Polarized sorting and trafficking in epithelial cells (original) (raw)
Abstract
The polarized distribution of proteins and lipids at the surface membrane of epithelial cells results in the formation of an apical and a basolateral domain, which are separated by tight junctions. The generation and maintenance of epithelial polarity require elaborate mechanisms that guarantee correct sorting and vectorial delivery of cargo molecules. This dynamic process involves the interaction of sorting signals with sorting machineries and the formation of transport carriers. Here we review the recent advances in the field of polarized sorting in epithelial cells. We especially highlight the role of lipid rafts in apical sorting.
Keywords: epithelial polarity, lipid rafts, polarized sorting
Introduction
Throughout the body, polarized epithelial cells are organized into sheets that line the surfaces and cavities of organs (for example, in the respiratory, urinary and digestive systems) to perform multiple physiological functions. The most important functions of these epithelia are protection and the maintenance of homeostasis by regulated exchange between the exterior and the interior milieus, as well as the build-up of most organs in the body. Exchange by vectorial transport for uptake and secretion is managed by a large array of transporters, channels and receptors that are distributed in distinct plasma membrane domains1. The plasma membrane of a polarized epithelial cell is subdivided into an apical and a basolateral domain by tight junctions2,3. The basolateral domain comprising spot desmosomes, gap junctions and adherent junction contacts with the basement membrane and neighboring cells mediates cell to cell contacts and communication. The apical domain confronts the external milieu and consists of planar regions and protrusions (microvilli and the primary cilium), mediating an exchange with the external environment. The apical surface has to be constructed such that it can withstand the threats from the outside. For instance, in the digestive tract, the secreted bile salts act as detergents and could potentially solubilize the apical membrane. Here, the asymmetric lipid composition of the apical and the basolateral plasma membrane domains comes into play. The apical membrane is enriched in sphingolipids, which together with cholesterol have the potential to form tightly packed membrane microdomains (lipid rafts) that help to form a robust bilayer4.
Establishment and maintenance of epithelial polarity are necessary for the normal physiological function of an epithelial cell. This requires complex sorting machineries that deliver proteins and lipids to their proper membrane domains. This review will describe the molecular mechanisms of polarized sorting by which epithelial surface polarity is established and maintained, mostly using Madin–Darby canine kidney (MDCK) cells as an experimental model system, focusing on the biogenesis of the apical membrane.
Establishment of epithelial polarity
Most eukaryotic cells are polarized. Some are polarized transiently, such as migrating fibroblasts, and others such as epithelial cells show a stably polarized phenotype. The polarization machinery has common features involving polarity protein complexes such as the Par proteins and the actin and tubulin dynamic networks5. Polarizing epithelial cells often use external cues to start the process, involving the extracellular matrix and integrins that define the basal pole6. In unpolarized MDCK cells, the microtubules are nucleated by the centrosome, like in fibroblasts, but upon polarization the microtubular network is re-organized, so that in the fully polarized state the bulk of the microtubules runs parallel to the apico-basal polarity axis with their minus ends underneath the apical surface7. There is also a horizontal network underlying the apical surface, where the actin organizes to form apical microvilli and an apical terminal web, involving villins and ezrins8. Along the lateral cell surface, actin has a different arrangement with E-cadherin playing a role as organizer9.
Three polarity complexes guide the polarization process: Crumbs, Par and Scribble5. Crumbs and Par collaborate to mark the apical domain, while Scribble defines the basolateral plasma membrane domain. It is not known as to how this is done exactly, but as soon as the domain markers are in place they exclude each other, so that the apical polarity proteins cannot enter the basolateral domain and vice versa5.
An important event10 in establishing epithelial polarity is the introduction of the junctional complexes11. The polarity proteins are involved in facilitating the assembly of the tight junctions that function both as a gate to regulate paracellular transport and as a fence to block the mixing of apical and basolateral proteins by lateral diffusion12. The tight junctions also act as a barrier for lipid diffusion but only in the extracellular bilayer leaflet13.
The primary cilium is a specialized part of the apical plasma membrane, having a unique lipid and protein composition. The ciliary membrane thus constitutes a separate domain in the apical membrane with a septin barrier that prevents the mixing of components14. Several proteins and protein complexes have been implicated in ciliogenesis. FAPP2, a phosphatidylinositol 4-phosphate adaptor protein, is involved in apical trafficking and its knockdown strongly delays ciliogenesis15. Annexin 13, syntaxin 316 and the polarity complex comprising PDZ domain-containing proteins Par3, Par6 and atypical protein kinase C17,18, all have been implicated in the establishment of the apical membrane during cell polarization and the biogenesis of the cilium. The same is true for the exocyst19,20, an octameric protein complex involved in the vesicle tethering before its fusion with the plasma membrane. Experiments showed that transport to the cilium requires the BBSome, a multiprotein complex of Bardet–Biedl Syndrome proteins, and functional Rab821,22,23. Recent research indicated that the BBSome constitutes a coat complex that sorts membrane proteins to primary cilia24.
Lipid analysis of the changes occurring during polarization of the MDCK cells provided an additional insight into this highly dynamic process25. The most striking changes were that the sphingolipids became longer, more hydroxylated and more glycosylated when compared with their counterparts in the not yet polarized epithelium. In parallel, the glycerophospholipids acquired longer and more unsaturated fatty acids. Most importantly, the Forssman glycosphingolipid, practically absent in the unpolarized MDCK cells, became the major sphingolipid in the fully polarized epithelium. Analogously, when the MDCK cells depolarized towards the mesenchymal state, the lipids changed back to that of the contact-naïve cells25. Observed changes in lipidomes of polarizing MDCK cells are in line with the composition of their purified apical membranes26. These changes are what one would expect when a lipid raft-enriched apical plasma membrane domain is introduced into the epithelial cell surface to form a robust and impermeable barrier facing the outer environment.
Trafficking routes in epithelial cells
To generate the asymmetric cell surface that characterizes epithelial cells, the apical and the basolateral proteins and lipids have to be transported from their site of synthesis to the correct final destination. In polarized epithelial cells, the trans-Golgi network (TGN) is considered to be the main sorting station for newly synthesized proteins and lipids destined for the cell surface27. Support for this hypothesis came from early biochemical and morphological studies that demonstrated that apically delivered influenza virus and basolaterally delivered vesicular stomatitis virus glycoproteins are still together at the trans-side of the Golgi complex and they separate just afterwards28,29,30. Live cell-imaging studies31,32,33 confirmed that distinct cargo-containing carriers were formed at the TGN and delivered to the plasma membrane without apparent detouring via endosomes. More experiments broadened the view and suggested that protein sorting is not confined to the TGN only but may occur at other locations along the biosynthetic pathway34,35,36,37,38. In epithelial cells, besides the TGN-sorting station, there are two distinct classes of early endosomes39: apical early endosomes and basolateral early endosomes (BEE) and at least two functionally distinct recycling endosomes37: apical recycling endosomes (ARE) and common recycling endosomes (CRE). Internalized transmembrane proteins that enter these compartments can be sorted for recycling to the cell surface, for transport to the lysosome, for transcytosis or for retrograde transport to the TGN. The complex trafficking routes connecting these sorting stations and the trafficking machineries involved were described in excellent reviews1,40.
In contrast to the direct delivery to the apical plasma membrane domain, some apically targeted cargoes are transported via the transcytotic pathway and reach their destination only after a detour to the basolateral membrane. One of the best-studied example of transcytosis in MDCK cells is the transport of polymeric immunoglobulins by the polymeric immunoglobulin receptor (pIgR)41, the cytoplasmic domain, which contains basolateral and endocytic sorting signals. Guided by the basolateral sorting signal, newly synthesized pIgR arrives at the basolateral membrane directly from the TGN or via the BEE42, where it can bind polymeric immunoglobulins. The complex is then internalized via clathrin-mediated endocytosis and traverses the CRE and ARE before arriving at the apical surface43, directed by specific transcytosis signals44. Transcytosis is stimulated by ligand binding and consequent signaling events45.
Another route to the apical membrane is dependent on a luminal clustering agent. Galectin-3 was identified to interact directly with the apical cargo lactase-phlorizin hydrolase (LPH) in a glycan-dependent manner46. Depletion of galectin-3 from MDCK cells resulted in missorting of apical membrane proteins, such as LPH and P75, to the basolateral domain. Intriguingly, high molecular weight clusters of apical glycoproteins were observed only in the presence of galectin-3, suggesting a role for the lectin in cluster formation. This cluster was found to be carbohydrate-dependent, because its formation and apical sorting were perturbed in glycosylation-deficient MDCK cells47. The cargo proteins that were shown to be dependent on galectin-3 were not detergent-resistant and thus this pathway was considered to be raft-independent. Taken together, these data support the model that binding of galectin-3 cross-links apical glycoproteins and/or glycolipids into clusters that can then be sorted into specific apical transport carriers. Interestingly, galectin-4, another member of the family, associates with sulfatides to form another type of sorting platform for the delivery of proteins to the apical domain in intestinal HT29 cells48,49.
Most recently, interaction of galectin-9 with Forssman glycosphingolipid was shown to be necessary for the maintenance of MDCK polarity50. The loss of epithelial polarity caused by galectin-9 knockdown could be rescued by the addition of recombinant galectin-9. The Forssman sphingolipid was identified as the surface receptor that mediates the cycling of galectin-9 between the Golgi apparatus and the apical domain in polarized MDCK cells. The identification of galectin-9 in apical membrane biogenesis has provided a missing link that could function as a clustering agent in apical raft sorting and could be a key to understanding the mechanism of protein and lipid sorting in the TGN of MDCK cells.
Additionally, several other proteins have been implicated in the apical sorting processes. Annexin 2 and annexin 13b are involved in apical transport in MDCK cells51,52,53, and annexins, including annexin 2, have been shown to form two-dimensional arrays54,55. Also myelin and lymphocyte (VIP17/MAL) proteolipids are membrane proteins having a role in the apical transport55,56. VIP17/MAL can form clusters that show lateral concentration of sphingolipid markers and exclusion of a fluorescent analogue of unsaturated phosphatidylethanolamine, making VIP17/MAL an interesting player in the organization of membrane domains and sorting platforms57. Interestingly, MAL2, like VIP17/MAL, is involved in apical transport and while the latter protein regulates direct apical transport from the TGN, MAL2 is a part of the transcytosis machinery58.
Why are there so many pathways and components involved in the polarized sorting in epithelial cells? What is their physiological significance? Multiple and highly regulated pathways are most likely required for eliciting specific cellular responses to extracellular signals. Signaling and sorting are highly interconnected. In addition, multiple pathways might enhance the fidelity of sorting. Moreover, the existence of many traffic routes to different destinations makes the trafficking systems more robust compared with one single route.
This latter aspect constitutes a challenge for investigators studying these complex delivery routes to the different cell-surface domains. The fact that they are interconnected means that they are often redundant. Therefore, interference with one machinery protein may not give raise to any phenotype in response to the change, because the cargo can also take another route59. In other cases, some specific phenotypes may be hard to interpret. For example, the outgrowth of the cilium represents the final stage in epithelial morphogenesis7, but is easily perturbed16. Therefore, it can be difficult to specify the exact reason for impaired ciliogenesis and to pinpoint the responsible pathways and mechanisms.
Apical sorting mechanisms
Apical sorting signals
Apical sorting signals are required to direct the transport of newly synthesized proteins to the apical cell surface. Remarkably, sorting signals have been localized to all the portions of apical proteins: extracellular, transmembrane and cytoplasmic domains8,60.
A well-studied apical sorting signal is the glycosylphosphatidylinositol (GPI) anchor. GPI-anchored proteins (GPI-APs) are preferentially localized to the apical membrane of epithelial cells61. Supporting evidence for the role of GPI anchors in apical localization comes from the fact that not only endogenous GPI-APs but also chimeric GPI-APs62,63,64 in polarized MDCK cells localize apically. However, the relative strength of this sorting signal and what determines in detail whether a GPI-AP will be routed to the apical membrane remain not completely understood. For example, the GPI-anchored prion protein was shown to localize basolaterally in MDCK cells65, and GPI-APs are preferentially targeted to the basolateral surface in Fischer rat thyroid epithelial cells66. Importantly, clustering of GPI-AP is necessary for efficient apical targeting67,68. Furthermore, the GPI-attachment sequences69 and the remodeling of the fatty-acid chains70 seem to play important roles in membrane targeting8.
_N_- and _O_-linked protein glycosylation are other apical sorting signals8,71,72. Glycan structures are extraordinarily diverse, thus having considerable information potential, nevertheless the molecular mechanism for apical sorting of glycosylated proteins has not been determined yet73, although their functional interactions with lectins during sorting at the TGN were postulated72. The sequential addition of one to five _N_-glycans to the basolaterally located Na+/K+-ATPase β1-subunit caused a gradual redirection of this subunit to the apical domain in HGT-1 cells74. Similarly, the _O_-glycosylated stalk domain in neurotrophin receptor p75 (p75NTR) is necessary for its apical targeting. An internal deletion of 50 amino acids that removes this stalk domain from p75NTR causes this protein to be sorted to the basolateral plasma membrane71. Oligomerization and apical sorting of glycosylated GPI-APs may not involve _N_- and _O_-glycans directly, but may depend on a lipid raft-associated glycosylated interactor75.
Also, proteoglycan-sorting determinants have been identified76. Proteoglycans with chondroitin sulfate are preferentially sorted to the apical membrane, while those carrying heparan sulfate are routed basolaterally.
Transmembrane apical sorting signals have been identified in influenza virus hemagglutinin (HA) and neuraminidase, but so far little work has been done to uncover the underlying sorting principles77,78.
Other apical sorting signals have been found, e.g., in rhodopsin79, megalin80, M2 muscarinic acetylcholine receptor81, the copper transporting P-type ATPase (ATP7B)82 and the Na-K-Cl cotransporter (NKCC2)83, and they ranged from short motifs of a few amino acids to up to 30 amino acids long stretches.
The diversity of apical sorting determinants implies that several different mechanisms are employed to route the apical proteins to their destination. One such mechanism in MDCK cells involves lipid rafts as apical sorting platform in the Golgi complex84.
Lipid rafts in apical sorting
A role of lipid rafts in polarized epithelial sorting was suggested long ago. This was the origin of the lipid raft concept: apical proteins were postulated to be sorted through their affinity for microdomains of glycosphingolipids and cholesterol, assembled in the Golgi complex to form apical transport carriers4,84. The concept was generalized into a dynamic sub-compartmentalization principle, making use of sphingolipids and sterols to form small fluid membrane entities (lipid rafts) with specific proteins included. Lipid rafts are now defined as dynamic, nanometer-sized, sterol-sphingolipid-enriched, tightly packed lipid–protein assemblies that fluctuate on a sub-second time scale85,86,87,88,89. These assemblies can be induced to cluster to form more stable, specific ordered lipid raft platforms, which exert functions in membrane trafficking, cell polarization, signaling and other membrane processes88,89.
The best studied apical cargo that employs lipid rafts to be delivered to the apical membrane is the influenza virus HA. HA becomes detergent-resistant after entering the Golgi complex90,91,92. Obviously, detergent-resistant membranes (DRMs) cannot be directly equated with lipid rafts, as has often been the case93,94, though DRM analysis is a useful method to determine a protein's raft association potential when changes in DRM composition are induced by biochemically/physiologically meaningful events94,95. However, HA lipid raft association was also demonstrated by several other studies involving different methods. First, depletion of raft lipids, such as cholesterol and sphingolipids, resulted in the missorting of HA on its way to the apical domain of MDCK cells96,97,98. Second, antibody-mediated cross-linking of HA, GPI-proteins or non-raft proteins led to cholesterol-dependent co-patching of HA with GPI-proteins, while excluding non-raft proteins99. Third, photonic force microscopy demonstrated that HA was moving as a cholesterol-dependent assembly with a size of 50 nm in the plasma membrane100. In these experiments, beads containing antibodies that bound the HA protein were immobilized by an optical trap. Although binding to more than one HA protein was prevented, the force field applied to the cell and the immobilization of the protein by the trap could have altered the lifetime of the nanoscale HA-protein assemblies and caused them to grow larger than in the resting state. Nevertheless, this was clear demonstration that the HA protein was associated with lipids. Fourth, studies employing quantitative electron microscopy and fluorescence spectroscopy also showed that HA was present in microdomains of different sizes, which could be modulated by cholesterol and sphingolipid depletion101,102. Fifth, fluorescence photoactivation localization microscopy demonstrated that HA was present in nanoscale domains of different sizes103. And finally sixth, FRET microscopy showed that HA clustered with GPI-proteins on the cell surface in a cholesterol-dependent manner104. Altogether, these various experiments demonstrated that the HA protein is present in dynamic cholesterol-dependent assemblies, which is in the agreement with the lipid raft concept.
What is still missing from showing that rafts are directly involved in transport from the TGN to the apical membrane is the demonstration that the apical transport carriers are enriched in raft lipids as predicted by the concept. In yeast, Klemm et al.105 used a lipid raft-associated plasma membrane protein as bait to isolate TGN-derived vesicles and subsequently characterized their lipid composition by mass spectrometry. Their results showed that yeast sphingolipids and ergosterol (the equivalent to cholesterol in animal cells) are sorted at the TGN and transported in specific secretory vesicles to the cell surface. This was the first time that a transport carrier involved in a lipid raft-dependent pathway has been isolated and characterized. The finding that raft lipids are enriched in these carriers brought convincing support to the raft concept as originally postulated. Further experiments with additional yeast plasma membrane proteins as baits showed that sorting of raft lipids is a generic feature of vesicles carrying transmembrane and GPI-protein cargoes to the plasma membrane106.
For a long time, a disturbing issue in the field has been the lack of genetic evidence for the lipid raft-sorting model in the generation and maintenance of the apical membrane. Why has all the work on different model organisms failed to identify lipid raft elements in the genetic screens of mutations affecting epithelial polarity? However, this gap has been closed recently. Through a combination of genetic screens, lipid analysis and imaging methods, it was established that glycosphingolipids indeed play a role in mediating apical sorting in the gut of Caenorhabditis elegans107.
Generation of apical transport carriers
After sorting in the plane of the membrane, cargo must be selectively incorporated into specific transport carriers. Membrane curvature has to be generated to form cargo-containing membrane buds or tubules, followed by subsequent scission to release the transport carrier from the donor membrane.
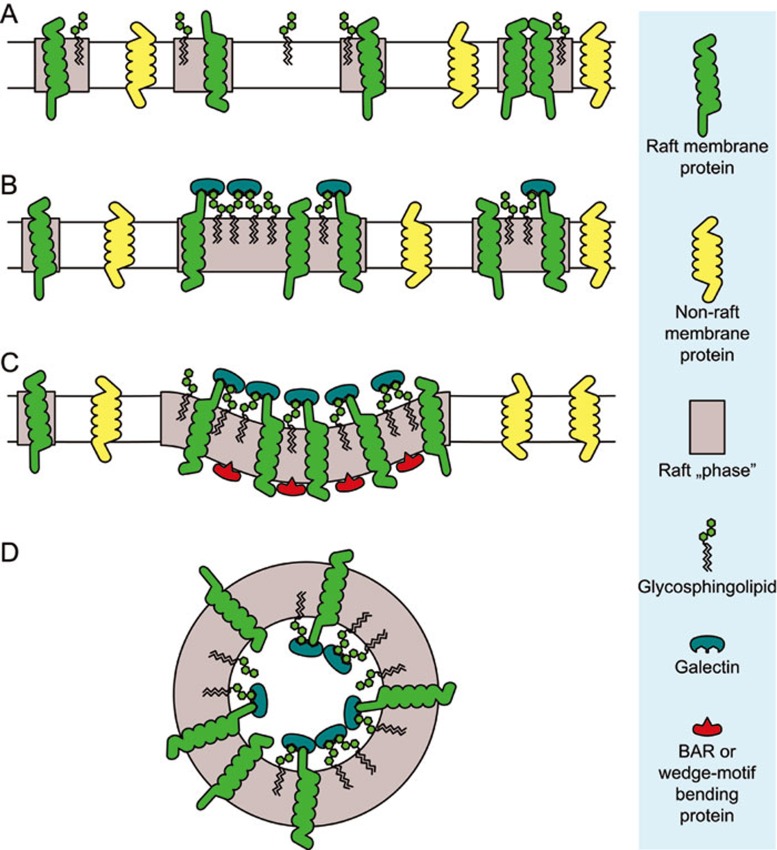
Since the advent of the lipid raft concept, raft clustering was postulated as a major driving force in the generation of transport carriers. In cellular membranes, nanoscale rafts are usually dispersed in a continuous non-raft phase108,109. In model membranes, coexistence of liquid-ordered and liquid-disordered phases results in line tension at the phase boundary, which arises from the immiscibility of membrane components that prefer different phases110. Clustering of small rafts into larger domains further increases the line tension, which in three dimensional system can be relieved by domain budding from the donor membrane, followed by fission at the phase boundaries, resulting in the generation of vesicles enriched in raft components60 (Figure 1). The growing curvature of a membrane close to the demixing point (phase separation) further induces lipid sorting based solely on their underlying connectivity, which is greatly amplified by their clustering111. Since curvature of a membrane can also drive protein sorting, a growing bud can generate a feedback system whereby curvature-preferring proteins would be recruited to a growing lipid raft platform, further increasing the propensity to generate curvature112,113,114,115. Once a curved membrane is generated, phase separation in membrane tubes can trigger membrane fission arising from the difference in elastic constants between the domains115,116.
Figure 1.
A scheme for apical transport carrier formation by domain-induced budding. (A) Nanoscale dynamic rafts surrounded by non-raft membrane. (B) Growing rafts are selectively induced by galectin–glycolipid–glycoprotein interactions into a budding domain, while non-raft components are excluded. Raft clustering results in increased line tension. (C) Insertion of hydrophobic or amphipathic protein domains (red) promotes membrane bending. FAPP2 could play this role for membrane deformation. (D) Fission at the domain boundary (possibly aided by fission proteins) results in the release of an apical transport carrier. For simplicity GPI-APs, cholesterol and other (e.g., palmitoylated proteins) proteins are not shown and cytoskeletal elements are omitted as well.
Supporting this domain-budding hypothesis, it was shown that the interaction of the B-subunit of Shiga toxin with the plasma membrane glycosphingolipid Gb3 is sufficient for clustering, which increases the bilayer order in these regions117. This, together with an asymmetric membrane stress imposed by the toxin, results in negative curvature of the membrane and induced tubule formation. Similarly, membrane invaginations are induced by Simian virus 40 binding to GM1 gangliosides118. Therefore, multivalent binding of specific lipids and clustering can result in membrane tube formation and a similar mechanism might be at work at the TGN.
Galectins, annexins and VIP17/MAL proteins, involved in apical trafficking and with a potential to cluster or array, are possible mediators of lipid clustering upon the exit from the TGN. For the raft-mediated pathway in MDCK cells, galectin-9 is the strongest candidate for a clustering function64 due to its binding to the Forssman glycolipid50.
The process of carrier generation probably does not rely solely on the lipid clustering. Bending proteins are likely to be essential for successful transport carrier formation (Figure 1). There are two principal mechanisms of protein-induced membrane curvature. BAR domain–containing proteins are 'banana-shaped' and thus confer curvature by direct membrane scaffolding119,120. They bind to membranes by their positively charged concave face and therefore are able to sense, stabilize, and generate membrane curvature. Recently, Willenborg et al. reported that the sorting nexin 18 (SNX-18), a BAR domain-containing protein, together with the Rab11 GTPase-binding protein FIP5, which enhances its tubulation potential, is involved in the formation of podocalyxin-containing apical carriers121. The other mechanism relies on the insertion of a small amphipathic or hydrophobic wedge to induce membrane asymmetry resulting in curvature122. Recently, the FAPP2 protein, involved in the transport of apical cargo in polarized MDCK cells, was shown to possess phosphatidylinositol 4-P-dependent membrane tubulation activity, which could be attributed to a hydrophobic wedge in its PH domain123,124.
Proteins secreted apically have been shown to depend on _N_-glycans to be sorted correctly125. Galectin-3 seems not to be involved in this pathway126. Whether other galectins such as galectin-9 in MDCK cells plays a role in transporting secretory proteins to the apical side of the epithelium remains to be analyzed. Also in the basolateral direction, binding proteins or sorting receptors would be needed. However, little is so far known about how this is accomplished. Probably, each basolaterally secreted protein will need its own receptor because so far no general sorting signals have been identified. However, for basolateral transmembrane proteins specific cytoplasmic sorting signals have been identified.
Basolateral sorting
Basolateral sorting signals
Basolateral sorting signals are indeed relatively well defined when compared with apical sorting signals. Mellman and co-workers127 reported the existence of basolateral signals in the cytoplasmic domain of the low-density lipoprotein receptor and subsequently showed that these signals were also transplantable. Basolateral signals are usually located in the cytoplasmic tail of cargo proteins. They include tyrosine-based YXXØ, NPXY motifs (where X can be any amino acid and Ø is a bulky hydrophobic residue) and di-hydrophobic-based sorting signals40,128,129,130. Recently, Weise et al.131 identified two basolateral targeting signals in the surface glycoproteins of the Nipah virus, involving tyrosine 525 in the F protein and a di-tyrosine motif at position 28/29 in the G protein. There are also basolateral signals constituted of a single leucine patch as in CD147132 or other sequences as identified in neural cell adhesion molecule133, pIgR134, epidermal growth-factor receptor135, epidermal growth-factor receptor 2136 and transforming growth factor β137. Recently, a 25-residue region within the C-terminal tail of the P2Y(1) receptor was identified, where the total number of charged residues was found to be crucial for basolateral targeting138.
Basolateral sorting of syntaxin 4 depends on its N-terminal domain and the AP1B clathrin adaptor139. Here, a short stretch between residues 24 and 29 (ALVVHP) was identified as the sorting determinant.
Most likely other basolateral sorting signals remain to be identified and characterized.
Basolateral sorting mechanisms
How do basolateral sorting signals specify the destination? The fact that many basolateral proteins contain the di-hydrophobic-based or the tyrosine-based sorting signals resembling the clathrin-dependent endocytosis motifs has long suggested that adaptor protein (AP)–clathrin complexes play an important role in basolateral sorting. Five AP complexes (AP-1, AP-2, AP-3, AP-4 and recently discovered AP-5)140 have been identified to localize along the exocytic and endocytic routes and function in recognizing cargo and mediating vesicle formation129,141.
AP-1B is the only clathrin-associated AP adaptor with a well-characterized role in basolateral sorting142 and differs from the ubiquitous adaptor AP-1A by a different medium subunit mu1B143. AP-1B is expressed in various polarized epithelial cell lines, including MDCK, Caco-2, HT-29, Hec-1-A and RL95-2 cells. Lack of the mu1B subunit in the kidney epithelial cell line LLC-PK1 results in missorting of many basolateral proteins to the apical surface, but proper basolateral trafficking can be restored by stable expression of mu1B. Also AP-4 is involved in basolateral sorting, but the detailed mechanism remains to be defined144.
In polarized epithelial cells, only recent functional experiments provided the evidence that clathrin is required for basolateral plasma-membrane protein sorting145,146. Knockdown of the clathrin heavy chain in MDCK cells depolarized most basolateral proteins, by interfering with their biosynthetic delivery and recycling, but did not affect the polarity of apical proteins. Quantitative live imaging showed that clathrin knockdown selectively slowed down the exit of basolateral proteins from the Golgi complex and promoted their missorting into apical carrier vesicles. However, so far it is not known exactly at which step in basolateral sorting clathrin comes into play.
Membrane trafficking to the polarized cell surface
The release of transport vesicles carrying apical cargoes or basolateral cargoes from the TGN or recycling endosomes must be coordinated with vesicle trafficking, docking and fusion with target membranes during the establishment and maintenance of epithelial cell polarity. Both microtubules and actin play an important role in these processes8.
Vesicle transport along microtubules in polarized epithelial cells is driven by motor proteins. It was shown that the minus-end kinesin KIFC3 delivers influenza HA and annexin 13b to the apical domain147. KIF5B and KIF17, plus-end microtubule motors, are involved in apical targeting of P75148,149. Dynein has a role in rhodopsin transport to the apical membrane150. Trafficking of transport carriers destined for the basolateral domain is driven by different microtubule motors130,151.
The actin network not only provides the epithelial cell with structure and shape but also is thought to contribute to vesicle trafficking in several other ways, including vesicle formation, scission and fusion and vesicle transport8,152,153,154. Also myosins have been implied in apical trafficking155, and myosin 5B was shown to be required for apical polarization156. It interacts with Rab11 and Rab8156, and the latter one seems to play a role in both apical and basolateral delivery, but how this is regulated is not known157,158. Myosins, for instance myosin VI, are also involved in basolateral trafficking159. When and how actin and myosin mechanistically carry out their functions are not yet understood.
The cargo carriers also have to include many of the proteins that are required for specific delivery to the apical or basolateral plasma membrane domains. These include SNAREs and Rab proteins160,161. The exocyst, a tethering complex at the plasma membrane, not only plays an important role in tethering and spatial targeting of post-Golgi vesicles to the basolateral membrane prior to vesicle fusion162,163 but also is involved in the formation of basolateral transport vesicles164. It has been shown that basolateral cargo, such as E-cadherin, interacts with the exocyst subunits, AP-1B, and basolateral SNAREs, ensuring that basolateral delivery is a coordinated process165.
These are just glimpses of the dynamic interactions that regulate the whole trafficking system and bind it together. More extensive reviews on basolateral sorting can be found elsewhere130,166.
Conclusions
Epithelial cells employ an elaborate trafficking system to control the distribution of proteins and lipids to their apical and basolateral surface domains, which is an important determinant of cell polarity. The complex nature of the underlying mechanisms is still far from being completely understood. More research is necessary to discover how the involved machineries are functioning in the Golgi apparatus and endosomal sorting and to gain a better understanding of aspects such as transport routes, sorting signals, the exact role of lipid rafts and transport carrier formation. Also the role of cytoskeletal elements and motor proteins has to be included to yield a more comprehensive view of epithelial protein sorting. Signaling pathways and their cross-talk with the trafficking machinery will contribute a further layer of complexity. To be able to fully unravel how the machinery for epithelial protein and lipid sorting works, it will be necessary to embark on biochemical studies that aim at reconstituting in vitro the steps that lead to the segregation of apical and basolateral cargoes.
Acknowledgments
We thank Ünal Coskun and Robert Ernst for their critical reading of this manuscript. This work was supported by DFG “Schwerpunktprogramm1175” grant (SI459/2-1), DFG “Transregio 83” grant (TRR83 TP02), ESF “LIPIDPROD” grant (SI459/3-1), BMBF “ForMaT” grant (03FO1212), the Klaus Tschira Foundation and grants for Scientific Research of BSKY (XJ201123) from the Anhui Medical University.
References
- Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- Caplan MJ. Membrane polarity in epithelial cells: protein sorting and establishment of polarized domains. Am J Physiol. 1997;272:F425–F429. doi: 10.1152/ajprenal.1997.272.4.F425. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Yeaman C. Protein trafficking in the exocytic pathway of polarized epithelial cells. Trends Cell Biol. 2001;11:483–486. doi: 10.1016/s0962-8924(01)02145-6. [DOI] [PubMed] [Google Scholar]
- Simons K, Van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Datta A, Bryant DM, Mostov KE. Molecular regulation of lumen morphogenesis. Curr Biol. 2011;21:R126–R136. doi: 10.1016/j.cub.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacallao RL, McNeill H. Cystic kidney diseases and planar cell polarity signaling. Clin Genet. 2009;75:107–117. doi: 10.1111/j.1399-0004.2008.01148.x. [DOI] [PubMed] [Google Scholar]
- Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122:4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays RW, Beck KA, Nelson WJ. Organization and function of the cytoskeleton in polarized epithelial cells: a component of the protein sorting machinery. Curr Opin Cell Biol. 1994;6:16–24. doi: 10.1016/0955-0674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959. doi: 10.1126/science.1072161. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- van Meer G, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986;5:1455–1464. doi: 10.1002/j.1460-2075.1986.tb04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Milenkovic L, Jin H, et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WL, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc Natl Acad Sci USA. 2006;103:18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkko JM, Manninen A, Schuck S, Simons K. Depletion of apical transport proteins perturbs epithelial cyst formation and ciliogenesis. J Cell Sci. 2008;121:1193–1203. doi: 10.1242/jcs.015495. [DOI] [PubMed] [Google Scholar]
- Fan S, Hurd TW, Liu CJ, et al. Polarity proteins control ciliogenesis via kinesin motor interactions. Curr Biol. 2004;14:1451–1461. doi: 10.1016/j.cub.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Wang Q, Margolis B. Apical junctional complexes and cell polarity. Kidney Int. 2007;72:1448–1458. doi: 10.1038/sj.ki.5002579. [DOI] [PubMed] [Google Scholar]
- Oztan A, Silvis M, Weisz OA, et al. Exocyst requirement for endocytic traffic directed toward the apical and basolateral poles of polarized MDCK cells. Mol Biol Cell. 2007;18:3978–3992. doi: 10.1091/mbc.E07-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Guo W, Lipschutz JH. The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell. 2009;20:2522–2529. doi: 10.1091/mbc.E08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loktev AV, Zhang Q, Beck JS, et al. A BBSome subunit links ciliogenesis, microtubule stability, and acetylation. Dev Cell. 2008;15:854–865. doi: 10.1016/j.devcel.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Omori Y, Zhao C, Saras A, et al. Elipsa is an early determinant of ciliogenesis that links the IFT particle to membrane-associated small GTPase Rab8. Nat Cell Biol. 2008;10:437–444. doi: 10.1038/ncb1706. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, et al. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell. 2010;141:1208–1219. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio JL, Gerl MJ, Klose C, et al. Membrane lipidome of an epithelial cell line. Proc Natl Acad Sci USA. 2011;108:1903–1907. doi: 10.1073/pnas.1019267108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerl MJ, Sampaio JL, Urban S, et al. Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J Cell Biol. 2012;196:213–221. doi: 10.1083/jcb.201108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Simons K. The trans-Golgi network: sorting at the exit site of the Golgi complex. Science. 1986;234:438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Misek DE, Bard E, Rodriguez-Boulan E. Biogenesis of epithelial cell polarity: intracellular sorting and vectorial exocytosis of an apical plasma membrane glycoprotein. Cell. 1984;39:537–546. doi: 10.1016/0092-8674(84)90460-4. [DOI] [PubMed] [Google Scholar]
- Pfeiffer S, Fuller SD, Simons K. Intracellular sorting and basolateral appearance of the G protein of vesicular stomatitis virus in Madin–Darby canine kidney cells. J Cell Biol. 1985;101:470–476. doi: 10.1083/jcb.101.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller SD, Bravo R, Simons K. An enzymatic assay reveals that proteins destined for the apical or basolateral domains of an epithelial cell line share the same late Golgi compartments. EMBO J. 1985;4:297–307. doi: 10.1002/j.1460-2075.1985.tb03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Toomre D, Diaz E, White J, Simons K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat Cell Biol. 2001;3:140–149. doi: 10.1038/35055042. [DOI] [PubMed] [Google Scholar]
- Hirschberg K, Miller CM, Ellenberg J, et al. Kinetic analysis of secretory protein traffic and characterization of Golgi to plasma membrane transport intermediates in living cells. J Cell Biol. 1998;143:1485–1503. doi: 10.1083/jcb.143.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer G, Schmoranzer J, Low SH, et al. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat Cell Biol. 2003;5:126–136. doi: 10.1038/ncb917. [DOI] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, et al. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresawn KO, Potter BA, Oztan A, et al. Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. EMBO J. 2007;26:3737–3748. doi: 10.1038/sj.emboj.7601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in recycling endosomes regulates the sorting and basolateral transport of E-cadherin. Mol Biol Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H, Mattila PE, Weisz OA. Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells. Traffic. 2009;10:972–981. doi: 10.1111/j.1600-0854.2009.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D, Tyteca D, van ISC. The subapical compartment: a traffic center in membrane polarity development. J Cell Sci. 2004;117:2183–2192. doi: 10.1242/jcs.01217. [DOI] [PubMed] [Google Scholar]
- Sheff DR, Kroschewski R, Mellman I. Actin dependence of polarized receptor recycling in Madin-Darby canine kidney cell endosomes. Mol Biol Cell. 2002;13:262–275. doi: 10.1091/mbc.01-07-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov KE. Transepithelial transport of immunoglobulins. Annu Rev Immunol. 1994;12:63–84. doi: 10.1146/annurev.iy.12.040194.000431. [DOI] [PubMed] [Google Scholar]
- Orzech E, Cohen S, Weiss A, Aroeti B. Interactions between the exocytic and endocytic pathways in polarized Madin-Darby canine kidney cells. J Biol Chem. 2000;275:15207–15219. doi: 10.1074/jbc.275.20.15207. [DOI] [PubMed] [Google Scholar]
- Brown PS, Wang E, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Definition of distinct compartments in polarized Madin-Darby canine kidney (MDCK) cells for membrane-volume sorting, polarized sorting and apical recycling. Traffic. 2000;1:124–140. doi: 10.1034/j.1600-0854.2000.010205.x. [DOI] [PubMed] [Google Scholar]
- Luton F, Hexham MJ, Zhang M, Mostov KE. Identification of a cytoplasmic signal for apical transcytosis. Traffic. 2009;10:1128–1142. doi: 10.1111/j.1600-0854.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T, Bryant DM, Luton F, et al. A kinase cascade leading to Rab11-FIP5 controls transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2010;12:1143–1153. doi: 10.1038/ncb2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacour D, Cramm-Behrens CI, Drobecq H, Le Bivic A, Naim HY, Jacob R. Requirement for galectin-3 in apical protein sorting. Curr Biol. 2006;16:408–414. doi: 10.1016/j.cub.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Delacour D, Greb C, Koch A, et al. Apical sorting by galectin-3-dependent glycoprotein clustering. Traffic. 2007;8:379–388. doi: 10.1111/j.1600-0854.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- Delacour D, Gouyer V, Zanetta JP, et al. Galectin-4 and sulfatides in apical membrane trafficking in enterocyte-like cells. J Cell Biol. 2005;169:491–501. doi: 10.1083/jcb.200407073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechly L, Morelle W, Dessein AF, et al. Galectin-4-regulated delivery of glycoproteins to the brush border membrane of enterocyte-like cells. Traffic. 2009;10:438–450. doi: 10.1111/j.1600-0854.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- Mishra R, Grzybek M, Niki T, Hirashima M, Simons K. Galectin-9 trafficking regulates apical-basal polarity in Madin-Darby canine kidney epithelial cells. Proc Natl Acad Sci USA. 2010;107:17633–17638. doi: 10.1073/pnas.1012424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R, Heine M, Eikemeyer J, et al. Annexin II is required for apical transport in polarized epithelial cells. J Biol Chem. 2004;279:3680–3684. doi: 10.1074/jbc.C300503200. [DOI] [PubMed] [Google Scholar]
- Lafont F, Lecat S, Verkade P, Simons K. Annexin XIIIb associates with lipid microdomains to function in apical delivery. J Cell Biol. 1998;142:1413–1427. doi: 10.1083/jcb.142.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oling F, Bergsma-Schutter W, Brisson A. Trimers, dimers of trimers, and trimers of trimers are common building blocks of annexin a5 two-dimensional crystals. J Struct Biol. 2001;133:55–63. doi: 10.1006/jsbi.2000.4337. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Martin-Belmonte F, Millan J, et al. The MAL proteolipid is necessary for normal apical transport and accurate sorting of the influenza virus hemagglutinin in Madin-Darby canine kidney cells. J Cell Biol. 1999;145:141–151. doi: 10.1083/jcb.145.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong KH, Zacchetti D, Schneeberger EE, Simons K. VIP17/MAL, a lipid raft-associated protein, is involved in apical transport in MDCK cells. Proc Natl Acad Sci USA. 1999;96:6241–6248. doi: 10.1073/pnas.96.11.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magal LG, Yaffe Y, Shepshelovich J, et al. Clustering and lateral concentration of raft lipids by the MAL protein. Mol Biol Cell. 2009;20:3751–3762. doi: 10.1091/mbc.E09-02-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco MC, Martin-Belmonte F, Kremer L, et al. MAL2, a novel raft protein of the MAL family, is an essential component of the machinery for transcytosis in hepatoma HepG2 cells. J Cell Biol. 2002;159:37–44. doi: 10.1083/jcb.200206033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay E, Schekman R. A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J Cell Biol. 2002;156:271–285. doi: 10.1083/jcb.200109077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, Simons K. Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J Cell Sci. 2004;117:5955–5964. doi: 10.1242/jcs.01596. [DOI] [PubMed] [Google Scholar]
- Mayor S, Riezman H. Sorting GPI-anchored proteins. Nat Rev Mol Cell Biol. 2004;5:110–120. doi: 10.1038/nrm1309. [DOI] [PubMed] [Google Scholar]
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Sargiacomo M, Graeve L, Saltiel AR, Rodriguez-Boulan E. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci USA. 1988;85:9557–9561. doi: 10.1073/pnas.85.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Caras IW, Davitz MA, Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989;109:2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnataro D, Paladino S, Campana V, Grassi J, Nitsch L, Zurzolo C. PrPC is sorted to the basolateral membrane of epithelial cells independently of its association with rafts. Traffic. 2002;3:810–821. doi: 10.1034/j.1600-0854.2002.31106.x. [DOI] [PubMed] [Google Scholar]
- Zurzolo C, Lisanti MP, Caras IW, Nitsch L, Rodriguez-Boulan E. Glycosylphosphatidylinositol-anchored proteins are preferentially targeted to the basolateral surface in Fischer rat thyroid epithelial cells. J Cell Biol. 1993;121:1031–1039. doi: 10.1083/jcb.121.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan LA, Lisanti MP, Rodriguez-Boulan E, Edidin M. Correctly sorted molecules of a GPI-anchored protein are clustered and immobile when they arrive at the apical surface of MDCK cells. J Cell Biol. 1993;120:353–358. doi: 10.1083/jcb.120.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladino S, Sarnataro D, Pillich R, Tivodar S, Nitsch L, Zurzolo C. Protein oligomerization modulates raft partitioning and apical sorting of GPI-anchored proteins. J Cell Biol. 2004;167:699–709. doi: 10.1083/jcb.200407094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladino S, Lebreton S, Tivodar S, Campana V, Tempre R, Zurzolo C. Different GPI-attachment signals affect the oligomerisation of GPI-anchored proteins and their apical sorting. J Cell Sci. 2008;121:4001–4007. doi: 10.1242/jcs.036038. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Fujita M, Maeda Y. Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: recent progress. J Biochem. 2008;144:287–294. doi: 10.1093/jb/mvn090. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol. 1997;139:929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Potter BA, Weixel KM, Bruns JR, Ihrke G, Weisz OA. N-glycans mediate apical recycling of the sialomucin endolyn in polarized MDCK cells. Traffic. 2006;7:146–154. doi: 10.1111/j.1600-0854.2005.00371.x. [DOI] [PubMed] [Google Scholar]
- Vagin O, Turdikulova S, Sachs G. Recombinant addition of N-glycosylation sites to the basolateral Na,K-ATPase beta1 subunit results in its clustering in caveolae and apical sorting in HGT-1 cells. J Biol Chem. 2005;280:43159–43167. doi: 10.1074/jbc.M508262200. [DOI] [PubMed] [Google Scholar]
- Catino MA, Paladino S, Tivodar S, Pocard T, Zurzolo C. N- and O-glycans are not directly involved in the oligomerization and apical sorting of GPI proteins. Traffic. 2008;9:2141–2150. doi: 10.1111/j.1600-0854.2008.00826.x. [DOI] [PubMed] [Google Scholar]
- Prydz K, Dalen KT. Synthesis and sorting of proteoglycans. J Cell Sci. 2000;113:193–205. doi: 10.1242/jcs.113.2.193. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Roth MG, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu A, Avalos RT, Sanderson CM, Nayak DP. Transmembrane domain of influenza virus neuraminidase, a type II protein, possesses an apical sorting signal in polarized MDCK cells. J Virol. 1996;70:6508–6515. doi: 10.1128/jvi.70.9.6508-6515.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang JZ, Sung CH. The cytoplasmic tail of rhodopsin acts as a novel apical sorting signal in polarized MDCK cells. J Cell Biol. 1998;142:1245–1256. doi: 10.1083/jcb.142.5.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzolo MP, Yuseff MI, Retamal C, et al. Differential distribution of low-density lipoprotein-receptor-related protein (LRP) and megalin in polarized epithelial cells is determined by their cytoplasmic domains. Traffic. 2003;4:273–288. doi: 10.1034/j.1600-0854.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- Chmelar RS, Nathanson NM. Identification of a novel apical sorting motif and mechanism of targeting of the M2 muscarinic acetylcholine receptor. J Biol Chem. 2006;281:35381–35396. doi: 10.1074/jbc.M605954200. [DOI] [PubMed] [Google Scholar]
- Braiterman L, Nyasae L, Guo Y, Bustos R, Lutsenko S, Hubbard A. Apical targeting and Golgi retention signals reside within a 9-amino acid sequence in the copper-ATPase, ATP7B. Am J Physiol Gastrointest Liver Physiol. 2009;296:G433–G444. doi: 10.1152/ajpgi.90489.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmosino M, Rizzo F, Procino G, et al. MAL/VIP17, a new player in the regulation of NKCC2 in the kidney. Mol Biol Cell. 2010;21:3985–3997. doi: 10.1091/mbc.E10-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Rafts defined: a report on the Keystone Symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- Lenne PF, Wawrezinieck L, Conchonaud F, et al. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J. 2006;25:3245–3256. doi: 10.1038/sj.emboj.7601214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Simons K, Gerl MJ. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol. 2010;11:688–699. doi: 10.1038/nrm2977. [DOI] [PubMed] [Google Scholar]
- Skibbens JE, Roth MG, Matlin KS. Differential extractability of influenza virus hemagglutinin during intracellular transport in polarized epithelial cells and nonpolar fibroblasts. J Cell Biol. 1989;108:821–832. doi: 10.1083/jcb.108.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Kobayashi T, Kurzchalia TV, Simons K. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry. 1993;32:6365–6373. doi: 10.1021/bi00076a009. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Kurzchalia TV, Hartmann E, Dupree P. Guilty by insolubility – does a protein's detergent insolubility reflect a caveolar location. Trends Cell Biol. 1995;5:187–189. doi: 10.1016/s0962-8924(00)88990-4. [DOI] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Detergent resistance as a tool in membrane research. Nat Protoc. 2007;2:2159–2165. doi: 10.1038/nprot.2007.294. [DOI] [PubMed] [Google Scholar]
- Coskun U, Simons K. Membrane rafting: from apical sorting to phase segregation. FEBS Lett. 2010;584:1685–1693. doi: 10.1016/j.febslet.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Hansen GH, Niels-Christiansen LL, Thorsen E, Immerdal L, Danielsen EM. Cholesterol depletion of enterocytes. Effect on the Golgi complex and apical membrane trafficking. J Biol Chem. 2000;275:5136–5142. doi: 10.1074/jbc.275.7.5136. [DOI] [PubMed] [Google Scholar]
- Keller P, Simons K. Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mays RW, Siemers KA, Fritz BA, Lowe AW, van Meer G, Nelson WJ. Hierarchy of mechanisms involved in generating Na/K-ATPase polarity in MDCK epithelial cells. J Cell Biol. 1995;130:1105–1115. doi: 10.1083/jcb.130.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T, Scheiffele P, Verkade P, Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pralle A, Keller P, Florin EL, Simons K, Horber JK. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvartsman DE, Kotler M, Tall RD, Roth MG, Henis YI. Differently anchored influenza hemagglutinin mutants display distinct interaction dynamics with mutual rafts. J Cell Biol. 2003;163:879–888. doi: 10.1083/jcb.200308142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess ST, Kumar M, Verma A, Farrington J, Kenworthy A, Zimmerberg J. Quantitative electron microscopy and fluorescence spectroscopy of the membrane distribution of influenza hemagglutinin. J Cell Biol. 2005;169:965–976. doi: 10.1083/jcb.200412058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess ST, Gould TJ, Gudheti MV, Maas SA, Mills KD, Zimmerberg J. Dynamic clustered distribution of hemagglutinin resolved at 40 nm in living cell membranes discriminates between raft theories. Proc Natl Acad Sci USA. 2007;104:17370–17375. doi: 10.1073/pnas.0708066104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S, Scolari S, Thaa B, et al. FLIM-FRET and FRAP reveal association of influenza virus haemagglutinin with membrane rafts. Biochem J. 2010;425:567–573. doi: 10.1042/BJ20091388. [DOI] [PubMed] [Google Scholar]
- Klemm RW, Ejsing CS, Surma MA, et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol. 2009;185:601–612. doi: 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surma MA, Klose C, Klemm RW, Ejsing CS, Simons K. Generic sorting of raft lipids into secretory vesicles in yeast. Traffic. 2011;12:1139–1147. doi: 10.1111/j.1600-0854.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Abraham N, Khan LA, Hall DH, Fleming JT, Gobel V. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat Cell Biol. 2011;13:1189–1201. doi: 10.1038/ncb2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior IA, Parton RG, Hancock JF. Observing cell surface signaling domains using electron microscopy. Sci STKE. 2003;2003:PL9. doi: 10.1126/stke.2003.177.pl9. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Lipowsky R. Domain-induced budding of fluid membranes. Biophys J. 1993;64:1133–1138. doi: 10.1016/S0006-3495(93)81479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorre B, Callan-Jones A, Manneville JB, et al. Curvature-driven lipid sorting needs proximity to a demixing point and is aided by proteins. Proc Natl Acad Sci USA. 2009;106:5622–5626. doi: 10.1073/pnas.0811243106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Vaz WL. Membrane curvature sorts lipids. Stabilized lipid rafts in membrane transport. EMBO Rep. 2005;6:418–419. doi: 10.1038/sj.embor.7400410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian A, Baumgart T. Sorting of lipids and proteins in membrane curvature gradients. Biophys J. 2009;96:2676–2688. doi: 10.1016/j.bpj.2008.11.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safouane M, Berland L, Callan-Jones A, et al. Lipid cosorting mediated by shiga toxin induced tubulation. Traffic. 2010;11:1519–1529. doi: 10.1111/j.1600-0854.2010.01116.x. [DOI] [PubMed] [Google Scholar]
- Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 2005;24:1537–1545. doi: 10.1038/sj.emboj.7600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain JM, Storm C, Roux A, Ben Amar M, Joanny JF. Fission of a multiphase membrane tube. Phys Rev Lett. 2004;93:158104. doi: 10.1103/PhysRevLett.93.158104. [DOI] [PubMed] [Google Scholar]
- Romer W, Berland L, Chambon V, et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- Ewers H, Romer W, Smith AE, et al. GM1 structure determines SV40-induced membrane invagination and infection Nat Cell Biol 20101211–18.sup pp 11–12. [DOI] [PubMed] [Google Scholar]
- Farsad K, De Camilli P. Mechanisms of membrane deformation. Curr Opin Cell Biol. 2003;15:372–381. doi: 10.1016/s0955-0674(03)00073-5. [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, et al. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science. 2004;303:495–499. doi: 10.1126/science.1092586. [DOI] [PubMed] [Google Scholar]
- Willenborg C, Jing J, Wu C, et al. Interaction between FIP5 and SNX18 regulates epithelial lumen formation. J Cell Biol. 2011;195:71–86. doi: 10.1083/jcb.201011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- Cao X, Coskun U, Rossle M, et al. Golgi protein FAPP2 tubulates membranes. Proc Natl Acad Sci USA. 2009;106:21121–21125. doi: 10.1073/pnas.0911789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Coskun U, Grzybek M, et al. Structural basis of wedging the Golgi membrane by FAPP pleckstrin homology domains. EMBO Rep. 2010;11:279–284. doi: 10.1038/embor.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Peranen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Mattila PE, Youker RT, Mo D, et al. Multiple biosynthetic trafficking routes for apically secreted proteins in MDCK cells. Traffic. 2012;13:433–442. doi: 10.1111/j.1600-0854.2011.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Edeling MA, Smith C, Owen D. Life of a clathrin coat: insights from clathrin and AP structures. Nat Rev Mol Cell Biol. 2006;7:32–44. doi: 10.1038/nrm1786. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Weise C, Erbar S, Lamp B, Vogt C, Diederich S, Maisner A. Tyrosine residues in the cytoplasmic domains affect sorting and fusion activity of the Nipah virus glycoproteins in polarized epithelial cells. J Virol. 2010;84:7634–7641. doi: 10.1128/JVI.02576-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deora AA, Gravotta D, Kreitzer G, Hu J, Bok D, Rodriguez-Boulan E. The basolateral targeting signal of CD147 (EMMPRIN) consists of a single leucine and is not recognized by retinal pigment epithelium. Mol Biol Cell. 2004;15:4148–4165. doi: 10.1091/mbc.E04-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall AH, Powell SK, Yeaman CA, Rodriguez-Boulan E. The neural cell adhesion molecule expresses a tyrosine-independent basolateral sorting signal. J Biol Chem. 1997;272:4559–4567. doi: 10.1074/jbc.272.7.4559. [DOI] [PubMed] [Google Scholar]
- Aroeti B, Kosen PA, Kuntz ID, Cohen FE, Mostov KE. Mutational and secondary structural analysis of the basolateral sorting signal of the polymeric immunoglobulin receptor. J Cell Biol. 1993;123:1149–1160. doi: 10.1083/jcb.123.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Hobert M, Friend L, Carlin C. The epidermal growth factor receptor juxtamembrane domain has multiple basolateral plasma membrane localization determinants, including a dominant signal with a polyproline core. J Biol Chem. 2002;277:38284–38293. doi: 10.1074/jbc.M104646200. [DOI] [PubMed] [Google Scholar]
- Dillon C, Creer A, Kerr K, Kumin A, Dickson C. Basolateral targeting of ERBB2 is dependent on a novel bipartite juxtamembrane sorting signal but independent of the C-terminal ERBIN-binding domain. Mol Cell Biol. 2002;22:6553–6563. doi: 10.1128/MCB.22.18.6553-6563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey PJ, Meise KS, Coffey RJ. Basolateral sorting of transforming growth factor-alpha precursor in polarized epithelial cells: characterization of cytoplasmic domain determinants. Exp Cell Res. 2003;285:159–174. doi: 10.1016/s0014-4827(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Wolff SC, Qi AD, Harden TK, Nicholas RA. Charged residues in the C-terminus of the P2Y1 receptor constitute a basolateral-sorting signal. J Cell Sci. 2010;123:2512–2520. doi: 10.1242/jcs.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reales E, Sharma N, Low SH, Folsch H, Weimbs T. Basolateral sorting of syntaxin 4 is dependent on its N-terminal domain and the AP1B clathrin adaptor, and required for the epithelial cell polarity. PloS One. 2011;6:e21181. doi: 10.1371/journal.pone.0021181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Barlow LD, Francisco GC, et al. The fifth adaptor protein complex. PLoS Biol. 2011;9:e1001170. doi: 10.1371/journal.pbio.1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DJ, Collins BM, Evans PR. Adaptors for clathrin coats: structure and function. Annu Rev Cell Dev Biol. 2004;20:153–191. doi: 10.1146/annurev.cellbio.20.010403.104543. [DOI] [PubMed] [Google Scholar]
- Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Ohno H, Tomemori T, Nakatsu F, et al. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- Simmen T, Honing S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- Deborde S, Perret E, Gravotta D, et al. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorino JJ, Deborde S, Deora A, et al. Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia. Traffic. 12:483–498. doi: 10.1111/j.1600-0854.2010.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda Y, Okada Y, Saito N, et al. KIFC3, a microtubule minus end-directed motor for the apical transport of annexin XIIIb-associated Triton-insoluble membranes. J Cell Biol. 2001;155:77–88. doi: 10.1083/jcb.200108042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaulin F, Xue X, Rodriguez-Boulan E, Kreitzer G. Polarization-dependent selective transport to the apical membrane by KIF5B in MDCK cells. Dev Cell. 2007;13:511–522. doi: 10.1016/j.devcel.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaulin F, Kreitzer G. KIF17 stabilizes microtubules and contributes to epithelial morphogenesis by acting at MT plus ends with EB1 and APC. J Cell Biol. 2010;190:443–460. doi: 10.1083/jcb.201006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai AW, Chuang JZ, Sung CH. Cytoplasmic dynein regulation by subunit heterogeneity and its role in apical transport. J Cell Biol. 2001;153:1499–1509. doi: 10.1083/jcb.153.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafont F, Burkhardt JK, Simons K. Involvement of microtubule motors in basolateral and apical transport in kidney cells. Nature. 1994;372:801–803. doi: 10.1038/372801a0. [DOI] [PubMed] [Google Scholar]
- Smythe E, Ayscough KR. Actin regulation in endocytosis. J Cell Sci. 2006;119:4589–4598. doi: 10.1242/jcs.03247. [DOI] [PubMed] [Google Scholar]
- Lanzetti L. Actin in membrane trafficking. Curr Opin Cell Biol. 2007;19:453–458. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Harris KP, Tepass U. Cdc42 and vesicle trafficking in polarized cells. Traffic. 2010;11:1272–1279. doi: 10.1111/j.1600-0854.2010.01102.x. [DOI] [PubMed] [Google Scholar]
- Altschuler Y, Hodson C, Milgram SL. The apical compartment: trafficking pathways, regulators and scaffolding proteins. Curr Opin Cell Biol. 2003;15:423–429. doi: 10.1016/s0955-0674(03)00084-x. [DOI] [PubMed] [Google Scholar]
- Roland JT, Bryant DM, Datta A, Itzen A, Mostov KE, Goldenring JR. Rab GTPase-Myo5B complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci USA. 2011;108:2789–2794. doi: 10.1073/pnas.1010754108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber LA, Pimplikar S, Parton RG, Virta H, Zerial M, Simons K. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Mushiake S, Kato Y, et al. The Rab8 GTPase regulates apical protein localization in intestinal cells. Nature. 2007;448:366–369. doi: 10.1038/nature05929. [DOI] [PubMed] [Google Scholar]
- Au JS, Puri C, Ihrke G, Kendrick-Jones J, Buss F. Myosin VI is required for sorting of AP-1B-dependent cargo to the basolateral domain in polarized MDCK cells. J Cell Biol. 2007;177:103–114. doi: 10.1083/jcb.200608126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Molr Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- He B, Guo W. The exocyst complex in polarized exocytosis. Curr Opin Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson M, Novick P. The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol. 2006;13:577–581. doi: 10.1038/nsmb1097. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Wright JR, Nelson WJ. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J Cell Biol. 2001;155:593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J Cell Biol. 2007;178:323–335. doi: 10.1083/jcb.200705094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield A, Caplan MJ, Muth TR. Protein trafficking in polarized cells. Int Rev Cell Mol Biol. 2008;270:145–179. doi: 10.1016/S1937-6448(08)01404-4. [DOI] [PubMed] [Google Scholar]