The evidence for a role of B cells in multiple sclerosis (original) (raw)
Abstract
Understanding the pathogenesis of complex immunologic disorders such as multiple sclerosis (MS) is challenging. Abnormalities in many different cell types are observed in the immune system and CNS of patients with MS and the identification of the primary and secondary events is difficult. Recent studies suggest that the model of MS as a disorder mediated only by T cells is overly simplistic and propose an important role for B cells in the propagation of the disease. B-cell activation in the form of oligoclonal bands (OCB) production is the most consistent immunologic finding in patients with MS. Notably, markers of B-cell activation within the CSF of patients with MS predict conversion from clinically isolated syndrome to clinically definite MS and correlate with MRI activity, onset of relapses, and disability progression. In addition, the main genetic risk factor in MS is associated with OCB production, and environmental agents associated with MS susceptibility (vitamin D and the Epstein-Barr virus) influence B-cell proliferation and function. Finally, the only cell-specific treatments that are effective in patients with MS are monoclonal antibodies targeting the B-cell antigen CD20, suggesting a potentially causative role for B cells. Based on current evidence there is no longer doubt that B cells are relevant to the etiology and pathogenesis of MS. Elucidating the role of B cells in MS will be a fruitful strategy for disease prevention and treatment._Neurology_® 2012;78:823–832
Multiple sclerosis (MS) is a debilitating disease of the CNS characterized by myelin loss, axonal pathology, and progressive neurologic dysfunction.1 Genetic, epidemiologic, and pathologic studies support the hypothesis that the neurologic manifestations of the disease arise from immune-mediated demyelination, which impairs neuronal transmission and results in axonal degeneration.2,3 However, understanding the factors driving this abnormal immune response is limited.
T cells and in particular CD4+ T helper cells (Th) have until recently been considered the primary immune drivers in MS. Evidence supporting this hypothesis includes that the main MS genetic risk factor resides in the major histocompatibility complex (MHC) class II region, which plays a central role in the development of T-cell central tolerance4,5 and that the animal model of MS, experimental autoimmune encephalomyelitis (EAE), can be adoptively transferred to mice by the injection of encephalitogenic myelin-specific Th cells.6
However, the notion of MS being primarily a Th-cell disease has been reassessed, and the role of CD8+ T cells, B cells, and innate immunity emphasized. We review here recent evidence that B cells are important in driving the pathologic immune response in MS.
LIMITATIONS OF THE CD4+ MODEL
Several features of MS plaque pathology cast doubt on the view that MS is a purely Th-cell–directed process. Lymphocytes may be absent in early MS lesions with extensive oligodendrocyte loss and demyelination.7,8 Conversely, inflammatory cuffs are often present in otherwise normal-appearing white matter contiguous with or distant from active MS lesions and unlike the lesions of EAE, CD4+ T cells are outnumbered by MHC class I–restricted, clonally expanded CD8+ cells in active MS lesions.9,10
The necessity to re-evaluate the role of different cell types in MS was also recently suggested when a phase II clinical trial testing the effect of Ustekinumab, an antibody directed against the common subunit of interleukin (IL)-12 and IL-23, relevant for Th1 and Th17 cell differentiation respectively, reported no benefit in relapsing-remitting MS (RRMS).11 Previously, an antibody directed against CD4, which successfully depleted CD4+ T cells, was also found to have no effect on disease activity or course in patients with MS.12 In clear contrast, analogous antibodies prevented the development of EAE.13,14 These trials demonstrate why, although animal models represent useful and essential tools in modern research, one needs to be aware of their distance from the human disease.15 This is especially true in complex immune disorders such as MS, in which primary causal and secondary events are difficult to separate.
OLIGOCLONAL BANDS: THE HALLMARK OF MS
The most consistent immunologic finding in patients with MS is the presence of oligoclonal bands (OCB) in the CSF. OCB arise from the intrathecal synthesis of clonal IgG and are present in more than 95% of patients with MS.16 Clonally expanded B cells are also located in brain parenchyma and are responsible for the production of OCB.17–19 Plasma cells are observed in large numbers in the perivascular spaces within subacute and chronic MS plaques, and it is likely that antigen processing and antibody synthesis takes place at these sites.8,20 A lymph node sinus-like organization of the perivascular spaces is seen within plaques of chronic patients with MS, with lymphocytes and resident macrophages located within, and plasma cells located outside, smooth-walled channels.20 Plasma cells are also present in smaller numbers in parenchymal plaque and periplaque white matter tissue, and the leptomeninges. This indicates a targeted B-cell response consequent to antigenic stimulation within the CNS.
The pathogenicity of these antibodies remains controversial. IgG deposition and complement-mediated demyelination are predominant features of brain lesions in patients with established disease.21 However, in newly forming lesions, oligodendrocyte apoptosis is a striking feature that does not appear to be triggered by immunoglobulin deposition, T cells, or macrophages.7,8 Additionally, the ability of these intrathecal antibodies to react against myelin antigens is unclear.22 For example, a recent study has shown that recombinant antibodies generated from clonally expanded plasma cell and B-cell clones can react against MS brain tissue.23 However, in a larger screen of more than 50 recombinant antibodies derived from the CSF of patients with MS, nearly all did not bind myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG), or proteolipid protein (PLP) (the main candidate autoantigens of MS), and hardly reacted with either control or MS brain tissues.24 This inconsistency indicates that autoantibody production within the CNS is probably not the initiating force in MS. However, there is no doubt the presence of OCB indicates abnormal B-cell activation within the CNS of patients with MS and suggests a role for B cells in the propagation of disease.
B CELLS AND DISEASE COURSE
B-cell homeostasis and function within the CNS are relevant for the development and course of clinically definite MS (CDMS). In 85% of patients with MS the initial presentation is defined as a clinically isolated syndrome (CIS). Only about 60% of CIS cases will develop a second demyelinating event and therefore be diagnosed with CDMS over 20 years.25 Identifying factors driving the fate of patients with CIS is relevant not only for a better prognosis and early intervention strategies but also for the understanding of the biological mechanisms behind the development of CDMS.
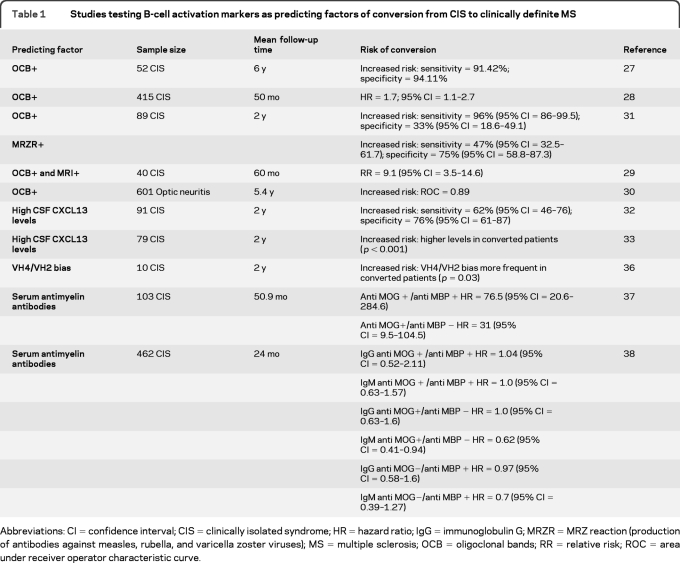
B-cell counts are increased in the CSF of patients with CIS and peripheral B-cell subsets show a markedly higher expression of the α4 subunit of the VLA-4 receptor, needed for their migration across the blood–brain barrier.26 Abnormal B-cell activity is not only present at the first manifestation of the disease, but also predicts conversion to CDMS (table 1). CIS cases with OCB and polyspecific production of antibodies against measles, rubella, and varicella zoster viruses (the so-called “MRZ reaction”) and high levels of the B-cell attractant chemokine CXCL13 in the CSF are at an increased risk of a second demyelinating event.27–33 Other studies have also shown an overrepresentation of certain variable heavy chain sequences (VH4 and VH2 families) in B and plasma cells collected from CSF and plaques of patients with MS and that this feature may influence conversion to CDMS.34–36
Table 1.
Studies testing B-cell activation markers as predicting factors of conversion from CIS to clinically definite MS
The hypothesis that the presence of antibodies directed against myelin antigens in the serum of CIS cases could predict conversion to MS has also been investigated. While Berger et al.37 reported that risk of conversion was greater in CIS cases with serum antibodies directed against MBP and MOG, these results have not been confirmed by a larger multicenter trial,38 suggesting that anti-myelin antibody production is unlikely to mediate the role of B cells in CIS conversion.
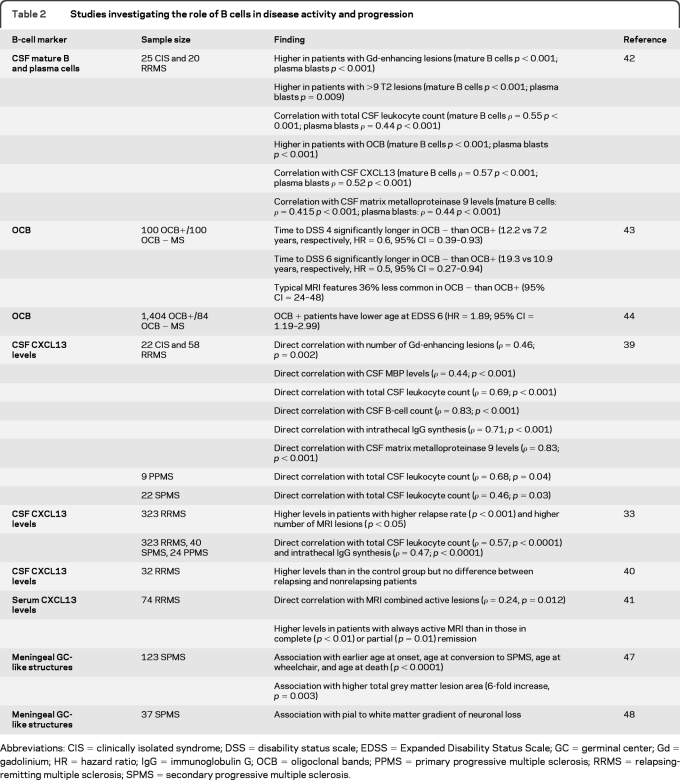
Markers of B-cell presence and activation also correlate with disease activity and progression in CDMS (table 2). CSF CXCL13 levels are augmented in CIS, RRMS, secondary progressive MS (SPMS), and primary progressive MS (PPMS) and correlate with CSF total leukocyte and B-cell counts, intrathecal IgG synthesis, markers of demyelination and blood–brain barrier leakage, MRI activity, and relapse rate.33,39,40 Serum levels of CXCL13 are also higher during periods of MRI activity.41 Mature B-cell and plasma blast CSF counts correlate with MRI lesions.42 Furthermore, disease progression is slower in OCB-negative (OCB−) than in OCB-positive (OCB+) patients.43,44 Further evidence for a role of B cells in disease progression comes from pathologic studies showing the presence of lymphoid neogenesis in a substantial percentage of SPMS cases. Inflammatory aggregates organized as B-cell germinal center (GC)–like structures were observed in the subarachnoid space of SPMS cases and associated with features indicative of a more severe disease course.45–47 Notably, a colocalization between these B-cell aggregates and subpial cortical lesions46,47 and a gradient of neuronal loss in the cortical layers48 were found, suggesting the release of soluble factors across the inflamed meninges may cause cortical damage.
Table 2.
Studies investigating the role of B cells in disease activity and progression
B CELLS AND MS RISK FACTORS
The role of genetics.
Complex disorders such as MS are conditions that have no single cause but result from a combination of genetic and environmental factors and their interactions.49 It would be logical to hypothesize that these factors would have a direct influence on the cell type triggering the abnormal immune response in MS. This does not prove causation but it is an unconditional requirement for plausibility.
The main genetic locus in MS is located within the MHC class II region and corresponds to the HLA-DRB1*1501 class II allele.4 This allele has been found to increase the risk of MS in most populations studied and its presence in homozygosity increases the risk of MS more than 6-fold.2,4
A further stratification of patients based on the presence or absence of OCB has demonstrated that the DRB1*1501 allele is strongly associated with the OCB+ subpopulation whereas the association tends to disappear in OCB− patients.50–52 It has therefore been hypothesized that OCB− patients may represent a phenotypically similar but immunologically distinct entity.50 However, OCB status can change during the course of MS,53 perhaps suggesting that OCB negativity represents particular phases of the disease, or individuals with a weaker tendency to B-cell activation rather than a distinct condition. The DRB1*1501 allele may be involved in this scenario, since MHC class II mediated presentation of antigens from B cells to CD4+ T cells is important for B-cell differentiation into GC B cells and plasma cells.54
Several non-MHC genes have been associated with MS susceptibility,55–57 some of which, such as CD40, CXCR4, and CXCR5, appear particularly relevant for B-cell homeostasis and function. The transmembrane protein CD40 is constitutively present on B cells. Its cognate receptor CD154 is expressed in a wide range of cell types (mainly activated CD4+ and CD8+ T cells) and its binding with CD40 induces B-cell proliferation, chemokine and cytokine production, GC formation, differentiation into memory B and plasma cells and B-cell–mediated T-cell activation by upregulating MHC class II and costimulatory molecules expression.58 In addition, the B-cell attractant CXCL13 acts through binding the receptor CXCR5, which is highly expressed on B cells and to a lesser extent T cells.59 Together with CXCR4, these 2 genes are directly involved in GC development and their finely regulated expression in centroblasts and centrocytes is critical for correct GC organization.60
The role of environment.
The environment also exerts a significant influence on MS susceptibility.e1 A wealth of studies strongly support vitamin D deficiency as a key factor for MS. The prevalence of MS correlates with latitude and UV radiatione2,e3 and both vitamin D intake and low vitamin D levels are inversely associated with risk of MS.e4,e5 Possibly the strongest evidence for a role for vitamin D is the association of 2 genes involved in vitamin D biology with MS.57
As in other immune cell types, vitamin D influences development and functionality of B cells.e6 This pleiotropic hormone plays an important role in B-cell homeostasis and function by decreasing cell proliferation, inducing apoptosis, and inhibiting plasma cell differentiation.e7
Using chromatin immunoprecipitation followed by massively parallel DNA sequencing (ChIP-seq) in B-cell lines, our group has shown the presence of 2,776 different vitamin D responsive elements (VDREs) through the entire genome bound by the vitamin D receptor (VDR). Genetic loci associated with MS are strikingly enriched for VDR binding sites. In other words, vitamin D regulates the expression of genes which are associated with MS susceptibility in B cells including HLA-DRB1, CD40, CXCR4, and CXCR5.e8
The Epstein-Barr virus (EBV) may also be involved in the pathophysiology of MS. Although a great majority (more than 90%) of the general population appears to encounter EBV at some point during life, nearly all patients with MS (>99.5%) have been infected with EBV.2 The risk of MS is increased in individuals with either high anti-EBV antibody titers or a history of infectious mononucleosis (IM).2e9 These observations do not arise from a shared genetic susceptibility since the HLA-DRB1*1501 class II allele is not associated with IM.e10
This is relevant as EBV is a DNA human γ herpesvirus which primarily infects B cells and is able to immortalize them in vitro and induce lymphoproliferative disorders in vivo.e11 Different proteins encoded by EBV, in particular members of the Epstein-Barr nuclear antigen (EBNA) and latent membrane protein (LMP) families, influence the expression of a number of genes involved in cell adhesion or signaling, transcription, RNA processing, immune processes, and cell-cycle regulation.e11-e14
Interestingly, a common thread seems to link EBV and vitamin D B-cell gene regulation pathways. One way EBV influences gene expression is by an EBNA-3 mediated blockage of the VDR.e15 As an exploratory analysis, we used the expression profiles of lymphoblastoid cell lines (LCLs) obtained by infecting primary B cells with an EBV mutant strain lacking the EBNA-3 genee13 and our VDR ChIP-Seq mape8 to explore to what extent EBNA-3 may influence the expression of vitamin D–responsive genes. Almost 30% of the genes which are regulated by EBNA-3 are characterized by the presence of a VDRE, which is much greater than expected by chance (p = 0.003). In a gene ontology analysis we found that these genes with both an EBNA-3 and VDR influence are involved in cell proliferation, apoptosis, and immune response.
Other findings supporting a link between B cells, EBV, and MS come from pathologic studies reporting the presence of markers of latent EBV infection in a very high percentage of brain-infiltrating B and plasma cells in nearly all MS samples examined. The persistence of EBV was particularly enriched in meningeal B-cell follicles where viral reactivation (as defined by the presence of the early lytic protein BFRF1) was also observed.e16 By using laser microdissection and preamplifying EBV transcripts, subsequent experiments significantly increased the sensitivity of EBV detection.e17 EBV-positive B cells were also found to express the B-cell activating factor, which previous studies had shown to be upregulated by EBV proteins in B-cell lines and overexpressed in MS brain.e17-e19
However, regardless of how attractive the underlying biological rationale of the EBV presence in the CNS of patients with MS is, caution is needed since other groups have not been able to replicate these findings.e20-e25 For example, Sargsyan et al.e25 did not detect any EBV transcript in MS CSF B and plasma cells or in most of the actively demyelinating MS plaques analyzed. A single EBV-specific transcript (EBER-1) was found in a subset of MS plaques previously shown to be EBV DNA positive indicating the absence of EBV reactivation or abnormal latency programs.e25 Differences in the ascertainment of cases, sample preparation, and methods may be possible confounding factors,e20,e26 but replication is still needed to claim the presence and activation of EBV in MS brain as consistent features of the disease.
Another observation that needs replication is decreased CD8+ T-cell reactivity to autologous EBV-infected lymphoblastoid cell lines in patients with MS compared with healthy subjects.e27 This may predispose to the development of MS by allowing the accumulation of EBV-infected B cells in the MS brain. However, these data are contrary to other findings of higher EBV-specific CD8+ T-cell response in the blood of patients with CIS and normal response in patients with MS.e28
B-CELL DEPLETION IN MS: INSIGHT INTO BIOLOGICAL MECHANISMS
The development of monoclonal antibodies has represented one of the most significant changes in MS therapy. To date, several different molecules have been tested and the therapeutic efficacy of many of them promises to be much greater than currently available treatments.e29 Although the antigens targeted by such molecules are known, the identification of the mechanism underlying their therapeutic action is unclear since generally these antibodies target a wide range of immune cells.
For example, the monoclonal antibody natalizumab acts by blocking the α4 subunit of the VLA-4 receptor, which is needed for most leukocyte migration across the blood–brain barrier. Similarly, the impressively effective alemtuzumab causes complete depletion of all cells expressing the CD52 molecule which comprises T, B, natural killer and dendritic cells, most monocytes, and macrophages.e29
Interestingly, the only cell-specific antibody which has proved to be highly efficient in RRMS, rituximab, acts on the B-cell compartment, particularly by depleting the B cells positive for the CD20 antigen.e30 This transmembrane protein is expressed in different stages of B-cell differentiation, from pre-B cells to naive and memory B cells, but is absent in earlier stages (pro-B) and plasma cells.e30,e31 Clinical trials in RRMS have shown that rituximab causes a nearly complete depletion of CD20+ B cells and reduces the number of new Gd-enhancing lesions and the proportion of patients experiencing relapses.e32-e34 Similar results have been obtained in trials testing the second generation of anti-CD20 molecules (ocrelizumab and ofatumumab).e30
The fact that treatments specifically targeting B cells are effective in RRMS provides not only further support for the pathogenic role of B cells in MS but also the opportunity to elucidate how B cells may be acting. Notably, anti CD20 treatment does not target plasma cells and the influence on CSF IgG levels and OCB is minimal or absent.e35,e36 Therefore, it is unlikely that autoantibody production mediates the pathogenic role of B cells in MS.
Rather than producing autoantibodies, B cells may play a central role in influencing the T-cell response in patients with MS. As described above, B cells can function as professional antigen-presenting cells able to initiate T-cell–specific responses.e37,e38 Studies have suggested that a proportion of peripheral B cells in patients with RRMS can elicit CD4+ T-cell activation and proliferation in response to myelin antigens via direct antigen presentation.e39 However, B cells could also influence the T-cell response through cytokine and chemokine production (bystander mechanisms). These hypotheses find support in both animal and human studies of B-cell depletion.
Interestingly, the effects of B cell depletion on EAE models range from EAE prevention to exacerbation depending on the timing of anti-CD20 treatment and the peptide used to induce CNS inflammation.e40,e41 These findings result from B cells having either regulatory or proinflammatory phenotypes and influencing the T-cell response through cytokine production and antigen presentation.e40,e41
Similarly to EAE, in human MS rituximab treatment influences the interplay between B and T cells. Studies have shown that rituximab administration reduces CSF counts of both B cells and T cells (95% and 50% mean reductions, respectively).e35,e42 This decrease in T-cell counts correlated with a reduction of CXCL13 levels between pre and post treatment CSF paired samples.e42 Since CXCR5 (the receptor for CXCL13) is found on B cells and ∼20% of CD4+ T cells in blood and CSF of patients with MS59 and B cells can promote maturation of follicular dendritic cells and CXCL13 production in positive feedback fashion,e43 the authors speculated that lack of B cells may decrease the number of T cells within the CNS by reducing chemokine secretion.e42
Another hypothesis is that B-cell depletion may impair T-cell activation and trafficking across the blood–brain barrier. In line with this, Bar Or et al. have shown that CD4+ and CD8+ T cells from patients with MS exhibited reduced proliferation and IFN-γ and IL-17 production after B cell depletion as compared to pretreatment levels. Notably, these effects could be partially reversed by adding the supernatant of activated B-cell cultures obtained from untreated patients, implicating the presence of soluble factors able to activate T cells. Further experiments identified the B-cell–produced molecules lymphotoxin and tumor necrosis factor–α as relevant in mediating this mechanism.e44
Although these observations indicate that T cells may be partly mediating the pathogenic role of B cells in MS, the secretion of soluble factors other than antibodies from B cells, located in either meninges or brain parenchyma, may directly induce inflammation and consequent demyelination. Notably, these hypotheses are not mutually exclusive and multiple mechanisms are likely to take place simultaneously.
DISCUSSION
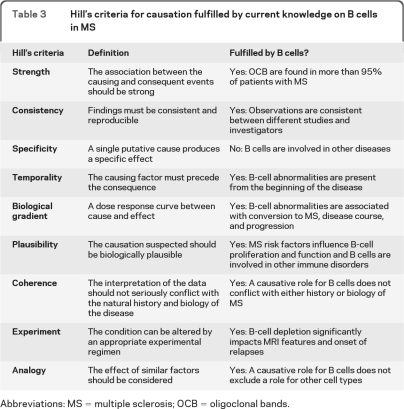
Establishing a causal relationship is not easy and requires a number of observations. In 1965, Sir Austin Bradford Hille45 outlined a number of criteria with the intent to define “what aspects of that association should we especially consider before deciding that the most likely interpretation of it is causation.”
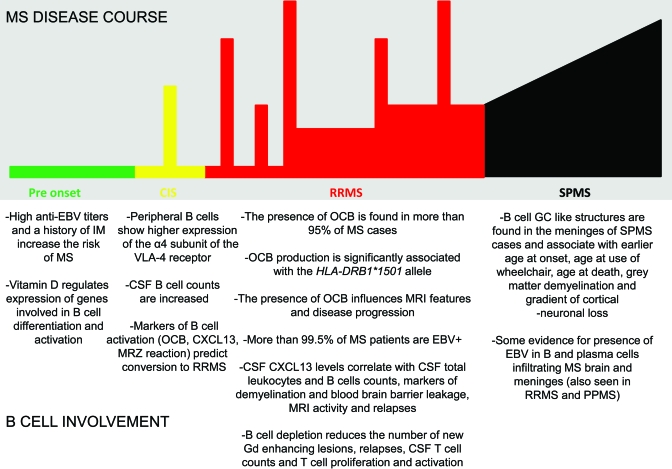
As Hill wanted to clarify, “none of these 9 viewpoints can bring indisputable evidence for or against the cause and effect hypothesis and none can be required as a sine qua non.” However, it is interesting to see how many and how well these criteria are met by B cells in MS today (figure and table 3). Strikingly, B cells satisfy 8 out of the 9 features of causation and in particular fulfill the main requirements that B-cell abnormalities are strongly associated with MS, are influenced by MS risk factors, precede its clinical onset, influence disease course, and can be modified by a specific intervention strategy.
Figure. B-cell involvement in different phases of multiple sclerosis (MS).
Environmental risk factors influence B cells prior to disease onset; B-cell abnormalities within the CNS are already present at time of clinically isolated syndrome (CIS) diagnosis and predict conversion to clinically definite MS; during relapsing-remitting MS (RRMS), markers of B-cell activation correlate with disease course and MRI activity while B-cell depletion significantly reduces MRI lesions and onset of relapses; organized B-cell structures are found in the meningeal space of patients with secondary progressive MS (SPMS) and their presence is associated with a worse outcome. EBV = Epstein-Barr virus; GC = germinal center; IM = infectious mononucleosis; OCB = oligoclonal bands.
Table 3.
Hill's criteria for causation fulfilled by current knowledge on B cells in MS
Current studies unequivocally indicate that the general view of MS as a condition mediated only by T cells is overly simplistic and that B cells influence the pathogenesis of MS. Although the understanding of MS causation will require the unification of all the pieces of a complex immunologic puzzle, particularly with regard to B–T cell interactions, these observations suggest that further elucidation of the role of B cells in MS will lead to the development of novel disease treatment and prevention strategies.
Supplementary Material
Data Supplement
GLOSSARY
CDMS
clinically definite multiple sclerosis
ChIP-seq
chromatin immunoprecipitation followed by massively parallel DNA sequencing
CIS
clinically isolated syndrome
EAE
experimental autoimmune encephalomyelitis
EBNA
Epstein-Barr nuclear antigen
EBV
Epstein-Barr virus
GC
germinal center
IL
interleukin
IM
infectious mononucleosis
LCL
lymphoblastoid cell line
LMP
latent membrane protein
MBP
myelin basic protein
MHC
major histocompatibility complex
MOG
myelin oligodendrocyte glycoprotein
MS
multiple sclerosis
OCB
oligoclonal bands
PLP
proteolipid protein
PPMS
primary progressive multiple sclerosis
RRMS
relapsing-remitting multiple sclerosis
SPMS
secondary progressive multiple sclerosis
Th
T helper cells
VDR
vitamin D receptor
VDRE
vitamin D responsive element
Footnotes
AUTHOR CONTRIBUTIONS
Study concept and design: Drs. Disanto and Ramagopalan. Drafting of the manuscript: Dr. Disanto. Critical revision of the manuscript for important intellectual content: Drs. Morahan, Barnett, Giovannoni, and Ramagopalan.
DISCLOSURE
Dr. Disanto is funded by a research fellowship FISM-Fondazione Italiana Sclerosi Multipla-Cod.: 2010/B/5. Dr. Morahan is funded by the MS Society of Australia and the UK. Dr. Barnett has served on scientific advisory boards for and received speaker honoraria from Bayer Schering Pharma, Merck Serono, Novartis, and sanofi-aventis; and received research support from Multiple Sclerosis Research Australia and the Nerve Research Foundation, The University of Sydney. Dr. Giovannoni serves on scientific advisory boards for Merck Serono and Biogen Idec and Vertex Pharmaceuticals; served on the editorial board of Multiple Sclerosis; has received speaker honoraria from Bayer Schering Pharma, Merck Serono, Biogen Idec, Pfizer Inc, Teva Pharmaceutical Industries Ltd.–sanofi-aventis, Vertex Pharmaceuticals, Genzyme Corporation, Ironwood, and Novartis; has served as a consultant for Bayer Schering Pharma, Biogen Idec, GlaxoSmithKline, Merck Serono, Protein Discovery Laboratories, Teva Pharmaceutical Industries Ltd.–sanofi-aventis, UCB, Vertex Pharmaceuticals, GW Pharma, Novartis, and FivePrime; serves on the speakers bureau for Merck Serono; and has received research support from Bayer Schering Pharma, Biogen Idec, Merck Serono, Novartis, UCB, Merz Pharmaceuticals, LLC, Teva Pharmaceutical Industries Ltd.–sanofi-aventis, GW Pharma, and Ironwood. Dr. Ramagopalan receives research support from the Multiple Sclerosis Society of Canada Scientific Research Foundation and the Multiple Sclerosis Society of the United Kingdom.
REFERENCES
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med 2000; 343: 938– 952 [DOI] [PubMed] [Google Scholar]
- 2.Ramagopalan SV, Dobson R, Meier UC, Giovannoni G. Multiple sclerosis: risk factors, prodromes, and potential causal pathways. Lancet Neurol 2010; 9: 727– 739 [DOI] [PubMed] [Google Scholar]
- 3.Lassmann H, Bruck W, Lucchinetti CF. The immunopathology of multiple sclerosis: an overview. Brain Pathol 2007; 17: 210– 218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramagopalan SV, Ebers GC. Multiple sclerosis: major histocompatibility complexity and antigen presentation. Genome Med 2009; 1: 105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol 2009; 9: 833– 844 [DOI] [PubMed] [Google Scholar]
- 6.Kasper LH, Shoemaker J. Multiple sclerosis immunology: the healthy immune system vs the MS immune system. Neurology 2010; 74 (suppl 1): S2– S8 [DOI] [PubMed] [Google Scholar]
- 7.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 2004; 55: 458– 468 [DOI] [PubMed] [Google Scholar]
- 8.Henderson AP, Barnett MH, Parratt JD, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol 2009; 66: 739– 753 [DOI] [PubMed] [Google Scholar]
- 9.Babbe H, Roers A, Waisman A, et al. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med 2000; 192: 393– 404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gay FW, Drye TJ, Dick GW, Esiri MM. The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis. Identification and characterization of the primary demyelinating lesion. Brain 1997; 120: 1461– 1483 [DOI] [PubMed] [Google Scholar]
- 11.Segal BM, Constantinescu CS, Raychaudhuri A, Kim L, Fidelus-Gort R, Kasper LH. Repeated subcutaneous injections of IL12/23 p40 neutralising antibody, ustekinumab, in patients with relapsing-remitting multiple sclerosis: a phase II, double-blind, placebo-controlled, randomised, dose-ranging study. Lancet Neurol 2008; 7: 796– 804 [DOI] [PubMed] [Google Scholar]
- 12.van Oosten BW, Lai M, Hodgkinson S, et al. Treatment of multiple sclerosis with the monoclonal anti-CD4 antibody cM-T412: results of a randomized, double-blind, placebo-controlled, MR-monitored phase II trial Neurology 1997; 49: 351– 357 [DOI] [PubMed] [Google Scholar]
- 13.Brok HP, van Meurs M, Blezer E, et al. Prevention of experimental autoimmune encephalomyelitis in common marmosets using an anti-IL-12p40 monoclonal antibody. J Immunol 2002; 169: 6554– 6563 [DOI] [PubMed] [Google Scholar]
- 14.Waldor MK, Sriram S, Hardy R, et al. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science 1985; 227: 415– 417 [DOI] [PubMed] [Google Scholar]
- 15.Handel AE, Lincoln MR, Ramagopalan SV. Of mice and men: experimental autoimmune encephalitis and multiple sclerosis. Eur J Clin Invest Epub 2011 . [DOI] [PubMed] [Google Scholar]
- 16.Freedman MS, Thompson EJ, Deisenhammer F, et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: a consensus statement. Arch Neurol 2005; 62: 865– 870 [DOI] [PubMed] [Google Scholar]
- 17.Owens GP, Ritchie AM, Burgoon MP, Williamson RA, Corboy JR, Gilden DH. Single-cell repertoire analysis demonstrates that clonal expansion is a prominent feature of the B cell response in multiple sclerosis cerebrospinal fluid. J Immunol 2003; 171: 2725– 2733 [DOI] [PubMed] [Google Scholar]
- 18.Lovato L, Willis SN, Rodig SJ, et al. Related B cell clones populate the meninges and parenchyma of patients with multiple sclerosis. Brain 2011; 134: 534– 541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obermeier B, Lovato L, Mentele R, et al. Related B cell clones that populate the CSF and CNS of patients with multiple sclerosis produce CSF immunoglobulin. J Neuroimmunol 2011; 233: 245– 248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prineas JW. Multiple sclerosis: presence of lymphatic capillaries and lymphoid tissue in the brain and spinal cord. Science 1979; 203: 1123– 1125 [DOI] [PubMed] [Google Scholar]
- 21.Breij EC, Brink BP, Veerhuis R, et al. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol 2008; 63: 16– 25 [DOI] [PubMed] [Google Scholar]
- 22.Weber MS, Hemmer B, Cepok S. The role of antibodies in multiple sclerosis. Biochim Biophys Acta 2011; 1812: 239– 245 [DOI] [PubMed] [Google Scholar]
- 23.von Budingen HC, Harrer MD, Kuenzle S, Meier M, Goebels N. Clonally expanded plasma cells in the cerebrospinal fluid of MS patients produce myelin-specific antibodies. Eur J Immunol 2008; 38: 2014– 2023 [DOI] [PubMed] [Google Scholar]
- 24.Owens GP, Bennett JL, Lassmann H, et al. Antibodies produced by clonally expanded plasma cells in multiple sclerosis cerebrospinal fluid. Ann Neurol 2009; 65: 639– 649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisniku LK, Brex PA, Altmann DR, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain 2008; 131: 808– 817 [DOI] [PubMed] [Google Scholar]
- 26.Lee-Chang C, Zephir H, Top I, et al. B-cell subsets up-regulate alpha4 integrin and accumulate in the cerebrospinal fluid in clinically isolated syndrome suggestive of multiple sclerosis onset. Neurosci Lett 2011; 487: 273– 277 [DOI] [PubMed] [Google Scholar]
- 27.Masjuan J, Alvarez-Cermeno JC, Garcia-Barragan N, et al. Clinically isolated syndromes: a new oligoclonal band test accurately predicts conversion to MS. Neurology 2006; 66: 576– 578 [DOI] [PubMed] [Google Scholar]
- 28.Tintore M, Rovira A, Rio J, et al. Do oligoclonal bands add information to MRI in first attacks of multiple sclerosis? Neurology 2008; 70: 1079– 1083 [DOI] [PubMed] [Google Scholar]
- 29.Ignacio RJ, Liliana P, Edgardo C. Oligoclonal bands and MRI in clinically isolated syndromes: predicting conversion time to multiple sclerosis. J Neurol 2010; 257: 1188– 1191 [DOI] [PubMed] [Google Scholar]
- 30.Skov AG, Skov T, Frederiksen JL. Oligoclonal bands predict multiple sclerosis after optic neuritis: a literature survey. Mult Scler 2011; 17: 404– 410 [DOI] [PubMed] [Google Scholar]
- 31.Brettschneider J, Tumani H, Kiechle U, et al. IgG antibodies against measles, rubella, and varicella zoster virus predict conversion to multiple sclerosis in clinically isolated syndrome. PLoS One 2009; 4: e7638 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brettschneider J, Czerwoniak A, Senel M, et al. The chemokine CXCL13 is a prognostic marker in clinically isolated syndrome (CIS). PLoS One 2010; 5: e11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khademi M, Kockum I, Andersson ML, et al. Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler 2011; 17: 335– 343 [DOI] [PubMed] [Google Scholar]
- 34.Owens GP, Kraus H, Burgoon MP, Smith-Jensen T, Devlin ME, Gilden DH. Restricted use of VH4 germline segments in an acute multiple sclerosis brain. Ann Neurol 1998; 43: 236– 243 [DOI] [PubMed] [Google Scholar]
- 35.Owens GP, Winges KM, Ritchie AM, et al. VH4 gene segments dominate the intrathecal humoral immune response in multiple sclerosis. J Immunol 2007; 179: 6343– 6351 [DOI] [PubMed] [Google Scholar]
- 36.Bennett JL, Haubold K, Ritchie AM, et al. CSF IgG heavy-chain bias in patients at the time of a clinically isolated syndrome. J Neuroimmunol 2008; 199: 126– 132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berger T, Rubner P, Schautzer F, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med 2003; 349: 139– 145 [DOI] [PubMed] [Google Scholar]
- 38.Kuhle J, Pohl C, Mehling M, et al. Lack of association between antimyelin antibodies and progression to multiple sclerosis. N Engl J Med 2007; 356: 371– 378 [DOI] [PubMed] [Google Scholar]
- 39.Sellebjerg F, Bornsen L, Khademi M, et al. Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology 2009; 73: 2003– 2010 [DOI] [PubMed] [Google Scholar]
- 40.Kalinowska-Lyszczarz A, Szczucinski A, Pawlak MA, Losy J. Clinical study on CXCL13, CCL17, CCL20 and IL-17 as immune cell migration navigators in relapsing-remitting multiple sclerosis patients J Neurol Sci 2011; 300: 81– 85 [DOI] [PubMed] [Google Scholar]
- 41.Festa ED, Hankiewicz K, Kim S, et al. Serum levels of CXCL13 are elevated in active multiple sclerosis. Mult Scler 2009; 15: 1271– 1279 [DOI] [PubMed] [Google Scholar]
- 42.Kuenz B, Lutterotti A, Ehling R, et al. Cerebrospinal fluid B cells correlate with early brain inflammation in multiple sclerosis. PLoS One 2008; 3: e2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joseph FG, Hirst CL, Pickersgill TP, Ben-Shlomo Y, Robertson NP, Scolding NJ. CSF oligoclonal band status informs prognosis in multiple sclerosis: a case control study of 100 patients. J Neurol Neurosurg Psychiatry 2009; 80: 292– 296 [DOI] [PubMed] [Google Scholar]
- 44.Imrell K, Greiner E, Hillert J, Masterman T. HLA-DRB115 and cerebrospinal-fluid-specific oligoclonal immunoglobulin G bands lower age at attainment of important disease milestones in multiple sclerosis. J Neuroimmunol 2009; 210: 128– 130 [DOI] [PubMed] [Google Scholar]
- 45.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Aloisi F. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 2004; 14: 164– 174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007; 130: 1089– 1104 [DOI] [PubMed] [Google Scholar]
- 47.Howell OW, Reeves CA, Nicholas R, et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain Epub 2011 . [DOI] [PubMed] [Google Scholar]
- 48.Magliozzi R, Howell OW, Reeves C, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol 2010; 68: 477– 493 [DOI] [PubMed] [Google Scholar]
- 49.Dempfle A, Scherag A, Hein R, Beckmann L, Chang-Claude J, Schafer H. Gene-environment interactions for complex traits: definitions, methodological requirements and challenges. Eur J Hum Genet 2008; 16: 1164– 1172 [DOI] [PubMed] [Google Scholar]
- 50.Imrell K, Landtblom AM, Hillert J, Masterman T. Multiple sclerosis with and without CSF bands: clinically indistinguishable but immunogenetically distinct. Neurology 2006; 67: 1062– 1064 [DOI] [PubMed] [Google Scholar]
- Romero-Pinel L, Martinez-Yelamos S, Bau L, et al. Association of HLA-DRB1*15 allele and CSF oligoclonal bands in a Spanish multiple sclerosis cohort. Eur J Neurol 2011. . [DOI] [PubMed] [Google Scholar]
- 52.Wu JS, Qiu W, Castley A, et al. Presence of CSF oligoclonal bands (OCB) is associated with the HLA-DRB1 genotype in a West Australian multiple sclerosis cohort. J Neurol Sci 2010; 288: 63– 67 [DOI] [PubMed] [Google Scholar]
- 53.Zeman AZ, Kidd D, McLean BN, et al. A study of oligoclonal band negative multiple sclerosis. J Neurol Neurosurg Psychiatry 1996; 60: 27– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shimoda M, Li T, Pihkala JP, Koni PA. Role of MHC class II on memory B cells in post-germinal center B cell homeostasis and memory response. J Immunol 2006; 176: 2122– 2133 [DOI] [PubMed] [Google Scholar]
- 55.ANZgene Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet 2009; 41: 824– 828 [DOI] [PubMed] [Google Scholar]
- 56.De Jager PL, Jia X, Wang J, et al. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet 2009; 41: 776– 782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sawcer S, Hellenthal G, Pirinen M, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 2011; 476: 214– 219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graham JP, Arcipowski KM, Bishop GA. Differential B-lymphocyte regulation by CD40 and its viral mimic, latent membrane protein 1. Immunol Rev 2010; 237: 226– 248 [DOI] [PubMed] [Google Scholar]
- 59.Krumbholz M, Theil D, Cepok S, et al. Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 2006; 129: 200– 211 [DOI] [PubMed] [Google Scholar]
- 60.Allen CD, Ansel KM, Low C, et al. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol 2004; 5: 943– 952 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement