Indirubin derivatives induce apoptosis of chronic myelogenous leukemia cells involving inhibition of Stat5 signaling (original) (raw)
Abstract
Indirubin is the major active anti‐tumor component of a traditional Chinese herbal medicine used for treatment of chronic myelogenous leukemia (CML). While previous studies indicate that indirubin is a promising therapeutic agent for CML, the molecular mechanism of action of indirubin is not fully understood. We report here that indirubin derivatives (IRDs) potently inhibit Signal Transducer and Activator of Transcription 5 (Stat5) protein in CML cells. Compound E804, which is the most potent in this series of IRDs, blocked Stat5 signaling in human K562 CML cells, imatinib‐resistant human KCL‐22 CML cells expressing the T315I mutant Bcr‐Abl (KCL‐22M), and CD34‐positive primary CML cells from patients. Autophosphorylation of Src family kinases (SFKs) was strongly inhibited in K562 and KCL‐22M cells at 5 μM E804, and in primary CML cells at 10 μM E804, although higher concentrations partially inhibited autophosphorylation of Bcr‐Abl. Previous studies indicate that SFKs cooperate with Bcr‐Abl to activate downstream Stat5 signaling. Activation of Stat5 was strongly blocked by E804 in CML cells. E804 down‐regulated expression of Stat5 target proteins Bcl‐xL and Mcl‐1, associated with induction of apoptosis. In sum, our findings identify IRDs as potent inhibitors of the SFK/Stat5 signaling pathway downstream of Bcr‐Abl, leading to apoptosis of K562, KCL‐22M and primary CML cells. IRDs represent a promising structural class for development of new therapeutics for wild type or T315I mutant Bcr‐Abl‐positive CML patients.
Keywords: Indirubin, SFKs, Stat5, Apoptosis, CML
Highlights
- We demonstrate that indirubin derivatives (IRDs) block Stat5 signaling in CML cells.
- Inhibition of activated Stat5 signaling is associated with induction of apoptosis.
- These findings suggest important pharmacological mechanism of action of indirubin.
- IRDs have potential as novel chemotherapeutic agents for treatment of CML patients.
Abbreviations
CML
chronic myelogenous leukemia
IRDs
indirubin derivatives
Stat5
Signal Transducer and Activator of Transcription 5
SFKs
Src family kinases
1. Introduction
Signal Transducer and Activator of Transcription (STAT) proteins have essential functions in normal cytokine signaling and are frequently constitutively activated in human tumor cells (Yu and Jove, 2004). STATs have key roles in regulating cell proliferation, survival, angiogenesis and immune function (Parsons and Parsons, 2004; Yu et al., 2009). One of seven different STAT family members, Stat5, is constitutively activated by non‐receptor tyrosine kinases (Herrington et al., 2000; Huang et al., 2002; Klejman et al., 2002; Nieborowska‐Skorska et al., 1999; Yu and Jove, 2004). Bcr‐Abl, an oncogenic non‐receptor tyrosine kinase activated in CML, induces persistent tyrosyl phosphorylation of Stat5 (Bromberg et al., 1999; Nelson et al., 2006; Quintas‐Cardama et al., 2007; Shah et al., 2004; Yu and Jove, 2004). Bcr‐Abl kinase cooperates with Src family kinases (SFKs) to activate Stat5 in CML cell transformation (Klejman et al., 2002; Wilson et al., 2002). SFKs, also non‐receptor tyrosine kinases, phosphorylate critical cellular substrates such STAT family members, including Stat5, thereby regulating oncogenic signaling pathways (Bromann et al., 2004; Parsons and Parsons, 2004; Silva, 2004; Yu and Jove, 2004). In particular, the SFKs, Lyn and Hck, have been shown to cooperate with Bcr‐Abl to activate Stat5 signaling in CML cells (Klejman et al., 2002; Lionberger et al., 2000; Wilson et al., 2002).
STAT signaling is currently being investigated as a new molecular target pathway for human cancer treatment (Yu and Jove, 2004; Yu et al., 2009). In Stat5 signaling, two phosphorylated Stat monomers dimerize through reciprocal phosphotyrosyl‐SH2 domain interactions (Bromberg et al., 1999; Yu and Jove, 2004). The phosphorylated Stat5 dimers then translocate to the nucleus and bind to the promoters of specific Stat5 responsive genes (Bromberg et al., 1999; Nelson et al., 2006; Yu and Jove, 2004). Persistent activation of Stat5 has a critical role in cell growth and survival in human hematopoietic malignancies (Carlesso et al., 1996; Yu and Jove, 2004). Constitutively‐activated Stat5 up‐regulates the expression of anti‐apoptotic genes encoding Mcl‐1 and Bcl‐xL proteins in human CML cells (Gesbert and Griffin, 2000; Horita et al., 2000; Nelson et al., 2006; Yu and Jove, 2004). In contrast, blockade of Stat5 signaling down‐regulates these downstream target genes of Stat5, associated with induction of apoptosis in CML cells (Horita et al., 2000; Shah et al., 2004; Yu and Jove, 2004).
Indirubin is the major active anti‐tumor ingredient of a traditional Chinese herbal medicine, Danggui Longhui Wan, which is a mixture of 11 herbal ingredients and used for CML treatment (Xiao et al., 2002). IRDs were shown to inhibit CDK1/cyclin B, CDK2/cyclinA, CDK2/cycling E, GSK 3β and CDK5/p25, leading to cell growth inhibition in human cancer cells (Hoessel et al., 1999; Marko et al., 2001; Vougogiannopoulou et al., 2008). IRDs also inhibit phosphorylation of Stat5 in acute myeloid leukemia cells (Zhou et al., 2009). Recently, we demonstrated that IRDs blocked constitutive Stat3 signaling in epithelial tumor cells such as breast and prostate cancer (Nam et al., 2005a). Previously, clinical studies indicated that indirubin is a promising anticancer therapeutic agent for CML treatment, showing low toxicity (Eisenbrand et al., 2004). However, the mechanism of action of IRDs in CML remains largely unknown. In this study, we report that IRDs inhibit SFK/Stat5 signaling, accompanied by induction of apoptosis in K562, imatinib‐resistant KCL‐22M and CD34‐positive primary CML cells. These findings indicate that IRDs induce apoptosis, involving inhibition of SFK/Stat5 signaling downstream of Bcr‐Abl, and are potential anticancer therapeutic agents for wild type or T315I mutant Bcr‐Abl‐positive CML patients.
2. Materials and methods
2.1. Cell lines and reagents
Human K562 CML cells were obtained from the American Type Culture Collection (ATCC). Cells were cultured in RPMI‐1640 media containing 10% fetal bovine serum (FBS). Imatinib‐resistant human KCL‐22 CML cells expressing the T315I mutant Bcr‐Abl (KCL‐22M) were derived from human KCL‐22 CML cells (Yuan et al., 2010). Cells were grown in RPMI 1640 media supplemented with 10% FBS. For primary CML cells, peripheral blood samples were obtained from newly diagnosed CML patients in chronic phase of the disease. Patient specimens were obtained with patient informed consent following an IRD approved protocol. CD34‐positive progenitors were isolated from patient specimens. Briefly, Mononuclear cells (MNCs) were isolated by Ficoll‐Hypaque (Sigma Diagnostics, St. Louis, MO) density gradient centrifugation (specific gravity, 1.077) for 30 min at 400 × g. CD34‐positive cells were selected by means of immunomagnetic column separation (Miltenyi Biotech, Auburn, CA) following the supplier's instructions. Monoclonal antibodies to Abl protein and phospho‐tyrosine (p‐Y) were obtained from BD Biosciences (San Diego, CA). Polyclonal antibodies to p‐Stat5 (Y694) and p‐Src family (Y419) were obtained from Cell Signaling Technologies (Cambridge, MA). Polyclonal antibodies to Stat5, Bcl‐xL, Mcl‐1, p‐Hck, Hck, Lyn and β‐actin were from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibody to Src was obtained from Millipore (Billerica, MA).
2.2. Immunoprecipitation and Western blot analyses
Immunoprecipitations and Western analyses were performed as described previously with minor modification (Nam et al., 2005b). Briefly, K562, KCL‐22M and CD34‐positive primary CML cells were treated with IRDs. Cell lysates (500 μg) were incubated with Abl and Lyn antibodies for 1 h at 4 °C followed by protein A/G‐agarose beads (Pierce, Rockford, IL). Immunoprecipitates or whole‐cell lysates were resolved by SDS‐PAGE and immunoblotted with specific antibodies. Primary phospho‐specific antibodies were incubated in TBS (pH 7.5) with 0.1% Tween‐20 and 5% BSA with gentle agitation overnight at 4 °C. Horseradish peroxidase‐conjugated secondary antibodies were incubated in TBS (pH 7.5) with 5% nonfat milk and 0.1% Tween‐20 at a 1:2000 dilution for 1 h at room temperature. Positive immuno‐reactive proteins were detected using the ECL system (Pierce, Rockford, IL).
2.3. Electrophoretic mobility shift assay (EMSA)
EMSAs were performed as described in detail previously (Huang et al., 2002; Nam et al., 2007). To assess Stat5 DNA‐binding activity, 8 μg of nuclear protein extract was incubated with 32P‐radiolabeled oligonucleotide probe containing the MGFe (mammary gland factor element), derived from the bovine β‐casein gene promoter (5′‐AGATTTCTAGGAATTCAA‐3′) (Huang et al., 2002). For supershifts, 1 μl of antibody to Stat5 was preincubated with nuclear extract for 30 min prior to addition of the 32P‐labeled MGFe probe. Resolution of protein‐DNA complexes was performed by 5% non‐denaturing PAGE and detected by autoradiography.
2.4. Viability and apoptosis assays
MTS assays were performed for cell viability as described by the supplier (Promega, Madison, WI). K562 CML cells were seeded in 96‐well plates (10,000/well), incubated overnight at 37 °C in 5% CO2, and exposed to E804 for the indicated times. Dimethyl sulfoxide (DMSO) was used as the vehicle control. Viable cell numbers were determined by tetrazolium conversion to its formazan dye and absorbance was measured at 490 nm using an automated ELISA plate reader.
Apoptosis assays based on loss of membrane integrity were carried out using Annexin V‐FITC as described by the supplier (BD Biosciences PharMingen, San Diego, CA). For apoptosis of CD34‐positive primary CML, cells were cultured in the presence or in the absence of E804 or imatinib at the indicated concentrations at 37 °C in a humidified atmosphere with 5% CO2 in serum‐free medium (SFEM) (Stem Cell Technologies, Vancouver, BC, Canada) supplemented with growth factors (GFs) at concentrations similar to that found in stroma‐conditioned medium from long‐term bone marrow cultures (200 pg/mL granulocyte‐macrophage colony‐stimulating factor [GM‐CSF]; 1 ng/mL G‐CSF; 200 pg/mL stem cell factor [SCF]; 50 pg/mL leukemia inhibitory factor [LIF]; 200 pg/mL macrophage‐inflammatory protein‐1α [MIP‐1α]; and 1 ng/mL interleukin 6 [IL6]).
Cells were harvested after 48 h and assayed in proliferation and apoptosis assays. Cells were analyzed using a FACScan flow cytometer to quantify fluorescence. Briefly, CD34‐positive progenitor cells were labeled with 5‐ and 6‐carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) as described previously (Holtz et al., 2002). CFSE labeled cells were cultured for 48 h in the presence or absence of inhibitors. At the end of the culture period, the cells were labeled with Annexin V‐PE (BD Pharmingen, San Diego, CA, USA). Cell division was analyzed on the basis of CFSE fluorescence measured by flow cytometry (FACScalibur, Becton Dickinson, San Jose, CA). The percentage of cells in different generations was enumerated and a proliferation index was generated using ModFit software (Verity, Topsham, ME). Apoptotic cells were defined as Annexin V‐PE positive.
3. Results
3.1. IRDs inhibit Stat5 activity
Bcr‐Abl/SFK signaling constitutively activates Stat5, which has a key role in cell proliferation, apoptosis, tumorigenesis and metastasis in CML cells (Benekli et al., 2003; Haura et al., 2005; Yu and Jove, 2004). To determine whether IRDs inhibit Stat5 DNA‐binding activity in Bcr‐Abl positive human K562 CML cells, cells were treated with 10 μM IRDs for 24 h and EMSA was performed with nuclear extracts. Compounds E728, E804 and E806 potently reduced Stat5 DNA‐binding activity (Figure 1). In particular, E804, which contains a 2,3‐dihydroxypropyl substituent at the R 1 position, demonstrated the strongest activity against Stat5 DNA‐binding (Figure 1). Thus, E804 was chosen for further characterization on K562, imatinib‐resistant KCL‐22M and CD34‐positive primary CML cells.
Figure 1.

IRDs inhibit Stat5 DNA‐binding activity in K562 CML cells. A. Structures of IRDs. B. K562 CML cells were treated with DMSO or 10 µM IRDs for 24 h. Nuclear extracts were incubated with radiolabeled MGFe probe and Stat5 DNA‐binding activities were determined with EMSA analysis as described in Materials and Methods. Supershift was performed with antibody to Stat5 as indicated. The electrophoretic gel mobilities of Stat5:Stat5 homodimers bound to DNA probe and supershift of Stat5 complexes are indicated by arrows.
3.2. E804 reduces levels of p‐Stat5 and inhibits Stat5 DNA‐binding activity
Western blot analysis with specific antibodies to p‐Stat5 was performed to evaluate the effects of E804 on phosphorylation of Stat5 in K562, KCL‐22M and CD34‐positive primary CML cells. Cells were treated with E804 in a dose‐dependent manner for 4 h and Western blot analysis was performed using whole‐cell lysates. E804 substantially inhibited tyrosyl phosphorylation of Stat5 at 5 μM, whereas total Stat5 levels were unchanged (Figure 2A top and middle panels). Time course studies indicate that the levels of p‐Stat5 were dramatically reduced as early as 30 min after treatment with 10 μM E804 in CML cells, whereas total Stat5 protein levels remained unchanged (Figure 2B top panel).
Figure 2.

E804 reduces levels of p‐Stat5 (Y694) and inhibits Stat5 DNA‐binding activity in CML cells. A. K562, KCL‐22M and CD34‐positive primary CML cells were treated with E804 in a dose‐dependent manner for 4 h. Whole‐cell lysates were immunoblotted with specific antibodies to p‐Stat5 (Y694) and total Stat5 (top and middle panels). For EMSA analysis (bottom panel), nuclear extracts from CML cells treated with E804 were prepared as in Figure 1. B. K562 CML cells were treated with 10 μM of E804 in a time‐dependent manner. Whole‐cell lysates were immunoblotted with specific antibodies to p‐Stat5 (Y694) and total Stat5 (top panel). EMSA analysis was conducted as in Figure 1B (bottom panel). Supershift was done with antibody to Stat5 as indicated.
Consistent with the reduction of p‐Stat5, E804 blocked Stat5 DNA‐binding activity in a dose‐ or time‐dependent manner (Figure 2A bottom and 2B bottom panels). These observations indicate that IRDs inhibit tyrosyl phosphorylation of Stat5, followed by blockade of Stat5 DNA‐binding activity in human K562, KCL‐22M and primary CML cells. In our previous study, we demonstrated that IRDs inhibit Src/Stat3 signaling (Nam et al., 2005a), associated with induction of apoptosis in solid tumor cells. Similarly, these results suggest that IRDs could directly target upstream kinases such as Bcr‐Abl and/or SFKs, which constitutively activate Stat5 via tyrosyl phosphorylation of Stat5 at Y694 in chronic leukemias.
3.3. E804 inhibits tyrosyl phosphorylation of Src and SFKs
Stat5 is often constitutively activated in hematopoietic cancers by non‐receptor tyrosine kinases such as Bcr‐Abl and SFKs (Klejman et al., 2002; Silva, 2004; Yu and Jove, 2004). Overexpression of Lyn, one of the SFKs, is involved in resistance to Imatinib in CML cells (Donato et al., 2003; Konig et al., 2008; Wu et al., 2008). We previously reported that E804 targets Src kinase activity with IC50 = 0.43 μM in vitro (Nam et al., 2005a). To examine the effects of E804 on autophosphorylation of Src and SFKs in K562, KCL‐22M and primary patient CML cells, Western blot analysis and immunoprecipitation were performed with specific antibodies to p‐SFK, Src, p‐Lyn, Lyn, p‐Hck and Hck. E804 caused strong reduction of autophosphorylation of Src or SFKs at 5 μM in K562 and KCL‐22M CML cells (Figure 3A, B and C), and at 10 μM in primary patient CML cells (Figure 3D).
Figure 3.
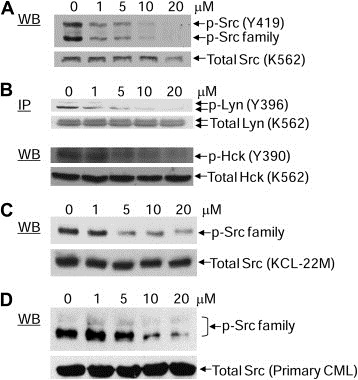
Effect of E804 on tyrosyl phosphorylation of Src and SFKs in K562, KCL‐22M and primary CML cells. A. K562 CML cells were treated with E804 in a dose‐dependent manner for 4 h. Whole‐cell lysates were immunoblotted with specific antibodies to p‐Src family (Y419) and Src. B. For Lyn immunoprecitation, cell lysates (500 μg) were incubated with specific antibody to Lyn. Samples were immunoblotted with p‐Src family antibody (Y419), which cross‐reacts with p‐Lyn (Y396). Whole‐cell lysates were immunoblotted with specific antibodies to p‐Hck and Hck. C. KCL‐22M cells were treated with E804 in a dose‐dependent manner for 4 h. Whole‐cell lysates were immunoblotted with specific antibodies to p‐Src family (Y419) and Src. D. CD34‐positive primary CML cells were treated with E804 in a dose‐dependent manner for 4 h. Western blot was performed with specific antibodies to p‐Src family (Y419) and Src.
3.4. Effect of E804 on Abl kinase activity and levels of p‐Bcr‐Abl
To determine whether E804 directly inhibits Abl kinase activity, kinase assays in vitro were performed with active recombinant Abl protein. E804 showed an inhibitory activity against Abl kinase with an IC50 = 8.7 μM (Supplementary Figure 1). Next, to address whether E804 inhibits tyrosyl phosphorylation of endogenous Bcr‐Abl in K562 human CML cells, immunoprecipitation of Bcr‐Abl was performed using lysates from K562 cells treated with E804 in a dose‐dependent manner for 4 h. Indeed, E804 reduced levels of p‐Bcr‐Abl at concentrations higher than 10–20 μM in cells (Figure 4). Indirubins are known to be ATP competitors and bind to the ATP binding pocket in the catalytic domain of CDKs (Hoessel et al., 1999). Likewise, the inhibitory activity E804 might result from ATP‐competitive binding into the Bcr‐Abl kinase binding pocket in CML cells.
Figure 4.
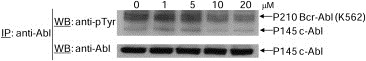
Effect of E804 on tyrosyl phosphorylation of Bcr‐Abl. For Bcr‐Abl immunoprecipitation, whole‐cell lysates (500 μg) were incubated with antibody to Abl at 4 °C. Immunoprecipitates were resolved by SDS‐PAGE and immunoblotted with specific antibodies to phospho‐tyrosine (p‐Y) and Abl.
3.5. E804 down‐regulates Mcl‐1 and Bcl‐xL
Inhibition of Stat5 signaling down‐regulates expression of Stat5 downstream gene products such as anti‐apoptotic proteins Mcl‐1 and Bcl‐xL (Horita et al., 2000; Shah et al., 2004; Yu and Jove, 2004). To determine the effects of E804 on anti‐apoptotic proteins such as Mcl‐1 and Bcl‐xL, CML cells were treated with E804 in a dose‐dependent manner for 48 h and whole‐cell lysates were used for Western blot analysis. Consistent with down‐regulation of p‐Stat5 and inhibition of Stat5 DNA‐binding activity (Figure 2), expression of the anti‐apoptotic proteins Mcl‐1 and Bcl‐xL was reduced at 5 μM (Figure 5A). In addition, down‐regulation of these anti‐apoptotic proteins correlates with inhibition of p‐Src and p‐SFKs as shown in Figure 3, associated with induction apoptosis by IRDs in CML cells (Figure 5C).
Figure 5.
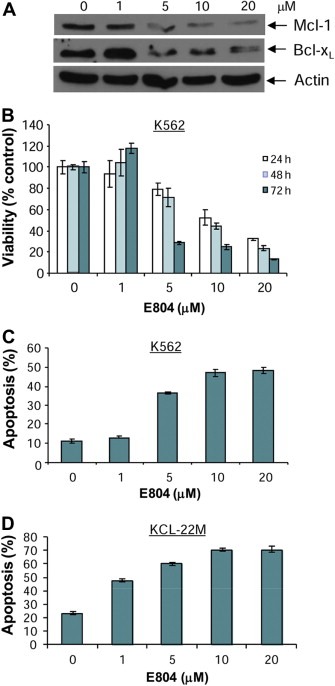
E804 down‐regulates anti‐apoptotic Mcl‐1 and Bcl‐xL proteins, associated with reduction of cell viability and induction of apoptosis in K562 CML cells. A. K562 CML cells were treated with E804 in a dose‐dependent manner for 48 h. Whole‐cell lysates were immunoblotted with specific antibodies to Mcl‐1, Bcl‐xL and β‐Actin. B. K562 CML cells were treated with E804 in a dose‐ or time‐dependent manner. Cell viability was determined using MTS assays as described in Materials and Methods. Each experiment was performed in quadruplicate. Data are mean ± SD. C and D. E804 induces apoptosis. Human K562 (C) and KCL‐22M (D) CML cells were treated with E804 in a dose‐dependent manner for 48 h. To determine induction of apoptosis, Annexin V‐FITC staining was used as an early marker of apoptosis. Each experiment was performed in quadruplicate. Data are mean ± SD.
3.6. E804 induces apoptosis of K562, KCL‐22M and CD34‐positive primary CML cells
IRDs inhibited constitutive activation of Stat5, followed by down‐regulation of survival proteins such as Mcl‐1 and Bcl‐xL in CML cells (1, 2 and Figure 5A). To assess the biological effects of E804 on K562, KCL‐22M and primary CML cells, MTS cell viability assays and apoptotic assays with Annexin V were performed. E804 reduced cell viability in a dose‐ and time‐dependent manner (Figure 5B). In addition, E804 induced apoptosis in a dose‐dependent manner 48 h after treatment in K562 (Figure 5C) and primary CML cells (Figure 6). In particular, E804 induced apoptosis of imatinib‐resistant KCL‐22M cells in the range of 1 μM–5 μM concentration (Figure 5D), while these cells extensively resist over 10 μM of imatinib (Yuan et al., 2010). These biological consequences of E804 treatment correlate well with inhibition of the SFK/Stat5 signaling pathway.
Figure 6.
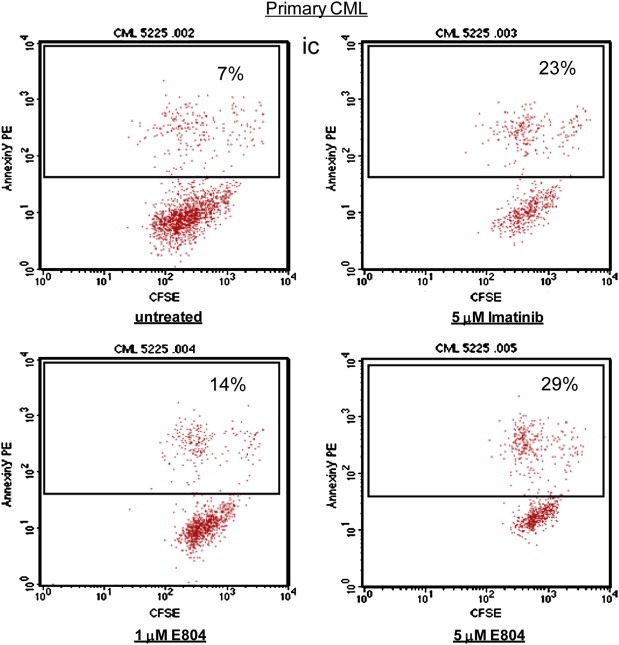
E804 induces apoptosis in primary CML cells. CD34‐positive primary CML cells were treated with E804 or Imatinib in a dose‐dependent manner for 48 h. Cells were labeled with Annexin V‐PE. Cells were analyzed using a FACScan flow cytometer to quantify fluorescence. Apoptotic cells were defined as Annexin V‐PE positive.
4. Discussion
Many structurally related series of IRDs are poorly water‐soluble and display low bioavailability in cells (Hoessel et al., 1999). Therefore, IRDs that contain hydrophilic substituents at the 3′‐oxime or the 5 position, including hydroxyalkyl, sugar, aminopolyol, or substituted glycine amide groups were synthesized to enhance bioavailability (Figure 1A). In a previous study, several synthetic IRDs showed potent anti‐tumor activities, blocking constitutive Stat3 signaling in human solid tumor cell lines (Nam et al., 2005a). The best activity was attributed to IRD compound E804 which contains a 2,3‐dihydroxypropyl substituent at R 1 position. In this study, E804 strongly inhibited Stat5 DNA‐binding activity in Bcr‐Abl positive human K562 CML cells. This is in accordance with its high inhibitory potency against Stat3 signaling found earlier (Nam et al., 2005a). In contrast, unbranched hydroxyalkyl (E246) or 2‐hydroxymethylethoxy (E564) or branched 2‐hydroxy‐2‐methylpropyl (E565) was less effective. This observation also applies to compounds with free oxime hydroxyl groups carrying hydrophilic substituents at the 5 position (E721, E728).
Considering the influence of 3′‐glycine amide‐oxime ether groups showed that the bis(2‐hydroxyethyl)‐glycine amide group (E806) conferred higher activity, approaching that of (E804) compared to its mono‐ (E805) or unsubstituted (E567) counterparts. Sterically more crowding hydrophilic substituents at the 3′‐oxime ether position are unfavorable in connection with 5‐substitution by iodine (E692) or a methoxy group (E729). This also applies to the 3′‐aminopolyol oxime ether (E673). The free, otherwise unsubstituted 3′‐oxime (indirubin‐3′‐oxime) is poorly active. These findings raise the possibility that inhibition of Stat5 signaling is at least partially responsible for biological effects of IRDs on CML cells.
In comparison of both of Src and Abl kinase activities in vitro, E804 inhibited Abl kinase activity at twenty‐fold higher concentration (Supplementary Figure 1) (Nam et al., 2005a). In addition, E804 reduced levels of p‐Bcr‐Abl at higher concentrations in cells (Figure 4). These findings suggest that IRDs inhibit SFK/Stat5 signaling more strongly than Bcr‐Abl/Stat5 signaling in CML cells. Recently, one study reported siRNA knockdown of Lyn induces apoptosis in drug‐resistant Bcr‐Abl positive blast cells, but not in normal blood cells, suggesting that Lyn could be a therapeutic target for treatment of drug‐resistant CML blast patients (Ptasznik et al., 2004). Interestingly, E804 down‐regulates autophosphorylation of Lyn at 5 μM in CML cells. These effects of E804 could be responsible for induction of apoptosis, suggesting that E804 and other IRDs may have potential as therapeutic agents in drug‐resistant CML cells.
Blockade of persistent STATs signaling has been shown to inhibit tumor cell survival in cell cultures and in vivo (Yu and Jove, 2004). Constitutively activated Stat5 up‐regulates genes of apoptosis inhibitors such as Mcl‐1 and Bcl‐xL, associated with oncogenesis in human CML cells (Buettner et al., 2002; Yu and Jove, 2004). It was reported that down‐regulation of Mcl‐1 by siRNA and antisense oligonucleotides dramatically reduces cell viability and induces apoptosis in K562 CML cells (Aichberger et al., 2005). In this study, down‐regulation of anti‐apoptotic protein Mcl‐1 by IRD suggests that Mcl‐1 could be a molecular therapeutic target for treatment of Bcr‐Abl‐dependent CML.
In summary, our findings demonstrate that IRDs inhibit SFK/Stat5 signaling, associated with induction of apoptosis on human K562 CML cells, imatinib‐resistant human KCL‐22 CML cells expressing the T315I mutant Bcr‐Abl, and CD34‐positive primary CML cells from patients. IRDs down‐regulate Mcl‐1, which is known to be a potential target for treatment of CML patients. These findings suggest a mechanism of pharmacological action of indirubins in CML cells that have important implications for CML treatment. In particular, IRDs represent a promising structural class for development of new therapeutics for wild type or T315I mutant Bcr‐Abl‐positive CML patients.
Funding
This study was supported by NIH grant R01 CA115674‐05 to RJ.
Supporting information
Supplementary Figure 1A. For Abl kinase assay in vitro, 20 ng of recombinant Abl protein were preincubated with DMSO, E804, or PD180970 as an Abl kinase inhibitor (1 μM) as positive control for 30 min. [γ‐32P] ATP (5 μCi) was added per 25 μL reaction mixture for 20 min. Twenty‐microliter aliquots were transferred onto the center of substrate‐binding phosphocellulose paper squares. Assay squares were transferred to vials with 5 mL of scintillation cocktail. After quantifying activity with a scintillation counter, radioactivity in assay squares was directly visualized by autoradiography. B. Abl kinase activities shown in A were quantified with ImageQuant software (Molecular Dynamics, Sunnydale, CA). The IC50 value of Abl kniase inhibition by E804 was determined.
Acknowledgments
We thank our lab colleagues for technical supports with experiments, critically discussing our findings and helping in the preparation of this manuscript. We also the thank Analytical Cytometry Core of City of Hope for apoptosis analysis.
Supplementary data 1.
1.1.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.molonc.2012.02.002.
Sangkil Nam, Scuto Anna, Yang Fan, Chen WenYong, Park Sungman, Yoo Hwa-Seung, Konig Heiko, Bhatia Ravi, Cheng Xinlai, Merz Karl-Heinz, Eisenbrand Gerhard, Jove Richard, (2012), Indirubin derivatives induce apoptosis of chronic myelogenous leukemia cells involving inhibition of Stat5 signaling, Molecular Oncology, 6, doi: 10.1016/j.molonc.2012.02.002.
Contributor Information
Sangkil Nam, Email: snam@coh.org.
Richard Jove, Email: rjove@coh.org.
References
- Aichberger, K.J. , Mayerhofer, M. , Krauth, M.T. , Skvara, H. , Florian, S. , Sonneck, K. , Akgul, C. , Derdak, S. , Pickl, W.F. , Wacheck, V. , Selzer, E. , Monia, B.P. , Moriggl, R. , Valent, P. , Sillaber, C. , 2005. Identification of mcl-1 as a BCR/ABL-dependent, target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 105, 3303–3311. [DOI] [PubMed] [Google Scholar]
- Benekli, M. , Baer, M.R. , Baumann, H. , Wetzler, M. , 2003. Signal transducer and activator of transcription proteins in leukemias. Blood. 101, 2940–2954. [DOI] [PubMed] [Google Scholar]
- Bromann, P.A. , Korkaya, H. , Courtneidge, S.A. , 2004. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 23, 7957–7968. [DOI] [PubMed] [Google Scholar]
- Bromberg, J.F. , Wrzeszczynska, M.H. , Devgan, G. , Zhao, Y. , Pestell, R.G. , Albanese, C. , Darnell, J.E. , 1999. Stat3 as an oncogene. Cell. 98, 295–303. [DOI] [PubMed] [Google Scholar]
- Buettner, R. , Mora, L.B. , Jove, R. , 2002. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res.. 8, 945–954. [PubMed] [Google Scholar]
- Carlesso, N. , Frank, D.A. , Griffin, J.D. , 1996. Tyrosyl phosphorylation and DNA binding activity of signal transducers and activators of transcription (STAT) proteins in hematopoietic cell lines transformed by Bcr/Abl. J. Exp. Med.. 183, 811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato, N.J. , Wu, J.Y. , Stapley, J. , Gallick, G. , Lin, H. , Arlinghaus, R. , Talpaz, M. , 2003. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 101, 690–698. [DOI] [PubMed] [Google Scholar]
- Eisenbrand, G. , Hippe, F. , Jakobs, S. , Muehlbeyer, S. , 2004. Molecular mechanisms of indirubin and its derivatives: novel anticancer molecules with their origin in traditional Chinese phytomedicine. J. Cancer Res. Clin. Oncol.. 130, 627–635. [DOI] [PubMed] [Google Scholar]
- Gesbert, F. , Griffin, J.D. , 2000. Bcr/Abl activates transcription of the Bcl-X gene through STAT5. Blood. 96, 2269–2276. [PubMed] [Google Scholar]
- Haura, E.B. , Turkson, J. , Jove, R. , 2005. Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat. Clin. Pract. Oncol.. 2, 315–324. [DOI] [PubMed] [Google Scholar]
- Herrington, J. , Smit, L.S. , Schwartz, J. , Carter-Su, C. , 2000. The role of STAT proteins in growth hormone signaling. Oncogene. 19, 2585–2597. [DOI] [PubMed] [Google Scholar]
- Hoessel, R. , Leclerc, S. , Endicott, J.A. , Nobel, M.E. , Lawrie, A. , Tunnah, P. , Leost, M. , Damiens, E. , Marie, D. , Marko, D. , Niederberger, E. , Tang, W. , Eisenbrand, G. , Meijer, L. , 1999. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nat. Cell Biol.. 1, 60–67. [DOI] [PubMed] [Google Scholar]
- Holtz, M.S. , Slovak, M.L. , Zhang, F. , Sawyers, C.L. , Forman, S.J. , Bhatia, R. , 2002. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood. 99, 3792–3800. [DOI] [PubMed] [Google Scholar]
- Horita, M. , Andreu, E.J. , Benito, A. , Arbona, C. , Sanz, C. , Benet, I. , Prosper, F. , Fernandez-Luna, J.L. , 2000. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. J. Exp. Med.. 191, 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M. , Dorsey, J.F. , Epling-Burnette, P.K. , Nimmanapalli, R. , Landowski, T.H. , Mora, L.B. , Niu, G. , Sinibaldi, D. , Bai, F. , Kraker, A. , Yu, H. , Moscinski, L. , Wei, S. , Djeu, J. , Dalton, W.S. , Bhalla, K. , Loughran, T.P. , Wu, J. , Jove, R. , 2002. Inhibition of Bcr-Abl kinase activity by PD180970 blocks constitutive activation of Stat5 and growth of CML cells. Oncogene. 21, 8804–8816. [DOI] [PubMed] [Google Scholar]
- Klejman, A. , Schreiner, S.J. , Nieborowska-Skorska, M. , Slupianek, A. , Wilson, M. , Smithgall, T.E. , Skorski, T. , 2002. The Src family kinase Hck couples BCR/ABL to STAT5 activation in myeloid leukemia cells. Embo J.. 21, 5766–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig, H. , Copland, M. , Chu, S. , Jove, R. , Holyoake, T.L. , Bhatia, R. , 2008. Effects of dasatinib on SRC kinase activity and downstream intracellular signaling in primitive chronic myelogenous leukemia hematopoietic cells. Cancer Res.. 68, 9624–9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionberger, J.M. , Wilson, M.B. , Smithgall, T.E. , 2000. Transformation of myeloid leukemia cells to cytokine independence by Bcr-Abl is suppressed by kinase- defective Hck. J. Biol. Chem.. 275, 18581–18585. [DOI] [PubMed] [Google Scholar]
- Marko, D. , Schatzle, S. , Friedel, A. , Genzlinger, A. , Zankl, H. , Meijer, L. , Eisenbrand, G. , 2001. Inhibition of cyclin-dependent kinase 1 (CDK1) by indirubin derivatives in human tumour cells. Br. J. Cancer. 84, 283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, S. , Buettner, R. , Turkson, J. , Kim, D. , Cheng, J.Q. , Muehlbeyer, S. , Hippe, F. , Vatter, S. , Merz, K.H. , Eisenbrand, G. , Jove, R. , 2005. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc. Natl. Acad. Sci. USA. 102, 5998–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, S. , Kim, D. , Cheng, J.Q. , Zhang, S. , Lee, J.H. , Buettner, R. , Mirosevich, J. , Lee, F.Y. , Jove, R. , 2005. Action of the Src family kinase inhibitor, dasatinib (BMS- 354825), on human prostate cancer cells. Cancer Res.. 65, 9185–9189. [DOI] [PubMed] [Google Scholar]
- Nam, S. , Williams, A. , Vultur, A. , List, A. , Bhalla, K. , Smith, D. , Lee, F.Y. , Jove, R. , 2007. Dasatinib (BMS-354825) inhibits Stat5 signaling associated with apoptosis in chronic myelogenous leukemia cells. Mol. Cancer Ther.. 6, 1400–1405. [DOI] [PubMed] [Google Scholar]
- Nelson, E.A. , Walker, S.R. , Li, W. , Liu, X.S. , Frank, D.A. , 2006. Identification of human STAT5-dependent gene regulatory elements based on interspecies homology. J. Biol. Chem.. 281, 26216–26224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieborowska-Skorska, M. , Wasik, M.A. , Slupianek, A. , Salomoni, P. , Kitamura, T. , Calabretta, B. , Skorski, T. , 1999. Signal transducer and activator of transcription (STAT)5 activation by BCR/ABL is dependent on intact Src homology (SH)3 and SH2 domains of BCR/ABL and is required for leukemogenesis. J. Exp. Med.. 189, 1229–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons, S.J. , Parsons, J.T. , 2004. Src family kinases, key regulators of signal transduction. Oncogene. 23, 7906–7909. [DOI] [PubMed] [Google Scholar]
- Ptasznik, A. , Nakata, Y. , Kalota, A. , Emerson, S.G. , Gewirtz, A.M. , 2004. Short interfering RNA (siRNA) targeting the Lyn kinase induces apoptosis in primary, and drug-resistant, BCR-ABL1(+) leukemia cells. Nat. Med.. 10, 1187–1189. [DOI] [PubMed] [Google Scholar]
- Quintas-Cardama, A. , Kantarjian, H. , Jones, D. , Nicaise, C. , O'Brien, S. , Giles, F. , Talpaz, M. , Cortes, J. , 2007. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood. 109, 497–499. [DOI] [PubMed] [Google Scholar]
- Shah, N.P. , Tran, C. , Lee, F.Y. , Chen, P. , Norris, D. , Sawyers, C.L. , 2004. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 305, 399–401. [DOI] [PubMed] [Google Scholar]
- Silva, C.M. , 2004. Role of STATs as downstream signal transducers in Src family kinase- mediated tumorigenesis. Oncogene. 23, 8017–8023. [DOI] [PubMed] [Google Scholar]
- Vougogiannopoulou, K. , Ferandin, Y. , Bettayeb, K. , Myrianthopoulos, V. , Lozach, O. , Fan, Y. , Johnson, C.H. , Magiatis, P. , Skaltsounis, A.L. , Mikros, E. , Meijer, L. , 2008. Soluble 3',6-substituted indirubins with enhanced selectivity toward glycogen synthase kinase -3 alter circadian period. J. Med. Chem.. 51, 6421–6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M.B. , Schreiner, S.J. , Choi, H.J. , Kamens, J. , Smithgall, T.E. , 2002. Selective pyrrolo-pyrimidine inhibitors reveal a necessary role for Src family kinases in Bcr- Abl signal transduction and oncogenesis. Oncogene. 21, 8075–8088. [DOI] [PubMed] [Google Scholar]
- Wu, J. , Meng, F. , Lu, H. , Kong, L. , Bornmann, W. , Peng, Z. , Talpaz, M. , Donato, N.J. , 2008. Lyn regulates BCR-ABL and Gab2 tyrosine phosphorylation and c-Cbl protein stability in imatinib-resistant chronic myelogenous leukemia cells. Blood. 111, 3821–3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Z. , Hao, Y. , Liu, B. , Qian, L. , 2002. Indirubin and meisoindigo in the treatment of chronic myelogenous leukemia in China. Leuk. Lymphoma. 43, 1763–1768. [DOI] [PubMed] [Google Scholar]
- Yu, H. , Jove, R. , 2004. The STATs of cancer–new molecular targets come of age. Nat. Rev. Cancer. 4, 97–105. [DOI] [PubMed] [Google Scholar]
- Yu, H. , Pardoll, D. , Jove, R. , 2009. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 9, 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, H. , Wang, Z. , Gao, C. , Chen, W. , Huang, Q. , Yee, J.K. , Bhatia, R. , 2010. BCR- ABL gene expression is required for its mutations in a novel KCL-22 cell culture model for acquired resistance of chronic myelogenous leukemia. J. Biol. Chem.. 285, 5085–5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Bi, C. , Janakakumara, J.V. , Liu, S.C. , Chng, W.J. , Tay, K.G. , Poon, L.F. , Xie, Z. , Palaniyandi, S. , Yu, H. , Glaser, K.B. , Albert, D.H. , Davidsen, S.K. , Chen, C.S. , 2009. Enhanced activation of STAT pathways and overexpression of survivin confer resistance to FLT3 inhibitors and could be therapeutic targets in AML. Blood. 113, 4052–4062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1A. For Abl kinase assay in vitro, 20 ng of recombinant Abl protein were preincubated with DMSO, E804, or PD180970 as an Abl kinase inhibitor (1 μM) as positive control for 30 min. [γ‐32P] ATP (5 μCi) was added per 25 μL reaction mixture for 20 min. Twenty‐microliter aliquots were transferred onto the center of substrate‐binding phosphocellulose paper squares. Assay squares were transferred to vials with 5 mL of scintillation cocktail. After quantifying activity with a scintillation counter, radioactivity in assay squares was directly visualized by autoradiography. B. Abl kinase activities shown in A were quantified with ImageQuant software (Molecular Dynamics, Sunnydale, CA). The IC50 value of Abl kniase inhibition by E804 was determined.