Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway (original) (raw)
Abstract
Body weight is regulated by coordinating energy intake and energy expenditure. Transforming growth factor β (TGFβ)/bone morphogenetic protein (BMP) signaling has been shown to regulate energy balance in lower organisms, but whether a similar pathway exists in mammals is unknown. We have previously demonstrated that BMP7 can regulate brown adipogenesis and energy expenditure. In the current study, we have uncovered a novel role for BMP7 in appetite regulation. Systemic treatment of diet-induced obese mice with BMP7 resulted in increased energy expenditure and decreased food intake, leading to a significant reduction in body weight and improvement of metabolic syndrome. Similar degrees of weight loss with reduced appetite were also observed in BMP7-treated ob/ob mice, suggesting a leptin-independent mechanism utilized by BMP7. Intracerebroventricular administration of BMP7 to mice led to an acute decrease in food intake, which was mediated, at least in part, by a central rapamycin-sensitive mTOR-p70S6 kinase pathway. Together, these results underscore the importance of BMP7 in regulating both food intake and energy expenditure, and suggest new therapeutic approaches for obesity and its comorbidities.—Townsend, K. L., Suzuki, R., Huang, T. L., Jing, E., Schulz, T. J., Lee, K., Taniguchi, C. M., Espinoza, D. O., McDougall, L. E., Zhang, H., He, T.-C., Kokkotou, E., Tseng, Y.-H. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway.
Keywords: hypothalamus, p70S6K
Obesity is currently a global pandemic, accompanied by high rates of metabolic syndrome and disorders, such as type 2 diabetes, cardiovascular disease, and some cancers. Therefore, developing prevention and treatment strategies for obesity and its comorbidities are currently of utmost importance for the health care and research communities. Obesity develops when energy intake exceeds energy expenditure; therefore, any treatment for obesity must reduce energy intake, increase energy expenditure, or have an effect on both. Despite this simple reality, treatment of obesity remains one of the most important challenges facing the health care system.
Bone morphogenetic proteins (BMPs), a family of developmental factors that belong to the transforming growth factor (TGF) β superfamily, regulate many aspects of organogenesis and patterning during embryonic development (1, 2). BMP-mediated effects on energy metabolism have just begun to emerge (3–7). The TGFβ/BMP signaling pathway has been shown to regulate food intake and quiescence in Caenorhabditis elegans (8) and to modulate metabolism in response to nutrient state in Drosophila (9). We and others have shown that BMPs may act as a molecular switch in regulating white vs. brown adipogenesis (10–13). BMP7, in particular, promotes brown adipocyte differentiation and thermogenic function in adipose progenitor cells and increases brown fat-mediated energy expenditure in vivo (12). Recently, several members of the BMP family of ligands and receptors have been found to associate with obesity-related traits in humans (5, 14–16); however, a detailed mechanism for how the BMPs affect energy balance and obesity remains to be elucidated.
The central nervous system (CNS) receives diverse inputs to coordinate appetite and energy expenditure and is the main control center for body weight regulation. The hypothalamus is the principal site for the homeostatic regulation of food intake (17, 18). Several BMP ligands are expressed in different regions of the brain. Among these, bioactive BMP7 has been found in cerebrospinal fluid (CSF; refs. 19, 20) and BMP7 is expressed in several brain regions, including the hypothalamus (21) and choroid plexus. In addition, different BMP receptor isoforms have been demonstrated throughout the brain (22), including the hypothalamus, indicating that BMP signaling may be involved in hypothalamic function.
Given that many factors that affect whole body energy balance act on central targets regulating food intake and energy expenditure (17, 23), here, we tested the hypothesis that BMP7 may play a role not only in energy expenditure via its direct effects on brown adipose tissue, as established previously by our group (12), but also in the central regulation of food intake, as is true for other factors such as leptin (24). We have now demonstrated that systemic administration of BMP7 is able to reverse obesity via an increase in energy expenditure as well as a reduction of appetite. In addition, this ameliorates the associated metabolic complications induced by a high-fat diet, even in the Agouti mice, which lack some melanocortin receptor activity, or in the absence of leptin (ob/ob mice), making BMP7 an attractive potential obesity therapeutic for diet-induced obesity and leptin-resistant situations. Intracerebroventricular (i.c.v.) administration of BMP7 reduces appetite, via the mammalian target of rapamycin (mTOR)-p70S6 kinase (p70S6K) pathway, and this effect was inhibited on pretreatment with the mTOR-p70S6K inhibitor rapamycin. Taken together, these data provide evidence for BMP7 as a novel anorectic factor, acting through leptin-independent mTOR pathways in the hypothalamus to reduce appetite and reverse obesity.
MATERIALS AND METHODS
Systemic BMP7 treatments
Mice were fed a high-fat, hypercaloric diet that provided 45% of calories from fat (Research Diets D12451; Research Diets, New Brunswick, NJ, USA). Adenoviruses were prepared as described previously (25). Mice were injected via tail vein with 2.5 × 1010 viral particles per gram of weight and sacrificed at 12–15 d after injection. We used a 50 times higher dosage of viruses per gram of body weight, which resulted in several hundred-fold higher levels of serum BMP7 in the obese mice compared with those used in our previous study (12). Blood was collected via cardiac puncture, and plasma insulin, leptin, and adiponectin were measured by ELISA at the Joslin Specialized Assay Core (Harvard Medical School).
Oxygen consumption
Oxygen consumption was measured in mice 7–10 d after adenoviral injection by indirect calorimetry in a comprehensive laboratory animal monitoring system (CLAMS; Columbus Instruments, Columbus, OH, USA), as described previously (12).
Brain immunohistochemistry and immunofluorescence
For immunohistochemical staining, primary antibodies recognized c-Fos (dilution 1:200; Cell Signaling Technologies, Beverly, MA, USA), α-melanocyte-stimulating hormone (αMSH; dilution 1:1000; Millipore, Bedford, MA, USA) or phospho-p70S6K (dilution 1:200; Cell Signaling Technologies). The signal was visualized using the Universal Vectorstain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). For immunofluorescence, ALK3 was detected by a Zymed antibody (1:200; Invitrogen, Carlsbad, Ca, USA), and ALK2 was detected by an Abcam antibody (1:200). Sections were analyzed by confocal microscopy on a Zeiss LSM-410 Invert Laser Scan Microscope (Carl Zeiss MicroImaging, Thornwood, NY, USA).
Quantitative-reverse transcription-polymerase chain reaction (Q-RT-PCR) analysis
This procedure was performed as described previously (12). Sequences of primers used in this study are listed in Supplemental Table S1.
In situ hybridization
Riboprobes were in vitro transcribed and labeled with digitoxin using DIG RNA Labeling Mix (Roche, Minneapolis, MN, USA) and the Riboprobe Combination System (Promega, Madison, WI, USA). cRNA sense and antisense probes to the region corresponding to nt 1037 to 1857 of bone morphogenetic protein receptor 2 (BMPRII) were generated. The specific signal was detected with anti-digoxigenin-AP, Fab fragments (1:500; Roche).
LacZ staining
Sections of 8 μm thickness were prepared and postfixed for 5 min in ice-cold 4% buffered formaldehyde. Tissues were rinsed (PBS, 0.02% Nonidet P-40, 0.01 sodium deoxycholate, 2 mM MgCl2) and stained (1 mg/ml X-Gal in rinse buffer) at 37°C overnight in the dark.
Acute feeding studies
Anesthetized mice were placed in a stereotactic device and a 26-gauge guide cannula (Plastics One, Roanoke, VA, USA) was inserted into the right lateral cerebral ventricle (1.0 mm posterior, 1.0 mm lateral, and 2.0 mm ventral to the bregma). After 1 wk recovery, cannulated mice were food deprived for 4 h prior the onset of the dark cycle. At 1–2 h prior to the onset of the dark cycle, mice received an i.c.v. injection (1 μl) of BMP7 (2 μg/μl; R&D Systems, Minneapolis, MN, USA), leptin (1.6 μg/μl), or vehicle using an internal cannula connected to a Hamilton microsyringe (Hamilton, Reno, NV, USA). Food was returned to the cages immediately after the injection. In combined treatments, food was removed 6 h before the onset of the dark cycle, and 0.5 μl of i.c.v. rapamycin (2.5 μg/μl) or DMSO was injected 2 h before the administration of BMP7 or buffer, as described above.
Glucose and insulin tolerance tests
Food was removed from the cages for 6 h, and mice were injected intraperitoneally with either 1 g/kg dextrose (for glucose tolerance test) or 2 U/kg insulin (Humulin; Eli Lilly, Indianapolis, IN, USA; for insulin tolerance test). Blood glucose was monitored at the indicated time points via tail bleeding and using a glucometer (Accu-Check; Roche).
Conditioned taste aversion and mouse malaise scoring
Conditioned taste aversion was conducted similar to Cannon et al. (26), with the choice of 2 water bottles (0.15% sodium saccharin or plain drinking water) following a period of training. Malaise scoring was based on methods described by the American Association for Laboratory Animal Science (http://www.iacuc.org), the National Academies Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (http://www.nap.edu), and the Institute for Laboratory Animal Research (http://dels-old.nas.edu/ilar_n/ilarjournal/41_2/Systematic.shtml).
Cell culture
Mouse embryonic hypothalamic cells (mHypoE-N25/2; Cellutions Biosystems, Toronto, ON, Canada) were grown in 6-cm dishes in DMEM-high-glucose media supplemented with 10% FBS and antibiotics. At 80% confluence, serum was replaced with 0.1% BSA, and after 16 h, cells were treated with 3.3 nM BMP7 (R&D Systems), 100 nM insulin, 100 nM rapamycin, or vehicle (4 mM HCl and 0.1% BSA) and harvested at various time points.
Western blot analysis
Protein lysates were analyzed as described previously (12) using antibodies against phospho-Sma and Mad-related family (SMAD) 1/5/8, phospho-p70S6K, phospho-signal transducer and activator of transcription 3 (STAT-3), phospho-p38, cyclophilin A, or β-tubulin (Cell Signaling Technology).
Statistical analysis
The effect of treatment was evaluated by Student's t test, ANOVA factorial followed by post hoc Fisher's protected least significant difference test, or ANOVA repeated measures using STAT View (SAS Institute, Cary, NC). Error bars indicate standard error; values of P < 0.05 were considered significant.
RESULTS
Systemic BMP7 treatment reduces appetite and promotes weight loss in mice with diet-induced obesity
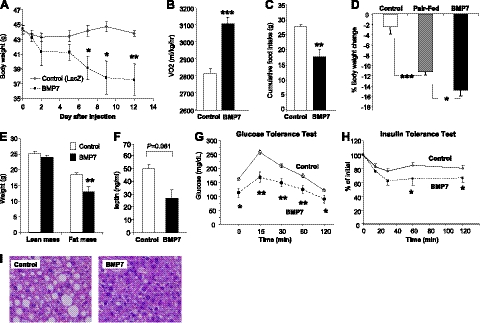
Following from our previous studies, which showed a role for BMP7 in promoting brown adipogenesis and increased energy expenditure (12, 13), we sought to investigate the effects of BMP7 on systemic energy metabolism and obesity-related metabolic disorders. C57BL/6 mice were made obese by consuming a high-fat, high-caloric diet (45% calories from animal fat) for 12 wk [diet-induced obese (DIO) mice]. This model closely resembles common dietary obesity in humans. DIO mice received a single tail-vein injection of BMP7-expressing adenovirus or LacZ-expressing adenovirus as a control. Interestingly, DIO mice receiving BMP7 adenovirus displayed a progressive weight loss (Fig. 1A). While mice receiving control LacZ adenovirus maintained stable body weight, BMP7-treated mice started to lose weight 2 d after adenovirus injection, and the weight loss reached statistical significance at d 7. At d 9, the BMP7-treated DIO mice had lost 14.8% of the initial body weight, and no more weight loss was observed at d 12, presumably due to the transient expression nature of the adenovirus.
Figure 1.
Systemic administration of BMP7 reduces appetite and body weight in DIO mice. A) Body weight monitoring of DIO mice receiving BMP7 or LacZ adenovirus (control), showing a weight-lowering effect of BMP7. B) DIO mice treated with BMP7 adenovirus had increased oxygen consumption as measured by CLAMS, compared to mice treated with LacZ adenovirus. C) DIO mice treated with BMP7 adenovirus consumed less food during a 12-d period, compared to mice treated with LacZ adenovirus. D) DIO mice treated with BMP7 adenovirus lost more weight compared to pair-fed animals, indicating that the effects of BMP7 on body weight reflect both increased energy expenditure and reduced food intake. E) BMP7-mediated weight loss in DIO mice was due to a decrease in fat mass, not lean mass, as measured by DEXA scanning. F) BMP7-treated DIO mice had lower serum leptin levels at the end of the study, consistent with reduced adiposity in these mice, compared to DIO mice treated with LacZ adenovirus. G, H) DIO mice treated with BMP7 adenovirus had improved glucose tolerance and increased insulin sensitivity as evaluated by glucose (G) and insulin (H) tolerance tests. I) H&E-stained liver sections of DIO mice treated with BMP7 or LacZ for 12 d. Mice treated with BMP7 (right panel) had reduced fat accumulation in their liver compared to control mice (left panel). *P < 0.05, **P < 0.01, ***P < 0.001.
Two factors potentially contribute to BMP7-mediated leanness in this mouse model: increased energy expenditure as previously demonstrated by our group (12) and reduced caloric intake. Indeed, BMP7 adenovirus-treated mice had a 10% increase in oxygen consumption without any changes in locomotor activity (Fig. 1B and Supplemental Fig. S1) and consumed 30% less food throughout the study, compared to the mice treated with control adenovirus (Fig. 1C). To delineate the relative contributions of reduced food intake and increased energy expenditure on BMP7's antiobesity effect, we included in our study a pair-fed group of DIO mice, which were treated with control adenovirus, but fed the same amount of food consumed the previous day by the BMP7-treated mice. This experiment revealed that ∼75% of the weight loss in the BMP7-treated obese mice was attributed to reduced food consumption, while increased energy expenditure accounted for the remaining 25% of the weight loss (Fig. 1D).
BMP7-induced leanness in DIO mice was primarily due to loss of fat, but not lean, mass (Fig. 1E). Consistent with reduced adiposity, BMP7-treated mice had a trend of lower serum leptin levels (Fig. 1F). The weight loss in DIO mice was also associated with improvements in additional parameters of the metabolic syndrome, including improved glucose tolerance (Fig. 1G) and insulin sensitivity (Fig. 1H) and reversed hepatic steatosis induced by high-fat feeding (Fig. 1I). DIO mice receiving adenoviral injections showed no sign of illness, and histological analysis of all major organs revealed no adverse pathology. Moreover, serum levels of alanine aminotransferase (ALT) and interleukin-6 (IL-6), markers of liver injury and inflammation, respectively, were not different in BMP7- vs. LacZ-treated mice (Supplemental Fig. S1_B_, C), suggesting that BMP7 adenovirus treatment did not cause any unwanted liver pathology or inflammation.
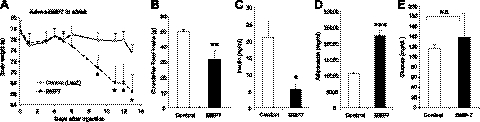
BMP7 treatment results in weight loss in leptin-deficient (ob/ob) mice
To interrogate whether the effects of BMP7 on appetite are leptin-dependent, we employed the paradigm described above to assess whether leptin-deficient (ob/ob) mice were responsive to BMP7 treatment. We found that systemic treatment of ob/ob mice with a BMP7-expressing adenovirus resulted in 15% weight loss (Fig. 2A) associated with a significant reduction in food intake (Fig. 2B). Most importantly, and consistent with their leaner phenotype, BMP7-treated ob/ob mice had reduced plasma insulin levels by 73.5% (Fig. 2C), a 2-fold increase in serum adiponectin levels (Fig. 2D) and retained their glucose levels (Fig. 2E), suggesting an overall improved insulin sensitivity in these mice. Similar to the DIO experiments, we found that ob/ob mice receiving BMP7 adenovirus did not have increased serum ALT or IL-6 (Supplemental Fig. S1_D_, E). Notably, the high circulating levels of ALT in ob/ob mice were significantly reduced in BMP7-treated mice, suggesting an improved liver function in these obese mice. Taken together, these results clearly indicate that BMP7 does not require leptin to mediate its appetite-lowering effects.
Figure 2.
Systemic effects of BMP7 are leptin-independent. A, B) Treatment of leptin-deficient ob/ob mice with BMP7 adenovirus resulted in significant weight loss (A) and reduced food consumption (B) compared to ob/ob mice that received LacZ adenovirus (control) during a 13-d study. C–E) BMP7 adenovirus-treated mice had lower plasma insulin levels (C), higher adiponectin levels (D), and no change in glucose levels (E), indicating increased insulin sensitivity compared to LacZ-adenovirus-treated ob/ob mice. N.S., not significant. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
BMP7 inhibits acute food intake when injected centrally
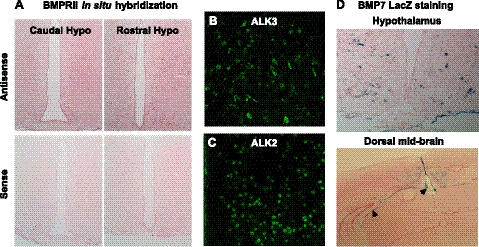
Given the role of the hypothalamus in homeostatic regulation of appetite, we first sought to confirm the presence of BMP7 ligand and receptors in mouse hypothalamus. The BMPs signal through a transmembrane receptor heterodimer comprising a type II receptor and a type I receptor. Among these different receptor isoforms, 3 type I receptors (ALK3/BMPR1a, ALK6/BMPR1b, and ALK-2) and 3 type II receptors (BMPRII, ActRIIa, and ActRIIb) mediate most of the effects of BMPs. We demonstrated hypothalamic mRNA expression of all 6 isoforms of the type I and type II BMP receptors by quantitative real-time PCR, as well as expression of BMP ligands 2, 4, 6, and 7 (data not shown). Furthermore, we verified the presence of BMPRII, ALK2, and ALK3 in hypothalamic areas known to regulate food intake by in situ hybridization and immunofluorescence staining, respectively (Fig. 3A–C). While BMPRII is widespread in the brain, type I receptors, such as ALK3 and ALK2, are more limited in distribution within the hypothalamus. Specifically, ALK3 is mainly expressed in the lateral hypothalamus and arcuate nucleus, and ALK2 is expressed in the medial-lateral hypothalamus. Using a transgenic mouse in which the BMP7 coding sequence has been replaced by LacZ (27), we localized BMP7 expression to the hypothalamus and choroid plexus (Fig. 3D), as well as other brain regions as previously reported (28).
Figure 3.
Expression of BMP7 ligand and receptors in the hypothalamus. A) In situ hybridization analysis of hypothalamic sections for BMPRII (pink), showing caudal hypothalamus (left panel) and rostral hypothalamus (right panel). Antisense represents specific staining (top panel), while sense staining represents background (bottom panel). BMPRII, a type 2 BMP receptor, was highly expressed throughout the hypothalamus. B, C) Immunofluorescence staining (green FITC signal) for the type 1 BMP receptors ALK3 (B) and ALK2 (C) in the mouse hypothalamus, along the third ventricle. ALK3 staining was most robust in the arcuate and paraventricular nuclei, while ALK2 staining was most robust in the dorsomedial and paraventricular nuclei. D) LacZ staining of mouse hypothalamus (top panel) and dorsal midbrain (including ventricular system and choroid plexus; bottom panel) showing BMP7-positive cells (blue) in BMP7 reporter mice. Arrowheads indicate choroid plexus surrounding the ventricles (bottom panel).
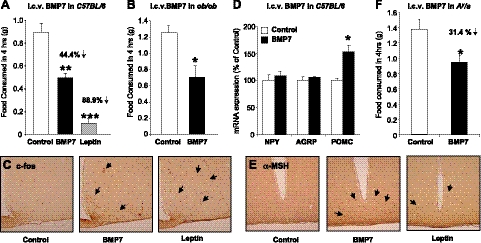
To examine potential effects of BMP7 on feeding behavior, we administered recombinant BMP7 intracerebroventricularly into the lateral ventricle of C57BL/6 mice. The injections took place within 2 h of the start of the dark cycle, when mice consume most of their food. Mice treated with BMP7 consumed 44.4% less food compared to vehicle treated mice (Fig. 4A), a finding which was replicated in 7 independent experiments. Likewise, mice treated with leptin, a strong suppressor of appetite, showed an 88.9% reduction in 4-h food consumption (Fig. 4A). Interestingly, in the same experimental setting, treatment with BMP2, another member of the family, had no effect on food intake (Supplemental Fig. S2_A_). The anorectic effect of BMP7 was not due to taste-aversion or illness, as mice receiving i.c.v. BMP7 displayed no malaise (Supplemental Fig. S2_B_), and saccharin preference was not affected by i.c.v. BMP7, as demonstrated in a conditioned taste aversion test (Supplemental Fig. S2_C_).
Figure 4.
Intracerebroventricular administration of BMP7 suppresses food intake. A) Intracerebroventricular administration of rhBMP7 acutely reduced food intake in C57BL/6 mice. Results are presented from a representative of 7 independent experiments. Leptin served as a positive control. B) Food intake 4 h after i.c.v. rhBMP7 or vehicle treatment of ob/ob mice, showing that leptin-deficient mice maintain an anorectic response to BMP7 administration. C) Hypothalamic staining for c-Fos immunoreactivity (brown) at 60 min after i.c.v. injection of mice with either vehicle (left panel), rhBMP7 (middle panel), or leptin (right panel), showing increased c-Fos expression in response to both leptin and BMP7 treatments in the arcuate and ventromedial nuclei. D) mRNA expression of hypothalamic neuropeptides at 4 h after i.c.v. rhBMP7, leptin, or vehicle as measured by Q-RT-PCR, showing a significant increase in POMC expression by BMP7 treatment. E) α-MSH immunostaining (brown) of hypothalamic sections 60 min after i.c.v. injection of mice with either vehicle (left panel), rhBMP7 (middle panel), or leptin (right panel), showing increased α-MSH expression in response to both leptin and BMP7 treatments, in the arcuate nucleus. F) Food intake 4 h after i.c.v. BMP7 or vehicle treatment in Agouti mice (KK, Cg-Ay/J), showing that Agouti mice maintain an anorectic response to BMP7 administration. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control.
Following from the systemic BMP7 treatment to ob/ob mice, and to dissociate the central from peripheral effects of BMP7 on food intake in the leptin-deficient state, ob/ob mice were also treated i.c.v. with a single injection of BMP7, and their food intake was monitored over time. We found that centrally administered BMP7 reduced food intake in the ob/ob mice (Fig. 4B), similar to levels seen in wild-type controls, further demonstrating that the central effect of BMP7 on suppression of food intake is leptin independent.
The arcuate nucleus (ARC), situated adjacent to the floor of the third ventricle in the mediobasal hypothalamus, contains neurons that exert potent effects on food intake and energy expenditure. The anorectic response to centrally administered BMP7 was accompanied by a significant increase in c-Fos-immunoreactive neurons, a marker of neuronal activation, in the hypothalamus and particularly in the ARC (Fig. 4C). It has been well established that leptin and other anorectic factors regulate food intake at the hypothalamic level, at least in part, by increasing expression of the anorexigenic neuropeptide α-MSH, one of the products of the proopiomelanocortin (POMC) gene, while also down-regulating expression of the orexigenic neuropeptide Y (NPY) and Agouti-related peptide (AgRP) in the ARC (18). We found a significant up-regulation of hypothalamic POMC expression in C57BL/6 mice that received BMP7 (Fig. 4D), along with increased hypothalamic αMSH immunoreactivity in the ARC (Fig. 4E). To determine whether the melanocortin pathway is necessary for the anorectic action of BMP7, we administered i.c.v. BMP7 to the Agouti yellow mutant (Ay/a) mice. These mice, which ectopically overexpress Agouti protein and display reduced melanocortin-4 receptor (MC4R) activity, are hyperphagic and obese (29, 30). Somewhat paradoxically, while BMP7 is able to increase POMC and α-MSH in the hypothalamus, the Ay/a mice retain responsiveness to the appetite-lowering effects of BMP7 (31.4% decrease; Fig. 4F), suggesting that MC4R may not be essential for BMP7's anorectic effect or that the increased αMSH is improving MC4R activity. These data led us to postulate that BMP7 might activate other anorectic pathways that are either parallel or downstream of the melanocortin pathway.
In a loss-of-function study, we knocked down BMPRII, the most abundantly expressed BMP receptor isoform in the brain, by delivering i.c.v. lentiviruses expressing shRNA against BMPRII or vector control. At 2 wk after injection, these mice were treated i.c.v. with BMP7 just prior to the start of the dark cycle, followed by acute food intake measurements. While control mice displayed reduced food intake in response to BMP7 treatment, mice with BMPRII knockdown did not respond to BMP7, indicating that this prevalent BMP receptor isoform in the brain is essential for BMP7's anorectic effect (Supplemental Fig. S3).
BMP7 activates the mTOR pathway to suppress food intake
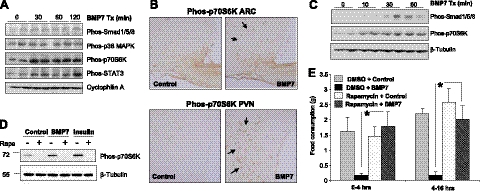
To map the signaling pathways utilized by BMP7 in the central regulation of food intake, we performed i.c.v. injections of BMP7 and evaluated activation of potential signaling candidates by Western blot analysis. The canonical BMP signaling pathways are the SMAD and p38 mitogen-activated protein kinase (MAPK) pathways; however, it has also been reported that BMPs are able to activate other signaling pathways, such as the mTOR (31) and STAT3 (32) pathways, in different cellular systems. Some of these pathways are well known to be involved in CNS regulation of appetite and energy expenditure. At 30 min, we observed a slight increase in SMAD1/5/8 phosphorylation and no change of phospho-p38 MAPK, both members of established pathways for BMP7 signaling in other tissues (33) (Fig. 5A). Notably, BMP7 treatment resulted in a robust and sustained increase in p70S6K phosphorylation, a downstream effector of mTOR signaling (Fig. 5A). Tyrosine phosphorylation of STAT3, a key mediator of leptin signaling, was also induced by BMP7 (Fig. 5A). Immunohistochemical staining confirmed that BMP7 induces activation of p70S6K in the ARC and the paraventricular nucleus (PVN) of the hypothalamus (Fig. 5B), two regions involved in the anorectic response.
Figure 5.
BMP7 utilizes the mTOR pathway to regulate food intake. A) Western blot analysis demonstrating that BMP7, when injected i.c.v. in mice, activated multiple signaling pathways in the hypothalamus, most notably the p70S6K and pSTAT3 pathways. Cyclophilin A serves as a loading control. B) Intracerebroventricular rhBMP7-activated phos-p70S6K (brown) in the ARC (left panels) and PVN (right panels), as detected by immunohistochemical staining of hypothalamic sections using a specific antibody against phospho-p70S6K at 30 min following injection of BMP7. Arrows indicate cells that are positive for phos-p70S6K. C) Activation of SMAD and p70S6K signaling pathways by BMP7 in the hypothalamic neuronal cell line N25/2, as evaluated by Western blot analysis using phospho-specific antibodies. D) Western blot analysis of phosphorylated p70S6K in response to 30 min of BMP7 or insulin stimulation in the hypothalamic neuronal cell line N25/2. The 70 kDa-band corresponding to p70S6K is completely blocked by 1 h rapamycin pretreatment. β-Tubulin serves as a loading control. E) Rapamycin, an mTOR inhibitor, reversed the anorectic effect of rhBMP7 injected i.c.v. into mice. Average food consumption at 4 h and 16 h post-treatment are presented. *P < 0.05.
To our knowledge, this is the first report of BMP-mediated activation of the mTOR pathway, a well-established sensor of nutrient availability, in the CNS. However, the effects of BMP7 on mTOR activation could be direct or indirect. To clarify this, we used a hypothalamic neuronal cell line (N25/2). Notably, in these neuronal cells, activation of p70S6K by BMP7 occurred earlier than that of SMAD activation (Fig. 5C), implying that the mTOR-p70S6K pathway may serve as a major signaling pathway in the CNS to mediate BMP7's effects on energy balance. The activation of this 70-kDa band by BMP7 or insulin (serving as a positive control) in N25/2 cells was completely blocked by the mTOR inhibitor rapamycin, confirming the specificity of the band (Fig. 5D).
Finally, to underscore the functional significance of BMP7-mediated mTOR activation in the regulation of food intake, we pretreated mice i.c.v. with rapamycin, an inhibitor of mTOR complex 1 (p70S6K) signaling, followed by BMP7 treatment. Indeed, rapamycin completely abolished the inhibitory effect of BMP7 on food intake (Fig. 5E), Taken together, these data provide a mechanistic explanation for how BMP7 regulates food intake and indicate that the mTOR pathway is essential for the central anorectic effects of BMP7.
DISCUSSION
Obesity develops as a result of positive energy balance due to increased nutrient intake and/or reduced energy expenditure. In previous studies, we have shown that systemic BMP7 delivery via adenovirus-mediated overexpression, leads to increased brown adipose tissue development and increased energy expenditure in lean mice (12). In the current study, we demonstrate, as predicted, that BMP7 is able to reverse diet-induced obesity, in part, by increasing energy expenditure. However, we also made the unexpected finding that systemic BMP7 administration also decreases appetite, and this reduction in food intake contributes to 75% of BMP7-mediated weight loss in DIO animals. We define a central mechanism involving the mTOR-p70S6K pathway utilized by BMP7 to directly regulate food intake. In addition to the findings in DIO mice, BMP7 is also able to exert its appetite-lowering and weight-reducing effects in a genetically obese mouse model lacking leptin, suggesting that BMP7's effect on appetite is leptin-independent. This finding is of particular importance for human obesity, which is associated with severe leptin resistance (34, 35). These studies provide exciting evidence that BMP7 may be a promising novel therapeutic target for the treatment of obesity and metabolic syndrome.
BMP7's anorectic effect necessitates mTOR-p70S6K signaling, as pretreatment with the inhibitor rapamycin renders the i.c.v. BMP7 treatments incapable of reducing acute food intake. The mTOR signaling pathway is now well recognized as an important component in the hypothalamic regulation of energy balance. Previous studies have highlighted the importance of mTOR signaling in the regulation of food intake by leptin and nutrients (36–40). The mTOR pathway has been shown to be activated by other BMPs in a lung cancer cell model (31); however, in this study, we demonstrate for the first time that BMP7 can directly activate the mTOR signaling pathway in the hypothalamus, as well as in a hypothalamic neuronal cell line. To the best of our knowledge, this is also the first report showing that BMP-mediated activation of the mTOR pathway is involved in appetite regulation. Interestingly, mTOR is also known to act as a regulator of neuronal remodeling (41). Thus, BMP7, a neurotrophic factor, may act through the noncanonical mTOR signaling pathway in the brain to regulate neuronal remodeling/synaptic plasticity, as well as appetite control.
The fact that BMP7 is able to suppress food intake in Ay/a (Agouti) mice further suggests that BMP7 may activate different signaling systems that are either parallel or downstream of the MCR4-dependent pathway utilized by α-MSH. Indeed, it has been previously shown that several anorectic peptides, including leptin, retain their effects in obese Ay/a or MC4R-deficient mice (42), and brain-derived neurotrophic factor (BDNF) is able to exert its anorectic effect downstream of the melanocortin receptors in the hypothalamus (43). Interestingly, BDNF is also able to activate mTOR signaling (44, 45), and mTOR signaling has been shown to act downstream of the MC4 receptor (46). This led us to postulate that BMP7 may act similarly to BDNF by acting downstream of MC4R to activate mTOR signaling, thereby reducing appetite. In addition, our findings that BMP7 is effective in states of leptin resistance (the DIO mice) and leptin deficiency (the ob/ob mice) indicate that BMP7 acts independently of leptin in suppressing food intake, as do several other appetite-regulating factors, such as ciliary neurotrophic factor (CNTF; 47). Interestingly, CNTF, BDNF, and BMP7 are all well-characterized neurotrophic factors, which have now all been linked to CNS regulation of energy balance (48–53). Thus, the anorectic effect of BMP7 discovered in this study suggests that BMP7 may functionally resemble BDNF and CNTF, which not only play an important role in neuronal development and plasticity, but also in regulating appetite and sympathetic outflow to energy-expending tissues, such as brown adipose tissue.
While these findings are novel and exciting, we acknowledge that much is still unknown about the physiological role of BMP signaling in the central regulation of energy balance. We have observed the presence of BMP7 ligand in a restricted pattern in the mouse brain, which includes the choroid plexus and the hypothalamus, while analysis of receptor distribution indicates that BMPRII is widespread in the brain, and type I receptors are more limited in distribution within the hypothalamus. At the mRNA level we can detect BMP 2, 4, 6, and 7 in the hypothalamus, but whether these BMPs exert unique vs. overlapping/complementary functions in this key brain region is still unknown. Thus far, we have shown that at least one other BMP, BMP2, is unable to exert appetite-lowering effects. While the molecular differences between BMP2 and BMP7 remain to be unraveled, these data suggest specific roles for different BMPs in the brain. In particular, loss-of-function studies using conditional knockout mouse models will clarify whether BMP7 or specific BMP receptors in the hypothalamus are required for physiological appetite regulation, and these studies warrant future investigation.
Accumulating evidence suggests that hypothalamic circuits are not the only players in the central regulation of food intake and energy balance. For instance, leptin also modulates hedonic aspects of food intake by acting on dopaminergic neurons of the mesolimbic system (54, 55). Moreover, it has been shown that leptin receptors present in the nucleus tractus solitarius and area postrema converge with gastrointestinal satiation signals and reduce food intake (56). Given the fact that BMP7 is present in the CSF circulation of the brain (19, 20) and the fact that both BMP7 and its receptors are expressed in multiple regions of the brain (22), BMP7 may act not only on the hypothalamus but also on extrahypothalamic sites to regulate food intake. Future studies are needed to determine BMP7's effect on the extrahypothalamic sites.
Originally identified as a bone-forming and developmental factor, BMP7 is now recognized as a cytokine with pleiotropic functions and has been offered as a treatment for cardiovascular (57), fibrotic (58), metabolic (12), and neurodegenerative diseases (59, 60) Data presented here identify BMP7 as a modulator of food intake when given either centrally or systemically, which is able to activate several well-established signaling pathways that orchestrate energy balance. This newly identified appetite-regulating mechanism could provide new directions in the development of therapeutics for obesity and eating disorders.
Supplementary Material
Supplemental Data
Acknowledgments
This work was supported in part by U.S. National Institutes of Health (NIH) grants NIH R01 DK077097 and UL1 RR 025758-01 (Harvard Catalyst/The Harvard Clinical and Translational Science Center) and research grants from the Eli Lilly Research Foundation and Harvard Stem Cell Institute (to Y.-H.T.) and NIH R01 DK080058 (to E.K.). K.L.T. was supported by NIH T32-DK007260-33 and F32-DK091996. The Joslin Physiology and Special Assay Cores were supported by the Joslin Diabetes and Endocrinology Center, grant P30-DK036836 from the National Institute of Diabetes and Digestive and Kidney Diseases. The authors thank C. R. Kahn (Joslin Diabetes Center) for helpful discussion, and Andre Kleinridders (Joslin Diabetes Center) for technical advice. The authors declare no conflicts of interest. K.L.T. wrote the manuscript and conducted several of the experiments. R.S. performed most of the i.c.v. experiments. T.L.H. performed several of the in vitro and in vivo experiments. E.J. did the immunohistochemical staining. T.J.S. performed some of the LacZ staining and assisted with some of the i.c.v. studies. K.L. did the in situ hybridization and part of the LacZ staining. D.O.E. performed some of the in vitro studies and assisted with some of the i.c.v. studies. C.M.T. did the adenovirus experiments. L.E.M. assisted with the animal studies and RNA extractions. T.-C.H. provided the adenovirus and technical expertise. E.K. and Y.-H.T. designed the studies, performed the key experiments, and wrote the manuscript.
Abbreviations:
αMSH
α-melanocyte-stimulating hormone
AgRP
Agouti-related peptide
ALT
alanine aminotransferase
ARC
arcuate nucleus
BDNF
brain-derived neurotrophic factor
BMP7
bone morphogenetic protein 7
BMPR1a
bone morphogenetic protein receptor 1a
BMPRII
bone morphogenetic protein receptor 2
CLAMS
comprehensive laboratory animal monitoring system
CSF
cerebrospinal fluid
CNS
central nervous system
CNTF
ciliary neurotrophic factor
DIO
diet-induced obese
IL-6
interleukin-6
MAPK
mitogen-activated protein kinase
MC4R
melanocortin-4 receptor
mTOR
mammalian target of rapamycin
NPY
neuropeptide Y
POMC
proopiomelanocortin
PVN
paraventricular nucleus
SMAD
Sma and Mad-related family
STAT3
signal transducer and activator of transcription 3
TGFβ
transforming growth factor β.
REFERENCES
- 1.Chen D., Zhao M., Mundy G. R. (2004) Bone morphogenetic proteins. Growth Factors 22, 233–241 [DOI] [PubMed] [Google Scholar]
- 2.Mira H., Andreu Z., Suh H., Lie D. C., Jessberger S., Consiglio A., San Emeterio J., Hortiguela R., Marques-Torrejon M. A., Nakashima K., Colak D., Gotz M., Farinas I., Gage F. H. (2010) Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7, 78–89 [DOI] [PubMed] [Google Scholar]
- 3.Schulz T. J., Tseng Y. H. (2009) Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 20, 523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobin J. F., Celeste A. J. (2006) Bone morphogenetic proteins and growth differentiation factors as drug targets in cardiovascular and metabolic disease. Drug Discov. Today 11, 405–411 [DOI] [PubMed] [Google Scholar]
- 5.Zamani N., Brown C. W. (2010) Emerging roles for the transforming growth factor-β superfamily in regulating adiposity and energy expenditure. Endocr. Rev. 32, 387–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnen H., Lin S., Kuffner T., Brown D. A., Tsai V. W., Bauskin A. R., Wu L., Pankhurst G., Jiang L., Junankar S., Hunter M., Fairlie W. D., Lee N. J., Enriquez R. F., Baldock P. A., Corey E., Apple F. S., Murakami M. M., Lin E. J., Wang C., During M. J., Sainsbury A., Herzog H., Breit S. N. (2007) Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 13, 1333–1340 [DOI] [PubMed] [Google Scholar]
- 7.Yadav H., Quijano C., Kamaraju A. K., Gavrilova O., Malek R., Chen W., Zerfas P., Zhigang D., Wright E. C., Stuelten C., Sun P., Lonning S., Skarulis M., Sumner A. E., Finkel T., Rane S. G. (2011) Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 14, 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You Y. J., Kim J., Raizen D. M., Avery L. (2008) Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: a model for satiety. Cell Metab. 7, 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballard S. L., Jarolimova J., Wharton K. A. (2010) Gbb/BMP signaling is required to maintain energy homeostasis in Drosophila. Dev. Biol. 337, 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang Q. Q., Otto T. C., Lane M. D. (2004) Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 101, 9607–9611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang E. A., Israel D. I., Kelly S., Luxenberg D. P. (1993) Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors 9, 57–71 [DOI] [PubMed] [Google Scholar]
- 12.Tseng Y. H., Kokkotou E., Schulz T. J., Huang T. L., Winnay J. N., Taniguchi C. M., Tran T. T., Suzuki R., Espinoza D. O., Yamamoto Y., Ahrens M. J., Dudley A. T., Norris A. W., Kulkarni R. N., Kahn C. R. (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz T. J., Huang T. L., Tran T. T., Zhang H., Townsend K. L., Shadrach J. L., Cerletti M., McDougall L. E., Giorgadze N., Tchkonia T., Schrier D., Falb D., Kirkland J. L., Wagers A. J., Tseng Y. H. (2011) Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl. Acad. Sci. USA 108, 143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schleinitz D., Kloting N., Bottcher Y., Wolf S., Dietrich K., Tonjes A., Breitfeld J., Enigk B., Halbritter J., Korner A., Schon M. R., Jenkner J., Tseng Y. H., Lohmann T., Drebetaler M., Stumvoll M., Bluher M., Kovacs P. (2011) Genetic and evolutionary analyses of the human bone morphogenetic protein receptor 2 (BMPRII) in the pathophysiology of obesity. PLoS ONE 6, e16155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Son J. W., Kim M. K., Park Y. M., Baek K. H., Yoo S. J., Song K. H., Son H. S., Yoon K. H., Lee W. C., Cha B. Y., Son H. Y., Kwon H. S. (2011) Association of serum bone morphogenetic protein 4 levels with obesity and metabolic syndrome in non-diabetic individuals. Endocr. J. 58, 39–46 [DOI] [PubMed] [Google Scholar]
- 16.Bottcher Y., Unbehauen H., Kloting N., Ruschke K., Korner A., Schleinitz D., Tonjes A., Enigk B., Wolf S., Dietrich K., Koriath M., Scholz G. H., Tseng Y. H., Dietrich A., Schon M. R., Kiess W., Stumvoll M., Bluher M., Kovacs P. (2009) Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes 58, 2119–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz M. W., Porte D., Jr. (2005) Diabetes, obesity, and the brain. Science 307, 375–379 [DOI] [PubMed] [Google Scholar]
- 18.Coll A. P., Farooqi I. S., O'Rahilly S. (2007) The hormonal control of food intake. Cell 129, 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charytoniuk D. A., Traiffort E., Pinard E., Issertial O., Seylaz J., Ruat M. (2000) Distribution of bone morphogenetic protein and bone morphogenetic protein receptor transcripts in the rodent nervous system and up-regulation of bone morphogenetic protein receptor type II in hippocampal dentate gyrus in a rat model of global cerebral ischemia. Neuroscience 100, 33–43 [DOI] [PubMed] [Google Scholar]
- 20.Dattatreyamurty B., Roux E., Horbinski C., Kaplan P. L., Robak L. A., Beck H. N., Lein P., Higgins D., Chandrasekaran V. (2001) Cerebrospinal fluid contains biologically active bone morphogenetic protein-7. Exp. Neurol. 172, 273–281 [DOI] [PubMed] [Google Scholar]
- 21.Ohyama K., Das R., Placzek M. (2008) Temporal progression of hypothalamic patterning by a dual action of BMP. Development 135, 3325–3331 [DOI] [PubMed] [Google Scholar]
- 22.Ebendal T, Bengtsson H, Soderstrom S. (1998) Bone morphogenetic proteins and their receptors: potential functions in the brain. J. Neurosci. Res. 51, 139–146 [DOI] [PubMed] [Google Scholar]
- 23.Lowell B. B., Spiegelman B. M. (2000) Towards a molecular understanding of adaptive thermogenesis. Nature 404, 652–660 [DOI] [PubMed] [Google Scholar]
- 24.Farooqi I. S., Bullmore E., Keogh J., Gillard J., O'Rahilly S., Fletcher P. C. (2007) Leptin regulates striatal regions and human eating behavior. Science 317, 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueki K., Kondo T., Tseng Y. H., Kahn C. R. (2004) Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc. Natl. Acad. Sci. USA 101, 10422–10427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cannon C. M., Scannell C. A., Palmiter R. D. (2005) Mice lacking dopamine D1 receptors express normal lithium chloride-induced conditioned taste aversion for salt but not sucrose. Eur. J. Neurosci. 21, 2600–2604 [DOI] [PubMed] [Google Scholar]
- 27.Godin R. E., Takaesu N. T., Robertson E. J., Dudley A. T. (1998) Regulation of BMP7 expression during kidney development. Development 125, 3473–3482 [DOI] [PubMed] [Google Scholar]
- 28.Soderstrom S., Ebendal T. (1999) Localized expression of BMP and GDF mRNA in the rodent brain. J. Neurosci. Res. 56, 482–492 [DOI] [PubMed] [Google Scholar]
- 29.Lu D., Willard D., Patel I. R., Kadwell S. H., Overton L., Kost T., Luther M., Chen W., Woychik R. P., Wilkison W. O., Cone R. D. (1994) Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature 371, 799–802 [DOI] [PubMed] [Google Scholar]
- 30.Shimizu H., Inoue K., Mori M. (2007) The leptin-dependent and -independent melanocortin signaling system: regulation of feeding and energy expenditure. J. Endocrinol. 193, 1–9 [DOI] [PubMed] [Google Scholar]
- 31.Langenfeld E. M., Kong Y., Langenfeld J. (2005) Bone morphogenetic protein-2-induced transformation involves the activation of mammalian target of rapamycin. Mol. Cancer Res. 3, 679–684 [DOI] [PubMed] [Google Scholar]
- 32.Rajan P., Panchision D. M., Newell L. F., McKay R. D. (2003) BMPs signal alternately through a SMAD or FRAP-STAT pathway to regulate fate choice in CNS stem cells. J. Cell Biol. 161, 911–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nohe A., Keating E., Knaus P., Petersen N. O. (2004) Signal transduction of bone morphogenetic protein receptors. Cell. Signal. 16, 291–299 [DOI] [PubMed] [Google Scholar]
- 34.Bluher S., Mantzoros C. S. (2009) Leptin in humans: lessons from translational research. Am. J. Clin. Nutr. 89, 991S–997S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjorbaek C., Elmquist J. K., Frantz J. D., Shoelson S. E., Flier J. S. (1998) Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol. Cell 1, 619–625 [DOI] [PubMed] [Google Scholar]
- 36.Blouet C., Ono H., Schwartz G. J. (2008) Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 8, 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cota D., Proulx K., Smith K. A., Kozma S. C., Thomas G., Woods S. C., Seeley R. J. (2006) Hypothalamic mTOR signaling regulates food intake. Science 312, 927–930 [DOI] [PubMed] [Google Scholar]
- 38.Mori H., Inoki K., Munzberg H., Opland D., Faouzi M., Villanueva E. C., Ikenoue T, Kwiatkowski D., MacDougald O. A, Myers M. G., Jr., Guan K. L. (2009) Critical role for hypothalamic mTOR activity in energy balance. Cell Metab. 9, 362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villanueva E. C., Munzberg H., Cota D., Leshan R. L., Kopp K., Ishida-Takahashi R., Jones J. C., Fingar D. C., Seeley R. J., Myers M. G., Jr. (2009) Complex regulation of mammalian target of rapamycin complex 1 in the basomedial hypothalamus by leptin and nutritional status. Endocrinology 150, 4541–4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alessi D. R., Pearce L. R., Garcia-Martinez J. M. (2009) New insights into mTOR signaling: mTORC2 and beyond. Sci. Signal. 2, e27. [DOI] [PubMed] [Google Scholar]
- 41.Stoica L., Zhu P. J., Huang W., Zhou H., Kozma S. C., Costa-Mattioli M. (2011) Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc. Natl. Acad. Sci. USA 108, 3791–3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marsh D. J., Hollopeter G., Huszar D., Laufer R., Yagaloff K. A., Fisher S. L., Burn P., Palmiter R. D. (1999) Response of melanocortin-4 receptor-deficient mice to anorectice and orexigenic peptides. Nat. Genet. 21, 119–122 [DOI] [PubMed] [Google Scholar]
- 43.Xu B., Goulding E. H, Zang K., Cepoi D., Cone R. D., Jones K. R., Tecott L. H., Reichardt L. F. (2003) Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 6, 736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liao L., Pilotte J, Xu T, Wong C. C., Edelman G. M., Vanderklish P., Yates J.R., III. (2007) BDNF induces widespread changes in synaptic protein content and up-regulates components of the translation machinery: an analysis using high-throughput proteomics. J. Prot. Res. 6, 1059–1071 [DOI] [PubMed] [Google Scholar]
- 45.Takei N., Inamura N., Kawamura M., Namba H., Hara K., Yonezawa K., Nawa H. (2004) Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J. Neurosci. 24, 9760–9769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chai B., Li J. Y., Zhang W., Wu X., Zhang C., Mulholland M. W. (2010) Melanocortin-4 receptor activation promotes insulin-stimulated mTOR signaling. Peptides 31, 1888–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cota D., Matter E. K., Woods S. C., Seeley R. J. (2008) The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J. Neurosci. 28, 7202–7208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lebrun B., Bariohay B., Moyse E., Jean A. (2006) Brain-derived neurotrophic factor (BDNF) and food intake regulation: a minireview. Auton. Neurosci. 126–127, 30–38 [DOI] [PubMed] [Google Scholar]
- 49.Vacher C. M., Crepin D., Aubourg A., Couvreur O., Bailleux V., Nicolas V., Ferezou J., Gripois D., Gertler A., Taouis M. (2008) A putative physiological role of hypothalamic CNTF in the control of energy homeostasis. FEBS Lett. 582, 3832–3838 [DOI] [PubMed] [Google Scholar]
- 50.Perides G., Jensen F. E., Edgecomb P., Rueger D. C., Charness M. E. (1995) Neuroprotective effect of human osteogenic protein-1 in a rat model of cerebral hypoxia/ischemia. Neurosci. Lett. 187, 21–24 [DOI] [PubMed] [Google Scholar]
- 51.Sabo J. K., Kilpatrick T. J., Cate H. S. (2009) Effects of bone morphogenic proteins on neural precursor cells and regulation during central nervous system injury. Neurosignals 17, 255–264 [DOI] [PubMed] [Google Scholar]
- 52.Cordeira J. W., Frank L., Sena-Esteves M., Pothos E. N., Rios M. (2010) Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J. Neurosci. 30, 2533–2541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rios M., Fan G., Fekete C., Kelly J., Bates B., Kuehn R., Lechan R. M., Jaenisch R. (2001) Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 15, 1748–1757 [DOI] [PubMed] [Google Scholar]
- 54.Fulton S., Pissios P., Manchon R. P., Stiles L., Frank L., Pothos E. N., Maratos-Flier E., Flier J. S. (2006) Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51, 811–822 [DOI] [PubMed] [Google Scholar]
- 55.Leinninger G. M., Opland D. M., Jo Y. H., Faouzi M., Christensen L., Cappellucci L. A., Rhodes C. J., Gnegy M. E., Becker J. B., Pothos E. N., Seasholtz A. F., Thompson R. C., Myers M. G., Jr. (2011) Leptin action via neurotensin neurons controls orexin, the mesolimbic dopamine system and energy balance. Cell Metab. 14, 313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hayes M. R., Skibicka K. P., Leichner T. M., Guarnieri D. J., DiLeone R. J., Bence K. K., Grill H. J. (2010) Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab. 11, 77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang C. F., Lin S. Z., Chiang Y. H., Morales M., Chou J., Lein P., Chen H. L., Hoffer B. J., Wang Y. (2003) Intravenous administration of bone morphogenetic protein-7 after ischemia improves motor function in stroke rats. Stroke 34, 558–564 [DOI] [PubMed] [Google Scholar]
- 58.Zeisberg M., Hanai J., Sugimoto H., Mammoto T., Charytan D., Strutz F., Kalluri R. (2003) BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 9, 964–968 [DOI] [PubMed] [Google Scholar]
- 59.Chou J., Harvey B. K., Chang C. F., Shen H., Morales M., Wang Y. (2006) Neuroregenerative effects of BMP7 after stroke in rats. J. Neurol. Sci. 240, 21–29 [DOI] [PubMed] [Google Scholar]
- 60.Boon M. R., van der H. G., van der P. G., Tamsma J. T., Smit J. W., Rensen P. C. (2011) Bone morphogenetic protein 7: A broad-spectrum growth factor with multiple target therapeutic potency. Cytokine Growth Factor Rev. 7, 1–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data