Stem cell therapy for cerebral ischemia: from basic science to clinical applications (original) (raw)
Abstract
Recent stem cell technology provides a strong therapeutic potential not only for acute ischemic stroke but also for chronic progressive neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis with neuroregenerative neural cell replenishment and replacement. In addition to resident neural stem cell activation in the brain by neurotrophic factors, bone marrow stem cells (BMSCs) can be mobilized by granulocyte-colony stimulating factor for homing into the brain for both neurorepair and neuroregeneration in acute stroke and neurodegenerative diseases in both basic science and clinical settings. Exogenous stem cell transplantation is also emerging into a clinical scene from bench side experiments. Early clinical trials of intravenous transplantation of autologous BMSCs are showing safe and effective results in stroke patients. Further basic sciences of stem cell therapy on a neurovascular unit and neuroregeneration, and further clinical advancements on scaffold technology for supporting stem cells and stem cell tracking technology such as magnetic resonance imaging, single photon emission tomography or optical imaging with near-infrared could allow stem cell therapy to be applied in daily clinical applications in the near future.
Keywords: cerebral ischemia, neuroregeneration, neurorepair, stem cell therapy
Despite numerous studies and active challenges for the treatment of major neurologic diseases such as ischemic stroke, Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and multiple sclerosis, only a few treatments have ameliorated the neurologic symptoms with conventional symptomatic therapies for neurorepair. Among various organs, brain is the most sensitive organ to various injuries such as ischemia, hypoglycemia, infection/inflammation, trauma, aging, and degeneration. In the brain, neurons are particularly sensitive to such injuries, and the vulnerability is different even within the neuronal populations (Abe et al, 1991, 1995; Abe and Kogure, 1993). These vulnerabilities of neurons make it difficult to cure patients suffered from the above diseases in clinical settings.
However, the stem cell approach could provide an alternative choice for neuroregeneration and disease-modifying therapy. For neuroregenerative therapy, the activation of intrinsic neural stem cells (NSCs) or the exogenous transplantation of NSCs/neural progenitor cells (NPCs) can be applied (Abe, 2000; Iwai et al, 2003). To support stem cell migration, an artificial scaffold can be implanted into the injured brain for promoting ischemic brain repair and regeneration (Deguchi et al, 2006). The addition of neurotrophic factors greatly enhanced the intrinsic migration or invasion of stem cells into the scaffold, which could provide a future regenerative potential against ischemic brain damage at the chronic stage (Zhang et al, 2008). Especially, granulocyte-colony stimulating factor (G-CSF) is regarded as a promising drug candidate which can reduce neuroinflammation and potentiate both neurogenesis and angiogenesis after ischemic stroke by promoting bone marrow (BM) cell migration into the ischemic brain (Sehara et al, 2007_a_, 2007_b_). Recent studies have demonstrated that cord blood mononuclear cells, BM mononuclear cells, and BM stromal cells (BMSCs) can survive in postischemia tissue, and reduce neuronal damage when transplanted into rodents subjected to cerebral infarction (Brenneman et al, 2010; Chen et al, 2006; Hokari et al, 2008; Prockop et al, 2003). Recent studies suggest an important interaction between neuronal cells and vascular component as neurovascular unit and a potential therapeutic target for ischemic stroke, Alzheimer's disease, and amyotrophic lateral sclerosis (del Zoppo, 2009; Kurata et al, 2011; Miyazaki et al, 2011; Yamashita et al, 2009; Zlokovic, 2010). In addition to necrosis and apoptosis, additional neuronal cell death mechanisms such as autophagy and transcriptional repression-induced atypical death have recently been pointed out as important for the therapeutic approach of both neurorepair and neuroregeneration (Morimoto et al, 2009; Tian et al, 2011). Potential therapeutic benefits of stem cell therapy on these neurovascular units and additional cell death mechanisms are future topics to be studied. The purpose of this review is to summarize the current progress of basic stem cell science and its early clinical applications for advanced stem cell therapy.
Part 1. Intrinsic neurogenesis and exogenic stem cell transplantation
Intrinsic Neural Stem Cells
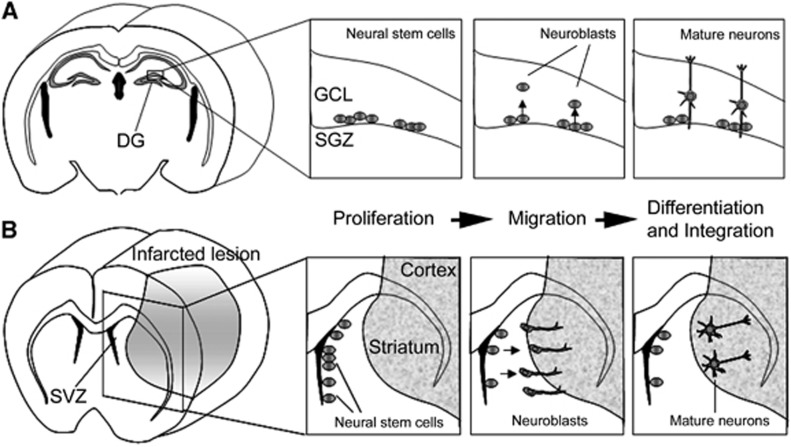
To supply new neurons into the infarcted brain, two tactics are proposed. One is the transplantation of extrinsic NSCs/NPCs derived from stem cells such as embryonic stem (ES) cells and induced pluripotent stem (iPS) cells. The other is the activation of intrinsic NSCs. It was already reported that persistent neurogenesis occurs in two restricted regions of the adult mammalian brain: the subgranular zone (SGZ) of the hippocampal dentate gyrus (Gage, 2000) and the subventricular zone (SVZ) of the lateral ventricle (Alvarez-Buylla and Garcia-Verdugo, 2002). In the SGZ, newly born neurons migrate into the granule cell layer and integrate into the existing neuronal network. In the SVZ, which is a thin cell layer in the lateral walls of lateral ventricles, NSCs continuously give rise to NPCs migrating into the olfactory bulb (Doetsch et al, 1999). To know whether the ischemic condition affects endogenous neurogenesis, we studied the temporal profile of NSC division, migration, and differentiation in the SGZ and the SVZ by using the transient forebrain ischemia gerbil model. We found that the ischemic condition increased the division of NSCs of the SGZ with a peak 10 days after ischemic induction, following which cells migrated into the granule cell layer and differentiated mainly into neuronal cells (Iwai et al, 2002; Figure 1A). Furthermore, we also found that transient forebrain ischemia enhances NSC proliferation in the SVZ with a peak 10 days after ischemia, leading to the migration of more NPCs into the olfactory bulb (Iwai et al, 2003). These results suggest that forebrain ischemia increased NSC number and resulted in increased neurogenesis, mostly in the two restricted lesions, the SGZ and the SVZ.
Figure 1.
The stage of endogenous neurogenesis can be divided into three steps: proliferation, migration, and differentiation. (A) Neural stem cells proliferate at the subgranular zone (SGZ), and the cells then migrate into the granule cell layer (GCL) and differentiate mainly into neuronal cells in the postischemic dentate gyrus (DG) of gerbils. (B) Neural stem cells proliferate at the subventricular zone (SVZ), and a sub-population of these cells can migrate toward the infarcted lesion, and finally differentiate into mature neurons, which can be integrated into the neighboring neuronal network.
Many researchers reported that newly born neurons can be found in the postinfarcted lesion including the striatum and cortex in another animal model, the transient focal ischemia model (Arvidsson et al, 2002; Teramoto et al, 2003), which is closer to the pathophysiology of human cardioembolic stroke. To clarify whether SVZ NSCs supply new neurons to areas injured by ischemia, several study groups have performed region-specific cell labeling and long-term tracing experiments. Subventricular zone-derived NPCs were also reported to migrate toward the injured striatum after middle cerebral artery occlusion. A long-term tracing study showed that the SVZ-derived NPCs differentiated into mature neurons in the striatum, in which they formed synapses with neighboring striatal cells (Yamashita et al, 2006; Figure 1B), implying that the SVZ has an important role as a cell source supplying newborn neurons to brain lesions damaged by focal ischemia. Recently, NPCs supplying GABAergic neurons were found even in the neocortical layer 1 of adult rats, and their proliferation was highly activated in the ischemic condition (Ohira et al, 2010).
In the postischemic brain, newly born neurons can be supplied from the SVZ, the SGZ, and the neocortical layer, but the number is too small for recovery of neurologic functions. For example, newly born neurons could replace only 0.2% of the dead striatal neurons even in the rat middle cerebral artery occlusion model (Arvidsson et al, 2002). Appropriate interventions need to be added to enhance the proliferation, survival, and neuronal maturation of intrinsic NSCs and their progeny, so as to use their intrinsic neural cell source for therapeutic purposes.
Cell Transplantation Therapy with Embryonic Stem, Induced Pluripotent Stem, and Induced Neuronal Cells
Human ES (hES) cells were first generated from the inner cell mass of blastocysts in 1998 (Thomson et al, 1998), and human-induced pluripotent stem (hiPS) cells were established by introducing four transcriptional factors (Oct3/4, Sox2, Klf4, and c-Myc) into human skin fibroblasts in 2007 (Takahashi et al, 2007). Both hES and hiPS cells are known as multipotent stem cells with pluripotency and high replication competence. Daadi et al (2009) transplanted NSCs derived from hES cells into the poststroke rat brain, and showed that transplanted cells could differentiate into neurons, oligodendrocytes, and astrocytes. Neural progenitor cells derived from murine or monkey ES cells were also reported to survive in stroke lesions of brain, and differentiated into mature neurons (Buhnemann et al, 2006; Hayashi et al, 2006). These results indicate that ES cells can be a promising cell source, but human fertilized eggs are needed to establish each hES cell line. This ethical problem interferes with the clinical application of hES cells. However, hiPS cells can be produced from each patient's skin fibroblasts, implying that iPS cells do not possess the same ethical barrier. In addition, many researchers have reported that hiPS cells could be differentiated into various kinds of neurons including glutaminergic, motor, and dopaminergic neurons (Table 1). It has also been reported that hNPCs derived from iPS cells could be transplanted into the murine brain, and survive as mature neurons (Chen et al, 2010). Therefore, hiPS cells are now regarded as a promising cell source for cell transplantation therapy to supply new neurons to repair a neuronal network disrupted by various kinds of neuronal diseases, including ischemic stroke.
Table 1. Scientific reports showing that each specific type of neuron could be generated from human skin fibroblasts.
| Induced neuron | How to induce? | Contents | Reference |
|---|---|---|---|
| Dopaminergic neuron | via iPS | Sporadic Parkinson's disease patient-derived iPS cells could differentiate into dopaminergic neurons | Soldner et al (2009) |
| Dopaminergic neuron | via iPS | Human iPS cells could differentiate into progenitors of a dopaminergic neuron. Their progenitors could survive and differentiate into mature dopaminergic neurons in 6-OHDA-treated rat striatum | Cai et al (2010) |
| Dopaminergic neuron | via iPS | Parkinson's disease patient-derived iPS cells could differentiate into a dopaminergic neuron. The dopaminergic neurons could survive and improve motor dysfunction in 6-OHDA-treated rats | Hargus et al (2010) |
| Dopaminergic neuron | Direct conversion | Functional dopaminergic neurons could be generated from human fibroblasts with Ascl1, Nurr1, and Lmx1a | Caiazzo et al (2011) |
| Glutamatergic neuron | via iPS | Human iPS cells could differentiate into glutamatergic neurons. Their cells showed typical ion channels and action potentials | Zeng et al (2010) |
| Glutamatergic neuron | Direct conversion | Functional glutaminergic neurons could be generated from human fibroblasts with Ascl1, Brn2, Myt1l, and NeuroD | Pang et al (2011) |
| Glutamatergic neuron | Direct conversion | Functional glutaminergic neurons could be generated from familial Alzheimer's disease patient skin fibroblasts. These cells showed selective deficits in vitro | Qiang et al (2011) |
| Motor neuron | via iPS | Spinal muscular atrophy patient-derived iPS cells could differentiate into motor neurons. These cells showed selective deficits in vitro | Ebert et al (2009) |
| Motor neuron | via iPS | Human iPS cells generate electrically active motor neurons in vitro | Karumbayaram et al (2009) |
To achieve iPS cell therapy in a clinical setting, iPS cells tumorigenicity is a critical problem that clearly needs to be overcome. Germline-competent chimera mice with iPS cells developed tumors in which the integrated exogenous c-Myc gene was reactivated. Attempts were made to establish iPS cells without c-Myc, but the induction ratio of iPS cells was significantly reduced (Nakagawa et al, 2008). Another research group also reported that Yamanaka four transcriptional factors, which are integrated into the genome by retrovirus vectors, can express in iPS-derived cells, alter their characteristics and also induce tumorigenesis (Soldner et al, 2009). We then compared the tumorigenicity of two different iPS cell lines established with or without retrovirus vectors, by transplanting into the intact or the ischemic murine brain. When iPS cells are virus free, there is no significant difference in tumor volume between the intact and the ischemic group (Yamashita et al, 2011). However, virus-induced iPS cells in the ischemic brain formed significantly larger teratomas than those in the intact brain (Kawai et al, 2010). These results suggest that integrated transcriptional factors might affect cell characteristics and enhance the outgrowth of transplanted iPS cells under the ischemic condition. In addition, secondary neurospheres from iPS cells also formed a teratoma in mouse brains at a constant rate (Miura et al, 2009), where a small number of undifferentiated iPS cells were suspected to be in a pluriopotent state even after a differentiation assay, and formed a teratoma. To realize safe cell transplantation therapy with iPS cells, we have to use an iPS cell line without exogenous gene integration, and confirm that there are no undifferentiated cells left. Moreover, we have to develop novel methodology to control and monitor neuronal differentiation and integration of transplanted cells in stroke-lesion areas. In addition, Zhao et al (2011) reported that transplanted undifferentiated iPS cells induced a T cell-dependent immune response even in syngenic mice, suggesting that immunogenicity can occur in iPS cells derived from each patient. However, in that study, the authors used only undifferentiated iPS cells for cell transplantation, which would never be used for a clinical setting. Therefore, it remains obscure whether differentiated cells derived from iPS cells can induce immune rejections, but the immunogenicity of used cells should be carefully evaluated before clinical application. Recently, functional dopaminergic or glutaminergic neurons have been reported to be directly converted from human skin fibroblasts without the need for passing through a pluripotent state (Table 1). Their cells were named as induced neuronal cells and may be safer with low tumorigenicity, compared with iPS cells. Much attention is now being paid to see whether the transplantation of induced neuronal cells can show a therapeutic effect without tumor formation or immune rejections in disease models. In addition, a clinical trial with ReN001, a genetically engineered NSC line, with chronic stroke patients has already started. This PISCES study (Pilot Investigation of Stem Cells in Stroke) is the first clinical trial of an NSC therapy for stroke patients, and its results will also draw attention (Mack, 2011).
Part 2. Granulocyte-colony stimulating factor in basic and clinical sciences
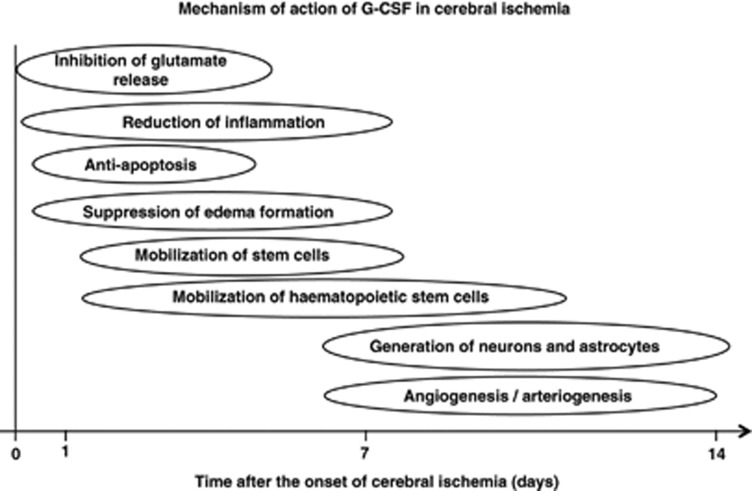
Research on stem and progenitor cells has the potential to yield new treatments for ischemic stroke, but transplantation of these cells faces a variety of problems, such as infection, rejection, and risk of malignancy, and there are also ethical and political issues (Lo and Parham, 2009). Granulocyte-colony stimulating factor, which is in widespread clinical use for treatment of chemotherapy-associated neutropenia (Cavallaro et al, 2000), is a new candidate for neuroprotection and neuroregeneration. As its profiles of pharmacological and adverse effects are well known, clinical application of G-CSF is expected to be straightforward, compared with stem/progenitor cell therapy. This review focuses on the neuroprotective and neuroregenerative properties of G-CSF in experimental ischemic models (Figure 2). The evidence from current clinical trials is also reviewed.
Figure 2.
Mechanism of action of granulocyte-colony stimulating factor (G-CSF) in cerebral ischemia.
Neuroprotective Mechanisms of Granulocyte-Colony Stimulating Factor in Cerebral Ischemia
It is well established that G-CSF reduces infarct volume in experimental cerebral ischemia (Schäbitz et al, 2003; Schneider et al, 2005; Six et al, 2003). However, most studies assessed transient rather than permanent ischemia, and short-term rather than prolonged ischemia (England et al, 2009). In the acute phase of cerebral ischemia, the neuroprotective mechanisms of G-CSF include inhibition of glutamate release, reduction of inflammation, antiapoptosis activity, and suppression of edema formation.
Ischemia causes impairment of brain energy metabolism, with the release of excessive amounts of glutamate into the extracellular space. Granulocyte-colony stimulating factor can attenuate the release of extracellular glutamate in transient focal ischemia (Han et al, 2008). Further, G-CSF has a direct protective effect against glutamate-induced excitotoxicity in cultured neurons (Schäbitz et al, 2003). Both G-CSF and G-CSF receptors are widely expressed by neurons in the central nervous system (CNS), and their expression is upregulated in ischemia, suggesting the involvement of an autocrine protective signaling mechanism (Schneider et al, 2005). At 6 hours after cerebral ischemia, endogenously released G-CSF is presumably active on the upregulated G-CSF receptor in the penumbra, and may provide protection against apoptotic cell death of neurons. After interaction with G-CSF receptor through JAK signaling, G-CSF activates several antiapoptotic pathways involving signal transducer and activation of transcription-3, extracellular signal-regulated kinase, and phophatidylinositol 3-kinase-Akt (Schneider et al, 2005). Furthermore, G-CSF upregulates Bcl-2 and Pim-1 expression, and downregulates cytochrome c reductase translocation to the cytosol, Bax translocation to mitochondria, and the level of cleaved caspase-3 in neurons (Solaroglu et al, 2006).
Microglia/macrophages are considered to be major sources of inflammatory cytokines in the ischemic brain. Granulocyte-colony stimulating factor decreased the migration of BM-derived monocytes/macrophages into the peri-infarct and core regions (Komine-Kobayashi et al, 2006; Lee et al, 2005). It also suppressed inducible nitric oxide synthase activity and nitrotyrosine expression (Komine-Kobayashi et al, 2006). At 24 and 72 hours after transient focal ischemia, G-CSF also reduced expression of tumor necrosis factor-α, transforming growth factor-β, and inducible nitric oxide synthase in the peri-infarct region (Sehara et al, 2007a). Recently, Gibson et al reported that administration of G-CSF during reperfusion reduced motor deficit and neuronal loss in inducible nitric oxide synthase gene-deficient mice, suggesting that the mechanism is partly independent of inducible nitric oxide synthase, perhaps involving decreased interleukin-1_β_ expression (Gibson et al, 2005, 2010; Solaroglu et al, 2009). In addition, G-CSF can suppress edema formation by decreasing matrix metalloproteinase 9, blood–brain barrier breakdown, and tissue injury in acute stroke (Sevimli et al, 2009).
In contrast, an increased amount of peripheral neutrophils may result in aggregate formation in cerebral microvasculature, leading to a breakdown of blood flow, and may worsen brain damage (del Zoppo and Mabuchi, 2003). A negative effect of G-CSF on outcome, associated with enhanced brain atrophy and an exaggerated inflammatory response, was also reported in permanent cerebral infarction (Taguchi et al, 2007). Although administration of G-CSF was accompanied by a significant increase of circulating neutrophils 2 days after ischemia, leukocytosis was restricted to the vessel compartment with no elevation of intraparenchymal neutrophil count, and there was no deleterious effect on lesion formation or functional recovery (Strecker et al, 2010).
Neuroregenerative Mechanisms of Granulocyte-Colony Stimulating Factor in Cerebral Ischemia
In the subacute phase (and partly also the chronic phase) of cerebral ischemia, neuroprotective mechanisms include the mobilization of hematopoietic stem cells, generation of neurons and astrocytes, angiogenesis and arteriogenesis.
Granulocyte-colony stimulating factor mobilizes stem cells from BM to circulating blood. CD34+ hematopoietic stem cells from BM migrated into the injured brain when administered intracerebrally or intravenously (Sykova and Jendelova, 2005). It improves the recovery of motor function in spinal cord injury models (Urdzikova et al, 2006). Piao et al (2009) reported that numerous BM-derived cells migrated into the brain parenchyma when G-CSF+ stem cell factor (SCF) was applied 16 weeks after ischemia. However, G-CSF+SCF treatment in the subacute phase predominantly increased BM-derived microglia, and slightly increased neuronal and endothelial cells, in the peri-infarct region (Kawada et al, 2006). The migration of BM-derived monocytes/macrophages was slightly reduced in the peri-infarct region after G-CSF treatment (Komine-Kobayashi et al, 2006). Thus, the capacity of BM-derived cells to restore function in the injured brain has been demonstrated, but there is some doubt as to whether mobilization of stem cells is the main neuroprotective mechanism of G-CSF.
Cerebral ischemia induces the generation of new neurons, which may potentially be used to restore brain function, from precursor cells. Administration of G-CSF immediately after middle cerebral artery occlusion increased newly generated neurons in the SGZ (Schneider et al, 2005; Sehara et al, 2007_b_). Administration of G-CSF+SCF in the subacute phase (days 11 to 20) after middle cerebral artery occlusion significantly stimulated proliferation of intrinsic NSCs/NPCs in the rostral SVZ, compared with administration in the acute phase (days 1 to 10) (Kawada et al, 2006). The subacute-phase treatment elevated the expression of IL-10 mRNA and antiinflammatory cytokines at 14 days after occlusion, suggesting that hematopoietic cytokine treatment in the subacute phase may provide a favorable microenvironment for neurogenesis (Morita et al, 2007). Even in the chronic phase of ischemia, significant functional improvement was seen at 1, 5, and 17 weeks after SCF+G-CSF treatment (Zhao et al, 2007). Thus, proliferation of intrinsic NSCs/NPCs is considered to be the main protective role of G-CSF rather than mobilization of stem cells.
Angiogenesis is a process in which new vessels arise from preexisting ones (Pandya et al, 2006). Lee et al (2005) found that G-CSF enhanced angiogenesis in stroke, measured in terms of endothelial cell proliferation, vascular surface area, number of branch points, and vascular length. Granulocyte-colony stimulating factor-induced angiogenesis may be caused by direct activation of brain endothelial cells (Bussolino et al, 1991) and mobilization of endothelial progenitor cells to the ischemic boundary zone. The effect of G-CSF was more pronounced when treatment was initiated earlier, but it remained even when treatment was delayed up to 7 days after ischemia (Lee et al, 2005). The G-CSF+SCF treatment during chronic stroke also elevated BM-derived endothelial cells (Piao et al, 2009), suggesting a prolonged therapeutic time window.
Arteriogenesis is a process in which preexisting collateral arterioles transform into functional collateral arteries. Granulocyte-colony stimulating factor promoted leptomeningeal collateral growth after common carotid artery occlusion, and increased circulating blood monocytes and Mac-2-positive cells, suggesting that mechanisms coupled to monocyte upregulation might be involved (Sugiyama et al, 2011_c_).
Current Clinical Trials of Granulocyte-Colony Stimulating Factor
There have been several clinical studies of G-CSF, as shown in Table 2. Shyu et al (2006) conducted a trial involving 10 patients with acute cerebral infarction using subcutaneous G-CSF injections (15 _μ_g/kg per day) for 5 days. They found no severe adverse effects, and there was a greater improvement in neurologic function in the G-CSF group than in the control group. Sprigg et al (2006) performed a dose-escalation, placebo-controlled trial of G-CSF (1 to 10 _μ_g/kg subcutaneously, 1 or 5 daily doses) in 36 patients with ischemic stroke (7 to 30 days after the onset). Granulocyte-colony stimulating factor increased CD34+ count in a dose-dependent manner, and appeared to be safe and well tolerated. Schäbitz et al (2010) performed a placebo-controlled dose-escalation study using 4 intravenous dose regimens (30 to 180 _μ_g/kg over the course of 3 days) of G-CSF in 44 stroke patients (within 12 hours after onset). They observed no serious adverse events, although the maximum leukocyte count reached around 80,000/_μ_L. There was no significant difference in the clinical outcome between treatment versus placebo, and there was a beneficial effect in patients with diffusion-weighted image (DWI) lesions >14 to 17 cm3. In chronic stroke patients (>4 months), subcutaneous G-CSF treatment (10 _μ_g/kg body weight/day for 10 days) was safe and reasonably well tolerated, although no primary efficacy was detected (Floel et al, 2011). Thus, although the feasibility and safety of G-CSF have been confirmed, its efficacy remains unproved.
Table 2. Current clinical trials of G-CSF for ischemic stroke.
| | Shyu et al (2006) | Sprigg et al (2006) | Schäbitz et al (2010) | Floel et al 2011 | | | -------------------------------- | ------------------------------------------------------------------------------------------------------------------------------------------------------------ | ------------------------------------------------------------------------------------------------------------------------------------------------------------------------- | ---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- | ----------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- | | Patients | 10 patients (G-CSF: _n_=7, placebo: _n_=3) | 36 patients (G-CSF: _n_=24, placebo: _n_=12) | 44 patients (G-CSF: _n_=30, placebo: _n_=14) | 41 patients (G-CSF: _n_=21 placebo: _n_=20) | | Methods | G-CSF 15 _μ_g/kg subcutaneously, >5 days | G-CSF 1–10 _μ_g/kg subcutaneously, 1 or 5 daily doses | G-CSF 30–180 _μ_g/kg drip intravenous injection over the course of 3 days | G-CSF 10 _μ_g/kg subcutaneously >10 days | | Time to medication | Within 7 days | 7–30 Days | <12 Hours | >4 Months | | Test points | 12 Months | 90 Days | 90 Days | 38 Days | | Results | No severe adverse effects. Greater improvement in neurological function between baseline and 12-month follow-up in the G-CSF group than in the control group | (1) G-CSF (5 days of 10 _μ_g/kg) increased CD34+ count dose dependently. (2) No difference between treatment groups in the number of patients with serious adverse events | (1) G-CSF treatment for acute stroke is safe even at high dosages (2) No significant difference in outcome between treatment and placebo groups (3) Dose-dependent beneficial effect of G-CSF in patients with diffusion-weighted image lesions >14–17 cm3 | (1) Adverse events were more frequent in G-CSF group, but were generally graded mild or moderate (2) Leukocyte count rose after day 2 of G-CSF dosing, reached a maximum on day 8, and returned to baseline 1 week after treatment cessation (3) No significant effect was detected at primary efficacy end point |
In conclusion, it is unclear whether G-CSF has a neuroprotective or neuroregenerative effect in stroke patients. To clarify the optimal time and dose of G-CSF administration, further clinical studies with a larger number of patients enrolled, uniform infarct size, similar stroke subtype and background factors, lower extent of leukocytosis, among other factors, will be needed.
Part 3. Basic and translational aspects of bone marrow stromal cell transplantation for ischemic stroke
Basic Aspects of Bone Marrow Stromal Cell Transplantation
Although a huge number of preclinical and clinical tests were performed over these past 50 years, there are few drugs that are effective to protect or repair the damaged CNS due to ischemic stroke (Savitz and Fisher, 2007). However, recent decade studies have strongly suggested that cell transplantation therapy may potentially promote functional recovery after various kinds of CNS disorders, including cerebral infarct. A variety of cell types have been studied as cell source of transplantation into animal models of CNS disorders, including ES cells, NSCs, iPS cells, and BMSCs. Of these, BMSCs may have the most promising potential because they can be harvested from patients without posing ethical or immunological difficulties (Bliss et al, 2007; Parr et al, 2007). Bone marrow stromal cells are known to support the homing and proliferation of hematopoietic cells in the BM (Kortesidis et al, 2005; Uccelli et al, 2011). They differentiate into fat, bone, and cartilage, but can also transdifferentiate into embryological unrelated tissues, including neural cells (Sanchez-Ramos et al, 2000; Uccelli et al, 2011; Woodbury et al, 2000).
There is increasing evidence that transplanted BMSCs aggressively migrate toward the damaged CNS tissue and promote the recovery of motor function after cerebral infarct. Recent studies have shown that BMSCs also improve cognitive function due to chronic cerebral ischemia (Shichinohe et al, 2010). Now, they are considered to provoke these beneficial effects through their differentiation into neural cells and production of various kinds of cytokines or growth factors that can rescue the host neurons (Kuroda et al, 2011; Prockop et al, 2003). Thus, BMSCs per se express the gene related to neuronal and glial cells (Nandoe Tewarie et al, 2011; Yamaguchi et al, 2006). They can also modify their gene expression profile under certain experimental conditions (Yamaguchi et al, 2006) and differentiate into the neurons without evidence of cell fusion (Hokari et al, 2008). They may also produce some neuroprotective or neurotrophic factors and support the survival of the host neural cells (Zhong et al, 2003). The conditioned medium of BMSCs significantly promotes neurite outgrowth from the dorsal root ganglion (Neuhuber et al, 2005). When BMSCs are cocultured with the neurons exposed to an excitotoxic amino acid, glutamate, they significantly increases their release of soluble neuroprotective factors such as nerve growth factor and brain-derived neurotrophic factor, and ameliorate neuronal injury (Hokari et al, 2008). They markedly promote extension of neurites from neurons in organotypic slices of the brain and spinal cord (Kamei et al, 2007; Shichinohe et al, 2008). According to these observations, BMSCs may consist of heterogeneous cell populations and protect and repair the damaged CNS through multiple mechanisms (Hokari et al, 2008). Interestingly, Uccelli et al (2008) proposed that BMSCs demonstrate bystander mechanisms in the CNS; they can rescue the neurons and promote the proliferation and maturation of local neural precursors through the release of trophic molecules but, however, they can have antiinflammatory and antiproliferative effects on microglia and astrocytes, providing a neuroprotective microenvironment.
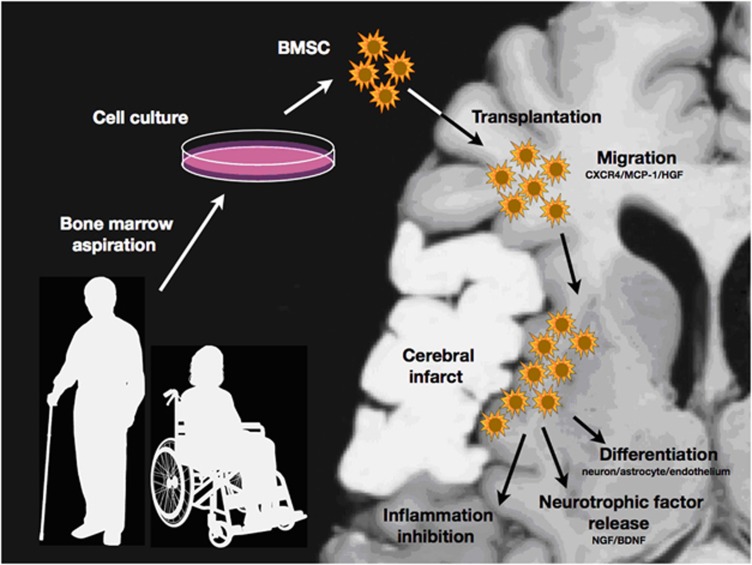
Recent in-vivo studies have gradually elucidated their behaviors in the infarcted brain. Thus, engrafted BMSCs maintain their aggressive proliferation property even after intracerebral transplantation into the infarcted brain (Yano et al, 2005). They migrate toward the infarcted tissue by chemokine systems such as monocyte chemoattractant protein-1, stromal cell-derived factor-1_α_, and hepatocyte growth factor (Shang et al, 2011; Shichinohe et al, 2007; Son et al, 2006; Wang et al, 2002). Numerous experimental studies have proven that engrafted BMSCs express the proteins specific for neurons, astrocytes, and endothelial cells in the peri-infarct area (Ito et al, 2011; Lee et al, 2003; Osanai et al, 2011; Shen et al, 2007; Shichinohe et al, 2004; Sugiyama et al, 2011_a_, 2011_b_). Alternatively, they may support the survival of the host neurons through their paracrine production of soluble factors (see above). However, it remains to be defined how the engrafted cells contribute to functional recovery after cerebral infarct. A recent study has shown that the engrafted BMSCs express GABA (_γ_-aminobutyric acid) receptor and improve the binding potential for 125I-iomazenil in the peri-infarct area (Shichinohe et al, 2006). They also improve glucose metabolism in response to sensory stimuli when transplanted into the rat cold injury model (Mori et al, 2005). According to recent work by Liu et al (2010), BMSCs may enhance axonal sprouting from surviving cortical neurons in the peri-infarct area. Hofstetter et al (2002) also transplanted BMSCs into an injured spinal cord and found that the engrafted cells were tightly associated with longitudinally arranged immature astrocytes and formed bundles bridging the epicenter of the injury. More recently, Chiba et al (2009) found that BMSCs are integrated into the neural circuits of the host spinal cord and promote functional recovery. Proposed biological features of BMSCs in the CNS are summarized in Figure 3.
Figure 3.
Scheme of bone marrow stromal cell (BMSC) transplantation for ischemic stroke. The engrafted cells migrate toward the peri-infarct area via chemokine interaction. They may rescue and repair the damaged central nervous system (CNS) tissue through the differentiation into the neural cells, the release of neurotrophic or neuroprotective factors, and the inhibition of inflammatory reactions.
Translational Aspects of Bone Marrow Stromal Cell Transplantation
As described above, the observations in basic experiments are encouraging. Some clinical trials of BMSC transplantation have already been initiated for patients with ischemic stroke. Bang et al (2005) intravenously injected autologous BMSCs into five patients with severe neurologic deficits due to ischemic stroke at 5 to 9 weeks after the onset, and concluded that autologous BMSC infusion is a feasible and safe therapy that may improve functional recovery. Honmou et al (2011) intravenously transplanted BMSCs into 12 patients with ischemic stroke 36 to 133 days after stroke. Lee et al (2010) performed an open-label, observer-blinded clinical trial of 52 patients with ischemic stroke, and followed them for up to 5 years. They concluded that intravenous transplantation of autologous BMSCs could be a safe and effective strategy for ischemic stroke. These studies indicate that BMSC transplantation may at least be safe and feasible for patients with ischemic stroke. However, we should clearly remind that there are no clinical trials that prove the clinical significance of cell-based therapy, including BMSC transplantation.
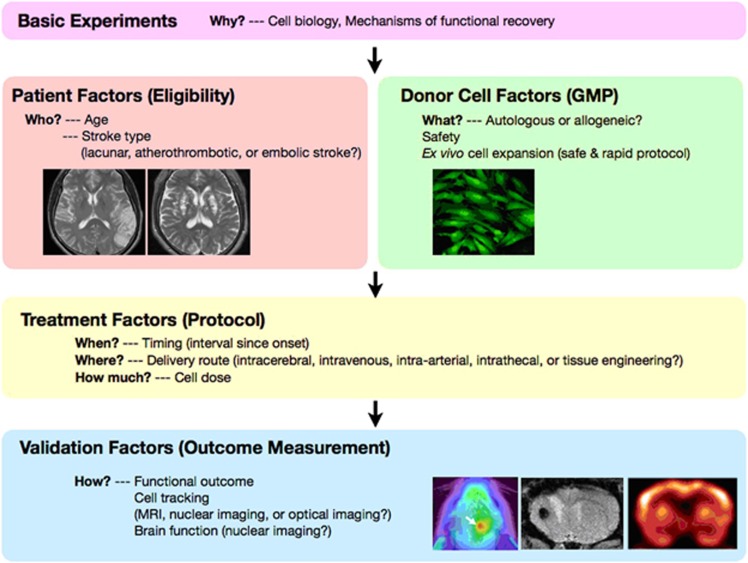
There are many variables that may affect the efficacy of BMSC transplantation in a clinical setting. As shown in Figure 4, these include donor cell factors (safety, autologous or allogeneic, ex-vivo cell expansion), patient factors (age and stroke type), treatment factors (interval since onset, delivery route and cell dose), and validation factors (neurologic assessment and imaging) (Borlongan et al, 2008; Dharmasaroja, 2009; Kuroda, 2008; Kuroda et al, 2011; Savitz et al, 2011). Now is the time to learn lessons from preclinical studies for the development of neuroprotective drugs (Feuerstein et al, 2008; Savitz and Fisher, 2007).
Figure 4.
‘Five Ws and two Hs (5W2H)' of cell therapy—the issues to be answered in preclinical studies and early-stage clinical trial of bone marrow stromal cell (BMSC) transplantation for ischemic stroke. GMP, good manufacturing practice; MRI, magnetic resonance imaging.
First, allogeneic cells would permit ‘off the shelf' use even within 24 hours after the onset, but force a long-term medication of immunosuppressant. Therefore, the use of autologous donor cells would be ideal for patients if the following issues were to be resolved. Autologous BMSCs from patients themselves would be ideal as donor cells for restorative medicine, but they would require several weeks for ex-vivo expansion. It would be critical to establish a feasible protocol to safely and rapidly expand BMSCs. Thus, BMSCs have been cultured in medium including fetal calf serum in the majority of animal experiments and even clinical trials (Bang et al, 2005). However, fetal calf serum carries the potential risk of contamination by prions, viruses, or zoonoses. Alternatively, autologous serum is used to expand BMSCs, but may require a large amount of serum (Honmou et al, 2011). Very recently, human platelet lysate has proven useful as an alternative substitute to expand BMSCs. Human BMSCs expanded with the fetal calf serum-free, platelet lysate-containing medium retain their capacity of migration, survival and differentiation, and significantly promote functional recovery when stereotactically transplanted into the infarcted brain. Therefore, platelet lysate may be a clinically valuable and safe substitute for fetal calf serum in expanding human BMSCs to regenerate the infarct brain (Ito et al, 2011; Shichinohe et al, 2011; Sugiyama et al, 2011b).
Second, BMSCs are transplanted within 24 hours or 7 days after the insults in the majority of animal studies, whereas they are usually transplanted several weeks (or even several months) after the onset in previous clinical trials (Bang et al, 2005; Honmou et al, 2011; Lee et al, 2010). Therefore, a considerable gap of treatment protocol exists between animal experiments and clinical trials, which may correspond to ‘inadequate preclinical testing' (Savitz and Fisher, 2007). Granulocyte-colony stimulating factor may be useful to speed up BMSC expansion and shorten the interval between onset and transplantation therapy. Thus, a certain concentration of G-CSFs significantly enhances their proliferation by modulating their cell cycle and also upregulates their production of nerve growth factor, hepatocyte growth factor, and stromal cell-derived factor-1_α_ (Hokari et al, 2009). However, it is well known that aging markedly reduces the self-renewal and differentiation capacity of various kinds of adult stem cells, including BMSCs. This fact would have a significant impact on the efficacy of BMSC transplantation for ischemic stroke, because most patients with ischemic stroke have an advanced age. A very recent study has clearly shown that G-CSF also activates their capacity of proliferation and neurotrophic factor release in aged animals (Chiba et al, 2011).
Third, BMSCs can be transplanted directly, intravenously, intraarterially, or intrathecally. Although direct (intracerebral) injection permits most efficient delivery of the donor cells to the damaged tissue, a less invasive procedure would be optimal. Intravenous or intrathecal transplantation is attractive because it is a noninvasive, safe technique for the host CNS, but has been reported to result in less pronounced cell migration and functional recovery than direct cell transplantation (Vaquero et al, 2006). Therefore, an optimal transplantation technique should be developed to serve maximally safe and efficient results. Alternatively, the intraarterial injection of BMSCs may be valuable to noninvasively deliver them to the damaged CNS (Osanai et al, 2011; Shen et al, 2006). There are a limited number of studies that directly compare the therapeutic effects of these delivery routes under the same conditions. It is urgent to test the effects of each delivery route on functional recovery after cerebral infarct (Kuroda et al, 2011). More interestingly, tissue-engineering technology may also provide an alternative route for cell delivery. Degradable biomaterials have been accepted as a valuable ‘scaffold' to fix and stabilize the transplanted cells in other organs such as bone, cartilage, heart, and skin. Until recently, however, there have only been a small number of studies that denote effective scaffolds for cell transplantation for CNS disorders (Lu et al, 2007). A recent study has shown that a fibrin matrix may improve the survival and migration of BMSCs, being a useful material for injured CNS tissue (Yasuda et al, 2010). A thermoreversible gelation polymer hydrogel may also be one candidate scaffold to provide a suitable environment for BMSCs (Osanai et al, 2010). However, no clinical trials have been performed to examine the efficacy of such technology in cell therapy for ischemic stroke.
Finally, it would be essential to develop techniques to serially and noninvasively track the fate of the transplanted cells in the host CNS. A cell tracking technique would also be important as a ‘biologically relevant end point' (Savitz and Fisher, 2007). Magnetic resonance imaging, nuclear imaging, and optical imaging are candidate modalities. Donor cells can be identified on magnetic resonance imaging by labeling with a superparamagnetic iron oxide agent (Hoehn et al, 2002; Ito et al, 2011; Jendelova et al, 2003). Magnetic resonance imaging can visualize intact, opaque organisms in three dimensions with good spatial resolution, but requires long imaging times and consequently slows data acquisition because of low sensitivity. Nuclear imaging can also detect the transplanted cells by labeling them with radioactive tracers. Correa et al (2007) recently labeled BM mononuclear cells with 99mTc-hexamethylpropylene (HMPAO), and injected them into a patient with ischemic stroke through a catheter. The transplanted cells were visualized on single photon emission tomography. Nuclear imaging can detect the target with high sensitivity, but has the difficulty to monitor donor cells for several weeks because of the relatively short half-life of clinically available tracers. Separately, optical imaging techniques may also serve future technology to visualize BMSCs engrafted in the damaged CNS. Quantum dot emits near-infrared fluorescence with a longer wavelength (800 nm) that can easily penetrate living tissue. A very recent study has shown that the quantum dot-labeled BMSCs can be clearly visualized under in-vivo fluorescence imaging through the skull and scalp for at least 8 weeks when transplanted into the infarcted brain of rats (Osanai et al, 2011; Sugiyama et al, 2011a). Imaging technology would be valuable to assess the effects of BMSC transplantation on the function of the host brain (Mori et al, 2005).
In conclusion, recent studies have strongly suggested the therapeutic potential of BMSC transplantation for ischemic stroke. However, further translational studies would be warranted to establish it as a scientifically proven strategy in a clinical setting. In addition, a cell-based therapy combined with other procedures such as recanalization strategies would provide additional benefits for patients.
In this review, we summarize the current progress of basic stem cell science and its early clinical applications for advanced stem cell therapy, with a focus on iPS cells, G-CSF, and BMSCs as current topics. Problems such as tumorigenicity of iPS cells and exaggerated inflammatory response of G-CSF need to be overcome. The mechanisms underlying functional recovery after cell transplantation, including of BMSCs, remain to be clarified. Although it may take time to realize a future therapy for human stroke, the current prospect supported by successful research looks promising.
The authors declare no conflict of interest.
Footnotes
This work was partly supported by Grant-in-Aid for Scientific Research (B) 21390267 and Challenging Research 22659260, and by Grants-in-Aid from the Research Committees (Mizusawa H, Nakano I, Nishizawa M, Sasaki H, and Sobue G) from the Ministry of Health, Labour, and Welfare of Japan.
References
- Abe K. Therapeutic potential of neurotrophic factors and neural stem cells against ischemic brain injury. J Cereb Blood Flow Metab. 2000;20:1393–1408. doi: 10.1097/00004647-200010000-00001. [DOI] [PubMed] [Google Scholar]
- Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y. Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke. 1995;26:1478–1489. doi: 10.1161/01.str.26.8.1478. [DOI] [PubMed] [Google Scholar]
- Abe K, Kogure K. Selective gene expression after brain ischemia. Prog Brain Res. 1993;96:221–236. doi: 10.1016/s0079-6123(08)63269-0. [DOI] [PubMed] [Google Scholar]
- Abe K, Tanzi RE, Kogure K. Induction of HSP70 mRNA after transient ischemia in gerbil brain. Neurosci Lett. 1991;125:166–168. doi: 10.1016/0304-3940(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. J Neurosci. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38:817–826. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Chopp M, Steinberg GK, Bliss TM, Li Y, Lu M, Hess DC, Kondziolka D. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regen Med. 2008;3:249–250. doi: 10.2217/17460751.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman M, Sharma S, Harting M, Strong R, Cox Jr, CS, Aronowski J, Grotta JC, Savitz SI. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhnemann C, Scholz A, Bernreuther C, Malik CY, Braun H, Schachner M, Reymann KG, Dihne M. Neuronal differentiation of transplanted embryonic stem cell-derived precursors in stroke lesions of adult rats. Brain. 2006;129:3238–3248. doi: 10.1093/brain/awl261. [DOI] [PubMed] [Google Scholar]
- Bussolino F, Ziche M, Wang JM, Alessi D, Morbidelli L, Cremona O, Bosia A, Marchisio PC, Mantovani A. In vitro and in vivo activation of endothelial cells by colony-stimulating factors. J Clin Invest. 1991;87:986–995. doi: 10.1172/JCI115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Yang M, Poremsky E, Kidd S, Schneider JS, Iacovitti L. Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem Cells Dev. 2010;19:1017–1023. doi: 10.1089/scd.2009.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo M, Dell'anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, Colciago G, Russo G, Carninci P, Pezzoli G, Gainetdinov RR, Gustincich S, Dityatev A, Broccoli V. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- Cavallaro AM, Lilleby K, Majolino I, Storb R, Appelbaum FR, Rowley SD, Bensinger WI. Three to six year follow-up of normal donors who received recombinant human granulocyte colony-stimulating factor. Bone Marrow Transplant. 2000;25:85–89. doi: 10.1038/sj.bmt.1702072. [DOI] [PubMed] [Google Scholar]
- Chen SH, Chang FM, Tsai YC, Huang KF, Lin CL, Lin MT. Infusion of human umbilical cord blood cells protect against cerebral ischemia and damage during heatstroke in the rat. Exp Neurol. 2006;199:67–76. doi: 10.1016/j.expneurol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Chang CM, Tsai SK, Chang YL, Chou SJ, Huang SS, Tai LK, Chen YC, Ku HH, Li HY, Chiou SH. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. 2010;19:1757–1767. doi: 10.1089/scd.2009.0452. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Kuroda S, Maruichi K, Osanai T, Hokari M, Yano S, Shichinohe H, Hida K, Iwasaki Y. Transplanted bone marrow stromal cells promote axonal regeneration and improve motor function in a rat spinal cord injury model. Neurosurgery. 2009;64:991–999. doi: 10.1227/01.NEU.0000341905.57162.1D. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Kuroda S, Osanai T, Shichinohe H, Houkin K, Iwasaki Y.2011Impact of ageing on biological features of bone marrow stromal cells (BMSC) in cell transplantation therapy for central nervous system disorders—Functional enhancement by granulocyte-colony stimulating factor (G-CSF) Neuropathologyadvance online publication, 10 October 2011 (e-pub ahead of print) [DOI] [PubMed]
- Correa PL, Mesquita CT, Felix RM, Azevedo JC, Barbirato GB, Falcao CH, Gonzalez C, Mendonca ML, Manfrim A, de Freitas G, Oliveira CC, Silva D, Avila D, Borojevic R, Alves S, Oliveira Jr, AC, Dohmann HF. Assessment of intra-arterial injected autologous bone marrow mononuclear cell distribution by radioactive labeling in acute ischemic stroke. Clin Nucl Med. 2007;32:839–841. doi: 10.1097/RLU.0b013e318156b980. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Li Z, Arac A, Grueter BA, Sofilos M, Malenka RC, Wu JC, Steinberg GK. Molecular and magnetic resonance imaging of human embryonic stem cell-derived neural stem cell grafts in ischemic rat brain. Mol Ther. 2009;17:1282–1291. doi: 10.1038/mt.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi K, Tsuru K, Hayashi T, Takaishi M, Nagahara M, Nagotani S, Sehara Y, Jin G, Zhang H, Hayakawa S, Shoji M, Miyazaki M, Osaka A, Huh NH, Abe K. Implantation of a new porous gelatin-siloxane hybrid into a brain lesion as a potential scaffold for tissue regeneration. J Cereb Blood Flow Metab. 2006;26:1263–1273. doi: 10.1038/sj.jcbfm.9600275. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–982. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16:12–20. doi: 10.1016/j.jocn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose Jr, FF, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England TJ, Gibson CL, Bath PM. Granulocyte-colony stimulating factor in experimental stroke and its effects on infarct size and functional outcome: a systematic review. Brain Res Rev. 2009;62:71–82. doi: 10.1016/j.brainresrev.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Zaleska MM, Krams M, Wang X, Day M, Rutkowski JL, Finklestein SP, Pangalos MN, Poole M, Stiles GL, Ruffolo RR, Walsh FL. Missing steps in the STAIR case: a translational medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J Cereb Blood Flow Metab. 2008;28:217–219. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- Floel A, Warnecke T, Duning T, Lating Y, Uhlenbrock J, Schneider A, Vogt G, Laage R, Koch W, Knecht S, Schäbitz WR. Granulocyte-colony stimulating factor (G-CSF) in stroke patients with concomitant vascular disease—a randomized controlled trial. PLoS One. 2011;6:e19767. doi: 10.1371/journal.pone.0019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Bath PM, Murphy SP. G-CSF reduces infarct volume and improves functional outcome after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:431–439. doi: 10.1038/sj.jcbfm.9600033. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Bath PM, Murphy SP. G-CSF administration is neuroprotective following transient cerebral ischemia even in the absence of a functional NOS-2 gene. J Cereb Blood Flow Metab. 2010;30:739–743. doi: 10.1038/jcbfm.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JL, Blank T, Schwab S, Kollmar R. Inhibited glutamate release by granulocyte-colony stimulating factor after experimental stroke. Neurosci Lett. 2008;432:167–169. doi: 10.1016/j.neulet.2007.07.056. [DOI] [PubMed] [Google Scholar]
- Hargus G, Cooper O, Deleidi M, Levy A, Lee K, Marlow E, Yow A, Soldner F, Hockemeyer D, Hallett PJ, Osborn T, Jaenisch R, Isacson O. Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci USA. 2010;107:15921–15926. doi: 10.1073/pnas.1010209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi J, Takagi Y, Fukuda H, Imazato T, Nishimura M, Fujimoto M, Takahashi J, Hashimoto N, Nozaki K. Primate embryonic stem cell-derived neuronal progenitors transplanted into ischemic brain. J Cereb Blood Flow Metab. 2006;26:906–914. doi: 10.1038/sj.jcbfm.9600247. [DOI] [PubMed] [Google Scholar]
- Hoehn M, Kustermann E, Blunk J, Wiedermann D, Trapp T, Wecker S, Focking M, Arnold H, Hescheler J, Fleischmann BK, Schwindt W, Buhrle C. Monitoring of implanted stem cell migration in vivo: a highly resolved in vivo magnetic resonance imaging investigation of experimental stroke in rat. Proc Natl Acad Sci USA. 2002;99:16267–16272. doi: 10.1073/pnas.242435499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokari M, Kuroda S, Chiba Y, Maruichi K, Iwasaki Y. Synergistic effects of granulocyte-colony stimulating factor on bone marrow stromal cell transplantation for mice cerebral infarct. Cytokine. 2009;46:260–266. doi: 10.1016/j.cyto.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Hokari M, Kuroda S, Shichinohe H, Yano S, Hida K, Iwasaki Y. Bone marrow stromal cells protect and repair damaged neurons through multiple mechanisms. J Neurosci Res. 2008;86:1024–1035. doi: 10.1002/jnr.21572. [DOI] [PubMed] [Google Scholar]
- Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, Waxman SG, Kocsis JD. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. 2011;134:1790–1807. doi: 10.1093/brain/awr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Kuroda S, Sugiyama T, Shichinohe H, Takeda Y, Nishio M, Koike M, Houkin K. Validity of bone marrow stromal cell expansion by animal serum-free medium for cell transplantation therapy of cerebral infarct in rats—a serial MRI study. Transl Stroke Res. 2011;2:294–306. doi: 10.1007/s12975-011-0098-9. [DOI] [PubMed] [Google Scholar]
- Iwai M, Sato K, Kamada H, Omori N, Nagano I, Shoji M, Abe K. Temporal profile of stem cell division, migration, and differentiation from subventricular zone to olfactory bulb after transient forebrain ischemia in gerbils. J Cereb Blood Flow Metab. 2003;23:331–341. doi: 10.1097/01.WCB.0000050060.57184.E7. [DOI] [PubMed] [Google Scholar]
- Iwai M, Sato K, Omori N, Nagano I, Manabe Y, Shoji M, Abe K. Three steps of neural stem cells development in gerbil dentate gyrus after transient ischemia. J Cereb Blood Flow Metab. 2002;22:411–419. doi: 10.1097/00004647-200204000-00005. [DOI] [PubMed] [Google Scholar]
- Jendelova P, Herynek V, DeCroos J, Glogarova K, Andersson B, Hajek M, Sykova E. Imaging the fate of implanted bone marrow stromal cells labeled with superparamagnetic nanoparticles. Magn Reson Med. 2003;50:767–776. doi: 10.1002/mrm.10585. [DOI] [PubMed] [Google Scholar]
- Kamei N, Tanaka N, Oishi Y, Ishikawa M, Hamasaki T, Nishida K, Nakanishi K, Sakai N, Ochi M. Bone marrow stromal cells promoting corticospinal axon growth through the release of humoral factors in organotypic cocultures in neonatal rats. J Neurosurg Spine. 2007;6:412–419. doi: 10.3171/spi.2007.6.5.412. [DOI] [PubMed] [Google Scholar]
- Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K, Wiedau-Pazos M, Kornblum HI, Lowry WE. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113:701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- Kawai H, Yamashita T, Ohta Y, Deguchi K, Nagotani S, Zhang X, Ikeda Y, Matsuura T, Abe K. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab. 2010;30:1487–1493. doi: 10.1038/jcbfm.2010.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komine-Kobayashi M, Zhang N, Liu M, Tanaka R, Hara H, Osaka A, Mochizuki H, Mizuno Y, Urabe T. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J Cereb Blood Flow Metab. 2006;26:402–413. doi: 10.1038/sj.jcbfm.9600195. [DOI] [PubMed] [Google Scholar]
- Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–3801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- Kurata T, Miyazaki K, Kozuki M, Morimoto N, Ohta Y, Ikeda Y, Abe K. Progressive neurovascular disturbances in the cerebral cortex of Alzheimer's disease-model mice: protection by atorvastatin and pitavastatin. Neuroscience. 2011;197:358–368. doi: 10.1016/j.neuroscience.2011.09.030. [DOI] [PubMed] [Google Scholar]
- Kuroda S. How should we bridge the missing steps in translational research for stroke therapy?—A critical review. Jpn J Stroke. 2008;30:875–880. [Google Scholar]
- Kuroda S, Shichinohe H, Houkin K, Iwasaki Y. Autologous bone marrow stromal cell transplantation for central nervous system disorders—recent progress and perspective for clinical application. J Stem Cell Regen Med. 2011;7:1–12. doi: 10.46582/jsrm.0701002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JB, Kuroda S, Shichinohe H, Ikeda J, Seki T, Hida K, Tada M, Sawada K, Iwasaki Y. Migration and differentiation of nuclear fluorescence-labeled bone marrow stromal cells after transplantation into cerebral infarct and spinal cord injury in mice. Neuropathology. 2003;23:169–180. doi: 10.1046/j.1440-1789.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Ko SY, Kim EH, Sinn DI, Lee YS, Lo EH, Kim M, Roh JK. Granulocyte colony-stimulating factor enhances angiogenesis after focal cerebral ischemia. Brain Res. 2005;1058:120–128. doi: 10.1016/j.brainres.2005.07.076. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, Savant-Bhonsale S, Chopp M. Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab. 2010;30:1288–1295. doi: 10.1038/jcbfm.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204–213. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Mahmood A, Qu C, Hong X, Kaplan D, Chopp M.2007Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury Neurosurgery 61596–602.discussion 602-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack GS. ReNeuron and StemCells get green light for neural stem cell trials. Nat Biotechnol. 2011;29:95–97. doi: 10.1038/nbt0211-95. [DOI] [PubMed] [Google Scholar]
- Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, Ohnuki M, Ogawa D, Ikeda E, Okano H, Yamanaka S. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol. 2009;27:743–745. doi: 10.1038/nbt.1554. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Ohta Y, Nagai M, Morimoto N, Kurata T, Takehisa Y, Ikeda Y, Matsuura T, Abe K. Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res. 2011;89:718–728. doi: 10.1002/jnr.22594. [DOI] [PubMed] [Google Scholar]
- Mori K, Iwata J, Miyazaki M, Nakao Y, Maeda M. Functional recovery of neuronal activity in rat whisker-barrel cortex sensory pathway from freezing injury after transplantation of adult bone marrow stromal cells. J Cereb Blood Flow Metab. 2005;25:887–898. doi: 10.1038/sj.jcbfm.9600083. [DOI] [PubMed] [Google Scholar]
- Morimoto N, Nagai M, Miyazaki K, Kurata T, Takehisa Y, Ikeda Y, Kamiya T, Okazawa H, Abe K. Progressive decrease in the level of YAPdeltaCs, prosurvival isoforms of YAP, in the spinal cord of transgenic mouse carrying a mutant SOD1 gene. J Neurosci Res. 2009;87:928–936. doi: 10.1002/jnr.21902. [DOI] [PubMed] [Google Scholar]
- Morita Y, Takizawa S, Kamiguchi H, Uesugi T, Kawada H, Takagi S. Administration of hematopoietic cytokines increases the expression of anti-inflammatory cytokine (IL-10) mRNA in the subacute phase after stroke. Neurosci Res. 2007;58:356–360. doi: 10.1016/j.neures.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Nandoe Tewarie RD, Bossers K, Ritfeld GJ, Blits B, Grotenhuis JA, Verhaagen J, Oudega M. Early passage bone marrow stromal cells express genes involved in nervous system development supporting their relevance for neural repair. Restor Neurol Neurosci. 2011;29:187–201. doi: 10.3233/RNN-2011-0591. [DOI] [PubMed] [Google Scholar]
- Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G, Fischer I. Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res. 2005;1035:73–85. doi: 10.1016/j.brainres.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, Miyakawa T, Kaneko T, Nakamura S. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 2010;13:173–179. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- Osanai T, Kuroda S, Sugiyama T, Kawabori M, Ito M, Shichinohe H, Kuge Y, Houkin K, Tamaki N, Iwasaki Y.2011Therapeutic effects of intra-arterial delivery of bone marrow stromal cells in traumatic brain injury of rats—in vivo cell tracking study by near-infrared fluorescence imaging Neurosurgeryadvance online publication, 3 August 2011 (e-pub ahead of print) [DOI] [PubMed]
- Osanai T, Kuroda S, Yasuda H, Chiba Y, Maruichi K, Hokari M, Sugiyama T, Shichinohe H, Iwasaki Y.2010Noninvasive transplantation of bone marrow stromal cells for ischemic stroke: preliminary study with a thermoreversible gelation polymer hydrogel Neurosurgery 661140–1147.discussion 7 [DOI] [PubMed] [Google Scholar]
- Pandya NM, Dhalla NS, Santani DD. Angiogenesis—a new target for future therapy. Vascul Pharmacol. 2006;44:265–274. doi: 10.1016/j.vph.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476:220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- Piao CS, Gonzalez-Toledo ME, Xue YQ, Duan WM, Terao S, Granger DN, Kelley RE, Zhao LR. The role of stem cell factor and granulocyte-colony stimulating factor in brain repair during chronic stroke. J Cereb Blood Flow Metab. 2009;29:759–770. doi: 10.1038/jcbfm.2008.168. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003;100 (Suppl 1:11917–11923. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, Vanti WB, Moreno H, Abeliovich A. Directed conversion of Alzheimer's disease patient skin fibroblasts into functional neurons. Cell. 2011;146:359–371. doi: 10.1016/j.cell.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Chopp M, Deans R, Carmichael ST, Phinney D, Wechsler L. Stem cell therapy as an emerging paradigm for stroke (STEPS) II. Stroke. 2011;42:825–829. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Fisher M. Future of neuroprotection for acute stroke: in the aftermath of the SAINT trials. Ann Neurol. 2007;61:396–402. doi: 10.1002/ana.21127. [DOI] [PubMed] [Google Scholar]
- Schäbitz WR, Kollmar R, Schwaninger M, Juettler E, Bardutzky J, Scholzke MN, Sommer C, Schwab S. Neuroprotective effect of granulocyte colony-stimulating factor after focal cerebral ischemia. Stroke. 2003;34:745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- Schäbitz WR, Laage R, Vogt G, Koch W, Kollmar R, Schwab S, Schneider D, Hamann GF, Rosenkranz M, Veltkamp R, Fiebach JB, Hacke W, Grotta JC, Fisher M, Schneider A. AXIS: a trial of intravenous granulocyte colony-stimulating factor in acute ischemic stroke. Stroke. 2010;41:2545–2551. doi: 10.1161/STROKEAHA.110.579508. [DOI] [PubMed] [Google Scholar]
- Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, Aronowski J, Maurer MH, Gassler N, Mier W, Hasselblatt M, Kollmar R, Schwab S, Sommer C, Bach A, Kuhn HG, Schäbitz WR. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehara Y, Hayashi T, Deguchi K, Zhang H, Tsuchiya A, Yamashita T, Lukic V, Nagai M, Kamiya T, Abe K. Decreased focal inflammatory response by G-CSF may improve stroke outcome after transient middle cerebral artery occlusion in rats. J Neurosci Res. 2007a;85:2167–2174. doi: 10.1002/jnr.21341. [DOI] [PubMed] [Google Scholar]
- Sehara Y, Hayashi T, Deguchi K, Zhang H, Tsuchiya A, Yamashita T, Lukic V, Nagai M, Kamiya T, Abe K. Potentiation of neurogenesis and angiogenesis by G-CSF after focal cerebral ischemia in rats. Brain Res. 2007b;1151:142–149. doi: 10.1016/j.brainres.2007.01.149. [DOI] [PubMed] [Google Scholar]
- Sevimli S, Diederich K, Strecker JK, Schilling M, Klocke R, Nikol S, Kirsch F, Schneider A, Schäbitz WR. Endogenous brain protection by granulocyte-colony stimulating factor after ischemic stroke. Exp Neurol. 2009;217:328–335. doi: 10.1016/j.expneurol.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Shang J, Deguchi K, Ohta Y, Liu N, Zhang X, Tian F, Yamashita T, Ikeda Y, Matsuura T, Funakoshi H, Nakamura T, Abe K. Strong neurogenesis, angiogenesis, synaptogenesis, and antifibrosis of hepatocyte growth factor in rats brain after transient middle cerebral artery occlusion. J Neurosci Res. 2011;89:86–95. doi: 10.1002/jnr.22524. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007;38:2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- Shichinohe H, Kuroda S, Lee JB, Nishimura G, Yano S, Seki T, Ikeda J, Tamura M, Iwasaki Y. In vivo tracking of bone marrow stromal cells transplanted into mice cerebral infarct by fluorescence optical imaging. Brain Res Brain Res Protoc. 2004;13:166–175. doi: 10.1016/j.brainresprot.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Shichinohe H, Kuroda S, Sugiyama T, Ito M, Kawabori M. Bone marrow stromal cell transplantation attenuates cognitive dysfunction due to chronic cerebral ischemia in rats. Dement Geriatr Cogn Disord. 2010;30:293–301. doi: 10.1159/000320486. [DOI] [PubMed] [Google Scholar]
- Shichinohe H, Kuroda S, Sugiyama T, Ito M, Kawabori M, Nishio M, Takeda Y, Kioke T, Houkin K. Biological features of human bone marrow stromal cells (hBMSC) cultured with animal protein-free medium—safety and efficacy of clinical use for neurotransplantation. Transl Stroke Res. 2011;2:307–315. doi: 10.1007/s12975-011-0088-y. [DOI] [PubMed] [Google Scholar]
- Shichinohe H, Kuroda S, Tsuji S, Yamaguchi S, Yano S, Lee JB, Kobayashi H, Kikuchi S, Hida K, Iwasaki Y. Bone marrow stromal cells promote neurite extension in organotypic spinal cord slice: significance for cell transplantation therapy. Neurorehabil Neural Repair. 2008;22:447–457. doi: 10.1177/1545968308315596. [DOI] [PubMed] [Google Scholar]
- Shichinohe H, Kuroda S, Yano S, Hida K, Iwasaki Y. Role of SDF-1/CXCR4 system in survival and migration of bone marrow stromal cells after transplantation into mice cerebral infarct. Brain Res. 2007;1183:138–147. doi: 10.1016/j.brainres.2007.08.091. [DOI] [PubMed] [Google Scholar]
- Shichinohe H, Kuroda S, Yano S, Ohnishi T, Tamagami H, Hida K, Iwasaki Y. Improved expression of gamma-aminobutyric acid receptor in mice with cerebral infarct and transplanted bone marrow stromal cells: an autoradiographic and histologic analysis. J Nucl Med. 2006;47:486–491. [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony-stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006;174:927–933. doi: 10.1503/cmaj.051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six I, Gasan G, Mura E, Bordet R. Beneficial effect of pharmacological mobilization of bone marrow in experimental cerebral ischemia. Eur J Pharmacol. 2003;458:327–328. doi: 10.1016/s0014-2999(02)02785-1. [DOI] [PubMed] [Google Scholar]
- Solaroglu I, Cahill J, Tsubokawa T, Beskonakli E, Zhang JH. Granulocyte colony-stimulating factor protects the brain against experimental stroke via inhibition of apoptosis and inflammation. Neurol Res. 2009;31:167–172. doi: 10.1179/174313209X393582. [DOI] [PubMed] [Google Scholar]
- Solaroglu I, Tsubokawa T, Cahill J, Zhang JH. Anti-apoptotic effect of granulocyte-colony stimulating factor after focal cerebral ischemia in the rat. Neuroscience. 2006;143:965–974. doi: 10.1016/j.neuroscience.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, Mitalipova M, Isacson O, Jaenisch R. Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell. 2009;136:964–977. doi: 10.1016/j.cell.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son BR, Marquez-Curtis LA, Kucia M, Wysoczynski M, Turner AR, Ratajczak J, Ratajczak MZ, Janowska-Wieczorek A. Migration of bone marrow and cord blood mesenchymal stem cells in vitro is regulated by stromal-derived factor-1-CXCR4 and hepatocyte growth factor-c-met axes and involves matrix metalloproteinases. Stem Cells. 2006;24:1254–1264. doi: 10.1634/stemcells.2005-0271. [DOI] [PubMed] [Google Scholar]
- Sprigg N, Bath PM, Zhao L, Willmot MR, Gray LJ, Walker MF, Dennis MS, Russell N. Granulocyte-colony-stimulating factor mobilizes bone marrow stem cells in patients with subacute ischemic stroke: the Stem cell Trial of recovery EnhanceMent after Stroke (STEMS) pilot randomized, controlled trial (ISRCTN 16784092) Stroke. 2006;37:2979–2983. doi: 10.1161/01.STR.0000248763.49831.c3. [DOI] [PubMed] [Google Scholar]
- Strecker JK, Sevimli S, Schilling M, Klocke R, Nikol S, Schneider A, Schäbitz WR. Effects of G-CSF treatment on neutrophil mobilization and neurological outcome after transient focal ischemia. Exp Neurol. 2010;222:108–113. doi: 10.1016/j.expneurol.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kuroda S, Osanai T, Shichinohe H, Kuge Y, Ito M, Kawabori M, Iwasaki Y.2011aNear-infrared fluorescence labeling allows noninvasive tracking of bone marrow stromal cells transplanted into rat infarct brain Neurosurgery 681036–1047.discussion 1047 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Kuroda S, Takeda Y, Nishio M, Ito M, Shichinohe H, Koike T. Therapeutic impact of human bone marrow stromal cells expanded by animal serum-free medium for cerebral infarct in rats. Neurosurgery. 2011b;68:1733–1742. doi: 10.1227/NEU.0b013e31820edd63. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Yagita Y, Oyama N, Terasaki Y, Omura-Matsuoka E, Sasaki T, Kitagawa K. Granulocyte colony-stimulating factor enhances arteriogenesis and ameliorates cerebral damage in a mouse model of ischemic stroke. Stroke. 2011c;42:770–775. doi: 10.1161/STROKEAHA.110.597799. [DOI] [PubMed] [Google Scholar]
- Sykova E, Jendelova P. Magnetic resonance tracking of implanted adult and embryonic stem cells in injured brain and spinal cord. Ann NY Acad Sci. 2005;1049:146–160. doi: 10.1196/annals.1334.014. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wen Z, Myojin K, Yoshihara T, Nakagomi T, Nakayama D, Tanaka H, Soma T, Stern DM, Naritomi H, Matsuyama T. Granulocyte colony-stimulating factor has a negative effect on stroke outcome in a murine model. Eur J Neurosci. 2007;26:126–133. doi: 10.1111/j.1460-9568.2007.05640.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Teramoto T, Qiu J, Plumier JC, Moskowitz MA. EGF amplifies the replacement of parvalbumin-expressing striatal interneurons after ischemia. J Clin Invest. 2003;111:1125–1132. doi: 10.1172/JCI17170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tian F, Morimoto N, Liu W, Ohta Y, Deguchi K, Miyazaki K, Abe K. In vivo optical imaging of motor neuron autophagy in a mouse model of amyotrophic lateral sclerosis. Autophagy. 2011;7:985–992. doi: 10.4161/auto.7.9.16012. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011;24:59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- Urdzikova L, Jendelova P, Glogarova K, Burian M, Hajek M, Sykova E. Transplantation of bone marrow stem cells as well as mobilization by granulocyte-colony stimulating factor promotes recovery after spinal cord injury in rats. J Neurotrauma. 2006;23:1379–1391. doi: 10.1089/neu.2006.23.1379. [DOI] [PubMed] [Google Scholar]
- Vaquero J, Zurita M, Oya S, Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: systemic or local administration. Neurosci Lett. 2006;398:129–134. doi: 10.1016/j.neulet.2005.12.072. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Y, Chen J, Gautam SC, Zhang Z, Lu M, Chopp M. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol. 2002;30:831–836. doi: 10.1016/s0301-472x(02)00829-9. [DOI] [PubMed] [Google Scholar]
- Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000;61:364–370. doi: 10.1002/1097-4547(20000815)61:4<364::AID-JNR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Kuroda S, Kobayashi H, Shichinohe H, Yano S, Hida K, Shinpo K, Kikuchi S, Iwasaki Y. The effects of neuronal induction on gene expression qprofile in bone marrow stromal cells (BMSC)-a preliminary study using microarray analysis. Brain Res. 2006;1087:15–27. doi: 10.1016/j.brainres.2006.02.127. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kamiya T, Deguchi K, Inaba T, Zhang H, Shang J, Miyazaki K, Ohtsuka A, Katayama Y, Abe K. Dissociation and protection of the neurovascular unit after thrombolysis and reperfusion in ischemic rat brain. J Cereb Blood Flow Metab. 2009;29:715–725. doi: 10.1038/jcbfm.2008.164. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Kawai H, Tian F, Ohta Y, Abe K. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant. 2011;20:883–891. doi: 10.3727/096368910X539092. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S, Kuroda S, Shichinohe H, Hida K, Iwasaki Y. Do bone marrow stromal cells proliferate after transplantation into mice cerebral infarct?—a double labeling study. Brain Res. 2005;1065:60–67. doi: 10.1016/j.brainres.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Kuroda S, Shichinohe H, Kamei S, Kawamura R, Iwasaki Y. Effect of biodegradable fibrin scaffold on survival, migration, and differentiation of transplanted bone marrow stromal cells after cortical injury in rats. J Neurosurg. 2010;112:336–344. doi: 10.3171/2009.2.JNS08495. [DOI] [PubMed] [Google Scholar]
- Zeng H, Guo M, Martins-Taylor K, Wang X, Zhang Z, Park JW, Zhan S, Kronenberg MS, Lichtler A, Liu HX, Chen FP, Yue L, Li XJ, Xu RH. Specification of region-specific neurons including forebrain glutamatergic neurons from human induced pluripotent stem cells. PLoS One. 2010;5:e11853. doi: 10.1371/journal.pone.0011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kamiya T, Hayashi T, Tsuru K, Deguchi K, Lukic V, Tsuchiya A, Yamashita T, Hayakawa S, Ikeda Y, Osaka A, Abe K. Gelatin-siloxane hybrid scaffolds with vascular endothelial growth factor induces brain tissue regeneration. Curr Neurovasc Res. 2008;5:112–117. doi: 10.2174/156720208784310204. [DOI] [PubMed] [Google Scholar]
- Zhao LR, Berra HH, Duan WM, Singhal S, Mehta J, Apkarian AV, Kessler JA. Beneficial effects of hematopoietic growth factor therapy in chronic ischemic stroke in rats. Stroke. 2007;38:2804–2811. doi: 10.1161/STROKEAHA.107.486217. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Zhong C, Qin Z, Zhong CJ, Wang Y, Shen XY. Neuroprotective effects of bone marrow stromal cells on rat organotypic hippocampal slice culture model of cerebral ischemia. Neurosci Lett. 2003;342:93–96. doi: 10.1016/s0304-3940(03)00255-6. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]