Spectraplakins: Master orchestrators of cytoskeletal dynamics (original) (raw)
Abstract
The dynamics of different cytoskeletal networks are coordinated to bring about many fundamental cellular processes, from neuronal pathfinding to cell division. Increasing evidence points to the importance of spectraplakins in integrating cytoskeletal networks. Spectraplakins are evolutionarily conserved giant cytoskeletal cross-linkers, which belong to the spectrin superfamily. Their genes consist of multiple promoters and many exons, yielding a vast array of differential splice forms with distinct functions. Spectraplakins are also unique in their ability to associate with all three elements of the cytoskeleton: F-actin, microtubules, and intermediate filaments. Recent studies have begun to unveil their role in a wide range of processes, from cell migration to tissue integrity.
Spectraplakins, an evolutionarily conserved spectrin subfamily
In most multicellular organisms, the cytoskeleton is composed of F-actin, microtubules (MTs), and intermediate filaments (IFs). Together, these filamentous networks orchestrate an integrated cytoplasmic scaffold that functions in intracellular trafficking, polarization, migration, adhesion, mechanical strength, and cellular shape. As such, it is not surprising that the cytoskeleton must be highly dynamic, able to remodel itself quickly during normal development and differentiation as well as in response to tissue injury. Spectraplakins function in these remodeling processes through their ability to cross-link and integrate different cytoskeletal networks with proteins that function in cell–cell interactions, motility, polarity, morphology, and mechanical strength (Broderick and Winder, 2005).
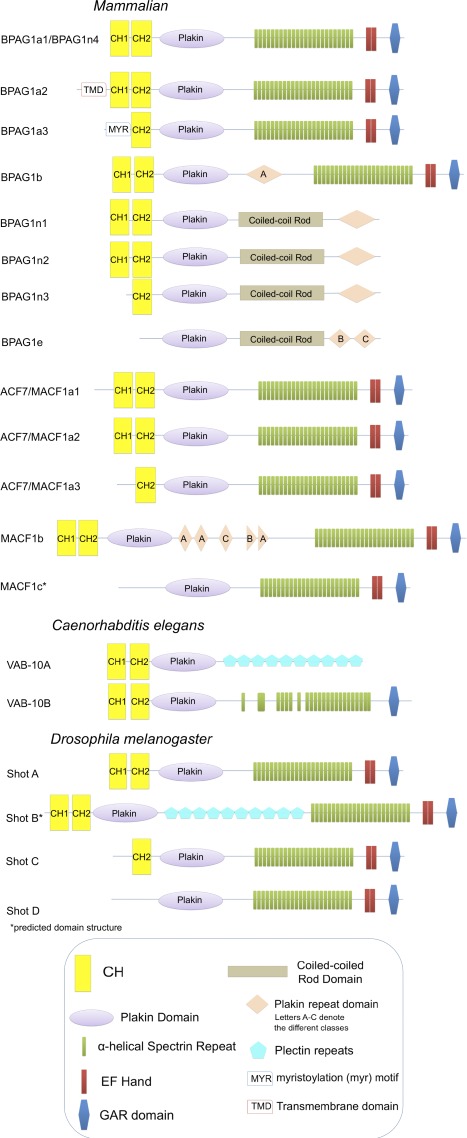
Spectraplakins are evolutionarily conserved enormous (>500 kD), multifunctional cytoskeletal proteins that coordinate cytoskeletal elements through direct binding to different cytoskeletal networks. The spectraplakin family presently consists of only two mammalian genes, MACF1, encoding ACF7 (actin cross-linking factor 7)/MACF1 (MT and actin cross-linking factor 1), and Dst, encoding BPAG1 (bullous pemphigoid antigen 1)/dystonin. The family also consists of a single gene in zebrafish, Magellan, a single Drosophila melanogaster gene, short stop (shot)/Kakapo, and a single gene in Caenorhabditis elegans, vab-10 (Fig. 1 and Table 1). Rather than creating diversity in gene number, spectraplakins generate diversity through differential promoter usage and differential splicing. Although unconventional in method, the ability to mix and match protein-interacting domains results in an incredibly rich repertoire of possible proteins able to selectively link and coordinate an enormous array of biological functions within different cell types.
Figure 1.
Mammalian and invertebrate spectraplakin isoforms. Seven types of functional domains can be found in this family of proteins: a calponin-type actin-binding domain composed of CH1 and CH2 regions, a plakin domain, an α-helical spectrin repeat domain, PRDs, plectin repeats, an EF hand, and a GAR domain. The BPAG1a2 isoform contains a sequence coding for a highly conserved N-terminal transmembrane domain (TMD), and BPAG1a3 contains a sequence coding for a conserved myristoylation (myr) motif. BPAG1b, BPAG1e, and MACF1b contain a variable number of PRDs. PRDs are grouped into three classes termed A, B, and C, which are connected by a linker subdomain. The number of spectrin repeats and plectin repeats shown here are descriptive in nature and, in reality, vary between the spectraplakins. The structures shown of MACF1c and Shot B are predicted domain structures. Please note that this figure is not drawn to scale.
Table 1.
Spectraplakins and their isoforms
| Isoform | Tissue | Description |
|---|---|---|
| BPAG1e; BPAG1 | Major epithelial variant | Contains plakin domain conserved in related plakin family of proteins; lacking functional actin-binding domain at N terminus |
| BPAG1a1; BPAG1n4 | Major neuronal variant; predominantly in sensory neurons | Contains unique short N-terminal region mediating actin binding |
| BPAG1a2 | Major neuronal variant; predominantly expressed in tissues affected in dt mice | Contains N-terminal transmembrane domain |
| BPAG1a3 | Isoform 3 is not expressed in appreciable levels in the brain but is the primary isoform expressed in the lung | Contains N-terminal conserved myristoylation motif |
| BPAG1b1, b2, and b3 | Predominant in muscle | Similar to BPAG1a1–3 but contains a 2,000–amino acid sequence coding for a novel PRD domain before the spectrin repeats |
| ACF7/MACF1a1 | Ubiquitously expressed | Contains a 100-kb uncharacterized sequence between the first exon and exons encoding the first CH domain |
| ACF7/MACF1a2 | Ubiquitously expressed | |
| ACF7/MACF1a3 | Ubiquitously expressed | Contains only CH2 domain and lacks CH1 |
| MACF1b | Ubiquitously expressed with especially high levels in the lung; localizes to Golgi complex | Identical to MACF1a but contains a region with plectin or plakin repeats in the middle of the molecule |
| MACF1c | Lacks N-terminal actin-binding domain | |
| VAB-10A | Restricted to fibrous organelles | Resembles BPAG1e and ends with the PRD |
| VAB-10B | Diffuse distribution in epidermis and muscle | Resembles BPAG1a |
| Shot A | Represents predominant isoform in central nervous system | Original shot isoform contains an actin-binding domain, a plakin domain, a series of spectrin repeats, and a GAR MT-binding domain |
| Shot B | Localizes at cell–cell junctions in the zonula adherens in embryonic epidermis and follicle epithelium | Contains a long stretch of plakin repeats inserted between the plakin domain and the spectrin repeats; note that the presence of plakin repeats is surprising, given that in other organisms, this repeat mediates interaction with IFs and Drosophila do not have cytoplasmic IFs |
| Shot C | – | Lacks the first CH domain |
| Shot D | – | Lacks both CH domains |
How did nature achieve this ingenious feat? Spectraplakins evolved from the spectrin family of proteins, the most ancient of which is α-actinin, present in fission but not budding yeast. The defining features of α-actinin and other members of the spectrin superfamily are a calponin homology (CH) domain for calcium regulation, an EF-hand domain for direct calcium sensing/binding, and a series of so-called spectrin repeats (Fig. 1). Other members of the spectrin superfamily, including spectrins, dystrophin, and spectraplakins, appear to be restricted to metazoans (Bennett and Baines, 2001). The existence of spectraplakins can be traced back to lower metazoans; the genome of sponge, Amphimedon queenslandica, encodes a gene entitled LOC100639902 that seems to be a homolog of ACF7/MACF1.
Metazoans lack the robust, indestructible cell walls of plants and bacteria. Their tissues and cell types rely on flexible cell–cell contacts and cell–substratum adhesions for their proper morphology, function, and survival, and this requires an elaborate cellular infrastructure. Spectrin family proteins emerged to resolve these problems, allowing cells to adopt shape and polarity and thereby enabling them to organize into multicellular tissues. Along the way, they acquired a myriad of additional protein–protein- and protein–membrane-interacting domains and isoforms, which endow family members with diverse cellular roles. Spectraplakin’s genes are typified by multiple tissue-specific promoters, large numbers of coding exons, and differentially spliced transcripts, resulting in a diversity of different isoforms, each with the ability to interact with different cytoskeletal and/or membrane components (Röper et al., 2002). In their divergence from α-actinin, spectrins, and dystrophin genes, spectraplakin genes endowed some of their encoded isoforms with abilities to bind to and dynamically regulate MTs as well as coordinate linkages between the cortical actin cytoskeleton and IFs (Röper et al., 2002).
Unique domain structure of spectraplakins
Actin-binding domains.
Most spectraplakin isoforms harbor a conserved sequence motif in their N-terminal domain that is also found in the spectrins and which enables them to associate directly with the actin cytoskeleton. The F-actin–binding domain consists of two CH domains, CH1 and CH2 (Fig. 1), found in many other F-actin–binding proteins, including filamins (Korenbaum and Rivero, 2002) and spectrins. The crystal structure of the F-actin–binding domain has been solved for the spectrin proteins dystrophin and utrophin and consists of a bundle of α helices arranged in a head-to-tail dimer (Keep et al., 1999).
Although CH1 alone can bind F-actin, affinity is greatly increased with addition of CH2 (Jefferson et al., 2004). The CH1-CH2 actin-binding domains of mammalian ACF7/MACF1 and BPAG1 and Drosophila Shot spectraplakins bind to F-actin strongly and with similar dissociation constants (Yang et al., 1996; Karakesisoglou et al., 2000; Röper et al., 2002). The F-actin–binding domain of BPAG1 also mediates homo- and heterodimerization (Geerts et al., 1999).
Some isoforms of BPAG1 and ACF7/MACF1 only have the CH2 domain. These isoforms have weaker F-actin interactions than those carrying both CH1 and CH2 (Leung et al., 1999b; Yang et al., 1999; Karakesisoglou et al., 2000).
Spectrin repeats.
The presence of a large number of spectrin repeats is what classifies spectraplakins as members of the spectrin superfamily (Fig. 1). Each repeat consists of ∼106 residues, which fold into three α helices. These helices have a heptad periodicity, characterized by a pattern of charged and hydrophobic residues, enabling them to fold into an antiparallel coiled coil (Jefferson et al., 2004).
The spectrin repeats are thought to endow these proteins with flexibility and enable them to respond elastically to applied extensions or mechanical forces. In addition, they act as a spacer region that separates the functional domains at the N and C termini. For α-actinin, which functions as an F-actin cross-linker, the number of spectrin repeats determines the spacing between actin filaments and hence the bundling of actin fibers. In dystrophin, the spectrin rod separates the F-actin–binding domain from the membrane-binding domain at the C terminus. The length of the rod domain is critical for optimal protein function: when even a few spectrin repeats are deleted in dystrophin, a mild form of muscular dystrophy results (Palmucci et al., 1994).
Although spectrin repeats have been thought to function predominantly as spacer regions within the spectrin family, an SH3 (Src homology-3) protein–protein-interacting domain resides within the ninth spectrin repeat of α-spectrin (Sonnenberg et al., 2007), raising speculation that this domain may have other, as yet undiscovered protein–protein interactions. Indeed, within the spectrin repeats of spectraplakins, such protein-binding functions have been described. Most notable is the existence of an ERM (erzin/radixin/moesin)-interacting domain within the spectrin repeat region of BPAG1a1/BPAG1n4 expressed by sensory neurons (Liu et al., 2003). Through the ability of ERM to bind to both BPAG1a1/BPAG1n4 and dynactin and through BPAG1a1/BPAG1n4’s additional ability to bind to the endosomal integral membrane protein retrolinkin, this spectraplakin isoform can act as an adapter to tether endosomal vesicles to dynactin/dynein, thereby facilitating retrograde vesicular transport and cytoplasmic trafficking within these neurons (Liu et al., 2003, 2007; Kakinuma et al., 2004).
EF hand and GAS2-related protein (GAR) domain.
Within the 3′ (C-terminal encoding) exons of spectraplakins are an EF-hand and a GAR domain (Fig. 1). EF-hand motifs are found in many members of the spectrin superfamily as well as in several unrelated proteins. These domains contain calcium-dependent EF hands proximally and calcium-independent EF hands distally. The calcium-dependent hands bind Ca2+, which, in spectrin, transforms the domain from a closed to open confirmation (Travé et al., 1995). The calcium-independent EF hand of α-spectrin amplifies the function of the F-actin–binding domain of its heterodimer binding partner, β-spectrin (Korsgren and Lux, 2010). Interestingly, the muscle-specific isoforms of the spectrin family lack the ability to bind calcium through their EF hand, likely an evolutionary mechanism to protect the muscle architecture from the destabilizing effects of calcium in muscle fiber contractions (Broderick and Winder, 2005).
In contrast, the GAR domain is restricted to the spectraplakins and is not found in other spectrin family members (Röper et al., 2002). Moreover, aside from the spectraplakins, the GAR domain has only been found in a few other proteins: GAS2 (growth arrest–specific protein 2), GAR17, and GAR22 (International Human Genome Sequencing Consortium, 2001). The GAR domain of spectraplakins associates with and stabilizes MTs, indicating that this domain evolved as a mechanism for spectraplakins to interact with MTs and link MTs to other components of the cytoskeletal network (Fig. 2; Leung et al., 1999b; Sun et al., 2001; Lee and Kolodziej, 2002). Moreover, the C-terminal GAR domain encoded by the Drosophila Kakapo/Shot gene can interact with EB1, a protein which binds to the growing (i.e., plus) ends of cytoplasmic MTs (Subramanian et al., 2003). Interestingly, deletion of the GAR domain in the GAS2 protein causes apoptotic-like rearrangements of the actin cytoskeleton (Brancolini et al., 1995), leaving open the possibility that this domain could have multiple functions.
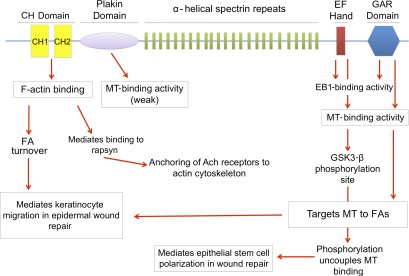
Figure 2.
The binding partners of full-length ACF7/MACF1. A schematic diagram that shows multiple domains of the full-length ACF7/MACF1 and their functions. ACF7/MACF1 binds to F-actin at the N-terminal CH actin-binding domain. F-actin binding of ACF7/MACF1 mediates FA turnover and keratinocyte migration in epidermal wound repair. ACF7/MACF1 links actin with rapsyn, which anchors Ach receptors to the actin cytoskeleton. The plakin domain has been shown to have weak MT-binding activity. EF-hand Ca2+-binding activity is responsible for ACF7/MACF1 binding to MT +TIP protein EB1 and, along with GAR domain, mediates ACF7/MACF1 binding to MTs. The C terminus contains the phosphorylation site of GSK3-β. ACF7/MACF1 binding to MTs is important for targeting MTs to FAs. Phosphorylation of ACF7/MACF1 uncouples MT binding. Coordination of ACF7/MACF1 binding to MTs is important for polarizing epithelial stem cells in wound repair.
The plakin domain.
The other signifying feature of spectraplakins is their plakin domain. Protein analysis of this domain, using tools such as SMART (Schultz et al., 1998) and Pfam (Sonnhammer et al., 1997), suggests that it originated from spectrin repeats (Röper et al., 2002; Jefferson et al., 2004). This has been confirmed by the crystal structures for the plakins of two other proteins with such domains, BPAG1 (Jefferson et al., 2007) and plectin (Sonnenberg et al., 2007; Ortega et al., 2011). Interestingly, the plakin domain is composed of six to nine spectrinlike repeats organized in a tandem array with an SH3 protein–protein-interacting domain embedded within the fifth spectrin repeat (Sonnenberg et al., 2007; Choi and Weis, 2011). These remarkable structural similarities suggest that plakin domains are evolutionarily derived from spectrins.
Proteins that contain plakin domains typically bind to membrane-associated junctional proteins (Jefferson et al., 2004). This feature holds not only for the spectraplakins but also a second family of plakin proteins that have an N-terminal plakin domain but do not contain spectrin repeats or the MT-interacting GAR domains. These plakins instead have a long central coiled-coil rod domain for dimerization and a C-terminal segment known as the plectin repeat domain (Janda et al., 2001; Jefferson et al., 2004). Such plakins are often expressed in tissues that experience mechanical stress (i.e., muscle and epithelia).
Although spectraplakins coordinate dynamic MT functions, plakins evolved as adaptors that link the IF cytoskeleton and membrane–protein complexes to adhesive junctions (Jefferson et al., 2004). An example is the isoform BPAG1e, an epidermally expressed plakin encoded by the BPAG1 spectraplakin gene. Through its association with β4-integrin, BPAG1e links the keratin IF network to hemidesmosomal cell–ECM junctions to strengthen the mechanical integrity at the base of the epidermis (Guo et al., 1995; Koster et al., 2003). Plectin plays a similar role, in which the plakin domain was shown to interact specifically not only with β4-integrin but also with the cytoplasmic domains of another hemidesmosomal protein, transmembrane collagen XVII (BPAG2; Rezniczek et al., 1998; Koster et al., 2003). Analogously, the plakin protein desmoplakin links the desmosomal cell–cell junctions to the IF network in muscle and epidermis (Stappenbeck et al., 1993; Kouklis et al., 1994). Other human plakin members include periplakin, envoplakin, and epiplakin (Röper et al., 2002). In contrast to spectraplakins, plakins have not been found in Drosophila and C. elegans, suggesting that they evolved from spectraplakins (Leung et al., 2002). For the spectraplakins BPAG1n3 and ACF7/MACF1, a weak MT-binding domain has been identified within this region, and their interactions render MTs resistant to depolymerization by drugs and cold (Fig. 2; Yang et al., 1999; Karakesisoglou et al., 2000).
Plakin repeat domain (PRD).
PRDs are unique to the plakin family and a few spectraplakin isoforms. The PRD contains a conserved central core region known as the plectin module, which is flanked by less-conserved linker sequences of variable lengths (Janda et al., 2001). The plectin module is a globular domain composed of 4.5 tandem repeats of 38 amino acid residues, called the plectin repeat (Janda et al., 2001; Jefferson et al., 2004). The 38 residues form a β-hairpin followed by two antiparallel α helices (Choi et al., 2002). Envoplakin has one PRD (Ruhrberg et al., 1996), desmoplakin has three (Green et al., 1990), plectin has six (Janda et al., 2001), and epiplakin has thirteen (Fujiwara et al., 2001). Among the spectraplakins, some BPAG1 isoforms have up to two, whereas MACF1b has five (Fig. 1). These domains confer the ability of these proteins, including plectin, periplakin, envoplakin, BPAG1, and desmoplakin, to interact with IFs (Wiche et al., 1993; Nikolic et al., 1996; Yang et al., 1996; Leung et al., 1999a; Choi et al., 2002; Karashima and Watt, 2002; Fontao et al., 2003; Lapouge et al., 2006).
The PRDs of BPAG1e have been shown to bind to IFs, linking them to hemidesmosomes (Guo et al., 1995). Similarly, the nematode plakin VAB-10A has been shown to affect the stability of IFs in fibrous organelles, an analogous structure to hemidesmosomes in C. elegans (Bosher et al., 2003). The PRDs of desmoplakin have also been shown to bind to IFs, linking them to hemidesmosomes and desmosomes (Choi et al., 2002). For plectin, IF binding seems to be mediated by the linker region between PRD 5 and 6 rather than the PRDs themselves (Nikolic et al., 1996).
Interestingly, Drosophila lacks cytoplasmic IFs, but they do have Kakapo/Short Stop, which contains conserved plectin repeats (Röper et al., 2002; Röper and Brown, 2003). The plectin repeats in the invertebrate spectraplakins are not organized into the groups of 4.5 tandem repeats that are so well conserved in the plakins, suggesting that they do not form the same PRD globular domain structure (Röper et al., 2002). Together, these features suggest that the PRD may have functions that extend beyond IF binding. Plectin has also been reported to bind not only IFs but also F-actin and MTs, even though it lacks the classical CH1/CH2 actin-binding domains or the GAR MT-binding domains of the spectraplakins (Foisner and Wiche, 1991; Janda et al., 2001).
Spectraplakins: Few genes, many isoforms
Although the number of spectrin family genes in the genome is limited, different tissue-specific promoters and alternative splicing have evolved to generate a variety of cytoskeletal binding proteins to fit the demands of a range of tissues (Table 1). Different transcription start sites in spectraplakin genes result in at least four different N termini in the single encoded Drosophila spectraplakin Shot and in mammalian BPAG1. At least three start sites are present in the other mammalian spectraplakin gene encoding the ACF7/MACF1 proteins (Fig. 1 and Table 1; Röper et al., 2002). In addition to altering their cytoskeletal associations, the alternate start sites also confer tissue specificity (Table 1). Drosophila Shot isoforms A and B, which contain full F-actin–binding domains, are expressed in the central nervous system, whereas isoforms C and D are not (Lee et al., 2000). BPAG1e is specific to the skin epidermis, whereas BPAG1a1 (also referred to as BPAG1n4) is highly expressed in the peripheral nervous system, and BPAG1b is specific to the muscle (Yang et al., 1996; Leung et al., 2001b; Groves et al., 2010; Steiner-Champliaud et al., 2010). Mammalian ACF7/MACF1 is also expressed broadly, including the central nervous system and the epidermis (Karakesisoglou et al., 2000).
Differential splicing of transcripts from the Dst/BPAG1 gene has led to several additional fascinating cytoskeletal linker proteins. Depending on promoter usage, the BPAG1 gene has been shown to be expressed in the epidermis, peripheral nervous system, and muscle, with tissue-specific patterns and roles for different isoforms. The first mammalian BPAG1 isoforms reported were BPAG1e followed by BPAG1n1 and n2. The two BPAG1n isoforms are derived from distinct neuronal transcriptional start sites that produce different short peptides preceding the F-actin–binding domain (Bernier et al., 1996; Yang et al., 1999; Lee et al., 2000). Based on RT-PCR and rapid amplification of cDNA ends, these isoforms are thought to have the C-terminal exon sequences of BPAG1e, namely a plakin, rod, and PRD domain, consistent with the domain structure of the plakin family of proteins. Such isoforms are predicted to link the neurofilament IF cytoskeleton to cortical actin lining the axon (Yang et al., 1996; Leung et al., 1999a). In contrast, the transcription start site encoding neuronal BPAG1n3 begins within the F-actin–binding domain and was otherwise thought to resemble BPAG1n1 and n2. Biochemical analyses further indicated that the BPAGn3 N-terminal half indeed lacks F-actin binding, but, with its weak MT-binding activity, this isoform along with BPAG1n1 and n2 appears to endow these sensory neuronal plakins with the combined potential to integrate all three cytoskeletal networks (Fig. 1; Yang et al., 1999).
Subsequent studies led to the identification of what appears to be spectraplakin sensory neural BPAG1 isoforms that are more robustly expressed than the BPAG1 neuronal plakins (Leung et al., 2001a; Young and Kothary, 2007). The three identified thus far, namely BPAG1a1/BPAG1n4, BPAG1a2, and BPAG1a3, are derived from the same N-terminal exons of BPAG1n1–3, respectively. In these cases, however, their C-terminal segments are derived from the exons encoding the spectrin repeat region and downstream EF hands and GAR domain (Fig. 1; Leung et al., 2001b; Jefferson et al., 2006).
The presence of the GAR domain, which mediates considerably stronger MT binding than the plakin domain (Sun et al., 2001), confers new functions for these isoforms. In addition to the aforementioned vesicular transport and cytoplasmic trafficking roles ascribed to BPAG1n4, these other spectraplakins have the ability to function in stabilizing the long MTs of the neuronal axons by tethering to the cortical actin network (BPAG1a2) and by interconnecting MTs (BPAG1a3). Finally, an additional study suggests that BPAG1e has isoforms BPAG1eA, BPAG1eS, and BPAG1eB that exist in muscle (Okumura et al., 2002). Collectively, the BPAG1 gene encodes a diverse array of plakin and spectraplakin isoforms, and, with >80 different exons, there are, no doubt, additional BPAG1 isoforms awaiting characterization.
The vab-10 locus in the nematode _C. elegan_s generates two sets of isoforms from a common 5′ start site but different 3′ regions resulting from alternate splicing (Fig. 1 and Table 1; Bosher et al., 2003). VAB-10A has a C terminus with plakin repeats, and VAB-10B has a C terminus with spectrin repeats. The two isoforms have different distributions in the epidermis. VAB-10A is essential for epidermis–ECM attachment, similar to BPAG1e; however, VAB-10B maintains a connection between the apical and basal epidermis and plasma membranes during morphogenesis, more similar to the Drosophila spectraplakin Shot (Gregory and Brown, 1998; Bosher et al., 2003). In line with these functions, VAB-10A closely resembles plectin and BPAG1e, whereas VAB-10B is more similar to ACF7/MACF1 and BPAG1a (Bosher et al., 2003).
Functions of spectraplakin proteins in invertebrate animals
As outlined in the previous section, Drosophila and C. elegans both contain a single spectraplakin gene, shortstop (shot)/kakapo and vab-10, respectively, and both genes have two major isoforms. Genetic analyses of vab-10 mutants reveal that VAB-10A is an essential component of fibrous organelles, which mediate muscle connection to the cuticle across the epidermis in C. elegans (Bosher et al., 2003). With loss of VAB-10A, the number of fibrous organelles is reduced, and the epidermis cannot attach to the underlying basement membrane enriched in ECM proteins. In contrast, loss of VAB-10B results in increased epidermal thickness. It has been proposed that VAB-10B may protect cells from mechanical shearing forces generated within a cell, and, given VAB-10B’s conserved actin- and MT-binding domains, it seems likely that VAB-10B attaches cortical actin and anchors it to the MT network (Bosher et al., 2003). In this model, isoform VAB-10A protects against external forces, whereas VAB-10B protects against internal forces within the epidermis. VAB-10B also functions in direct nuclear migration and gonadal distal tip cell migration in C. elegans by regulating the polarized alignment of MTs (Kim et al., 2011).
Mutations in Drosophila shot result in a wide variety of cellular and tissue defects. These include perturbations in actin–MT organization, cell–cell adhesion and integrin-mediated epidermal attachment to muscle (Röper and Brown, 2003), developmental aberrations in the foregut and tracheal tubes (Lee et al., 2003), defects in neuronal growth, defects in formation and maintenance of tendon cells, and defects in MT association with the fusome during oogenesis (Röper and Brown, 2004).
The actin- and MT-binding domains of Shot are essential for axon extension; mutant Shot axons navigate along the sensory axon substrate but then terminate prematurely (Lee et al., 2000; Sanchez-Soriano et al., 2009). The actin-binding CH domain is essential for Shot function in the nervous system but dispensable in tendon cells (Bottenberg et al., 2009). Similarly, the plakin domain of Shot is necessary for compartmentalization in neurons and axonal growth but is not essential in tendon cells (Bottenberg et al., 2009). It is possible that Shot plays a role in targeting MTs from the apical to basal surface during tendon cell development. Shot has been shown to associate with EB1 and APC1 (Subramanian et al., 2003), and these molecules are known to regulate cortical targeting of MTs. Tendon cells have highly organized arrays of stable MTs, and it is also possible that Shot may mediate this organization via stabilizing and bundling MTs, a known function of ACF7/MACF1 (Sun et al., 2001).
Shot also regulates filopodia formation. Interestingly, this function is not dependent on Shot binding to F-actin but instead reliant on its EF-hand motifs (Sanchez-Soriano et al., 2009). Sanchez-Soriano et al. (2009) show that Shot EF-hand motifs interact with a translational regulator, Kra/eIF5C. They have proposed that this interaction might lead to local translation events that influence subcellular concentrations of actin and actin regulators, which could govern polarized induction of new filopodia. Lastly, Shot is enriched between adherens junctions and the apical side of MTs in developing Drosophila photoreceptors at the zone known as the rhabdomere terminal web. It is thought that Shot acts as an actin–MT cross-linker during photoreceptor morphogenesis (Mui et al., 2011).
Functions of spectraplakins in vertebrate animals
The presence of two spectraplakin genes in the mammalian genome has allowed for additional diversification of cytoskeletal dynamics, a feature exploited by these higher organisms. Like their invertebrate counterparts, mammalian spectraplakins display their most essential functions in muscle, neurons, and skin epithelium, i.e., tissues which maintain elaborate yet dynamic cytoskeletal networks (Röper et al., 2002; Bosher et al., 2003; Jefferson et al., 2004; Wu et al., 2008). For example, mice lacking BPAG1 display skin blistering and sensory neuron and muscle degeneration, with each cell type manifesting gross defects in cytoskeletal organization. Termed dystonia musculorum (dt/dt), Dst/BPAG1 mutant mice begin to display defect features at birth, and by the second week of life, signs of global sensory neuron degeneration and loss-of-limb coordination become striking (Duchen et al., 1964; Brown et al., 1995; Guo et al., 1995). Dst/BPAG1 knockout mice also display marked accumulation of intracellular vesicles in sensory neurons and severely disrupted retrograde transport (Guo et al., 1995; Liu et al., 2003).
Ultrastructural analysis of dt/dt dorsal root ganglion revealed disorganized neurofilaments and a disrupted MT network (Dalpé et al., 1998). Loss of BPAG1 in dt/dt neurons results in short, disorganized, unstable MTs defective in axonal transport (Yang et al., 1999). Increasing evidence suggests that despite the presence of neurofilament aggregates in the degenerating axons of _Dst/BPAG1_-null mice and the ability of some BPAG1 isoforms to bind neurofilaments (Yang et al., 1996), these interactions do not contribute to the dt/dt phenotype. Thus, mice lacking neurofilaments appear to be phenotypically normal, and, when these mice are crossed with the Dst/BPAG1 knockout mouse, the resultant double knockout mice are indistinguishable phenotypically from the Dst/BPAG1 single knockout (Yang et al., 1999). These findings suggest that a key function of BPAG1 is to orchestrate the organization and stabilization of the MT network of sensory neurons to maintain axonal transport (Yang et al., 1999; Liu et al., 2003). In summary, these data point to the critical functions of additional BPAG1 isoforms in the nervous system.
Another contributor to the neuronal phenotype of the dt/dt mice is BPAG1a2, which associates with the ER (Young and Kothary, 2008). F-actin strongly associates with BPAG1a2, expressed in Cos-1 cells, at the ER, and expression of an ER chaperone protein is altered in dt/dt sensory neurons. Young and Kothary (2008) have posited that BPAG1a2 links actin filaments to the ER membrane, likely through its N-terminal actin-binding domain, and that this functions critically in maintaining neuronal integrity. Loss of expression of BPAG1a2 results in ultrastructural defects at the ER in sensory neurons and in vivo induction of ER stress proteins, leading to neurodegeneration through induction of a proapoptotic caspase cascade (Ryan et al., 2012b). Moreover, BPAG1a2 contains a transmembrane region responsible for targeting the protein to the perinuclear region of the cell, and this isoform may be involved in cytoskeletal organization and membrane attachment in the region of the nucleus (Young et al., 2006).
A recently uncovered function of BPAG1a2 in protein trafficking has been identified. BPAG1a2 associates with MT-associated protein 1B around the centromere, promoting MT acetylation. Stabilized MTs maintain the cis-Golgi, promoting anterograde trafficking of motor proteins (Ryan et al., 2012a). These findings suggest that spectraplakins may mediate axonal transport by regulating organelle organization. Although it is clear that BPAG1a2 and BPAG1a1/1n4 are vital players in sensory neurons, many questions still remain regarding the full repertoire of BPAG1 isoforms in the sensory nervous system, their localizations and interactions, and their relative importance in understanding the neuronal degeneration seen in dt/dt mutant mice.
In the epidermis, loss of BPAG1e imparts a selective fragility to the base of the columnar basal cells. Upon mechanical pressure, the cells break, leading to internal cell degeneration, resulting in blisters and compromised wound healing (Guo et al., 1995). The epidermal-specific features of the dt/dt mutant mice resemble those of bullous pemphigoid, a human disorder in which patients produce autoantibodies against BPAG1 (Labib et al., 1986; Mueller et al., 1989; Diaz et al., 1990). Autoantibodies produced in this disorder are targeted to a protein region specific to BPAG1e and not found in the neuronal isoforms (Guo et al., 1995). Additionally, a BPAG1e-specific mutation has been found in humans with an inherited skin fragility disorder (Groves et al., 2010). This mutation within the coiled-coil domain of BPAG1e is associated with loss of the hemidesmosomal inner plaque but not the outer plaque. Analogous to the skin blistering seen in _BPAG1_-null epidermis (Guo et al., 1995), this resulted in an intraepidermal fragility of the basal cells and an autosomal recessive form of epidermolysis bullosa simplex (Groves et al., 2010; Liu et al., 2012). Most cases of epidermolysis bullosa simplex are autosomal dominant and involve dominant-negative mutations in keratins 5 and 14, the basal keratins constituting the IF network that binds to both hemidesmosomes and desmosomes within this layer (Fuchs and Cleveland, 1998).
Hamill et al. (2009) showed that BPAG1e is an essential component of the signaling pathway in which β4-integrin determines front-to-rear cell polarity and processivity of cell migration. Moreover, loss of BPAG1e leads to decreased activity of Rac1 and cofilin and to the amount of Rac1 that associates with β4-integrin in keratinocytes. Overexpression of constitutively active Rac1 or cofilin can rescue the polarity defects in BPAG1e mutant cells (Hamill et al., 2009). In wild-type (WT) keratinocytes, BPAG1e, Rac1, and cofilin are recruited to the leading edges, causing localized remodeling of the actin network, lamellipodia formation, and promoting cell migration (Hamill et al., 2009).
Among spectraplakins, mammalian ACF7/MACF1 has been the most extensively studied with regards to cytoskeletal coordination. Originally named for its function as an actin cross-linking factor, ACF7 plays a major role in integrating coordinated MT and actin dynamics in mammalian cells and hence has also been referred to as MACF1 (MT–actin cross-linking factor 1). ACF7/MACF1 enhances MT–actin colocalization in vitro (Leung et al., 1999b). ACF7/MACF1 is an MT plus-end–tracking protein (+TIP) that mediates cortical interactions through association of MT ends with the actin cytoskeleton and the plasma membrane (Akhmanova and Steinmetz, 2008; Gupta et al., 2010). Moreover, ACF7/MACF1 directly binds the +TIP protein EB1 and exhibits EB1-dependent plus-end tracking of MTs in vivo (Fig. 2; Slep et al., 2005).
In _MACF1_-null primary endodermal cells, extending MTs fail to coalign with F-actin at the plasma membrane (Kodama et al., 2003). In HeLa cells, ACF7/MACF1 is required for the cortical localization of MT tip-binding protein CLASP2, suggesting that ACF7/MACF1 has a role in MT stabilization and cell motility (Drabek et al., 2006). The consequences of loss of ACF7/MACF1 are less-stable, long MTs with skewed cytoplasmic trajectories. This is disastrous for the developing mouse embryo, where the full ACF7/MACF1 knockout causes preimplantation lethality (Kodama et al., 2003).
In mammalian cells, F-actin bundling provides stabilizing forces for capture, growth, and guidance of MTs (Kaverina et al., 2002; Kodama et al., 2003). ACF7/MACF1 deficiency compromises the growth of MTs along F-actin to focal adhesions (FAs), thus impairing FA dynamics (Fig. 2; Wu et al., 2008). ACF7/MACF1’s effect on FA dynamics in epidermal cells is reliant on these polarized MTs that are stabilized by the underlying actin fibers. The stabilized MTs at sites of FAs are thought to serve as macromolecular tracks to deliver factors such as dynamin that promote FA turnover (Kaverina et al., 1998, 1999; Krylyshkina et al., 2002, 2003; Ezratty et al., 2005). Thus, when ACF7/MACF1 is missing and MT ends that normally converge at peripheral FA are unable to do so, FAs become highly stabilized and refractile to the normal dynamics required for efficient cell migration (Wu et al., 2008).
Exactly how ACF7/MACF1 functions in targeting plus-ended MT growth along actin fibers to polarized FAs is still not fully clear. However, ACF7/MACF1’s binding domains for F-actin, MTs, and MT plus-end proteins are not sufficient to rescue the defects in FA cytoskeletal dynamics and migration functions of _ACF7/MACF1_-null keratinocytes in vitro. One additional element in this equation is an intrinsic actin-regulated ATPase domain in ACF7/MACF1 (Wu et al., 2008). Although further experiments will be required to define its precise role, it might help to maintain essential +TIP proteins at the plus ends of MTs during their coordinated growth along F-actin.
A related cellular process that relies on ACF7/MACF1 is the transport of vesicles from the TGN to the cell periphery along cytoskeletal tracks (Kakinuma et al., 2004). Kakinuma et al. (2004) showed that the TGN protein p230, which is anchored to TGN membranes, interacts with ACF7/MACF1, allowing for transport of glycophosphatidylinositol-anchored proteins along the MT and actin cytoskeleton.
ACF7/MACF1 isoforms are broadly expressed and present early in embryonic development. In the epidermis, BPAG1e and ACF7/MACF1 are both expressed in the basal keratinocytes, and, yet, loss of each of these genes in epidermis leads to very different abnormalities, most likely a result of the distinct isoforms expressed by these genes. Whether the severe phenotypes in the nervous system and muscle lacking BPAG1 or ACF7/MACF1 are also reflective of isoform-specific functions or whether they are attributable to differences in temporal or cell type–specific expression patterns is not yet fully resolved.
Another interesting twist for ACF7/MACF1, not yet shown for any of the BPAG1 isoforms, is a role in the Wnt signaling pathway (Chen et al., 2006). In one strain, _MACF1_−/− embryos formed a neural plate but not a primitive streak, node, or mesoderm (Chen et al., 2006). This phenotype was very similar to that described for loss of Wnt3, the earliest-acting Wnt in mammals (Liu et al., 1999), and for loss of the Wnt coreceptors LPR5 and LPR6 in LRP5/6 double knockout embryos (Kelly et al., 2004). Chen et al. (2006) provided evidence suggesting that ACF7/MACF1 can bind to Axin, a component of the APC–Axin–GSK3-β–β-catenin complex, which normally promotes β-catenin phosphorylation and degradation and prevents Wnt signaling. By binding to Axin, Chen et al. (2006) surmise that ACF7/MACF1 might be a positive regulator in translocating Axin to Wnt receptors Fzd/LRP5/6 at the cell membrane and sequestering GSK3-β kinase activity and stabilizing β-catenin. T cell factor/β-catenin transcription assays are in agreement with this notion.
Goryunov et al. (2010) underscored the importance of ACF7/MACF1 in nervous system development by generating a conditional knockout (cKO). MACF1 cKO brains displayed disorganized cerebral cortex, heterotopia of the hippocampal pyramidal layer, aplasia of the corpus callosum, anterior and hippocampal commissures, altered shapes of the lateral ventricles, and hypotrophic thalamocortical fibers. Mutant forms of ACF7/MACF1c that lack the actin-binding domain cannot compensate for loss of ACF7/MACF1a, arguing that the actin-binding capacity of ACF7/MACF1 is essential in the brain. Consistent with this notion, the MACF1a cKO phenotype is accompanied by defects in cell-autonomous migration and noncell-autonomous guidance (Goryunov et al., 2010). Loss of ACF7/MACF1 also results in a 25–35% decrease in axon length (Sanchez-Soriano et al., 2009), and the defects in axon outgrowth suggest that axon guidance is also disrupted in these animals (Goryunov et al., 2010).
ACF7/MACF1 may also play a role in the peripheral nervous system. Results from in vitro cotransfection experiments suggest that acetylcholine receptors are anchored to the membrane in mammalian skeletal muscle via binding to rapsyn, which interacts with the actin-binding domain of ACF7/MACF1, tethering acetylcholine receptors to the actin cytoskeleton (Antolik et al., 2007). Whether this may also be a function of BPAG1 isoforms remains to be addressed.
Wu et al. (2008, 2011) elucidated the function of ACF7/MACF1 in mammalian skin by specifically deleting the MACF1 gene in skin epidermal cells. MACF1 cKO animals showed no gross morphological changes in skin or hair coat; however, when challenged to respond to injury, cKO skin exhibited a significant delay in repairing full-thickness wounds. The delayed wounding response is rooted in impaired epidermal migration, as also indicated by monolayer scratch assays performed on cultured primary keratinocytes isolated from cKO backskin and WT littermate controls (Fig. 2). Moreover, knockout keratinocytes displayed ∼60% decreased mean speed on a fibronectin matrix as compared with WT keratinocytes (Wu et al., 2008). This aberrant migration seen in _MACF1_-null keratinocytes results from more stable FAs and enhanced adherence to the underlying ECM substratum (Wu et al., 2011). Together, these findings suggest that ACF7/MACF1 regulates migration by promoting FA dynamics through its ability to coordinate MT/F-actin dynamics.
In addition to promoting cell motility, ACF7/MACF1 also participates in establishing proper cellular polarity during skin wound repair. Wu et al. (2011) showed that GSK3-β directly phosphorylates ACF7/MACF1, further substantiating the link between Wnt signaling and ACF7/MACF1. Moreover, GSK3-β phosphorylation of sites within the MT-binding domain of ACF7/MACF1 uncouples its ability to bind to MTs. Thus, when localized Wnt signaling occurs upon wounding, the polarized inhibition of GSK3-β enables ACF7/MACF1–MT binding to be stabilized at the migrating front of the stem cells streaming from hair follicles during wound repair. Mutagenesis experiments reveal that functional ACF7/MACF1, but not GSK3-β–refractile ACF7/MACF1, is able to restore polarized MT growth and stem cell migration to _MACF1_-null backskin (Wu et al., 2011). Collectively, these findings suggest that ACF7/MACF1 may facilitate directed migration of bulge stem cells in response to wounding through its ability to coordinate MT dynamics and polarization of hair follicle stem cells (Fig. 2). Consistent with these findings, it has been shown that ErbB2-induced repression of GSK3-β is required for MT capture and targeting of ACF7/MACF1 to the plasma membrane in breast carcinoma cells (Zaoui et al., 2010).
Another interesting and novel dimension to the multifaceted functions of spectraplakins comes from studies on Magellan, the single spectraplakin found in zebrafish (Dosch et al., 2004). During oogenesis, this fish spectraplakin plays a critical role in the formation of the animal vegetal axis (Gupta et al., 2010). The Balbiani body, a transient structure composed of organelles including mitochondria, ER, and Golgi, is the first marker of asymmetry in the fish oocyte. In Magellan mutants, the Balbiani body is abnormally large and does not localize to the oocyte cortex as it does in the WT (Gupta et al., 2010). Magellan could directly regulate Balbiani size and localization through binding to cytoskeletal elements within the Balbiani body. Alternatively, loss of Magellan could impair MT network stability and indirectly prevent Balbiani body localization to the vegetal cortex of the oocyte (Gupta et al., 2010). Moreover, the nucleus in the mutant oocyte is asymmetrically localized. This is interesting given that ACF7/MACF1a3 has been shown to localize to the outer nuclear envelope and may have a role in tethering the nucleus to the MT network (Gupta et al., 2010).
Conclusion and prospects
The research on spectraplakins over this past decade has catapulted this fascinating group of multifunctional cytoskeletal linker proteins to the forefront of proteins that function in such diverse processes as cell migration, cell signaling, tissue integrity and maintenance, and axonal extension. Spectraplakins contain domain structures related to two protein superfamilies, spectrins and plakins. The complex gene structure allows for a diverse array of promoter usage and exon splicing, yielding a plethora of context-, cell type–, and temporally specific ways of assembling functional domains to suit the specific cytoskeletal–junctional requirements of polarized cells within multicellular organisms. Thus, the relative paucity of spectraplakin genes is compensated for by the richness of their encoded isoforms.
Defects in spectraplakins have led to an array of different degenerative disorders, and, yet, defects in coordinated cytoskeletal dynamics and cellular polarity are also features of tumorigenesis. In this regard, it may be noteworthy that serum BPAG1 autoantibody has been identified as a novel marker for human melanoma (Shimbo et al., 2010). Although initially established as a gene expressed by keratinocytes, BPAG1 is now known to be expressed in many cell types, including mouse F10 and human (AA375 and G361) melanoma cell lines and tumors as well as normal human melanocytes (Shimbo et al., 2010). Which BPAG1 isoforms are expressed by melanocytes, how this might change in melanoma, and whether there is a direct relevance between loss of BPAG1 and melanoma progression await future explorations.
Although ACF7/MACF1 has not been implicated in tumorigenesis, recent studies suggest it may nevertheless play a role in human cancer. In a comprehensive mutational analysis of human cancer, ACF7/MACF1 was identified as a candidate cancer gene in breast cancer (Sjöblom et al., 2006). It is important to note that the identification by Sjöblom et al. (2006) of new genes has been questioned after reanalysis of their data with different statistical methods and background mutation rate assumptions (Rubin and Green, 2007). That said, a more recent study detected alternative exons in ACF7/MACF1 transcripts of adenocarcinoma tumors from patients with nonsmall cell lung carcinoma (Misquitta-Ali et al., 2011). Given ACF7/MACF1’s reported function in the Wnt signaling pathway, it will be interesting to see whether the increased inclusion of the alternative exon contributes to the altered Wnt signaling often associated with lung cancer. With their exceptional size, domain modularity, and isoform variability, spectraplakins harbor the diversity that it takes not only to govern normal cellular processes but in addition to create a variety of abnormal phenotypes when mutated. Further study of spectraplakins is likely to continue to unravel interesting and exciting new functions in both physiological and pathological settings.
Acknowledgments
We’d like to thank our colleagues in this field for their many wonderful contributions that have helped to illuminate the importance of spectraplakins. R. Kothary, N. Brown, G. Wiche, R. Foisner, K. Green, A. Sonnenburg, M. Labouesse, R. Liem, W. Franke, and V. Bennett are just a few of the colleagues whose work has been instrumental in the field. In addition, we are grateful to the many Fuchs’ laboratory members past and present who have contributed passionately to this research, in particular Y.-M. Yang, A. Karakesisoglu, and A. Kodama.
Support for this work was provided by The National Institutes of Health (R01-AR27883) and the Howard Hughes Medical Institute. X. Wu was supported by fellowships from the American Association for Cancer Research and the Jane Coffin Childs Memorial Fund.
Footnotes
Abbreviations used in this paper:
CH
calponin homology
cKO
conditional knockout
FA
focal adhesion
IF
intermediate filament
MT
microtubule
PRD
plakin repeat domain
WT
wild type
References
- Akhmanova A., Steinmetz M.O. 2008. Tracking the ends: A dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9:309–322 10.1038/nrm2369 [DOI] [PubMed] [Google Scholar]
- Antolik C., Catino D.H., O’Neill A.M., Resneck W.G., Ursitti J.A., Bloch R.J. 2007. The actin binding domain of ACF7 binds directly to the tetratricopeptide repeat domains of rapsyn. Neuroscience. 145:56–65 10.1016/j.neuroscience.2006.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Baines A.J. 2001. Spectrin and ankyrin-based pathways: Metazoan inventions for integrating cells into tissues. Physiol. Rev. 81:1353–1392 [DOI] [PubMed] [Google Scholar]
- Bernier G., Mathieu M., De Repentigny Y., Vidal S.M., Kothary R. 1996. Cloning and characterization of mouse ACF7, a novel member of the dystonin subfamily of actin binding proteins. Genomics. 38:19–29 10.1006/geno.1996.0587 [DOI] [PubMed] [Google Scholar]
- Bosher J.M., Hahn B.S., Legouis R., Sookhareea S., Weimer R.M., Gansmuller A., Chisholm A.D., Rose A.M., Bessereau J.L., Labouesse M. 2003. The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J. Cell Biol. 161:757–768 10.1083/jcb.200302151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottenberg W., Sanchez-Soriano N., Alves-Silva J., Hahn I., Mende M., Prokop A. 2009. Context-specific requirements of functional domains of the Spectraplakin Short stop in vivo. Mech. Dev. 126:489–502 10.1016/j.mod.2009.04.004 [DOI] [PubMed] [Google Scholar]
- Brancolini C., Benedetti M., Schneider C. 1995. Microfilament reorganization during apoptosis: The role of Gas2, a possible substrate for ICE-like proteases. EMBO J. 14:5179–5190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick M.J., Winder S.J. 2005. Spectrin, alpha-actinin, and dystrophin. Adv. Protein Chem. 70:203–246 10.1016/S0065-3233(05)70007-3 [DOI] [PubMed] [Google Scholar]
- Brown A., Bernier G., Mathieu M., Rossant J., Kothary R. 1995. The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat. Genet. 10:301–306 10.1038/ng0795-301 [DOI] [PubMed] [Google Scholar]
- Chen H.J., Lin C.M., Lin C.S., Perez-Olle R., Leung C.L., Liem R.K. 2006. The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt signaling pathway. Genes Dev. 20:1933–1945 10.1101/gad.1411206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.J., Weis W.I. 2011. Crystal structure of a rigid four-spectrin-repeat fragment of the human desmoplakin plakin domain. J. Mol. Biol. 409:800–812 10.1016/j.jmb.2011.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.J., Park-Snyder S., Pascoe L.T., Green K.J., Weis W.I. 2002. Structures of two intermediate filament-binding fragments of desmoplakin reveal a unique repeat motif structure. Nat. Struct. Biol. 9:612–620 [DOI] [PubMed] [Google Scholar]
- Dalpé G., Leclerc N., Vallée A., Messer A., Mathieu M., De Repentigny Y., Kothary R. 1998. Dystonin is essential for maintaining neuronal cytoskeleton organization. Mol. Cell. Neurosci. 10:243–257 10.1006/mcne.1997.0660 [DOI] [PubMed] [Google Scholar]
- Diaz L.A., Ratrie H., III, Saunders W.S., Futamura S., Squiquera H.L., Anhalt G.J., Giudice G.J. 1990. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J. Clin. Invest. 86:1088–1094 10.1172/JCI114812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch R., Wagner D.S., Mintzer K.A., Runke G., Wiemelt A.P., Mullins M.C. 2004. Maternal control of vertebrate development before the midblastula transition: Mutants from the zebrafish I. Dev. Cell. 6:771–780 10.1016/j.devcel.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Drabek K., van Ham M., Stepanova T., Draegestein K., van Horssen R., Sayas C.L., Akhmanova A., Ten Hagen T., Smits R., Fodde R., et al. 2006. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr. Biol. 16:2259–2264 10.1016/j.cub.2006.09.065 [DOI] [PubMed] [Google Scholar]
- Duchen L.W., Strich S.J., Falconer D.S. 1964. Clinical and pathological studies of an hereditary neuropathy in mice (dystonia musculorum). Brain. 87:367–378 10.1093/brain/87.2.367 [DOI] [PubMed] [Google Scholar]
- Ezratty E.J., Partridge M.A., Gundersen G.G. 2005. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat. Cell Biol. 7:581–590 10.1038/ncb1262 [DOI] [PubMed] [Google Scholar]
- Foisner R., Wiche G. 1991. Intermediate filament-associated proteins. Curr. Opin. Cell Biol. 3:75–81 10.1016/0955-0674(91)90168-X [DOI] [PubMed] [Google Scholar]
- Fontao L., Favre B., Riou S., Geerts D., Jaunin F., Saurat J.H., Green K.J., Sonnenberg A., Borradori L. 2003. Interaction of the bullous pemphigoid antigen 1 (BP230) and desmoplakin with intermediate filaments is mediated by distinct sequences within their COOH terminus. Mol. Biol. Cell. 14:1978–1992 10.1091/mbc.E02-08-0548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Cleveland D.W. 1998. A structural scaffolding of intermediate filaments in health and disease. Science. 279:514–519 10.1126/science.279.5350.514 [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Takeo N., Otani Y., Parry D.A., Kunimatsu M., Lu R., Sasaki M., Matsuo N., Khaleduzzaman M., Yoshioka H. 2001. Epiplakin, a novel member of the Plakin family originally identified as a 450-kDa human epidermal autoantigen. Structure and tissue localization. J. Biol. Chem. 276:13340–13347 10.1074/jbc.M011386200 [DOI] [PubMed] [Google Scholar]
- Geerts D., Fontao L., Nievers M.G., Schaapveld R.Q., Purkis P.E., Wheeler G.N., Lane E.B., Leigh I.M., Sonnenberg A. 1999. Binding of integrin α6β4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J. Cell Biol. 147:417–434 10.1083/jcb.147.2.417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goryunov D., He C.Z., Lin C.S., Leung C.L., Liem R.K. 2010. Nervous-tissue-specific elimination of microtubule-actin crosslinking factor 1a results in multiple developmental defects in the mouse brain. Mol. Cell. Neurosci. 44:1–14 10.1016/j.mcn.2010.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K.J., Parry D.A., Steinert P.M., Virata M.L., Wagner R.M., Angst B.D., Nilles L.A. 1990. Structure of the human desmoplakins. Implications for function in the desmosomal plaque. J. Biol. Chem. 265:11406–11407 [PubMed] [Google Scholar]
- Gregory S.L., Brown N.H. 1998. kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J. Cell Biol. 143:1271–1282 10.1083/jcb.143.5.1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves R.W., Liu L., Dopping-Hepenstal P.J., Markus H.S., Lovell P.A., Ozoemena L., Lai-Cheong J.E., Gawler J., Owaribe K., Hashimoto T., et al. 2010. A homozygous nonsense mutation within the dystonin gene coding for the coiled-coil domain of the epithelial isoform of BPAG1 underlies a new subtype of autosomal recessive epidermolysis bullosa simplex. J. Invest. Dermatol. 130:1551–1557 10.1038/jid.2010.19 [DOI] [PubMed] [Google Scholar]
- Guo L., Degenstein L., Dowling J., Yu Q.C., Wollmann R., Perman B., Fuchs E. 1995. Gene targeting of BPAG1: Abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 81:233–243 10.1016/0092-8674(95)90333-X [DOI] [PubMed] [Google Scholar]
- Gupta T., Marlow F.L., Ferriola D., Mackiewicz K., Dapprich J., Monos D., Mullins M.C. 2010. Microtubule actin crosslinking factor 1 regulates the Balbiani body and animal-vegetal polarity of the zebrafish oocyte. PLoS Genet. 6:e1001073 10.1371/journal.pgen.1001073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill K.J., Hopkinson S.B., DeBiase P., Jones J.C. 2009. BPAG1e maintains keratinocyte polarity through beta4 integrin-mediated modulation of Rac1 and cofilin activities. Mol. Biol. Cell. 20:2954–2962 10.1091/mbc.E09-01-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium 2001. Initial sequencing and analysis of the human genome. Nature. 409:860–921 (published erratum appears in Nature. 2001. 411:720) 10.1038/35057062 [DOI] [PubMed] [Google Scholar]
- Janda L., Damborský J., Rezniczek G.A., Wiche G. 2001. Plectin repeats and modules: Strategic cysteines and their presumed impact on cytolinker functions. Bioessays. 23:1064–1069 10.1002/bies.1151 [DOI] [PubMed] [Google Scholar]
- Jefferson J.J., Leung C.L., Liem R.K. 2004. Plakins: Goliaths that link cell junctions and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 5:542–553 10.1038/nrm1425 [DOI] [PubMed] [Google Scholar]
- Jefferson J.J., Leung C.L., Liem R.K. 2006. Dissecting the sequence specific functions of alternative N-terminal isoforms of mouse bullous pemphigoid antigen 1. Exp. Cell Res. 312:2712–2725 10.1016/j.yexcr.2006.04.025 [DOI] [PubMed] [Google Scholar]
- Jefferson J.J., Ciatto C., Shapiro L., Liem R.K. 2007. Structural analysis of the plakin domain of bullous pemphigoid antigen1 (BPAG1) suggests that plakins are members of the spectrin superfamily. J. Mol. Biol. 366:244–257 10.1016/j.jmb.2006.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinuma T., Ichikawa H., Tsukada Y., Nakamura T., Toh B.H. 2004. Interaction between p230 and MACF1 is associated with transport of a glycosyl phosphatidyl inositol-anchored protein from the Golgi to the cell periphery. Exp. Cell Res. 298:388–398 10.1016/j.yexcr.2004.04.047 [DOI] [PubMed] [Google Scholar]
- Karakesisoglou I., Yang Y., Fuchs E. 2000. An epidermal plakin that integrates actin and microtubule networks at cellular junctions. J. Cell Biol. 149:195–208 10.1083/jcb.149.1.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima T., Watt F.M. 2002. Interaction of periplakin and envoplakin with intermediate filaments. J. Cell Sci. 115:5027–5037 10.1242/jcs.00191 [DOI] [PubMed] [Google Scholar]
- Kaverina I., Rottner K., Small J.V. 1998. Targeting, capture, and stabilization of microtubules at early focal adhesions. J. Cell Biol. 142:181–190 10.1083/jcb.142.1.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O., Small J.V. 1999. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J. Cell Biol. 146:1033–1044 10.1083/jcb.146.5.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O., Beningo K., Anderson K., Wang Y.L., Small J.V. 2002. Tensile stress stimulates microtubule outgrowth in living cells. J. Cell Sci. 115:2283–2291 [DOI] [PubMed] [Google Scholar]
- Keep N.H., Winder S.J., Moores C.A., Walke S., Norwood F.L., Kendrick-Jones J. 1999. Crystal structure of the actin-binding region of utrophin reveals a head-to-tail dimer. Structure. 7:1539–1546 10.1016/S0969-2126(00)88344-6 [DOI] [PubMed] [Google Scholar]
- Kelly O.G., Pinson K.I., Skarnes W.C. 2004. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 131:2803–2815 10.1242/dev.01137 [DOI] [PubMed] [Google Scholar]
- Kim H.S., Murakami R., Quintin S., Mori M., Ohkura K., Tamai K.K., Labouesse M., Sakamoto H., Nishiwaki K. 2011. VAB-10 spectraplakin acts in cell and nuclear migration in Caenorhabditis elegans. Development. 138:4013–4023 10.1242/dev.059568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama A., Karakesisoglou I., Wong E., Vaezi A., Fuchs E. 2003. ACF7: An essential integrator of microtubule dynamics. Cell. 115:343–354 10.1016/S0092-8674(03)00813-4 [DOI] [PubMed] [Google Scholar]
- Korenbaum E., Rivero F. 2002. Calponin homology domains at a glance. J. Cell Sci. 115:3543–3545 10.1242/jcs.00003 [DOI] [PubMed] [Google Scholar]
- Korsgren C., Lux S.E. 2010. The carboxyterminal EF domain of erythroid alpha-spectrin is necessary for optimal spectrin-actin binding. Blood. 116:2600–2607 10.1182/blood-2009-12-260612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster J., Geerts D., Favre B., Borradori L., Sonnenberg A. 2003. Analysis of the interactions between BP180, BP230, plectin and the integrin alpha6beta4 important for hemidesmosome assembly. J. Cell Sci. 116:387–399 10.1242/jcs.00241 [DOI] [PubMed] [Google Scholar]
- Kouklis P.D., Hutton E., Fuchs E. 1994. Making a connection: Direct binding between keratin intermediate filaments and desmosomal proteins. J. Cell Biol. 127:1049–1060 10.1083/jcb.127.4.1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylyshkina O., Kaverina I., Kranewitter W., Steffen W., Alonso M.C., Cross R.A., Small J.V. 2002. Modulation of substrate adhesion dynamics via microtubule targeting requires kinesin-1. J. Cell Biol. 156:349–359 10.1083/jcb.200105051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylyshkina O., Anderson K.I., Kaverina I., Upmann I., Manstein D.J., Small J.V., Toomre D.K. 2003. Nanometer targeting of microtubules to focal adhesions. J. Cell Biol. 161:853–859 10.1083/jcb.200301102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib R.S., Anhalt G.J., Patel H.P., Mutasim D.F., Diaz L.A. 1986. Molecular heterogeneity of the bullous pemphigoid antigens as detected by immunoblotting. J. Immunol. 136:1231–1235 [PubMed] [Google Scholar]
- Lapouge K., Fontao L., Champliaud M.F., Jaunin F., Frias M.A., Favre B., Paulin D., Green K.J., Borradori L. 2006. New insights into the molecular basis of desmoplakin- and desmin-related cardiomyopathies. J. Cell Sci. 119:4974–4985 10.1242/jcs.03255 [DOI] [PubMed] [Google Scholar]
- Lee M., Lee S., Zadeh A.D., Kolodziej P.A. 2003. Distinct sites in E-cadherin regulate different steps in Drosophila tracheal tube fusion. Development. 130:5989–5999 10.1242/dev.00806 [DOI] [PubMed] [Google Scholar]
- Lee S., Kolodziej P.A. 2002. Short Stop provides an essential link between F-actin and microtubules during axon extension. Development. 129:1195–1204 10.1242/dev.00159 [DOI] [PubMed] [Google Scholar]
- Lee S., Harris K.L., Whitington P.M., Kolodziej P.A. 2000. short stop is allelic to kakapo, and encodes rod-like cytoskeletal-associated proteins required for axon extension. J. Neurosci. 20:1096–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C.L., Sun D., Liem R.K. 1999a. The intermediate filament protein peripherin is the specific interaction partner of mouse BPAG1-n (dystonin) in neurons. J. Cell Biol. 144:435–446 10.1083/jcb.144.3.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C.L., Sun D., Zheng M., Knowles D.R., Liem R.K. 1999b. Microtubule actin cross-linking factor (MACF): A hybrid of dystonin and dystrophin that can interact with the actin and microtubule cytoskeletons. J. Cell Biol. 147:1275–1286 10.1083/jcb.147.6.1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C.L., Liem R.K., Parry D.A., Green K.J. 2001a. The plakin family. J. Cell Sci. 114:3409–3410 [DOI] [PubMed] [Google Scholar]
- Leung C.L., Zheng M., Prater S.M., Liem R.K. 2001b. The BPAG1 locus: Alternative splicing produces multiple isoforms with distinct cytoskeletal linker domains, including predominant isoforms in neurons and muscles. J. Cell Biol. 154:691–697 10.1083/jcb.200012098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C.L., Green K.J., Liem R.K. 2002. Plakins: A family of versatile cytolinker proteins. Trends Cell Biol. 12:37–45 10.1016/S0962-8924(01)02180-8 [DOI] [PubMed] [Google Scholar]
- Liu J.J., Ding J., Kowal A.S., Nardine T., Allen E., Delcroix J.D., Wu C., Mobley W., Fuchs E., Yang Y. 2003. BPAG1n4 is essential for retrograde axonal transport in sensory neurons. J. Cell Biol. 163:223–229 10.1083/jcb.200306075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.J., Ding J., Wu C., Bhagavatula P., Cui B., Chu S., Mobley W.C., Yang Y. 2007. Retrolinkin, a membrane protein, plays an important role in retrograde axonal transport. Proc. Natl. Acad. Sci. USA. 104:2223–2228 10.1073/pnas.0602222104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Dopping-Hepenstal P.J., Lovell P.A., Michael M., Horn H., Fong K., Lai-Cheong J.E., Mellerio J.E., Parsons M., McGrath J.A. 2012. Autosomal recessive epidermolysis bullosa simplex due to loss of BPAG1-e expression. J. Invest. Dermatol. 132:742–744 10.1038/jid.2011.379 [DOI] [PubMed] [Google Scholar]
- Liu P., Wakamiya M., Shea M.J., Albrecht U., Behringer R.R., Bradley A. 1999. Requirement for Wnt3 in vertebrate axis formation. Nat. Genet. 22:361–365 10.1038/11932 [DOI] [PubMed] [Google Scholar]
- Misquitta-Ali C.M., Cheng E., O’Hanlon D., Liu N., McGlade C.J., Tsao M.S., Blencowe B.J. 2011. Global profiling and molecular characterization of alternative splicing events misregulated in lung cancer. Mol. Cell. Biol. 31:138–150 10.1128/MCB.00709-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S., Klaus-Kovtun V., Stanley J.R. 1989. A 230-kD basic protein is the major bullous pemphigoid antigen. J. Invest. Dermatol. 92:33–38 10.1111/1523-1747.ep13070476 [DOI] [PubMed] [Google Scholar]
- Mui U.N., Lubczyk C.M., Nam S.C. 2011. Role of spectraplakin in Drosophila photoreceptor morphogenesis. PLoS ONE. 6:e25965 10.1371/journal.pone.0025965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic B., Mac Nulty E., Mir B., Wiche G. 1996. Basic amino acid residue cluster within nuclear targeting sequence motif is essential for cytoplasmic plectin-vimentin network junctions. J. Cell Biol. 134:1455–1467 10.1083/jcb.134.6.1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura M., Yamakawa H., Ohara O., Owaribe K. 2002. Novel alternative splicings of BPAG1 (bullous pemphigoid antigen 1) including the domain structure closely related to MACF (microtubule actin cross-linking factor). J. Biol. Chem. 277:6682–6687 10.1074/jbc.M109209200 [DOI] [PubMed] [Google Scholar]
- Ortega E., Buey R.M., Sonnenberg A., de Pereda J.M. 2011. The structure of the plakin domain of plectin reveals a non-canonical SH3 domain interacting with its fourth spectrin repeat. J. Biol. Chem. 286:12429–12438 10.1074/jbc.M110.197467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmucci L., Doriguzzi C., Mongini T., Restagno G., Chiadò-Piat L., Maniscalco M. 1994. Unusual expression and very mild course of Xp21 muscular dystrophy (Becker type) in a 60-year-old man with 26 percent deletion of the dystrophin gene. Neurology. 44:541–543 [DOI] [PubMed] [Google Scholar]
- Rezniczek G.A., de Pereda J.M., Reipert S., Wiche G. 1998. Linking integrin α6β4-based cell adhesion to the intermediate filament cytoskeleton: Direct interaction between the β4 subunit and plectin at multiple molecular sites. J. Cell Biol. 141:209–225 10.1083/jcb.141.1.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper K., Brown N.H. 2003. Maintaining epithelial integrity: A function for gigantic spectraplakin isoforms in adherens junctions. J. Cell Biol. 162:1305–1315 10.1083/jcb.200307089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röper K., Brown N.H. 2004. A spectraplakin is enriched on the fusome and organizes microtubules during oocyte specification in Drosophila. Curr. Biol. 14:99–110 [PubMed] [Google Scholar]
- Röper K., Gregory S.L., Brown N.H. 2002. The ‘spectraplakins’: Cytoskeletal giants with characteristics of both spectrin and plakin families. J. Cell Sci. 115:4215–4225 10.1242/jcs.00157 [DOI] [PubMed] [Google Scholar]
- Rubin A.F., Green P. 2007. Comment on “The consensus coding sequences of human breast and colorectal cancers”. Science. 317:1500 10.1126/science.1138956 [DOI] [PubMed] [Google Scholar]
- Ruhrberg C., Hajibagheri M.A., Simon M., Dooley T.P., Watt F.M. 1996. Envoplakin, a novel precursor of the cornified envelope that has homology to desmoplakin. J. Cell Biol. 134:715–729 10.1083/jcb.134.3.715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S.D., Bhanot K., Ferrier A., De Repentigny Y., Chu A., Blais A., Kothary R. 2012a. Microtubule stability, Golgi organization, and transport flux require dystonin-a2–MAP1B interaction. J. Cell Biol. 196:727–742 10.1083/jcb.201107096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan S.D., Ferrier A., Sato T., O’Meara R.W., De Repentigny Y., Jiang S.X., Hou S.T., Kothary R. 2012b. Neuronal dystonin isoform 2 is a mediator of endoplasmic reticulum structure and function. Mol. Biol. Cell. 23:553–566 10.1091/mbc.E11-06-0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Soriano N., Travis M., Dajas-Bailador F., Gonçalves-Pimentel C., Whitmarsh A.J., Prokop A. 2009. Mouse ACF7 and drosophila short stop modulate filopodia formation and microtubule organisation during neuronal growth. J. Cell Sci. 122:2534–2542 10.1242/jcs.046268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J., Milpetz F., Bork P., Ponting C.P. 1998. SMART, a simple modular architecture research tool: Identification of signaling domains. Proc. Natl. Acad. Sci. USA. 95:5857–5864 10.1073/pnas.95.11.5857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimbo T., Tanemura A., Yamazaki T., Tamai K., Katayama I., Kaneda Y. 2010. Serum anti-BPAG1 auto-antibody is a novel marker for human melanoma. PLoS ONE. 5:e10566 10.1371/journal.pone.0010566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöblom T., Jones S., Wood L.D., Parsons D.W., Lin J., Barber T.D., Mandelker D., Leary R.J., Ptak J., Silliman N., et al. 2006. The consensus coding sequences of human breast and colorectal cancers. Science. 314:268–274 10.1126/science.1133427 [DOI] [PubMed] [Google Scholar]
- Slep K.C., Rogers S.L., Elliott S.L., Ohkura H., Kolodziej P.A., Vale R.D. 2005. Structural determinants for EB1-mediated recruitment of APC and spectraplakins to the microtubule plus end. J. Cell Biol. 168:587–598 10.1083/jcb.200410114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Rojas A.M., de Pereda J.M. 2007. The structure of a tandem pair of spectrin repeats of plectin reveals a modular organization of the plakin domain. J. Mol. Biol. 368:1379–1391 10.1016/j.jmb.2007.02.090 [DOI] [PubMed] [Google Scholar]
- Sonnhammer E.L., Eddy S.R., Durbin R. 1997. Pfam: A comprehensive database of protein domain families based on seed alignments. Proteins. 28:405–420 [DOI] [PubMed] [Google Scholar]
- Stappenbeck T.S., Bornslaeger E.A., Corcoran C.M., Luu H.H., Virata M.L., Green K.J. 1993. Functional analysis of desmoplakin domains: Specification of the interaction with keratin versus vimentin intermediate filament networks. J. Cell Biol. 123:691–705 10.1083/jcb.123.3.691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner-Champliaud M.F., Schneider Y., Favre B., Paulhe F., Praetzel-Wunder S., Faulkner G., Konieczny P., Raith M., Wiche G., Adebola A., et al. 2010. BPAG1 isoform-b: Complex distribution pattern in striated and heart muscle and association with plectin and alpha-actinin. Exp. Cell Res. 316:297–313 10.1016/j.yexcr.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Subramanian A., Prokop A., Yamamoto M., Sugimura K., Uemura T., Betschinger J., Knoblich J.A., Volk T. 2003. Shortstop recruits EB1/APC1 and promotes microtubule assembly at the muscle-tendon junction. Curr. Biol. 13:1086–1095 10.1016/S0960-9822(03)00416-0 [DOI] [PubMed] [Google Scholar]
- Sun D., Leung C.L., Liem R.K. 2001. Characterization of the microtubule binding domain of microtubule actin crosslinking factor (MACF): Identification of a novel group of microtubule associated proteins. J. Cell Sci. 114:161–172 [DOI] [PubMed] [Google Scholar]
- Travé G., Lacombe P.J., Pfuhl M., Saraste M., Pastore A. 1995. Molecular mechanism of the calcium-induced conformational change in the spectrin EF-hands. EMBO J. 14:4922–4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Gromov D., Donovan A., Castañón M.J., Fuchs E. 1993. Expression of plectin mutant cDNA in cultured cells indicates a role of COOH-terminal domain in intermediate filament association. J. Cell Biol. 121:607–619 10.1083/jcb.121.3.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Kodama A., Fuchs E. 2008. ACF7 regulates cytoskeletal-focal adhesion dynamics and migration and has ATPase activity. Cell. 135:137–148 10.1016/j.cell.2008.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Shen Q.T., Oristian D.S., Lu C.P., Zheng Q., Wang H.W., Fuchs E. 2011. Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3β. Cell. 144:341–352 10.1016/j.cell.2010.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Dowling J., Yu Q.C., Kouklis P., Cleveland D.W., Fuchs E. 1996. An essential cytoskeletal linker protein connecting actin microfilaments to intermediate filaments. Cell. 86:655–665 10.1016/S0092-8674(00)80138-5 [DOI] [PubMed] [Google Scholar]
- Yang Y., Bauer C., Strasser G., Wollman R., Julien J.P., Fuchs E. 1999. Integrators of the cytoskeleton that stabilize microtubules. Cell. 98:229–238 10.1016/S0092-8674(00)81017-X [DOI] [PubMed] [Google Scholar]
- Young K.G., Kothary R. 2007. Dystonin/Bpag1—a link to what? Cell Motil. Cytoskeleton. 64:897–905 10.1002/cm.20235 [DOI] [PubMed] [Google Scholar]
- Young K.G., Kothary R. 2008. Dystonin/Bpag1 is a necessary endoplasmic reticulum/nuclear envelope protein in sensory neurons. Exp. Cell Res. 314:2750–2761 10.1016/j.yexcr.2008.06.021 [DOI] [PubMed] [Google Scholar]
- Young K.G., Pinheiro B., Kothary R. 2006. A Bpag1 isoform involved in cytoskeletal organization surrounding the nucleus. Exp. Cell Res. 312:121–134 10.1016/j.yexcr.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Zaoui K., Benseddik K., Daou P., Salaün D., Badache A. 2010. ErbB2 receptor controls microtubule capture by recruiting ACF7 to the plasma membrane of migrating cells. Proc. Natl. Acad. Sci. USA. 107:18517–18522 10.1073/pnas.1000975107 [DOI] [PMC free article] [PubMed] [Google Scholar]