cell 16: 493–506 [DOI] [PubMed] [Google Scholar]
- Caviston JP, Longtine M, Pringle JR, Bi E (2003) The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol Biol Cell 14: 4051–4066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Howell AS, Robenson A, Lew DJ (2011) Dynamics of septin ring and collar formation in Saccharomyces cerevisiae. Biol Chem 392: 689–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SJ, Lee H, Dutta S, Song J, Walikonis R, Moon IS (2011) Septin 6 regulates the cytoarchitecture of neurons through localization at dentritic branch points and bases of protrusions. Mol Cells 32: 89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D, Yang Z, Castaldi M, Simmons N, Oktay MH, Coniglio S, Fazzari MJ, Verdier-Pinard P, Montagna C (2011a) Septin 9 isoform expression, localization and epigenetic changes during human and mouse breast cancer progression. Breast Cancer Res 13: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly D, Abdessekam I, Verdier-Pinard P, Montagna C (2011b) Septin roles in tumorigenesis. Biol Chem 392: 725–738 [DOI] [PubMed] [Google Scholar]
- DeMay BS, Bai X, Howard L, Occhipinti P, Meseroll RA, Spiliotis ET, Oldenbourg R, Gladfelter AS (2011) Septin filaments exhibit a dynamic, paired organization that is conserved from yeast to mammals. J Cell Biol 193: 1065–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent EW, Merriam EB, Hu X (2011) The dynamic cytoskeleton: backbone of dendritic spine plasticity. Curr Opin Neurobiol 21: 175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere J, Gentry MS, Hallberg RL, Barral Y (2003) Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev Cell 4: 345–357 [DOI] [PubMed] [Google Scholar]
- Engmann O, Hortobagyi T, Thompson AJ, Guadagno J, Troakes C, Soriano S, Al-Sarraj S, Kim Y, Giese KP (2011) Cyclin-dependent kinase 5 activator p25 is generated during memory formation and is reduced at an early stage in Alzheimers disease. Biol Psychiatry (in the press). [DOI] [PubMed] [Google Scholar]
- Estey MP, Di Ciano-Oliveira C, Froese CD, Bejide MT, Trimble WS (2010) Distinct roles of septins in cytokinesis: SEPT9 mediates midbody abscission. J Cell Biol 191: 741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field SJ, Madson N, Kerr ML, Galbraith KA, Kennedy CE, Tahiliani M, Wilkins A, Cantley LC (2005) PtdIns(4,5)P2 functions at the cleavage furrow during cytokinesis. Curr Biol 15: 1407–1412 [DOI] [PubMed] [Google Scholar]
- Flannagan RS, Cosio G, Grinstein S (2009) Antimicrobial mechanisms of phagocytes and bacterial evasion strategies. Nat Rev Microbiol 7: 355–366 [DOI] [PubMed] [Google Scholar]
- Francis SS, Sfakianos J, Lo B, Mellman I (2011) A hierarchy of signals regulates entry of membrane proteins into the ciliary membrane domain in epithelial cells. J Cell Biol 193: 219–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishima K, Kiyonari H, Kurisu J, Hirano T, Kengaku M (2007) Targeted disruption of Sept3, a heteromeric assembly partner of Sept5 and Sept7 in axons, has no effect on developing CNS neurons. J Neurochem 102: 77–92 [DOI] [PubMed] [Google Scholar]
- Garrenton LS, Stefan CJ, McMurray MA, Emr SD, Thorner J (2010) Pheromone-induced anisotropy in yeast plasma membrane phosphatidylinositol-4,5-bisphosphate distribution is required for MAPK signaling. Proc Natl Acad Sci USA 107: 11805–11810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden J, Krummel MF (2010) Control of cortical rigidity by the cytoskeleton: emerging roles for septins. Cytoskeleton 67: 477–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladfelter AS (2010) Guides to the final frontier of the cytoskeleton: septins in filamentous fungi. Curr Opin Microbiol 13: 720–726 [DOI] [PubMed] [Google Scholar]
- Gonzalez ME, Peterson EA, Privette LM, Loffreda-Wren JL, Kalikin LM, Petty EM (2007) High SEPT9_v1 expression in human breast cancer cells is associated with oncogenic phenotypes. Cancer Res 67: 8554–8564 [DOI] [PubMed] [Google Scholar]
- Gouin E, Welch MD, Cossart P (2005) Actin-based motility of intracellular pathogens. Curr Opin Microbiol 8: 35–45 [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Tanaka Y, Hikawa R, Morone N, Kusumi A, Kimura H, Kinoshita M (2011) Submembranous septins as relatively stable components of actin-based membrane skeleton. Cytoskeleton (in the press). [DOI] [PubMed] [Google Scholar]
- Hall PA, Russell SE (2004) The pathobiology of the septin gene family. J Pathol 204: 489–505 [DOI] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ (2010) A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science 329: 436–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YW, Yan M, Collins RF, Diciccio JE, Grinstein S, Trimble WS (2008) Mammalian septins are required for phagosome formation. Mol Biol Cell 19: 1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara M et al. (2005) Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell 8: 343–352 [DOI] [PubMed] [Google Scholar]
- Ihara M et al. (2007) Sept4, a component of presynaptic scaffold and Lewy bodies, is required for the suppression of α-synuclein neurotoxicity. Neuron 53: 519–533 [DOI] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV (2010) The conserved Bardet–Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141: 1208–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John CM et al. (2007) The Caenorhabditis elegans septin complex is nonpolar. EMBOJ 26: 3296–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo E, Surka MC, Trimble WS (2007) Mammalian SEPT2 is required for scaffolding nonmuscle myosin II and its kinases. Dev Cell 13: 677–690 [DOI] [PubMed] [Google Scholar]
- Kim SK et al. (2010) Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science 329: 1337–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M (2003) Assembly of mammalian septins. J Biochem 134: 491–496 [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Field CM, Coughlin ML, Straight AF, Mitchison TJ (2002) Self- and actin-templated assembly of mammalian septins. Dev Cell 3: 791–802 [DOI] [PubMed] [Google Scholar]
- Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR, Steller H (2005) The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell 8: 353–364 [DOI] [PubMed] [Google Scholar]
- Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W (2010) Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA 107: 6346–6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer BE, Haystead T, Macara IG (2005) Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol Biol Cell 16: 4648–4659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer BE, Adang LA, Macara IG (2007) Septins regulate actin organization and cell-cycle arrest through nuclear accumulation of NCK mediated by SOCS7. Cell 130: 837–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch J, Meyer A, Snyder MP, Barral Y (2002) Microtubule capture by the cleavage apparatus is required for proper spindle positioning in yeast. Genes Dev 16: 1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmermann T, Sixt M (2009) Mechanical modes of 'amoeboid' cell migration. Curr Opin Cell Biol 21: 636–644 [DOI] [PubMed] [Google Scholar]
- Li X, Serwanski DR, Miralles CP, Nagata K, De Blas AL (2009) Septin 11 is present in GABAergic synapses and plays a functional role in the cytoarchitecture of neurons and GABAergic synaptic connectivity. J Biol Chem 284: 17253–17265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Shen S, Chen F, Yu W, Yu L (2010) Linking the septin expression with carcinogenesis. Mol Biol Rep 37: 3601–3608 [DOI] [PubMed] [Google Scholar]
- Lokuta MA, Senetar MA, Bennin DA, Nuzzi PA, Chan KT, Ott VL, Huttenlocher A (2007) Type Iγ PIP kinase is a novel uropod component that regulates rear retraction during neutrophil chemotaxis. Mol Biol Cell 18: 5069–5080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedeke C, Frei SB, Sbazarini I, Schwarz H, Spang A, Barral Y (2005) Septin-dependent compartmentalization of the endoplasmic reticulum during yeast polarized growth. J Cell Biol 169: 897–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MA, Thorner J (2009) Reuse, replace, recycle. Specificity in subunit inheritance and assembly of higher-order septin structures during mitotic and meiotic division in budding yeast. Cell Cycle 8: 195–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray MA, Bertin A, Garcia G 3rd, Lam L, Nogales E, Thorner J (2011) Septin filament formation is essential in budding yeast. Dev Cell 20: 540–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S, Cossart P (2011) Septins as key regulators of actin based processes in bacterial infection. Biol Chem 392: 831–835 [DOI] [PubMed] [Google Scholar]
- Mostowy S, Nam Tham T, Danckaert A, Guadagnini S, Boisson-Dupuis S, Pizarro-Cerda J, Cossart P (2009) Septins regulate bacterial entry into host cells. PLoS ONE 4: e4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S et al. (2010) Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe 8: 433–444 [DOI] [PubMed] [Google Scholar]
- Mostowy S, Janel S, Forestier C, Roduit C, Kasas S, Pizarro-Cerda J, Cossart P, Lafont F (2011) A role for septins in the interaction between the Listeria monocytogenes invasion protein InlB and the Met receptor. Biophys J 100: 1949–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV et al. (2007) A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213 [DOI] [PubMed] [Google Scholar]
- Nagata K et al. (2003) Filament formation of MSF-A, a mammalian septin, in human mammary epithelial cells depends on interactions with microtubules. J Biol Chem 278: 18538–18543 [DOI] [PubMed] [Google Scholar]
- Norden C, Liakopoulos D, Barral Y (2004) Dissection of septin actin interactions using actin overexpression in Saccharomyces cerevisiae. Mol Microbiol 53: 469–483 [DOI] [PubMed] [Google Scholar]
- Oegema K, Savoian MS, Mitchison TJ, Field CM (2000) Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J Cell Biol 150: 539–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono R et al. (2005) Disruption of Sept6, a fusion partner gene of MLL, does not affect ontogeny, leukemogenesis induced by MLL-SEPT6, or phenotype induced by the loss of Sept4. Mol Cell Biol 25: 10965–10978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando K, Sun X, Zhang J, Lu T, Yokomizo L, Wang P, Guo W (2011) Exo-endocytic trafficking and the septin-based diffusion barrier are required for the maintenance of Cdc42p polarization during budding yeast asymmetric growth. Mol Biol Cell 22: 624–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F, Malmberg RL, Momany M (2007) Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol 7: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB (2008) Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet 40: 871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng XR, Jia Z, Zhang Y, Ware J, Trimble WS (2002) The septin CDCrel-1 is dispensable for normal development and neurotransmitter release. Mol Cell Biol 22: 378–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EA, Petty EM (2010) Conquering the complex world of human septins: implications for health and disease. Clin Genet 77: 511–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne D, Gao L, Bi E, Bretscher A (2004) Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol Biol Cell 15: 4971–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian CN, Knol J, Igarashi P, lin F, Zylstra U, The BT, Williams BO (2005) Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem 280: 3938–3945 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Escudero I, Roelants FM, Thorner J, Nombela C, Molina M, Cid VJ (2005) Reconstitution of the mammalian PI3K/PTEN/Akt pathway in yeast. Biochem J 390: 613–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Snell WJ (2010) The ciliary membrane. Curr Opin Cell Biol 22: 541–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu J, Liu L, Wong TP, Wu DC, Burette A, Weinberg R, Wang YT, Sheng M (2006) A critical role for myosin IIb in dendritic spine morphology and synaptic function. Neuron 49: 175–182 [DOI] [PubMed] [Google Scholar]
- Sellin ME, Sandblad L, Stenmark S, Gullberg M (2011) Deciphering the rules governing assembly order of mammalian septin complexes. Mol Biol Cell (in the press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar J, Messenberg A, Chan J, Underhill TM, Foster LJ, Nabi IR (2010) Pseudopodial actin dynamics control epithelial–mesenchymal transition in metastatic cancer cells. Cancer Res 70: 3780–3790 [DOI] [PubMed] [Google Scholar]
- Shinoda T, Ito H, Sudo K, Iwamoto I, Morishita R, Nagata K (2010) Septin 14 is involved in cortical neuronal migration via interaction with Septin 4. Mol Biol Cell 21: 1324–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A (2007) Structural insight into filament formation by mammalian septins. Nature 449: 311–315 [DOI] [PubMed] [Google Scholar]
- Sorokin S (1962) Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol 15: 363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET (2010) Regulation of microtubule organization and functions by septin GTPases. Cytoskeleton 67: 339–345 [DOI] [PubMed] [Google Scholar]
- Spiliotis ET, Kinoshita M, Nelson WJ (2005) A mitotic septin scaffold for mammalian chromosome congression and segregation. Science 307: 1781–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiliotis ET, Hunt SJ, Hu Q, Kinoshita M, Nelson WJ (2008) Epithelial polarity requires septin coupling of vesicle transport to polyglutamylated microtubules. J Cell Biol 180: 295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steels JD, Estey MP, Froese CD, Reynaud D, Pace-Asciak C, Trimble WS (2007) Sept12 is a component of the mammalian sperm tail annulus. Cell Motil Cytoskel 64: 794–807 [DOI] [PubMed] [Google Scholar]
- Surka MC, Tsang CW, Trimble WS (2002) The mammalian septin MSF localizes with microtubules and is required for completion of cytokinesis. Mol Biol Cell 13: 3532–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki G et al. (2009) Sept5 deficiency exerts pleiotropic influence on affective behaviors and cognitive functions in mice. Hum Mol Genet 18: 1652–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T, Simonetta A, Batterton M, Kinoshita M, Edbauer D, Sheng M (2007) Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr Biol 17: 1752–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD (2000) Plasma membrane compartmentalization in yeast by messenger mRNA transport and a septin diffusion barrier. Science 290: 341–344 [DOI] [PubMed] [Google Scholar]
- Tooley AJ, Gilden J, Jacobelli J, Beemiller P, Trimble WS, Kinoshita M, Krummel MF (2009) Amoeboid T lymphocytes require the septin cytoskeleton for cortical integrity and persistent motility. Nat Cell Biol 11: 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toure A, Rode B, Hunnicutt GR, Escalier D, Gacon G (2011) Septins at the annulus of mammalian sperm. Biol Chem 392: 799–803 [DOI] [PubMed] [Google Scholar]
- Tsang CW, Fedchyshyn M, Harrison J, Xie H, Xue J, Robinson PJ, Wang LY, Trimble WS (2008) Superfluous role of mammalian septins 3 and 5 in neuronal development and synaptic transmission. Mol Cell Biol 28: 7012–7029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez-Taubas J, Pelham HR (2003) Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic recycling. Curr Biol 13: 1636–1640 [DOI] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K (2006) FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin–Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA 103: 18556–18561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabioiu AM, Mitchison TJ (2006) Structural insights into yeast septin organization from polarized fluorescence microscopy. Nature 443: 466–469 [DOI] [PubMed] [Google Scholar]
- Weirich CS, Erzberger JP, Barral Y (2008) The septin family of GTPases: architecture and dynamics. Nat Rev Mol Cell Biol 9: 478–489 [DOI] [PubMed] [Google Scholar]
- Wiens CJ et al. (2010) Bardet–Biedl syndrome-associated small GTPase ARL6 (BBS3) functions at or near the ciliary gate and modulates Wnt signaling. J Biol Chem 285: 16218–16230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL et al. (2011) MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol 192: 1023–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Vessey JP, Konecna A, Dahm R, Macchi P, Kiebler MA (2007) The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr Biol 17: 1746–1751 [DOI] [PubMed] [Google Scholar]
- Yang YM, Fedchyshyn MJ, Grande G, Aitoubah J, Tsang CW, Xie H, Ackerley CA, Trimble WS, Wang LY (2010) Septins regulate developmental switching from microdomain to nanodomain coupling of Ca(2+) influx to neurotransmitter release at a central synapse. Neuron 67: 100–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Kong C, Xie H, McPherson PS, Grinstein S, Trimble WS (1999) Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr Biol 9: 1458–1467 [DOI] [PubMed] [Google Scholar]