Distinct and separable activities of the endocytic clathrin coat components Fcho1/2 and AP-2 in developmental patterning (original) (raw)
. Author manuscript; available in PMC: 2012 Nov 1.
Published in final edited form as: Nat Cell Biol. 2012 Apr 8;14(5):488–501. doi: 10.1038/ncb2473
Abstract
Clathrin-mediated endocytosis occurs at multiple independent import sites on the plasma membrane, but how these positions are selected and how different cargo is simultaneously recognized is obscure. FCHO1 and FCHO2 are early-arriving proteins at surface clathrin assemblies and are speculated to act as compulsory coat nucleators, preceding the core clathrin adaptor AP-2. Here, we show the μ-homology domain (μHD) of FCHO1/2 represents a novel endocytic interaction hub. Translational silencing of fcho1 in zebrafish embryos causes strong dorsoventral patterning defects analogous to Bmp signal failure. The Fcho1 μHD interacts with the Bmp receptor Alk8, uncovering a new endocytic component that positively modulates Bmp signal transmission. Still, the fcho1 morphant phenotype is distinct from severe embryonic defects apparent when AP-2 is depleted. Our data thus contradict the primacy of FCHO1/2 in coat initiation.
INTRODUCTION
Clathrin-mediated endocytosis is a major mechanism for the selective internalization of cell surface components and extracellular macromolecules1,2. The import sites contain clathrin triskelia assembled into a polygonal lattice3. As the lattice curves by incorporating pentagonal facets and projects into the cell interior, select cargo is packaged into the clathrin-coated invagination. Preferential retention of cargo within the bud depends on cytosol-oriented sorting signals4-6. A heterotetrameric AP-2 adaptor complex and numerous clathrin-associated sorting proteins (CLASPs) identify structurally disparate sorting signals6; this recognition allows non-competitive grouping of dissimilar cargo into single clathrin-coated buds. The processes of coat assembly, cargo capture and budding takes less than a minute, and eukaryotic cells have hundreds of spatially-discrete clathrin-coated structures forming on the surface3. Precisely how buds initiate at defined locations is unclear7,8. Certainly, phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) is pivotal since depleting this lipid triggers swift dissolution of surface coats9,10. Because AP-2 and numerous CLASPs and clathrin accessory proteins bind physically to PtdIns(4,5)P2, current models invoke stochastic but simultaneous encounters of these molecules with PtdIns(4,5)P2, themselves, cargo and clathrin to begin coat polymerization on a patch of membrane6,11. Recently, however, on the basis of two PtdIns(4,5)P2-binding proteins invariantly preceding the arrival of AP-2 and clathrin at nascent bud sites, FCH domain only 1 (FCHO1) and FCHO2 were proposed to be functionally redundant pioneer proteins demarcating sites of future clathrin assembly12.
Here, we examine the endocytic activity of the modular FCHO1 and FCHO2 proteins to address the following questions: What molecular interactions distinguish the various protein domains? What role do Fcho1 and Fcho2 play in zebrafish embryonic development? Are Fcho1 and Fcho2 functionally interchangeable? And, if FCHO1/2 is obligatory for clathrin-coat nucleation, does the phenotype of Fcho1+2-compromised embryos parallel that of AP-2 morphants? We find Fcho1 operates during dorsoventral (DV) patterning of the embryo and associates with activin receptor-like kinase 8 (Alk8/lost-a-fin), a type I BMP receptor involved in signaling ventral cell fates13,14. Yet AP-2 depletion causes a much more penetrant, broadly severe and earlier developmental phenotype, indicating AP-2 function is not dependent on Fcho1/2.
RESULTS
FCHO1 and FCHO2 are members of the muniscin subfamily of EFC domain proteins evolutionarily conserved from unicellular eukaryotes to mammals15,16. These paralogues play a functionally redundant role beginning at the earliest assembly stages of clathrin-mediated endocytosis7,12,15,17,18. HeLa cells express transcripts for both FCHO1 and FCHO2 but endogenous FCHO1 protein is undetectable with antibodies19. Transient expression of GFP-tagged FCHO1 reveals scattered bright puncta on the ventral surface, but a diffuse membrane-tethered population becomes evident with higher over-expression (Fig. S1a). The heterogeneously sized fluorescent structures in cells expressing low-level GFP-FCHO1 colocalize with AP-2 but are spatially distinct from peripheral APPL1-positive endosomes or more centrally positioned EEA1-positive endosomes (Fig. S1b-e)20. More abundant endogenous FCHO2 populates similar punctate structures to GFP-FCHO1 at steady state with considerable (~75%) overlap, although not perfect coincidence, with AP-2 (Fig. S1f-i). These findings confirm FCHO1/2 operate at clathrin-coated structures at the cell surface.
The FCHO endocytic hub
FCHO1 and FCHO2 are modular proteins (Fig. S1j). The N-terminal EFC domain is connected to a ~280 residue C-terminal μHD by a linker of differing length in the paralogues. The first 270 residues of human FCHO1 and FCHO2, encoding a helical EFC domain monomer21, are 58% identical. Accordingly, dose-dependent association of purified FCHO1 EFC domain with PtdIns(4,5)P2-containing liposomes parallels that of the FCHO2 (Fig. S1k)12,21. Binding to liposomes lacking PtdIns(4,5)P2 is ~10-fold less efficient. The membrane binding and curvature sensing/inducing properties21 of the antiparallel EFC domain dimer in FCHO1 and FCHO2 are thus similar.
The linker region
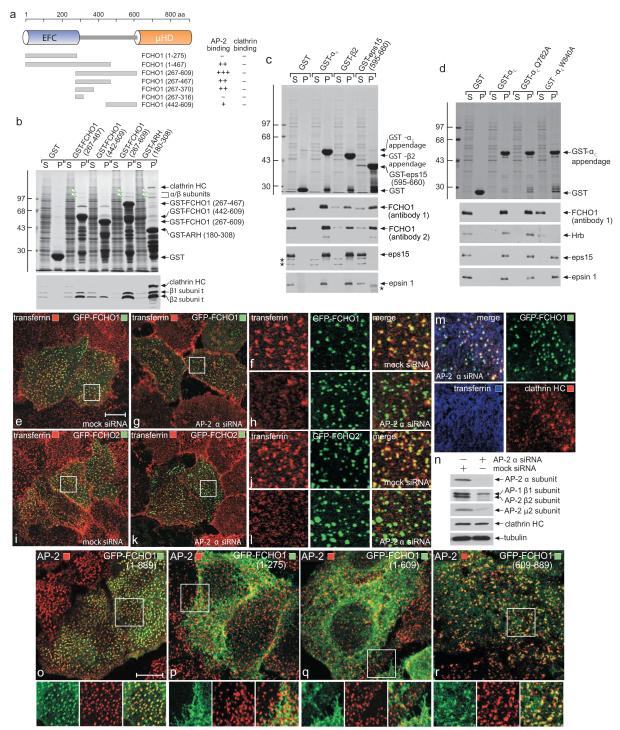
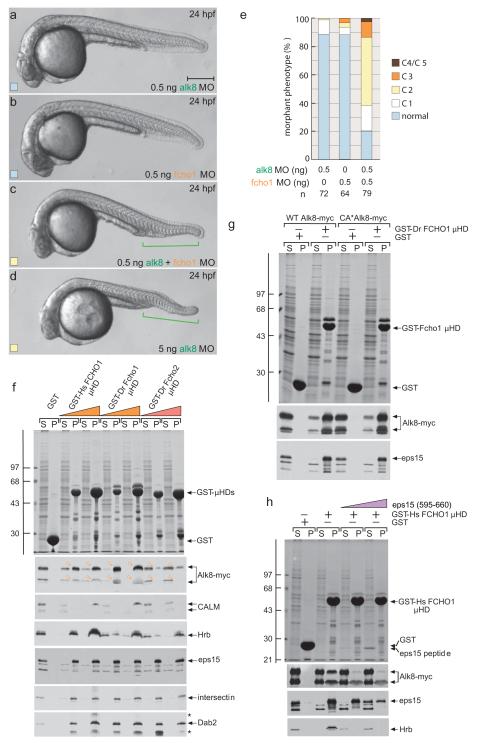
Protein disorder predictions indicate the EFC- and μHD-separating residues (300-600) of FCHO1 comprise a low-complexity, largely unstructured segment. Intrinsic disorder is a hallmark of numerous endocytic factors that bind AP-2 utilizing short, tandemly-arrayed peptide motifs in the flexible region5,8,22. GST-fusion proteins encompassing the central portion of FCHO1 engage AP-2 in pull-down assays (Fig. 1a, b). The apparent affinity of this association with AP-2 is weaker than the C-terminal segment of ARH, which engages AP-2 with a _K_D of ~1 μM23,24. The major AP-2 interaction determinant(s) in FCHO1 is between residues 267-442 (Fig. 1a, b). Unlike ARH, the central segment of FCHO1 does not bind clathrin directly (Fig. 1b). FCHO2 does not associate with AP-2 (Fig. S2a); the shorter central linker in FCHO2 displays <30% identity with FCHO1, probably explaining the absence of an AP-2 interaction motif(s).
Figure 1.
Binding properties of FCHO1
(a) Cartoon of FCHO1 with the location and relative binding properties of the various truncations tested.
(b) Coomassie-stained gel and blot of supernatant (S) and pellet (P) fractions of a GST pull-down assay with brain cytosol and immobilized GST or the indicated GST-FCHO1 or GST-ARH fragments. Immunoblotted with anti-clathrin heavy chain (HC) and AP-1/2 β1/2-subunit antibodies. Molecular mass standards (kDa) are shown, and large adaptor subunits (arrowheads) indicated.
(c) Pull-down assay with FCHO1 overexpressing HeLa cell lysate and immobilized GST or the indicated GST-fusion proteins. Two independent anti-FCHO1 antibodies used for detection. Non-specific bands (asterisks) are indicated.
(d) Pull-down assay with FCHO1 overexpressing HeLa lysates and immobilized GST, GST-αC appendage or the indicated αC appendage point mutants.
(e-l) Mock or AP-2 α-subunit siRNA-treated HeLa cells transfected with either GFP-FCHO1 or GFP-FCHO2 as indicated were incubated with Alexa568 transferrin on ice before fixation. Representative confocal images show AP-2 silencing leads to loss of transferrin clusters on the surface. Insets (f,h,i,l) provide color-separated and merged enlargements of boxed regions. Scale bar: 10 μm.
(m) Confocal image of a region of an AP-2 α-subunit siRNA transfected HeLa cell also expressing GFP-FCHO1. Prior to fixation and staining with an anti-clathrin HC mAb, the silenced cells were incubated at 37°C with 25 μg/ml Alexa633-labeled transferrin.
(n) Biochemical assessment of AP-2 siRNA silencing in HeLa cell lysates by immunoblotting.
(o-r) Representative confocal images of the intracellular localization of full-length (1-889), EFC domain (1-275), ΔμHD (1-609) or μHD (609-889) GFP-tagged FCHO1 compared with AP-2 (α subunit). Insets provide color-separated and merged enlargements of boxed regions. Scale bar: 10 μm.
FCHO1 binds AP-2 through the globular appendage domain of the large α subunit, but some association with the β2-subunit appendage is also evident (Fig. 1c). The contact site upon the α appendage is the platform subdomain since a W840A substitution abolishes binding (Fig. 1d); a sandwich subdomain disruption (Q782A)25,26 has no effect on binding. Yet RNAi suppression of AP-2, causing pronounced accumulation of dispersed transferrin receptors at the cell surface27,28, is still compatible with surface-clustered FCHO1 or -2 puncta (Fig. 1e-l) also containing clathrin (Fig. 1m) and eps15 (Fig. S2b), in line with these proteins being constituents of a pioneer unit7,12,17. AP-2 depletion is seen on immunoblots (Fig. 1n). Thus, despite binding AP-2, the heterotetramer is not necessary for FCHO1 (or FCHO2) surface puncta. This is consistent with FCHO2 not contacting AP-2 directly and also supported by truncation analysis; while full-length GFP-FCHO1 (1-889) masses at AP-2 patches, GFP-FCHO1 (1-275) and (1-609) are diffusely plasma membrane associated (Fig. 1o-q) while GFP-FCHO1 (265-467) and (265-609) are cytosolic (Fig. S2). By contrast, GFP-μHD (609-889) populates clathrin spots, albeit not as efficiently as the full-length protein (Fig. 1r). Precise deposition of FCHO1 at clathrin foci therefore depends, in part, on the C-terminal μHD.
The μHD
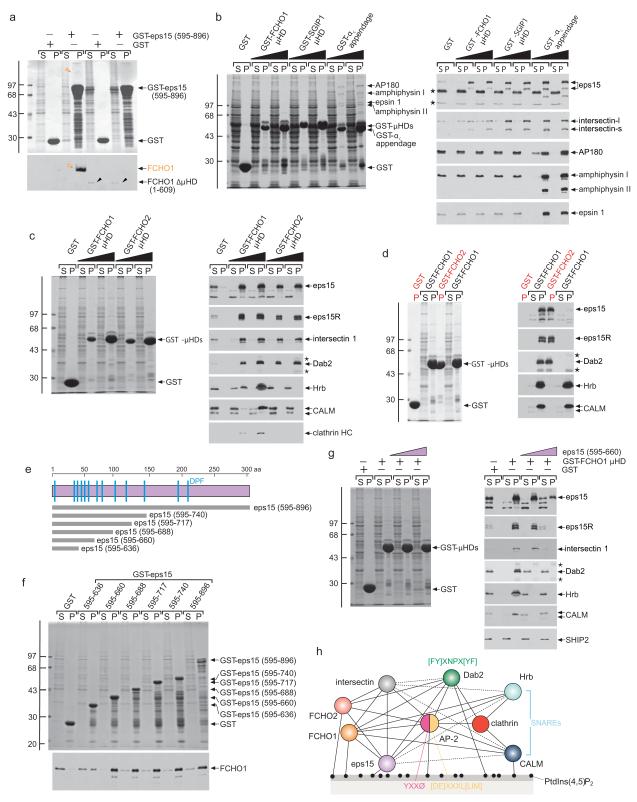
Muniscins associate with eps1512,15,29. Reciprocal pull-down assays using a GST fusion with the C-terminus of eps15 (595-896) confirm binding requires the μHD (Fig. 2a). μHDs also associate with intersectin 1 (Fig. 2b), another EH (and SH3) domain scaffold protein involved in clathrin-mediated endocytosis30,31 that heterooligomerizes with eps1532. Yet, unlike the AP-2 α-subunit appendage, which also binds eps15 and intersectin to a similar extent, the μHDs do not interact with endocytic factors like AP180, amphiphysin or epsin 1. The capacity of FCHO1/2 to associate with membranes in a PtdIns(4,5)P2-dependent manner and engage AP-2 and other core endocytic components (eps15, intersectin) distinguishes these proteins as dedicated endocytic factors. This is consistent with positioning of FCHO1/2 at clathrin structures on the plasma membrane (Fig. 1).
Figure 2.
The μHD interaction hub
(a) Coomassie-stained gel and blot of supernatant (S) and pellet (P) fractions of a pull-down assay with full-length or ΔμHD (1-609) FCHO1 overexpressing HeLa lysates and immobilized GST or GST-eps15 (595-896). Immunoblot with anti-FCHO1. Molecular mass standards (kDa) and intact FCHO1 (orange) and ΔμHD (black arrowheads) FCHO1 are indicated.
(b) Pull-down assay using brain cytosol and either GST or 50 or 100 μg of the GST-μHD fusions indicated or the GST- αC appendage. Replicate immunoblots probed with the indicated antibodies with non-specific bands (asterisks) indicated.
(c) Pull-down assay using HeLa lysate and either GST or 50 or 100 μg of the GST-μHD fusions indicated. Non-specific bands (asterisks) are indicated. All the GST-μHDs bind to eps15, eps15R, intersectin and Dab2 but only the FCHO1 μHD associates with Hrb, CALM, and clathrin. Note that different eps15 splice isoforms are present in HeLa lysates compared with brain (b).
(d) Stained gel and replicate blots of first-stage assay pellets (red P) from incubations of brain cytosol with either GST or GST-FCHO2 μHD compared with supernatant (S) and pellets (P) from subsequent second-stage pull-downs with the supernatant fractions resulting from the first-stage incubations.
(e) Cartoon of the eps15 C terminus with the relative positioning of the various truncations used indicated.
(f) Pull-down assay using FCHO1 overexpressing HeLa lysate and the indicated GST-eps15 C-terminal fusions. Immunoblot with anti-FCHO1.
(g) Stained gel and replicate blots from pull-down assays with GST or GST-FCHO1 μHD and HeLa lysates supplemented with 5 or 25 μM eps15 (595-660) peptide as indicated.
(h) Interaction diagram for FCHO1/2 and select endocytic pioneer coat components. Presumptive contacts are indicated with dotted lines.
The FCHO1/2 μHDs also associate with eps15, eps15R, and intersectin in HeLa lysates. In addition, Dab2, Hrb and CALM, three tandem Asn-Pro-Phe-containing CLASPs bind the μHDs in a concentration-dependent manner (Fig. 2c). Three observations rule out that these associations are indirect and mediated by Asn-Pro-Phe sequences binding to the EH domain scaffolds (eps15/R and intersectin). First, Dab2, Hrb and CALM binding does not parallel binding of the EH domain proteins to GST-μHDs. Second, Hrb and CALM bind efficiently to only the FCHO1 μHD and neither FCHO2 nor SGIP1, despite all exhibiting robust eps15 and intersectin interactions. Third, preincubating HeLa lysates with GST-FCHO2 μHD removes the majority of eps15 and intersectin, but remaining Hrb and CALM engage GST-FCHO1 μHD in second-stage incubations indistinguishably from GST-preincubated lysate (Fig. 2d). This reveals that association of Dab2, Hrb and CALM with the FCHO1 μHD is independent of EH domain proteins and highlights a functional difference between FCHO1 and FCHO2/SGIP1 μHDs.
The Asp-Pro-Phe tripeptide-rich C terminus of eps15 was used to delineate the FCHO1 μHD-binding region (Fig. 2e, f). A limited tract positioned between eps15 residues ~600 and 660 is recognized by the μHD. Since this short eps15 (595-660) peptide efficiently competes with cytosolic Hrb, CALM and Dab2 in assays at lower concentrations than required to displace eps15R, intersectin or eps15 (Fig. 2g), all likely engage the FCHO1 μHD through a common or partly overlapping interaction surface, but with the scaffolds binding with higher apparent affinity. Numerous important accessory proteins and CLASPs thus converge on the FCHO1 μHD (Fig. 2h) and, therefore, like the AP-2 appendage domains6,8, this domain represents a novel endocytic hub.
FCHO1 affects embryogenesis
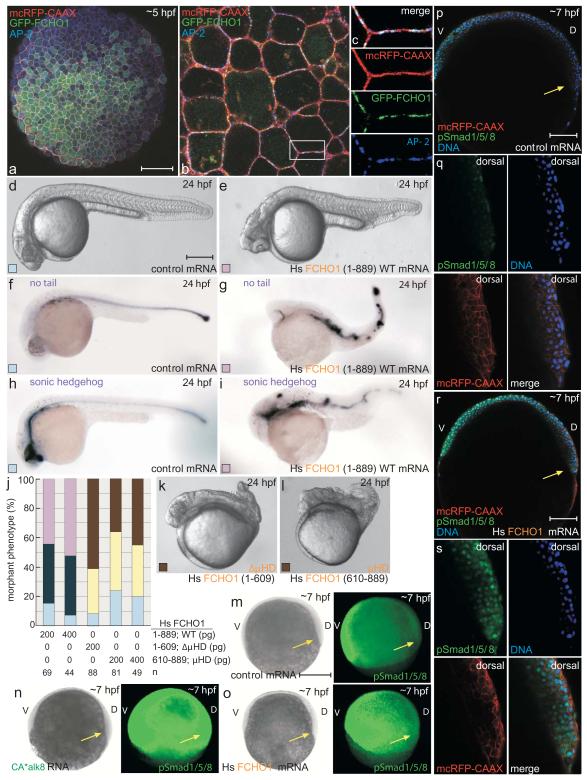
Misexpression of mammalian FCHO1 in zebrafish confirms the μHD importance. On microinjecting synthetic GFP-FCHO1 (1-889) mRNA into 1-cell stage embryos, fluorescence is visible during the early gastrula period, ~5 hours post fertilization (hpf) (Fig. 3a). Coinjection of a membrane-tethered RFP marker shows GFP-FCHO1 concentrates at the blastomere plasma membrane, but in discontinuous puncta as opposed to the uninterrupted RFP. The punctate GFP-FCHO1 pattern overlaps with endogenous AP-2 (Fig. 3b, c). By 24 hpf, microinjected ectopic mammalian FCHO1 produces a gain-of-function phenotype. Unlike controls, the FCHO1-expressing embryos are cyclopic and mildly ventralized, with small head regions, misshapen trunk somites, and loss of notochord tissue in severely affected embryos (Fig. 3d-j). Comparative in situ localization of transcripts for dorsally specified axial mesoderm, no-tail and sonic hedgehog, confirms disruption of dorsal patterning. Strikingly, misexpression of similar amounts of a μHD-truncated FCHO1 (1-609; ΔμHD) mRNA leads to strong dorsalization (Fig. 3k). Embryos now show posteriorly shortened and twisted body axes, loss of the yolk extension and tail. The most severe resemble C4/C5 category snailhouse (bmp7) and swirl (bmp2b) mutant embryos33,34. Injection of FCHO1 μHD (609-889) mRNA alone phenocopies FCHO1 (1-609) overexpression (Fig. 3j, l) indicating that the dominant-negative effect derives from uncoupling the linked domains and that the normal operation of FCHO1 requires physical connection of the EFC and μHD regions. These reciprocal dysmorphic effects suggest muniscins participate in early embryogenesis. In fact, because DV patterning is governed in part by Bmp signaling35, Fcho1 may assist operation of Bmp receptors during gastrulation. The Smad1/5/8 transcription factors are direct phosphotargets of the type I Bmp receptor Alk836, and phosphoSmad (pSmad1/5/8) localization is grossly misplaced dorsally in constitutively-active (CA*) alk8 mRNA-injected embryos (Fig. 3m, n). Likewise, ectopic FCHO1 expression drives notable, but weaker, dorsal expansion of nuclear pSmad1/5/8 localization in gastrulas (Fig. 3o-s), indicating hyperactive Bmp signaling.
Figure 3.
Developmental defects upon FCHO1 overexpression
(a-c) Representative confocal optical section of 30% epiboly-stage (~5 hpf) embryo injected with a mcRFP-CAAX surface marker (80 pg) and GFP-FCHO1 (400 pg) mRNA after fixation and staining with an anti-AP-2 β2 subunit antibody. Subcellular localization (b) and color-separated enlargements (c) of the boxed region. Scale bar: 125 μm.
(d-i) Representative morphology of 80 pg control or 200 pg human (Hs) FCHO1 mRNA-injected embryos at 24 hpf with no tail and sonic hedgehog mRNA expression patterns. Scale bar: 250 μm.
(j) Quantitation of normal (pale blue), mild (navy blue) and moderate (violet) ventralized, or mild (yellow) and severe (brown) mRNA overexpression-induced dorsalized phenotypes, color-coded as in d-i. See Fig. 4 and 6 for complete categorization of the dorsalized and ventralized phenotypic classes.
(k-l) Representative morphology of severely affected 200 pg truncated FCHO1 mRNA-expressing embryos.
(m-s) pSmad1/5/8 localization in control (m, p, q), CA*alk8 (5 pg; n) or FCHO1 (400 pg; o, r, s) mRNA-injected early gastrulation embryos co-injected with 50 pg mcRFP-CAAX. Wide field (m-o; left) and fluorescence images (m-o; right)) or representative confocal sections (p, r) with color separated and merged enlargements (q, s) of the dorsal organizer region (arrows) shown. D, dorsal; V, ventral. Scale bars: 250 μm (m-o) or 125 μm (p, r).
D. rerio Fcho1 and Fcho2 in DV patterning
A single gene encodes Fcho1 (LOC565812) and Fcho2 (ZDB-GENE-050522-228) in zebrafish. The structural and topological features are conserved in the teleost proteins (Fig. S3) and maternally deposited transcripts for both Fcho1 and Fcho2 are present; RT-PCR confirms the presence of the appropriate gene-specific amplicons while in situ hybridization shows localization in four-cell embryos through shield stage (when gastrulation begins) at ~6 hpf (Fig. S3). After the onset of general zygotic transcription (~3 hpf), both fcho1 and fcho2 transcripts are still detectable and increase gradually through the segmentation period to 24 hpf. Both fcho1 and fcho2 messages are broadly distributed but, by 24 hpf, regional differences are apparent; expression patterns are therefore overlapping but not identical.
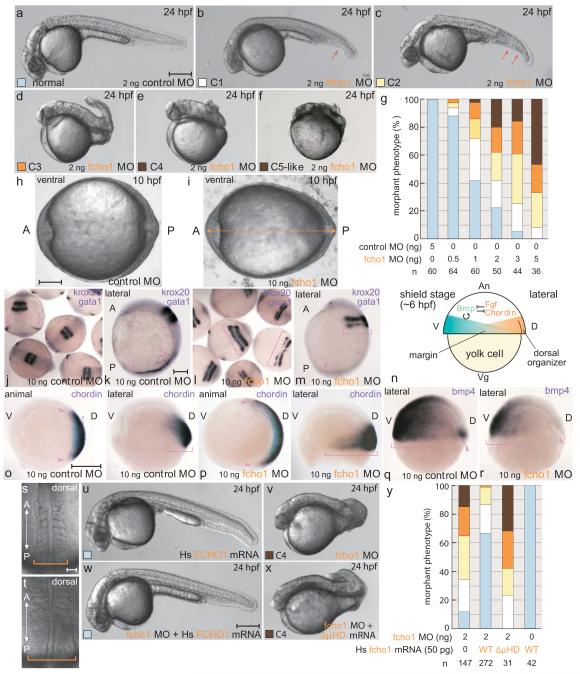
Injecting an initiation codon-targeting fcho1 antisense morpholino oligonucleotide (MO) (Fig. S4) at the 1-cell stage causes a strong dorsalized phenotype at 24 hpf, where structures originating from the dorsal side predominate with accompanying loss of ventral tissues (Fig. 4a-g). Again, severely affected embryos mirror snailhouse and swirl mutants. The phenotype is dose dependent with increased lethality at higher MO concentrations. MO efficacy is verified by silencing of an appropriate _fcho1_-GFP reporter (Fig. S4) and the phenotype is not markedly different in p53 MO-coinjected embryos37. Earlier in development, at the end of gastrulation (~10 hpf), the fcho1 morphants are oblong (Fig, 4h, i), typical of dorsalization33,34. These morphological abnormalities accompany gene expression pattern changes diagnostic for dorsalization: the positioning of the hindbrain marker krox20 reveals laterally-expanded stripes compared with controls, while, in the same embryos, expression of gata1, a marker of the ventrally-specified erythroid lineage, is diminished (Fig. 4j-m). Also in line with expansion of dorsal cell fates, regional mRNA expression of the dorsally positioned Bmp antagonist Chordin (Fig. 4n) is widened ventrally in shield stage fcho1 morphants (Fig. 4o, p). Reciprocally, the lateral bmp4 expression field is variably reduced ventrally, consistent with suppression of positive feedback of bmp expression by Bmp signaling in the ventral region (Fig. 4q, r)38. During segmentation, the fcho1 morphants have laterally broadened somitic mesoderm (Fig. 4s, t) that could reflect delayed/abnormal convergence–extension movements. Defective convergence likely reflects a second function of the Bmp gradient in the gastrula: proper locomotion of cells to the midline by modulating cell adhesion39.
Figure 4.
Fcho1 participates in early embryonic development
(a-f) Representative images of the morphant phenotypic range on injection with 2 ng control or 2 ng fcho1 MO. C5-like morphants are rare and are grouped with the C4 class. Arrows: ventral fin defects. Scale bar: 250 μm.
(g) Quantitation of dose-dependent fcho1 morphant phenotypes at 24 hpf, color coded as in a-f.
(h-i) Typical vegetal pole view of 10 ng control or 10 ng fcho1 early-stage morphants at 10 hpf. A, anterior; P, posterior. Arrow: expanded A-P axis. Scale bar: 125 μm.
(j-m) Representative mRNA expression patterns of both krox20 (brackets) and gata1 (arrows) in control (j, k) or 10 ng fcho1 MO-injected (l, m) 5-somite stage embryos. A, anterior; P, posterior. Group dorsal survey view: j, l; individual lateral view: k, m. Scale bar: 225 μm (j and l) or 125 μm (k, m).
(n) Schematic cartoon depiction of the general location of opposing morphogen gradients that dictate DV patterning at shield stage. An, animal pole; Vg, vegetal pole; D, dorsal; V, ventral.
(o-r) Representative mRNA expression patterns of indicated genes in 10 ng control (o and q) or fcho1 (p and r) shield-stage morphants. Relative expression zones (arrowhead in animal and bracket in lateral views) shown. Scale bar: 250 μm.
(s-t) Dorsal view of gross morphology of developing notochord and somite regions of live 10 ng control (R) and fcho1 (S) morphants at the 6-somite stage. Scale bar: 50 μm.
(u-x) representative phenotypes of 2 ng fcho1 morphants coinjected with indicated human (Hs) FCHO1 mRNA. Scale bar: 250 μm.
(y) Quantitation of morphant phenotypes at 24 hpf.
Administering full-length FCHO1 mRNA together with the fcho1 MO increases morphologically normal 24 hpf embryos to 67% from 12% with the MO alone (Fig. 4u-y). There is >5 bp mismatch with the FCHO1 RNA (Fig. S4) excluding the coinjected transcript titrates out the fcho1 MO. Injection of the ΔμHD-encoding (1-610) transcript does not similarly correct development in fcho1 morphants arguing that the basis for the DV patterning defect is Fcho1 insufficiency and that the intact protein is necessary for proper function.
Because FCHO1 and FCHO2 are functionally redundant12 a translation-blocking fcho2 MO was also analyzed. The fcho2 morphants differ strikingly from fcho1 despite globally similar expression profiles during early embryogenesis (Fig. S5). The largely distinct developmental defects of the morphants indicate that Fcho1 and Fcho2 regulate separate events during zebrafish gastrulation and segmentation, but may also reflect temporal and regional differences in the expression of the two paralogues. There is evidence of weak overlapping function during embryogenesis: although fcho1+2 MOs combined do not provoke a gross phenotype distinct from fcho1 MO alone, the krox20 lateral expansion, elliptical morphology, and spectrum of dorsalized embryos is slightly more severe in _fcho1+2+p53_-injected embryos than in _fcho1+p53_- treated ones (Fig. S5l-r).
Fcho1 binds to Alk8
As the general fcho1 MO phenotype mirrors Bmp antagonism, we investigated interaction between Fcho1 and Alk8, an early DV patterning type I receptor for Bmp2b–Bmp714,40,41. A genetic link between fcho1 and alk8 is revealed by co-injection of MOs targeting the two transcripts at levels (0.5 ng) that, alone, do not produce many dorsalized embryos (Fig. 5a-e). When combined, mildly dorsalized (C2) embryos result with defective ventral tail fin development that resembles 5 ng alk8 morphants, suggesting that Alk8 and Fcho1 operate along the same pathway. This conclusion is directly substantiated biochemically. C-terminally myc-tagged Alk8 in detergent lysates from transfected HeLa cells is bound by GST-FCHO1 μHD dose-dependently, along with the other binding partners (Fig. 5f-h). Also, Alk8-myc interacts with the immobilized cognate GST-Fcho1 μHDs (~55% identical) while neither the Fcho2 (Fig. 5f), FCHO2 nor SGIP1 μHD (Fig. S6) bind similarly, despite equivalent eps15 interactions. The strong, differential interaction of the Fcho1/FCHO1 μHD with Alk8 is clearly consistent with the differing phenotypes in fcho1 or fcho2 MO embryos. There is no obvious preference of the Fcho1 μHD for binding to CA*Alk8 Q204D mutant41 over the wild-type receptor (Fig. 5g), whereas the related Alk1 interacts with the μHD comparatively weakly (Fig. S6). The μHD interaction with Alk8 is similar to Hrb binding and both are displaced by the eps15 (595-660) peptide at low concentrations (Fig. 5h); so Alk8 does not appear to use a tyrosine-based sorting signal to engage the Fcho1 μHD.
Figure 5.
The Fcho1–Alk8 association
(a-d). Representative images of live 24 hpf embryos after injection of 0.5 or 5 ng alk8 MO, 0.5 ng fcho1 MO or 0.5 ng each of alk8+fcho1 MOs. Defective ventral tail fin is bracketed (green). Scale bar: 250 μm.
(e) Quantitation of single and double morphant phenotypes at 24 hpf.
(f) Coomassie-stained gel and replicate blots from a pull-down assay using Alk8-myc overexpressing HeLa cell lysate and 150 μg immobilized GST or 50 or 150 μg human (Hs) GST-FCHO1 μHD or D. rerio (Dr) GST-Fcho1 or GST-Fcho2 μHDs. Alk8 (arrowheads in pellet (P) fractions) immunoblot with anti-myc. Note both bands of expressed Alk8-myc, probably differentially _N_-glycosylated forms, bind to the FCHO1/Fcho1 μHD. Non-specific bands (asterisks) are indicated.
(g) Pull-down assay using wild-type (WT) or CA*Alk8-myc overexpressing HeLa cell lysate and immobilized GST or GST-Dr Fcho1 μHD.
(h) Pull-down assay using Alk8-myc overexpressing HeLa cell lysate and immobilized GST or GST-Hs FCHO1 μHD. Addition of the eps15 (595-660) competitor peptide (5 or 25 μM) is indicated.
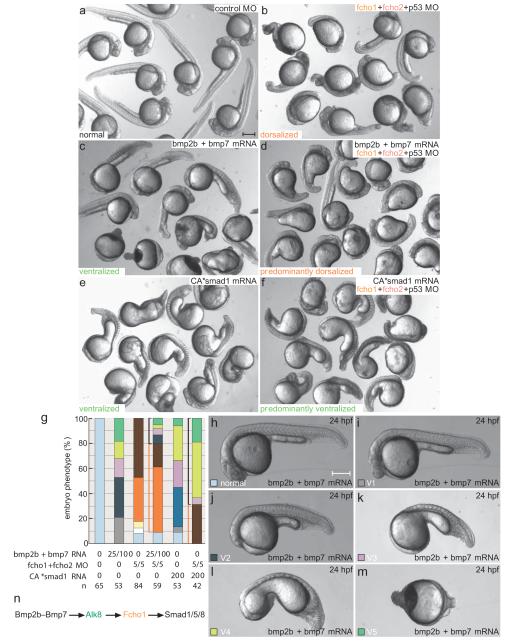
Injecting bmp2b+bmp7 mRNA elicits strong ventralization although coinjection with fcho1+2 MOs results in genetic suppression, with primarily dorsalized embryos resulting (Fig. 6). Ectopic mRNA encoding a phosphomimetic CA*Smad1 also strongly ventralizes, even in the presence of fcho1+2 MOs (Fig. 6e-g). These epistasis-type experiments place Fcho1 within a genetically specified sequence for Bmp signaling (Fig. 6n). Overall, our data imply that Fcho1 positively regulates ventral specification during embryogenesis by bridging Alk8 and the clathrin machinery, consistent with expanded pSmad1/5/8 staining in FCHO1 mRNA-injected embryos.
Figure 6.
Fcho1 operates in a genetic Bmp-to-Smad signaling pathway
(a-f) Group survey views of comparative morphology of injected control, 25 pg bmp2b with 100 pg bmp7 mRNA_,_ 5 ng each of fcho1+2+p53 MOs, bmp2b+bmp7 mRNA along with the fcho1+2+p53 MOs, 200 pg CA*smad1 mRNA, or the CA*smad1 mRNA along with the fcho1+2+p53 MOs-injected embryos at 24 hpf. Scale bar: 250 μm.
(g) Quantitation of mRNA injected gain-of function (ventralized; black bracketed) and morphant (dorsalized; orange bracketed) phenotypes. The relevant unit for injected mRNA is pg and for MO is ng.
(h-m) Representative images of the range and classification of ventralization phenotypes induced by injection of 25 pg bmp2b with 100 pg bmp7 mRNA at the 1-cell stage. Scale bar: 250 μm.
(n) Schematic of the linear Bmp-dependent signaling pathway including the relative positioning of Fcho1.
AP-2 loss-of-function
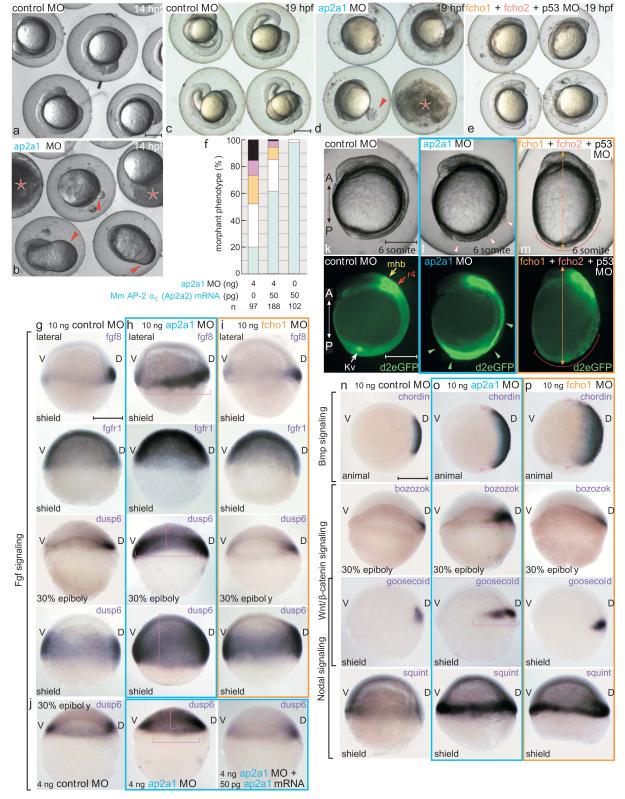
FCHO1/2 are argued to be master actuators of endocytosis, remodeling the initial membrane patch and recruiting eps15 and intersectin prior to AP-212. If FCHO1/2 are compulsory for clathrin-coated vesicle formation, one testable prediction is that the early dorsalizing effect of fcho1+2 MOs is wholly reflective of clathrin-mediated endocytosis dysfunction in developing embryos. Like Fcho1/2, in D. rerio the AP-2 α subunit is encoded by a single gene (ap2a1) and is maternally deposited. The transcript is broadly expressed in blastula-, gastrula- and segmentation-stage embryos (Fig. S7a-c) like fcho1/2.
Translation blocking ap2a1 MO (Fig. S4) produces pleomorphic early patterning and axis formation abnormalities and many embryos do not survive to 24 hpf (Fig. 7a-e, S5). Phenotypes of surviving morphants at 24 hpf are classified from mild to arrested (Fig. S7d-i). In the most severe embryos, the epiboly movement of blastomeres toward the vegetal pole slows and blastopore closure with incomplete epiboly results in dysmorphic bulging of the yolk (Fig. 7b, d). This defect persists beyond bud stage (10 hpf) and yolk rupture typically follows 14-19 hpf (Fig. 7d). Surviving arrested embryos are not developmentally delayed because there is no further morphological progression by 24 hpf (Fig. S6h). ap2a1+p53 MO-coinjected embryos still arrest, while simultaneous injection of the ap2a1 MO and mouse ap2a2 mRNA reverts the wild-type morphology (Fig. 7f). A second ap2a1 and an AP-2 μ2-subunit-targeting MO both cause an analogous phenotype. Epiboly arrest may be due, in part, to defective yolk cell endocytosis as the blastoderm margin moves toward the vegetal pole42. Later, in surviving embryos, ectoderm delaminates and gross morphology is indicative of severe patterning defects (Fig. 7l, S6f-h) consistent with the major effect of μ_2_ MO in early Xenopus embryos43.
Figure 7.
AP-2 morphants are unlike fcho1+2 double morphants
(a-e) Representative survey views of comparative morphology of 10 ng control, ap2a1 or fcho1+2+p53 morphants at 14 (a, b) and 19 (c-e) hpf. Extruded yolk (arrowheads) and exploded/disintegrating (asterisk) embryos shown. Scale bars: 250 μm.
(f) Quantitation of ap2a1 MO rescue with mouse (Mm) αC-subunit (Ap2a2) mRNA.
(g-i) mRNA expression patterns of Fgf signaling genes in control (g), ap2a1 (h) or fcho1 (i) shield- or 30% epiboly-stage morphants. The zones of expanded expression (brackets) are indicated; D, dorsal; V, ventral. Scale bar: 250 μm.
(j) Restoration of dusp6 expression in 30% epiboly (~5 hpf) ap2a1 MO embryos by coinjection of mouse Ap2a2 mRNA. Zone of expansion without rescue (brackets) indicated.
(k-m) Fgf-dependent GFP expression in live embryos injected with 10 ng control, ap2a1 or fcho1+fcho2+p53 MOs. Delaminating ectoderm, broadly expanded GFP expression (arrowheads) in ap2a1, and diminished ventral tissue (bracket) in fcho1+2 morphants shown. A, anterior; P, posterior; mhb, mid-hindbrain boundary; r4, rhombomere 4; Kv, Kupfer’s vesicle. Elliptical fcho1 morphant (orange arrow) shown in m. Scale bar: 250 μm.
(n-p) mRNA expression profiles of early patterning signaling components in control (n), ap2a1 (o) or fcho1 (p) shield- or 30% epiboly-stage morphants. Arrowheads and brackets show zones of expanded expression. Scale bar: 250 μm.
A survey of standard target genes for early development35 shows serious expression abnormalities. When gastrulation begins Fgf is normally produced from the dorsal organizer and weakly along the margin, but in ap2a1 (but not fcho1) MO embryos fgf8 transcripts are expanded toward both the ventral and animal poles (Fig. 7g-i). Improper fgf8 transcription is evident at 30% epiboly (~4.7 hpf). At this stage, there is also precocious hyperactiviation of the phosphatase Dusp6, an Fgf target gene44. Abnormal and mislocalized production of dusp6 in ap2a1 morphants persists to shield stage and, strikingly, expands far beyond the zone of fgf8 ligand expression. Coinjecting AP-2 α-subunit mRNA restores the normal dusp6 expression pattern (Fig. 7j). Expansion of Fgf signaling range in AP-2 compromised embryos is consistent with endocytosis shaping and maintaining this morphogen gradient in the zebrafish gastrula45,46. Clear Fgf signaling and phenotypic differences between ap2a1 and fcho1+2+p53 morphants are apparent in live transgenic Tg(Dusp6:d2EGFP)pt6 reporter embryos47 at the 6-somite stage (Fig. 7k-m). As the ap2a1 MO embryos are still round, suppressing AP-2 expression must cause additional cellular/regional defects because Fgf receptor hyperactivation typically induces dorsalization44,48. Importantly, the GFP reporter rules out that abnormal activation of Fgf signaling underlies the fcho1 MO dorsalization.
Other body plan patterning pathways are anomalous in _ap2a1_-silenced embryos when gastrulation starts: distribution of chordin, bmp4, bozozok, goosecoid, and squint transcripts is abnormal (Fig. 7n-p). Thus, AP-2 appears to function generally in multiple developmental pathways during early development as seriously defective body patterning information results without a functional adaptor. Because the effect on transducing Fgf and other inductive signals in ap2a1 morphants is distinct from how fcho1+2 MO alters the fate map, we conclude AP-2 function is not invariably dependent on upstream Fcho1/2 during embryogenesis.
DISCUSSION
Proper embryonic patterning depends on precise temporal integration of spatially complex signaling events. Endocytosis modulates signaling by constantly adjusting surface protein abundance49 and here we show that this process is vital for zebrafish development. Our results are consistent with Fcho1 operating with Alk8 and Bmp2b–Bmp7 heterodimers to signal ventral fates. Because FCHO1/2 are incontestably clathrin-coat constituents, the simplest model is that clathrin-mediated endocytosis promotes Bmp signaling. The precise basis for this remains to be elucidated; one possibility is that Fcho1/2 with Alk8 shapes the ventral Bmp gradient, as does type I BMP receptor Thick veins in Drosophila50. Thick veins, the Drosophila Decapentaplegic receptor, binds directly to another intersectin-binding EFC domain protein, Nervous wreck51. Yet Nervous wreck has a C-terminal SH3 domain substituted for the μHD, so the basis for the interaction cannot be structurally similar, and, critically, the consequence of the association is negative feedback on Decapentaplegic signaling. It thus appears remote that muniscins govern routing of Alk8 receptors to lysosomes because Fcho1 positively modulates Bmp signaling in zebrafish embryos.
Receptors can require prior internalization and recycling to gain signaling competence52. Another possibility then is that by promoting uptake of uncomplexed type I receptors (Alk8), the level of type I–II functional heterodimers is optimized. Alternatively, Fcho1 could be involved in forming Bmp gradients by decreasing available receptor/ligand in dorsally positioned cells, but that would give rise to ventralization, not dorsalization, upon MO injection. More likely, clathrin-mediated endocytosis provides access to an endosomal signaling station for cytosolic propagation and refinement of the Bmp signal. Spatial separation between TGF-β/BMP receptor activation at the plasma membrane and signal propagation from endosomes is apparent53,54. In cultured cells, the extent of activin-triggered Smad phosphorylation decreases when internalization is blocked55. Optimal phosphorylation of R-Smads by TGF-β receptors requires SARA, an endosome-associated PtdIns3P-binding protein56-59. The R-Smad binding FYVE domain protein Endofin plays an analogous role during BMP signaling60. Fcho1 could drive the delivery of Alk8 to endosomes for an encounter with Endofin then.
Zebrafish are characterized by very rapid developmental progression; tissue specific cell clones are evident during gastrulation (>6 hpf) and a recognizable body plan and differentiating organs apparent before 12 hpf61. Perhaps Fcho1/2 has been previously missed because the time constraints for signal propagation in other systems are dissimilar to D. rerio early development. An association between FCHO1 and ACVR1, the mammalian Alk8 ortholog, has been mapped by high-throughput affinity-capture proteomics62. Another hallmark of zebrafish embryogenesis is brisk and expansive cell movements. Maximal signal transmission from an endosomal extension of the cell surface may allow proper ‘memory’ signaling in cells that have moved away from the initial, fate-determining morphogen gradient63.
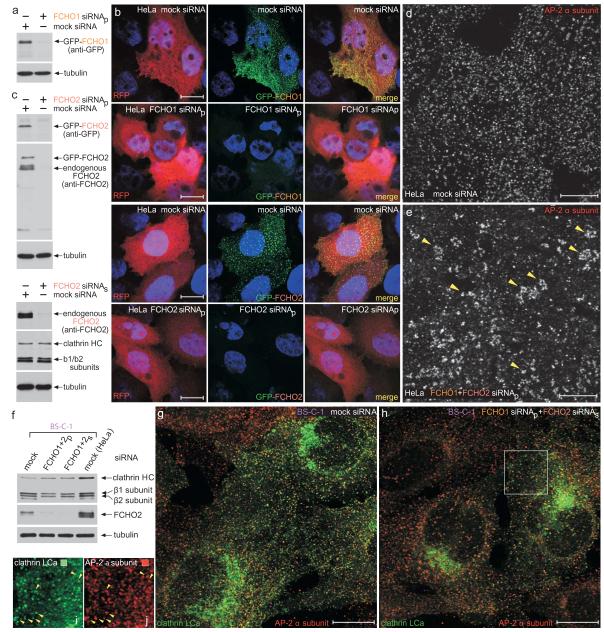
On the basis of MOs recapitulating maternal effect phenotypes33 it is clear the translational silencing approach ablates maternal gene expression. Why then are fcho1, fcho2 and ap2a1 morphants not more severely affected? Targeted gene disruption of the AP-2 μ2 subunit is lethal64 but ap2a1 MO in zebrafish allows visualization of early embryonic processes prior to dysmorphic arrest due to protein insufficiency. This is because MO-based silencing cannot obstruct maternally deposited protein. Substantial clathrin coat machinery must be provisioned in the oocyte as massive cortical granule exocytosis following fertilization is balanced by compensatory clathrin-dependent endocytosis65. Clathrin-coated buds are also seen at the cleavage furrow during the initial rounds of cell division following fertilization66. Still, an early and severe developmental defect on ap2a1 silencing is fully consistent with AP-2 μ2 subunit morphants in Xenopus43. Strikingly the phenotypic outcome of extinguishing AP-2 or Fcho1/2 transcripts is not identical. If Fcho1/2 are obligatory for the nucleation of endocytic clathrin coats, this is unexpected. One possibility is that the perdurance of the muniscins is greater than the AP-2 heterotetramer in early embryos. However, simultaneous RNAi silencing of both FCHO1 and FCHO2 in neither HeLa nor BS-C-1 cells causes loss of AP-2-positive clathrin-coated structures at the cell surface (Fig. 8). This is fully consistent with the very mild phenotype in syp1Δ yeast15,17,18.
Figure 8.
AP-2–clathrin coats persist in FCHO1 and FCHO2 siRNA-treated HeLa and BS-C-1 cells.
(a) Biochemical validation of FCHO1 transcript silencing in GFP-FCHO1-expressing HeLa cells by immunoblot analysis of lysates from mock or FCHO1 siRNA transfected cells. An ON-TARGET plus SMART pool (siRNAp; Dharmacon) was used. Replicate immunoblot with an anti-β tubulin mAb to verify equivalent loading of lysates.
(b) HeLa cells subject to mock, FCHO1 or FCHO2 siRNAp silencing and also ectopically expressing GFP-FCHO1 (along with RFP to mark co-transfected cells) were fixed and stained with Hoechst 33258. Representative, color-separated or merged, confocal optical sections are show. Note that both the FCHO1 and FCHO2 siRNAp sets efficiently quench the GFP-FCHO1 fluorescence. Scale bar: 10 μm.
(c) Biochemical verification of FCHO2 siRNAp- or siRNAs-mediated silencing in HeLa cells by immunoblotting. SDS-PAGE resolved lysates from cells subjected to the indicated treatments were immunoblotted with antibodies against either GFP or FCHO2 and β tubulin as a loading control. Notice that the FCHO2 siRNAp suppresses both the transfected GFP-FCHO2 as well as the endogenous protein in these cell populations highly efficiently.
(d-e) Representative single confocal optical sections of HeLa cells subjected to mock (d) or combined FCHO1+FCHO2 siRNAp (e) followed by fixation and immunodetection of the endogenous AP-2 α subunit using mAb AP.6. Note that although the roughly regular patterning and surface density of AP-2–clathrin structures is diminished upon knockdown of FCHO1/2, AP-2-positive puncta still persist and, generally appear to increase in size and are more irregularly deposited (arrowheads).
(f) Biochemical verification of FCHO1+FCHO2 siRNAp- or siRNAs-mediated silencing in BS-C-1 cells by immunoblotting. Notice, again, that the Stealth (Invitrogen) FCHO2 siRNAs very effectively extinguishes the endogenous FCHO2 in these populations of cells. Mock transfected HeLa cells included for comparison.
(g-j) Representative single confocal optical sections of clathrin LCa-GFP expressing BS-C-1 cells subjected to mock (g) or combined FCHO1 siRNAp+FCHO2 siRNAs (h) followed by fixation and immunodetection of the endogenous AP-2 α subunit using mAb AP.6. The insets (i, j) show a color-separated enlarged region corresponding to the boxed area in h. Surface AP-2-positive puncta clearly persist in these FCHO1+2-silenced BS-C-1 cells as well (arrowheads). Note that only a subset of clathrin-labeled structures is AP-2 positive in both the mock and FCHO1+2-silenced cells, as clathrin normally assembles on other intracellular structures as well. Scale bar: 10 μm.
The structure, dynamics7,12,67 and extensive interactions shared by FCHO1 and FCHO2 suggest muniscins advance clathrin endocytosis. This may explain the weak additive effect of fcho2 MO on the fcho1 morphant phenotype, reflecting a degree of functional overlap between the paralogues. If part of a pioneer module7,12, how do clathrin coats persist without these factors? A topological feature of the clathrin interactome is redundancy2,6. Surplus connections make the network tolerant to perturbations; AP-2 can be largely extinguished in cultured cells but rapid uptake of certain cargo continues27,28. As the μHD engages other pioneer scaffolds (Fig. 2h) collective establishment of this preponderance of links, even without Fcho1/2, permits positioning of AP-2 at the first encounter zone of a bud site. Cargo selective defects are instead manifest, highlighting that all known μHD-bearing proteins are involved in gathering cargo15.
METHODS
Molecular cloning and constructs
The human FCHO1, FCHO2, mouse Fcho2 and zebrafish Fcho2 cDNA clones were obtained from Open Biosystems. The sequences encoding full-length FCHO1, FCHO1 (1-609), FCHO1 (267-609), FCHO1 (442-609), FCHO1 μHD (609-889), full-length Fcho2 and Fcho2 μHD (527-809) were PCR amplified and cloned into pEGFP-C1 or pGEX-4T-1 for expression of either an N-terminal GFP or GST fusion protein. The full-length human FCHO1, FCHO1 (1-609) and zebrafish fcho2 were similarly cloned into pCS2+ for mRNA synthesis or transient transfections. Stop codons were introduced at appropriate sites using QuikChange (Stratagene) site-directed mutagenesis to construct FCHO1 (1-275), FCHO1 (1-609), Fcho2 (1-302) and the various other truncation mutants described in the text. The GST-β2 appendage (rat residues 701-937), GST-αC appendage (mouse residues 701-938), GST-eps15 (595-896) and GST-ARH constructs have been described previously15,25,68. The 25-bp regions at the 5′ end of zebrafish fcho1, fcho2 and ap2a1 used to design antisense morpholinos were cloned in-frame into pCS2-GFP-N1 to generate respective 5′MO-GFP constructs that were subsequently used to test the effectiveness of translational silencing by fcho1, fcho2 and ap2a1 MOs in vivo. The coding sequence of the zebrafish fcho1 μHD with flanking 5′ and 3′ regions was first amplified by RT-PCR from 1,000-cell stage zebrafish embryonic cDNA using a set of PCR primers designed from EST clones encoding the 5′ (GenBank accession AL920021) or the 3′ (GenBank AL921007) ends of the μHD. Then, the complete coding sequence of the μHD was PCR amplified from the RT-PCR product and cloned into pGEX-4T-1. The pCS2-alk8, pCS2-bmp2b and pCS2-bmp7 plasmids13 were kindly provided by Dr. Mary Mullins while the pCS2-CA*smad1 construct (470SVS of zebrafish Smad1 mutated to 470DVD ) was a gift from Dr. Beth Roman. The alk8 coding sequence was PCR amplified from pCS2-alk8 and subcloned into the pCS2-myc6 vector to obtain pCS2-_alk8_-myc6. A Q204D mutation yielded the CA*Alk8. All constructs were sequence-verified and full details of plasmids and primers (Integrated DNA Technologies) are available upon request.
RT-PCR and 5′ RACE
Total RNA (5 μg) extracted from embryos at different developmental stages using TRIZOL method (Invitrogen) was used as template for cDNA preparation. cDNA was generated using an oligo-(dT) primer and Thermoscript reverse transcriptase (Invitrogen) according to the manufacturer’s directions. Subsequently, the cDNA was amplified with gene specific primers for fcho1, fcho2, and ap2a1 (as listed in the Supplementary Table). β actin primers were used as an internal control for the quality of cDNA synthesis. The amplified DNA was subjected to agarose gel electrophoresis and exon-specific bands identified both by size and by sequencing. 5′ RACE was performed by standard procedures (Invitrogen). Total RNA from 1-cell and 1,000-cell stage embryos was reverse transcribed to cDNA using an antisense gene specific primer (GSP1) near the 5′ end. The template cDNA was tailed at 3′ end using dCTP and terminal deoxynucleotidyl transferase (New England Biolabs). The tailed cDNA was PCR amplified with a set composed of a 5′ RACE anchor adaptor primer and a second antisense gene specific primer (GSP2). The primer sequences used for 5′ RACE are provided in the Supplementary Table. The amplicon was purified, confirmed by sequencing and the sequence deposited in GenBank (Accession number JN412732) at the NCBI.
Antibodies
The affinity-purified rabbit anti-FCHO1 antibody 1 (1:2,500), anti-FCHO2 (1:2,500), anti-Dab2 (1:1,000), anti-epsin 1 (1:10,000) and anti-AP-1/2 β1/β2-subunit GD/1 (1:2,500) antibodies were produced commercially for our laboratory. A second affinity-purified anti-FCHO2 (1:2500) antibody was kindly provided by Dr. Harvey McMahon. Affinity-purified rabbit anti-eps15 polyclonal antibody was a gift from Dr. Ernst Ungewickell, the anti-clathrin HC mAbs TD.1 (1:5,000) and X22 and the anti-AP-2 α-subunit mAb AP.6 were generously provided by Dr. Frances Brodsky, the anti-CALM mAb (1:1,000) kindly provided by Dr. Jeong-Ah Kim, rabbit R11-29 anti-AP-2 μ2-subunit (1:3,000) antiserum kindly provided by Dr. Juan Bonifacino and the rabbit anti-intersectin 1 (1:2,000) antibody a gift from Dr. Peter McPherson. The affinity-purified rabbit anti-APPL1 (1:50) was kindly provided by Dr. David Kaplan. The goat anti-Hrb C-19 (1:1000; sc-1424), rabbit anti-eps15 C-20 (1:500; sc-534) polyclonal antibodies and rabbit anti-SHIP2 mAb E-2 (1:500; sc-166641) from Santa Cruz Biotechnology, rabbit anti-eps15R (1:5,000; EP-1145Y) antibody and rabbit anti-FCHO1 antibody 2 (1:1,000; ab84740) from Abcam, and the mouse anti-myc mAb 9E10 (1: 1,000; MMS-150P) from Covance were used. The anti-β-tubulin mAb E7 (1:2,500) was purchased from the DSHB, mAbs directed against the AP-2 α subunit clone 8/Adaptin α (1:1,000; 610502), AP180 clone 34 (1:250; A41820), amphiphysin I clone 15 (1:5,000; A59420), EEA1 clone C14/EEA1 (1:250; 610457) and intersectin clone 29 (1:250; 611574) were from BD Transduction Laboratories. The secondary antibodies used were donkey anti-rabbit (1:5,000; NA934V) or anti-mouse (1:5,000; NA931V) horseradish peroxidase conjugates from GE Healthcare or rabbit anti-goat (1:5,000; A4174) peroxidase conjugate from Sigma.
Cell culture and transfections
HeLa SS6 cells were cultured in DMEM supplemented with 10% fetal calf serum and 2 mM L-glutamine at 37°C in an atmosphere of 5% CO2. BS-C-1 cells stably expressing GFP-tagged clathrin light chain a were generously provided by Tomas Kirchhausen. The BS-C-1 cells were similarly grown in DMEM, 10% fetal calf serum, 2 mM L-glutamine supplemented with 0.4 mg/ml G418. Cells were routinely transfected with plasmids using Lipofectamine 2000 (Invitrogen) or siRNA oligonucleotides (listed in Supplementary Table) using Oligofectamine (Invitrogen) according to the manufacturer’s instructions. For all RNAi treatments, cells were transfected twice, normally 48 h apart and with reseeding the cells before the second siRNA application, at which time the relevant transiently transfected plasmid was also included. Typically, 18-24 h after the second transfection, cells were fixed with 4% paraformaldehyde, blocked and permeabilized in 10% normal goat serum, 0.2% saponin in PBS. For transferrin binding and uptake assays, cells were incubated in DMEM, 25 mM Hepes, 0.5% BSA for 1 h at 37°C to remove bound transferrin. Alternatively, lysates from parental HeLa cells or HeLa cells expressing (from pCS2+) full-length (1-889) or truncated (1-609) H. sapiens FCHO1, or myc-tagged WT Alk8 or CA*Alk8 were prepared from transiently-transfected (24-48 h) cells collected by trypsinization. After washing, cell pellets were solubilized on ice for 30 min in 25 mM Hepes-KOH (pH 7.2), 125 mM potassium acetate, 5 mM magnesium acetate, 2 mM EDTA, 2mM EGTA, and 2 mM dithiothreitol (assay buffer) supplemented with 1% Triton X-100, 1 mM PMSF and Complete protease inhibitor cocktail (Roche). Lysates were centrifuged at 20,000 X _g_max before use in binding assays.
Zebrafish maintenance, morpholinos and mRNA injections
The Oregon AB* and Tg(Dusp6:d2EGFP)pt6 strains were maintained under standard conditions at the University of Pittsburgh School of Medicine in accordance with Institutional and Federal guidelines for use, care and maintenance of experimental animal models and with University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) approval. Embryos from natural matings were obtained and developmentally staged61. The sequence of the various morpholinos used in this study, custom synthesized by Gene Tools, are listed in the Supplementary Table. The translation-blocking ATG specific fcho1 morpholino was designed on the basis of the authentic 5′ sequence of zebrafish embryonic fcho1 transcript mapped by 5′ RACE. A standard control MO and p53 MO was obtained from Gene Tools as detailed elsewhere69. The desired concentrations of the appropriate morpholinos were microinjected at 5 nl/embryo into the yolk at 1- to 2-cell stage. Capped mRNAs for misexpression and rescue experiments were transcribed in vitro from linearized pCS2+ constructs using SP6 mMessage mMachine kit (Ambion) and microinjected at 1 nl into the blastomere at 1-cell stage. After injections, the embryos were incubated in E3 medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM MgSO4, 0.01% methylene blue) at 28°C until the desired stage.
Whole-mount in situ hybridization and immunofluorescence
The EST clones for fcho1 (GenBank AL920021) and ap2a1 (GenBank AL920300) in pBluescript SK+ were obtained from Open Biosystems, linearized and directly used for riboprobe synthesis. For fcho2, an 880-bp fragment encoding the C-terminal end was PCR amplified (using fcho2 T7 sense and antisense primers) from the zebrafish fcho2 EST clone (GenBank BC095680). All other probes used in this study (no tail, sonic hedgehog, bmp4, chordin, krox20, gata1, goosecoid, bozozok, squint, fgf8, fgfR1 and dusp6) were made similarly by linearizing corresponding vectors containing a T7 RNA polymerase promoter, using appropriate restriction enzymes as described elsewhere47,69. Riboprobe synthesis for in situ hybridization was performed using DIG RNA labeling mix and T7 RNA polymerase (Roche) according to the manufacturer’s recommendations. Zebrafish embryos at appropriate developmental stages were fixed in 4% paraformaldehyde at 4°C overnight, washed once in PBS and stored in methanol at -20°C. Whole-mount in situ hybridizations were performed by standard procedures69. Embryos were washed several times in PBS and mounted in glycerol. Alternatively, live embryos, either within or removed from the chorion, were mounted in 3% methylcellulose and overlaid with E3 containing 0.016% tricaine (pH 7.0). Oriented embryos were viewed using a Leica MZ16FA stereo fluorescence microscope with a 1x (NA 0.14) objective and bright field or fluorescence images collected with a QImaging Retiga-EXi Fast 1394 digital camera.
For immunofluorescence, embryos fixed overnight in 4% paraformaldehyde at 4°C, were permeabilized in 1% Triton-X-100, blocked in 10% sheep serum, 1% DMSO, 0.1% Triton X-100 in PBS and probed overnight with rabbit anti-phosphoSmad (pSmad) 1/5/8 (1:100; 9511) from Cell Signaling Technology or GD/1 (1:100) antibody. The immunizing peptide for the GD/1 antibody (GDLLNLLGPPV) is 100% conserved in the D. rerio AP-2 β2 subunit (ap2b1). Primary antibodies were followed with goat anti-rabbit Alexa488-or Cy5-conjugated antibody (1:500; Molecular Probes) and Hoechst 33258 nuclear stain before mounting for confocal microscopy. For GFP-FCHO1/AP-2 immunofluorescence, embryos at 5 hpf were embedded in 1% low melting point agarose in E3 medium on poly-D-lysine coated dishes (MatTek) with the animal pole oriented toward the glass bottom. For pSmad 1/5/8 immunofluorescence, embryos at 7 hpf were embedded with dorsal shield side toward the right. Images were acquired on an Olympus Fluoview1000 confocal microscope using an UplanSapo 20X (NA 0.75) or an UPlanFLN 40X (NA 1.3) oil objective. Data was acquired using the FV10-ASW software. Similar procedures were used for immunofluorescence analysis of cultured mammalian cells.
Supplementary Material
1
5
6
7
8
9
10
11
12
13
14
2
3
4
Acknowledgements
We are indebted to our many gracious colleagues for generously providing important reagents that were essential for this study. Supported by NIH grants R01 HL088016 (MT) and R01 GM60979 (BW) and R01 DK53249 (LMT).
Footnotes
Contributions P.K.U., S.S., J.R.T., S.C., L.M.T. designed, performed and interpreted various experiments. B.W. and M.T. provided intellectual input, contributed to experimental design and advised on data interpretation. L.M.T. conceived and directed the overall project and wrote the manuscript with comments from all the authors.
Competing financial interests The authors declare no competing financial interests.
REFERENCES
- 1.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 2.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. doi:nrm3151 [pii] 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 3.Kirchhausen T. Imaging endocytic clathrin structures in living cells. Trends Cell Biol. 2009;19:596–605. doi: 10.1016/j.tcb.2009.09.002. doi:S0962-8924(09)00192-5 [pii] 10.1016/j.tcb.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly BT, Owen DJ. Endocytic sorting of transmembrane protein cargo. Curr Opin Cell Biol. 2011 doi: 10.1016/j.ceb.2011.03.004. doi:S0955-0674(11)00021-4 [pii] 10.1016/j.ceb.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Reider A, Wendland B. Endocytic adaptors - social networking at the plasma membrane. J Cell Sci. 2011;124:1613–1622. doi: 10.1242/jcs.073395. doi:124/10/1613 [pii] 10.1242/jcs.073395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat. Rev. Mol. Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 7.Taylor MJ, Perrais D, Merrifield CJ. A high precision survery of the molecular dynamics of mammalian clathrin mediated endocytosis. PLoS Biol. 2011;9:e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- 9.Boucrot E, Saffarian S, Massol R, Kirchhausen T, Ehrlich M. Role of lipids and actin in the formation of clathrin-coated pits. Exp. Cell Res. 2006;312:4036–4048. doi: 10.1016/j.yexcr.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zoncu R, et al. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc. Natl. Acad. Sci. U S A. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson LP, et al. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell. 2010;141:1220–1229. doi: 10.1016/j.cell.2010.05.006. doi:S0092-8674(10)00542-8 [pii] 10.1016/j.cell.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henne WM, et al. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328 doi: 10.1126/science.1188462. doi:science.1188462 [pii] 10.1126/science.1188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Little SC, Mullins MC. Bone morphogenetic protein heterodimers assemble heteromeric type I receptor complexes to pattern the dorsoventral axis. Nat. Cell Biol. 2009;11:637–643. doi: 10.1038/ncb1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mintzer KA, et al. Lost-a-fin encodes a type I BMP receptor, Alk8, acting maternally and zygotically in dorsoventral pattern formation. Development. 2001;128:859–869. doi: 10.1242/dev.128.6.859. [DOI] [PubMed] [Google Scholar]
- 15.Reider A, et al. Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J. 2009;28:3103–3016. doi: 10.1038/emboj.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katoh M. Identification and characterization of human FCHO2 and mouse Fcho2 genes in silico. Int. J. Mo.l Med. 2004;14:327–331. [PubMed] [Google Scholar]
- 17.Stimpson HE, Toret CP, Cheng AT, Pauly BS, Drubin DG. Early-arriving Syp1p and Ede1p function in endocytic site placement and formation in budding yeast. Mol. Biol. Cell. 2009 doi: 10.1091/mbc.E09-05-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boettner DR, et al. The F-BAR protein Syp1 negatively regulates WASp-Arp2/3 complex activity during endocytic patch formation. Curr. Biol. 2009;19:1979–1987. doi: 10.1016/j.cub.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uezu A, et al. Characterization of the EFC/F-BAR domain protein, FCHO2. Genes Cells. 2011 doi: 10.1111/j.1365-2443.2011.01536.x. doi:10.1111/j.1365-2443.2011.01536.x. [DOI] [PubMed] [Google Scholar]
- 20.Zoncu R, et al. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell. 2009;136:1110–1121. doi: 10.1016/j.cell.2009.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henne WM, et al. Structure and analysis of FCHo2 F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure. 2007;15:839–852. doi: 10.1016/j.str.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Edeling MA, Smith C, Owen D. Life of a clathrin coat: insights from clathrin and AP structures. Nat. Rev. Mol. Cell Biol. 2006;7:32–44. doi: 10.1038/nrm1786. [DOI] [PubMed] [Google Scholar]
- 23.Edeling MA, et al. Molecular switches involving the AP-2 β2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev. Cell. 2006;10:329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Schmid EM, et al. Role of the AP2 β-appendage hub in recruiting partners for clathrin coated vesicle assembly. PLoS Biol. 2006;4:e262. doi: 10.1371/journal.pbio.0040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra SK, et al. Dual-engagement regulation of protein interactions with the AP-2 adaptor α appendage. J. Biol. Chem. 2004;279:46191–46203. doi: 10.1074/jbc.M408095200. [DOI] [PubMed] [Google Scholar]
- 26.Praefcke GJ, et al. Evolving nature of the AP2 α-appendage hub during clathrin-coated vesicle endocytosis. EMBO J. 2004;23:4371–4383. doi: 10.1038/sj.emboj.7600445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinrichsen L, Harborth J, Andrees L, Weber K, Ungewickell EJ. Effect of clathrin heavy chain- and α-adaptin specific small interfering RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 2003;278:45160–45170. doi: 10.1074/jbc.M307290200. [DOI] [PubMed] [Google Scholar]
- 28.Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 2003;162:909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uezu A, et al. SGIP1α is an endocytic protein that directly interacts with phospholipids and Eps15. J. Biol. Chem. 2007;282:26481–26489. doi: 10.1074/jbc.M703815200. [DOI] [PubMed] [Google Scholar]
- 30.Yamabhai M, et al. Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J. Biol. Chem. 1998;273:31401–31407. doi: 10.1074/jbc.273.47.31401. [DOI] [PubMed] [Google Scholar]
- 31.Koh TW, et al. Eps15 and Dap160 control synaptic vesicle membrane retrieval and synapse development. J. Cell Biol. 2007;178:309–322. doi: 10.1083/jcb.200701030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sengar AS, Wang W, Bishay J, Cohen S, Egan SE. The EH and SH3 domain Ese proteins regulate endocytosis by linking to dynamin and Eps15. EMBO J. 1999;18:1159–1171. doi: 10.1093/emboj/18.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imai Y, Talbot WS. Morpholino phenocopies of the bmp2b/swirl and bmp7/snailhouse mutations. Genesis. 2001;30:160–163. doi: 10.1002/gene.1055. [DOI] [PubMed] [Google Scholar]
- 34.Mullins MC, et al. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- 35.Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Annu. Rev. Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- 36.Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. doi:S1534-5807(07)00424-8 [pii] 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robu ME, et al. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. doi:10.1038/43092. [DOI] [PubMed] [Google Scholar]
- 39.von der Hardt S, et al. The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr. Biol. 2007;17:475–487. doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Bauer H, Lele Z, Rauch GJ, Geisler R, Hammerschmidt M. The type I serine/threonine kinase receptor Alk8/Lost-a-fin is required for Bmp2b/7 signal transduction during dorsoventral patterning of the zebrafish embryo. Development. 2001;128:849–858. doi: 10.1242/dev.128.6.849. [DOI] [PubMed] [Google Scholar]
- 41.Payne TL, Postlethwait JH, Yelick PC. Functional characterization and genetic mapping of alk8. Mech. Dev. 2001;100:275–289. doi: 10.1016/s0925-4773(00)00541-4. [DOI] [PubMed] [Google Scholar]
- 42.Solnica-Krezel L. Gastrulation in zebrafish — all just about adhesion? Curr. Opin. Genet. Dev. 2006;16:433–441. doi: 10.1016/j.gde.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Borner GH, et al. CVAK104 is a novel regulator of clathrin-mediated SNARE sorting. Traffic. 2007;8:893–903. doi: 10.1111/j.1600-0854.2007.00576.x. doi:TRA576 [pii] 10.1111/j.1600-0854.2007.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsang M, et al. A role for MKP3 in axial patterning of the zebrafish embryo. Development. 2004;131:2769–2779. doi: 10.1242/dev.01157. [DOI] [PubMed] [Google Scholar]
- 45.Yu SR, et al. Fgf8 morphogen gradient forms by a source-sink mechanism with freely diffusing molecules. Nature. 2009;461:533–536. doi: 10.1038/nature08391. [DOI] [PubMed] [Google Scholar]
- 46.Scholpp S, Brand M. Endocytosis controls spreading and effective signaling range of Fgf8 protein. Curr. Bio.l. 2004;14:1834–1841. doi: 10.1016/j.cub.2004.09.084. doi:S0960982204007481 [pii] 10.1016/j.cub.2004.09.084. [DOI] [PubMed] [Google Scholar]
- 47.Molina GA, Watkins SC, Tsang M. Generation of FGF reporter transgenic zebrafish and their utility in chemical screens. BMC Dev. Biol. 2007;7:62. doi: 10.1186/1471-213X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furthauer M, Van Celst J, Thisse C, Thisse B. Fgf signalling controls the dorsoventral patterning of the zebrafish embryo. Development. 2004;131:2853–2864. doi: 10.1242/dev.01156. doi:10.1242/dev.01156 dev.01156 [pii] [DOI] [PubMed] [Google Scholar]
- 49.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 2009;10:609–922. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Belenkaya TY, et al. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 2004;119:231–244. doi: 10.1016/j.cell.2004.09.031. doi:S0092867404009390 [pii] 10.1016/j.cell.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 51.O’Connor-Giles KM, Ho LL, Ganetzky B. Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth. Neuron. 2008;58:507–518. doi: 10.1016/j.neuron.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W, Struhl G. Distinct roles for Mind bomb, Neuralized and Epsin in mediating DSL endocytosis and signaling in Drosophila. Development. 2005;132:2883–2894. doi: 10.1242/dev.01860. [DOI] [PubMed] [Google Scholar]
- 53.Hartung A, et al. Different routes of bone morphogenic protein (BMP) receptor endocytosis influence BMP signaling. Mol Cell Biol. 2006;26:7791–7805. doi: 10.1128/MCB.00022-06. doi:MCB.00022-06 [pii] 10.1128/MCB.00022-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heining E, Bhushan R, Paarmann P, Henis YI, Knaus P. Spatial segregation of BMP/Smad signaling affects osteoblast differentiation in C2C12 cells. PLoS One. 2011;6:e25163. doi: 10.1371/journal.pone.0025163. doi:10.1371/journal.pone.0025163 PONE-D-11-09166 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Y, et al. Receptor internalization-independent activation of Smad2 in activin signaling. Mol. Endocrinol. 2004;18:1818–1826. doi: 10.1210/me.2004-0079. [DOI] [PubMed] [Google Scholar]
- 56.Tsukazaki T, Chiang TA, Davison AF, Attisano L, Wrana JL. SARA, a FYVE domain protein that recruits Smad2 to the TGFb receptor. Cell. 1998;95:779–791. doi: 10.1016/s0092-8674(00)81701-8. doi:S0092-8674(00)81701-8 [pii] [DOI] [PubMed] [Google Scholar]
- 57.Lin HK, Bergmann S, Pandolfi PP. Cytoplasmic PML function in TGF-beta signalling. Nature. 2004;431:205–211. doi: 10.1038/nature02783. doi:10.1038/nature02783 nature02783 [pii] [DOI] [PubMed] [Google Scholar]
- 58.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat. Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 59.Hayes S, Chawla A, Corvera S. TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J. Cell Biol. 2002;158:1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi W, et al. Endofin acts as a Smad anchor for receptor activation in BMP signaling. J Cell Sci. 2007;120:1216–1224. doi: 10.1242/jcs.03400. doi:jcs.03400 [pii] 10.1242/jcs.03400. [DOI] [PubMed] [Google Scholar]
- 61.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 62.Barrios-Rodiles M, et al. High-throughput mapping of a dynamic signaling network in mammalian cells. Science. 2005;307:1621–1625. doi: 10.1126/science.1105776. [DOI] [PubMed] [Google Scholar]
- 63.Jullien J, Gurdon J. Morphogen gradient interpretation by a regulated trafficking step during ligand-receptor transduction. Genes Dev. 2005;19:2682–2694. doi: 10.1101/gad.341605. doi:gad.341605 [pii] 10.1101/gad.341605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitsunari T, et al. Clathrin adaptor AP-2 is essential for early embryonal development. Mol. Cell. Biol. 2005;25:9318–9323. doi: 10.1128/MCB.25.21.9318-9323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hart NH, Collins GC. An electron-microscope and freeze-fracture study of the egg cortex of Brachydanio rerio. Cell Tissue Res. 1991;265:317–328. doi: 10.1007/BF00398079. [DOI] [PubMed] [Google Scholar]
- 66.Feng B, Schwarz H, Jesuthasan S. Furrow-specific endocytosis during cytokinesis of zebrafish blastomeres. Exp. Cell Res. 2002;279:14–20. doi: 10.1006/excr.2002.5579. doi:S0014482702955795 [pii] [DOI] [PubMed] [Google Scholar]
- 67.von Kleist L, et al. Role of the clathrin terminal domain in regulating coated pit dynamics revelaed by small molecule inhibition. Cell. 2011;146:471–484. doi: 10.1016/j.cell.2011.06.025. doi:S0092-8674(11)00667-2 [pii] 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 68.Thieman JR, et al. Clathrin regulates the association of PIPKIγ661 with the AP-2 adaptor β2 appendage. J. Biol. Chem. 2009;284:13924–13939. doi: 10.1074/jbc.M901017200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edeling MA, et al. Structural requirements for PACSIN/Syndapin operation during zebrafish embryonic notochord development. PLoS One. 2009;4:e8150. doi: 10.1371/journal.pone.0008150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1
5
6
7
8
9
10
11
12
13
14
2
3
4