Prion-Like Behavior and Tau-dependent Cytotoxicity of Pyroglutamylated β-Amyloid (original) (raw)
. Author manuscript; available in PMC: 2012 Nov 30.
Published in final edited form as: Nature. 2012 May 2;485(7400):651–655. doi: 10.1038/nature11060
Abstract
Extracellular plaques of β-amyloid (Aβ) and intraneuronal neurofibrillary tangles made from tau are the histopathological signatures of Alzheimer’s disease (AD). Plaques comprise Aβ fibrils that assemble from monomeric and oligomeric intermediates, and are prognostic indicators of AD. Despite the significance of plaques to AD, oligomers are considered to be the principal toxic forms of Aβ1,2. Interestingly, many adverse responses to Aβ, such as cytotoxicity3, microtubule loss4, impaired memory and learning5, and neuritic degeneration6, are greatly amplified by tau expression. N-terminally truncated, pyroglutamylated (pE) forms of Aβ7,8 are strongly associated with AD, are more toxic than Aβ1–42 and Aβ1–40, and have been proposed as initiators of AD pathogenesis9,10. We now report a mechanism by which pE-Aβ may trigger AD. Aβ3(pE)-42 co-oligomerizes with excess Aβ1–42 to form metastable low-n oligomers (LNOs) that are structurally distinct and far more cytotoxic to cultured neurons than comparable LNOs made from Aβ1–42 alone. Tau is required for cytotoxicity, and LNOs comprising 5% Aβ3(pE)-42 plus 95% Aβ1–42 (5% pE-Aβ) seed new cytotoxic LNOs through multiple serial dilutions into Aβ1–42 monomers in the absence of additional Aβ3(pE)-42. LNOs isolated from human AD brain contained Aβ3(pE)-42, and enhanced Aβ3(pE)-42 formation in mice triggered neuron loss and gliosis at 3 months, but not in a tau null background. We conclude that Aβ3(pE)-42 confers tau-dependent neuronal death and causes template-induced misfolding of Aβ1–42 into structurally distinct LNOs that propagate by a prion-like mechanism. Our results raise the possibility that Aβ3(pE)-42 acts similarly at a primary step in AD pathogenesis.
pE-Aβ peptides contain an N-terminal pyroglutamate, whose modification from glutamate is catalyzed by glutaminyl cyclase (QC)10. The most prominent pE-Aβ species in vivo are Aβ3(pE)-40, Aβ3(pE)-42, Aβ11(pE)-40 and Aβ11(pE)-428 (Supplementary Fig. 1), with Aβ3(pE)-42 being most abundant11. pE-Aβ is more cytotoxic12 and aggregates more rapidly13,14 than conventional Aβ, and QC activity and pE-Aβ levels are increased several-fold in AD brain10. AD mouse models also imply a role for pE-Aβ in initiating AD pathology: oral administraton of a QC inhibitor led to improved memory and learning, and reduced levels of pE-Aβ and conventional Aβ10. These data imply that pE-Aβ potentiates the neurotoxicity of conventional Aβ, but leave open the issue of molecular mechanisms. To address that issue, we compared oligomerization of Aβ3(pE)-42, Aβ1–42, and mixtures of the peptides in vitro, and analyzed responses of primary cultured neurons and glial cells (Supplementary Fig. 2) to the oligomers.
At 5 μM peptide, 5% pE-Aβ aggregated faster than Aβ3(pE)-42 or Aβ1–42 alone based on thioflavin T fluorescence shifts15 (Supplementary Fig. 3). The OD450/OD490 ratio for Aβ3(pE)-42 rose and peaked more rapidly than for Aβ1–42, but peaked at an ~25% lower level. The fastest rise in the OD450/OD490 ratio was for 5% pE-Aβ, which peaked similarly to Aβ3(pE)-42. Aβ3(pE)-42, Aβ1–42 and 5% pE-Aβ thus oligomerized by different pathways.
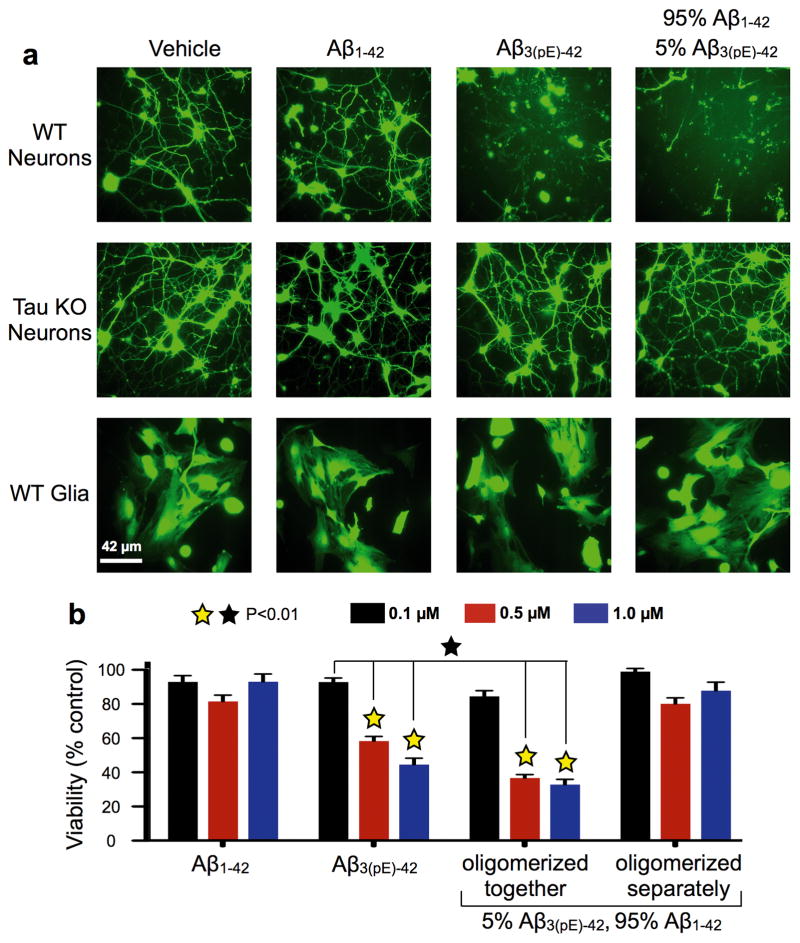
To test whether distinct biological activities were coupled to these oligomerization differences, we compared cytotoxicity of the peptides towards cultured neurons or glia using calcein-AM and fluorescence microscopy16. Twelve hours of Aβ1–42 exposure had little effect on cell viability for wild type (WT) or tau knockout (KO) neurons, or WT glial cells (Fig. 1a). Contrastingly, most WT neurons died and detached from the substrate after exposure to Aβ3(pE)-42 or 5% pE-Aβ. Tau KO neurons and WT glia, which express little tau, were resistant to Aβ3(pE)-42 and 5% pE-Aβ.
Figure 1. Tau-dependent cytotoxicity of oligomers formed by co-incubation of Aβ3(pE)-42 and Aβ1–42.
Primary mouse wild type (WT) and tau knockout (KO) forebrain neurons, and secondary cultures of WT mouse glia were treated for 12 hours with Aβ1–42, Aβ3(pE)-42, or 5% Aβ3(pE)-42 plus 95% Aβ1–42, which were oligomerized for 24 hours at 5 μM before dilution into culture media. a, Cells were exposed to calcein-AM and imaged live by epifluorescence microscopy to assay viability16. Extensive death and detachment of cells were observed only for WT neurons treated with Aβ3(pE)-42 or the 5% Aβ3(pE)-42 plus 95% Aβ1–42. b, Following peptide treatment, cell viability was analyzed by the XTT plate reader assay17. Note the robust cytotoxicity of Aβ3(pE)-42 containing solutions at concentrations as low as 0.5 μM, unless Aβ3(pE)- 42 and Aβ1–42 were incubated separately during oligomerization (p<0.01; yellow [ ] stars signify statistical significance of the indicated bar graphs versus vehicle controls; black [□] stars signify statistical significance between the indicated bar graph pairs; mean ± SEM, n= 9 replicates from 3 independent experiments).
] stars signify statistical significance of the indicated bar graphs versus vehicle controls; black [□] stars signify statistical significance between the indicated bar graph pairs; mean ± SEM, n= 9 replicates from 3 independent experiments).
Cytotoxicity dose-dependence was examined by incubating WT neurons for 24 hours in oligomers comprising 0.1, 0.5 or 1 μM peptides, and using the XTT reduction assay17 (Fig. 1b). Cells were unaffected by Aβ1–42, but Aβ3(pE)-42 and 5% pE-Aβ had substantial cytotoxicity at 0.5 μM and even more at 1.0 μM. Cytotoxicity of 5% pE-Aβ required Aβ3(pE)-42 and Aβ1–42 to incubate together for 24 hours before being added to cells. When they were incubated separately for 24 hours and mixed together at a 1:19 molar ratio immediately before being applied to cells, they were not cytotoxic. A small amount of Aβ3(pE)-42 can thus dramatically enhance the cytotoxicity of a large excess of Aβ1–42, provided the two peptides oligomerize together.
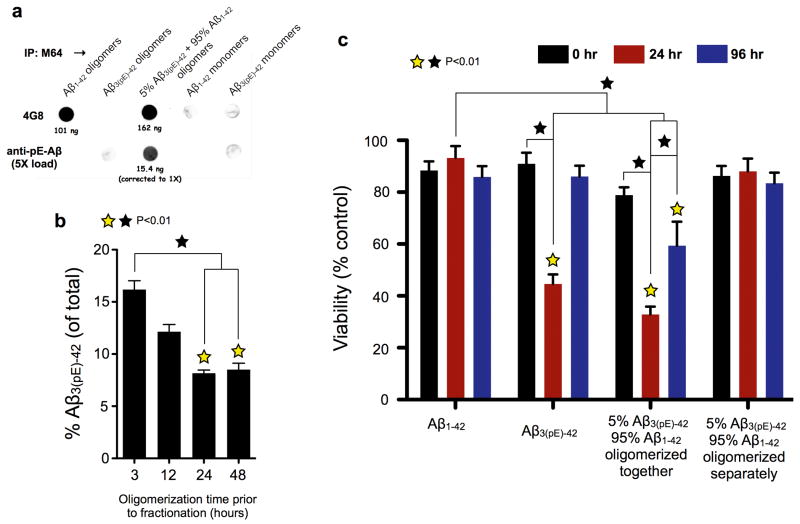
Evidence for hybrid oligomers came from immunoprecipitation (IP) of various forms of Aβ using aggregation-dependent M64, which does not recognize Aβ3pE-42 (see Supplementary Fig, 4 for characterization of all anti-Aβ antibodies used, including M64). IPs were analyzed on dot blots using 4G8, which equally recognizes Aβ3(pE)-42 and Aβ1–42, and anti-pE-Aβ, which does not react with Aβ1–42. M64 IP’d oligomers made from Aβ1–42 or 5% pE-Aβ, but it did not IP Aβ3(pE)-42 oligomers, nor monomers of either peptide (Fig. 2a). Because anti-pE-Aβ reacted with material IP’d out of 5% pE-Aβ, M64 pulled down hybrid peptide oligomers. Aβ3(pE)-42 accounted for ~16% of the Aβ in gel filtered cytotoxic oligomers after 3 hours of oligomerization, and steadily dropped to ~8% by 24 hours (Fig. 2b). Aβ3(pE)-42 thus acts as a template that initiates formation of cytotoxic oligomers.
Figure 2. Aβ3(pE)-42 and Aβ3(pE)-42 form metastable, cytotoxic, hybrid oligomers.
a, Aβ3(pE)-42 and Aβ1–42 were incubated together at a 1:19 molar ratio (5% pE-Aβ) for 24 hours at 1 μM total Aβ, and then were IP’d with M64, a rabbit monoclonal antibody that specifically recognizes residues 3–7 (EFRH) of Aβ1–40 oligomers or fibrils. Additional samples that were IP’d included otherwise identically treated oligomers made from pure Aβ3(pE)-42 or Aβ1–42, and monomeric versions of the two peptides. IP’d oligomers were converted to monomers by lyophilization, solubilization with HFIP and dilution into PBS, and along with the other samples were dot blotted onto nitrocellulose and analyzed using 4G8, a mouse monoclonal antibody that recognizes Aβ3(pE)-42 andAβ1–42 equally well, and an antibody that specifically recognizes pE-Aβ (see Supplemental Fig. 4 for characterization of all antibodies used here). Quantitation of the dot blots using a LI-COR Odyssey imaging station indicated that the oligomers that were IP’d from the mixed peptide solution contained both Aβ3(pE)-42 and Aβ1–42, at a molar ratio of ~1:10. b, Solutions containing 5% pE-Aβ3(pE)-42 and 95% Aβ1–42 were incubated for the indicated times, and then were fractionated by gel filtration. At each time point, fractions that eluted at 12.5 ml, where most cytotoxicity resided (see Figure 3b) were IP’d using anti-human amyloid β (N), an amino terminal-specific antibody that does not react with pE-Aβ (data not shown). The IPs were then lyophilized, re-solubilized with HFIP, and quantitatively analyzed on dot blots with 4G8 and anti-pE-Aβ using the LI-COR Odyssey. The time-dependent decrease in the Aβ3(pE)-42 content of the IP’d oligomers implies that Aβ3(pE)-42 initiated formation of hybrid peptide oligomers. c, Aβ3pE-42 and Aβ1–42 oligomerized for 0, 24 and 96 hours either separately or together as 1:19 mixtures, and then were added to primary WT neuron cultures for 24 hours at a final concentration of 1 μM total Aβ. Following peptide treatment, cell viability was analyzed by the XTT plate reader assay17. The most cytotoxic species observed were the hybrid oligomers after 24 hours of oligomerization (p<0.01; yellow [ ] stars signify statistical significance of the indicated bar graphs versus vehicle controls; black [□] stars signify statistical significance between the indicated bar graph pairs; mean ± SEM, n= 6 or 9 replicates from 3 independent experiments for panel b or c, respectively).
] stars signify statistical significance of the indicated bar graphs versus vehicle controls; black [□] stars signify statistical significance between the indicated bar graph pairs; mean ± SEM, n= 6 or 9 replicates from 3 independent experiments for panel b or c, respectively).
Cytotoxicity was sensitive to oligomerization time (Fig. 2c). Baseline cytotoxicity was observed at alltime points for Aβ1–42, and for 5% pE-Aβ solutions in which Aβ3(pE)-42 and Aβ1–42 oligomerized separately. Pure Aβ3(pE)-42 killed ~50% of the cells after 24 hours of oligomerization, but was virtually non-toxic at 0 hours and after 96 hours of oligomerization. The most cytotoxic solutions were 5% pE-Aβ in which the constituent peptides co-oligomerized for 24 hours. These solutions killed ~60% of the cells within 24 hours, and lower, but robust cytotoxicity was observed at 96 hours. Even the 0 hour co-oligomers of 5% pE-Aβ exhibited low, significant cytotoxicity. Co-incubated mixtures of 5% Aβ3(pE)-42 and 95% Aβ1–42 can therefore form oligomers whose cytotoxicity is both greater and more enduring than oligomers formed by Aβ3(pE)-42 alone.
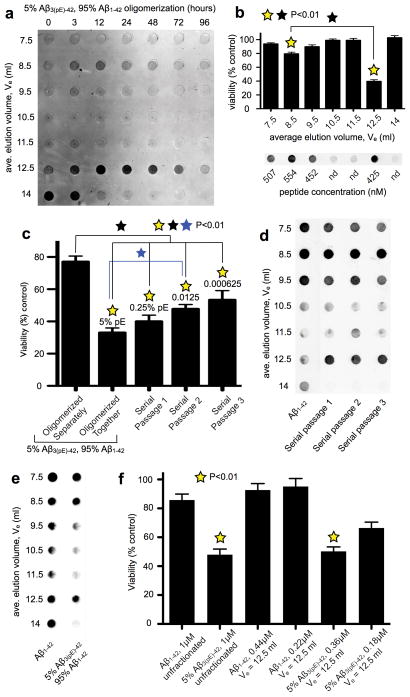
To identify the co-oligomer size(s) that were cytotoxic, Aβ solutions were oligomerized for various times from 0–96 hours before fractionation by gel filtration. Total Aβ in all fractions was determined using 4G8 dot blots, which as shown in Fig. 3a (for 5% pE-Aβ) and Supplementary Fig. 5 (for Aβ1–42 and Aβ3(pE)-42), illustrate the full fractionation range of the column but exclude most void volume fractions. Presumptive monomeric Aβ1–42 dominated initially and persisted at 3 hours, but was nearly undetectable after 12 hours. 3 hours also marked the appearance of Aβ1–42 oligomers, which gradually increased in size over the next 93 hours. Aβ3(pE)-42 and 5% pE-Aβ oligomerized differently. Putative monomers were present at 0 hours for both samples, when slightly larger species, LNOs that possibly corresponded to dimers/trimers (Supplementary Fig. 6), were also present. These persisted as the main species for 24 hours for Aβ3(pE)-42 and for nearly 72 hours for 5% pE-Aβ, and later time points were dominated by larger aggregates that eluted in void volume fractions. Cytotoxicity was assayed for individual fractions of 5% pE-Aβ that oligomerized for 24 hours (Fig. 3b). Most cytotoxicity was associated with the possible dimers/trimers that eluted at 12.5 ml, which at 425 nM peptide killed more than 60% of the cells. Low cytotoxicity was also observed at 554 nM peptide for the larger oligomers that eluted at 8.5 ml.
Figure 3. The cytotoxic species are low-n, prion-like oligomers.
a, Gel filtration chromatography was used to fractionate 5% pE-Aβ after oligomerization at 5 μM at 37° C for 0–96 hours. The resulting fractions were then converted to monomers using HFIP and analyzed on dot blots using monoclonal antibody 4G8. Note the metastable oligomers with an average elution volume (Ve) of 12.5 ml. b, Isolated gel filtration fractions from the 24 hour time point were added to WT neuron cultures for 24 hours, after which the cells were assayed for cell viability using XTT17. Robust cytotoxicity was associated only with the Ve = 12.5 ml fraction, although the Ve = 8.5 ml fraction had low, but statistically significant cell killing activity (p<0.01; mean ± SEM, n= 9 replicates from 3 independent experiments). c, Cytotoxic hybrid oligomers made by co-incubating a 1:19 ratio of Aβ3(pE)-42:Aβ1–42 for 24 hours at 5 μM were diluted into a 19-fold molar excess of freshly dissolved, monomeric Aβ1–42, which was then incubated at 5 μM for another 24 hours to yield Serial Passage 1. Two further iterations of this strategy yielded Serial Passages 2 and 3. The starting material and its serially passaged derivatives were added to WT neurons at 1 μM peptide for 24 hours, after which cells were analyzed using the XTT assay for cell viability17. Only a gradual loss of cytotoxicity was observed with each successive serial passage. d, Each serially passaged sample, as well as otherwise identically prepared oligomers made from pure Aβ1–42, were fractionated by gel filtration and analyzed on dot blots with 4G8. Note that all serially passaged samples contained metastable low-n oligomers of Ve = 12.5 ml, which were absent from the pure Aβ1–42 samples. e, Aβ1–42 (10 μM) that was oligomerized for 30 minutes at 4° C, and 5% Aβ3pE-42 plus 95% Aβ1–42 (5 μM) that was oligomerized for 24 hours at 37° C were fractionated by gel filtration and analyzed on dot blots exactly using 4G8. Note the isolation of fractions with Ve = 12.5 ml from both preparations. f, WT neurons were assayed for viability using the XTT plate reader assay17 following 24 hours of exposure to the indicated Aβ preparations. Note the minimal cytotoxicity of unfractionated Aβ1–42 and Aβ1–42 with Ve = 12.5 ml (p<0.01, mean ± SEM, n= 9 replicates from 3 independent experiments). (p<0.01; yellow [ ] stars signify statistical significance of the indicated bar graphs versus vehicle controls; black [□] star signifies statistical significance between the indicated bar graph pairs; mean ± SEM, n= 9 replicates from 3 independent experiments for panels b, c and f).
] stars signify statistical significance of the indicated bar graphs versus vehicle controls; black [□] star signifies statistical significance between the indicated bar graph pairs; mean ± SEM, n= 9 replicates from 3 independent experiments for panels b, c and f).
The dramatic enhancement of Aβ1–42 cytotoxicity by Aβ3(pE)-42 suggested a prion-like templating mechanism of Aβ1–42 misfolding initiated by Aβ3(pE)-42. To test that hypothesis, 5% pE-Aβ that oligomerized for 24 hours was diluted into 19 volumes of monomeric Aβ1–42. A 24 hour incubation of this mixture yielded “serial passage 1”, which was followed by two equivalent, sequential dilutions into monomeric Aβ1–42 to yield serial passages 2 and 3. A gradual loss of cytotoxicity was observed with successive passages, but even passage 3, which contained only 0.000625% Aβ3(pE)-42, killed ~50% of the neurons within 24 hours (Fig. 3c). Serially passaged gel filtration samples contained abundant material that eluted at 12.5 ml in passages 1–3, despite the progressive dilution of Aβ3(pE)-42 (Fig. 3d). Aβ3(pE)-42 can therefore template formation of metastable, cytotoxic LNOs from excess Aβ1–42, yielding potent bioactivity that can be serially passaged multiple times into monomeric Aβ1–42 without further addition of Aβ3(pE)-42.
One possible explanation for why Aβ1–42 LNOs were inert is they lacked sufficient properly sized oligomers. Accordingly, we altered the oligomerization protocol from 5 μM peptide for 24 hours at 37° C to 10 μM peptide for 30 minutes at 4° C to obtain abundant Aβ1–42 oligomers that eluted at 12.5 ml (Fig. 3e). These LNOs were not cytotoxic (Fig. 3f), implying they were structurally distinct from the putative dimers/trimers initiated by Aβ3(pE)-42. This was confirmed by dot blots using M87, a conformation-sensitive anti-Aβ antibody, to compare the putative dimers/trimers used for the cytotoxicity assays shown in Fig. 3f. We first lyophilized aliquots of all the Aβ solutions, resuspended them with hexafluoroisopropanol (HFIP) to restore them to monomers, and then analyzed them using 4G8. When parallel samples that were not lyophilized but were otherwise identical were analyzed using M87, immunoreactivity was ~2X as strong with LNOs made from Aβ1–42 versus those made from 5% pE-Aβ (Supplementary Fig. 7). Cytotoxic LNOs of 5% pE-Aβ are thus structurally distinct from comparably sized LNOs of Aβ1–42.
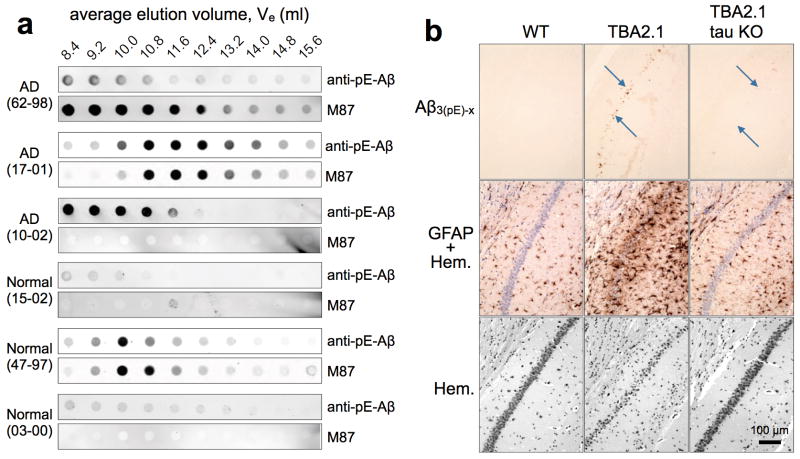
Several lines of evidence demonstrate in vivo relevance for the data described so far. First, we identified LNOs containing Aβ3(pE)-42 in 3 out of 3 AD samples, based on gel filtration of human brain extracts followed by dot blots of resulting fractions with anti-pE-Aβ and M87. In contrast, only 1 of 3 age-matched samples with normal neuropathological diagnoses was positive for Aβ3(pE)-42. (Fig. 4a and Supplementary Fig. 8). Secondly, we crossed TBA2.1 mice18 into a tau KO background19. By 3 months, TBA2.1 mice accumulated small amounts (40–100 ng/g brain weight) of Aβ3(pE)-42, which formed primarily intraneuronal aggregates, and was associated with massive hippocampal neuron loss and gliosis18. Knocking out tau provided almost complete protection against neuron loss and glial activation (Fig. 4b). Additional in vivo data are shown in Supplementary Fig. 9. Long term potentiation (LTP) of mouse hippocampal neurons in slice cultures was potently and equally inhibited by oligomers made from 5% Aβ3(pE)-42 or 100% Aβ3(pE)-42, whereas Aβ1–42 oligomers had no effect on LTP. 1% Aβ3(pE)-42 provoked mild, but statistically insignificant LTP impairment (Supplementary Fig. 9a). To evaluate effects of increased Aβ3(pE)-42 in animal models, we crossed mice with neuron-specific expression of human APP harboring Swedish and London mutations (hAPPSL)20, with mice expressing human QC21. Nine month old double (hAPPSL/hQC) and single (hAPPSL) transgenic mice were indistinguishable in terms of insoluble and soluble Aβx-42 levels, but the double transgenics had ~2-fold more insoluble Aβ3(pE)-42 and ~9-fold more soluble Aβ3(pE)-42 than single transgenics (Supplementary Fig. 9b). Further analysis of the soluble Aβx-42 by the A4 assay22 revealed an ~8-fold excess of oligomers in the double, versus single transgenics (Supplementary Fig. 9c). Double transgenics performed more poorly in Morris water maze tests (Supplementary Fig. 9d) and had reduced hippocampal immunoreactivity for the synapse marker, synaptophysin (Supplementary Fig. 9e). Finally, peri-hippocampal injection of 5% pE-Aβ at 5 μM into APPSwDI/NOS2−/− AD model mice23 led 3–5 months later to the presence of plaques containing both pE-Aβ and conventional Aβ. Comparable plaques were rarely seen in sham injected AD mice or in WT mice injected with 5% pE-Aβ (Supplementary Fig. 9f). These collective in vivo results emphasize the physiological significance of the companion biochemical and cultured cell results.
Figure 4. Aβ3(pE)-42 in vivo.
a, Cytosol obtained from human AD and similarly aged normal brains (Supplementary Fig. 9) were fractionated by gel filtration, and analyzed by dot blotting with anti-pE-Aβ and M87. Note the appearance of pE-Aβ in low-n oligomer fractions, including those that eluted at 12.4 ml, especially in the AD samples. b, 3 month old TBA2.1 mice generating Aβ3(pE-42)18 show Aβ-deposits (arrows), massive astrogliosis (GFAP) and neuron loss (Hem; hematoxylin nuclear staining), none of which are evident in comparably aged WT mice or TBA2.1/tau KO19 hybrids.
Our studies provide new insights into AD pathogenesis by demonstrating that hypertoxic Aβ oligomers can be triggered by small quantities of a specifically truncated and post-translationally modified version of Aβ. Although some previous studies demonstrated that pE modification of Aβ significantly enhances its aggregation kinetics13,14,24, toxicity12,25 and resistance to degradation12, a mechanistic explanation for the unique properties of pE-Aβ has been lacking until now. Prior studies suggest coincident appearance of Aβ3(pE)-42 with development or progression of human AD26,27. Co-localization of QC and Aβ3(pE)-42 was found in cored plaques of vulnerable regions in AD, and evidence was provided for axonal transport of Aβ3(pE)-x from QC-rich neuronal populations of the entorhinal cortex and locus coeruleus28. Since LNOs containing Aβ3(pE)-42 are reasonably stable (Fig. 3a), they might initiate tau-dependent cytotoxicity intracellularly during axonal transport29 or extracellularly following release at remote hippocampal synapses30 of projection neurons28. The Aβ3(pE)-42 induced formation of toxic mixed oligomers provides a rationale for these previous observations, and the tau-dependent cytotoxicity of 5% pE-Aβ establishes a new functional connection between Aβ and tau in AD pathogenesis.
Methods Summary
The online Methods section provides full descriptions of thioflavin T assays, cell culture, cell viability assays, procedures for oligomerization of Aβ peptides and their fractionation by gel filtration chromatography, production and specificity of rabbit monoclonal anti-Aβ antibodies, immunoprecipitation, dot blots and western blots, generation of hAPPSL/hQC transgenic mice, LTP measurements of mouse hippocampal slice cultures, peri-hippocampal injection of 5% pE-Aβ into AD model mice, cultured cell and brain immunohistochemistry, and collection of human brain extracts.
Supplementary Material
1
Acknowledgments
The authors are grateful for support from the following sources: the Alzheimer’s Association (grant 4079 to GSB); the Owens Family Foundation (GSB); the Cure Alzheimer’s Fund (GSB, CGG); NIH/NIGMS training grant T32 GM008136, which funded part of JMN’s Ph.D. training; NIH/NIA grant R01 AG033069 (CGG); and the German Federal Department of Science and Technology grant 03IS2211F (HUD). We also thank Drs. Hana Dawson and Michael Vitek of Duke University for providing the tau KO mice. This work fulfilled part of the requirements for the Ph.D. earned by JMN at the University of Virginia. The technical assistance of Hans-Henning Ludwig, Eike Scheel and Katrin Schulz is gratefully acknowledged.
Footnotes
Full Methods and relevant references will be available in the online Supplementary Information accompanying this paper at http://www.nature.com/nature.
Author Contributions: J.M.N. performed most of the biochemical and cell biological experiments; S.S. was the principal force behind the experiments involving hAPPSL/hQC and TBA2.1/tau KO mice, and was aided by B.H.-P., H.C.; A.S. and T.W. fractionated and analyzed human brain extracts; E.S., K.Y. and B.W. performed the peri-hippocampal injection experiments; A.H. and C.G.G. produced and characterized the M64 and M87 antibodies; R.R. and K.R. performed the electrophysiology experiments; A.A., W.J. and S.G. performed and analyzed the immunohistochemical experiments on TBA2.1 and Tau-KO/TBA2.1 mice; G.S.B. and H.-U.D. initiated and directed the project; G.S.B. was the principal writer of the paper; all of the authors participated in the design and analysis of experiments, and in editing of the paper.
References
- 1.Gandy S, et al. Days to criterion as an indicator of toxicity associated with human Alzheimer amyloid-beta oligomers. Ann Neurol. 2010;68:220–2302. doi: 10.1002/ana.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007;101:1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 3.Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A. Tau is essential to β-amyloid-induced neurotoxicity. Proc Natl Acad Sci USA. 2002;99:6364–6369. doi: 10.1073/pnas.092136199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King ME, et al. Tau-dependent microtubule disassembly initiated by pre-fibrillar β-amyloid. J Cell Biol. 2006;175:541–546. doi: 10.1083/jcb.200605187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberson ED, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer’s disease mouse model. Science. 2007;316:750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- 6.Jin M, et al. Soluble amyloid {beta}-protein dimers isolated from Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic degeneration. Proc Natl Acad Sci USA. 2011;108:5819–5824. doi: 10.1073/pnas.1017033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori H, Takio K, Ogawara M, Selkoe DJ. Mass spectrometry of purified amyloid beta protein in Alzheimer’s disease. J Biol Chem. 1992;267:17082–17086. [PubMed] [Google Scholar]
- 8.Saido TC, et al. Dominant and differential deposition of distinct beta-amyloid peptide species, A beta N3(pE), in senile plaques. Neuron. 1995;14:457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- 9.Jawhar S, Wirths O, Bayer TA. Pyroglutamate amyloid-beta (Abeta): a hatchet man in Alzheimer disease. J Biol Chem. 2011;286:38825–38832. doi: 10.1074/jbc.R111.288308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schilling S, et al. Glutaminyl cyclase inhibition attenuates pyroglutamate Abeta and Alzheimer’s disease-like pathology. Nature Med. 2008;14:1106–1111. doi: 10.1038/nm.1872. [DOI] [PubMed] [Google Scholar]
- 11.Tabaton M, et al. Soluble amyloid beta-protein is a marker of Alzheimer amyloid in brain but not in cerebrospinal fluid. Biochem Biophys Res Commun. 1994;200:1598–1603. doi: 10.1006/bbrc.1994.1634. [DOI] [PubMed] [Google Scholar]
- 12.Russo C, et al. Pyroglutamate-modified amyloid beta-peptides--AbetaN3(pE)--strongly affect cultured neuron and astrocyte survival. J Neurochem. 2002;82:1480–1489. doi: 10.1046/j.1471-4159.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- 13.Schilling S, et al. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro) Biochem. 2006;45:12393–12399. doi: 10.1021/bi0612667. [DOI] [PubMed] [Google Scholar]
- 14.Schlenzig D, et al. Pyroglutamate formation influences solubility and amyloidogenicity of amyloid peptides. Biochem. 2009;48:7072–7078. doi: 10.1021/bi900818a. [DOI] [PubMed] [Google Scholar]
- 15.LeVine H., 3rd Thioflavine T interaction with synthetic Alzheimer’s disease beta-amyloid peptides: detection of amyloid aggregation in solution. Protein Sci. 1993;2:404–410. doi: 10.1002/pro.5560020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XM, et al. A new microcellular cytotoxicity test based on calcein AM release. Human Immunol. 1993;37:264–270. doi: 10.1016/0198-8859(93)90510-8. [DOI] [PubMed] [Google Scholar]
- 17.Scudiero DA, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–4833. [PubMed] [Google Scholar]
- 18.Alexandru A, et al. Selective hippocampal neurodegeneration in transgenic mice expressing small amounts of truncated Abeta is induced by pyroglutamate-Abeta formation. J Neurosci. 2011;31:12790–12801. doi: 10.1523/JNEUROSCI.1794-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nature Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- 20.Rockenstein E, Mallory M, Mante M, Sisk A, Masliaha E. Early formation of mature amyloid-beta protein deposits in a mutant APP transgenic model depends on levels of Abeta(1–42) J Neurosci Res. 2001;66:573–582. doi: 10.1002/jnr.1247. [DOI] [PubMed] [Google Scholar]
- 21.Jawhar S, et al. Overexpression of glutaminyl cyclase, the enzyme responsible for pyroglutamate A{beta} formation, induces behavioral deficits, and glutaminyl cyclase knock-out rescues the behavioral phenotype in 5XFAD mice. J Biol Chem. 2011;286:4454–4460. doi: 10.1074/jbc.M110.185819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanghe A, et al. Pathological Hallmarks, Clinical Parallels, and Value for Drug Testing in Alzheimer’s Disease of the APP[V717I] London Transgenic Mouse Model. Inter J Alzheimer’s Dis. 2010;2010 doi: 10.4061/2010/417314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilcock DM, et al. Progression of amyloid pathology to Alzheimer’s disease pathology in an amyloid precursor protein transgenic mouse model by removal of nitric oxide synthase 2. J Neurosci. 2008;28:1537–1545. doi: 10.1523/JNEUROSCI.5066-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W, Barrow CJ. The A beta 3-pyroglutamyl and 11-pyroglutamyl peptides found in senile plaque have greater beta-sheet forming and aggregation propensities in vitro than full-length A beta. Biochem. 1999;38:10871–10877. doi: 10.1021/bi990563r. [DOI] [PubMed] [Google Scholar]
- 25.Wirths O, et al. Intraneuronal pyroglutamate-Abeta 3–42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol. 2009;118:487–496. doi: 10.1007/s00401-009-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guntert A, Dobeli H, Bohrmann B. High sensitivity analysis of amyloid-beta peptide composition in amyloid deposits from human and PS2APP mouse brain. Neurosci. 2006;143:461–475. doi: 10.1016/j.neuroscience.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 27.Piccini A, et al. beta-amyloid is different in normal aging and in Alzheimer disease. The J Biol Chem. 2005;280:34186–34192. doi: 10.1074/jbc.M501694200. [DOI] [PubMed] [Google Scholar]
- 28.Hartlage-Rubsamen M, et al. Glutaminyl cyclase contributes to the formation of focal and diffuse pyroglutamate (pGlu)-Abeta deposits in hippocampus via distinct cellular mechanisms. Acta Neuropathol. 2011;121:705–719. doi: 10.1007/s00401-011-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vossel KA, et al. Tau reduction prevents Abeta-induced defects in axonal transport. Science. 2010;330:198. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilcox KC, Lacor PN, Pitt J, Klein WL. Abeta oligomer-induced synapse degeneration in Alzheimer’s disease. Cell Mol Neurobiol. 2011;31:939–948. doi: 10.1007/s10571-011-9691-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
1