Receptor Clustering Is Involved in Reelin Signaling (original) (raw)
Abstract
The Reelin signaling cascade plays a crucial role in the correct positioning of neurons during embryonic brain development. Reelin binding to apolipoprotein E receptor 2 (ApoER2) and very-low-density-lipoprotein receptor (VLDLR) leads to phosphorylation of disabled 1 (Dab1), an adaptor protein which associates with the intracellular domains of both receptors. Coreceptors for Reelin have been postulated to be necessary for Dab1 phosphorylation. We show that bivalent agents specifically binding to ApoER2 or VLDLR are sufficient to mimic the Reelin signal. These agents induce Dab1 phosphorylation, activate members of the Src family of nonreceptor tyrosine kinases, modulate protein kinase B/Akt phosphorylation, and increase long-term potentiation in hippocampal slices. Induced dimerization of Dab1 in HEK293 cells leads to its phosphorylation even in the absence of Reelin receptors. The mechanism for and the sites of these phosphorylations are identical to those effected by Reelin in primary neurons. These results suggest that binding of Reelin, which exists as a homodimer in vivo, to ApoER2 and VLDLR induces clustering of ApoER2 and VLDLR. As a consequence, Dab1 becomes dimerized or oligomerized on the cytosolic side of the plasma membrane, constituting the active substrate for the kinase; this process seems to be sufficient to transmit the signal and does not appear to require any coreceptor.
Correct positioning of neurons of the cortical plate depends on Reelin, an extracellular matrix protein produced by Cajal-Retzius cells (10), on the Reelin receptors apolipoprotein E receptor 2 (ApoER2) and very-low-density-lipoprotein receptor (VLDLR) (35), and on the intracellular adaptor protein disabled 1 (Dab1) (30). Mutations in the corresponding genes, i.e., the Reelin gene (as in the reeler mouse) (12) and the Dab1 gene (as in the scrambler and yotari mice) (16, 32, 37), and deletions of the genes for both ApoER2 and VLDLR (35) result in identical cortical layering defects, suggesting that the gene products are part of the same signaling pathway. The current working model proposes that Reelin binds to ApoER2 and VLDLR (11, 14). Subsequent phosphorylation of Dab1 is a key event leading to the ultimate cell responses required for correct positioning of newly generated neurons (17, 18). Dab1 was originally identified as an interaction partner of Src (15) and contains a phosphotyrosine binding domain which interacts with the unphosphorylated NPXY motif present in the cytoplasmic domains of ApoER2 and VLDLR (19, 34). Phosphorylation of Dab1 induced by Reelin is dependent on the presence of ApoER2 and VLDLR (5) and occurs on Tyr198 and Tyr220 (20). Recent studies demonstrated that members of the Src family of nonreceptor tyrosine kinases (SFKs) are involved in Dab1 phosphorylation in neurons (2, 6). Coreceptors, such as members of the family of cadherin-related neuronal receptors (CNRs), have been proposed to be involved in this pathway (31). Neuronal migration is also regulated by cyclin-dependent kinase 5 (27, 28), but whether this pathway is connected to the Reelin pathway is still not fully explored. Very little is known about the signaling cascade downstream of Dab1; however, recent results demonstrated that Reelin activates SFKs (2, 6) and modulates phosphoinositide 3-kinase-mediated phosphorylation of protein kinase B (PKB)/Akt (4) by a direct interaction of Dab1 with the regulatory subunit p85α (7).
An interesting mechanistic aspect of the function of Reelin was recently elucidated. Reelin molecules form higher-order complexes in vitro and in vivo (36). This observation was further refined by showing that Reelin is secreted in vivo as a disulfide-linked homodimer (22). Deletion of a short region, called the CR-50 epitope, located at the N terminus of the molecule abolishes oligomerization, and the mutated Reelin fails to induce Dab1 phosphorylation in primary mouse neurons. These results are in accordance with earlier observations that an antibody against the CR-50 epitope antagonizes Reelin function in vitro and in vivo (25, 26).
Here we show that clustering of ApoER2 and/or VLDLR induces Dab1 phosphorylation and downstream events including activation of SFKs and modulation of PKB/Akt. Furthermore, modulation of long-term potentiation (LTP), one of the biological effects of Reelin, is also mediated by Reelin-independent receptor clustering. These results strongly suggest that receptor-induced dimerization or oligomerization of Dab1 is sufficient for its phosphorylation and downstream events without the need for an additional coreceptor providing tyrosine kinase activity.
MATERIALS AND METHODS
Antibodies.
Antibodies against the entire ligand binding domains of ApoER2 (Ab 186) and VLDLR (Ab 187) were raised in rabbits by using the corresponding maltose binding protein (MBP) fusion proteins as antigens. Rabbit anti-ApoER2 (Ab 20), which is directed against the intracellular domain of the receptor (33), rabbit anti-Dab1 (2720) (35), and rabbit anti-receptor-associated protein (anti-RAP) (24) are described in the indicated references. Monoclonal mouse anti-Dab1 (D4), mouse anti-Reelin (G10), and mouse anti-VLDLR (6G6) were kind gifts from Andre Goffinet (University of Louvain, Brussels, Belgium). The following antibodies were purchased from the indicated sources: mouse antiphosphotyrosine (PY99), Santa Cruz; horseradish peroxidase (HRP)-coupled anti-V5 antibody, Invitrogen; phosphorylation site-specific antibodies against phospho-SFK (Y418), BioSource, Camarillo, Calif.; phospho-Akt (Ser473; catalog no. 44-622), BioSource; anti-PKB/Akt (catalog no. 9272), Cell Signaling Technology; and anti-Fyn, Upstate Biotechnology. Mouse anti-myc (9E10) was used as the supernatant of the hybridoma cell line 9E10 from the American Type Culture Collection at a dilution of 1:100. The phosphotyrosine-specific anti-Dab antibodies (anti-Dab1-PY198 and anti-Dab1-PY220) were generous gifts from Tom Curran (Department of Developmental Neurobiology, St. Jude's Children's Research Hospital, Memphis, Tenn.).
Expression of recombinant proteins, preparation of cell extracts, electrophoresis, and Western blotting.
Reelin was expressed in 293T cells, and conditioned media were prepared as described previously (8). Reelin-conditioned medium was concentrated by ultrafiltration by using Ultrafree-15 (Millipore). Preparation of myc-tagged RAP and MBP fusion proteins containing the entire ligand binding domains of ApoER2 (ApoER2Δ4-6-MBP/His) and VLDLR (VLDLR1-8-MBP/His) was performed as described in reference 21.
The expression plasmid coding for Fc-RAP was constructed using a PCR fragment coding for full-length rat RAP lacking the endoplasmic reticulum signal HNEL and the stop codon which was amplified using the following primer pair: 3′-RAPrev, 5′-GCCCTCTAGACTCCGAGCCCTTGAGACCCTGCT-3′, and 5′-RAPforw, 5′-CGTGGATCCACCATGCCGCCTCTTAGAGACAGG-3′. ThePCR fragment was cloned into pcDNA3.1/V5/His-TOPO. The insert was released with _Bam_HI and _Xba_I and cloned into the backbone of the VLDLR-FC expression vector (14) cleaved with the same enzymes. A stable cell line expressing the Fc-RAP construct was created as follows. HEK293 cells in four 100-mm-diameter dishes (8 × 105 cells/dish) were transfected using the MBS kit (Stratagene). G418 selection was started 24 h later and was continued for 15 days (0.8 mg of active G418/ml). Twenty surviving colonies were tested for expression and secretion of Fc-RAP. Secreted RAP-V5-Fc samples from two clones with high expression levels were tested by ligand blotting for their ability to bind to lipoprotein receptor-related protein and megalin.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method of Laemmli (23), and proteins were transferred onto nitrocellulose membranes by semidry blotting. For Western blotting with antibodies 20, 186, 187, and G10, nitrocellulose membranes were blocked for 1 h in Tris-buffered saline (TBS)-0.1% Tween (pH 7.4) containing 5% milk powder. For Western blotting using PY99, D4, anti-phospho-Akt, and anti-phospho-SFK, 5% bovine serum albumin instead of milk powder was used. Appropriate HRP-conjugated secondary antibodies (1:20,000; Jackson Immuno Research) were used for detection with enhanced chemiluminescence (Pierce).
Dab1 phosphorylation assay.
The Dab1 phosphorylation assay was performed essentially as described previously (11, 14). Briefly, brains from embryonic-day-15 mouse embryos were homogenized in Hank's balanced salt solution, centrifuged (200 × g for 4 min), resuspended in medium (Dulbecco's modified Eagle medium-nutrient mixture F-12 [Ham] containing B27 supplement [Gibco BRL], 10 mM glutamine, and antibiotics), and plated onto poly-l-ornithine-coated 6-cm-diameter dishes. After 3 days in culture, the cells were washed with Hank's balanced salt solution and incubated with different media containing the indicated ligands (see figures). After 20 min at 37°C, cells were washed again, scraped into 350 μl of radioimmunoprecipitation assay (RIPA) buffer (10 mM sodium phosphate [pH 7.4], 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 2 mM Na3VO4, 1% β-mercaptoethanol, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, and complete protease inhibitor cocktail [Roche]), and lysed for 30 min on ice. The lysates were centrifuged at 20,000 × g for 30 min, and the supernatants were immediately used for immunoprecipitation of Dab1 with 4 μl of 2720 antiserum. After 2 h at 4°C, 20 μl of a suspension containing protein A beads (Amersham) was added for 2 h at 4°C. The beads were washed with RIPA buffer and boiled in reducing Laemmli buffer prior to SDS-PAGE and Western blotting.
Eukaryotic expression constructs.
Constructs Dab wt-1FKBP, containing wild-type (wt) Dab1 and one copy of FK506 binding protein (FKBP12), and Dab 5F-1FKBP, containing FKBP and a mutated version of Dab1 with the relevant five tyrosine residues replaced by phenylalanines (Dab1-5F), were prepared using a dimerizer kit from ARIAD Pharmaceuticals. Briefly, cDNAs for Dab1 and Dab1-5F (18) were amplified by PCR as _Xba_I-_Spe_I fragments and cloned into the _Xba_I site of the pC4-Fv1E plasmid that contains FKBP12 harboring an F36V mutation and a C-terminal hemagglutinin epitope. To generate constructs with wt Dab1 and two copies of FKBP (Dab1 wt-2FKBP), Dab wt-1FKBP plasmids were digested with _Xba_I/_Spe_I and the resulting fragment was again cloned into the _Xba_I site of pC4-Fv1E.
Dab1 dimerization assay.
For expression of wt Dab1, Dab wt-1FKBP, Dab wt-2FKBP, and Dab1 5F-2FKBP, the human embryonic kidney cell line 293 was transfected with the respective constructs using Lipofectin reagent (Life Technologies, Inc.) according to the manufacturer's protocol. For dimerization of FKBP12 chimeras, cells were treated 48 h after transfection with the chemical dimerizer AP20187 (ARIAD Pharmaceuticals) for 20 min at 37°C. AP20187 was used at a final concentration of 40 or 100 nM. Cells were subsequently washed, scraped into RIPA buffer, and lysed on ice for 30 min. Lysates were centrifuged at 21,000 × g for 30 min, and the supernatants were subjected to SDS-PAGE and Western blotting
Phosphorylation of Akt/PKB and SFKs.
Phosphorylation of Akt/PKB and SFKs was measured directly in crude cell extracts derived from primary embryonic rat neurons as described previously (6). Briefly, equal amounts of protein (10 μg) from cell lysates from neuronal cultures treated with control medium, Reelin-conditioned medium, or Ab 186 were separated by 4 to 15% gradient SDS gel electrophoresis, transferred onto nitrocellulose membranes, and blocked in Blotto (5% milk in phosphate-buffered saline with 0.05% Tween 20, pH 7.4; Sigma) for 1 h. Membranes were incubated overnight at 4°C with polyclonal or monoclonal antibodies directed against Dab1, phospho-Akt/PKB (Ser473), and phospho-SFK (Y418). After washing, secondary HRP-linked antibodies (Amersham Biosciences) were applied at 1:20,000 in phosphate-buffered saline-Tween for 1 h, washed, developed with SuperSignal West Pico chemiluminescent substrate (Pierce), and exposed to X-Omat Blue XB-1 film (Eastman Kodak Co.).
Hippocampal slice preparation and electrophysiology.
Adult mice were sacrificed by decapitation, and brains were rapidly removed and briefly submerged in ice-cold cutting saline (110 mM sucrose, 60 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 28 mM NaHCO3, 0.5 mM CaCl2, 5 mM d-glucose, and 0.6 mM ascorbate). All solutions used were saturated with 95% O2 and 5% CO2. Whole brains were dissected on cutting solution-soaked filter paper mounted on a glass platform resting on ice. Hippocampal slices (400 μm) were made using a vibratome and allowed to equilibrate in a 50% cutting saline-50% artificial cerebrospinal fluid (ACSF) solution (125 mM NaCl, 2.5 mM KCl, 1.24 mM NaH2PO4, 25 mM NaHCO3, 10 mM d-glucose, 2 mM CaCl2, and 1 mM MgCl2) at room temperature for a minimum of 30 min. Slices were transferred into an interface chamber supported by a nylon mesh and allowed to recover for a minimum of 1 h prior to recording. Extracellular field recordings were obtained from the area CA1 stratum radiatum. Stimulation was given using a bipolar Teflon-coated platinum electrode, and recordings were obtained with the use of a glass microelectrode filled with ACSF (resistance, 1 to 4 MΩ). The 100-Hz stimulation protocol consisted of two trains of 100-Hz frequency stimulation for 1 s with each train separated by a 20-s interval. Stimulus intensities were adjusted to give population excitatory postsynaptic potentials (pEPSP) with slopes that were ≤50% that of the maximum determined from an input-output curve. The calculated 50% maximum stimulus intensity was used for the 100-Hz LTP-inducing protocol. Potentiation was measured as the normalized increase of the mean pEPSP following tetanic stimulation normalized to the mean pEPSP for the duration of the baseline recording. Experimental results were obtained from those slices that exhibited stable baseline synaptic transmission for a minimum of 30 min prior to the delivery of the LTP-inducing stimulus. Fc-Rap, Fc, or control medium was diluted in oxygenated ACSF and perfused onto hippocampal slices at 1 ml/min.
Solid phase binding assay.
One hundred microliters of TBS (2 mM CaCl2) containing 10 μg of ApoER2Δ4-6-MBP/His or VLDLR1-8-MBP/His/ml was incubated on a 96-well plate overnight at 4°C. All further incubations were carried out at room temperature for 1 h, and Reelin or antibodies were diluted in blocking solution (2% bovine serum albumin in TBS, 2 mM CaCl2, 0.05% Tween). After blocking and binding of Reelin, anti-Reelin antibody (G10) followed by HRP-conjugated secondary antibody was used for detection of bound Reelin. For the color reaction, 0.1 mg of 3,3′,5,5′-tetramethylbenzidine/ml was used in 0.1 M sodium acetate, pH 6.0, containing 10 mM H2O2. The reaction was stopped after 5 min by addition of 0.3 M H2SO4, and bound secondary antibody was photometrically quantified at 450 nm. For the sandwich binding assay, plates were coated with ApoER2Δ4-6-MBP/His as described above. Plates were overlaid with concentrated (100×) Reelin-containing medium followed by the addition of 10 μg of VLDLR1-8-MBP/His/ml. Bound receptor fragments were detected by the addition of a monoclonal antibody against VLDLR (6G6) in combination with an anti-mouse immunoglobulin G (IgG) coupled to HRP.
RESULTS
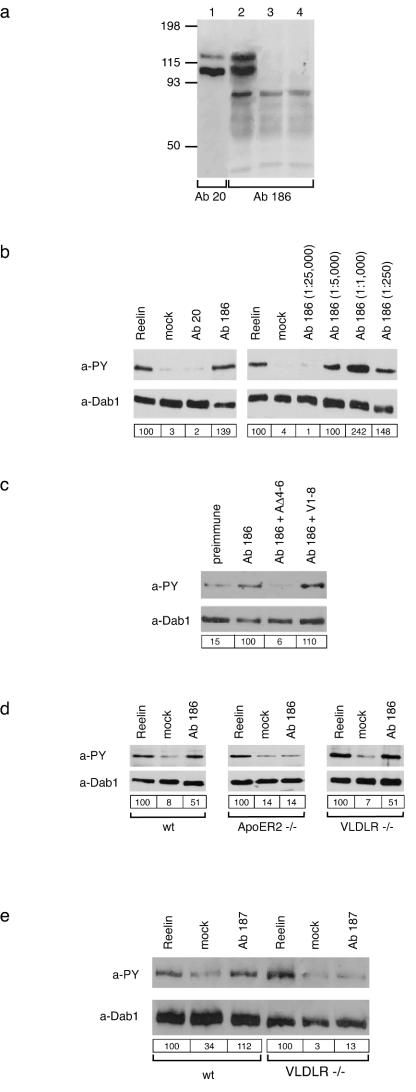
Recent results demonstrated that Reelin is secreted as a disulfide-linked homodimer (22) and that a truncated version of Reelin, which lacks the N-terminal CR-50 epitope, does not form oligomers and fails to induce Dab1 phosphorylation (36). In analogy to growth factor receptor signaling, these results are compatible with a mechanism in which Reelin dimers may transmit the signal by receptor dimerization or receptor clustering on the surface of target neurons. To test this possibility, we designed specific bivalent ligands for ApoER2 and VLDLR and tested their ability to mimic Reelin signaling in primary mouse neurons. First, we expressed RAP fused to a V5 epitope and the Fc portion of human IgG in 293T cells (Fig. 1a). RAP is a specialized chaperone for endocytic receptors which binds to most members of the low-density-lipoprotein receptor (LDLR) family (39). Due to two disulfide bonds between the Fc portions, the recombinant fusion protein is secreted as a dimer with a molecular mass of approximately 85 to 90 kDa (Fig. 1b), representing a bivalent ligand for ApoER2 and VLDLR. As a control, we used monomeric myc-RAP (Fig. 1a and b) which binds with high affinity to both receptors (21). Demonstrated in Fig. 1c, Fc-RAP binds to both receptors with an affinity similar to that described for myc-RAP (21). myc-RAP added simultaneously with Reelin to primary neurons abolishes Reelin-induced Dab1 phosphorylation by competing for receptor binding sites (Fig. 1d). Furthermore, myc-RAP reduces the baseline phosphorylation of Dab1, which is sustained by Reelin produced by a small portion of neurons in the culture (21). In contrast, addition of Fc-RAP is able to induce Dab1 phosphorylation which reaches the intensity of that induced by Reelin in the presence of protein A (Fig. 1d). These experiments demonstrate that binding of a bivalent or multivalent (Fc-RAP plus protein A) ligand to the Reelin receptors is sufficient to transduce a Reelin-like signal, suggesting that dimerization or oligomerization of the receptors plays a role in this process. Since RAP does not discriminate between ApoER2 and VLDLR, it is not clear from this experiment whether both receptors must be recruited into such a signaling complex. To answer this question, we expressed the ligand binding domains of both receptors in Escherichia coli (21) and developed specific polyclonal antibodies against the receptor fragments. Due to their structure, antibodies could mimic bivalent ligands, inducing clustering of receptor molecules. To test for the specificity of Ab 186, which was raised against the ligand binding domain of ApoER2, the full-length cDNA for ApoER2 was expressed in 293T cells (Fig. 2a, lanes 1 and 2). Ab 186 (lane 2) recognized the same double band as Ab 20 (lane 1), which was previously prepared against the intracellular domain of the receptor (33). The double band originates from differential glycosylation of the receptor in 293T cells. Analysis of VLDLR-expressing cells (lane 3) and mock-transfected cells (lane 4) demonstrated that Ab 186 is specific for ApoER2 and does not cross-react with VLDLR, which has a structurally related ligand binding domain. In addition to these analyses, the specificity of the antibody was confirmed by fluorescence microscopy using neurons from ApoER2−/− mice (data not shown). To test whether the antibody-mediated clustering of ApoER2 is sufficient to induce Dab1 phosphorylation, primary neurons were incubated with Ab 186 and Dab1 phosphorylation was monitored. As shown in Fig. 2b, this antibody induced a signal comparable to that of Reelin. In contrast, Ab 20 had no effect. The effect of Ab 186 was dose dependent, showing maximal Dab1 phosphorylation at an antiserum dilution of 1:1,000 (final dilution of antibody-containing serum in the culture medium) (Fig. 2b). This effect is significantly higher than that of Reelin. However, at higher concentrations of the antibody, the phosphorylation signal decreased. This observation is compatible with the concept of receptor clustering, which decreases at nonstoichiometric concentrations of the interacting partners. To test for the specificity of the effect, we incubated neurons with the optimal concentration of Ab 186 (1:1,000) in the presence of the soluble recombinant ligand binding domain of ApoER2 or VLDLR. As demonstrated in Fig. 2c, the stimulatory effect of Ab 186 was abolished by the ligand binding domain of ApoER2 but not by the corresponding domain of VLDLR.
FIG. 1.
A dimeric form of RAP induces tyrosine phosphorylation of Dab1 in primary embryonic neurons. (a) Structure of the dimeric Fc-RAP and the monomeric myc-RAP. hu-Fc, human Fc. (b) 293T cells were transfected with the cDNA encoding Fc-RAP. Ten microliters of the respective cell supernatant (1, 3) as well as 10 μl of control supernatant (2, 4) was analyzed by Western blotting using the indicated antibodies. myc-RAP expressed in E. coli (5) was analyzed by Western blotting using an anti-RAP antibody. Molecular size markers are shown to the left of each panel. a-hu Fc, anti-human Fc; a-V5, anti-V5; a-RAP, anti-RAP. (c) Microtiter plates were coated with ApoER2Δ4-6-MBP/His or VLDLR1-8-MBP/His. After incubationwith the indicated amounts of Fc-RAP (top) or myc-RAP (bottom), bound Fc-RAP was detected with protein A-HRP and myc-RAP was detected with 9E10 and an appropriate HRP-conjugated secondary antibody. OD450, optical density at 450 nm. (d) Primary mouse neurons were incubated with different conditioned or control media as indicated. myc-RAP was used at a concentration of 100 μg/ml. Cells were processed for immunoprecipitation with anti-Dab1 antibody, and Western blotting was subsequently performed using antiphosphotyrosine (a-PY) and anti-Dab1 (a-Dab1) antibodies. Bands were scanned with a personal densitometer (Molecular Dynamics), and the antiphosphotyrosine signal was normalized to the anti-Dab1 band. The level of Dab1 phosphorylation induced by Reelin was set at 100% in each individual experiment. Relative intensities are shown boxed in the corresponding panels. Prot. A, protein A; +, present; −, absent.
FIG. 2.
Polyclonal antibodies directed against the ligand binding domains of ApoER2 and VLDLR induce Dab1 phosphorylation in primary embryonic neurons. (a) 293T cells were transfected with constructs encoding ApoER2 (ApoER2Δ4-6) (lanes 1 and 2) or VLDLR (lane 3) or with the empty vector (lane 4). The corresponding cell extracts were separated using SDS-8% PAGE under reducing conditions, and Western blotting was performed using Ab 20 (lane 1) or Ab 186 (lanes 2 to 4). Molecular size markers are shown at the left. (b) Primary mouse neurons were incubated with Reelin-conditioned medium, control medium, or antiserum 20 or 186 (1:1,000) (left panel). Neurons were stimulated with different concentrations of Ab 186 (right panel). Cells were processed for immunoprecipitation with anti-Dab1,and Western blotting was subsequently performed using antiphosphotyrosine (a-PY) or anti-Dab1 (a-Dab1) antibody as indicated. (c) Primary neurons were incubated with the preimmune serum from the rabbit producing Ab 186 or with Ab 186 alone or in the presence of an excess of soluble ligand binding domains of ApoER2 (AΔ4-6) or VLDLR (V1-8). Immunoprecipitation and Western blot analysis were performed as described for panel b. (d) Primary neurons from wt, ApoER2−/−, and VLDLR−/− mice were stimulated with Reelin, control medium, or Ab 186 (1:1,000). Immunoprecipitation and Western blot analysis were performed as described for panel b. (e) Primary mouse neurons derived from wt or VLDLR−/− mice were incubated with Reelin-conditioned medium, control medium, or serum from the rabbit producing Ab 187 at a dilution of 1:800. Cells were processed for immunoprecipitation with anti-Dab1 antibody, and Western blotting was subsequently performed using anti-PY and anti-Dab1 antibodies as indicated. Bands were scanned with a personal densitometer (Molecular Dynamics), and the antiphosphotyrosine signal was normalized to the anti-Dab1 band. The level of Dab1 phosphorylation induced by Reelin was set at 100% in each individual experiment. Relative intensities are shown boxed in the corresponding panels.
To genetically test the specificity of the effect of Ab 186 on Dab1 phosphorylation, primary neurons derived from wt, ApoER2−/−, or VLDLR−/− mice were stimulated with Ab 186. As demonstrated in Fig. 2d, Reelin induced Dab1 phosphorylation in all three types of neurons. However, Ab 186 induced the signal only in neurons derived from wt mice and from VLDLR−/− mice but not in neurons derived from ApoER2−/− mice. These experiments demonstrated that the effect of Ab 186 is indeed transmitted by ApoER2 and that clustering of both or one receptor, namely, ApoER2, is sufficient to transduce the signal.
Next, we tested an antibody against the ligand binding domain of VLDLR (Ab 187). The specificity of this antibody was tested by Western blot assays and immunoprecipitation experiments (data not shown). This antibody stimulates Dab1 phosphorylation in a manner similar to that of Ab 186 (Fig. 2e). Using neurons from VLDLR−/− mice demonstrated that the effect of Ab 187 is also specific and depends on the presence of VLDLR (Fig. 2e).
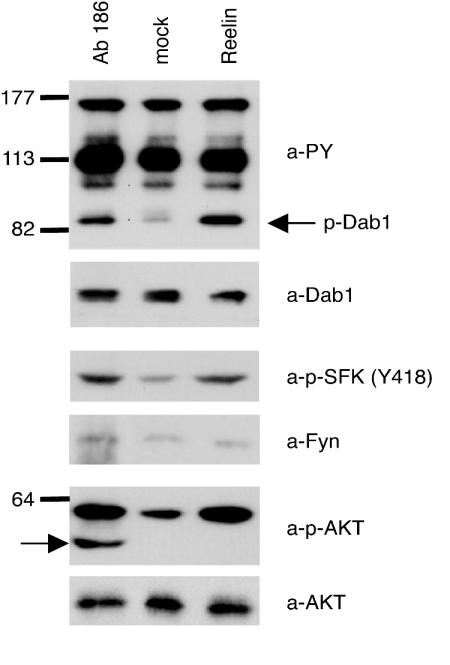
As recently demonstrated, activation of members of the SFKs (2, 6) and modulation of PKB/Akt phosphorylation (4, 6) are downstream effects of Reelin-induced Dab1 phosphorylation. To test whether these effects can also be mimicked by the clustering of ApoER2 and/or VLDLR on the cell surface, we incubated primary neurons with increasing concentrations of Ab 186 and measured SFK and PKB/Akt phosphorylation. As a control, Dab1 phosphorylation was monitored directly by blotting the cell extract with an antiphosphotyrosine antibody (4). As shown in Fig. 3, addition of Ab 186 not only induces Dab1 phosphorylation but also stimulates SFK and Akt phosphorylation without affecting total levels of Fyn and Akt. These results demonstrate that the specific antibody is able to mimic Reelin effects downstream of Dab1 phosphorylation.
FIG. 3.
Ab 186 induces activation of SFKs and PKB/Akt in primary embryonic neurons. Primary rat neurons were incubated with Reelin-conditioned medium, control medium, or Ab 186, and the phosphorylation of Dab1, SFKs, and PKB/Akt was analyzed by Western blotting of crude cell lysates using the indicated antibodies. The IgG heavy chain marked by the arrow in the phospho-Akt immunoblot originates from Ab 186 added in this experiment. Molecular size markers are shown at the left. a-PY, antiphosphotyrosine; p-Dab1, phospho-Dab1; a-Dab1, anti-Dab1; a-p-SFK, anti-phospho-SFK; a-Fyn, anti-Fyn; a-p-AKT, anti-phospho-Akt; a-AKT, anti-Akt.
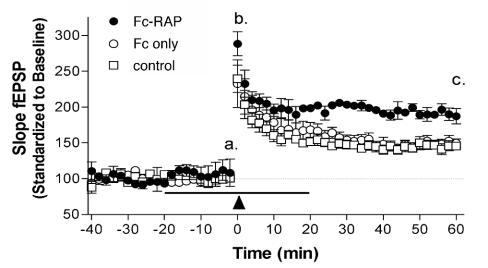
Reelin modulates synaptic plasticity in the adult brain by enhancing LTP induction and maintenance (38). To test whether induced receptor clustering is sufficient to mimic this biologic effect of Reelin, we examined the effect of Fc-RAP on hippocampal synaptic plasticity. As demonstrated in Fig. 4, perfusion with 10 μg of Fc-RAP/ml enhanced LTP induction and maintenance compared to perfusion with control Fc or nontreatment of slices. Baseline synaptic responses were unchanged in the presence of Fc-RAP or control Fc protein (Fig. 4, section a). Fc-RAP-treated slices showed an elevated potentiation immediately following high-frequency stimulation (HFS) (Fig. 4, section b) (Fc-RAP, 288.4% ± 16.6% [n = 5]; Fc only, 239.5% ± 19.1% [n = 5]; no treatment, 232.7% ± 33.4% [n = 5]; P = 0.246). LTP induction was significantly enhanced in hippocampus slices perfused with Fc-RAP 60 min posttetanus (Fig. 4, section c) (187.3% ± 10.6%; n = 5) compared to that in slices perfused with Fc only (145.4% ± 5.0%; n = 5) or that in untreated slices (149.6% ± 5.9%; n = 5) (P = 0.0038). These effects are comparable to the effects seen with Reelin (compare Fig. 5 in reference 38) and demonstrate that receptor clustering with a synthetic ligand is sufficient for this effect of Reelin.
FIG. 4.
Perfusion with Fc-RAP enhances hippocampal LTP induction. Hippocampal slices were perfused with Fc-RAP (10 μg/ml), Fc (10 μg/ml), or control medium. Baseline synaptic responses (a) and potentiation immediately following HFS (b) and up to 60 min after HFS (c) were recorded. The arrowhead represents LTP induced with two trains of 1-s-long, 100-Hz stimulation, separated by 20 s. The horizontal line indicates application of Fc-RAP, Fc, or control medium. Results are shown as means ± standard errors of the mean. fEPSP, field excitatory postsynaptic potential.
FIG. 5.
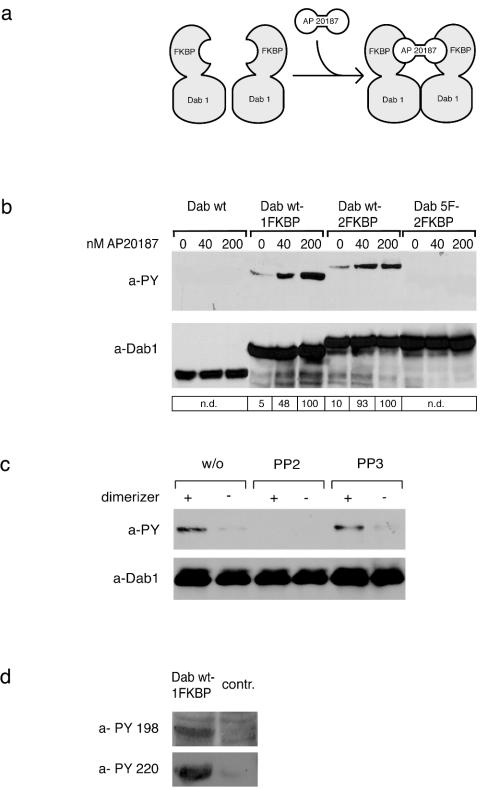
Dimerization and oligomerization of Dab1 lead to receptor-independent phosphorylation in 293 cells. (a) Cartoon demonstrating dimerization of the Dab1-FKBP fusion protein by addition of AP20187. (b) Wt Dab1, fusion proteins containing wt Dab1 and one (Dab wt-1FKBP) or two (Dab wt-2FKBP) copies of FKBP, and a fusion protein containing a mutated version of Dab1 with the relevant five tyrosine residues replaced by phenylalanines and containing two copies of FKBP (Dab 5F-2FKBP) were expressed in 293 cells and treated with the indicated amounts of the FKBP-dimerizing agent AP20187 for 20 min. Dab1 phosphorylation was analyzed by Western blotting with the indicated antibodies by using crude cell lysates. a-PY, antiphosphotyrosine; a-Dab1, anti-Dab1; n.d., not determined. (c) 293 cells expressing Dab wt-1FKBP were treated for 1 h with or without (w/o) 10 μM PP2 or PP3 prior to the addition of 50 nM AP20187 or medium. After 20 min, Dab1 phosphorylation was analyzed by Western blotting with the indicated antibodies by using crude cell lysates. +, present; −, absent. (d) 293 cells expressing Dab wt-1FKBP or control cells (contr.) were treated with 50 nM AP20187, and after 20 min Dab1 phosphorylation was analyzed by Western blotting with the indicated antibodies by using crude cell lysates. Bands were scanned with a personal densitometer (Molecular Dynamics), and the antiphosphotyrosine signal was normalized to the anti-Dab1 band. The level of Dab1 phosphorylation induced by the highest concentration of AP20187 was set at 100% in each individual experiment. Relative intensities are shown boxed in the corresponding panels.
To explore the possibility that receptor-independent dimerization of Dab1 is sufficient for its phosphorylation, we used an inducible homodimerization system (ARIAD) (9). Dab1 was fused to either one or two copies of FKBP12 and expressed in 293 cells. Addition of a cell-permeable synthetic ligand (AP20187) induces dimerization or multimerization (dependent on whether one or two copies of FKBP are present) of the fusion protein by linking the FKBP moieties (Fig. 5a). As shown in Fig. 5b, expression of wt Dab in these cells does not result in detectable amounts of phosphorylated Dab1. However, expression of the fusion proteins (Dab wt-1FKBP or Dab wt-2FKBP) resulted in weak but detectable phosphorylation of the fusion proteins even in the absence of AP20187. Addition of the dimerizer to the cells induced a dramatic increase in the phosphorylation of the fusion proteins but did not result in the phosphorylation of wt Dab1. As a control, we used a corresponding fusion protein containing a mutated version of Dab1 in which the five tyrosine residues involved in Reelin-induced phosphorylation were replaced by phenylalanine (18). As shown in Fig. 5b, this fusion protein (Dab1 5F-2FKBP) was not phosphorylated under the same experimental conditions. To test whether phosphorylation of Dab1 induced by its dimerization in 293 cells is mediated by the same family of kinases which phosphorylates Dab1 in neurons upon Reelin stimulation (2, 6), we carried out the same experiment in the presence of PP2 [4-amino-5-(4-chlorophenyl)-7-(_t_-butyl)pyrazolo(3,4-d)pyramidine], which selectively inhibits Dab1 phosphorylation induced by members of the Src family and Abl (2). As demonstrated in Fig. 5c, preincubation of transfected 293 cells with PP2 completely abolished dimerization-induced Dab1 phosphorylation. Addition of PP3, a structural analogue of PP2 which does not inhibit Src family kinases, is without effect. Next we tested whether the same tyrosines of Dab1 which are phosphorylated by Reelin-induced stimulation of neurons (20) become phosphorylated by forced dimerization of Dab1 in 293 cells. Western blotting with phosphotyrosine-specific antibodies (20) demonstrated that Dab wt-1FKBP indeed becomes phosphorylated at positions 198 and 220 upon addition of AP20187 (Fig. 5d). These results suggest that dimerization-induced phosphorylation of Dab1 in 293 cells is caused by a mechanism similar to that in Reelin-stimulated neurons. Taken together, these experiments demonstrate that under certain conditions, tyrosine phosphorylation of Dab1 can be induced by dimerization or oligomerization of the protein independently of its interaction with the Reelin receptors, ApoER2 and VLDLR.
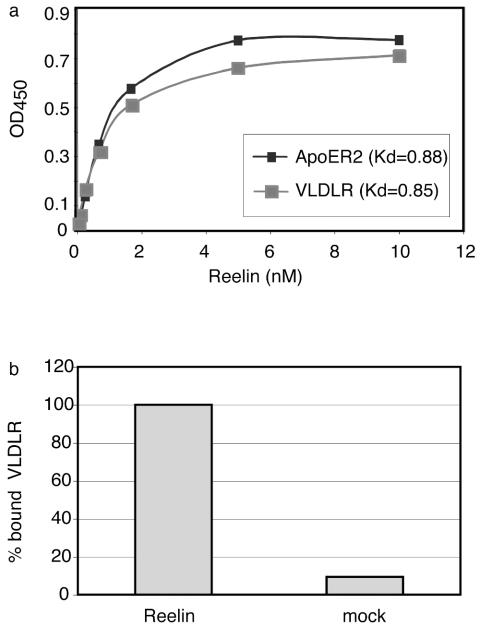
These results are compatible with a model in which Dab1 phosphorylation and downstream events of this pathway are induced in target neurons by clustering of ApoER2 and/or VLDLR by Reelin dimers. To test whether Reelin indeed is a bi- or polyvalent ligand for the receptors, we used a recently developed enzyme-linked immunosorbent assay-based binding assay which allows quantitative evaluation of binding parameters for ApoER2 and VLDLR (21). As demonstrated in Fig. 6a, Reelin has very similar affinities to both receptors. To test the polyvalent character of Reelin, we coated the plates with ApoER2 and added Reelin at a concentration of approximately 200 nM. This amount is well above the concentration at which maximal binding of the receptor is achieved (Fig. 6a). Under these conditions, free binding sites must be available on the bound Reelin if more than one binding site is present on the dimeric Reelin molecule. Indeed, soluble VLDLR fragments could be bound to Reelin bound to ApoER2 under these conditions (Fig. 6b). As a control, medium from mock-transfected cells was used instead of Reelin. This experiment demonstrates that Reelin is able to bind to more than one receptor molecule, suggesting that it is indeed able to cluster the receptors on the cell surface.
FIG. 6.
Reelin acts as a multivalent ligand for ApoER2 and VLDLR. (a) Microtiter plates were coated with ApoER2Δ4-6-MBP/His or VLDLR1-8-MBP/His (10 μg/ml). After incubation with the indicated amounts of Reelin, bound protein was detected with anti-Reelin antibody (G10) and HRP-conjugated secondary antibody as described in Materials and Methods. OD450, optical density at 450 nm; Kd, dissociation constant. (b) Microtiter plates were coated with ApoER2Δ4-6-MBP/His and incubated with Reelin (200 nM) or concentrated mock medium. After the plates were washed, VLDLR1-8-MBP/His (10 μg/ml) was added and bound VLDLR was detected using a monoclonal anti-VLDLR antibody (6G6) and HRP-conjugated secondary antibody.
DISCUSSION
Reelin, an extracellular matrix protein, initiates a signal cascade in neurons which plays a key role in the positioning of neuronal layers in the central nervous system during embryonic development. Ample genetic and biochemical evidence defines this pathway as follows. Reelin is secreted by specialized neurons and binds to ApoER2 and VLDLR, two members of the LDLR family. This leads to the phosphorylation of Dab1, which binds to the cytoplasmic domains of both receptors. Further downstream events are still poorly defined but have been suggested to involve the activation of Src family members (2, 6), modulation of PKB/Akt phosphorylation (4, 7), and redistribution of Nckβ from the cell soma into distal sites of neuronal processes (29). It is unclear how the phosphorylation of Dab1 is generated at the receptor level. Since neither ApoER2 nor VLDLR have detectable kinase activity, coreceptors like CNRs (31) or integrins (13) have been postulated. However, direct involvement of these receptors in Dab1 phosphorylation has not yet been demonstrated. In vivo, Reelin is secreted as a disulfide-linked homodimer (22). In this regard, it is of interest that a truncated version of Reelin, which lacks the N-terminal CR-50 epitope, does not form oligomers and fails to induce Dab1 phosphorylation (36). If dimerization of the Reelin molecule is the key to its signaling function, receptor clustering on the surface of target cells may be the primary effect of ligand binding. We reasoned that mimicking Reelin action with artificial dimeric or multivalent ligands for ApoER2 and VLDLR would help us understand the action of Reelin at the molecular level. Thus, we constructed a bivalent RAP molecule in which two RAP moieties were fused together via a human Fc domain. RAP acts as a molecular chaperone for members of the LDLR family by preventing their intracellular interaction with ligands present in the secretory pathway (39). RAP binds with high affinity to the ligand binding domains of these receptors and inhibits binding of all known cognate ligands in vitro and in vivo. In contrast to the RAP monomer, which inhibits Reelin signaling by inhibiting Reelin binding to ApoER2 and VLDLR, the dimeric Fc-RAP construct mimics the Reelin signal by inducing Dab1 phosphorylation in primary embryonic neurons. This experiment demonstrates that binding of a monovalent ligand to the receptors is not sufficient to induce a Reelin-like signal. Interaction of the receptors with a bivalent agent, however, is sufficient to induce Dab1 phosphorylation. Since we cannot exclude a priori that RAP also interacts with other putative coreceptors, we produced polyclonal antibodies against the ligand binding domains of ApoER2 and VLDLR. Both antibodies induce Dab1 phosphorylation in primary embryonic neurons. Using neurons from ApoER2−/− and VLDLR−/− mice, we demonstrated that the capacity of these agents to mimic Reelin signaling is specific and indeed transduced by these receptors. The observation that the effect is concentration dependent points to clustering of the receptors as an important mechanistic aspect. Due to the specificity of the antibodies, we conclude that coreceptors are not required for the primary action of Reelin, i.e., induction of Dab1 phosphorylation. Furthermore, the antibody induces phosphorylation of SFKs and PKB/Akt. Especially in the case of Akt, these experiments confirm recent reports demonstrating that activation of Akt is a direct consequence of Dab1 phosphorylation and does not involve additional phosphoinositide 3-kinase activation by another pathway (3, 7). In addition, Fc-RAP mimics the effect of Reelin on LTP modulation in hippocampal slices. This demonstrates that bivalent synthetic ligands are able to mimic several Reelin-induced actions without the necessity for another receptor.
How can Reelin-induced receptor dimerization lead to the phosphorylation of Dab1? Since both receptors bind Dab1 via a shared sequence motif in their intracellular domains, the clustering of the receptors may lead to dimerization or oligomerization of Dab1. As the results of Fig. 5 show, dimerization of Dab1 is sufficient for its phosphorylation in 293 cells. In contrast, no phosphorylation was observed under the same experimental conditions with a mutant version of Dab1 which lacks the tyrosine residues that become phosphorylated in response to Reelin. Furthermore, a specific inhibitor of Src family kinases (PP2) inhibits phosphorylation of the dimer in 293 cells, and using phosphotyrosine-specific antibodies (20) demonstrates that Dab wt-1FKBP becomes phosphorylated at the same positions as in the case of Reelin stimulation of primary neurons. These results show that ApoER2 and VLDLR together are not per se necessary for inducing the phosphorylation of Dab1 but serve to induce dimerization or oligomerization of Dab1 upon binding of Reelin. This mode of action implies that Reelin associates with more than one receptor molecule simultaneously. In fact, as our results presented in Fig. 6 demonstrate, Reelin has more than one binding site for ApoER2 and VLDLR. This finding is supported by a recent study which also demonstrates that Reelin associates with two or more receptor molecules simultaneously (1).
In summary, these data allow us to postulate the following model for Reelin action. Reelin is secreted by specialized neurons as an oligomeric protein which binds to ApoER2 and VLDLR on the cell surface, thereby inducing homo- or heterodimerization or clustering of the receptors. As a consequence, Dab1 becomes dimerized or oligomerized on the cytosolic side of the plasma membrane and thereby constitutes an active substrate for Src family members or an as yet unidentified kinase. Subsequent tyrosine phosphorylation of Dab1 triggers the intracellular part of the complex Reelin pathway. Thus, Reelin regulates the actual amount of Dab1 dimers within target neurons by binding to ApoER2 and VLDLR, and as revealed here, this process is independent of coreceptors. However, it cannot be excluded that the interaction of Reelin with CNRs or other receptors is needed for the induction of parallel pathways which act independently of Dab1 phosphorylation. Thus, clustering of ApoER2 and VLDLR may not be sufficient to mimic all effects of Reelin, but it seems to be the only requirement for the induction of Dab1 phosphorylation.
Acknowledgments
This work was supported by Austrian Science Foundation grants P13931-MOB, F606, and F608 and the Herzfelder'sche Familienstiftung. D.F. was supported by the Austrian Academy of Science (DOC-FFORT/21282). H.H.B. was supported by a postdoctoral fellowship from the Deutsche Forschungsgemeinschaft. J.H. is supported by grants from the National Institutes of Health, the Alzheimer Association, and the Humboldt Foundation.
Antibodies against Reelin and Dab1 were generous gifts from Andre Goffinet (Medical School, University of Louvain). The expression plasmid for Reelin and the phosphorylation site-specific antibodies against Dab1 (anti-P198/200 and anti-P220) were generously provided by Tom Curran (Department of Developmental Neurobiology, St. Jude's Children's Research Hospital, Memphis, Tenn.).
REFERENCES
- 1.Andersen, O. M., D. Benhayon, T. Curran, and T. E. Willnow. 2003. Differential binding of ligands to the apolipoprotein E receptor 2. Biochemistry 42**:**9355-9364. [DOI] [PubMed] [Google Scholar]
- 2.Arnaud, L., B. A. Ballif, E. Forster, and J. A. Cooper. 2003. Fyn tyrosine kinase is a critical regulator of Disabled-1 during brain development. Curr. Biol. 13**:**9-17. [DOI] [PubMed] [Google Scholar]
- 3.Ballif, B. A., L. Arnaud, and J. A. Cooper. 2003. Tyrosine phosphorylation of Disabled-1 is essential for Reelin-stimulated activation of Akt and Src family kinases. Mol. Brain Res. 117**:**152-159. [DOI] [PubMed] [Google Scholar]
- 4.Beffert, U., G. Morfini, H. H. Bock, H. Reyna, S. T. Brady, and J. Herz. 2002. Reelin-mediated signaling locally regulates PKB/Akt and GSK-3β. J. Biol. Chem. 277**:**49958-49964. [DOI] [PubMed] [Google Scholar]
- 5.Benhayon, D., S. Magdaleno, and T. Curran. 2003. Binding of purified Reelin to ApoER2 and VLDLR mediates tyrosine phosphorylation of Disabled-1. Mol. Brain Res. 112**:**33-45. [DOI] [PubMed] [Google Scholar]
- 6.Bock, H. H., and J. Herz. 2003. Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 13**:**18-26. [DOI] [PubMed] [Google Scholar]
- 7.Bock, H. H., Y. Jossin, P. Liu, E. Forster, P. May, A. M. Goffinet, and J. Herz. 2003. Phosphatidylinositol 3-kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J. Biol. Chem. 278**:**38772-38779. [DOI] [PubMed] [Google Scholar]
- 8.Brandes, C., L. Kahr, W. Stockinger, T. Hiesberger, W. J. Schneider, and J. Nimpf. 2001. Alternative splicing in the ligand binding domain of mouse ApoE receptor-2 produces receptor variants binding Reelin but not alpha2-macroglobulin. J. Biol. Chem. 276**:**22160-22169. [DOI] [PubMed] [Google Scholar]
- 9.Clackson, T., W. Yang, L. W. Rozamus, M. Hatada, J. F. Amara, C. T. Rollins, L. F. Stevenson, S. R. Magari, S. A. Wood, N. L. Courage, X. Lu, F. Cerasoli, M. Gilman, and D. A. Holt. 1998. Redesigning an FKBP-ligand interface to generate chemical dimerizers with novel specificity. Proc. Natl. Acad. Sci. USA 95**:**10437-10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curran, T., and G. D'Arcangelo. 1998. Role of Reelin in the control of brain development. Brain Res. Rev. 26**:**285-294. [DOI] [PubMed] [Google Scholar]
- 11.D'Arcangelo, G., R. Homayoundi, L. Keshvara, D. S. Rice, M. Sheldon, and T. Curran. 1999. Reelin is a ligand for lipoprotein receptors. Neuron 24**:**471-479. [DOI] [PubMed] [Google Scholar]
- 12.D'Arcangelo, G., G. G. Miao, S. C. Chen, H. D. Soares, J. I. Morgan, and T. Curran. 1995. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374**:**719-723. [DOI] [PubMed] [Google Scholar]
- 13.Dulabon, L., E. C. Olson, M. G. Taglienti, S. Eisenhuth, B. McGrath, C. A. Walsh, J. A. Kreidberg, and E. S. Anton. 2000. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron 27**:**33-44. [DOI] [PubMed] [Google Scholar]
- 14.Hiesberger, T., M. Trommsdorff, B. W. Howell, A. Goffinet, M. C. Mumby, J. A. Cooper, and J. Herz. 1999. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosin phosphorylation of the adaptor protein disabled-1 and modulates tau phosphorylation. Neuron 24**:**481-489. [DOI] [PubMed] [Google Scholar]
- 15.Howell, B. W., F. B. Gertler, and J. A. Cooper. 1997. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 16**:**121-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howell, B. W., R. Hawkes, P. Soriano, and J. A. Cooper. 1997. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature 389**:**733-737. [DOI] [PubMed] [Google Scholar]
- 17.Howell, B. W., T. M. Herrick, and J. A. Cooper. 1999. Reelin-induced tryosine phosphorylation of disabled 1 during neuronal positioning. Genes Dev. 13**:**643-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howell, B. W., T. M. Herrick, J. D. Hildebrand, Y. Zhang, and J. A. Cooper. 2000. Dab1 tyrosine phosphorylation sites relay positional signals during mouse brain development. Curr. Biol. 10**:**877-885. [DOI] [PubMed] [Google Scholar]
- 19.Howell, B. W., L. M. Lanier, R. Frank, F. B. Gertler, and J. A. Cooper. 1999. The disabled 1 phosphotyrosine-binding domain binds to the internalization signals of transmembrane glycoproteins and to phospholipids. Mol. Cell. Biol. 19**:**5179-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keshvara, L., D. Benhayon, S. Magdaleno, and T. Curran. 2001. Identification of Reelin-induced sites of tyrosyl phosphorylation on disabled 1. J. Biol. Chem. 276**:**16008-16014. [DOI] [PubMed] [Google Scholar]
- 21.Koch, S., V. Strasser, C. Hauser, D. Fasching, C. Brandes, T. M. Bajari, W. J. Schneider, and J. Nimpf. 2002. A secreted soluble form of ApoE receptor 2 acts as dominant negative receptor and inhibits Reelin signaling. EMBO J. 21**:**5996-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubo, K., K. Mikoshiba, and K. Nakajima. 2002. Secreted Reelin molecules form homodimers. Neurosci. Res. 43**:**381-388. [DOI] [PubMed] [Google Scholar]
- 23.Laemmli, U. K. 1970. Cleavage of structural proteins during assembly of the head of the bacteriophage T4. Nature 227**:**680-685. [DOI] [PubMed] [Google Scholar]
- 24.Lindstedt, K. A., M. G. Mahon, R. Foisner, M. Hermann, J. Nimpf, and W. J. Schneider. 1997. Receptor-associated protein in an oviparous species is correlated with the expression of a receptor variant. J. Biol. Chem. 272**:**30221-30227. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima, K., K. Mikoshiba, T. Miyata, C. Kudo, and M. Ogawa. 1997. Disruption of hippocampal development in vivo by CR-50 mAb against Reelin. Proc. Natl. Acad. Sci. USA 94**:**8196-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogawa, M., T. Miyata, K. Nakajima, K. Yagyu, M. Seike, K. Ikenaka, H. Yamamoto, and K. Mikoshiba. 1995. The reeler gene-associated antigen on Cajal-Retzius neurons is a crucial molecule for laminar organization of cortical neurons. Neuron 14**:**899-912. [DOI] [PubMed] [Google Scholar]
- 27.Ohshima, T., M. Ogawa, Veeranna, M. Hirasawa, G. Longenecker, K. Ishiguro, H. C. Pant, R. O. Brady, A. B. Kulkarni, and K. Mikoshiba. 2001. Synergistic contributions of cyclin-dependant kinase 5/p35 and Reelin/Dab1 to the positioning of cortical neurons in the developing mouse brain. Proc. Natl. Acad. Sci. USA 98**:**2764-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohshima, T., J. M. Ward, C. G. Huh, G. Longenecker, Veeranna, H. C. Pant, R. O. Brady, L. J. Martin, and A. B. Kulkarni. 1996. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc. Natl. Acad. Sci. USA 93**:**11173-11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pramatarova, A., P. G. Ochalski, K. Chen, A. Gropman, S. Myers, K. T. Min, and B. W. Howell. 2003. Nck beta interacts with tyrosine-phosphorylated disabled 1 and redistributes in Reelin-stimulated neurons. Mol. Cell. Biol. 23**:**7210-7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice, D. S., M. Sheldon, G. D'Arcangelo, K. Nakajima, D. Goldowitz, and T. Curran. 1998. Disabled-1 acts downstream of Reelin in a signaling pathway that controls laminar organization in the mammalian brain. Development 125**:**3719-3729. [DOI] [PubMed] [Google Scholar]
- 31.Senzaki, K., M. Ogawa, and T. Yagi. 1999. Proteins of the CNR family are multiple receptors for Reelin. Cell 99**:**635-647. [DOI] [PubMed] [Google Scholar]
- 32.Sheldon, M., D. S. Rice, G. D'Arcangelo, H. Yoneshima, K. Nakajima, K. Mikoshiba, B. W. Howell, J. A. Cooper, D. Goldowitz, and T. Curran. 1997. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature 389**:**730-733. [DOI] [PubMed] [Google Scholar]
- 33.Stockinger, W., E. Hengstschläger-Ottnad, S. Novak, A. Matus, M. Hüttinger, J. Bauer, H. Lassmann, W. J. Schneider, and J. Nimpf. 1998. The LDL receptor gene family: differential expression of two α2-macroglobulin receptors in the brain. J. Biol. Chem. 273**:**32213-32221. [DOI] [PubMed] [Google Scholar]
- 34.Trommsdorff, M., J.-P. Borg, B. Margolis, and J. Herz. 1998. Interaction of cytosolic adaptor proteins with neuronal apoE receptors and the amyloid presursor proteins. J. Biol. Chem. 273**:**33556-33565. [DOI] [PubMed] [Google Scholar]
- 35.Trommsdorff, M., M. Gotthardt, T. Hiesberger, J. Shelton, W. Stockinger, J. Nimpf, R. Hammer, J. A. Richardson, and J. Herz. 1999. Reeler/Disabled-like disruption of neuronal migration in knock out mice lacking the VLDL receptor and apoE receptor-2. Cell 97**:**689-701. [DOI] [PubMed] [Google Scholar]
- 36.Utsunomiya-Tate, N., K. Kubo, S. Tate, M. Kainosho, E. Katayama, K. Nakajima, and K. Mikoshiba. 2000. Reelin molecules assemble together to form a large protein complex, which is inhibited by the function-blocking CR-50 antibody. Proc. Natl. Acad. Sci. USA 97**:**9729-9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ware, M. L., J. W. Fox, J. L. Gonzalez, N. M. Davis, C. Lambert de Rouvroit, C. J. Russo, S. C. Chua, Jr., A. M. Goffinet, and C. A. Walsh. 1997. Aberrant splicing of a mouse disabled homolog, mdab1, in the scrambler mouse. Neuron 19**:**239-249. [DOI] [PubMed] [Google Scholar]
- 38.Weeber, E. J., U. Beffert, C. Jones, J. M. Christian, E. Forster, J. D. Sweatt, and J. Herz. 2002. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 277**:**39944-39952. [DOI] [PubMed] [Google Scholar]
- 39.Willnow, T. E. 1998. Receptor-associated protein (RAP): a specialized chaperone for endocytic receptors. Biol. Chem. 379**:**1025-1031. [PubMed] [Google Scholar]