Discovery and prioritization of somatic mutations in diffuse large B-cell lymphoma (DLBCL) by whole-exome sequencing (original) (raw)
Abstract
To gain insight into the genomic basis of diffuse large B-cell lymphoma (DLBCL), we performed massively parallel whole-exome sequencing of 55 primary tumor samples from patients with DLBCL and matched normal tissue. We identified recurrent mutations in genes that are well known to be functionally relevant in DLBCL, including MYD88, CARD11, EZH2, and CREBBP. We also identified somatic mutations in genes for which a functional role in DLBCL has not been previously suspected. These genes include MEF2B, MLL2, BTG1, GNA13, ACTB, P2RY8, PCLO, and TNFRSF14. Further, we show that BCL2 mutations commonly occur in patients with BCL2/IgH rearrangements as a result of somatic hypermutation normally occurring at the IgH locus. The BCL2 point mutations are primarily synonymous, and likely caused by activation-induced cytidine deaminase–mediated somatic hypermutation, as shown by comprehensive analysis of enrichment of mutations in WRCY target motifs. Those nonsynonymous mutations that are observed tend to be found outside of the functionally important BH domains of the protein, suggesting that strong negative selection against BCL2 loss-of-function mutations is at play. Last, by using an algorithm designed to identify likely functionally relevant but infrequent mutations, we identify KRAS, BRAF, and NOTCH1 as likely drivers of DLBCL pathogenesis in some patients. Our data provide an unbiased view of the landscape of mutations in DLBCL, and this in turn may point toward new therapeutic strategies for the disease.
Keywords: next-generation sequencing, human genetics, activation-induced deaminase
Diffuse large B-cell lymphoma (DLBCL) is an aggressive non-Hodgkin lymphoma that affects 30,000 new patients in the United States every year (1, 2). The standard of care for the treatment of most cases of DLBCL is the R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) consisting of multiagent chemotherapy plus a therapeutic antibody directed against CD20, a marker of B lymphocytes. The 3-year event-free survival rate is approximately 60%, with the majority of the remaining 40% dying of their disease (3). To date, treatment strategies to improve outcome have largely included increased doses of standard agents in the context of autologous stem cell transplantation (4). Therefore, there is a great medical need to define the genetic abnormalities that are associated with DLBCL to define novel targets for therapy.
Germinal centers (GCs) in lymphoid tissues are sites of clonal expansion and editing of the Ig receptor in B lymphocytes, and this GC reaction is a physiological component of the humoral immune response. Somatic hypermutation (SHM) is part of the GC reaction, and its dysregulation contributes to the accumulation of somatic mutations in oncogenes and tumor-suppressor genes in B lymphocytes.
Traditionally, DLBCL has been classified by the morphology and immunophenotype of the malignant B-cells but more recently, molecular classifications have been reported. Specifically, gene expression-based classification of DLBCL has been proposed (5, 6), and the prognostic relevance for this has been demonstrated (7). It has been suggested that distinct signal transduction pathways are affected in the subtypes that are defined in this way, and that certain genetic defects preferentially occur in specific subtypes defined by the presumed cell of origin of the tumors (8–12).
However, comprehensive understanding of the genomic landscape of DLBCL is lacking. In particular, a key question is which mutations and pathways drive DLBCL pathogenesis. We report here the unbiased sequencing of all protein-coding exons in 55 DLBCL patients, comparing each to its patient-matched normal control. We uncover mutations that provide insights into mechanisms of lymphomagenesis.
Results
Whole-Exome Sequencing Reveals Recurrent Mutations in DLBCL.
We performed solution-phase hybrid capture and whole-exome sequencing on paired tumor and germline (i.e., normal) DNA samples from 55 patients with primary DLBCL. We achieved 150-fold mean sequence coverage of targeted exonic regions, with an average of 97% of bases covered per patient (range, 91–98%). Such high coverage is important because tumor samples are often contaminated with normal cells (e.g., fibroblasts, immune cells), which can obscure the identification of somatically mutated alleles unless very deep coverage is achieved.
We excluded six samples from further analysis because of extremely low apparent mutation rates, most consistent with extensive stromal contamination. Of the remaining 49 patients, the mean nonsynonymous mutation rate was 3.2 mutations per megabase, with mutation rates varying widely (0.6–8.7 mutations per megabase), which is higher than the estimated mutation rate in other hematopoietic malignancies, such as chronic lymphocytic leukemia and other leukemias (<1 per megabase) (13, 14), and multiple myeloma (1.3 per megabase) (15) (Fig. 1_A_). There was no correlation between patient-specific mutation rate and frequency of particular types of mutation (e.g., *CpG→T, Cp(A/C/T)→T, A→G, transversions, or indels; Fig. 1_C_). Similarly, there was no correlation between observed mutation rate and average allelic fraction of those mutations observed in each patient, suggesting that the variation in mutation rate was not simply a function of variability in extent of stromal contamination (Fig. S1).
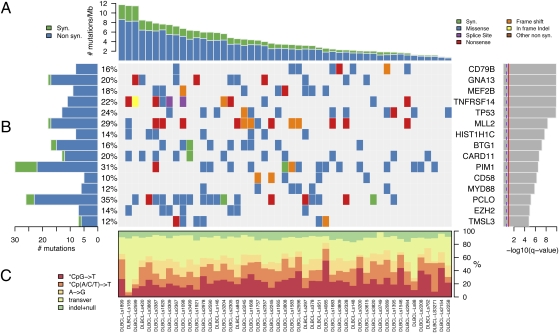
Fig. 1.
Significantly mutated genes in 49 patients with DLBCL. (A) The rate of synonymous and nonsynonymous mutations is displayed as mutations per megabase, with individual DLBCL samples ranked by total number of mutations. (B) The heat map represents individual mutations in 49 patient samples, color-coded by type of mutation. Only one mutation per gene is shown if multiple mutations were found in a sample. Left: Histogram shows the number of mutations in each gene. Percentages represent the fraction of tumors with at least one mutation in the specified gene. Right: The 15 genes with the lowest _q_1-value, ranked by level of significance. (C) Base substitution distribution of individual samples, ranked in the same order as in A.
To define significantly mutated genes in DLBCL, we applied the MutSig algorithm to identify genes harboring mutations at a higher frequency than expected by chance (15, 16). To better estimate the significance of observed mutations, MutSig takes into account (i) the sample-specific mutation rate, (ii) the ratio of nonsynonymous to synonymous mutations in a given gene, and (iii) the median expression level of each gene in DLBCL [based on gene expression profiling datasets (7)]. This approach revealed 58 statistically significant genes with a false discovery rate cutoff of 0.1 (_q_1 ≤ 0.1; Fig. 1_B_, Table 1, and Tables S1 and S2). We independently validated selected mutations by targeted resequencing in a subset of patients and obtained 97.9% validation rate (Table S5).
Table 1.
Significantly mutated genes and genes with mutations in clusters and at conserved sites
| Genes | Mutations | Patients | Sites | Silent | q1 | q2 |
|---|---|---|---|---|---|---|
| CD79B | 8 | 8 | 5 | 0 | 1.22 × 10-10 | 0.025 |
| GNA13 | 17 | 10 | 16 | 1 | 1.22 × 10-10 | |
| MEF2B | 9 | 9 | 9 | 0 | 1.22 × 10-10 | |
| TNFRSF14 | 11 | 11 | 11 | 0 | 1.22 × 10-10 | |
| TP53 | 13 | 12 | 12 | 0 | 1.36 × 10-10 | 0.025 |
| MLL2 | 17 | 14 | 17 | 1 | 4.95 × 10-9 | |
| HIST1H1C | 8 | 7 | 6 | 0 | 1.83 × 10-8 | |
| BTG1 | 15 | 8 | 14 | 2 | 5.95 × 10-8 | |
| CARD11 | 12 | 10 | 11 | 1 | 1.92 × 10-7 | 0.035 |
| PIM1 | 22 | 15 | 19 | 8 | 2.90 × 10-7 | 0.077 |
| CD58 | 5 | 5 | 5 | 0 | 5.51 × 10-7 | |
| MYD88 | 6 | 6 | 3 | 0 | 1.35 × 10-6 | <1 × 10-6 |
| PCLO | 23 | 17 | 23 | 3 | 6.65 × 10-6 | |
| EZH2 | 7 | 7 | 4 | 0 | 0.000011 | 0.035 |
| TMSL3 | 6 | 6 | 6 | 1 | 0.000015 | |
| CD70 | 5 | 5 | 5 | 0 | 0.000018 | |
| P2RY8 | 8 | 6 | 8 | 0 | 0.000024 | |
| KRTAP5-5 | 4 | 4 | 1 | 0 | 0.000033 | 0.0089 |
| HIST1H3B | 5 | 5 | 3 | 1 | 0.000062 | |
| ACTB | 5 | 5 | 4 | 0 | 0.00022 | |
| CREBBP | 9 | 8 | 9 | 0 | 0.0004 | |
| NFKBIA | 4 | 4 | 4 | 0 | 0.00048 | |
| B2M | 5 | 5 | 4 | 1 | 0.00054 | |
| SOCS1 | 4 | 3 | 4 | 1 | 0.00078 | |
| HLA-A | 5 | 4 | 5 | 0 | 0.0024 | |
| UBE2A | 3 | 3 | 3 | 0 | 0.0032 | |
| CCND3 | 3 | 3 | 3 | 0 | 0.0048 | |
| POU2F2 | 4 | 4 | 1 | 0 | 0.0051 | 0.0037 |
| OR6K3 | 3 | 3 | 3 | 0 | 0.0093 | |
| LOC153328 | 3 | 3 | 2 | 0 | 0.0097 | |
| UNC5D | 5 | 5 | 5 | 0 | 0.0097 | |
| PASD1 | 4 | 4 | 4 | 0 | 0.0099 | |
| STAT3 | 5 | 5 | 5 | 0 | 0.011 | |
| CIITA | 7 | 5 | 7 | 0 | 0.015 | |
| SYN2 | 3 | 3 | 1 | 0 | 0.017 | 0.00037 |
| PDGFC | 3 | 3 | 3 | 0 | 0.017 | |
| TBL1XR1 | 3 | 3 | 3 | 0 | 0.02 | |
| HIST1H1E | 10 | 7 | 10 | 3 | 0.021 | |
| UNC5C | 5 | 5 | 5 | 0 | 0.021 | |
| SRPX | 3 | 3 | 2 | 1 | 0.032 | |
| PCDHB6 | 4 | 4 | 4 | 0 | 0.033 | |
| S1PR2 | 4 | 3 | 4 | 0 | 0.033 | |
| KLHL6 | 5 | 4 | 5 | 0 | 0.034 | |
| ETV6 | 3 | 3 | 3 | 0 | 0.042 | |
| SLITRK6 | 4 | 4 | 4 | 0 | 0.042 | |
| DUSP2 | 4 | 3 | 3 | 1 | 0.045 | |
| SLC38A8 | 3 | 3 | 3 | 0 | 0.048 | |
| H1FOO | 2 | 2 | 1 | 0 | 0.05 | |
| HLA-B | 3 | 3 | 3 | 0 | 0.052 | |
| CPS1 | 5 | 5 | 5 | 0 | 0.052 | |
| BCR | 7 | 5 | 7 | 1 | 0.066 | 0.041 |
| TNF | 3 | 3 | 3 | 1 | 0.066 | |
| HIST1H2AL | 2 | 2 | 2 | 0 | 0.073 | |
| HIST1H2BC | 4 | 3 | 4 | 1 | 0.081 | |
| GABRA1 | 3 | 3 | 3 | 0 | 0.094 | |
| HIST1H2BO | 2 | 2 | 2 | 0 | 0.096 | |
| LRRIQ3 | 3 | 3 | 3 | 0 | 0.1 | |
| APOBEC2 | 2 | 2 | 2 | 0 | 0.1 | |
| ERBB2IP | 3 | 1 | 3 | 0 | <1 × 10-6 | |
| STAT6 | 2 | 2 | 2 | 0 | <1 × 10-6 | |
| MEF2C | 5 | 2 | 5 | 0 | 0.0061 | |
| SGK1 | 9 | 5 | 9 | 2 | 0.0061 | |
| ADAM10 | 2 | 1 | 2 | 0 | 0.0098 | |
| PABPC1 | 3 | 3 | 2 | 0 | 0.011 | |
| KIF1B | 2 | 2 | 1 | 0 | 0.014 | |
| ATP2C2 | 2 | 1 | 2 | 0 | 0.025 | |
| RGS12 | 2 | 2 | 2 | 0 | 0.025 | |
| TTC18 | 2 | 2 | 2 | 1 | 0.025 | |
| DIAPH1 | 2 | 1 | 2 | 0 | 0.041 | |
| ZNF830 | 3 | 2 | 3 | 0 | 0.041 | |
| CALR | 2 | 1 | 2 | 0 | 0.050 | |
| NEB | 3 | 3 | 3 | 0 | 0.051 | |
| PGAP2 | 2 | 1 | 2 | 0 | 0.074 | |
| SYK | 2 | 2 | 2 | 0 | 0.082 |
Among the significantly mutated genes were those for which a functional role in the pathogenesis of DLBCL has accumulated over many years. These include CD79B, TP53, CARD11, MYD88, and EZH2 (8–11). In addition, we discovered mutations in genes for which a pathogenic role in DLBCL has been suggested recently (17, 18). These include MLL2, TNFRSF14, BTG1, MEF2B, and GNA13, which are discussed in greater detail later.
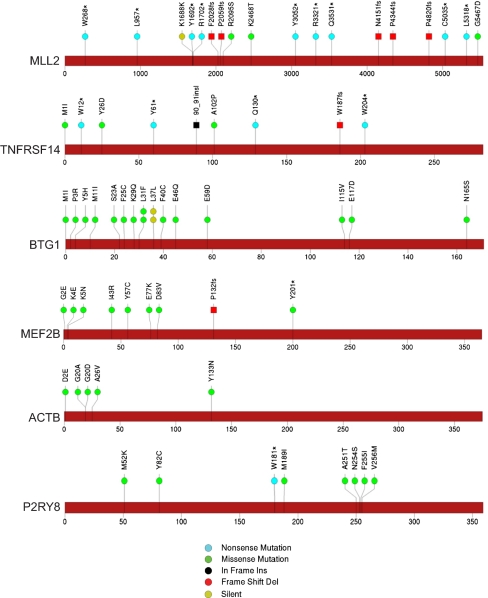
We discovered mutations in genes not previously recognized as drivers of cancer. For example, we found β-actin (ACTB) mutations in five patients (Fig. 2). Actins are highly conserved proteins of the cytoskeleton and are involved in B lymphocyte activation (19). By using the xvar algorithm (based on evolutionary conservation and the nature of the observed amino acid change), the predicted functional consequence of the ACTB mutations observed in DLBCL is high. We also note a preponderance of ACTB mutations toward the amino terminus of the protein, but the functional significance of this remains unknown.
Fig. 2.
Somatic mutations in DLBCL affect genes of various classes. Sites of somatic mutations in significantly mutated genes called by analysis pipeline and passing manual review. A diagram of the relative positions of somatic mutations is shown for MLL2, TNFRSF14, BTG1, MEF2B, ACTB, and P2RY8. The type of the mutation is indicated in the key (Bottom). The overall validation rate of mutation calls was 97.9% [of 47 selected mutations tested, only one (ACTB G20A) failed to validate; see Table S5].
Another unexpectedly recurrently mutated gene is P2RY8, encoding a G protein-coupled purinergic receptor whose normal function has not been extensively characterized (20). P2RY8 is most notable for its involvement in a chromosomal translocation with CRLF2 in 7% of patients with B-progenitor acute lymphoblastic leukemia (ALL) and 53% of individuals with ALL and Down syndrome (21). The presumed mechanism of action of such P2RY8/CRLF2 fusions is activation of CRLF2 by its coming under the control of the P2RY8 promoter, which is highly active in B lymphoid cells (21). However, we note that P2RY8 has itself been reported to function as an oncogene in experimental models (22). In DLBCL, we identified six patients with coding mutations in P2RY8, two of whom harbored two mutations (Fig. 2). In three patients, the observed allelic fraction of these mutations is greater than 0.5, suggestive of deletion of the WT allele or amplification of the mutant allele. The functional consequence of P2RY8 mutation in DLBCL remains to be determined.
We also observed very common mutations in the gene PCLO (Piccolo), encoding a protein that functions as part of the presynaptic cytoskeletal matrix thought to be involved in regulating neurotransmitter release (23). A role for PCLO in calcium sensing has also been suggested (24), but a role in cancer has not been reported. We found a total of 23 nonsynonymous mutations in 17 patients (35%; Fig. S2), but the observed ratio of nonsynonymous to synonymous mutations (23:3) is consistent with that expected by chance, given that most of the observed mutations (12 of 23) are transversion mutations, which favor nonsynonymous outcomes by a ratio of nearly 5:1. It is thus possible that, for unknown reasons, the local rate of mutation at the PCLO locus is unusually high, giving rise to passenger mutations of no functional consequence in DLBCL. Additional work is clearly needed to resolve the role, if any, of PCLO mutations in DLBCL and other cancers.
Histone 1 (H1) family proteins are linker histone proteins that bind to the DNA entering and exiting the nucleosomal cores. Different forms are expressed at different stages of the cell cycle in different tissue types and are involved in chromatin compaction and possibly transcription (25). We observed a striking accumulation of mutations in H1 family proteins, with 59 nonsynonymous and 35 synonymous mutations among 31 histone H1 proteins in 34 patients (69%; Table S2). The functional significance of these mutations remains to be explored, but hotspot analysis as outlined later suggests that HIST1H3B and possibly other core histone proteins are subject to activation-induced cytidine deaminase (AID)–mediated SHM.
We also identified mutations in genes that were recently reported to be significantly mutated (17, 18). MLL2 is a histone methyltransferase of the SET1 family that is responsible for histone H3-lysine 4 trimethylation (H3K4me3) during oogenesis and early development (26). Inactivating mutations have been reported in medulloblastoma (27) and multiple myeloma (15), and chromosomal translocations involving the MLL family member MLL1 are well described in acute leukemias (28). The MLL2 mutations we observed in DLBCL are highly biased toward truncating events, the large majority being nonsense mutations and frameshift-inducing insertions and deletions (Fig. 2). As has been suggested previously, our data suggest that MLL2 may function as an important tumor suppressor in DLBCL (17, 29).
TNFRSF14, also known as LIGHT Receptor, belongs to the TNF-receptor superfamily most extensively studied in T cells. Interestingly, it can convey opposing signals based on its specificity for diverse ligands. In our data, five of nine mutations suggest loss of function (n = 4 nonsense mutations and n = 1 frameshift deletion), with an additional in-frame insertion and three missense mutations. No synonymous mutations were seen (Fig. 2). As has been suggested, these results strongly suggest a tumor-suppressive role of TNFRSF14 in DLBCL (17). It has been proposed that LIGHT-mediated triggering of TNFRSF14 renders B-cell lymphomas more immunogenic and sensitive to FAS-induced apoptosis (30)—a potential mechanism by which TNFRSF14 can act as a tumor suppressor.
Mutations in BTG1 were also observed to be relatively common (15 nonsynonymous and two synonymous mutations). BTG1 belongs to the BTG/Tob family of proteins that regulate cell cycle progression in a variety of cells. BTG1 is thought to confer DNA binding of sequence-specific transcription factors (31). Whether mutations in BTG1 result in aberrations in chromatin structure (as with MLL-family mutations) as opposed to nonhistone targets remains to be determined. Interestingly, we observed several patients with more than one mutation in BTG1, including two patients with three mutations and one patient with four mutations. In the latter patient, two sets of adjacent mutations (L37L plus L31F and P3R plus M1I) were never found in the same sequencing read, suggesting that they occurred on different alleles, i.e., in trans. We note that two patients had the identical silent mutation at codon 31, suggesting that this mutation, although it did not affect protein coding sequence, may alter codon use or mRNA stability. Overall, the nature of BTG1 mutations does not clearly point toward a gain-of-function or loss-of-function mechanism, although the biallelic involvement seen in some patients tends to favor loss of function (Fig. 2).
MEF2B mutations were observed in 18% of patients with DLBCL, similar to data reported recently (17, 18), predominantly in the MADS box or MEF2 domains. MEF2B belongs to a family of calcium-regulated transcription factors that recruit histone-modifying enzymes. Further supporting an oncogenic role for MEF2 proteins, MEF2C has been identified as a T-cell acute lymphoblastic leukemia oncogene that is activated by chromosomal rearrangement (32).
Negative Selection Against Deleterious BCL2 Mutations.
A hallmark of cancer genes (i.e., genes that harbor “driver” mutations, which contribute to the formation and progression of cancer) is the preponderance of nonsynonymous mutations compared with synonymous mutations (typically with an expected ratio of ∼2.8:1). Curiously, we observed a striking opposite effect in the BCL2 gene—a known driver in some DLBCLs. We observed a very high mutation rate in BCL2, but with a depletion of nonsynonymous mutations, with 18 nonsynonymous mutations and 28 synonymous mutations, for a ratio of 0.64, far below that expected by chance (P = 8.8 × 10−6; Fig. 3). We hypothesized that this phenomenon might be caused by two processes: first, a locally high mutation rate at the BCL2 locus occurring via SHM, and second, negative selection against functionally deleterious mutations.
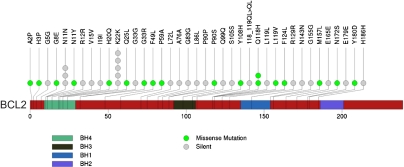
Fig. 3.
Selection for functionally inconsequential mutations in BCL2 and sites of somatic mutations in BCL2. Nonsynonymous mutations in BCL2 are preferentially located outside of BH-domains.
We first explored the possibility that the high rate of BCL2 mutations observed in our patients might be related to SHM. This physiologic process in B lymphocytes has been best studied at Ig gene loci, where it facilitates the development of antibody diversity (33), but other genes have been shown to be subject to aberrant SHM (including the PIM1 gene, in which we also observed hypermutation with 30 mutations in 16 patients; Fig. S2) (34). Chromosomal translocations of the BCL2 gene into the Ig heavy chain (IgH) locus occur in approximately 20% of DLBCL tumors, and the resulting BCL2 overexpression provides an antiapoptotic signal to the lymphoma cells (35). We hypothesized that BCL2 hypermutation in our patients might be explained by the BCL2 gene adopting the IgH locus's normal process of SHM as a result of the translocation (36). If this hypothesis were correct, those tumors with elevated BCL2 mutation rates would be expected to also harbor BCL2/IgH translocations. We tested for BCL2/IgH translocation in 26 patients with DLBCL (13 with BCL2 mutation and 13 others selected randomly). As predicted, the vast majority of patients with BCL2 hypermutation (10 of 13, 77%) had BCL2/IgH translocation, whereas only one of 13 patients (8%) lacking BCL2 mutation had the translocation (P = 0.0005, one-sided Fisher exact test; Fig. S3). To determine whether BCL2 or other genes may be targets of SHM, which is mediated by AID, we asked whether mutations occur preferentially in the context of WRCY motifs, which are known target sequences of AID. This analysis indeed revealed that BCL2, as well as other genes, including PIM1, have significant enrichment of mutations in WRCY motifs (Table S3).
Although these results likely explain the hypermutation at the BCL2 locus, they do not explain the preponderance of synonymous mutations observed in these patients. We hypothesized that they represent a vestige of negative selection against deleterious mutations in BCL2, on which the tumor cells are dependent for survival. If this hypothesis were correct, we would expect that any nonsynonymous mutations would be confined to functionally nonessential domains of the protein, whereas the synonymous mutations would not. To address this, we focused on the BCL2 BH domains that mediate interactions with proapoptotic proteins (37). We observed 18 nonsynonymous mutations, but only three of these fell within BH domains, whereas 15 of 28 synonymous mutations fell within BH domains (Fig. 3). We used permutations to determine the probability of nonsynonymous mutations being located within versus outside BH domains, corrected for domain size. To increase the power of this analysis, we included the BCL2 mutations observed in the present study and those recently reported (17). This analysis indicated a significant enrichment of nonsynonymous mutations falling outside of BH domains (P = 0.041). These results argue that the nonsynonymous mutations preserve BCL2 function by avoiding critical BH domains. On the contrary, we found the synonymous mutations to be preferentially located within BH domains (P = 0.02). This can be explained by nonuniform distribution of these mutations along the gene (P = 0.045) with preferential clustering at the 5′ end. This is consistent with previous demonstrations of aberrant SHM preferentially occurring closer to the 5′ end (34).
Our results are most consistent with purifying selection (i.e., negative selection) against mutations that inhibit or decrease BCL2 function. Such mutations likely result in loss of or impaired tumor cell viability, leaving behind only those mutations that are synonymous or that affect nonrelevant domains. Interestingly, we note the presence of recurrent synonymous mutations at codons N11 and K22 (Fig. 3), suggesting a preference for certain sites to acquire mutations. Whether there is a functional consequence of these synonymous mutations, for example by affecting mRNA stability, remains to be determined.
Identifying Functionally Relevant but Rare Mutations.
The preceding sections focused on the identification of cancer genes based on their mutation frequency across the whole genome. However, additional genes may harbor functionally important mutations that fall short of statistical significance (in the standard mutation frequency test) given the modest size of our dataset. Some of these genes are recognizable based on their known importance in the pathogenesis of other malignancies. For example, we observed two mutations in KRAS (G13D), four mutations in NOTCH1, and two mutations in BRAF, often involving specific amino acids documented to be sites of recurrent mutation in the Catalogue of Somatic Mutations in Cancer (COSMIC) database. It therefore seems likely that these mutations similarly play a causal role in DLBCL, albeit at a low frequency. To perform this search in a rigorous manner, we also tested each gene whether it has an increased mutation rate only in sites previously reported in the COSMIC database. Table S4 shows a list of the most significant genes identified by this test.
We also sought to prioritize rare mutations based on the clustering of mutations within particular regions of the coding sequence. Mutations occurring by chance (i.e., passenger mutations) would be expected to be scattered randomly across the protein, whereas functionally consequential mutations may cluster within critical domains. First, we assumed that mutations have a higher likelihood of functional impact if they are found at the same site or in close proximity to each other. For example, highly clustered mutations are observed in MYD88, CARD11, CD79B, and EZH2 (Fig. S4). Second, we assumed that mutated genes are more likely to be functionally relevant if the affected amino acid residue is more conserved across species. Our algorithm thus returns a list of mutated genes that is rank-ordered by a composite score reflecting sequence conservation and clustering of mutation sites (Table 1). This analysis prioritized genes known to be mutated in DLBCL and to play a role in its pathogenesis and also novel genes that would not otherwise be considered as likely drivers. For example, the tyrosine kinase SYK was identified as a likely driver based on these criteria, and it has recently been demonstrated that SYK inhibitors have significant clinical activity in non-Hodgkin lymphoma, including DLBCL (38, 39). Similarly, the serine/threonine kinase SGK1 was identified by this analysis despite its not reaching statistical significance based on frequency alone (_q_1 > 0.1), but a recent study confirms the recurrent nature of SGK1 mutation in DLBCL (17).
Discussion
We performed whole-exome sequencing on tumor samples and matched normal samples from 55 patients with DLBCL to identify the spectrum of mutations associated with the disease. This analysis provides a rich description of the DLBCL genome and forms the basis for future discovery and therapeutic target identification. In this single study, we rediscovered the genes previously discovered to be important drivers of DLBCL, and identified candidates deserving of functional follow-up.
Near the completion of our study, two groups reported their analysis of the DLBCL genome (17, 18). Remarkably, the recurrently mutated genes identified in these studies are highly overlapping [of our 58 significantly mutated genes, 20 were also reported as frequently mutated by Morin et al. (17), and 14 were reported by Pasqualucci et al. (18)]. Such overlap provides strong evidence that the mutations identified by our significance thresholds are indeed recurrent in DLBCL. The fact that these studies identified largely the same genes at similar frequency suggests that these are the most common targets of somatic mutation in DLBCL. Although our results and interpretation are largely concordant with those recently published, we differ in our interpretation of the frequent mutations seen in the BCL2 gene. Whereas Morin et al. (17) suggest that these are likely indicative of positive selection for nonsynonymous variants, we suggest the mutations are in fact passenger mutations and observe a depletion of damaging mutations in BCL2. Thus, whereas BCL2/IgH translocation is likely indeed a driver of DLBCL, it also increases the overall mutation rate in BCL2 (via AID), and we believe that the resulting point mutations are likely noncontributory to the pathogenesis of the disease. However, it is interesting to speculate that the striking recurrence of silent mutations at two distinct residues may indicate a selective advantage that is conferred by silent mutations.
More generally, a unique feature of B-cell malignancies is the role of SHM in increasing mutation frequency. Under normal conditions, such SHM promotes affinity maturation of antibodies, but the enzymes that mediate this effect, such as AID, may also erroneously cause somatic mutations and structural abnormalities in oncogenes and tumor-suppressor genes. High mutation rates observed at particular loci in the genome may therefore indicate the presence of dysfunctional SHM, rather than indicating positive selective growth advantage conferred by such mutations. Our analyses suggest that BCL2, as well as PIM1 and other genes, are subject to enrichment of mutations in WRCY hotspots, and therefore likely to represent AID targets. Although, in the case of BCL2, SHM can be explained as a result of translocation to the IgH locus (40), this is less clear for other genes. Whole-genome sequencing approaches may reveal whether hypermutation and chromosome translocation events are related more generally. A complete elucidation of those frequently mutated genes that are contributory to the malignant phenotype, versus those that are simply frequent passenger mutations caused by local SHM will require future studies.
Our list of significantly mutated genes is based on the frequency of the occurrence of mutations, corrected for the size of the gene and its expression level, the sample-specific mutation rate, and the ratio of nonsynonymous to synonymous mutations. However, some mutations may be functionally important despite not meeting statistical significance based on these criteria alone. As an additional tool to discover these mutations, we analyzed the clustering of mutations in hotspots of individual genes, evolutionary conservation, and overlap with mutated genes reported in the COSMIC database. These analyses reveal several genes that are rarely mutated, but that may nevertheless play an important role in the pathophysiology of DLBCL. Future studies may benefit from an even more thorough computational assessment of the likely functional consequence of observed mutations.
With a first draft of the genomic landscape of DLBCL now defined, the next step for the field should be to establish the functional consequence of the observed mutations. In many cases, the consequence is already known (e.g., activating the B-cell receptor pathway or activating the NF-κB pathway). In others, however (particularly in the case of rare mutations), there is no current insight into the role of those gene products in cancer pathogenesis. Although the traditional approach to this problem has been to attack such candidate genes one by one, we propose that new, systematic approaches to the functional characterization of candidate oncogenes and tumor suppressors are needed. Systematic studies to connect such genes to known pathways and/or processes will help to extend the utility of cancer genome studies and accelerate the pace at which genetic findings are translated into therapeutic impact.
Materials and Methods
Sample Selection and Massively Parallel Sequencing.
A total of 55 patients with DLBCL provided DNA for this study. This study was reviewed and approved by the human subjects review board of the Mayo Clinic, the University of Iowa, and the Broad Institute, and written informed consent was obtained from all participants. DNA was extracted from lymph node samples (tumor) and blood (normal) as previously described and processed as detailed in SI Materials and Methods.
Calculation of Sequence Coverage, Mutation Calling, and Significance Analysis.
Massively parallel sequencing data were processed by using two consecutive pipelines developed at the Broad Institute: “Picard” generates a single BAM file representing the sample; “Firehose” starts with the BAM files for each DLBCL sample and matched normal sample from peripheral blood (hg19) and performs various analyses (15, 16). We evaluated the fraction of all bases suitable for mutation calling whereby a base is defined as covered if at least 14 and eight reads overlapped the base in the tumor and in the germline sequencing, respectively. Subsequent analysis is described in more detail in SI Materials and Methods.
PCR.
A PCR assay was used for detection of the t(14;18) translocation, which targets the joining region of the IgH gene and distinct regions of the BCL2 locus (InVivoScribe Technologies). Details are provided in SI Materials and Methods.
Supplementary Material
Supporting Information
Acknowledgments
We thank the members of the T.R.G. laboratory, Maria Cortez, Jadwiga Grabarek, Ami S. Bhatt, Niall J. Lennon, and all members of the Broad Institute's Biological Samples Platform; Genetic Analysis Platform; and Genome Sequencing Platform, without whom this work would not have been possible. This work was conducted as part of the Slim Initiative for Genomic Medicine (SIGMA), a joint US–Mexico project funded by the Carlos Slim Health Institute, and was also supported by National Cancer Institute Grants 5P01 CA092625-07 and P50 CA97274.
Footnotes
The authors declare no conflict of interest.
References
- 1.Abramson JS, Shipp MA. Advances in the biology and therapy of diffuse large B-cell lymphoma: moving toward a molecularly targeted approach. Blood. 2005;106:1164–1174. doi: 10.1182/blood-2005-02-0687. [DOI] [PubMed] [Google Scholar]
- 2.Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362:1417–1429. doi: 10.1056/NEJMra0807082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfreundschuh M, et al. German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: A randomised controlled trial (RICOVER-60) Lancet Oncol. 2008;9:105–116. doi: 10.1016/S1470-2045(08)70002-0. [DOI] [PubMed] [Google Scholar]
- 4.Glass B, et al. German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) High-dose therapy followed by autologous stem-cell transplantation with and without rituximab for primary treatment of high-risk diffuse large B-cell lymphoma. Ann Oncol. 2010;21:2255–2261. doi: 10.1093/annonc/mdq235. [DOI] [PubMed] [Google Scholar]
- 5.Alizadeh AA, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 6.Monti S, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–1861. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 7.Lenz G, et al. Lymphoma/Leukemia Molecular Profiling Project Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis RE, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ngo VN, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenz G, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319:1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 11.Morin RD, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181–185. doi: 10.1038/ng.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasqualucci L, et al. Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature. 2011;471:189–195. doi: 10.1038/nature09730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puente XS, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenman C, et al. Patterns of somatic mutation in human cancer genomes. Nature. 2007;446:153–158. doi: 10.1038/nature05610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chapman MA, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morin RD, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303. doi: 10.1038/nature10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasqualucci L, et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet. 2011;43:830–837. doi: 10.1038/ng.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harwood NE, Batista FD. The cytoskeleton coordinates the early events of B-cell activation. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cantagrel V, et al. Disruption of a new X linked gene highly expressed in brain in a family with two mentally retarded males. J Med Genet. 2004;41:736–742. doi: 10.1136/jmg.2004.021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mullighan CG, et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet. 2009;41:1243–1246. doi: 10.1038/ng.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiwara S, et al. Transforming activity of purinergic receptor P2Y, G protein coupled, 8 revealed by retroviral expression screening. Leuk Lymphoma. 2007;48:978–986. doi: 10.1080/10428190701225882. [DOI] [PubMed] [Google Scholar]
- 23.Leal-Ortiz S, et al. Piccolo modulation of Synapsin1a dynamics regulates synaptic vesicle exocytosis. J Cell Biol. 2008;181:831–846. doi: 10.1083/jcb.200711167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujimoto K, et al. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2. Piccolo complex in cAMP-dependent exocytosis. J Biol Chem. 2002;277:50497–50502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- 25.Izzo A, Kamieniarz K, Schneider R. The histone H1 family: Specific members, specific functions? Biol Chem. 2008;389:333–343. doi: 10.1515/BC.2008.037. [DOI] [PubMed] [Google Scholar]
- 26.Andreu-Vieyra CV, et al. MLL2 is required in oocytes for bulk histone 3 lysine 4 trimethylation and transcriptional silencing. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons DW, et al. The genetic landscape of the childhood cancer medulloblastoma. Science. 2011;331:435–439. doi: 10.1126/science.1198056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coenen EA, et al. Prognostic significance of additional cytogenetic aberrations in 733 de novo pediatric 11q23/MLL-rearranged AML patients: Results of an international study. Blood. 2011;117:7102–7111. doi: 10.1182/blood-2010-12-328302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng SB, et al. Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet. 2010;42(9):790–793. doi: 10.1038/ng.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costello RT, et al. Stimulation of non-Hodgkin's lymphoma via HVEM: an alternate and safe way to increase Fas-induced apoptosis and improve tumor immunogenicity. Leukemia. 2003;17:2500–2507. doi: 10.1038/sj.leu.2403175. [DOI] [PubMed] [Google Scholar]
- 31.Winkler GS. The mammalian anti-proliferative BTG/Tob protein family. J Cell Physiol. 2010;222:66–72. doi: 10.1002/jcp.21919. [DOI] [PubMed] [Google Scholar]
- 32.Homminga I, et al. Integrated transcript and genome analyses reveal NKX2-1 and MEF2C as potential oncogenes in T cell acute lymphoblastic leukemia. Cancer Cell. 2011;19:484–497. doi: 10.1016/j.ccr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Klein U, Dalla-Favera R. Germinal centres: Role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 34.Pasqualucci L, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 35.Iqbal J, et al. BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res. 2011;17(24):7785–7795. doi: 10.1158/1078-0432.CCR-11-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito M, et al. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc Natl Acad Sci USA. 2009;106:11294–11299. doi: 10.1073/pnas.0903854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 38.Friedberg JW, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111:2230–2237. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka S, Louie DC, Kant JA, Reed JC. Frequent incidence of somatic mutations in translocated BCL2 oncogenes of non-Hodgkin's lymphomas. Blood. 1992;79:229–237. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information