IFN-αβ and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions (original) (raw)
. Author manuscript; available in PMC: 2011 Dec 6.
Published in final edited form as: J Immunol. 2010 Jun 30;185(3):1419–1428. doi: 10.4049/jimmunol.1001140
Abstract
Non-virus-specific bystander CD8 T cells bathe in an inflammatory environment during viral infections. To determine if bystander CD8 T cells are affected by these environments, we examined P14, HY, and OT-I TCR transgenic CD8 T cells sensitized in vivo by IFN-αβ-inducing viral infections or by poly(I:C). These sensitized cells rapidly exerted effector functions such as IFN-γ production and degranulation on contact with their high affinity cognate antigen. Sensitization required self-MHC I and indirect effects of IFN-αβ, which together up-regulated the t-box transcription factor Eomesodermin, potentially enabling the T cells to rapidly transcribe CTL effector genes and behave like memory rather than naïve T cells. IL-12, IL-15, IL-18, and IFN-γ were not individually required for sensitization to produce IFN-γ, but IL-15 was required for up-regulation of granzyme B. These experiments indicate that naïve CD8 T cells receive signals from self-MHC and IFN-αβ and that by this process CD8 T cell responses to viral infection can undergo distinct differentiation pathways, depending on the timing of antigen encounter during the virus-induced IFN response.
Introduction
Many viral infections induce robust CD8 T cell responses that result in the lysis of virus-infected cells, secretion of antiviral cytokines, and the clearance of the virus. Virus-specific CD8 T cells undergo a programmed pathway of differentiation that is tightly coupled to proliferation (1, 2). After several rounds of division, effector CD8 T cells gain the ability to secrete cytokines and chemokines, including IFN-γ, MIP-1β, and Rantes, and acquire the ability to lyse virus-infected or peptide-pulsed target cells after differentiating into CTL (2–4). Effector functions of CTL are tightly regulated to diminish the potential immune pathology associated with inflammatory cytokines and cytolysis. Naïve CD8 T cells normally require approximately three days to start expressing IFN-γ, whereas effector and memory CD8 T cells can rapidly turn on _IFN_-γ transcription as early as 1 hour on re-encountering their cognate ligands (4–6). Importantly, virus-specific CTL are not continuously producing IFN-γ during infections, but they turn on its expression when re-encountering antigen in a local environment of infected tissue (7).
_IFN_-γ gene transcription, like that of other CTL effector genes such as granzymes and perforin, is regulated by chromatin accessibility and the expression of appropriate transcription factors. The T box transcription factors T-bet and Eomesodermin (Eomes) are the principle transcription factors regulating CTL effector gene transcription (8–13), and the expression of these transcription factors is low in naïve CD8 T cells. They are both up-regulated in effector CTL, with T-bet being the prominent transcription factor present, whereas Eomes is up-regulated further in memory CD8 T cells (10, 14). Knockout and knockdown studies of both transcription factors have revealed partially overlapping and compensatory functions in CD8 T cells (8, 11–13, 15). Thus, CD8 T cells lacking both transcription factors have the most pronounced defect in effector function capabilities; lymphocytic choriomeningitis virus (LCMV)-specific CD8 T cells lacking both T-bet and Eomes express very little IFN-γ, perforin, and granzyme B (GrzB), and instead express an aberrantly high amount of IL-17 (9).
It was once highly debated whether bystander T cells contributed to the large pool of CD8 T cells at the peak of an immune response, but sophisticated techniques and models to detect virus-specific and bystander T cells have, for the most part, quelled this theory (16–18). Despite the lack of direct participation of bystander T cells during anti-viral immune responses, it was conceivable that these cells still received signals by the inflammatory milieu of cytokines and chemokines or by non-viral peptide-MHC-TCR interactions. Additionally, virus-specific T cells recruited later in the immune response, or “latecomer” T cells, may also be affected by inflammatory signals prior to antigen stimulation, and it is likely that a combination of T cells with different signaling sequences from cytokine receptors and TCR constitute the total T cell response to pathogens.
We sought to understand how bystander and thus possibly latecomer CD8 T cells were affected by ongoing anti-viral immune responses by generating several in vivo models using P14 (LCMV glycoprotein-specific), HY (male antigen-specific), and OT-I (ovalbumin-specific) transgenic CD8 T cells. We found that, during acute viral infections or after stimulation with type 1 IFN (IFN-αβ) inducers, some bystander CD8 T cells were sensitized to up-regulate GrzB in vivo and immediately exert effector functions such as IFN-γ production and degranulation upon stimulation with high affinity cognate antigen in vitro. Sensitization of naïve CD8 T cells required self-MHC I and indirect effects of IFN-αβ, while IL-12, IL-18, and IFN-γ were not individually required. IL-15 was not required for the rapid expression of IFN-γ, but was required for up-regulation of GrzB. Sensitized naive CD8 T cells up-regulated the t-box transcription factor Eomes, which can regulate these rapid effector functions.
Materials and methods
Mice
C57BL/6J mice, B6.129P2-B2m tm1Unc/J (β2m KO), B6.129S7-IFNg/J (IFN-γ KO), and B6.129P2-Il18tm1Aki/J (IL-18 KO) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). C57BL/6-H-2KbH-2Db (KbDb KO), C57BL/6-H-2Kb (Kb KO), C57BL/6-H-2Db (Db KO), C57BL/6NTac-IL15N5 (IL-15 KO) (19) and TCR-LCMV P14/Rag2 knockout (P14) mice were purchased from Taconic Farms (Germantown, NY). B6.SJL (Ly5.1+) male and female mice were purchased from Taconic Farms or bred within the Department of Animal Medicine at the University of Massachusetts Medical School (UMMS). TCR transgenic mice (P14 (20), HY (21), and OT-I (22)), IFN-αβ receptor KO mice (IFN-αβR KO) (23), and B6.129-IL-12β (IL-12 p40 KO) mice were bred at UMMS. TCR transgenic P14 mice were crossed to IFN-αβR KO mice and were screened via surface expression of Vα2+ TCR on CD8 T cells and genomic PCR for the knockout IFN-αβR locus. All mice were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of UMMS.
Virus stocks and inoculations
LCMV, strain Armstrong, Pichinde virus (PV), strain AN3739, and vesicular stomatitis virus (VSV), strain Indiana, were propagated in baby hamster kidney cells (BHK21), as previously described (24, 25). VV, strain WR, was propagated in NCTC 929 cells and purified over a sucrose gradient (26). Mice were inoculated i.p. with 5 × 104 pfu LCMV, 1.5 × 107 pfu PV, or 1 × 106 pfu VV. To induce IFN-αβ in vivo, mice were inoculated with 200 µg poly(I:C) (InvivoGen, San Diego, CA) i.p. unless otherwise described. To deplete NK cells, mice were inoculated i.v. with 25 µg anti-NK1.1 or IgG2a (Cl.18.4) isotype control (BioXcell, West Lebanon, NH), and cells were stained with NK1.1 (PK136; BD Pharmingen) to assess depletion. To deplete CD4 T cells, mice were inoculated i.v. with 100 µg anti-CD4 (GK1.5) antibody or IgG2b (LTF-2) isotype control (BioXcell) and cells were stained with anti-CD4 (RM4-4; BD Pharmingen) to assess depletion.
Adoptive transfers
Spleens were harvested from TCR transgenic mice (P14, HY, or OT-I) and single cell suspensions were prepared. Red blood cells were lysed with a 0.84% NH4Cl solution, and lymphocytes were washed with HBSS. Where described, cells were labeled with the fluorescent dye CFSE by incubation in 2 µM CFSE in HBSS (Invitrogen, Carlsbad, CA) at 37°C for 15 minutes. 5 × 105 – 1 × 106 TCR transgenic CD8 T cells were injected into congenic recipient mice i.v.
Synthetic peptides
Synthetic peptides were used to stimulate T cell responses. All peptides were purchased from 21st Century Biochemicals (Marlboro, MA) and were purified with reverse phase-HPLC to 90% purity. For ex vivo stimulations, P14 transgenic T cells were stimulated with the LCMV epitope GP33–41 (KAVYNFATC) (20), HY transgenic T cells were stimulated with the Y-chromosome-encoded Smcy epitope (KCSRNRQYL) (27), and OT-I transgenic T cells were stimulated with OVA257–264 (SIINFEKL) (22).
Intracellular cytokine and effector molecule staining
Cytokine production was evaluated after stimulation with peptides using the Cytofix/Cytoperm Kit Plus (with GolgiPlug; BD Pharmingen). Spleen leukocytes (2–4 × 106) were plated in replicates (as many as 10 wells/spleen) in 96-well plates with 5 µM synthetic peptide, 10 U/ml human rIL-2, and 0.2 µl GolgiPlug (BD Pharmingen) for 5 hours at 37°C. For positive controls, splenocytes were stimulated with 1 µg purified anti-mouse CD3ε mAb (145-2c11; BD Pharmingen). Following stimulations, splenocytes were washed in Flow Cytometry Buffer (2% FCS in HBSS) and blocked with α-Fc (2.4G2; BD Pharmingen) for 15 minutes at 4°C. Splenocytes were then stained with a combination of fluorescently-labeled monoclonal antibodies (mAb) specific for CD8 (53-6.7; BD Pharmingen), Ly5.2/CD45.2 (104; BD Pharmingen), Ly5.1/CD45.1 (A20; eBioscience (San Diego, CA) or BioLegend (San Deigo, CA)), Thy1.2/CD90.2 (53-2.1; BD Pharmingen), Thy1.1/CD90.1 (H1S51; eBioscience), Vα2 TCR (B20.1; eBioscience), HY TCR (T3.70; eBioscience), CD44 (IM7; BD Pharmingen), CD122 (TM-β1; BD Pharmingen), CD62L (MEL-14, BD Pharmingen), and CD43 (1B11; BioLegend) for 20 minutes at 4°C. Subsequent fixation and permeabilization was performed via Cytofix/Cytoperm for 20 minutes at 4°C. Following permeabilization, cells were stained with fluorescently-labeled mAbs specific for IFN-γ (XMG1.2; BD Pharmingen or eBioscience), TNF (MP6-XT22; BD Pharmingen), and/or granzyme B (GB11; Invitrogen). Eomes protein was stained with anti-mouse/human Eomes (Dan11mag; eBioscience) after fixation and permeabilization with the FoxP3 staining buffer kit (eBioscience) as per manufacturer’s instruction. To assay the ability of CD8 T cells to undergo antigen-driven degranulation, splenocytes were stimulated with synthetic peptides, as stated above, with the addition of 0.5 µl/well anti-CD107a (1D4B; BD Pharmingen) and anti-CD107b (ABL-93; BD Pharmingen) FITC-labeled antibodies and 0.2 µl/well of GolgiStop (BD Pharmingen).
Flow Cytometry
Freshly stained and previously fixed samples were acquired using a BD Biosciences LSRII with FACS Diva software and analyzed with FlowJo software (Treestar Inc, Ashland, OR). In order to analyze enough bystander TCR transgenic CD8 T cells, the threshold for acquisition was set to CD8+ events only, and the storage and stoppage gates were set on CD8+ events only. By setting these parameters, FACS Diva ignored all other (CD8−) events that ran through the cytometer and allowed for the acquisition of up to approximately 3–4 × 106 CD8 T cell events/sample. For most experiments in this study 3–6 × 105 CD8+ T cell events were collected/sample.
Functional IFN bioassay
Functional IFN-αβ was measured using a standard virus inhibition bioassay (28). Briefly, serum collected from mice and control human rIFN-α (PBL Interferon Source, Piscataway, NJ) were serially diluted (2-fold) across a 96-well flat bottom plate. Each well was seeded with 2 × 104 L-929 cells (NCTC clone 929). The following day, cells were infected with 7.5 × 105 pfu VSV. Cell morphology and cytopathic effects (CPE) were monitored 2 days post infection, and the amount of functional IFN was measured as the last dilution of serum or control rIFN-α to provide approximately 50% protection from VSV-mediated CPE. Due to the 2-fold serial dilutions, the Log2 of the reciprocal of the serum dilution that provided 50% protection from VSV-mediated CPE was graphed.
RNA isolation and quantitative real-time PCR
P14 transgenic CD8 T cells (7-AAD−, CD8+, Vα2+, and congenic marker+) were sorted to 93–99% purity on a Mo-Flo sorter (Cytomation, Fort Collins, CO). RNA was isolated from sorted P14 CD8 T cells with an RNeasy kit (Qiagen, Valencia, CA) and evaluated spectrophotometrically at 260 nm to determine concentration. cDNA was generated using the SuperScript III first-strand synthesis system (Invitrogen) on a PTC-200 Thermo Cycler (MJ Research, Waltham, MA) at 25°C for 10 minutes followed by 50°C for 50 minutes. Relative mRNA concentrations were determined by quantitative real-time PCR using SYBR Green PCR core reagent kit (Applied Biosystems, Foster City, CA) on an iCycler iQ (Bio-Rad, Hercules, CA). The following primers were used: 18S rRNA sense 5’-TGGTGGAGGGATTTGTCTGG-3’ and anti-sense 5’-TCAATCTCGGGTGGCTGAAC-3’, eomesodermin sense 5’TGAATGA ACCTTCCAAGACTCAGA-3’ and anti-sense 5’-GGCTTGAGGCAAAGTGTTGACA-3’, T-bet sense 5’-TTCCCATTCCTGTCCTTCACC-3’ and anti-sense 5’TGCCTTCTGCCTTTCCAC AC-3’. For the generation of standard curves, cDNA clones of 18S rRNA, eomesodermin, and t-bet were used.
Statistical Analyses
Where appropriate, student’s t tests were calculated using GraphPad InStat software. Significance was set at p<0.05 and denoted as *p<0.05, **p<0.005, and ***p<0.0005. All results are expressed as the mean ± standard deviation.
Results
Naïve bystander CD8 T cells are transiently sensitized to exert rapid effector functions during acute viral infection or after poly(I:C) treatment
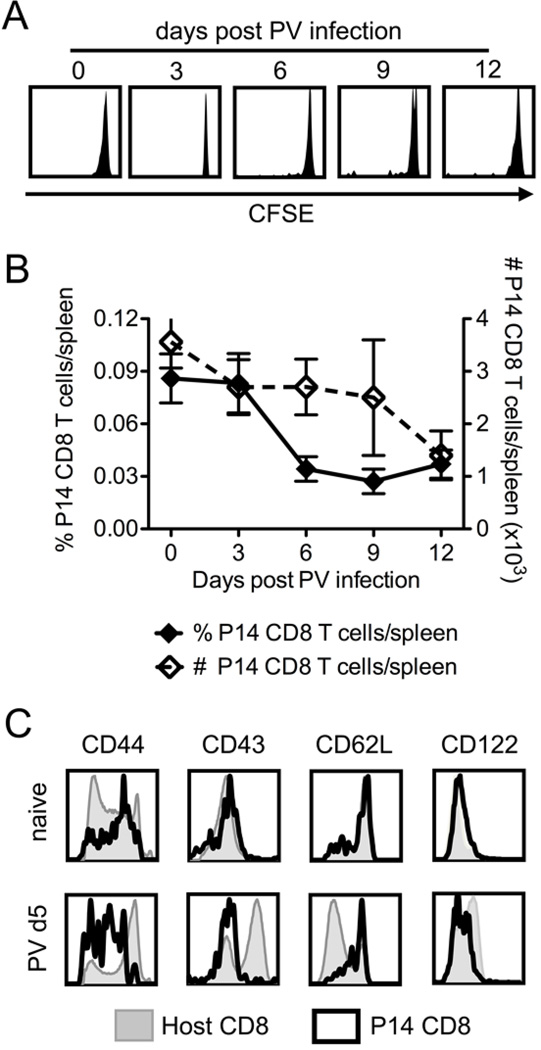
In order to study how bystander and latecomer CD8 T cells are affected by acute viral infections, we developed in vivo models to track and specifically activate bystander CD8 T cells. We tested three TCR transgenic CD8 T cell types and defined bystander as a transgenic CD8 T cell population that did not divide or proliferate (lose CFSE or increase in cell number) or alter the expression of activation markers (CD44, CD43 (1B11), CD62L, and CD122) during the viral infection. An example of this bystander phenotype is shown in Figure 1 for P14 transgenic CD8 T cells during PV infection. Figure 1A shows that there is no loss of CFSE, B shows no increase in frequency or cell number, and C shows the expression of activation antigens on P14 CD8 T cells and the host polyclonal CD8 T cells, which will include PV-specific CD8 T cells in the PV-infected mice. We used these phenotypes to define P14 cells as bystander cells also during VV infection. HY and OT-I transgenic CD8 T cells were similarly defined as bystander cells during LCMV, PV, and VV infections (data not shown).
Figure 1. P14 CD8 T cells do not divide or proliferate and remain phenotypically naive during PV infection.
P14 transgenic CD8 T cells were adoptively transferred into naïve congenic recipients followed by infection with 1.5×107 pfu PV i.p. A, CFSE profiles of donor P14 CD8 T cells at 0, 3, 6, 9, and 12 days post PV infection from individual mice representative of > 10 mice from 3 independent experiments. B, Frequency (left axis, closed diamonds) and number (right axis, open diamonds) of P14 CD8 T cells during PV infection, average of 3 mice/group, representative of 3 independent experiments. C, At day 0 (naïve) and day 5 post PV infection, splenocytes were harvested and stained for the surface markers shown. Representative examples of host CD8 T cells (shaded) and P14 CD8 T cells (thick black) are overlaid from the same host, representative of > 10 independent experiments.
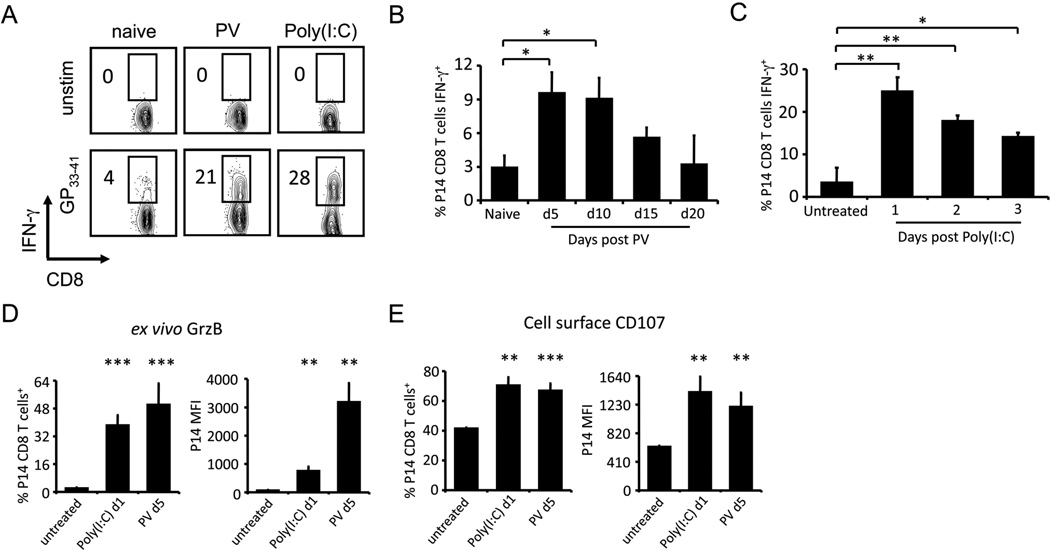
Once the in vivo bystander CD8 T cell models were established, we asked how naïve P14 CD8 T cells would respond when activated with cognate peptide GP33–41 during the early acute phase of PV infection. Spleen leukocytes from P14-implanted mice were harvested at day 5 post PV infection and stimulated with GP33–41 for 5 hours ex vivo. As predicted, naive P14 CD8 T cells isolated from untreated mice produced very little IFN-γ in response to GP33–41 stimulation. However, a substantial frequency of the P14 CD8 T cells from the PV-infected mice rapidly expressed IFN-γ after GP33–41 stimulation (Fig. 2A). Likewise, P14 cells isolated from mice one day after poly(I:C) inoculation also rapidly synthesized IFN-γ upon GP33–41 stimulation (Fig. 2A). The PV- or poly(I:C)-induced sensitization to rapid IFN-γ expression was transient, as the ability to rapidly express IFN-γ in response to cognate antigen was decreased at day 15 and was down to background levels by day 20 post PV infection (Fig. 2B); it was similarly decreased by 2–3 days after poly(I:C) inoculation (Fig. 2C).
Figure 2. PV infection and poly(I:C) treatment transiently sensitize bystander P14 CD8 T cells to rapid effector functions upon cognate antigen stimulation.
P14 transgenic CD8 T cells were adoptively transferred into naïve congenic recipients followed by infection with 1.5×107 pfu of PV or inoculation with 200 µg poly(I:C) i.p. A, At day 5 of PV infection or day 1 after poly(I:C) treatment, splenocytes were stimulated with GP33–41 peptide ex vivo. P14 transgenic CD8 T cells were gated, and intracellular accumulation of IFN-γ was assessed. B, At days 0 (naïve), 5, 10, 15, and 20 post PV infection, splenocytes were stimulated with GP33–41 peptide ex vivo and the frequency of P14 CD8 T cells producing IFN-γ was assessed. C, At days 0 (untreated), 1, 2, and 3 post poly(I:C) inoculation, splenocytes were stimulated with GP33–41 peptide ex vivo and the frequency of P14 CD8 T cells producing IFN-γ was assessed. D, At day 0 (untreated), day 1 post poly(I:C), or day 5 post PV GrzB expression was assessed by intracellular staining directly ex vivo and E, degranulation (CD107 surface expression) was assessed after GP33–41 peptide stimulation. The frequency of P14 CD8 T cells staining positive and MFI for both molecules were graphed. Representative experiments with 3–5 mice/group are shown, experiments were independently performed > 10 times. *p<0.05, **p<0.005, ***p<0.0005
We next questioned whether these naïve bystander CD8 T cells would also have the ability to be cytolytic. We measured their expression of GrzB immediately ex vivo and their ability to undergo antigen-driven degranulation in vitro by staining for the surface expression of LAMP-1 and LAMP-2 (CD107a and CD107b) in response to GP33–41 stimulation. In the absence of exposure to cognate GP33–41 ligand, GrzB was induced in P14 CD8 T cells after poly(I:C) treatment in terms of the frequency of P14 cells expressing GrzB and the relative amount of GrzB per cell (MFI) (Fig. 2D). The ability of P14 CD8 T cells to undergo antigen-driven degranulation, as measured by surface expression of CD107, was also significantly enhanced for P14 CD8 T cells after poly(I:C) treatment and PV infection (Fig. 2E). PV infection and poly(I:C) inoculation can thus sensitize naive P14 CD8 T cells such that they will up-regulate GrzB prior to cognate antigen stimulation in vivo and prime cells for IFN-γ production and degranulation upon ligand exposure in vitro.
Sensitization varies with the virus and the transgenic T cell
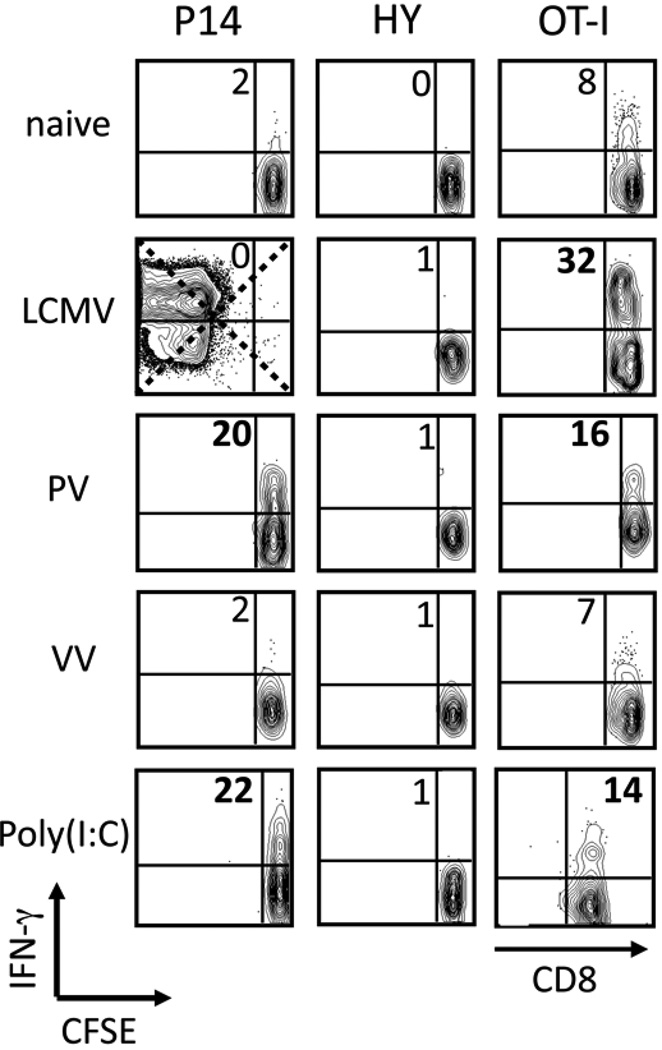
To determine whether other viral infections could sensitize P14 CD8 T cells and whether TCR transgenic CD8 T cells of other specificities could be sensitized, we examined the sensitization of P14, HY, and OT-I transgenic CD8 T cells by LCMV, PV, VV, and poly(I:C). Examples from 8 independent experiments depicting the IFN-γ production of cognate peptide-stimulated TCR transgenic CD8 T cells from all of these models are shown in Figure 3. The cumulative data generated from >30 experiments using all of these models are listed in Table I, which depicts the average ratio of cognate peptide-induced IFN-γ production for TCR transgenic CD8 T cells in infected/treated mice over the IFN-γ production for transgenic CD8 T cells from control mice. P14 CD8 T cells were sensitized to rapidly express IFN-γ in response to cognate peptide stimulation by PV and poly(I:C), but not by VV. HY transgenic CD8 T cells were not sensitized by any of the stimuli, and OT-I CD8 T cells were sensitized well by LCMV and moderately by PV and poly(I:C) (Fig. 3 and Table I).
Figure 3. Not all virus infections or pro-inflammatory stimuli sensitize bystander CD8 T cells.
P14, HY, or OT-I transgenic CD8 T cells were adoptively transferred into naive congenic recipients followed by inoculation with 5×104 pfu LCMV, 1.5×107 pfu PV, 1×106 pfu VV, or 200 µg poly IC i.p. At days 0 (naive) and 5 post infection or day 1 post poly(I:C), splenocytes were stimulated with GP33–41, Smcy, or SIINFEKL peptides ex vivo. Each transgenic population was gated and the ability of the transgenic CD8 T cells to produce IFN-γ in response to their cognate peptide was assessed. IFN-γ vs. CFSE is shown from representative mice from 8 independent experiments and the numbers depict the frequency of CFSEhi IFN-γ+ events, except for OT-I + poly(I:C) which shows IFN-γ vs CD8.
Table I.
Not all virus infections or pro-inflammatory stimuli sensitize bystander CD8 T cells1
| Virus infection or pro-inflammatory stimulus | ||||
|---|---|---|---|---|
| TCR Tg | LCMV | PV | VV | Poly(I:C) |
| P14 | N/A | 5.8±4 | 1.1±0.09 | 5.8±5 |
| HY | 0.93±0.13 | 0.96±0.04 | 0.5±0.5 | 0.66±0.3 |
| OT-I | 2.9±2 | 1.7±0.6 | 1.2±0.4 | 2.1±0.2 |
Indirect role for IFN-αβ
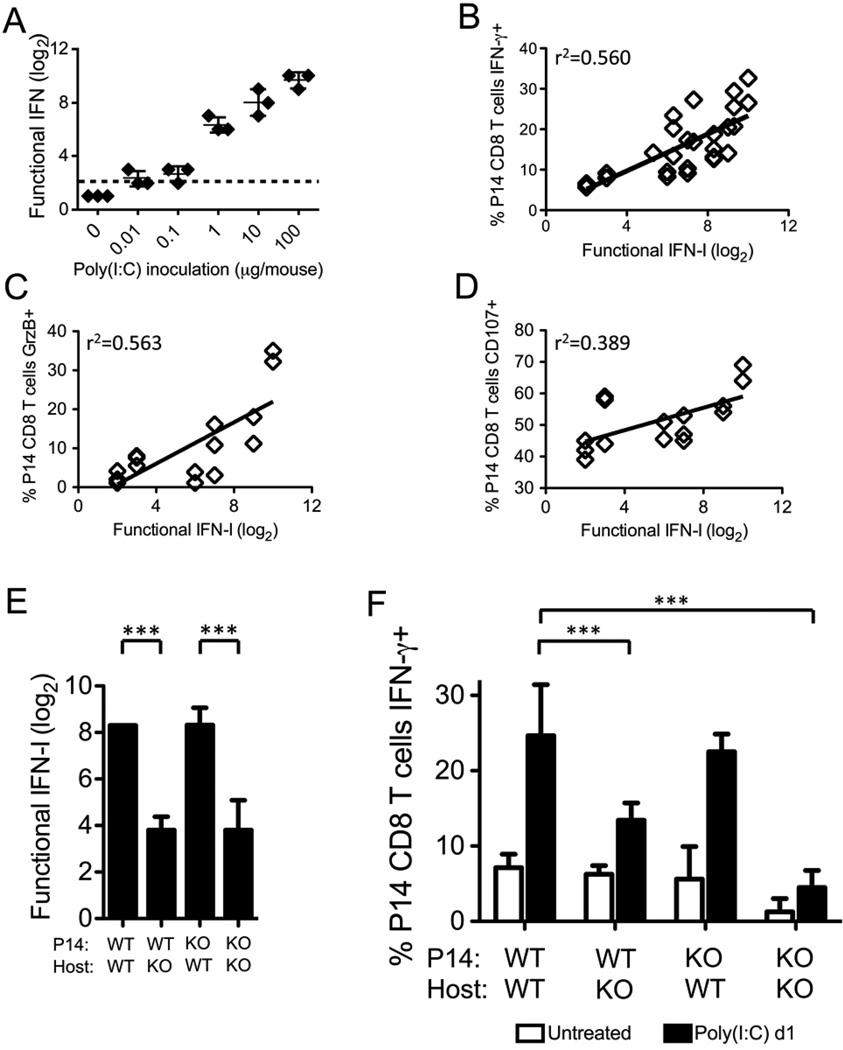
Due to the ability of LCMV, PV, and poly(I:C), all good IFN-αβ inducers, to sensitize naïve CD8 T cells and the inability of VV, a poor inducer of IFN-αβ, to do so, we questioned whether IFN-αβ levels correlated with the sensitization process. Mice were thus inoculated with 10-fold dilutions of poly(I:C) from 0.01–100 µg/mouse. Decreasing the dose of poly(I:C) resulted in lower induction of total functional IFN, as assessed by a VSV-mediated cytopathic effect inhibition bioassay (Fig. 4A), and there was a linear correlation between the amount of functional IFN induced and the ability of P14 CD8 T cells to rapidly express IFN-γ in response to GP33–41 stimulation (r2=0.560) (Fig. 4B). Likewise, we also found correlations between the induction of functional IFN and the up-regulation of GrzB (r2=0.563) and the ability of T cells to undergo enhanced antigen-driven degranulation (r2=0.389) (Fig. 4C, D).
Figure 4. Indirect role for IFN-αβ in the sensitization of P14 CD8 T cells after poly(I:C) treatment.
P14 CD8 T cells were adoptively transferred into congenic recipients followed by inoculation with 0–100 µg poly(I:C) i.p. One day post poly(I:C) inoculation, serum was collected for bioassay and splenocytes were collected for stimulation. A, Functional IFN for each individual mouse was assessed via VSV-induced CPE bioassay, and the log2 of the reciprocal of the serum dilution that provided 50% protection was graphed. Dashed line depicts the level of detection for the assay. B, The level of functional IFN was plotted against the ability of P14 T cells in each individual mouse to express IFN-γ in response to GP33–41 peptide stimulation from 2 independent experiments. n= 34 mice, r2=0.560, and p<0.005. C, The level of functional IFN was plotted against the expression of granzyme B in P14 T cells in each individual mouse. n= 18 mice, r2=0.563, and p<0.005. D, The level of functional IFN was plotted against the ability of P14 T cells in each individual mouse to degranulate in response to GP33–41 peptide stimulation. n=18 mice, r2=0.389, and p<0.05. E–F, WT or IFN-αβR KO P14 CD8 T cells were adoptively transferred into WT or IFN-αβR KO congenic recipients, followed by inoculation with 200 µg poly(I:C) i.p. At days 0 (untreated) and 1 post poly(I:C) inoculation, serum was collected for bioassay and splenocytes were stimulated with GP33–41 peptide ex vivo. E, Functional IFN in all poly(I:C)-treated groups was graphed. F, The ability of P14 CD8 T cells to rapidly express IFN-γ was assessed. Cumulative data from 5 independent experiments is depicted. ***p<.0005
We next asked if IFN-αβ was required for the sensitization of naïve P14 CD8 T cells by using IFN-αβR KO mice, which synthesize less total functional IFN than WT mice (Fig. 4E), likely due to the inhibition of a positive feedback loop initiated by IFN-α4 and IFN-β signaling (29, 30). Further, in order to address whether direct IFN-αβ signals were required for sensitization, we crossed the P14 transgenic mice to the IFN-αβR KO mice to generate P14 CD8 T cells that did not express the IFN-αβR and thus could not respond directly to IFN-αβ signals. WT or IFN-αβR KO P14 CD8 T cells were adoptively transferred into congenic WT or IFN-αβR KO recipients, which were then inoculated with poly(I:C). WT or IFN-αβR KO P14 CD8 T cells in the IFN-αβR KO host mice (WT→KO and KO→KO) were much less efficiently sensitized by poly(I:C) than either type of P14 cells in WT host mice (WT→WT and KO→WT) (Fig. 4F). The most pronounced defect in IFN-γ production was the KO→KO group, but the ratio of IFN-γ+ P14 CD8 T cells for the poly(I:C)-treated mice over the untreated mice in this group was similar to the WT→KO group. These data indicated that IFN-αβ is required for sensitization, but direct IFN-αβ signals on the P14 CD8 T cells are not required for their sensitization.
Influence of other cytokines and cytokine-producing cells on sensitization
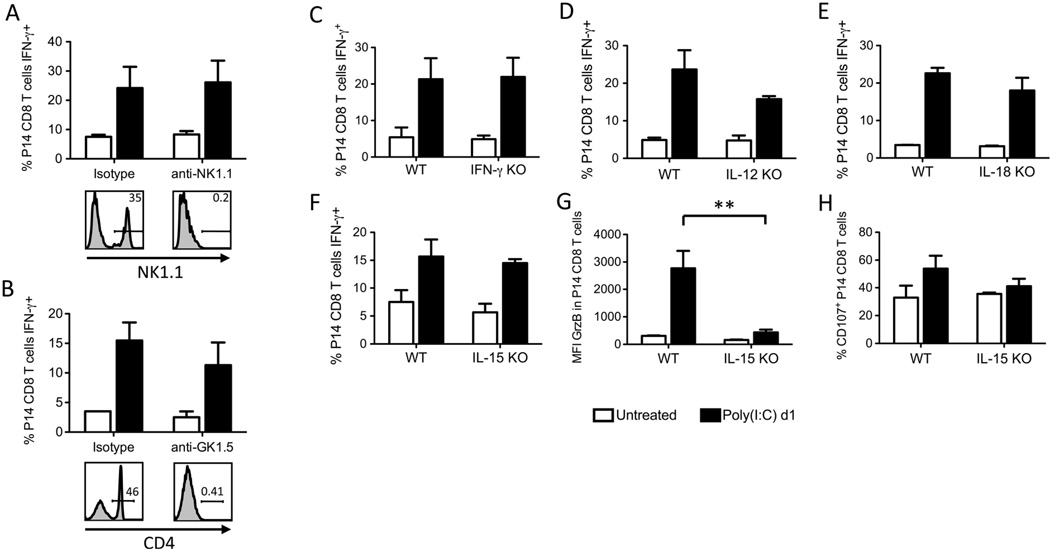
Due to the indirect requirement for IFN-αβ in the sensitization of naïve P14 CD8 T cells, we questioned whether IFN-αβ was inducing sensitization by way of another mediator. NK cells are activated by IFN-αβ (31), and CD4 T cells can be Th1-skewed by IFN-αβ (32), so it was possible that NK cells or CD4 T cells, perhaps by secreting IFN-γ, were mediating the sensitization of the P14 CD8 T cells. However, depletion of NK cells or CD4 T cells did not affect the sensitization of P14 CD8 T cells after poly(I:C) treatment (Fig. 5A,B). Further, P14 CD8 T cells were sensitized by poly(I:C) in IFN-γ KO mice (Fig. 5C).
Figure 5. NK cells, CD4 T cells, IFN-γ, IL-12, IL-18, and IL-15 are not required for the sensitization to rapid IFN-γ production after poly(I:C) treatment.
A–B, P14 CD8 T cells were adoptively transferred into WT congenic recipients, followed by i.v. inoculation with A, anti-NK1.1 or IgG2a, or B, anti-GK1.5 or IgG2b to deplete NK cells or CD4 T cells respectively. One day post antibody treatment, mice were inoculated with 200 µg poly(I:C) i.p. At days 0 (untreated) and 1 post poly IC inoculation, splenocytes were stimulated with GP33–41 peptide ex vivo and the intracellular accumulation of IFN-γ was assessed. Splenocytes were also stained with anti-NK1.1 clone PK136 (gated on CD3− DX5+) and anti-CD4 clone RM4-4 (gated on CD3+) of different clones than the depletion antibodies to assess depletion. C–H, P14 CD8 T cells were adoptively transferred into WT, IFN-γ KO (C), IL-12 KO (D), IL-18 KO (E), or IL-15 KO (F–H) congenic recipients. One day post adoptive transfer, mice were inoculated with 200 µg poly(I:C) i.p. At days 0 (untreated) and 1 post poly(I:C) inoculation, splenocytes were stimulated with GP33–41 peptide ex vivo and the intracellular accumulation of IFN-γ (C–F), GrzB (G), or degranulation (H) was assessed.
We next questioned whether another cytokine induced by viral infections and poly(I:C) could be mediating sensitization. Since IL-12, IL-18, and IL-15 have been shown to induce IFN-γ expression by effector and memory CD8 T cells (33–36), we tested the requirements for these cytokines by using cytokine knockout mice. We found that none of these cytokines, at least individually, was required for the sensitization of naive P14 CD8 T cells to rapidly express IFN-γ in response to peptide stimulation after poly(I:C) treatment (Fig. 5D–F). Each knockout mouse group induced a slightly lower frequency of IFN-γ-producing P14 CD8 T cells than did WT counterparts, but these differences were not statistically significant. Interestingly, IL-15 was required for up-regulation of GrzB (Fig. 5G) and may also play a role in the enhanced degranulation in response to cognate peptide stimulation. Antigen-driven degranulation of P14 CD8 T cells was reduced in the poly(I:C)-treated IL-15 KO mice in 2 independent experiments, but these results did not reach statistical significance (Fig. 5H and data not shown).
MHC I is required for the sensitization of naïve bystander CD8 T cells
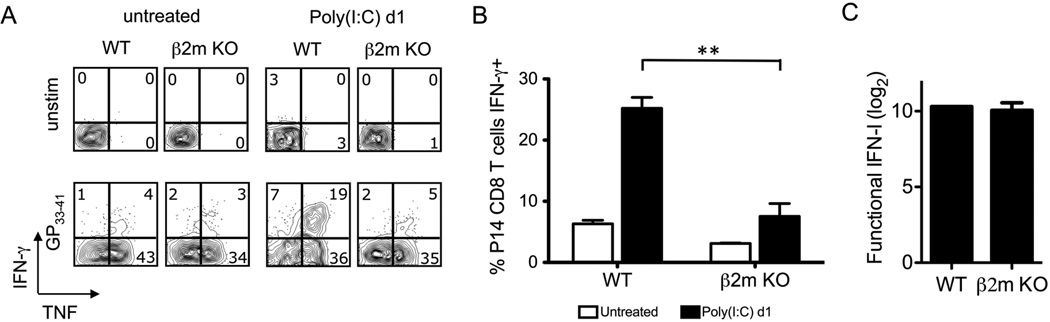
The fact that CD8 T cells of different specificities would be sensitized differently by poly(I:C) suggested that their TCR may play a role in sensitization. T cells are selected in the thymus for their low avidity to self antigens, and naïve T cells require MHC-presented self antigens to undergo homeostatic proliferation (37–39). Notably, female HY transgenic CD8 T cells, which recognize a male-encoded antigen, are known to be poor at homeostatic proliferation in female mice (38, 40) and could not be sensitized in our systems. This suggested that low affinity cryptically cross-reactive self-antigen in the presence of pro-inflammatory signals may sensitize P14 and OT-I CD8 T cells, which readily undergo homeostatic proliferation. If this were the case, CD8 T cell sensitization would require TCR signaling by class I MHC. To test for this, we first sought to determine if P14 CD8 T cells could be sensitized by poly(I:C) in β2m KO mice, which poorly express class I MHC (41, 42). We compensated for the lack of MHC I antigen presentation during the ex vivo T cell stimulation by providing congenic naïve splenocytes to efficiently present the GP33–41 peptide to the P14 CD8 T cells. Naïve T cells from untreated mice make TNF but not IFN-γ on exposure to their MHC-displayed ligand (43), and this set up allowed us to control for TCR stimulation in vitro, as under these conditions P14 cells taken from β2m KO mice produced TNF in response to GP33–41 (Fig. 6A). However, these P14 CD8 T cells were unable to rapidly express IFN-γ in response to peptide stimulation (Fig. 6A,B), suggesting that they required class I antigen presentation in vivo for sensitization. This difference between β2m KO and WT mice was not due to a defect in IFN-αβ induction, because similar levels of total functional IFN were induced in both strains of mice (Fig. 6C).
Figure 6. MHC I is required for the sensitization of P14 CD8 T cells after poly(I:C) treatment.
P14 transgenic CD8 T cells were adoptively transferred into naïve WT or β2m KO congenic recipients followed by inoculation with 200 µg poly(I:C) i.p. At days 0 (untreated) and 1 post poly(I:C), splenocytes were stimulated with GP33–41 peptide + exogenous WT splenocytes ex vivo. A, P14 CD8 T cells were gated and intracellular accumulation of IFN-γ and TNF was assessed. B, The frequency of IFN-γ producing P14 cells was graphed (n=4/group). C, Functional IFN production was graphed. Data are representative of 4 independent experiments. **p<0.005
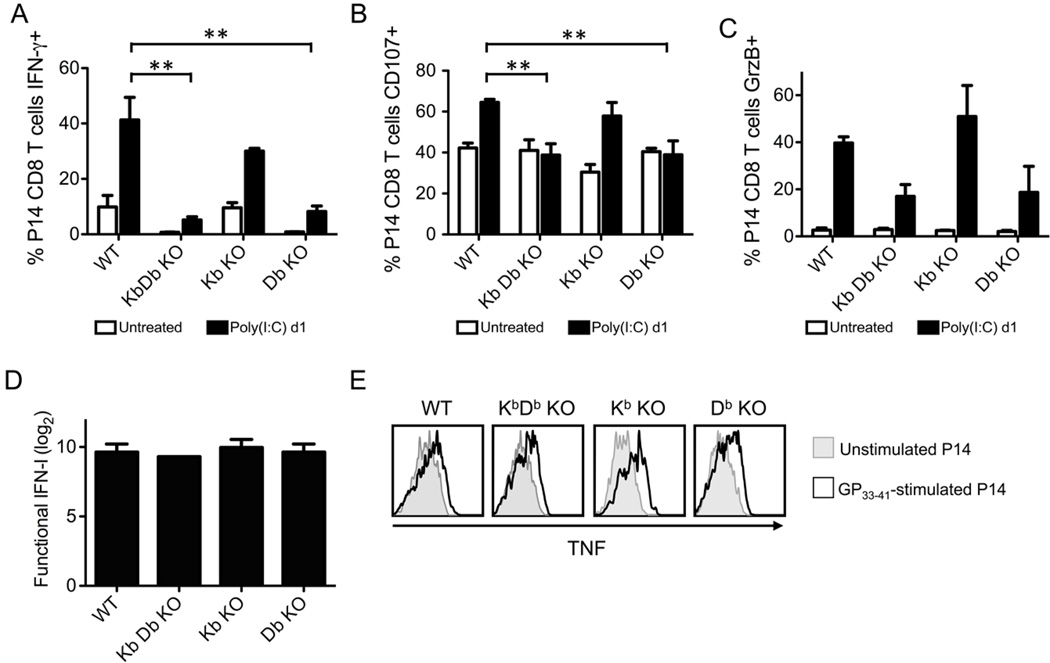
We also questioned whether cognate MHC (H2Db for P14) was required for sensitization or if any MHC I could sensitize the P14 CD8 T cells. To address this, we adoptively transferred P14 CD8 T cells into congenic WT, KbDb KO, Kb KO, and Db KO C57BL/6 mice, followed by inoculation with poly(I:C). As shown in Figure 7, P14 CD8 T cells were only sensitized in mice expressing H2Db (WT and Kb KO). Their ability to rapidly express IFN-γ and undergo enhanced degranulation in response to cognate peptide stimulation was abrogated in mice lacking H2Db but still expressing H2Kb (Fig. 7A,B). The immediate ex vivo expression of GrzB was up-regulated in the P14 CD8 T cells after poly(I:C) inoculation in all of the mice, but this up-regulation was reduced in those mice lacking H2Db (Fig. 7C), suggesting that there was both MHC I-dependent and -independent regulation of GrzB expression. Importantly, the inability of P14 CD8 T cells to be sensitized in mice lacking H2Db was not due to a defect in IFN induction (Fig. 7D) or due to an inability to be activated in vitro, as the P14 CD8 T cells from all recipient mice could synthesize TNF in response to GP33–41 stimulation (Fig. 7E). These data suggest that cognate MHC (H2Db) displaying cryptic (i.e. unidentified) self peptides was required for the sensitization of naïve P14 CD8 T cells during poly(I:C) treatment.
Figure 7. H2Db is required for the sensitization of P14 CD8 T cells after poly(I:C) treatment.
P14 transgenic CD8 T cells were adoptively transferred into naïve WT, KbDb KO, Kb KO, or Db KO congenic recipients followed by inoculation with 200 µg poly(I:C) i.p. At days 0 (untreated) and 1 post poly(I:C), splenocytes were stimulated with GP33–41 peptide + exogenous WT splenocytes ex vivo. The frequency of IFN-γ-producing (A), CD107+ (B), and GrzB+ (C) P14 CD8 T cells were graphed. D, Functional IFN production was graphed. E, TNF production by unstimulated (grey histograms) and GP33–41-stimulated (black lined histograms) P14 CD8 T cells was assessed. Data depict 3–4 mice/group and is representative of 3 independent experiments.
Induction of Eomes in sensitized P14 CD8 T cells during PV infection and poly(I:C) treatment
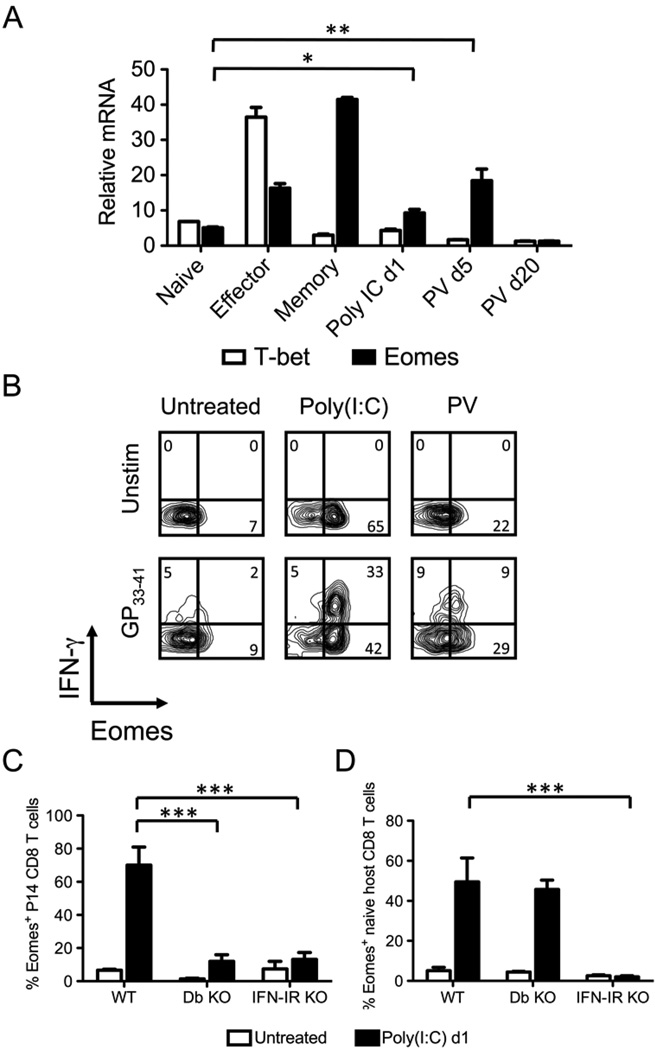
IFN-γ gene transcription is regulated by the transcription factors T-bet and Eomes (8–13), and we questioned whether T-bet or Eomes mRNA was induced in bystander-sensitized CD8 T cells. P14 CD8 T cells from naïve, LCMV day 6 (effector), LCMV day 40 (memory), poly(I:C) day 1, PV day 5, and PV day 20 infected mice were purified by cell sorting immediately ex vivo and without any exposure to cognate ligand. Their RNA was then extracted and used for quantitative real-time PCR to quantify mRNA levels of the transcription factors. As shown in Figure 8A and consistent with published reports, effector CD8 T cells up-regulated mRNA for T-bet and Eomes, and memory CD8 T cells further up-regulated Eomes but not T-bet mRNA (10, 14). The sensitized bystander P14 CD8 T cells up-regulated Eomes, but not T-bet mRNA, after poly(I:C) inoculation and PV infection (Fig. 8A). Importantly, the high expression of Eomes mRNA in bystander CD8 T cells was transient, and by day 20 after PV infection, at a time in which the P14 CD8 T cells could no longer rapidly express IFN-γ (Fig. 2B), the level of Eomes mRNA was below that detected in naïve CD8 T cells.
Figure 8. Induction of Eomes, but not T-bet, in bystander P14 CD8 T cells during acute PV infection and poly(I:C) treatment.
A, P14 transgenic CD8 T cells were adoptively transferred into naïve congenic recipients followed by inoculation with 5×104 pfu LCMV, 1.5×107 pfu PV, or 200 µg poly(I:C) i.p. P14 CD8 T cells were sorted from naïve P14 mice (Naïve), day 6 post LCMV (Effector), day 42 post LCMV (Memory), day 1 post poly(I:C), day 5 post PV, or day 20 post PV. RNA was extracted and converted to cDNA, followed by real-time PCR using primers for T-bet and Eomes. Standardized mRNA relative to 18S rRNA was calculated from an individual experiment that is representative of 4 independent experiments. B–D, P14 transgenic CD8 T cells were adoptively transferred into naïve congenic WT, H2Db KO, or IFN-αβR KO recipients followed by inoculation with 1.5×107 pfu PV or 200 µg poly(I:C) i.p. At day 5 of PV infection or day 1 after poly(I:C) treatment, splenocytes were stimulated with GP33–41 peptide ex vivo. B, P14 transgenic CD8 T cells were gated, and IFN-γ production and Eomes expression was assessed from representative mice for each group. C, The frequency of Eomes+ P14 CD8 T cells in WT, H2Db KO, and IFN-αβR KO mice was graphed. D, The frequency of Eomes+ CD44lo naïve host CD8 T cells WT, H2Db KO, and IFN-αβR KO mice was graphed. Data in (B–D) are representative of 3 independent experiments.
Eomes protein was also induced in sensitized P14 CD8 T cells after PV infection and poly(I:C) treatment and was expressed directly ex vivo without a requirement for exposure to GP33–41 (Fig. 8B,C). Strikingly, most of the P14 CD8 T cells up-regulated Eomes protein after poly(I:C) treatment (71% ± 7.7, n = 8), a frequency nearly twice that (38% ± 9, n = 8) of the cells that rapidly synthesized IFN-γ in response to GP33–41 stimulation ex vivo (Fig. 8B). Importantly, about half of the polyclonal naïve CD8 T cells in WT B6 mice also up-regulated Eomes expression after poly(I:C) treatment (Fig. 8D), arguing that this up-regulation is a common event and not restricted to only a rare transgenic T cell population.
Since MHC I and indirect effects of IFN-αβ were required for the sensitization of P14 CD8 T cells to rapidly express IFN-γ in response to cognate antigen, we also tested whether these signals were required for the induction of Eomes. To address this, we measured the induction of Eomes protein in P14 and naïve polyclonal host CD8 T cells in H2Db KO and IFN-αβR KO mice. As shown in Figure 8C, P14 CD8 T cells did not up-regulate Eomes protein in response to poly(I:C) treatment in H2Db KO mice, supporting the concept that recognition of MHC was needed for sensitization. In contrast, induction of Eomes protein in naïve polyclonal CD8 T cells in the H2Db KO mice was like that of wild-type (Fig. 8D), indicating that these host CD8 T cells, which had been selected in a MHC H2Kb environment, received sufficient stimulation for sensitization. Neither donor P14 cells nor polyclonal host naïve cells were sensitized in mice lacking receptors for IFN-αβ (Fig. 8C,D). Taken together, these data show that IFN-αβ and cognate MHC I are required for the up-regulation of Eomes in bystander-sensitized CD8 T cells, and we suggest that the expression of this transcription factor allows for the rapid synthesis of IFN-γ in response to cognate antigen stimulation.
Discussion
We demonstrate here that IFN-αβ-inducing acute viral infections and TLR agonists sensitize naïve phenotype bystander CD8 T cells such that they will up-regulate GrzB prior to cognate antigen stimulation in vivo and prime cells for IFN-γ production and degranulation upon ligand exposure in vitro (Fig. 2). Associated with this acquisition of effector functions was the up-regulation of the t-box transcription factor Eomes, known to regulate CTL effector functions (Fig. 8). Hence, these naïve bystander T cells were conditioned to behave like memory cells on exposure to high affinity cognate ligand and thus had entered a distinct differentiation pathway when activated by cognate antigen in the presence of this IFN-αβ stimulus.
The sensitization likely required low affinity MHC-TCR interactions that did not fully activate the T cells, because if MHC I was reduced, absent, or of the wrong allotype, sensitization did not occur (Figs. 6–7). Additionally, if the T cells expressed a TCR with very low self-reactivity, as is the case with the HY TCR transgenic cells (38, 40), they were not sensitized (Fig. 3 and Table I). About half of naïve host polyclonal CD8 T cells synthesized Eomes protein after poly(I:C) (Fig. 8D), arguing that a substantial proportion of the T cells may become sensitized by virus-induced cytokines and that once sensitized, their response to cognate ligand is altered. Sensitization also required IFN-αβ but not direct IFN-αβ signaling on the CD8 T cells (Fig. 4). IFN-αβ did not sensitize CD8 T cells by way of NK cells or CD4 T cells, nor were IFN-γ, IL-12, IL-15, or IL-18 required, at least individually, for sensitization (Fig. 5). Nevertheless, it is possible that combinations of IFN-γ, IL-12, IL-15, and IL-18 signals sensitize P14 CD8 T cells, because combinations of these cytokines can promote IFN-γ production by effector and memory CD8 T cells better than any of them individually (33, 34). Due to the requirement for MHC I, it is probable that one indirect role of IFN-αβ during PV infection or poly(I:C) treatment is to up-regulate expression of MHC I. T cells are positively and then negatively selected in the thymus under conditions when IFN is not up-regulating MHC, and it has been a mystery why T cells selected at one threshold of MHC do not become auto-aggressive during viral infections which induce high levels of MHC expression throughout the host (44). Here we show that these T cells may become sensitized but not fully activated by the enhanced expression of self-MHC during acute viral infections. Full activation only occurs on exposure to their high affinity ligand and not to cryptically cross-reactive self ligands present in the host. The simplest explanation of our results would be that the IFNαβ-induced up-regulation of class I MHC was all that was needed to sensitize the T cells. We cannot, however, rule out that other indirect IFN-induced events modulate this process.
IFN-γ transcription is a tightly controlled process, regulated by chromatin accessibility and the expression of transcription factors. The t-box transcription factors T-bet and Eomes play an essential role in the induction of IFN-γ transcription in virus-specific CD8 T cells (9, 11–13). We show here that Eomes is transiently induced in bystander-sensitized CD8 T cells (Fig. 8). This probably imparts the ability to rapidly express IFN-γ in response to high affinity cognate antigen. Additionally, another known target of Eomes is the β chain of the IL-2R and IL-15R, CD122 (11), which was not up-regulated to the level on virus-specific CD8 T cells, but was moderately induced on bystander P14 and naïve polyclonal CD8 T cells during acute viral infection and after poly(I:C) treatment (Fig. 1C), suggesting that Eomes expression in bystander-sensitized CD8 T cells may also induce IL-15 responsiveness. This is pertinent, given the result that IL-15 was required for the up-regulation of GrzB and possibly for enhanced degranulation of bystander-sensitized P14 CD8 T cells after poly(I:C) treatment (Fig. 5G,H).
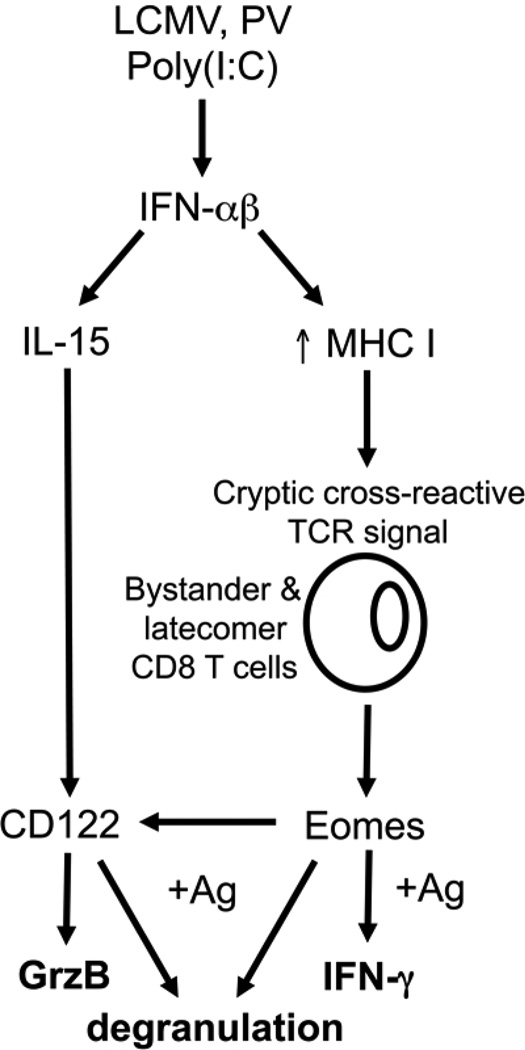
Taken together, we propose the model shown in Figure 9, whereby the arenaviruses LCMV and PV and the TLR agonist poly(I:C) induce IFN-αβ (31, 45). IFN-αβ has pleotropic effects on many cell types, including the up-regulation of MHC I and the induction of cytokines, including IL-15 (46–48). We propose that the enhanced expression of MHC I during these inflammatory conditions results in TCR signaling by low affinity cryptically cross-reactive self peptide-MHC. These signals up-regulate the t-box transcription factor Eomes, which allows for the rapid expression of IFN-γ upon cognate antigen stimulation. Eomes-induced expression of CD122 and concomitant IL-15 responsiveness also regulates the expression of GrzB and perhaps the ability of bystander or latecomer CD8 T cells to undergo enhanced antigen-driven degranulation. Therefore, inflammatory signals during acute viral infections initiate the sensitization of naive bystander and latecomer CD8 T cells such that they will rapidly exert effector functions upon cognate antigen stimulation.
Figure 9. Model of the mechanisms that sensitize bystander CD8 T cells during acute viral infections.
IFN-αβ induced by viral infection or TLR agonist poly(I:C) induces expression of IL-15 and up-regulation of MHC I. Up-regulation of MHC I enhances presentation of self and virus-encoded peptides, which signal through the TCR of bystander CD8 T cells, inducing Eomes expression. Eomes induces expression of CD122, which confers IL-15 responsiveness and induces GrzB expression. In response to cognate antigen, the expression of Eomes allows for the rapid synthesis of IFN-γ. IL-15 expression is also required for the enhanced cognate antigen-driven degranulation.
These biochemical changes in sensitized T cells that enable them to become immediate effector cells might also affect their proliferation potential. It is difficult to initiate new immune responses during viral infections, and in a separate study using the same transgenic models described herein, we have found that these sensitized bystander cells proliferate poorly in vivo in response to their cognate antigen (H.D.M. and R.M.W, manuscript in preparation). It has been suggested that high inflammatory environments occurring during viral infection may favor the expansion of short term effector cells that poorly develop into memory cells (49, 50), and that could be reflecting the IFN-αβ-induced sensitization observed in the present study. It is also intriguing to speculate that chronic viral infections and inflammatory diseases may impact naïve CD8 T cell responses, but whether sensitization could be maintained under such conditions is currently unknown.
The rules for T cell differentiation events determined by studying naïve T cells from unstimulated mice would thus be different for T cells derived from already inflamed environments. It could be predicted that latecomer T cells recruited at later stages of an immune response would behave differently than those stimulated at the beginning of a response. In this regard, latecomer T cells may be immediately able to produce effector cytokines and to lyse virus-infected cells without having to proliferate and may quickly assist in clearing the pathogen. Further, because different responses were seen in transgenic T cells of different specificities, it could be argued that this bystander sensitization may provide a spectrum of T cells receiving a variety of strength of signals from TCR and cytokine receptors and perhaps ultimately inducing different fates.
Acknowledgements
The authors would like to thank Drs. Michael Brehm, Kapil Bahl, and Stephen Waggoner for technical assistance and helpful discussions, and Keith Daniels for manuscript preparation.
Nonstandard abbreviations
LCMV
lymphocytic choriomeningitis virus
Eomes
eomesodermin
GrzB
granzyme B
PV
Pichinde virus
VV
vaccinia virus
Footnotes
1
This work was supported by U.S.P.H.S. research grants NIH AI-017672 and AI081675 (R.M.W.), NIH training grant T32AIO7349-16 (H.D.M.), and an NIH Diabetes and Endocrinology Center Research Grant DK32520.
References
- 1.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oehen S, Brduscha-Riem K. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J Immunol. 1998;161:5338–5346. [PubMed] [Google Scholar]
- 3.Jenkins MR, Mintern J, La Gruta NL, Kedzierska K, Doherty PC, Turner SJ. Cell cycle-related acquisition of cytotoxic mediators defines the progressive differentiation to effector status for virus-specific CD8+ T cells. J Immunol. 2008;181:3818–3822. doi: 10.4049/jimmunol.181.6.3818. [DOI] [PubMed] [Google Scholar]
- 4.Zimmermann C, Prevost-Blondel A, Blaser C, Pircher H. Kinetics of the response of naive and memory CD8 T cells to antigen: similarities and differences. Eur J Immunol. 1999;29:284–290. doi: 10.1002/(SICI)1521-4141(199901)29:01<284::AID-IMMU284>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 5.Aune TM, Penix LA, Rincon MR, Flavell RA. Differential transcription directed by discrete gamma interferon promoter elements in naive and memory (effector) CD4 T cells and CD8 T cells. Mol Cell Biol. 1997;17:199–208. doi: 10.1128/mcb.17.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slifka MK, Rodriguez F, Whitton JL. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature. 1999;401:76–79. doi: 10.1038/43454. [DOI] [PubMed] [Google Scholar]
- 7.Liu F, Whitton JL. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J Immunol. 2005;174:5936–5940. doi: 10.4049/jimmunol.174.10.5936. [DOI] [PubMed] [Google Scholar]
- 8.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 9.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz-Guilloty F, Pipkin ME, Djuretic IM, Levanon D, Lotem J, Lichtenheld MG, Groner Y, Rao A. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J Exp Med. 2009;206:51–59. doi: 10.1084/jem.20081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russc AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 12.Mayer KD, Mohrs K, Reiley W, Wittmer S, Kohlmeier JE, Pearl JE, Cooper AM, Johnson LL, Woodland DL, Mohrs M. Cutting edge: T-bet and IL-27R are critical for in vivo IFN-gamma production by CD8 T cells during infection. J Immunol. 2008;180:693–697. doi: 10.4049/jimmunol.180.2.693. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takemoto N, Intlekofer AM, Northrup JT, Wherry EJ, Reiner SL. Cutting Edge: IL-12 inversely regulates T-bet and eomesodermin expression during pathogen-induced CD8+ T cell differentiation. J Immunol. 2006;177:7515–7519. doi: 10.4049/jimmunol.177.11.7515. [DOI] [PubMed] [Google Scholar]
- 15.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarozinski CC, Welsh RM. Minimal bystander activation of CD8 T cells during the virus-induced polyclonal T cell response. J Exp Med. 1997;185:1629–1639. doi: 10.1084/jem.185.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Masopust D, Murali-Krishna K, Ahmed R. Quantitating the magnitude of the lymphocytic choriomeningitis virus-specific CD8 T-cell response: it is even bigger than we thought. J Virol. 2007;81:2002–2011. doi: 10.1128/JVI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murali-Krishna K, Altman JD, Suresh M, Sourdive D, Zajac A, Ahmed R. In vivo dynamics of anti-viral CD8 T cell responses to different epitopes. An evaluation of bystander activation in primary and secondary responses to viral infection. Adv Exp Med Biol. 1998;452:123–142. doi: 10.1007/978-1-4615-5355-7_14. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 21.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 22.Barnden MJ, Heath WR, Rodda S, Carbone FR. Peptide antagonists that promote positive selection are inefficient at T cell activation and thymocyte deletion. Eur J Immunol. 1994;24:2452–2456. doi: 10.1002/eji.1830241029. [DOI] [PubMed] [Google Scholar]
- 23.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 24.Yang HY, Dundon PL, Nahill SR, Welsh RM. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;142:1710–1718. [PubMed] [Google Scholar]
- 25.Welsh RM, Seedhom MO. Lymphocytic choriomeningitis virus (LCMV): propagation, quantitation, and storage. Curr Protoc Microbiol. 2008;Chapter 15(Unit 15A):11. doi: 10.1002/9780471729259.mc15a01s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–1715. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 28.Rubinstein S, Familletti PC, Pestka S. Convenient assay for interferons. J Virol. 1981;37:755–758. doi: 10.1128/jvi.37.2.755-758.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998;441:106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- 30.Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Welsh RM., Jr Cytotoxic cells induced during lymphocytic choriomeningitis virus infection of mice. I. Characterization of natural killer cell induction. J Exp Med. 1978;148:163–181. doi: 10.1084/jem.148.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-Krishna K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J Immunol. 2007;178:4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raue HP, Brien JD, Hammarlund E, Slifka MK. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J Immunol. 2004;173:6873–6881. doi: 10.4049/jimmunol.173.11.6873. [DOI] [PubMed] [Google Scholar]
- 34.Berg RE, Cordes CJ, Forman J. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur J Immunol. 2002;32:2807–2816. doi: 10.1002/1521-4141(2002010)32:10<2807::AID-IMMU2807>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 35.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001;166:1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 36.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seddon B, Legname G, Tomlinson P, Zamoyska R. Long-term survival but impaired homeostatic proliferation of Naive T cells in the absence of p56lck. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 38.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 39.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin SJ, Peacock CD, Bahl K, Welsh RM. Programmed death-1 (PD-1) defines a transient and dysfunctional oligoclonal T cell population in acute homeostatic proliferation. J Exp Med. 2007;204:2321–2333. doi: 10.1084/jem.20062150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 42.Lehmann-Grube F, Dralle H, Utermohlen O, Lohler J. MHC class I molecule-restricted presentation of viral antigen in beta 2-microglobulin-deficient mice. J Immunol. 1994;153:595–603. [PubMed] [Google Scholar]
- 43.Brehm MA, Daniels KA, Welsh RM. Rapid production of TNF-alpha following TCR engagement of naive CD8 T cells. J Immunol. 2005;175:5043–5049. doi: 10.4049/jimmunol.175.8.5043. [DOI] [PubMed] [Google Scholar]
- 44.Bukowski JF, Welsh RM. Interferon enhances the susceptibility of virus-infected fibroblasts to cytotoxic T cells. J Exp Med. 1985;161:257–262. doi: 10.1084/jem.161.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merigan TC, Oldstone MB, Welsh RM. Interferon production during lymphocytic choriomeningitis virus infection of nude and normal mice. Nature. 1977;268:67–68. doi: 10.1038/268067a0. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 47.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pham NL, Badovinac VP, Harty JT. A default pathway of memory CD8 T cell differentiation after dendritic cell immunization is deflected by encounter with inflammatory cytokines during antigen-driven proliferation. J Immunol. 2009;183:2337–2348. doi: 10.4049/jimmunol.0901203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]