Retrospective on the Early Development of Cryoelectron Microscopy of Macromolecules and a Prospective on Opportunities for the Future (original) (raw)
. Author manuscript; available in PMC: 2012 Mar 1.
Published in final edited form as: J Struct Biol. 2008 Jun 19;163(3):214–223. doi: 10.1016/j.jsb.2008.06.004
Abstract
Methods for preserving specimen hydration in protein crystals were pursued in the early 1970s as a prerequisite for protein crystallography using an electron microscope. Three laboratories approached this question from very different directions. One built a differentially pumped hydration chamber that could maintain the crystal in a liquid water environment, a second maintained hydration by rapidly freezing the protein crystal and examining it in a cold stage, and the third replaced the water of hydration by using glucose in the same way as one had previously used “negative stains”. Each of these early efforts succeeded in preserving the structures of protein crystals at high resolution within the vacuum of the electron microscope, as demonstrated by electron diffraction patterns. The next breakthrough came in the early 1980s when a technique was devised to preserve noncrystalline specimens by freezing them within vitreous ice. Since then, with the development of high stability cold stages and transfer mechanisms compatible with many instrument platforms, and by using commercially provided low-dose imaging techniques to avoid radiation damage, there has been an explosion of applications. These now include single particles, helical filaments, 2-D arrays and even whole cells, where the most exciting recent applications involve cryoelectron tomography. These achievements and possibilities generate a new set of research opportunities associated with increasing the reliability and throughput with which specimens can be studied by cryoEM.
Background
From the time of the first construction of an electron microscope (EM), the desire to examine biological specimens was high, dampened only by the two difficulties of making the specimen thin enough to get an electron beam through it, and preserving and contrasting the various structural elements of the material in the high vacuum of the microscope. The very early days of biological EM have been described (Marton, 1968). Because of the technical difficulties associated with maintaining hydration in a high vacuum environment, the early development of specimen preservation for biological EM concentrated on fixation, dehydration, embedding and sectioning combined with application of heavy metals to provide contrast in tissue samples.
The goal of visualizing protein molecules either alone or in large complexes was not successfully accomplished until the development of the negative stain technique, the early history of which has been described (Horne and Wildy, 1979). Negative staining, which still remains a widely used technique today, solved both the preservation and contrasting problem by replacing the surrounding water with a dried solution of a heavy metal salt. From that point on, the possibility of determining the structures of macromolecular assemblies was within reach, the only apparent obstacle being a framework for converting projections to 3-D images, a problem that was solved in 1968 (DeRosier and Klug, 1968).
A new barrier emerged during the early 1970’s with the realization that organic specimens could not be visualized directly in the electron microscope because either the electron beam destroyed the specimen or, if the image was recorded without damaging the specimen, it contained too few electrons to be interpreted directly (Glaeser, 1971; Glaeser, 2008). Negative stain had the useful property that, when exposed to high doses of electrons, specimens reached a relatively stable end point in which the protein would be obliterated but its outlines were still retained.
Once thin protein crystals were examined, however, it was clear that conventional negative stains fell far short of the ability to preserve the crystalline order at the resolution needed to visualize and trace the polypeptide chain. What was needed was a method for maintaining protein crystal hydration in the high vacuum of the microscope. Microscopes at that time had the capability of resolving features as fine as the polypeptide chain, but such structures could not be seen in early negative stain micrographs, even with the application of low dose techniques (Williams and Fisher, 1970). The vision of using the electron microscope for protein structure determination would remain unrealized unless the native order present in a protein crystal could be preserved.
The Specimen Hydration Problem
The problem of maintaining specimen hydration in an electron microscope was an old one. Early attempts, dating back as early as 1944 used thin window chambers to achieve this (Abrams and McBain, 1944), but these had the disadvantage that thin windows would inevitably break when irradiated. Windows that were thicker and sufficiently resilient had the disadvantage that image contrast was reduced due to excessive electron scattering. Beginning in the early 1970s, Dr. Don Parsons and coworkers were developing an alternative idea that used a windowless, differentially pumped hydration stage (Parsons et al., 1974). This device does not isolate the hydrated specimen from the column vacuum, and it thus avoids the problems of the sealed-window chambers. Instead, the specimen is placed into a chamber that has openings to the microscope vacuum above and below, provided by two pairs of collinear apertures. A steady flow of gas saturated with water vapor flows over the specimen but is removed by differential pumping. Despite the apparent complexity of balancing the flow rate and humidity against the risks of either evaporation or condensation, the device worked successfully. Atomic-resolution electron diffraction patterns were obtained of catalase crystals, a specimen that is extremely sensitive to even partial drying making it the ideal test specimen for hydration methods (Matricardi et al., 1972; Dorset and Parsons, 1975). The device was also capable of supporting image acquisition of fully hydrated whole cells in a high voltage electron microscope (Parsons, 1974). In so far as we are aware, however, no images of catalase crystals were ever produced that revealed the arrangement of molecules in the lattice.
Another approach replaced the water of hydration with glucose, which allowed the specimen to be dried in the vacuum of the EM while retaining periodic order to near atomic resolution (Unwin and Henderson, 1975). This method is thought to work by replacing the bulk water with glucose, while possibly retaining the strongly bound water at the protein surface. At the time, the chief advantage of glucose (and other polar compounds that work equally well) was that specimens could be examined on the conventional (room-temperature) EM specimen stage, which was far better suited for imaging than the then-available cryostages, and remained so for many years. The chief _dis_advantage of glucose embedding was that dried glucose closely matches the contrast of protein, so that the periodic arrangement of material within the crystals was virtually invisible without image processing. That was only a small disadvantage, however, since image averaging would be essential in any case, to take advantage of the excellent preservation. Glucose embedding led ultimately to the first atomic resolution structure of a 2-D crystalline array determined entirely by EM (Henderson et al., 1990).
The third approach was initially pursued by two groups, ourselves at Berkeley and Dr. Hans Gunther Heide at the Fritz-Haber Institute in Berlin. Frozen hydrated specimens offered superior possibilities for recording images, provided that thin, prefrozen specimens could be made and introduced into the electron microscope. Heide’s efforts went so far as to build a specimen-transfer device and to produce images of frozen hydrated bacteria (Heide and Grund, 1974), but little biological work was published thereafter.
Frozen Hydrated Specimens – The Berkeley Approach
Our goal was simply to develop a method that would preserve crystalline order in protein crystals within the EM so that ultimately structures could be determined at atomic resolution using electron crystallography, but using images as the “phasing” method. Freezing the specimens and observing them at low temperature appeared to be the simpliest approach. The early 1970s saw development of the freeze fracture/etch technique, and with it a high awareness by electron microscopists of the ice crystallization artifacts that can be produced in these specimens if freezing is too slow. Thus our effort was undertaken on a background in which it was widely assumed that a protein crystal embedded in an aqueous solvent could not be frozen in liquid nitrogen and still retain its crystallinity. There was also the wide belief that the contrast in frozen hydrated specimens would be too low to be useful, the argument being that electron scattering by the atoms in proteins would be similar to that by water.
Early attempts to freeze protein crystals for X-ray crystallography without using cryoprotectants or fixatives were largely unsuccessful (Low et al., 1966). Later it was shown that X-ray diffraction quality protein crystals could be readily frozen in the presence of a suitable cryoprotectant (Haas, 1968; Haas and Rossmann, 1970). Another study showed that 50% aqueous gelatin gels could be frozen at rates that were as slow as 1°/sec without there being evidence of crystalline ice in their X-ray diffraction diagrams (Dowell et al., 1962). We avoided using concentrated cryoprotectants in our experiments because we believed that the use of viscous buffers would make it difficult to make thin specimens. It is worth keeping in mind that all of the background work on freezing biological specimens was done on what could be considered bulk samples, in the sense that they were much larger than any specimen that could be visualized directly by EM. Our effort would be on very thin specimens, and that proved to be the crucial factor.
Starting literally from scratch, as we were doing at that time, there was a long list of problems that had to be addressed before frozen-hydrated specimens could even be examined in our EMs. These included (1) making thin aqueous films in which to suspend the crystal, (2) obtaining a cryostage suitable for transmission EM and (3) finding a way of transferring a prefrozen specimen onto the electron microscope cold stage.
The first problem to be overcome was the issue of freezing a suspension of protein crystals in a thin film of water. The specimen of choice was crystalline catalase, which could be purchased in pure crystalline suspension and recrystallized to produce thin plates (Wrigley, 1968) that were suitable for electron imaging. Later a preparation method developed by Douglas Dorset (Dorset and Parsons, 1975) produced thin square plates that were much better suited for electron diffraction.
The preparation of thin, aqueous films that extended over a large area required hydrophilic support films. We opted to use SiO deposited onto a thin carbon film as a way to make the surface hydrophilic. To control the thickness of these films, we built a small device that could be placed in a vacuum evaporator (Taylor and Glaeser, 1973), with which thin films could be reliably produced and their thickness could be monitored from the interference fringes produced on a glass microscope slide that was placed closer to the SiO evaporation-source.
Freezing a suitably thin specimen before the thin layer of water evaporated was a different problem, since we desired aqueous films only a few hundred Ångstroms thick. Although quantitative information on evaporation rates of water was difficult to come by, what was available indicated that at room temperature, under favorable circumstances of high humidity and still air, that the rates would be hundreds of Ångstroms/sec, although rates on the order of tens of Ångstroms/sec could be achieved if blotting and freezing was done in a cold room. We therefore devised a freezing chamber that contained a phase contrast light microscope built into a Lucite box where the water film thickness (we used crystals suspended in distilled water) could be monitored while the grid was held on the forceps and filter paper was applied to the edges. When the crystals became stationary and interference fringes appeared around the perimeter of the grid squares, the grid was removed from the microscope and frozen by plunging manually into liquid nitrogen, held in an adjacent chamber that could be accessed simply by removing a cover. We started with folding grids and later were able to make “open face” specimens using a single grid. This was all done in a cold room.
The microscope available to us at the time was a Hitachi HU-11 but it lacked a cold stage. However, Dr. Gareth Thomas in the Material Science Department at U. C. Berkeley had a cold stage for his Hitachi 650 keV microscope, and while it was too large to fit the HU-11 column, the design was easily adapted to the smaller space of the HU-11. Thermal conducting leads for the cold stage consisted of a stack of aluminum foil ribbons connected to the anticontamination dewar. There was no anticontamination device below the specimen.
Inserting a frozen specimen was another issue. Most cold stages at the time were designed to accept a warm specimen and cool it within the column. We needed the stage to accept a pre-frozen, cold specimen. The HU-11 airlock design required that the specimen holder be inserted into the airlock top first. The bottom of the holder, which contained the specimen, thus extended out into room air. This meant that the specimen would be in direct contact with air during the transfer and during airlock evacuation. To solve this problem, we built a copper cold shield/frost protector that would surround the specimen and cryospecimen holder during transfer and air lock evacuation (Taylor, 1975). Thus, we were able to produce thin aqueous films and observe thin, frozen catalase crystals for the first time in the high vacuum of the electron microscope. It was during the examination of these specimens that we first noticed the “bubbling” phenomenon, in which radiation damage from the electron beam, which was at normal viewing brightness, destroyed the crystal while the surrounding ice remained apparently untouched (Taylor and Glaeser, 1973). This observation suggested that direct imaging would be difficult without a low dose method, which could not be implemented on the HU-11. In addition, the inherent order in the frozen hydrated catalase crystals could not be assessed by low angle electron diffraction in this instrument. Instead, the attempt to do so was described by one of us at the time of the 1973 EMSA meeting in New Orleans as akin to “running a mule in the Kentucky Derby”.
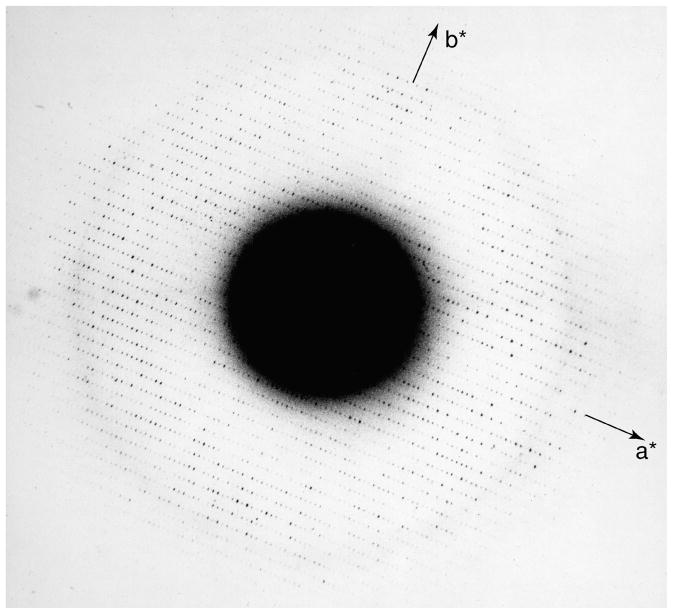
Fortunately, installation soon began of a new JEOL JEM-100B that would replace the HU-11. The microscope came with a top entry cold stage especially built for it. The cold stage had a rated lattice resolution of about 7Å, which was quite good for the time. However, the microscope lacked a transfer mechanism for frozen-hydrated specimens, although it did have an anticontaminator below the specimen. The JEM-100B had a rotating drum with sockets for six specimen holders in the door to the airlock. Using the same cold shield/frost protector concept devised for the HU-11, we built a similar design into which the rather long top-entry specimen holder for the 100B could be inserted. We then built a new airlock door and rotating drum that was long enough to hold the cold shield and that had a separate socket built of plastic to receive the cold specimen from the microscope (Taylor and Glaeser, 1975). The main disadvantage of the system was the large temperature differential between the specimen holder when inserted into the airlock (−196°C) and that of the cold stage, which would reach only −120°C. This temperature was well below the point where sublimation of the ice would be a problem, but far above the temperature that we now know is needed to maintain vitreous ice. If the specimen was transferred to the cold stage too quickly, the temperature differential would be too great and the holder would be “captured” as it expanded while warming to the higher temperature of the cold stage. Such an event essentially ended the experiment for the day, as the microscope would have to be brought to air and the specimen chamber opened to free the holder. We quickly learned not to be too fast with the transfer from the airlock to the stage. Almost immediately the microscope and the modified airlock door became operational, we obtained for the first time electron diffraction patterns from frozen hydrated specimens showing diffraction spots to near atomic resolution (Taylor and Glaeser, 1974). One of our best diffraction patterns is shown as Figure 1.
Figure 1.
Probably the best electron diffraction pattern of frozen hydrated catalase that the authors obtained in the early 1970’s. The crystals were grown by the method originally described by Dr. Douglas Dorset (Dorset and Parsons, 1975), which produced relatively square-shaped thin plates.
Once it was established that suitable frozen specimens could be made and inserted into the electron microscope, the two most important questions to be addressed were (1) would there be any contrast in the image and (2) how resistant to electron irradiation would the frozen specimens be. Electron diffraction is generally easier to do than high resolution electron imaging, one of the factors being that the incident electron dose rates can be kept low and exposure times can be arbitrarily long. This is not true for imaging, where the exposure times needed to be as short as possible to minimize effects of specimen drift. We knew, from our work with specimens on the HU-11 microscope, that directly visualizing the crystals was out of the question. Robley C. Williams had worked out a minimal dose procedure whereby the image center could be moved to the front edge of the fluorescent screen using a pair of projector lens alignment knobs located in the front of the column. The binoculars were then aimed at the front edge of the main screen but off the area that would be recorded. After thus focusing on an area adjacent to the one to be recorded, a film was moved into the camera and the beam was spread to cover the area of interest. This method proved to be exactly what we needed for low dose image recording of the ice embedded specimens.
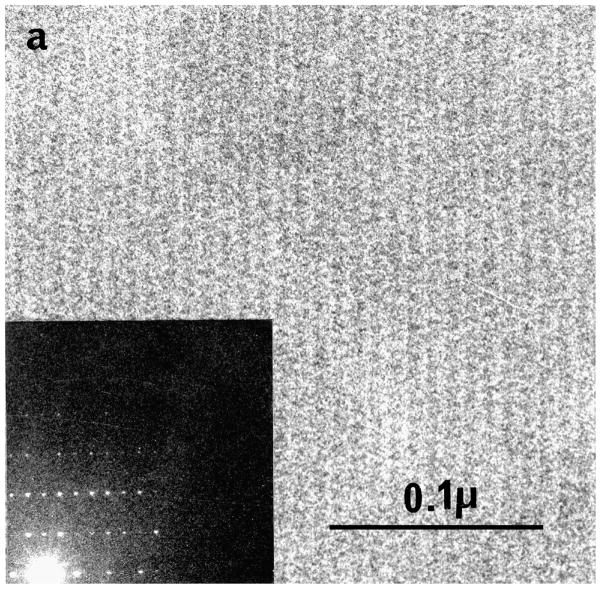
Our first images showing the crystalline periods in frozen hydrated catalase were surprisingly detailed (Taylor and Glaeser, 1975), but with experience and effort we were able to obtain even better images that showed optical diffraction that extended to 11 Å (Fig. 2) (Taylor and Glaeser, 1976). Densitometer traces across the images showed that the contrast was ~10%, certainly adequate for low resolution imaging (Taylor, 1975).
Figure 2.
Image of a frozen hydrated catalase crystal and its optical diffraction pattern. It was from such specimens that we were encouraged that protein structures could be determined from frozen hydrated specimens. From (Taylor, 1978) with permission.
The second question was the lifetime of the crystals in the electron beam. In this we were pleasantly surprised, in that the “crystal lifetime” was an order of magnitude better than we expected based on the results published by Parsons and coworkers for hydrated catalase at room temperature (27 e−/Å2 vs. 2 e−/Å2) (Taylor and Glaeser, 1976). That comparison had too many experimental intangibles to be a wholly accurate, however, since it came from two different labs using different equipment. A better comparison, which we could make ourselves, was an improvement of ~3-fold for glucose embedded catalase between room temperature and −120 °C. The definition of crystal lifetime that we were using, namely the dose for complete damage, was not the most useful comparison, however. It remained for the late Steve Hayward to compare the fading rate of particular reflections, a much better comparison, which showed about a 6-fold improvement for the low resolution reflections (Hayward and Glaeser, 1979). Our value for complete fading at 100 keV, however, corresponds pretty closely to the limiting dose used in cryoelectron tomography, where typically tilt-series images are collected right up to the bubbling point. The improvements in lifetime, however, were comparable to what X-ray crystallographers were finding with protein crystals cooled below −50°C (Haas and Rossmann, 1970; Petsko, 1975).
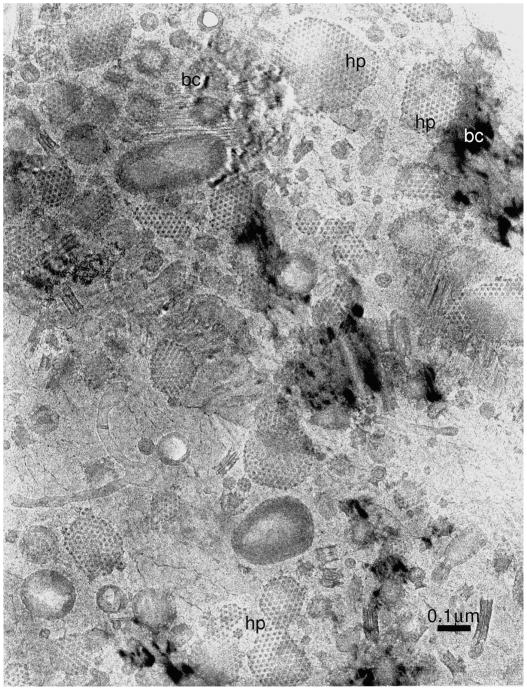
After the initial success with catalase crystals, we examined several other specimens, particularly a cell wall from Spirillum serpens. Images obtained from this specimen (Taylor, 1978) were much more dramatic with regard to showing what could be seen in an unstained, frozen hydrated specimen (Fig. 3). It is likely that these images of a highly irregular specimen, more so than the crystalline catalase results, encouraged others to take up the technique because it has structures in it that correspond more closely in size to those in many biological specimens, not just crystals.
Figure 3.
Image of a preparation of cell walls from Spirillum serpens that had been kindly provided by Dr. Wah Chiu. From (Taylor, 1978) with permission. “hp” indicates the location of the hexagonally packed surface layer (S-layer) proteins, “bc” denote the locations of bend contours in the surrounding crystalline ice. This type of image, although it too contains small periodic arrays, probably had wider influence on the further development of cryoEM of frozen hydrated specimens by others than did the images of crystalline catalase.
Vitrified Frozen Hydrated Specimens
Any retrospective on the development of cryoEM of frozen hydrated specimens would be incomplete without describing the tremendous contribution that Dr. Jacques Dubochet and his coworkers at the EMBL made to the technique. The major disadvantage of the method, at the stage to which we had developed it, was that freezing rates were too slow to prevent crystalline ice formation. While crystalline ice did not appear to propagate through the crystal lattice, wider application of the method to particle suspensions appeared to be doubtful. One of the main problems would be that particles in suspension would be squeezed between propagating crystals of hexagonal ice, where they would endure both a transient increase in solute concentration as solutes were excluded from the growing ice and possibly mechanical stresses comparable to those of a boat trapped in an arctic ice field. If frozen hydrated specimens were limited to crystalline objects, wide application was unlikely because there were very few crystalline specimens available at the time that were suitable for electron crystallography.
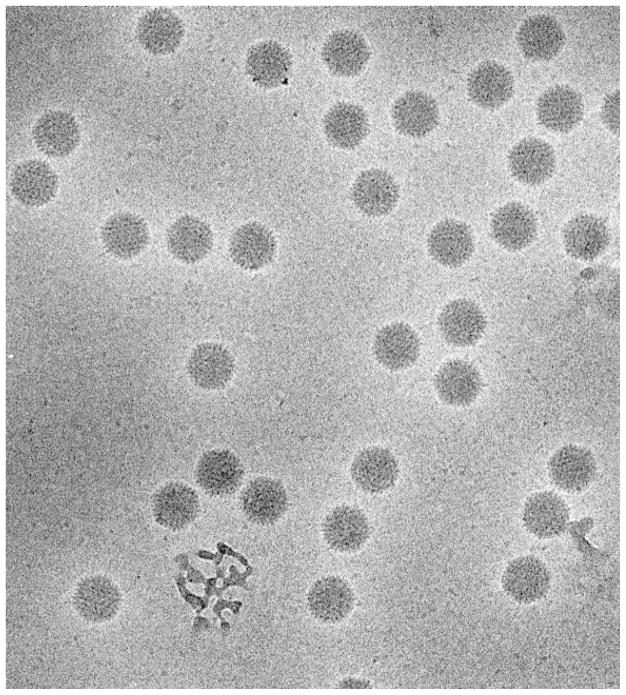
Dubochet and colleagues set about producing vitrified biological specimens once they had first shown that thin films of water could be frozen in a vitrified state. Their ultimate success has been described in detail in two very important review articles (Dubochet et al., 1981; Dubochet et al., 1988). Probably the article with the most impact, however, is the one that appeared in Nature (Adrian et al., 1984), which paved the way for the explosion of icosahedral virus structures obtained by cryoEM. Figure 4 shows a micrograph of adenovirus type 2 from that work. The entire field of cryoEM of vitrified samples owes its existence to Dubochet’s pioneering work.
Figure 4.
Electron micrograph of adenovirus type 2 suspended in vitrified ice from the same set described in (Adrian et al., 1984). Note the absence of crystalline ice features such as bend contours (see Figure 3) showing that indeed vitrification has been achieved. Micrograph courtesy of Dr. Jacques Dubochet.
The Emergence of CryoEM as a Structural Tool
The maturation of cryoEM of unstained, frozen hydrated specimens as a tool for structural investigations occurred when 3-D image reconstructions began to appear. The first of these was from naturally occurring gap junction arrays that had been frozen without stain in liquid nitrogen (Unwin and Ennis, 1984). Subsequently, a number of helical reconstructions appeared, mostly of specimens that had previously been done in negative stain but this time were examined without stain in vitrified ice (Lepault and Leonard, 1985; Mandelkow and Mandelkow, 1985; Trinick et al., 1986). The first single particle reconstructions, done on highly symmetrical specimens preserved unstained in vitrified ice, included an icosahedral virus reconstruction (Vogel et al., 1986) and clathrin coated vesicles (Vigers et al., 1986). The first 3-D reconstruction of an asymmetric structure by cryoEM appeared 5 years later (Frank et al., 1991). By today’s standards, the resolution of these early reconstructions was modest, but they showed that preservation was greatly improved when the specimen was not dried and they opened the way to the extensive list of 3-D structures of frozen-hydrated specimens that were to come.
Other Technologies Important for the Current Status of CryoEM
CryoEM would not be at the state where it is today without several other important advances, particularly in the construction of high resolution cryostages. The early development of cryoEM was hampered by the lack of good cold stages that could maintain vitreous ice and yield resolutions of 3.5 Å. Those investigators that made the most progress either built or had access to working cold stages. In the Glaeser lab, Steve Hayward built a stage based on the JEOL design (Hayward and Glaeser, 1980) that was capable of routinely achieving this resolution, but it could only maintain a temperature of −90°C, suitable for work with glucose embedded purple membrane. Specialty microscopes, such as one in Germany that was equipped with a superconducting objective lens system (Dietrich et al., 1977), could also routinely obtain high resolution images of purple membrane. Both of these instruments contributed to the first high resolution purple membrane structure (Henderson et al., 1990).
During the late 1970’s while a postdoc at the MRC Laboratory of Molecular Biology in Cambridge, UK, K. A. T. and Chris Raeburn worked on a design for a cold stage for a Philips EM300, but it was Dr. Nigel Unwin who pushed this design through to a working model (Taylor et al., 1984). This cold stage was used to determine the gap junction structure in states of low and high Ca2+ (Unwin and Ennis, 1984).
Gatan Inc. deserves credit for enabling the wide spread use of cryoEM through the introduction of their side entry Model 626 cryoholder. A related design (Henderson et al., 1991) was marketed as the popular Oxford Instruments holder. These holders, which incorporated both a cryo-transfer system and a capability of routinely resolving 3.4 Å, not only made cryoEM available to most laboratories, but they also simplified the task of collecting data over a range of tilt-angles up to 60°.
One of the most advanced cryoEM systems was a liquid-helium cooled design developed by Dr. Yoshinori Fujiyoshi (Fujiyoshi et al., 1991). This instrument and its successors have contributed to many of the more recent membrane protein structures including one that achieved a resolution of 1.9 Å (Gonen et al., 2005) and the structure of the acetylcholine receptor (Miyazawa et al., 2003).
Another very important advance was the development of easy to use low dose imaging capabilities. All of the early work on imaging unstained specimens, both glucose embedded and frozen hydrated, utilized rather elaborate schemes to avoid preirradiating the areas of interest prior to photographing it on film. In the late 1970s, Philips Electron Optics introduced an elegant electronic low dose kit for recording images of radiation-sensitive specimens that made this simple and routine. The design incorporated a fast electronic shutter just below the gun chamber. Focusing and astigmatism correction could be done on an area adjacent to the one to be recorded, using suitable combinations of deflection coils. The basic design is still in use today.
Frozen-hydrated specimen preparation has always been demanding because of the necessity to freeze the specimen in the thinnest possible layer of water. As mentioned above, the evaporation rates of water, even in high-humidity environments, are quite rapid. As a result, samples that are made too thin after blotting may dry out completely before freezing, while those that are made thick enough to allow for the unavoidable evaporation may experience unwanted changes in ionic strength and even changes in pH. The development of computerized freezing devices (Braet et al., 2003; Frederik and Hubert, 2005), in which thermal gradients are minimized and the relative humidity within an enclosed work space can approach 100% if desired, thus promises to improve on the yield of good specimens.
Revisiting Basic Principles: Preparation of Ideal CryoEM Specimens May Still Require Further Innovation
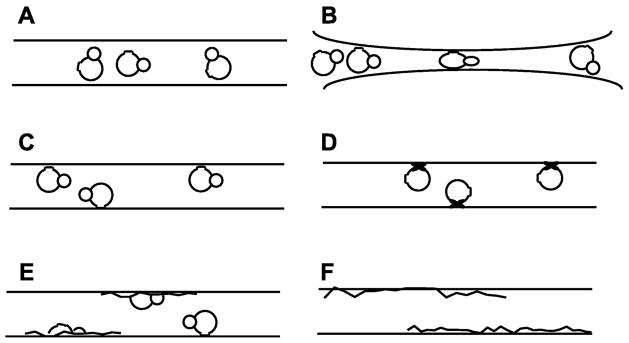
Cross sectional views of cryoEM specimens may not always look like what we wish that they did. The usually conceived model of a vitrified particle suspension (Figure 5A) pictures a macromolecular particle nicely situated in the middle of a uniform film of vitreous ice, the thickness of which is only slightly greater than the diameter of the particle. In this picture, the structure of the particle is literally “frozen” in a state that is exactly what it was like in its native buffer at the instant prior to vitrification. Different instances of the same particle may even be imagined to be trapped in different, functional conformations, corresponding to their being at different points in the biochemical cycle. What might be wrong with this picture?
Figure 5.
Cartoon-representations of the differences between what one would like a cryoEM preparation of a biological macromolecule to look like, (A), and the variety of outcomes that may actually occur in individual cases, (B – F). A. Particles are embedded within thin films of vitreous ice whose thickness is only slightly greater than the diameter of the particle itself. B. Particles remain fully embedded within a film of vitreous ice, but in this case some of them may be partially “flattened” when the thickness of the film becomes comparable to that of the particle. In other cases, the particles may be expelled from the thinnest region of the film, causing them to accumulate against the rim of the hole in a carbon support film. C. Particles are adsorbed to the air-water interface with one or more preferred orientations. In this case adsorption is due to the insertion of hydrophobic surface patches into the interface. D. Particles are again adsorbed to the air-water interface, but in this case it is imagined that one or another domains of the particle are either partially or completely denatured at the interface when interior hydrophobic residues are inserted into the interface. E. A more extreme alternative envisions that more or less “half” of a particle may denature and spread at the air-water interface, leaving the other “half” still intact in the aqueous subphase. This apparently unlikely scenario is proposed as a possible explanation of the fact that some particles are frequently seen to have about the correct size and even some of the expected internal features, but the contrast of the particle is much more faint than that of others in the population. F. In the most extreme case, virtually no particles are seen even though an appropriate concentration of protein was applied to the support film, and the hole in the carbon support film is filled with vitreous ice of the appropriate thickness. One possible explanation in this case is that all of the protein that was within the area of the hole has adsorbed to and become fully denatured at the air-water interface.
Cartoons like the one shown in Figure 5B often do illustrate at least two of the possibilities of what can go wrong. In some cases, for example, it is expected that particles may become flattened between the opposing air-water interfaces when the water layer surrounding the particle becomes too thin. In other cases the particles may segregate to the rim of the hole in the carbon support film as the water layer in the middle of the hole becomes too thin. In most cases, however, one can recognize areas where either such problems might occur, and simply avoid them for data collection.
Adsorption of particles to the air-water interface is another common worry. Adsorption to the air-water interface may occur with no more damage to the particle than is caused by running it through a hydrophobic interaction column, of course, as is imagined in Figure 5C. Rarely do such cartoons allow another possibility, however, namely that adsorbed particles are either partially damaged or even completely spread at the interface as a denatured-protein monolayer. Quite simple physical arguments, presented below, suggest that adsorption and denaturation may actually be the rule rather than the exception. It is thus worthwhile to ask how one might easily determine whether adsorption is a serious factor for any given sample, and if adsorption is a problem, what options may be available to avoid it.
It is a common part of training in biochemistry to be taught to not agitate the sample to the extent that long-lived bubbles are formed, the reason being that these bubbles – stabilized by a monolayer of denatured protein – have a disproportionate surface-to-volume ratio. As a result, formation of bubbles can destroy a significant fraction of the protein. Adsorption and denaturation of the same protein can occur equally well as soon as it is confined to the thin aqueous film that one prepares on an EM grid. There is a risk, therefore, that all of the protein confined in the aqueous film of a newly blotted grid might become denatured at the air-water interface.
The critical questions at this point are (1) how long does it take for a protein to hit the interface by diffusion, relative to the interval between blotting and plunging, and (2) how fast is the unfolding reaction relative to the residence time during which the protein touches the interface. The answer to the first question is that particles the size of a ribosome are likely to hit the air-water interface within a millisecond of formation of a 100 nm thick aqueous film. This estimate is based on their having a diffusion coefficient in the range of 2×10−7 cm2/s (Tissieres et al., 1959; Hill et al., 1969). Regarding the second question, it is less clear how frequently such collisions result in benign sticking, as pictured in Figure 5C; denaturation of just a single domain, as pictured in Figure 5D; disruption of part but not all of the complex, as pictured in Figure 5E; or complete spreading of all subunits and domains, as pictured in Figure 5F. It is reasonable to expect that the answer will be very different for one protein than it is for another. The question then becomes what should one look for in order to evaluate which of these alternatives apply to the sample at hand.
The question whether adsorption is benign or damaging can be answered for helical assemblies such as tobacco mosaic virus (TMV) particles, since the “reflections” on different layer lines can be assigned to the front and back halves of the helix. If the “front” and “back” portions of the calculated Fourier transform are not equivalent to each other, then it is almost certainly the case that the particle has adsorbed to the air-water interface, and that the half that touches the interface has been damaged.
Although no equivalent test for partial damage exists for non-helical particles, adsorption itself can be indicated in a number of ways. In some cases particles show a strongly preferred orientation, and this clearly implies that adsorption to the interface has occurred. Adsorption can also be inferred by either comparing the number of particles per unit area to what one expects from the ideal model shown in Figure 5A, or alternatively by observing whether the number of particles per unit area increases with the length of time that the sample is “incubated” on the grid before blotting.
If the mean distance between particles is significantly closer than the cartoon in Figure 5A would allow for a given protein concentration, and if significant evaporation did not occur, then many if not all of the particles that one sees on the grid must have bound to the air-water interface within the holes of the holey carbon film while the sample was “incubated” on the grid. Another suggestion for testing whether particles are adsorbed to the interface is to do tomography on a typical grid.
Whether adsorption of single particles is benign or causes some serious structural damage may become apparent when one computes a map at high enough resolution. Greater disorder at the surface relative to the interior of a particle might indicate that adsorption resulted in a situation similar to that pictured in Figure 5D or even that pictured in Figure 5E. There are alternative explanations for a radial fall off of the resolution, however, such as there being greater conformational heterogeneity at the surface of a particle even when it is suspended in bulk water; or there having been too high a level of error in the assignment of Euler angles. If partial disorder is confined to one domain (or one subunit) of a 3-D reconstruction, of course, and if the position of that domain/subunit correlates with preferential orientation in the expected way, then it would be natural to conclude that the domain or subunit is, indeed, damaged by interaction with the interface.
Although rarely discussed in the literature, many authors complain that it is impossible for them to prepare cryoEM samples of their protein over “bare” holes in a carbon film. Adsorption of particles onto the surrounding carbon is then often suspected as being the problem, and in some cases it is even easy to see that abundant adsorption to the carbon does occur. The absence of particles from the holes may be due to a more sinister reason, however, namely that the “bottom” interface becomes coated with a denatured monolayer of protein immediately upon applying the sample to a holey carbon film. Any native protein that is trapped between the bottom interface and the newly created “top” interface will then become denatured at the top interface, since – as we have pointed out above – that can happen within milliseconds of blotting. One test for this possibility is that images of the bare holes may show more phase-contrast granularity (stronger Thon rings), due to the presence of a film of denatured protein, than does a control sample that is prepared from the same buffer, but without added protein.
Many, if not most cryoEM samples show rather frequent examples of particles that have the same size as an undamaged particle, and some may even have characteristic internal structure (such as the bands seen in side views of GroEL), but the particle contrast is quite clearly – and mysteriously – only a fraction of the contrast of the “best” particles. Some have suggested that the higher-contrast particles actually protrude through the air-water interface, out into vacuum. This cannot happen while water is in the liquid state, of course, and it is highly implausible that it happens later, e.g. by sublimation of water. One must thus consider the alternative that a large part of the structure has denatured and spread at the air-water interface, as is pictured in Figure 5E, leaving the rest of the particle relatively intact in the subphase.
Other explanations for the appearance of “low contrast” particles include the possibility that a subpopulation was somehow biochemically damaged during the purification stage. While this is perhaps the explanation that the microscopist would think of first, it is prudent to use other experimental methods to confirm that it is actually the correct explanation. While checking the sample in negative stain should be among the first tests to try, negatively stained samples may also show great heterogeneity – for other reasons – even when the biochemical preparation itself is structurally homogeneous. Cross linking with gluteraldehyde (Kastner et al., 2008) may protect samples from partial or complete denaturation, but on the other hand it can be difficult to achieve quantitative cross linking, and crosslinking can also capture extreme motions of molecules, thus locking in unrepresentative conformational states.
Many, if not most cryoEM samples also show a rather high distribution of particle sizes, some of them smaller than the correct size – which can be called broken particles – and some of them larger than the correct size – which may be aggregated particles or aggregated pieces of broken particles. Once again, the assumption may be that both broken and aggregated particles were present in the biochemical preparation. Before jumping to such a conclusion, however, one should carry out control experiments similar to those mentioned in the previous paragraph. In the case of pyruvate:ferredoxin oxidoreductase (PFOR) from Desulfovibrio vulgaris Hildenborough, for example, Garczarek et al. found that the octomeric complex formed by this enzyme is beautifully preserved when negatively stained with ammonium molybdate plus trehalose, but that it is almost completely disassembled into dimers in cryoEM samples. When cross linked with gluteraldehyde, this sample also remained much more intact in cryoEM than did the native protein complex (Garczarek et al., 2007).
When damage at the air-water interface is suspected, the current alternative is to immobilize samples on continuous carbon before blotting and freezing. While this method of sample preparation is effective in preventing the protein from contacting the air-water interface, the adsorption of particles to carbon can also result in a high fraction of broken or aggregated particles. The use of particle adsorption to carbon films may thus be a pragmatic solution, but it is clearly not an ideal one.
Kelly et al. have very recently described a more biochemically oriented approach that may provide a nearly perfect solution to the problem of interaction with the air-water interface (Kelly et al., 2008). This solution simply involves the binding of particles to a lipid monolayer, and it is thus identical to the technique used to grow 2-D crystals at the air-water interface (for a recent review see (Chiu et al., 1997)). The goal in this case, however, is to bind particles in randomly distributed locations and with randomly distributed orientations. The advantage of this technique is obvious: if the lipid monolayer is formed at a high surface pressure, it is not possible for proteins in the subphase to insert into the monolayer and become denatured. At the same time, once bound to the polar headgroups of the monolayer, the protein molecules cannot diffuse to the second interface when it is newly formed by blotting. The only interfacial interactions that the particles experience are relatively mild ones, essentially identical to those that they experience during protein purification. Vitrification of hydrated lipid monolayer films recovered on holey carbon films is actually quite simple, and large areas the grid can be covered with suitably thin ice (Taylor et al., 2007).
In its initial implementation, a Ni-NTA-derivatized lipid was used to bind his-tagged protein particles (Kelly et al., 2008). One of the further advantages of binding to an affinity tag is that the lipid layer can be used for one-step, in situ purification (pull-down) of particles containing the desired protein, starting with just the crude cell lysate. The approach thus promises to greatly simplify the preparation of samples for single-particle electron microscopy. The speed and gentleness of the approach could even facilitate the study of transient multiprotein complexes and those that do not survive the conditions required for traditional purification protocols. One can imagine extending the method to a variety of other affinity ligands, of course, and even to less specific binding of purified protein complexes onto charged surfaces that mimic the interfaces used for ion-exchange chromatography.
Many Other Prospects Have Emerged for Future Improvements in CryoEM
At the time we were developing the frozen hydrated specimen technique, our vision hardly extended beyond its application to thin crystalline arrays because of the necessity to average images of many individual molecules in order to achieve high resolution. Helical filaments looked possible based on some of the early images of S. serpens cell walls (Glaeser et al., 1980), but images of T4 bacteriophage that we also obtained generally failed to show any helical diffraction. Single-particle EM (with negatively stained samples) and electron tomography (with plastic sections) were in their infancy. Both of these would eventually blossom, but only after it became possible to visualize vitrified specimens. Many other technologies have also appeared in the intervening 30 years, of course, and as a result the opportunities for new research developments are greater now than they have ever been in the past.
Although cryoEM tomography has already given extraordinary insight into the supramolecular organization within whole cells, one knows that the resolution that is routinely achievable, without averaging, has still not reached the physical limitations of what should be possible. One of the current frontiers on which important progress will surely be made is the development of detectors with larger numbers of pixels, a better modulation transfer function, and near-perfect detective quantum efficiency. The value of such detectors is that they would allow one to extend dose-fractionation closer to the limit that has been demonstrated in simulations (McEwen et al., 1995). The ability to align images recorded with fewer electrons per frame, which would be made possible with noise-free detectors, could be invaluable. The allowed electron exposure could thus be fractionated such that the number of images in a tilt series would be less limiting for tomogram resolution.
Cellular tomography will always be a competition between the desire to reconstruct as much of a cells volume as possible at the highest resolution possible, but resolution is ultimately tied to specimen thickness. Thus, improvements are also needed in the technology of cutting cryo-sections from thick, but nevertheless properly frozen specimens (Al-Amoudi et al., 2004; Gruska et al., 2007; Ladinsky and Howell, 2007). Cutting artifacts and adhesion of the sections to the grid are current roadblocks (Al-Amoudi et al., 2005). The use of ion-beam technologies to either mill down (Marko et al., 2007) or cut out thin specimens also holds promise for producing samples that are thinner than one mean-free-path for inelastic scattering, i.e. thin enough to be compatible with a resolution goal of 2 or 3 nm.
Even better for tomography would be to develop ways to collect and productively use inelastically scattered electrons. In principle, this would more than double the number of electron counts contributing to the image, but would be restricted to the low resolution features, which is the realm in which electron tomography is currently applicable. Chromatic aberration correctors are identified by some as possibly being the way to “rescue” electron counts that would otherwise have been discarded by an energy filter. Proper theoretical treatment (e.g. simulation) of image formation and 3-D reconstruction for thicker specimens would need to be pursued in order provide a better foundation for this suggestion. The same is also true for the use of scanning transmission electron microscopy (STEM) in tomography of thick biological specimens. In this case one can imagine the use of sophisticated detector technologies to collect nearly every scattered electron and characterize both its scattering angle and its energy loss, information-bearing details that would be lost in an aberration-corrected TEM.
As cryoEM tomography moves in the direction of higher resolution, one will have to confront the issue of differences in focus that exist from one end of a tilted specimen to the other, the focus gradient. One of the great strengths of STEM tomography is the possibility of dynamic focus correction along the defocus gradient. For TEM, two computational approaches to correction of the focus gradient have been reported (Winkler and Taylor, 2003; Fernandez et al., 2006), but there is room for new ideas, particularly in the determination of defocus in images with very low electron counts, which are the typical kind recorded in a tilt series of ice embedded specimens.
Within the realm of single-particle cryoEM there are many exciting opportunities that can be mentioned as ways to push the frontier forward. One of the key challenges on the computational side is to be able to recognize particles that should be assigned to different conformational subgroups. Related to this is the possibility that cryoEM might then be able to provide unique information about the modes of motion of a particle, and thus the role that its flexibility may play in its function. New theoretical tools and experimental implementations are also needed in order to obtain initial models of structures about which one currently knows little if anything. New tools are also needed for the validation of structures after they have been refined.
The recent application of Zernike-type phase contrast to produce images of GroEL (Danev and Nagayama, 2008) promises to open a new era in cryoEM of single particles. The large increase in contrast that is shown in these images suggest that it may be possible to work with particles that have been too small to image by defocus-based phase contrast. Still to be determined is whether in-focus phase contrast will also aid in the identification of particles that are in different conformational states.
Automated single-particle data collection has now been demonstrated to be fully successful (Suloway et al., 2005; Stagg et al., 2006). Not as well advanced, but nevertheless on its way, is the corresponding automation on the computational side of single-particle EM. The large increase in the size of data sets that automation provides are expected to lead to major improvements in the resolution of the corresponding 3-D reconstructions. In the long term, however, it would be even better if improvements could be made in the image quality, bringing it closer to the level that physics would allow (Henderson, 1995; Glaeser, 1999). If experimental methods could be devised to reduce the amount of beam-induced movement that normally occurs, data collection would be faster (because fewer images would be needed) and computational times would also be greatly reduced.
Summary and Conclusions
The breadth of applications of cryoEM to structural biology today is much greater than what we felt would be possible when we started work with frozen hydrated specimens in the early 1970s. Although no technique is perfect and error free, it is nevertheless amazing to see how much information can be gleaned from such a small amount of material using images that are so far from optimal. Today, good, though perhaps imperfect vitrified specimens are easy to make, automated electron microscopy can collect several orders of magnitude more images in a few days than were collected in years using film. Computers are so much more powerful than 30 years ago with the result that image analysis algorithms treat image parameters more exactly, and many more and ever larger images can be processed. This impacts all kinds of 3-D imaging. Electron tomography provides us with the ability to look at the thin margins of vitrified cells and holds the prospect of identifying many structures in situ and in action. This has all been made possible by the many workers in the cryoEM and structural biology fields and those in industry who imagined that so much more could be done with the simple idea of viewing a frozen specimen.
Acknowledgments
Much of K. A. T.’s work on frozen hydrated specimens was done during the tenure of a predoctoral traineeship from the NIH. The assistance of E. F. Dowling, who supervised the Donner Laboratory machine shop where as a student, K. A. T. was able to build the various devices described in this paper and more fully described in his dissertation, is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrams IM, McBain JW. A closed cell for electron microscopy. J Appl Phys. 1944;15:607–609. doi: 10.1126/science.100.2595.273. [DOI] [PubMed] [Google Scholar]
- Adrian M, Dubochet J, Lepault J, McDowall AW. Cryo-electron microscopy of viruses. Nature. 1984;308:32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Al-Amoudi A, Chang JJ, Leforestier A, McDowall A, Salamin LM, Norlen LP, Richter K, Blanc NS, Studer D, Dubochet J. Cryo-electron microscopy of vitreous sections. EMBO J. 2004;23:3583–3588. doi: 10.1038/sj.emboj.7600366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Amoudi A, Studer D, Dubochet J. Cutting artefacts and cutting process in vitreous sections for cryo-electron microscopy. J Struct Biol. 2005;150:109–121. doi: 10.1016/j.jsb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Braet F, Bomans PH, Wisse E, Frederik PM. The observation of intact hepatic endothelial cells by cryo-electron microscopy. J Microsc. 2003;212:175–185. doi: 10.1046/j.1365-2818.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- Chiu W, Avila-Sakar AJ, Schmid MF. Electron crystallography of macromolecular periodic arrays on phospholipid monolayers. Adv Biophys. 1997;34:161–172. doi: 10.1016/s0065-227x(97)89638-4. [DOI] [PubMed] [Google Scholar]
- Danev R, Nagayama K. Single particle analysis based on Zernike phase contrast transmission electron microscopy. J Struct Biol. 2008;161:211–218. doi: 10.1016/j.jsb.2007.10.015. [DOI] [PubMed] [Google Scholar]
- DeRosier DJ, Klug A. Reconstruction of three dimensional structures from electron micrographs. Nature. 1968;217:130–134. doi: 10.1038/217130a0. [DOI] [PubMed] [Google Scholar]
- Dietrich I, Fox F, Knapek E, Lefranc G, Nachtrieb K, Weyl R, Zerbst H. Improvements in electron microscopy by application of superconductivity. Ultramicroscopy. 1977;2:241–249. doi: 10.1016/s0304-3991(76)91487-x. [DOI] [PubMed] [Google Scholar]
- Dorset DL, Parsons DF. Electron diffraction from single, fully hydrated, ox liver catalase crystals. Acta Crystallogr A. 1975;31:210–215. [Google Scholar]
- Dowell LG, Moline SW, Rinfret AP. A low-temperature X-ray diffraction study of ice structures formed in aqueous gelatin gels. Biochim Biophys Acta. 1962;59:158–167. doi: 10.1016/0006-3002(62)90706-0. [DOI] [PubMed] [Google Scholar]
- Dubochet J, Adrian M, Chang JJ, Homo JC, Lepault J, McDowall AW, Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988;21:129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Dubochet J, Booy FP, Freeman R, Jones AV, Walter CA. Low temperature electron microscopy. Annu Rev Biophys Bioeng. 1981;10:133–149. doi: 10.1146/annurev.bb.10.060181.001025. [DOI] [PubMed] [Google Scholar]
- Fernandez JJ, Li S, Crowther RA. CTF determination and correction in electron cryotomography. Ultramicroscopy. 2006;106:587–596. doi: 10.1016/j.ultramic.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Frank J, Penczek P, Grassucci R, Srivastava S. Three-dimensional reconstruction of the 70s Escherichia coli ribosome in ice: The distribution of ribosomal RNA. J Cell Biol. 1991;115:597–605. doi: 10.1083/jcb.115.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederik PM, Hubert DH. Cryoelectron microscopy of liposomes. Methods Enzymol. 2005;391:431–448. doi: 10.1016/S0076-6879(05)91024-0. [DOI] [PubMed] [Google Scholar]
- Fujiyoshi Y, Mizusaki T, Morikawa K, Yamagishi H, Aoki Y, Kihara H, Harada Y. Development of a superfluid helium stage for high-resolution electron microscopy. Ultramicroscopy. 1991;38:241–251. [Google Scholar]
- Garczarek F, Dong M, Typke D, Witkowska HE, Hazen TC, Nogales E, Biggin MD, Glaeser RM. Octomeric pyruvate-ferredoxin oxidoreductase from desulfovibrio vulgaris. J Struct Biol. 2007;159:9–18. doi: 10.1016/j.jsb.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Glaeser RM. Limitations to significant information in biological electron microscopy as a result of radiation damage. J Ultrastruct Res. 1971;36:466–482. doi: 10.1016/s0022-5320(71)80118-1. [DOI] [PubMed] [Google Scholar]
- Glaeser RM. Review: Electron crystallography: present excitement, a nod to the past, anticipating the future. J Struct Biol. 1999;128:3–14. doi: 10.1006/jsbi.1999.4172. [DOI] [PubMed] [Google Scholar]
- Glaeser RM. Retrospective: radiation damage and its associated “information limitations”. J Struct Biol. 2008;163:271–276. doi: 10.1016/j.jsb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Glaeser RM, Chiu W, Grano D, Taylor KA. Morphological model of the surface-layer in Spirillum serpens. In: Baumeister W, Vogell W, editors. Electron Microscopy at Molecular Dimensions: State of the Art and Strategies for the Future. Berlin: Springer-Verlag; 1980. pp. 22–26. [Google Scholar]
- Gonen T, Cheng Y, Sliz P, Hiroaki Y, Fujiyoshi Y, Harrison SC, Walz T. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 2005;438:633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruska M, Medalia O, Baumeister W, Leis A. Electron tomography of vitreous sections from cultured mammalian cells. J Struct Biol. 2007;161:384–392. doi: 10.1016/j.jsb.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Haas D. X-ray studies on lysozyme crystals at −50 °C. Acta Crystallogr. 1968;B24:604. [Google Scholar]
- Haas DJ, Rossmann MG. Crystallographic studies on lactate dehydrogenase at −75 °C. Acta Crystallogr B. 1970;26:998–1004. doi: 10.1107/s0567740870003485. [DOI] [PubMed] [Google Scholar]
- Hayward SB, Glaeser RM. Radiation damage of purple membrane at low temperature. Ultramicroscopy. 1979;04:201–210. doi: 10.1016/s0304-3991(79)90211-0. [DOI] [PubMed] [Google Scholar]
- Hayward SB, Glaeser RM. High resolution cold stage for the JEOL 100B and 100C electron microscopes. Ultramicroscopy. 1980;5:3–8. doi: 10.1016/0304-3991(80)90005-4. [DOI] [PubMed] [Google Scholar]
- Heide HG, Grund S. deep-freeze link for transport of water-containing biological objects to the electron microscope. J Ultrastruct Res. 1974;48:259–268. doi: 10.1016/s0022-5320(74)80081-x. [DOI] [PubMed] [Google Scholar]
- Henderson R. The potential and limitations of neutrons, electrons and X-rays for atomic resolution microscopy of unstained biological molecules. Q Rev Biophys. 1995;28:171–193. doi: 10.1017/s003358350000305x. [DOI] [PubMed] [Google Scholar]
- Henderson R, Baldwin JM, Ceska TA, Zemlin F, Beckmann E, Downing KH. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990;213:899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Henderson R, Raeburn C, Vigers G. A side-entry cold holder for cryo-electron microscopy. Ultramicroscopy. 1991;35:45–53. doi: 10.1016/0304-3991(91)90043-6. [DOI] [PubMed] [Google Scholar]
- Hill WE, Rossetti GP, Van Holde KE. Physical studies of ribosomes from Escherichia coli. J Mol Biol. 1969;44:263–277. doi: 10.1016/0022-2836(69)90174-0. [DOI] [PubMed] [Google Scholar]
- Horne RW, Wildy P. An historical account of the development and applications of the negative staining technique to the electron microscopy of viruses. J Microsc. 1979;117:103–122. doi: 10.1111/j.1365-2818.1979.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Kastner B, Fischer N, Golas MM, Sander B, Dube P, Boehringer D, Hartmuth K, Deckert J, Hauer F, Wolf E, et al. GraFix: Sample preparation for single particle electron cryomicroscopy. Nature Methods. 2008;5:53–55. doi: 10.1038/nmeth1139. [DOI] [PubMed] [Google Scholar]
- Kelly DF, Dukovski D, Walz T. Monolayer purification: A rapid method for isolating protein complexes for single-particle electron microscopy. Proc Natl Acad Sci U S A. 2008;105:4703–4708. doi: 10.1073/pnas.0800867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky MS, Howell KE. Electron tomography of immunolabeled cryosections. Methods Cell Biol. 2007;79:543–558. doi: 10.1016/S0091-679X(06)79021-5. [DOI] [PubMed] [Google Scholar]
- Lepault J, Leonard K. Three-dimensional structure of unstained, frozen-hydrated extended tails of bacteriophage T4. J Mol Biol. 1985;182:431–441. doi: 10.1016/0022-2836(85)90202-5. [DOI] [PubMed] [Google Scholar]
- Low BW, Chen CC, Berger JE, Singman L, Pletcher JF. Studies of insulin crystals at low temperatures: Effects on mosaic character and radiation sensitivity. Proc Natl Acad Sci U S A. 1966;56:1746–1750. doi: 10.1073/pnas.56.6.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Unstained microtubules studied by cryo-electron microscopy. Substructure, supertwist and disassembly. J Mol Biol. 1985;181:123–135. doi: 10.1016/0022-2836(85)90330-4. [DOI] [PubMed] [Google Scholar]
- Marko M, Hsieh C, Schalek R, Frank J, Mannella C. Focused-ion-beam thinning of frozen-hydrated biological specimens for cryo-electron microscopy. Nat Methods. 2007;4:215–217. doi: 10.1038/nmeth1014. [DOI] [PubMed] [Google Scholar]
- Marton L. Early History of the Electron Microscope. San Francisco Press Inc; San Francisco: 1968. [Google Scholar]
- Matricardi VR, Moretz RC, Parsons DF. Electron diffraction of wet proteins: Catalase. Science. 1972;177:268–270. doi: 10.1126/science.177.4045.268. [DOI] [PubMed] [Google Scholar]
- McEwen BF, Downing KH, Glaeser RM. The relevance of dose-fractionation in tomography of radiation-sensitive specimens. Ultramicroscopy. 1995;60:357–373. doi: 10.1016/0304-3991(95)00082-8. [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- Parsons DF. Structure of wet specimens in electron microscopy. Improved environmental chambers make it possible to examine wet specimens easily. Science. 1974;186:407–414. doi: 10.1126/science.186.4162.407. [DOI] [PubMed] [Google Scholar]
- Parsons DF, Matricardi VR, Moretz RC, Turner JN. Electron microscopy and diffraction of wet unstained and unfixed biological objects. Adv Biol Med Phys. 1974;15:161–270. doi: 10.1016/b978-0-12-005215-8.50012-7. [DOI] [PubMed] [Google Scholar]
- Petsko GA. Protein crystallography at sub-zero temperatures: Cryo-protective mother liquors for protein crystals. J Mol Biol. 1975;96:381–388. doi: 10.1016/0022-2836(75)90167-9. [DOI] [PubMed] [Google Scholar]
- Stagg SM, Lander GC, Pulokas J, Fellmann D, Cheng A, Quispe JD, Mallick SP, Avila RM, Carragher B, Potter CS. Automated cryoEM data acquisition and analysis of 284742 particles of GroEL. J Struct Biol. 2006;155:470–481. doi: 10.1016/j.jsb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Suloway C, Pulokas J, Fellmann D, Cheng A, Guerra F, Quispe J, Stagg S, Potter CS, Carragher B. Automated molecular microscopy: The new Leginon system. J Struct Biol. 2005;151:41–60. doi: 10.1016/j.jsb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Taylor DW, Kelly DF, Cheng A, Taylor KA. On the freezing and identification of lipid monolayer 2-d arrays for cryoelectron microscopy. J Struct Biol. 2007;160:305–312. doi: 10.1016/j.jsb.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA. PhD. University of California; Berkeley, CA: 1975. Electron microscopy and electron diffraction of frozen hydrated biological specimens. [Google Scholar]
- Taylor KA. Structure determination of frozen, hydrated, crystalline biological specimens. J Microsc. 1978;112:115–125. doi: 10.1111/j.1365-2818.1978.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Glaeser RM. Hydrophilic support films of controlled thickness and composition. Rev Sci Instrum. 1973;44:1546–1547. doi: 10.1063/1.1685999. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Glaeser RM. Electron diffraction of frozen, hydrated protein crystals. Science. 1974;186:1036–1037. doi: 10.1126/science.186.4168.1036. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Glaeser RM. Modified airlock door for the introduction of frozen specimens into the JEM 100B electron microscope. Rev Sci Instrum. 1975;46:985–986. [Google Scholar]
- Taylor KA, Glaeser RM. Electron microscopy of frozen hydrated biological specimens. J Ultrastruct Res. 1976;55:448–456. doi: 10.1016/s0022-5320(76)80099-8. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Milligan RA, Raeburn C, Unwin PNT. A cold stage for the Philips EM300 electron microscope. Ultramicroscopy. 1984;13:185–190. doi: 10.1016/0304-3991(84)90197-9. [DOI] [PubMed] [Google Scholar]
- Tissières A, Watson JD, Schlessinger D, Hollingworth BR. Ribonucleoprotein particles from Eschericia coli. J Mol Biol. 1959;1:221–233. [Google Scholar]
- Trinick J, Cooper J, Seymour J, Egelman EH. Cryo-electron microscopy and three-dimensional reconstruction of actin filaments. J Microsc. 1986;141:349–360. doi: 10.1111/j.1365-2818.1986.tb02728.x. [DOI] [PubMed] [Google Scholar]
- Unwin PN, Ennis PD. Two configurations of a channel-forming membrane protein. Nature. 1984;307:609–613. doi: 10.1038/307609a0. [DOI] [PubMed] [Google Scholar]
- Unwin PNT, Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975;94:425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Vigers GP, Crowther RA, Pearse BM. Three-dimensional structure of clathrin cages in ice. EMBO J. 1986;5:529–534. doi: 10.1002/j.1460-2075.1986.tb04242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel RH, Provencher SW, von Bonsdorff CH, Adrian M, Dubochet J. Envelope structure of Semliki forest virus reconstructed from cryo-electron micrographs. Nature. 1986;320:533–535. doi: 10.1038/320533a0. [DOI] [PubMed] [Google Scholar]
- Williams RC, Fisher HW. Electron microscopy of tobacco mosaic virus under conditions of minimal beam exposure. J Mol Biol. 1970;52:121–123. doi: 10.1016/0022-2836(70)90181-6. [DOI] [PubMed] [Google Scholar]
- Winkler H, Taylor KA. Focus gradient correction applied to tilt series image data used in electron tomography. J Struct Biol. 2003;143:24–32. doi: 10.1016/s1047-8477(03)00120-5. [DOI] [PubMed] [Google Scholar]
- Wrigley NG. The lattice spacing of crystalline catalase as an internal standard of length in electron microscopy. J Ultrastruct Res. 1968;24:454–464. doi: 10.1016/s0022-5320(68)80048-6. [DOI] [PubMed] [Google Scholar]