Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance (original) (raw)
Abstract
Ablation of the kinases Mst1 and Mst2, orthologs of the Drosophila antiproliferative kinase Hippo, from mouse intestinal epithelium caused marked expansion of an undifferentiated stem cell compartment and loss of secretory cells throughout the small and large intestine. Although median survival of mice lacking intestinal Mst1/Mst2 is 13 wk, adenomas of the distal colon are common by this age. Diminished phosphorylation, enhanced abundance, and nuclear localization of the transcriptional coactivator Yes-associated protein 1 (Yap1) is evident in Mst1/Mst2-deficient intestinal epithelium, as is strong activation of β-catenin and Notch signaling. Although biallelic deletion of Yap1 from intestinal epithelium has little effect on intestinal development, inactivation of a single Yap1 allele reduces Yap1 polypeptide abundance to nearly wild-type levels and, despite the continued Yap hypophosphorylation and preferential nuclear localization, normalizes epithelial structure. Thus, supraphysiologic Yap polypeptide levels are necessary to drive intestinal stem cell proliferation. Yap is overexpressed in 68 of 71 human colon cancers and in at least 30 of 36 colon cancer-derived cell lines. In colon-derived cell lines where Yap is overabundant, its depletion strongly reduces β-catenin and Notch signaling and inhibits proliferation and survival. These findings demonstrate that Mst1 and Mst2 actively suppress Yap1 abundance and action in normal intestinal epithelium, an antiproliferative function that frequently is overcome in colon cancer through Yap1 polypeptide overabundance. The dispensability of Yap1 in normal intestinal homeostasis and its potent proliferative and prosurvival actions when overexpressed in colon cancer make it an attractive therapeutic target.
Mst1 and Mst2 are class II GC kinases (1) that are the closest mammalian homologs of the Drosophila Hippo protein kinase. Hippo is the central component of an antiproliferative pathway that responds to signals arising from cell–cell contact to regulate negatively the oncogenic transcriptional coactivator, yorkie. Loss of Hippo function results in a yorkie-dependent accelerated proliferation, resistance to apoptosis, and massive organ overgrowth (2, 3). In mouse liver, Mst1 and Mst2 act in a redundant manner to maintain hepatocyte proliferative quiescence. Acute inactivation of both Mst1 and Mst2 in the adult liver results in the immediate onset of hepatocyte proliferation, a doubling of liver mass within a week progressing to a four- to fivefold increase, followed within weeks by multifocal hepatocellular carcinoma (HCC) (4). Albumin-Cre mediated inactivation of Mst1 and Mst2 in liver is accompanied by expansion of both the hepatocytes and the bipotential adult liver progenitors known as “oval cells”; in addition to HCCs and cholangiocarcinomas, these livers exhibit many tumors with mixed cellularity, presumably reflecting an origin from the Mst1/Mst2-deficient oval cells (4–6). The Mst1/Mst2-deficient livers exhibit loss the inhibitory phosphorylation of Yes-associated protein 1 (Yap1), the mammalian ortholog of yorkie, and a marked increase in overall and nuclear Yap1 abundance. Tetracycline-induced overexpression of transgenic Yap1 in liver also induces hepatocyte proliferation and massive enlargement of the organ that is reversible (7, 8) but if sustained results in the development of HCCs (8). In HCC cell lines derived from Mst1/Mst2-null livers, depletion of Yap1 causes growth inhibition and extensive apoptosis, findings that support the view that Yap1 activation is the major mechanism underlying the liver overgrowth seen with Mst1/Mst2 inactivation (4).
These findings indicate that, as with Hippo, Mst1/Mst2 negatively regulates Yap1 in mammalian liver; however, such a relationship does not prevail in all mammalian tissues. Thus, in mouse embryo fibroblasts (MEFs), cell–cell contact results in Yap1 phosphorylation and nuclear exclusion equally well in wild-type and Mst1/Mst2-null MEFs (4); in mouse keratinocytes, Yap inactivation during cellular differentiation occurs independently of Mst1 and Mst2 (9). Conversely, Mst1 negatively regulates the proliferative response of naïve T cells to antigen receptor stimulation through a Yap1-independent process (10). Thus, it appears that the wiring upstream of Yap1 and downstream of Mst1/Mst2 has been diversified considerably in mammals compared with the Drosophila Hippo pathway. The intestinal epithelial cell, like the hepatocyte, is of endodermal origin; however the self-renewal mechanisms of these two cells are radically different. Hepatocyte self-renewal is mediated by the division of fully differentiated adult hepatocytes that emerge from replicative quiescence approximately once per year (11). In contrast, the epithelial lining of the small intestine turns over completely every 4–5 d through the continuous division of intestinal stem cells located in the crypts of Lieberkühn. These intestinal stem cells differentiate into a transient amplifying compartment and thereafter into four types of mature cells (enterocytes, goblet cells, enteroendocrine cells, and Paneth cells). Except for the Paneth cells, the maturing cells migrate toward the tip of the villus, from which they are shed (12–14). Previous work has shown that overexpression of Yap1 in this epithelium results in the enhanced proliferation of the stem cell compartment accompanied by the disappearance of all differentiated cell types (7). We wished to determine the role of Mst1 and Mst2 in intestinal epithelial homeostasis and whether these kinases suppress Yap1 activation in this cellular milieu.
Results
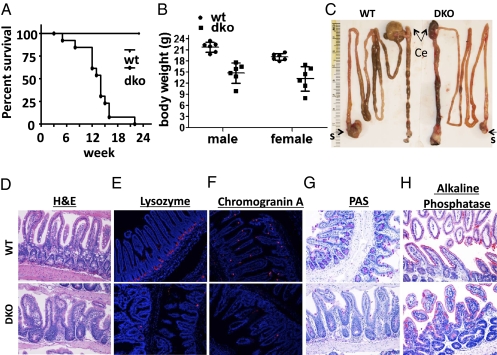
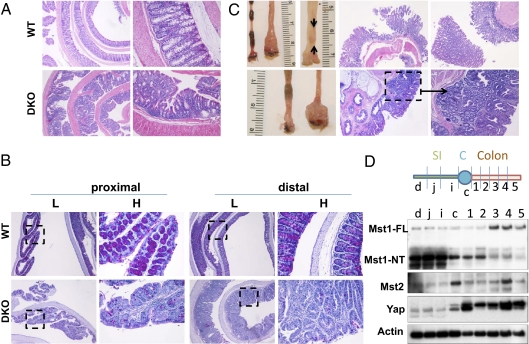
The intestine of Mst1-null and Mst2-null mice is indistinguishable from wild-type, whereas mice deficient in both Mst1 and Mst2 exhibit embryonic lethality at embryonic day 8.5–9.5 (4, 15), Mst1null/Mst2ff/villin-Cre mice are born in Mendelian ratios and appear normal at birth. However, these mice exhibit a median survival of only 13 wk (Fig. 1_A_) accompanied by severe wasting (Fig. 1_B_). The length of their small and large intestine is unaltered from wild type, but cecal size is greatly reduced, and the rectum usually is enlarged markedly (Fig. 1_C_ and SI Appendix, Fig. S1). The small intestinal mucosa of Mst1null/Mst2ff/villin-Cre mice is dysplastic: Villus structure is disorganized because of a marked increase in undifferentiated proliferating cells (Fig. 1_D_) with a dramatic decrease in cells of the secretory lineages (Fig. 1 E_–_G), although the abundance of absorbtive enterocytes is better preserved (Fig. 1_H_). The colonic epithelium also is highly dysplastic, with expansion of undifferentiated cells (Fig. 2 A and B) and severe loss of goblet cells (Fig. 2_B_). Although cecal size is reduced (Fig. 1_C_), the cecal mucosa is hyperproliferated and dysplastic (SI Appendix, Fig. S1), and hyperproliferation of epithelia throughout the colon is marked, especially near the rectum (Fig. 2_C_, Lower, arrows in second panel from left). In the distal colon there is especially exuberant overgrowth of dysplastic epithelia and the frequent presence of one or more adenomas extending into the lumen (Fig. 2_C_, Upper). Both the small and large intestine exhibit a marked expansion in the number of cells expressing high levels of CD133 (SI Appendix, Fig. S2 A and B), a marker of intestinal stem cells and transiently amplifying cells (16, 17), as well as increased abundance of the well-documented stem cell markers Leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5) and Achaete-scute complex homolog 2 (Ascl2) (Fig. 3_A_) (14) and increased numbers of cells expressing CD44 and CD24, markers associated with colon cancer stem cells (SI Appendix, Fig. S2 A and B) (18).
Fig. 1.
Mst1null/Mst2ff/villin-Cre mice exhibit early mortality and impaired epithelial differentiation in the small intestine. (A) A Kaplan–Meier survival curve from eight wild-type and 12 Mst1null/Mst2ff/villin-Cre (double-knockout, dko) mice, the latter with a average median survival of 13 wk. (B) Body weight of Mst1null/Mst2ff/villin-Cre (dko) and wild-type mice at age 6 wk. (C) Representative examples of intestinal tract from Mst1null/Mst2ff/villin-Cre (DKO) and wild-type mice at age 6 wk (n = 3 per group). Ce, cecum; s, stomach. (D_–_H) Representative samples showing absence of secretory cell lineages in the small intestine of Mst1null/Mst2ff/villin-Cre mice (DKO) compared with wild-type mice (n = 3 per group).
Fig. 2.
Mst1null/Mst2ff/villin-Cre (DKO) mice exhibit loss of goblet cells, epithelial dysplasia, and adenomas in the colon. (A) Hyperproliferation and dysplasia of the colonic epithelium in 6-wk-old Mst1null/Mst2ff/villin-Cre (DKO) mice. (B) Loss of goblet cells in proximal and distal colon of 6-wk-old Mst1null/Mst2ff/villin-Cre mice. Tissues shown are representative of three mutant and three wild-type mice. Boxed areas in low-magnification (L) views are shown to the right at higher magnification (H). Histologic views of the cecum are shown in SI Appendix, Fig. S1. (C) Formation of epithelial hyperplasia, dysplasia, and adenoma (arrows, Upper right) in the distal colon in two Mst1null/Mst2ff/villin-Cre mice, age 15 wk (Upper) and 20 wk (Lower). (D) Expression of the Mst1, Mst2, and Yap1 polypeptides in the mouse intestinal tract. C, colon; c, cecum; d, duodenum; i, ileum, j, jejunum; SI, small intestine.
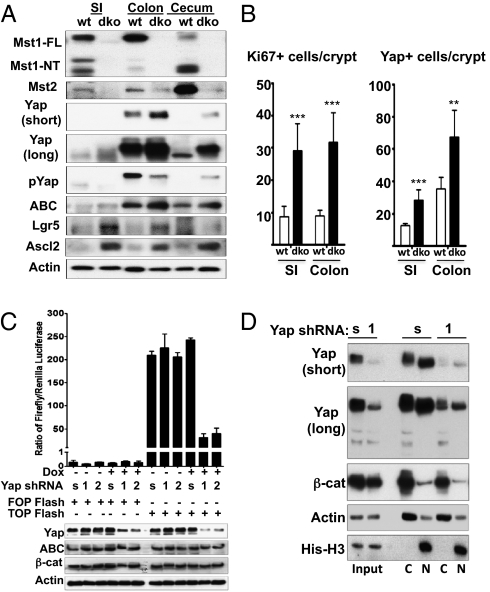
Fig. 3.
Expression of Mst1, Mst2, Yap, and Wnt targets in the intestine of Mst1null/Mst2ff/villin-Cre and wild-type mice. (A) Western blot of mucosal epithelium from the indicated intestinal segments of 6-wk-old Mst1null/Mst2ff/villin-Cre and wild-type mice for Mst1, Mst2 Yap, Yap(Ser127P), and the Wnt target polypeptides as indicated. ABC, activated β-catenin. SI, small intestine. (B) Quantitation of the number of Yap1+ and Ki67+ cells detected by immunofluorescence staining of small intestine (SI) and colon of Mst1null/Mst2ff/villin-Cre and wild-type mice. These results represent analyses of three mice of each genotype ± SE. (n = 20 crypts per section, 3 mice of each genotype; **P < 0.01 and ***P < 0.001 vs. WT). A representative immunofluorescent image of Yap1, Ki67, and β-catenin staining is shown in SI Appendix, Fig. S3. (C) SW480 cells stably expressing lentiviral-encoded, doxycycline-inducible scrambled (s) or either of two Yap1-directed shRNAs (1 and 2) were treated with doxycycline or carrier at time 0, transfected with plasmids encoding either TOPflash or FOPflash and Renilla luciferase 24 h thereafter, and were extracted 48 h after addition of doxycycline or carrier. Luciferase activities were assayed. Firefly luciferase activity normalized for Renilla luciferase is shown. (D) SW480 cells as in Fig. 4_C_ expressing scrambled (s) or Yap-directed shRNA (1) were harvested 48 h after addition of doxycycline or carrier, and cytosolic and nuclear fractions were immunoblotted for Yap, β-catenin, actin, and histone H3.
In 6-wk-old wild-type mice, Mst1 and Mst2 polypeptides, visualized by immunoblot, are expressed in all segments of the small and large intestine. In the small intestine, cecum, and proximal colon Mst1 is present predominantly as a 36-kDa polypeptide (Fig. 2_D_), corresponding in size to the constitutively active, caspase-cleaved catalytic fragment that is generated in cells undergoing apoptosis (19) but which also is predominant in normal quiescent mouse liver (4). In the mid and distal colon, the full-length Mst1 polypeptide predominates, as is true for Mst2 throughout the intestine. Mst1 is absent from the small and large intestine of the Mst1null/Mst2ff/villin-Cre mice, and the expression of Mst2 is severely reduced in these mice (Fig. 3_A_). Available Mst1 and Mst2 antibodies are unsuitable for immunocytochemistry, so the relative contribution of Mst2 from nonepithelial cell types is not known; however, the marked decrease in Mst2 observed in the intestine of Mst1null/Mst2ff/villin-Cre mice indicates that the majority of Mst2 is contributed by the epithelia (Fig. 3_A_, Top two panels). Yap1 polypeptide exhibits relatively low expression in the small intestine but is highly abundant in the colon, especially in the distal segments (Fig. 2_D_ and Fig. 3_A_, Panels 3–5 from top). Yap1 immunocytochemistry in the wild-type small intestine (SI Appendix, Fig. S3, Farthest left column, top two rows) and colon (SI Appendix, Fig. S3, Third column from the left, top two rows) shows strongest staining in crypts that appear largely or entirely extranuclear in both regions. In addition there is a clear attenuation in overall Yap stain in cells closer to the villus tip. Yap1 abundance is greatly enhanced by elimination of Mst1 and Mst2 in all regions of the intestine (Fig. 3 A, Panels 3 and 4 from top and B, Right and SI Appendix, Fig. S3, Rows 1 and 2 from top). In the colon, where Yap1 abundance normally is greatest, the loss of Mst1 and Mst2 results in a decrease in Yap1(Ser127) phosphorylation (Fig. 3_A_, Panel 5 from top) despite the marked up-regulation of Yap1 polypeptide. Consistent with increased Yap1 abundance and diminished Yap1(Ser127) phosphorylation, extensive Yap1 nuclear localization is evident in the epithelia of the small and large intestine (SI Appendix, Fig. S3, Columns 2 and 4, top two rows). Moreover, compared with the wild-type intestine, cells reactive with anti-Ki67 are substantially more abundant in the Mst1/Mst2-deficient intestine, both in the crypts and extending further toward the lumen (Fig. 3_B_, Left and SI Appendix, Fig. S3, Rows 3 and 4).
The hyperproliferation of undifferentiated cells and the loss of the secretory cell populations resulting from elimination of Mst1 and Mst2 in the intestine (Figs. 1 and 2) led us to examine the status of the Wnt and Notch pathways. Activation of the Wnt pathway is thought to be the primary driver of proliferation in the intestinal stem cell compartment (14, 20), acting synergistically with Notch (21). Elimination of Mst1 and Mst2 is accompanied by little or no change in the overall abundance of β-catenin, nor is there a convincing difference in the abundance of nuclear β-catenin in the Mst1/Mst2-null intestine (SI Appendix, Fig. S3, Bottom two panels). A modest increase in the abundance of the activated form of β-catenin (dephosphoSer37/Thr41) is evident in the Mst1/2-deficient small and especially large intestine (Fig. 3_A_, Panel 6 from top) whereas the abundance of the Wnt targets and stem cell markers Lgr5 (22) and Ascl2 (20) (Fig. 3_A_, Panels 7 and 8 from top) are increased more markedly, suggesting enhanced β-catenin transcriptional efficacy. We therefore inquired whether Yap1 overexpression enhances β-catenin action. SW480 cells contain high levels of β-catenin because of the inactivation of adenomatosis polyposis coli (APC) (23) and show modest overexpression of Yap1. The T-cell factor (TCF) reporter plasmid TOPflash (24) was transiently expressed in SW480 colon cancer cells engineered to express scrambled or Yap1-directed shRNAs in a tetracycline-stimulated manner (Fig. 3_C_). TOPflash-directed expression of firefly luciferase is ∼1,000 fold greater than that directed by the FOPflash vector, which encodes a mutant inactive TCF promoter. Induction of Yap1 shRNA reduces TOPflash-directed luciferase expression by more than 80% without altering β-catenin overall or intranuclear (Fig. 3_D_) abundance, strongly supporting the view that Yap1, at least when overexpressed, enhances the transcriptional efficacy of β-catenin.
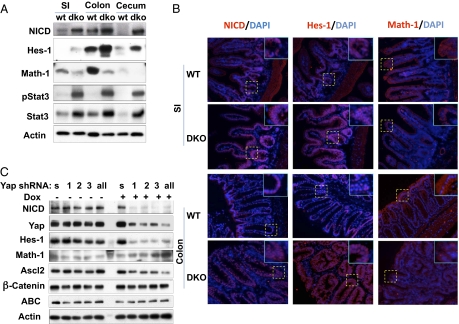
Strong activation of the Notch pathway also is evident in the Mst1/Mst2-deficient intestines (Fig. 4). The abundance of Hairy and enhancer of split 1 (Hes1), a transcriptional target of Notch, is up-regulated in small and large intestines (Fig. 4_A_, Panel 2 from top); in contrast to its predominant localization in crypts and transient amplifying cells in wild-type intestine, prominent intranuclear localization of Hes1 is evident along the entire epithelial surface of the Mst1/Mst2-deficient small and, especially, large bowel (Fig. 4_B_, Center column). The likelihood that the enhanced expression of Hes1 is driven by Notch is supported by the presence of increased amounts of intranuclear Notch intracellular domain (Fig. 4_A_, Top) along the entire epithelial surface of the Mst1/Mst2-deficient small and large bowel (Fig. 4_B_, Left). Thus, activation of the Wnt pathway, acting synergistically with the strong up-regulation of Notch signaling, probably accounts for the increased proliferation and expansion of Lgr5+ stem cells and transiently amplifying cell compartments in the Mst1/Mst2-deficient intestinal epithelium.
Fig. 4.
Notch signaling in the intestine of Mst1null/Mst2ff/villin-Cre and wild-type mice and in Yap-depleted HCT116 cells. (A) Western blot of mucosal epithelium from the indicated intestinal segments of 6-wk-old Mst1null/Mst2ff/villin-Cre and wild-type mice for Notch intracellular domain (NICD), the Notch targets Hes1 and Math1, Stat3, and PY-Stat3. SI, small intestine. (B) Immunofluorescence staining small intestine and colon of Mst1null/Mst2ff/villin-Cre and wild-type mice for Notch intracellular domain, Hes1, and Math1, each with DAPI. The areas in the dashed boxes are shown at higher magnification in Insets. Results in A and B are representative of three mice of each genotype. (C) HCT116 cells were engineered to express the following tetracycline-inducible shRNAs stably: a scrambled shRNA (s), one of three shRNAs each directed at a different segment of the Yap mRNA [Yap-sh1 (1); Yap-sh2 (2); or Yap-sh3 (3)], or all three shRNAs together (all). Extracts were subjected to immunoblot for the polypeptides shown 24 h after addition of doxycycline (+) or carrier (−).
Notch acts independently of Wnt to control intestinal epithelial differentiation through Hes1 (25), which is known to inhibit transcription of Atonal homolog1 (Atoh1/Math1) (26, 27), 2a transcription factor that is required for the differentiation of intestinal secretory cell lineages (28). In the Mst1/Mst2-deficient intestine, expression of the Notch targets Hes1 and Hairy/enhancer-of-split related with YRPW motif 1 (Hey1) is strongly up-regulated (SI Appendix, Fig. S4_A_), and Math1 expression is strongly reduced (Fig. 4 A, Panel 3 from top and B, Right). Thus, overactivation of the Notch pathway, in addition to synergizing with wingless-related MMTV integration site (Wnt) to drive stem cell proliferation, also is responsible for the failure of differentiation of the secretory lineages. Transgenic overexpression of Yap(Ser127Ala) in the mouse intestine was shown previously to be sufficient to cause Notch activation and a loss of cellular differentiation (7). We examined the contribution of Yap1 to the activation of Notch signaling in the colon cancer-derived cell line HCT116, which has a wild-type APC but a mutant allele of β-catenin caused by in-frame deletion of Ser45 (29) and mild overexpression of Yap1. Yap depletion in HCT116 cells does not alter the abundance of β-catenin, but Ascl2 polypeptide (Fig. 4_C_) is diminished strongly, indicating that, as in the SW480 cells, Yap1 directly or indirectly promotes β-catenin–stimulated gene expression. Additionally, shRNA suppression of endogenous Yap is accompanied by a strong decrease in the abundance of the Notch intracellular domain and in Hes1 expression, accompanied by an increase in Math1, all consistent with down-regulation of Notch activation (Fig. 4_C_). As to the mechanism of Notch activation in the Mst1/Mst2-deficient intestine, the mRNA levels of Notch1 are not altered, but there is a fourfold increase in the abundance of the mRNA encoding the Notch ligand Jagged 1 (SI Appendix, Fig. S4_A_), and immunoblot of small intestinal mucosal extracts verifies the presence of increased Jagged 1 polypeptide with intense Jagged 1 staining of crypts in the Mst1/Mst2-deficient small intestine (SI Appendix, Fig. S4_B_). Although Jagged 1, unlike connective tissue growth factor, is not known to be a direct transcriptional target of Yap1 (30), Jagged 1 is a direct target of β-catenin/TCF (31, 32). Thus, Notch activation in the Mst1/Mst2-deficient intestine is likely a consequence, at least in part, of Yap-stimulated β-catenin signaling; the contributions of other Mst1/Mst2- and/or Yap1-regulated pathways remain to be evaluated.
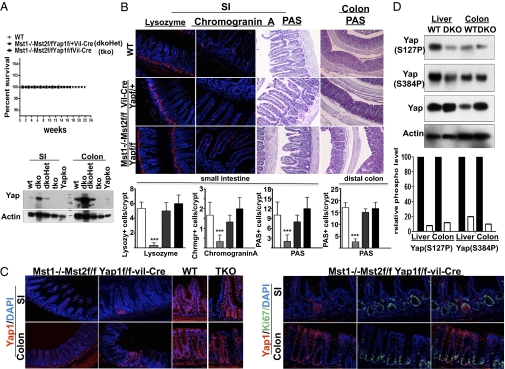
It was reported recently (33) that elimination of Yap1 from the intestinal epithelium has no significant effect on epithelial development or function in the unstressed mouse. We confirm this finding and observe that elimination of Yap does not affect the abundance of Ki67+ cells in mouse colon (SI Appendix, Fig. S5). Consequently, we sought to verify the importance of Yap1 to the phenotype of the Mst1/Mst2-deficient intestine. We therefore crossed the Mst1null/Mst2ff mice with Yap1ff and Yap1f+ mice and examined the impact of elimination of one or both Yap1 alleles on the consequences of Mst1/Mst2 deficiency in the intestine. Removal of one or both Yap1 alleles eliminates the premature mortality exhibited by mice lacking expression of Mst1 and Mst2 in the intestinal epithelium (Fig. 5_A_) and restores epithelial differentiation (Fig. 5_B_). Moreover, in the intestine of the Mst1null/Mst2ff/Yap1ff/villin-Cre mice, there are small patches where loxP excision failed to occur (Fig. 5_C_). These patches enable side-by-side comparison of epithelia that are Mst1 null but otherwise normal with epithelia lacking Mst1/Mst2 and Yap1. These two epithelia are morphologically indistinguishable, and the abundance of Ki67+ cells in the crypts is unaffected by the presence or absence of Yap1 (Fig. 5_C_ and SI Appendix, Fig. S5). These data demonstrate that, despite the presence of Yap1 in the intestinal stem cells and transiently amplifying cell compartments, Yap1 is entirely dispensable to the proliferative activity of these cells; nevertheless, removal of the Mst1 and Mst2 kinases initiates a Yap-dependent hyperproliferation. The ameliorative effect of the inactivation of a single Yap1allele emphasizes the importance of Yap1 overexpression to the phenotype incurred by Mst1/Mst2 deficiency. Thus, the level of Yap polypeptide in the colon of wild-type and Mst1null/Mst2ff/Yapf+ /villin-Cre mice is very similar (Fig. 5_A_), although in the absence of Mst1/Mst2, Yap is certainly underphosphorylated compared with wild type and predominantly is intranuclear. Nevertheless, the colonic morphology of the Mst1null Mst2ff Yapf+/villin-Cre mice is normal. Inasmuch as a wild-type level of Yap1 in intestinal epithelium, even when predominantly intranuclear, is insufficient to promote β-catenin signaling and Notch activation and the consequent hyperproliferation and loss of differentiation seen in the Mst1/Mst2-deficient intestine, it is clear that the ability of Mst1/Mst2 to regulate Yap1 abundance negatively is critical to their antiproliferative function. As to the mechanism underlying the increase in Yap polypeptide is the Mst1/Mst2-deficient intestine, Yap1 mRNA abundance is unaltered (SI Appendix, Fig. S4_A_). Zhao et.al. (34) reported that Yap polypeptide ubiquitination by β-transducin repeat-containing protein (β-TrCP) and subsequent degradation requires phosphorylation by Large tumor suppressor homologs 1 and 2 (Lats1/2) at Yap1(Ser381), enabling casein kinase 1 to catalyze a processive phosphorylation starting at Ser384. We find that elimination of Mst1/Mst2 markedly reduces Yap1(Ser384) phosphorylation (Fig. 5_D_), consistent with the view that diminished Yap degradation accounts for the increase in Yap abundance, in part or in whole.
Fig. 5.
The effect of deleting one or both Yap alleles on the morphology of the Mst1/Mst2-deficient intestinal epithelium. (A) A Kaplan–Meier plot comparing the survival of wild-type, Mst1null/Mst2ff/Yapf+/villin-Cre (dkoHet), and Mst1null/Mst2ff/Yapff/villin-Cre (tko) mice. Small intestine and colonic mucosa scrapings from these and from Mst1null/Mst2ff/villin-Cre and from Yapff/villin-Cre (Yapko) mice were extracted and immunoblotted for Yap. (B) Sections of small intestine (SI) (ileum) and colon from wild-type (Top), Mst1null/Mst2ff/Yapf+/villin-Cre (Middle), and Mst1null/Mst2ff/Yapff/villin-Cre (Bottom) mice were stained for lysozyme-, chromogranin-, or periodic acid Schiff (PAS)-positive material. Quantitation of the number of cells positive for these markers is shown in the bar graphs; data are ± SE. Open bars represent WT mice; light gray bars represent Mst1/Mst2ff/villin-Cre mice; dark gray bars represent Mst1null/Mst2ff/Yapf+/villin-Cre mice; black bars represent Mst1null/Mst2ff/Yapff/villin-Cre mice (n = 20 crypts per section, 3 mice of each genotype; ***P < 0.001 vs. WT). (C) Section of colon from wild-type and Mst1null/Mst2ff/Yapff/villin-Cre (TKO) mice stained for Yap with or without Ki67. The TKO panels in the upper row on the far right are from segments in which villin-Cre excision failed to occur, enabling a side-by side comparison of epithelia that are Mst1null but Mst2ff and Yap sufficient with epithelia that lack Mst1, Mst2, and Yap1; these samples are morphologically indistinguishable. The bottom rows show that the abundance of Ki67+ cells is similar in Mst-null and in Mst1null/Mst2ff/Yapff villin-Cre epithelium. (D) Extracts of normal mouse liver and colonic mucosa, and from Mst1null/Mst2ff/albumin-Cre mouse liver and from Mst1null/Mst2ff/villin-Cre colonic mucosa were immunoblotted for Yap and for the Yap phosphorylation sites indicated. The intensity of the Yap polypeptide, normalized for actin intensity, was divided into the value for each of the Yap-P signals; the relative phosphorylation at the two sites is shown in the bar graph with values for WT liver and colon set to 1.
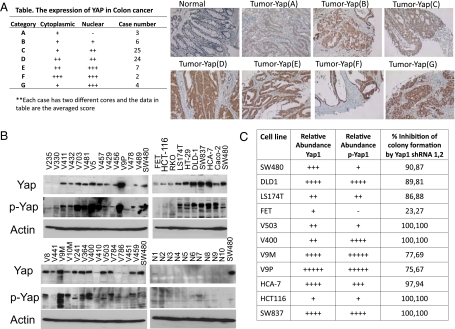
We examined the expression of Yap in human colon cancers and in colon cancer-derived cell lines and sought to evaluate Yap's role in sustaining the proliferation of these lines in vitro. Yap overexpression has been reported in a variety of cancers; Steinhardt et.al. (35) reported that widespread Yap cytoplasmic and nuclear staining is evident in ∼80% of 28 colonic cancers and 44% of 16 normal colons, in which staining is localized to the crypts (and submucosal smooth muscle cells). We performed Yap immunohistochemistry on 71 colon carcinomas using a Yap antibody (4), different from that used by Steinhardt et.al (35), which in our hands exhibits high specificity (Fig. 5_C_) and greater sensitivity. Two cores from each tumor were examined and compared with normal colon. As in the mouse, normal human colon exhibits occasional Yap+ cells located at the base of the crypt (Fig. 6_A_, Upper left). In comparison, 68 of 71 tumor samples exhibited a substantial increase in overall Yap expression and in the number of Yap+ cells, although intratumoral heterogeneity was evident, with interspersed regions of Yap− tumor cells (SI Appendix, Fig. S7_A_). In the majority of these tumors, Yap was present in both the nucleus and the cytoplasm. The distribution of Yap staining intensity (averaged estimate from both cores) is shown in the table in Fig. 6_A_.We also carried out immunoblots for Yap1 polypeptide and Yap1(Ser127P) using extracts from 36 colonic adenoma- and cancer-derived cell lines and from epithelial cells collected from the mucosa of 10 normal colons (Fig. 6_B_). We used blots of extracts from SW480 cells for visual normalization of the blots obtained from extracts of other cells. Yap polypeptide expression is greatly increased in the majority (at least 30/36) of colonic cancer cell lines in comparison with normal colonic epithelial cells, which show little or no Yap immunoreactivity under these immunoblotting conditions (Fig. 6_B_). Thus, Yap overexpression is very common in colonic cancer and in colonic cancer-derived cell lines.
Fig. 6.
The expression of Yap in human colon cancer tissue and cell lines and the effect of Yap shRNA on the proliferation of colon cancer-derived cell lines. (A) Yap immunohistochemistry of colon cancer tissue microarrays. (Right) A section of normal colon shows an occasional Yap+ cell in the crypt. Yap+ cells also are visualized in the lamina propria. Two cores were obtained from different regions of 71 colonic cancers; histologic sections were examined for the intensity and subcellular localization of Yap staining and scored as in described in Materials and Methods. (Left) The averaged score of the two samples from each tumor is shown in the table. Examples of the intratumoral heterogeneity of Yap staining are shown in SI Appendix, Fig. S5_A_). (B) Western blotting analysis of YAP expression and Yap(Ser127) phosphorylation in human colon cancer cell lines and mucosal epithelium isolated from 10 normal colons. (C) A qualitative estimate of the relative abundance of Yap polypeptide and Yap(Ser127) immunoreactivity (p-Yap1) in selected colon cancer cell lines and the extent of inhibition of colony formation of these lines after infection with lentiviruses encoding two different Yap shRNAs. Data shown are the average of two experiments). The primary data are shown in SI Appendix, Fig. S7.
The anti-Yap(Ser127P) antibody has a greater sensitivity than the anti-Yap polypeptide antibody: Immunoblot of the same extracts with anti-Yap(Ser127P) demonstrates the presence of Yap(Ser127) phosphorylation in 8/10 extracts from normal colonic epithelial cells and very much stronger signals in nearly all (∼27/30) the colon cancer cell lines that exhibit up-regulated Yap polypeptide immunoreactivity. This result indicates that rarely is Yap(Ser127) phosphorylation lost entirely in these colonic cancer cell lines; however, whether this result reflects the Yap(Ser127) phosphorylation state of the primary tumor is not known, because we have observed the reappearance of Yap(Ser127) phosphorylation on later passages of HCC-derived cell lines obtained from HCCs in Mst1/Mst2-null livers that exhibited no Yap(Ser127) phosphorylation in vivo or during early passages in vitro (4).
We next sought to determine whether Yap overexpression is functionally important to the proliferative capacity in vitro of these colonic cancer cell lines. We selected 11 lines reflecting a broad range of up-regulated Yap expression, only one of which, FET, exhibits no Yap polypeptide or Yap(Ser127P) immunoreactivity. Each line was infected with lentivirus pLKO.1 vectors encoding two different Yap-directed shRNAs or a scrambled shRNA control and was grown for 10 d under puromycin selection. A Yap immunoblot and quantitative PCR analysis of the SW480 line demonstrated ∼80% depletion of Yap mRNA and polypeptide with both Yap shRNAs. All lines infected with virus encoding Yap shRNA showed a marked inhibition in colony formation, except for the FET line, in which the colony count was reduced only modestly (Fig. 6_C_ and SI Appendix, Fig. S7 B_–_M). Thus, it appears that Yap, when overexpressed, contributes strongly to the in vitro proliferative capacity of colonic cancer cell lines.
Discussion
Elimination of the protein kinases Mst1 and Mst2 from the intestinal epithelial compartment results in an expansion of stem-like undifferentiated cells and the almost complete disappearance of all secretory lineages. There is a marked increase in the abundance of the Yap1 with a decrease in the extent of Yap1(Ser127) phosphorylation, a site known to be regulated by Mst1/Mst2 through an intermediating protein kinase, usually Lats1 and/or Lats2 (36), as well as a decrease in Ser384 phosphorylation, a CK1 site whose phosphorylation enables β-TrCP–mediated ubiquitination. Yap1 immunoreactivity, which in the wild-type small intestine is evident only in the crypts and is predominantly or entirely cytoplasmic, exhibits intense nuclear localization in the Mst1/Mst2-deficient small intestine. In the wild-type colon, Yap is evident largely in colonic crypt cells, and nuclear localization is detectable; in the Mst1/Mst2-deficient intestine, Yap1 is present at much higher abundance throughout the epithelium with prominent nuclear localization. Phosphorylation of Yap1 (at Ser127) promotes Yap1 nuclear exit and also is required to initiate its ubiquitin-dependent degradation (34). Thus, the loss of Mst1/Mst2 causes an increased abundance and enhanced nuclear translocation of Yap1. It was shown previously that overexpression of a constitutively nuclear form of Yap1 in the intestinal epithelium [i.e., Yap1(Ser127Ala)] is sufficient to cause an expansion of the stem cell compartment and a loss of differentiated cell types (7) similar to that seen with elimination of Mst1 and Mst2 in the present study. The primacy of Yap activation and overexpression to the phenotype of the Mst1/Mst2-null intestinal epithelium is demonstrated by the ability of inactivation of a single Yap allele to reverse entirely the hyperproliferation and loss of differentiation caused by the Mst1/Mst2 deficiency.
The ability of Yap nuclear overexpression to promote the hyperproliferation of intestinal stem cells and to inhibit intestinal epithelial differentiation is attributable, in large part and perhaps entirely, to an enhancement of β-catenin action and an activation of Notch signaling. The primary effect of Yap1 overexpression appears to be an enhancement of β-catenin transcriptional activity rather than an increase in the overall or nuclear abundance of β-catenin. Whether the Yap1 enhancement of β-catenin directed transcription reflects a direct binding of Yap1 and its action as a coactivator at β-catenin/TCF-regulated transcriptional sites or a more indirect action of Yap1 is not known; however, concurrent binding of Yap and β-catenin at regulatory sites in the Sex determining region Y (SRY)-box 2 (Sox2) and Snail homolog 2 (Snai2) genes in developing mouse heart was reported recently (37). In addition to the enhancement of β-catenin transcriptional activity, a modest increase in the abundance of the dephosphorylated form of β-catenin is detectable in the Mst1/Mst2-deficient intestinal epithelium. The mechanism responsible for this mild but reproducible β-catenin activation is not known. In Drosophila the Yap1 ortholog yorkie has been shown to bind and presumably to sequester Dishevelled (Dsh) in the cytoplasm, thereby interfering with the ability of Wnt to disrupt the β-catenin destruction complex (38). The loss of phosphorylation and enhanced nuclear retention of yorkie that occurs with deletion of hippo is proposed to decrease cytoplasmic yorkie, releasing Dsh and so facilitating Wnt's ability to disrupt the β-catenin destruction complex and thereby promote activation of Armadillo, the fly β-catenin ortholog. It is not known if such a mechanism of Yap action in the cytoplasm is operative in intestinal epithelia or, if so, whether the loss of Yap1(Ser127) phosphorylation and enhanced nuclear retention of Yap1 seen with Mst1/Mst2 deletion actually reduces Yap1 cytoplasmic abundance sufficiently to disinhibit Dishevelled.
Notch activation in the Mst1/Mst2-deficient intestine also is likely attributable, at least in part, to loss of Yap phosphorylation and consequent nuclear overabundance, inasmuch as transgenic overexpression of Yap(Ser127Ala) in the mouse intestine is sufficient to cause Notch activation and a loss of cellular differentiation that can be reversed partially by treatment with a γ-secretase inhibitor (7). The increased expression of Jagged in the Mst1/Mst2-deficient intestine, mediated in part through up-regulated Wnt signaling (31, 32), probably contributes to the activation of Notch. Although it is likely that Yap mediates the activation of both Wnt and Notch signaling in the Mst1/Mst2-deficient intestine, the extent of Yap's contribution compared with other, yet-to-be defined outputs of Mst1/Mst2 remains to be established.
The robust proliferative response elicited by elimination of Mst1 and Mst2 indicates that in the normal colonic epithelium one or both of these kinases actively represses Yap1 function. Consistent with this view is the high abundance in the small intestine and proximal colon of the 36-kDa Mst1 polypeptide that is known in other settings to be constitutively active. Unfortunately, immunoblot with the phospho-specific activation loop antibody, anti-Mst1/2(Thr183P/180P) (19), has been unsuccessful thus far. Direct evidence that Mst1 and/or Mst2 is active in the unperturbed colon is the finding that the phosphorylation of Mob1, a highly specific substrate of Mst1/Mst2 in vitro (39), whose phosphorylation is lost in Mst1-null T cells (4) and Mst1/Mst2-deficient liver (4), also is lost in the Mst1/Mst2-deficient colon (SI Appendix, Fig. S6_A_). The mechanisms regulating Mst1/Mst2 activation in this compartment are unknown, however, as is true of Mst1/Mst2 regulation in most mammalian cells, apart from T cells, where Mst1, through its association with Ras association (RalGDS/AF-6) domain family member 5B (Rassf5B/Nore1B/RAPL), is activated by Rap-GTP (40). Genetic and some biochemical evidence from Drosophila indicates that Hippo is activated by inputs arising from several varieties of cell adhesion elements, e.g., the atypical cadherins Fat and Dachsous involved in planar polarity; the apical–basal polarity assemblies such as the Crumbs, atypical protein kinase C, and partition-defective (Par) complexes; and the basolateral discs large-Lethal giant larvae-scribble complexes, each of which may signal to Hippo through the cortical actin-associated elements Kibra, Expanded, and Merlin (2, 3, 41). The contribution of such elements to Mst1/Mst2 regulation in mammalian intestine remains to be defined.
Indirect evidence for the loss of active Mst1/Mst1 in the wild-type intestine is the widespread and intense nuclear localization of Yap1 that prevails in the Mst1/Mst2-deficient intestine. In the wild-type colon, where Yap is visualized reliably because of its high abundance, nuclear localization of Yap1 is evident in crypt cells. However, as one moves away from the crypt, Yap1 staining, although persistent, weakens and is visualized primarily in the cytosol. This pattern suggests that Mst1/Mst2 activity may be relatively lower in the crypts and more activated as cells move toward the lumen. Notably, despite the apparent presence of nuclear Yap1 in crypt cells of the distal colon, elimination of Yap1 does not diminish the abundance of Ki67+ cells in this compartment (Fig. 5_C_ and SI Appendix, Fig. S5_A_), consistent with the view that Yap1, at the abundance found in the wild-type colon, makes little or no contribution to epithelial proliferation. This notion is consistent with the findings of Cai et.al. (33), who first reported that villin-Cre–induced deletion of Yap1 did not alter colonic development. In contrast, villin-Cre–induced deletion of Salvador/ww45, an Mst1/Mst2 scaffold protein, induces a modest Yap1-dependent expansion and hyperproliferation of colonic crypt cells. The Savff/villin-Cre mice also develop distal colonic polyps at ages greater than 12 mo, a phenotype similar to but much milder than that of the Mst1null/Mst2ff/villin-Cre mice. Salvador homolog 1 (Sav1) knockout in the liver (5, 42) also gives a much milder phenotype than Mst1/Mst2 double knockout in the liver (4–6). Thus, the phenotype of both the Mst1/Mst1 and Sav1 intestinal knockouts indicate that Mst1 and/or Mst2 are active in the normal intestinal epithelium and actively restrain Yap1 transcriptional regulatory activity in that compartment to a level that is insufficient to promote proliferation. Thus, despite the evidence indicating an active role for Yap in maintaining the phenotype of ES cells and induced pluripotent stem cells (43), Yap1 does not contribute to the proliferative capacity of the colonic stem cells and transient amplifying compartments during normal mucosal turnover.
Mst1/Mst2-null intestines develop colonic adenomas within 3 mo of birth. In contrast to the polyps described in the Sav1-deficient colon (33), the polypoid lesions in the Mst1/Mst2-deficient colon do not exhibit a sawtooth/serrated architecture but appear simply as hyperproliferative adenomas. Consistent with this conventional appearance, molecular analysis indicates that an activation of β-catenin is the dominant alteration in these lesions. This finding perhaps is not surprising in view of the highly proproliferative milieu created by Mst1/Mst2 deficiency, reflected by the increased expression of cMyc and cyclin E and diminished expression of the p21 cyclin-dependent kinase inhibitor (SI Appendix, Fig. S6 B and C). In addition, IL-6 expression is up-regulated, perhaps accounting in part for the robust tyrosine phosphorylation of Signal transducer and activator of transcription 3 (Stat3); activated Stat3 is known to be a crucial contributant to the development of colonic cancers, e.g., in the Dextran Sulfate sodium/azoxymethane murine model and presumably in inflammation-associated colon cancer (44). The early mortality of the Mst1/Mst2ff/villin-Cre mice leaves open the question of whether these polyps will evolve into invasive and/or metastatic cancers.
The very high prevalence of Yap1 overexpression in human colonic cancers and derived cell lines is striking. We find a 95% prevalence of Yap1 overexpression in colonic cancer specimens, exceeding the 78% prevalence observed by Steinhardt et.al (35). In addition, however, our finding that depletion of Yap1 in colon cancer-derived cell lines that overexpress Yap1 results in a strong inhibition of their proliferation in vitro provides strong evidence that Yap1 is likely to be an important driver in this common cancer. The basis for Yap1 overexpression in human colon cancer is not known; a preliminary estimate using RNA extracted from tissue microarrays indicates that Yap mRNA abundance is increased approximately twofold (SI Appendix, Fig. S7_N_), an increase that seems far less than the increase in Yap polypeptide. Nevertheless, loss of Yap1(Ser127) phosphorylation appears to be an uncommon occurrence, and additional studies will be needed to determine the contributions of increased transcription versus diminished degradation. In addition, the outputs of Yap1 critical to its proproliferative effect in human colon cancer remain to be defined. However, it is likely that the ability of Yap1 overexpression to enhance β-catenin transcriptional activity is one important factor. The frequent overexpression and proproliferative impact of Yap1 in colon cancer cell lines is of particular interest, because, if Yap1 plays little or no role in normal colonic epithelial turnover, interference with its expression and/or outputs may provide attractive therapeutic targets distinct from those of the Wnt and Notch pathways (45, 46), whose outputs are indispensable for normal intestinal homeostasis (12–14).
Materials and Methods
Mice.
Mst1−/−Mst2ff mice were generated as described previously (4). The generation of Yap1ff mice is described in ref. 10. Villin-Cre mice were purchased from the Jackson Laboratory. Mst1-null mice with Mst2 deleted in the intestinal epithelium were generated by breeding Mst1-null/Mst2ff mice with villin-Cre mice. Animal protocols were approved by the Institutional Animal Care and Use Committee of Massachusetts General Hospital.
Tissue Microarray and Histological Analysis.
Colonic tissues were collected under an Institutional Review Board-approved protocol at University Hospitals of Cleveland. Tissue microarray blocks, two cores from each tumor, were sectioned at 5 μm. After deparaffinization and rehydration, the antigens were retrieved by boiling the slides in Target Retrieval solution (Dakocytomation) for 20 min and visualized by the Envision+ System-HRP kit (Dakocytomation) following the manufacturer's instructions and using anti-Yap antibody (1:200) (4). Slides were counterstained with hematoxylin to visualize the nuclei before microscopic analysis. Relative Yap expression and subcellular localization were classified into seven groups based on the intensity of Yap staining and its distribution between cytoplasm and nuclei, as shown in the table in Fig. 6.
Additional materials and methods are described in SI Appendix.
Supplementary Material
Supporting Information
Acknowledgments
We thank James K. V. Willson for the VACO colon cancer cell lines, R. Polakiewicz (Cell Signaling Inc.) for the antiYap1(Ser384P) antiserum, and R. T. Bronson for mouse histologic analyses. This work was supported in part by National Institutes of Health Grants RO1 DK17776 (to J.A.), CA136567 (to J.A.), and CA127306 (to S.D.M.), by a grant from the Stand Up to Cancer-American Association for Cancer Research initiative (to F.D.C.), and by institutional funds. F.D.C. is a Pew Scholar in the Biomedical Sciences. D.Z. is supported by the Fundamental Research Funds for the Central Universities of China (T32DK007028, Project Number 2010111079) and by the Science Planning Program of Fujian Province (Project Number 2009J1010).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Author Summary on page 19463.
References
- 1.Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 2.Halder G, Johnson RL. Hippo signaling: Growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou D, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu L, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song H, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Dong J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou D, et al. The Nore1B/Mst1 complex restrains antigen receptor-induced proliferation of naïve T cells. Proc Natl Acad Sci USA. 2008;105:20321–20326. doi: 10.1073/pnas.0810773105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlegelmilch K, et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponder KP. Analysis of liver development, regeneration, and carcinogenesis by genetic marking studies. FASEB J. 1996;10:673–682. doi: 10.1096/fasebj.10.7.8635684. [DOI] [PubMed] [Google Scholar]
- 12.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 13.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 14.Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–670. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 15.Oh S, et al. Crucial role for Mst1 and Mst2 kinases in early embryonic development of the mouse. Mol Cell Biol. 2009;29:6309–6320. doi: 10.1128/MCB.00551-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snippert HJ, et al. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology. 2009;136:2187–2194. doi: 10.1053/j.gastro.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Zhu L, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermeulen L, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381:453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Flier LG, et al. Transcription factor Achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Fre S, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci USA. 2009;106:6309–6314. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 23.Morin PJ, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 24.Veeman MT, Slusarski DC, Kaykas A, Louie SH, Moon RT. Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Curr Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. [DOI] [PubMed] [Google Scholar]
- 25.Jarriault S, et al. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- 26.Jensen J, et al. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 27.Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127:4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- 28.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 29.Sekine S, Shibata T, Sakamoto M, Hirohashi S. Target disruption of the mutant beta-catenin gene in colon cancer cell line HCT116: Preservation of its malignant phenotype. Oncogene. 2002;21:5906–5911. doi: 10.1038/sj.onc.1205756. [DOI] [PubMed] [Google Scholar]
- 30.Zhao B, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodilla V, et al. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci USA. 2009;106:6315–6320. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pannequin J, et al. The Wnt target jagged-1 mediates the activation of notch signaling by progastrin in human colorectal cancer cells. Cancer Res. 2009;69:6065–6073. doi: 10.1158/0008-5472.CAN-08-2409. [DOI] [PubMed] [Google Scholar]
- 33.Cai J, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(β-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinhardt AA, et al. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao B, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varelas X, et al. The Hippo pathway regulates Wnt/β-catenin signaling. Dev Cell. 2010;18:579–591. doi: 10.1016/j.devcel.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311–321. doi: 10.1016/j.cub.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 41.Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol. 2010;20:R574–R582. doi: 10.1016/j.cub.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 42.Lee KP, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lian I, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 46.Dikic I, Schmidt MH. Notch: Implications of endogenous inhibitors for therapy. Bioessays. 2010;32:481–487. doi: 10.1002/bies.200900140. [DOI] [PubMed] [Google Scholar]
Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19463–19464.
Author Summary
The Hippo signaling pathway was discovered in the fruit fly Drosophila and functions primarily to phosphorylate and inhibit Yorkie, a coactivator of gene transcription that promotes cell proliferation and inhibits cell death (1). Phosphorylation is the addition of a phosphate group to a molecule such as protein, and the enzyme Hippo is a protein kinase that phosphorylates an intermediate protein kinase that in turn phosphorylates Yorkie. When components of the Hippo pathway upstream of Yorkie are functionally inactivated, Yorkie becomes underphosphorylated, its abundance increases because of diminished degradation, and it shifts from residing in the cytoplasm to the nucleus, a shift that is accompanied by massive organ overgrowth. Therefore the Hippo pathway is considered essential to the determination of proper organ size. The components of the pathway are conserved and duplicated in mammals, and their functions have been diversified. Thus, the protein kinases Mst1 and Mst2, the mammalian orthologs of the Hippo kinase, act as redundant tumor suppressors in the liver through inhibition of Yes-associated protein 1 (Yap1), the Yorkie ortholog; the combined inactivation of Mst1 and Mst2 in the liver results in diffuse liver overgrowth and in the rapid development of hepatocellular carcinomas (2). In contrast, Mst1 in T lymphocytes, a type of immune cell, acts to suppress proliferation and promote cell adhesion and migration (3) through Yap1-independent mechanisms. The role of the Hippo pathway in the development and cell turnover of most other mammalian tissues remains poorly understood, as is the contribution of the Yap1 oncogene to other common human cancers.
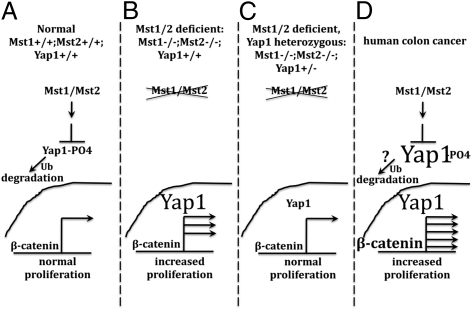
Hepatocytes turn over roughly once per year by replication of differentiated cells; in contrast, the epithelial cells of the intestinal lining turn over every 4 or 5 d, renewed from a stem cell compartment. Mst1, Mst2, and Yap1 are expressed in mouse intestinal epithelium, with Yap1 located predominantly in the cytoplasm. Previously, when a nonphosphorylatable Yap1 mutant was overexpressed in the intestinal epithelium of transgenic (i.e., genetically modified) mice, it was localized largely in the nucleus and resulted in the expansion of the stem cell compartment and the loss of differentiated (mature) cell types (4). This finding, however, did not reveal the normal function of the pathway in intestinal homeostasis. Therefore, we examined the impact of inactivating the genes encoding the Mst1, Mst2, and Yap1 proteins in the intestinal epithelium. Global inactivation of either Mst1 or Mst2 had no effect on the intestinal epithelium; however, combined elimination of both Mst1 and Mst2, specifically in the intestine, resulted in a marked expansion of undifferentiated stem-like cells and a complete loss of all intestinal secretory cell types, defects that strongly resembled those elicited by the overexpression of mutant nonphosphorylatable Yap1. Loss of Mst1 and Mst2 was accompanied by greatly diminished Yap1 phosphorylation and a marked increase in the abundance of Yap1, now predominantly located in the nucleus. The proliferation of intestinal stem cells is driven primarily by a pathway called the “Wnt” pathway, acting synergistically with another pathway, the Notch pathway. The Wnt pathway controls intestinal stem cell proliferation through β-catenin, a protein that regulates gene transcription (5). The Mst1/Mst2-deficient intestinal epithelium exhibited evidence of strong activation of gene expression as directed by β-catenin but without altered β-catenin abundance. Increased levels of the Notch intracellular domain and activation of Notch transcriptional responses also were evident. Thus, the active inhibition of Yap1 through Mst1 and Mst2 is required to prevent overactivation of Wnt and Notch signaling, so as to enable proper epithelial development (Fig. P1 A and B).
Fig. P1.
Operation of the mammalian Hippo pathway in mouse intestinal epithelium and human colon cancer. (A) Normal mouse intestinal epithelium. (B) Absence of Mst1 and Mst2. (C) Inactivation of a single copy of the Yap1 gene in the Mst1/Mst2-deficient epithelium. (D) Inappropriate activation of β-catenin in human colon cancer.
Surprisingly, inactivating both copies of the Yap1 gene in the intestinal epithelium caused no detectable aberration in intestinal development, indicating that Yap1 is dispensable for normal intestinal stem cell proliferation and development. Nevertheless, elimination of a single copy of the Yap1 gene from the Mst1/Mst2-deficient intestine, which reduces Yap1 protein expression to nearly wild-type or normal levels, completely restored the morphology and development of the Mst1/Mst2-deficient epithelium to normal. Although overall Yap1 protein levels in this circumstance were near those of wild-type, the Yap1 protein in the Mst1/Mst2-deficient intestinal epithelium was hypophosphorylated (under-phosphorylated) and predominantly within the nucleus; nevertheless, excessive proliferation and failed development in the Mst1/Mst2-deficient intestinal epithelium was reversed completely (Fig. P1_C_). This outcome indicates that wild-type levels of Yap1, even when the protein is fully within the nucleus, are insufficient to promote intestinal stem cell overproliferation and loss of differentiation. In essence, increased abundance of the Yap1 protein beyond endogenous levels is necessary to engage these responses. Moreover, Mst1 and Mst2, through their ability to promote Yap1 phosphorylation, actively inhibit both Yap1 abundance and Yap1 nuclear residence in the normal intestinal epithelium.
Yap1 polypeptide was found to be overabundant in more than 95% of 71 human colon cancers examined by a standard technique, immunohistochemistry. Yap1 mRNA levels in these cancers were increased consistently, although to a variable extent and often markedly less than Yap1 protein levels. Yap1 polypeptide also was overabundant in 30 of the 36 colonic adenoma and cancer cell lines examined, although Yap1 phosphorylation usually was preserved. In the colon cancer cell lines SW480 and HCT116, which exhibit moderate Yap1 overabundance, depletion of Yap1 by shRNA-induced silencing of gene expression strongly inhibited gene transcription directed by β-catenin, without altering the abundance of total or nuclear β-catenin. Yap1 depletion in HCT116 colon cancer cells also strongly reduced the abundance of the Notch intracellular domain and Notch-directed transcription. Concurrently, in these and other colon cancer lines that overexpress Yap1, Yap1 depletion greatly inhibited proliferation in culture (Fig. P1_D_).
In conclusion, Yap1, when overexpressed beyond the levels normally present in intestinal epithelium, drives the proliferation of intestinal stem and/or transiently amplifying cells and interdicts normal epithelial differentiation. The overabundance and nuclear localization of Yap1 normally is prevented by the activity of the Mst1 and Mst2 kinases. At a molecular level, the effects of Yap1 likely result from the ability of high levels of Yap1 within the nucleus to activate β-catenin transcriptional activity synergistically. Nevertheless, Yap1 is entirely dispensable for normal intestinal epithelial development, and the circumstances that reduce Mst1/Mst2 activity so as to recruit Yap1 in intestinal epithelia remain to be defined. Overabundance of Yap1 is a ubiquitous feature of human colon cancer, where it serves to augment substantially the already inappropriately high levels of β-catenin signaling to drive proliferation and promote survival. Given the dispensability of Yap1 for normal intestinal homeostasis, interference with Yap1 expression and/or outputs may provide attractive therapeutic targets for this common cancer.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See full research article on page E1312 of www.pnas.org.
References
- 1.Halder G, Johnson RL. Hippo signaling: Growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou D, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katagiri K, Imamura M, Kinashi T. Spatiotemporal regulation of the kinase Mst1 by binding protein RAPL is critical for lymphocyte polarity and adhesion. Nat Immunol. 2006;7:919–928. doi: 10.1038/ni1374. [DOI] [PubMed] [Google Scholar]
- 4.Camargo FD, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 5.Fre S, et al. Notch and Wnt signals cooperatively control cell proliferation and tumorigenesis in the intestine. Proc Natl Acad Sci USA. 2009;106:6309–6314. doi: 10.1073/pnas.0900427106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary Materials
Supporting Information