Dephosphorylation of Cdc20 is required for its C-box-dependent activation of the APC/C (original) (raw)
Abstract
The anaphase-promoting complex/cyclosome (APC/C) ubiquitin ligase is tightly regulated to ensure programmed proteolysis in cells. The activity of the APC/C is positively controlled by cyclin-dependent kinase (CDK), but a second level of control must also exist because phosphorylation inactivates Cdc20, a mitotic APC/C co-activator. How Cdc20 is dephosphorylated specifically, when CDK is high, has remained unexplained. Here, we show that phosphatases are crucial to activate the APC/C. Cdc20 is phosphorylated at six conserved residues (S50/T64/T68/T79/S114/S165) by CDK in Xenopus egg extracts. When all the threonine residues are phosphorylated, Cdc20 binding to and activation of the APC/C are inhibited. Their dephosphorylation is regulated depending on the sites and protein phosphatase 2A, active in mitosis, is essential to dephosphorylate the threonine residues and activate the APC/C. Consistently, most of the Cdc20 bound to the APC/C in anaphase evades phosphorylation at T79. Furthermore, we show that the ‘activation domain’ of Cdc20 associates with the Apc6 and Apc8 core subunits. Our data suggest that dephosphorylation of Cdc20 is required for its loading and activation of the APC/C ubiquitin ligase.
Keywords: APC/C, Cdc20, CDK, cell cycle, phosphatase
Introduction
Selective proteolysis is a central mechanism in controlling numerous cellular processes, which is achieved by the ubiquitin-proteasome system. The anaphase-promoting complex/cyclosome (APC/C) is a multi-subunit E3 ubiquitin ligase and carries out programmed proteolysis in key events during the cell cycle, such as chromosome segregation, mitotic exit and entry into S phase. The APC/C also plays a role in meiotic regulation, and has been implicated in post-mitotic functions including axonal morphogenesis in neurons (Harper et al, 2002; Peters, 2006; Thornton and Toczyski, 2006; Pesin and Orr-Weaver, 2008).
The APC/C is composed of at least 11 conserved subunits forming two separable subcomplexes that associate independently with the largest subunit, Apc1 (Thornton et al, 2006; Barford, 2010). The cullin subunit Apc2 and RING H2 domain subunit Apc11 form the catalytic core subcomplex (Gmachl et al, 2000; Leverson et al, 2000; Tang et al, 2001). The second subcomplex mainly consists of tetratricopeptide repeat (TPR) domain subunits Apc3/Cdc27, Apc6/Cdc16, Apc8/Cdc23, Apc5 and Apc7 and is believed to confer substrate specificity for APC/C-dependent ubiquitylation. Due to the size and complexity of the APC/C, the structural information of the APC/C has been limited until very recently. Last year, high-resolution single-particle electron microscopy revealed the structural basis for the subunit assembly of the APC/C and the substrate recognition (da Fonseca et al, 2011; Schreiber et al, 2011). Three conserved TPR subunits in the substrate recognition subcomplex, Apc3, Apc6 and Apc8 each forms a superhelical homo-dimer resulting all together in hexameric architectures, which bind to Apc1 via Apc4 and Apc5. Apc1 functions as a scaffolding-like assembly subunit as it also binds to the catalytic core subcomplex. Intriguingly, Apc10 seems to interact with both subcomplexes and locate within the central cavity together with Cdh1, a co-activator of the APC/C, where Apc10 and Cdh1 function as a co-receptor of the destruction box (D-box), a destruction motif of APC/C substrates (Buschhorn et al, 2011; da Fonseca et al, 2011). The TPR domain of Apc3 is responsible for the binding to the C-terminal isoleucine-arginine (IR) motif of the Fizzy/Cdc20 family of APC/C co-activators (Vodermaier et al, 2003; Burton et al, 2005; Kraft et al, 2005), allowing the adjacent WD40 repeat domain of the co-activator to recruit substrates into the APC/C core. Recently, another TPR protein Apc8 has been reported to be essential to recruit Cdc20 in particular when the spindle assembly checkpoint (SAC) is active (Izawa and Pines, 2011). Thus, the TPR-containing subcomplex seems to provide docking sites for substrate recognition and recruitment; however, its involvement in catalytic activation and the roles of the remaining subunits are still poorly understood.
The temporal regulation of the APC/C is mainly achieved through the association of the Fizzy/Cdc20 family of co-activators (Peters, 2006; Thornton and Toczyski, 2006; Yu, 2007; Barford, 2010). Fizzy/Cdc20 (hereafter referred to as Cdc20) activates the APC/C in metaphase and anaphase to degrade substrates such as cyclin B and securin and then an alternative co-activator Cdh1 takes over to degrade APC/C substrates in late anaphase and G1. The association of these co-activators with the APC/C is subject to control by phosphorylation. Cdc20 binds and activates mitotically phosphorylated APC/C (Fang et al, 1998; Shteinberg et al, 1999; Kramer et al, 2000; Rudner and Murray, 2000; Yudkovsky et al, 2000). In contrast, Cdh1 is able to activate both interphase and mitotic APC/C, regardless of APC/C phosphorylation, but this activity is inhibited by its own cyclin-dependent kinase (CDK)-dependent phosphorylation (Visintin et al, 1998; Zachariae et al, 1998). This ensures that when CDK is high in mitosis, Cdc20 is the predominant co-activator but when CDK activity is low during interphase, activation depends upon Cdh1. In animal cells, Cdh1 is also regulated by Rca1 or Emi1, an inhibitor of the interphase APC/C (Grosskortenhaus and Sprenger, 2002; Di Fiore and Pines, 2007). However, another layer of phosphorylation-dependent regulation has also been reported. Phosphorylation of Cdc20 by CDK or mitogen-activated protein kinase (MAPK) is inhibitory and stimulates Cdc20 binding to the SAC proteins such as Mad2 (Yudkovsky et al, 2000; Chung and Chen, 2003; D'Angiolella et al, 2003). Therefore, a mechanism antagonistic to Cdc20 phosphorylation is likely to be involved in mitotic activation of the APC/C, however whether and how it may regulate the process remains to be elucidated.
We have previously demonstrated that Cdc20 has not only a substrate recruitment role but also an APC/C activation role through the C-box (conserved in Cdc20 family proteins) in the N-terminal domain (Schwab et al, 2001; Kimata et al, 2008). Here, we have studied the molecular mechanisms of the C-box-dependent activation of the APC/C and uncovered another regulatory element in the N-terminal domain. We have identified and characterized dephosphorylation of Cdc20 required for its activation. Furthermore, we show how the N-terminal domain interacts with the TPR-containing APC/C subcomplex and activates the APC/C ubiquitin ligase.
Results
Phosphorylation of the N-terminal domain of Cdc20 inhibits its activation role
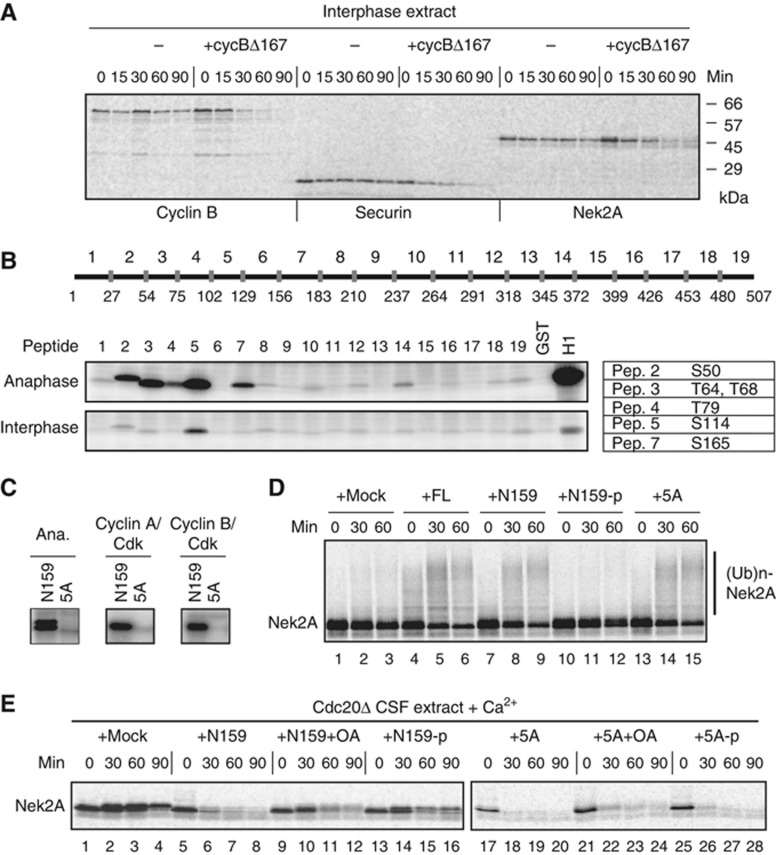
CDK1 (Cdc2) is thought to activate the APC/C ubiquitin ligase through phosphorylation of the APC/C subunits (Shteinberg et al, 1999; Kramer et al, 2000; Rudner and Murray, 2000). However, how phosphorylation of the APC/C co-activator Cdc20 is regulated and controls the activation of the APC/C remains unclear. To address this question, we first monitored the activity of the APC/C in a cell-free system reconstituted in Xenopus egg extracts by following the destruction of typical APC/C substrates such as cyclin B, securin and Nek2A. In Xenopus egg extracts, mitotic anaphase can be induced by the addition of non-degradable cyclin B (cycBΔ167) to interphase extracts. All the APC/C substrates were stable in interphase, but became unstable after incubation with cycBΔ167 (hereafter called anaphase extracts) (Figure 1A), suggesting that the APC/C is converted from an inactive to an active state by CDK. Next, we sought to determine the phosphorylation sites of Cdc20 in anaphase extracts. Cdc20 was phosphorylated at six conserved sites by CDK (S50, T64, T68, T79, S114, S165) (Figure 1B and C; Supplementary Figure S1 and S2). Since the phosphorylation sites are exclusively located around the C-box in the N-terminal domain, we hypothesized that the C-box-dependent activation function might be regulated by phosphorylation. Nek2A, which directly binds the APC/C, serves as a model substrate to study the ‘activation role’ of the Cdc20 N-terminal domain (N159) (Kimata et al, 2008). First, we investigated whether the phosphorylation of N159 affects its ability to support Nek2A ubiquitylation. As reported before, both Cdc20 full length (FL) and N159-WT supported Nek2A ubiquitylation, however N159 that had been phosphorylated by CDK failed to ubiquitylate Nek2A (Figure 1D, lanes 4–12), suggesting that phosphorylation of Cdc20 is inhibitory towards the activation of the APC/C. N159-5A was able to support the level of ubiquitylation of Nek2A seen with N159-WT (Figure 1D, lanes 13–15).
Figure 1.
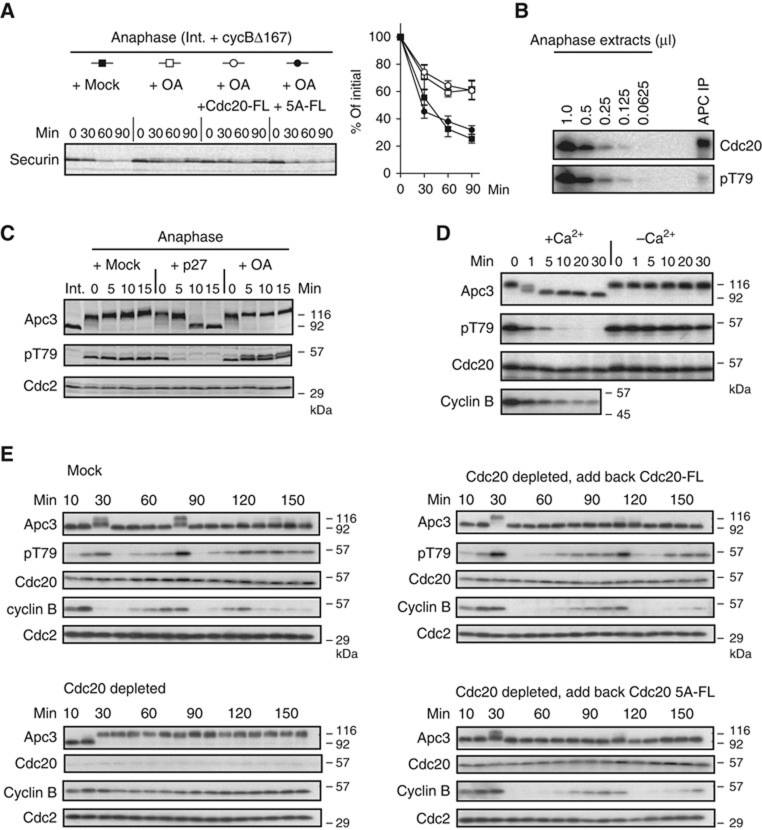
CDK phosphorylation of Cdc20 blocks its activation role. (A) CDK-cyclin B activates interphase APC/C. APC/C-dependent destruction assays were performed in Xenopus interphase extracts and interphase extracts incubated with GST-cyclinBΔ167 (2 μM) for 30 min. 35S-labelled _in vitro_-translated cyclin B (fission yeast Cdc13), securin and Nek2A were used as substrates. Samples were taken at the indicated time points and analysed by SDS–PAGE and autoradiography. (B) Phosphorylation of 19 Cdc20 peptides. (Top panel) A schematic diagram shows the 19 short peptides used as substrates. (Bottom panel) The GST-fused peptides were incubated with Xenopus interphase or anaphase extracts in the presence of [γ-32P]-ATP for 20 min, and then analysed by SDS–PAGE and autoradiography. The table lists the possible phosphorylation sites identified in each peptide. (C) GST-fused Cdc20-N159 or the same fragment with all the CDK sites (S50, T64, T68, T79, S114) mutated to alanine (5A) was incubated with anaphase extracts as well as recombinant CDK-cyclin A or CDK-cyclin B kinases. (D) APC/C was purified from Xenopus mitotic extracts depleted of endogenous Cdc20 and used for in-vitro ubiquitylation assays with buffer (+mock), _in vitro_-translated full-length Cdc20 (+FL), recombinant GST-N159-WT or -5A mock treated (+N159 or 5A) or phosphorylated by recombinant CDK (+N159-p). 35S-labelled _in vitro_-translated Nek2A was used as a substrate. (E) Nek2A destruction was examined in Cdc20-depleted CSF extracts supplemented with N159 or 5A in the presence or absence of OA (2 μM) or phosphorylated by recombinant CDK. Samples were taken at the indicated time points after addition of CaCl2 and analysed by SDS–PAGE and autoradiography.
Next, we wanted to investigate the impact of N159 phosphorylation on Nek2A destruction in Cdc20-depleted Xenopus egg extracts. The cell cycle of Xenopus laevis eggs is arrested at meiotic metaphase II with high CDK1-cyclin B by the activity of cytostatic factor (CSF). Extracts prepared from these eggs are called CSF-arrested extracts. At fertilization, a transient increase in cytoplasmic calcium triggers APC/C activation by degrading the APC/C inhibitor Erp1/Emi2 and activating calcineurin (Liu and Maller, 2005; Rauh et al, 2005; Tung et al, 2005; Mochida and Hunt, 2007). Hence, addition of calcium into CSF extracts degrades APC/C substrates such as cyclin B and securin and causes exit to interphase, whereas in the absence of calcium the activity of CDK in CSF extracts is high and progress to interphase prevented. Nek2A was degraded in Cdc20-depleted extract only when supplemented with N159 and calcium (Figure 1E, lanes 5–8), however, addition of okadaic acid (OA) reduced the activation role of N159 (Figure 1E, lanes 9–12). Similarly, N159, that had been phosphorylated by CDK before its addition to the extract, could poorly support Nek2A destruction (Figure 1E, lanes 13–16), suggesting that phosphorylation of N159 blocks its activation role. In agreement with this idea, CDK non-phosphorylatable N159-5A efficiently degraded Nek2A regardless of OA treatment or preincubation with CDK and ATP (Figure 1E, lanes 17–28). We also used Cdc20 full-length (Cdc20-FL) in order to address the relationship between CDK phosphorylation and its activation role (Supplementary Figure S3). Phosphorylated Cdc20-FL failed to degrade cyclin B and securin, whereas phosphorylation of 5A-FL did not show any inhibition. These results confirm the importance of Cdc20 N-terminal dephosphorylation in the activation of the APC/C.
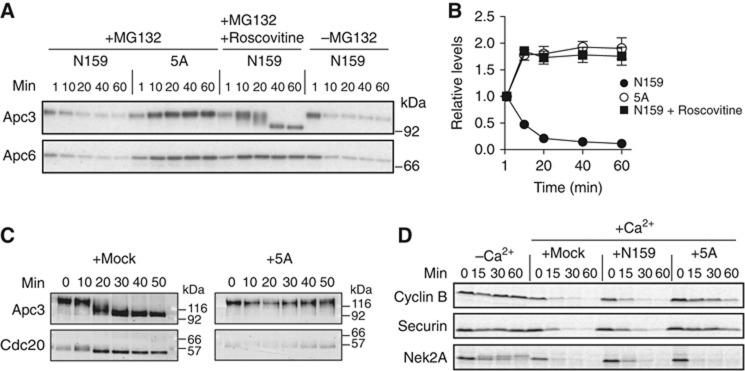
Dephosphorylation of the N-terminal domain is essential for Cdc20 to bind the APC/C
To evaluate whether the dephosphorylation of Cdc20 (N159) is directly involved in the association with the APC/C, we incubated N159 with Xenopus CSF extracts and monitored its association with the APC/C using Apc3 and Apc6 antibodies. A proteasome inhibitor, MG132 was added to ensure that CSF extracts remain arrested at metaphase II with high CDK activity. N159 was able to bind the APC/C immediately after incubation with CSF extracts; however, the binding was reduced depending on the incubation time with the extract. In contrast, non-phosphorylatable N159-5A efficiently bound the APC/C. Consistently, when we added roscovitine, a potent CDK inhibitor in egg extracts, levels of Apc3 and Apc6 co-precipitating with N159 dramatically increased (Figure 2A and B). We also observed an increased interaction between Cdc20 and the APC/C in more physiological conditions (Figure 2C). When APC/C was immunopurified with anti-Apc3 antibody after calcium addition in CSF extracts, more endogenous Cdc20 bound to the APC/C at 20–30 min when Cdc20 starts to be dephosphorylated as seen by a mobility shift (Figure 2C, left panel). If N159-5A was included as a competitor, then the binding of endogenous Cdc20 to APC/C was minimal (Figure 2C, right panel), suggesting that N159-5A has higher affinity for the APC/C than endogenous Cdc20 and thus prevents the latter from associating with the APC/C. Similarly, we found that N159-5A had a dominant-negative effect on endogenous Cdc20-mediated destruction of APC/C substrates. Cyclin B and securin were efficiently degraded in either mock or N159-added CSF extracts upon calcium addition, but if N159-5A was added, then degradation of cyclin B and securin was severely compromised (Figure 2D) since N159-5A lacking the C-terminal WD40 domain cannot recruit those substrates to the APC/C. In contrast, destruction of Nek2A was not affected, because Nek2A can directly bind the APC/C and N159-5A is sufficient to activate the APC/C.
Figure 2.
Phosphorylation of the N-terminal domain of Cdc20 blocks the association with the APC/C. (A) APC/C binding assays with the Cdc20 N-terminal domain. Purified N159-WT or -5A was bound to GSH Sepharose and then incubated with Xenopus CSF extract at 23°C for the indicated time. A proteasome inhibitor, MG132 (150 μM) and a CDK inhibitor, roscovitine (500 μM) were added as indicated. The amounts of bound APC/C were analysed by immunoblotting. (B) Quantification of the Apc6 immunoblot in (A). Error bars, s.e.m. from three independent experiments. (C) The amounts of endogenous Cdc20 bound to APC/C in Xenopus extract were examined in the presence of buffer (mock) or recombinant N159-5A. APC/C was purified using anti-Apc3 mAb AF3.1 at the indicated time points after addition of CaCl2 into CSF extract and analysed by immunoblotting. (D) Destruction of 35S-labelled APC/C substrates was examined in the presence of mock, Cdc20-N159-WT or 5A in a cell-free destruction assay. Cyclin B, securin and Nek2A were used as substrates. Samples were taken at the indicated time points and analysed by SDS–PAGE and autoradiography. CaCl2 was added to initiate proteolysis.
Threonine 64/68/79 phosphatase(s) is active in mitosis
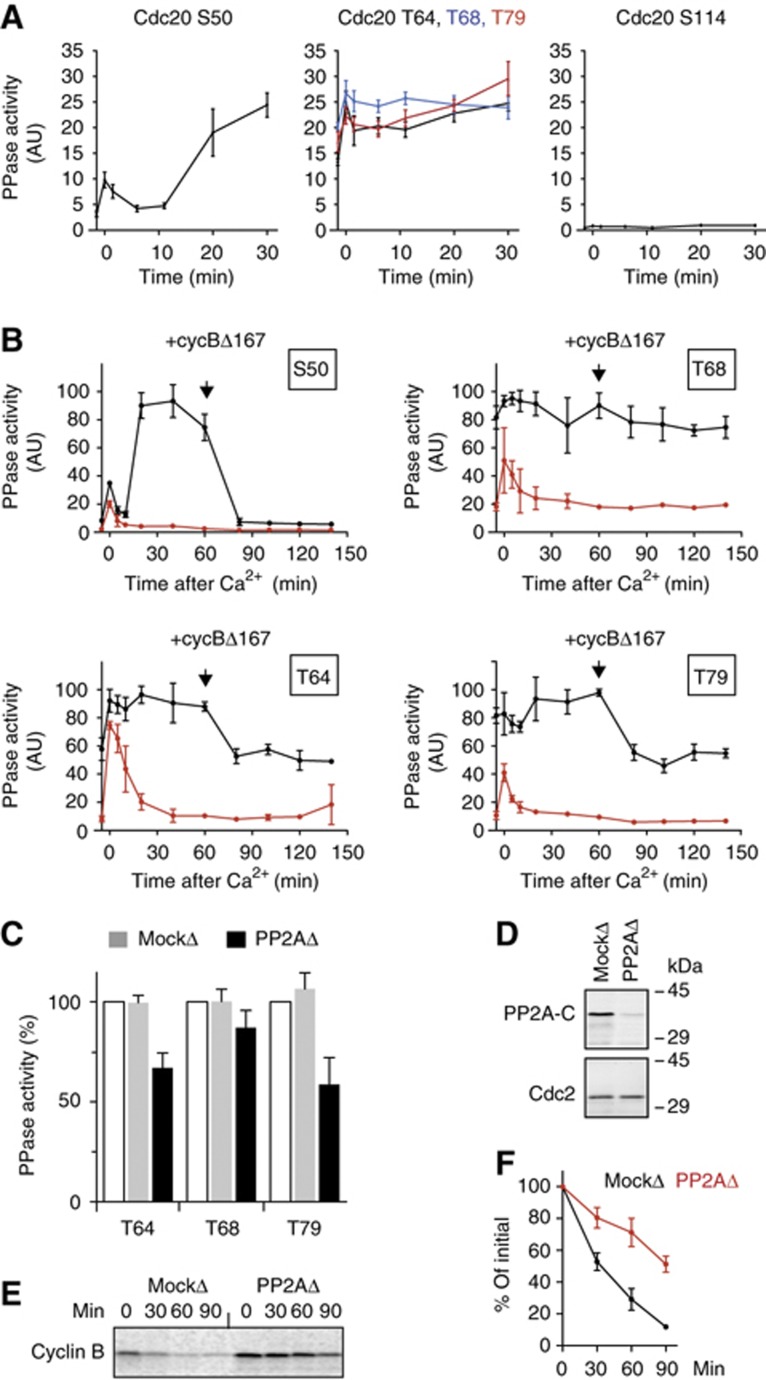
Next, we investigated how the dephosphorylation of Cdc20 is controlled during the cell cycle. To measure phosphate turnover from individual CDK sites on Cdc20 in Xenopus egg extracts, we have set up a phosphatase assay using five different substrates (Supplementary Figure S4). We confirmed previous findings (Mochida and Hunt, 2007) that S50 dephosphorylation was very low in CSF-arrested extracts but was transiently induced by calcineurin after the addition of calcium. This was then followed by a second wave of phosphatase activity in interphase that was abolished by the addition of OA (Figure 3A left panel; Supplementary Figure S5). In strong contrast, the activity of T64, T68 and T79 phosphatases was all relatively high in CSF-arrested extract. After calcium addition, a transient activation, by calcineurin, was observed and the phosphatase activity remained at high levels through the end of meiosis and during interphase (Figure 3A, middle panel). This is distinctly different from PP2A-B55δ activity which has been reported to dephosphorylate S50 in Cdc20 exclusively in interphase (Mochida et al, 2009). The activity of the S114 phosphatase was, if any, very little and did not change during the cell cycle. To further investigate the T64/68/79 phosphatase activity, mitosis was induced by the addition of non-degradable cyclin B (cycBΔ167) into interphase extracts (Figure 3B). Consistent with Figure 3A, S50 phosphatase activity was decreased upon the addition of cycBΔ167, whereas T64/68/79 phosphatase(s) remained high, although there was some decrease observed, depending on the individual phosphorylated sites. The phosphatase(s) responsible for T64/68/79 was inhibited by OA, although the inhibition of T68 phosphatase was slightly weaker than the others (Figure 3B).
Figure 3.
The activity of Cdc20 phosphatases during the cell cycle. (A) Cdc20 substrates containing only one CDK site were phosphorylated with CDK-cyclin A and [γ-32P]ATP. The substrates (S50, T64, T68, T79 and S114) were added individually into CSF extract released by the addition of CaCl2. Release of 32PO4 was measured at intervals after addition of CaCl2. The first time point is before CaCl2 addition (−1.5 min) and the 0-min time point is immediately after CaCl2 addition. Error bars, s.e.m. from three independent experiments. (B) Phosphatase assays using Cdc20 substrates S50, T64, T68 and T79 were performed after addition of CaCl2 to CSF extract in the presence (red circle) or absence (black circle) of OA (2.5 μM). At 60 min, non-degradable cyclin B (400 nM cycBΔ167) was added to the interphase egg extracts and individual Cdc20 phosphatase assays were continuously performed. Error bars, s.e.m. from three independent experiments. (C) PP2A was removed from anaphase egg extracts by immunodepletion using anti-PP2A A subunit mAb 6F9. Cdc20 T64, T68 and T79 phosphatase activities were measured in mock- or PP2A-depleted extracts. The activities are expressed as a percentage of the phosphatase activity of the untreated anaphase extracts (white bar). Error bars, s.e.m. from three independent experiments. (D) PP2A depletion was confirmed by immunoblotting with anti-PP2A catalytic subunit antibody. Cdc2 was used to normalize the quantification. (E) Destruction of 35S-labelled cyclin B (fission yeast Cdc13) was examined in mock- or PP2A-depleted anaphase extracts. (F) Quantification of (E). Error bars, s.e.m. from three independent experiments.
PP2A is essential for the activation of the APC/C ubiquitin ligase
To address whether PP2A is the OA-sensitive protein phosphatase responsible for the T64/68/79 dephosphorylation, we used the 6F9 monoclonal antibody against the A subunit of PP2A (Kremmer et al, 1997) to deplete the PP2A holoenzyme. Over 80% of PP2A C subunit was depleted and ∼40% of Cdc20 T64 and T79 phosphatase activities were removed from anaphase extracts by the 6F9 antibody (Figure 3C and D). This result confirms that PP2A is involved in Cdc20 dephosphorylation. To ascertain the role of PP2A, we examined the activity of the APC/C in the PP2A-depleted anaphase extracts by monitoring destruction of cyclin B. When PP2A was depleted, cyclin B was substantially stabilized (Figure 3E and F).
Threonine 64/68/79 residues of Cdc20 have to be dephosphorylated to activate the APC/C
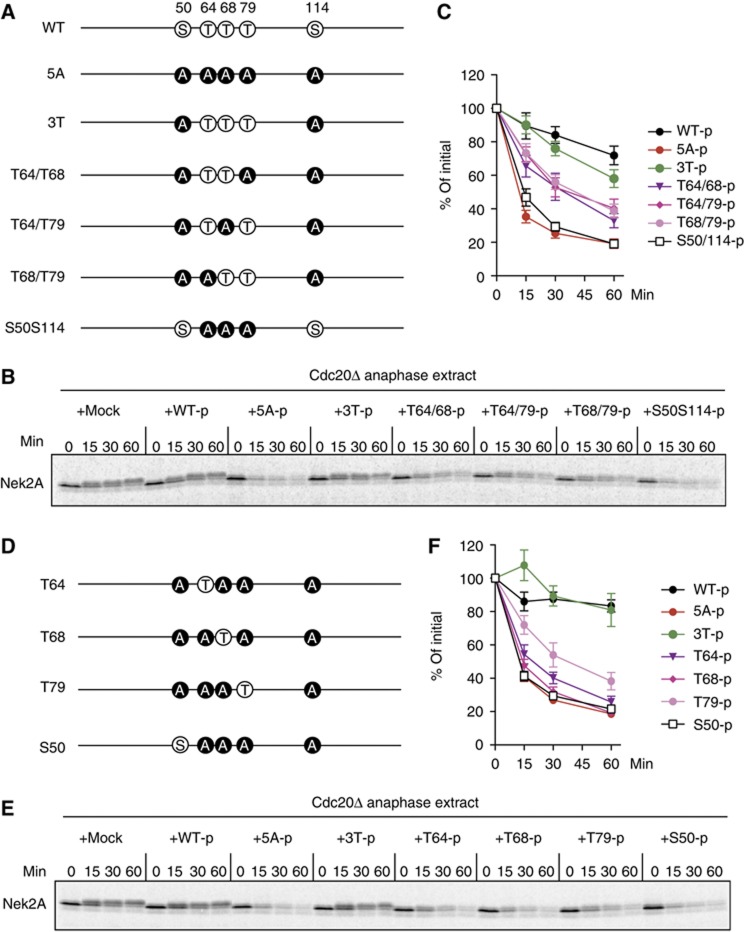
The fact that T64/68/79 phosphatase(s) is active in anaphase prompted us to examine whether these sites are critical to regulate the activity of Cdc20. We prepared Cdc20 N159-3T, in which T64/T68/T79 are intact, but S50 and S114 are mutated to alanine (Figure 4A). In support of this notion, when N159-3T had been phosphorylated by CDK, its ability to degrade Nek2A in Cdc20-depleted egg extracts was significantly inhibited (Figure 4B, C and F). We next wanted to investigate the effect of phosphorylation of two sites out of three (Figure 4A). We prepared T64/68, T64/79 and T68/79 that had been phosphorylated by CDK, and added these back to Cdc20-depleted anaphase extracts. Phosphorylation of two threonine residues could inhibit the activation role of Cdc20; however, the inhibition was not as efficient as that observed with 3T-p (Figure 4B and C). We also examined whether any single site might be more important than the others using N159 constructs where only one site can be phosphorylated. Among the three (T64/68/79), phosphorylation of T79 showed slightly stronger inhibition than the others (Figure 4D–F). All together, these results indicate that the phosphorylation status of the threonine residues (T64/68/79) is crucial for controlling the activity of Cdc20.
Figure 4.
Dephosphorylation of threonine residues is required for the activation of the APC/C. (A) The diagram shows the different phosphorylation site mutants of Cdc20 (N159) used. (B) Phosphorylation of 3T (T64/68/79) largely inhibits the activation role of Cdc20. Destruction of 35S-labelled Nek2A was examined in Cdc20-depleted anaphase extracts in the presence of OA (2.5 μM) after purified N159 proteins phosphorylated by recombinant CDK were added to the extracts. Samples were taken at the indicated time points after N159 addition and analysed by SDS–PAGE and autoradiography. OA was added to prevent dephosphorylation of N159 during the experiment. (C) Quantification of the destruction assay of (B). Error bars, s.e.m. from four independent experiments. (D) The diagram shows single site mutants of N159 used. (E) The same as (B), but single site mutants of N159 were used. (F) Quantification of the destruction assay of (E). Error bars, s.e.m. from five independent experiments.
Phosphorylation of Cdc20 is in a dynamic state in anaphase
Both APC/C and Cdc20 are phosphorylated in anaphase extracts, but we hypothesized that the phosphorylation status of Cdc20 might be in a dynamic state controlled by phosphorylation and dephosphorylation, and a fraction of dephosphorylated Cdc20 may bind and activate mitotic APC/C. To explore this model, we prepared anaphase extracts in the presence/absence of OA, and examined the destruction of securin (an APC/C substrate) (Figure 5A). In this experiment, endogenous Cdc20 activates the APC/C, depending on its phosphorylation state. Consistent with our model, securin was degraded in anaphase extracts but upon addition of OA the ability of the APC/C to destroy securin was reduced (Figure 5A). However, adding back 5A-FL, but not Cdc20-FL, rescued securin degradation by the APC/C (Figure 5A). This result suggests that as long as the N-terminal CDK sites on Cdc20 are not phosphorylated, it can activate mitotic APC/C to initiate ubiquitylation, regardless of any hyperphosphorylation of APC/C subunits.
Figure 5.
Threonine 79 on Cdc20 bound to the APC/C is dephosphorylated in anaphase. (A) Left panel: Overphosphorylation of Cdc20, but not APC/C, abolishes its activity. Destruction of 35S-labelled securin was examined in anaphase extracts in the presence of OA (2.5 μM) and _in vitro_-translated Cdc20 full length (Cdc20-FL) or its 5A mutant (5A-FL), as indicated. Untreated anaphase extracts (+mock) served as positive controls. Right panel: quantification of the destruction assay. Error bars, s.e.m. from four independent experiments. (B) T79 on Cdc20 bound to the APC/C is barely phosphorylated. The APC/C was purified from 50 μl of anaphase egg extracts using anti-Apc3 mAb AF3.1 and analysed by immunoblotting with anti-Cdc20 mAb (BA8) and anti-p-T79 mAb (BT2.1). In the same gel, indicated amounts of anaphase egg extracts were run and analysed under the same conditions. (C) Anaphase extracts in the presence of mock, p27 (2 μM) or OA (2.5 μM) were examined by immunoblotting with anti-Apc3, pT79 (BT2.1) or anti-Cdc2 antibodies. (D) CaCl2 was added to release CSF-arrested extracts and initiate anaphase. Apc3/Cdc27, pT79, Cdc20 and cyclin B were monitored by immunoblotting. (E) Fresh ‘cycling extract’ was treated with Dynabeads-Protein A conjugated with anti-Cdc20 antibodies. Then, to a Cdc20-depleted extract, Cdc20-FL or 5A-FL was added back to give approximately the same levels as endogenous Cdc20. These extracts were incubated at 23°C to monitor progress through the cell cycle. Samples were taken at 10 min intervals for immunoblotting of Apc3/Cdc27, pT79 (BT2.1), Cdc20, cyclin B2 and Cdc2. Untreated cycling extract (mock) and the Cdc20-depleted extract served as positive and negative controls, respectively.
Another prediction from our model would be that anaphase Cdc20 bound to the APC/C is either all or mostly dephosphorylated. To investigate this possibility, we made an anti-phospho-specific monoclonal antibody against T79 (BT2.1) (see Supplementary Figure S6) and used this antibody to quantify the relative levels of phosphorylated T79-containing Cdc20 within the APC/C complex. Figure 5B shows that >80% of Cdc20 bound to the APC/C complex was dephosphorylated at T79, compared with total Cdc20 in anaphase extracts, which showed phosphorylation at T79. In addition, we examined whether the phosphorylation status of T79 is in a dynamic state of phosphorylation and dephosphorylation. The CDK activity and phosphatase(s) activity can be blocked by p27 and OA, respectively. In agreement with our model, the BT2.1 signal (pT79 status) was constant in mock-treated anaphase extracts, whereas phosphorylated T79 immediately disappeared when p27 was added. Conversely, when OA was added, the BT2.1 signal was dramatically increased (Figure 5C). Moreover, we compared the kinetics of phosphate removal from 32P-labelled S50, T79 or S114 of N159 fragments incubated in anaphase extracts (Supplementary Figure S7). In support of our model, the loss of radioactivity from T79 was much faster than that from S50 and S114.
We have investigated whether the T79 site is dephosphorylated in physiological conditions using anti-phospho-T79 mAb (BT2.1). In CSF-mediated metaphase II arrested extract, phosphorylation of T79 was detected by BT2.1. On addition of calcium, which triggers anaphase (as observed with the band shifts of Apc3/Cdc27), the signal disappeared (Figure 5D). Without calcium addition, the extract remained in metaphase II and the BT2.1 signal remained constant. We confirmed this result using a cycling extract (Figure 5E). The extract entered mitosis at around 30 and 80 min and returned to interphase 10 min later, judging from the hyper-phosphorylation status (band shift) of Apc3/Cdc27 as well as cyclin destruction (Figure 5E, mock). In the Cdc20-depleted extract, however, mitotic entry occurred at the same time, but the extract remained in mitosis. We prepared Cdc20 full-length construct (Cdc20-FL) and its 5A derivative (5A-FL) and added these to Cdc20-depleted extract to approximately the same level as that of endogenous Cdc20. When Cdc20-FL or 5A-FL was added, the cycling oscillation of Cdc20-depleted extract was significantly restored, although the kinetics was slower than the original mock extract. Intriguingly, 5A-FL seems to trigger cyclin proteolysis slightly earlier than Cdc20-FL (Figure 5E right panels; Supplementary Figure S8) during the second mitotic cycle (90–120 min). All together, we conclude that the phosphorylation status of T79 is likely to be regulated in vivo and plays a role in controlling the activity of the APC/C.
Apc6 and Apc8 subunits associate with the N-terminal domain of Cdc20
Finally, we investigated the relationship between the C-box (Cb)- and dephosphorylation-dependent association of Cdc20 with the APC/C. We prepared GST-fused N159 with ΔCb, 5A, 5A-ΔCb and WT (Figure 6A), incubated these with CSF extracts and monitored the interaction with the APC/C using Apc3 and Apc6 antibodies. As expected, WT, but not ΔCb, bound the APC/C and pulled down the APC/C complex (Figure 6B). In contrast, the same fragment with 5A showed a much higher affinity and could pull down >10 times more APC/C than WT (Figure 6B). Intriguingly, even 5A-ΔCb was able to bind more APC/C than WT, although it lacks the C-box. This result suggests that the non-phosphorylated sites on Cdc20-5A and the C-box might interact with different subunit(s) of the APC/C; therefore, we sought to identify the APC/C core subunit(s) responsible for Cdc20 dephosphorylation and the C-box-dependent interactions. Since Cdc20 has been shown to bind Apc3 through its C-terminal IR motif, the neighbouring subunits, Apc6 and Apc8 were investigated. We set up a cell-free Cdc20 binding assay and incubated GST-fused N159-5A with Apc6 or Apc8-expressing Sf9 cell lysates (Figure 6C). The same fragment with phospho-mimic residue, glutamate (5E) was used as a negative control. GST-N159-5A bound both Apc6 and Apc8, whereas 5E failed to bind Apc6, but was able to bind Apc8 as efficiently as 5A, suggesting that Apc6 binds to the dephosphorylated N-terminal domain of Cdc20. Since both 5A and 5E bind the Apc8 subunit, we investigated how the N-terminal fragments interact with the Apc8 subunit. Both GST-N159 WT and 5A fragments bound Apc8 equally; however, the interaction was abolished if the C-box was mutated (Figure 6D). To confirm the interaction between the C-box and Apc8, we introduced a point mutation in the TPR domain of Apc8 (N321A) that had previously been shown to reduce binding to the Fizzy/Cdc20 family (Matyskiela and Morgan, 2009; Izawa and Pines, 2011). Figure 6E shows that the point mutation of the TPR in Apc8 eliminated the C-box-dependent binding.
Figure 6.
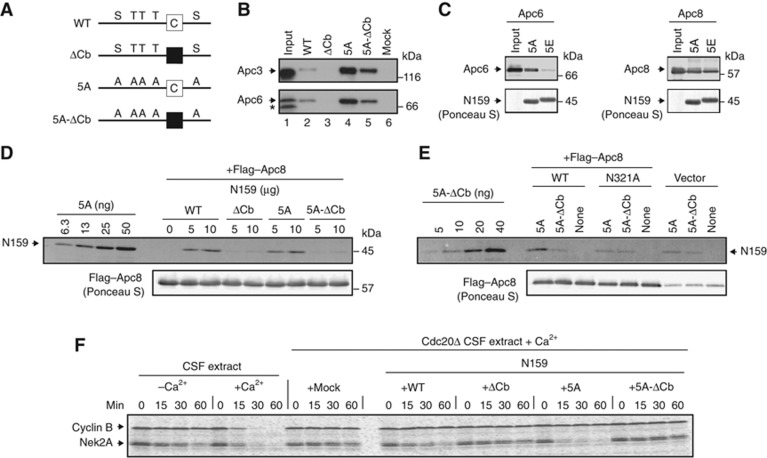
Apc6 and Apc8 associate with the activation domain of Cdc20. (A) A schematic diagram shows GST-fused Cdc20-N159 (WT) and its variants. The white box indicates an intact C-box motif whereas the black box indicates a mutated C-box motif. (B) Dephosphorylation-driven binding is independent of the C-box. The purified GST-N159 WT, ΔCb, 5A or 5A-ΔCb was bound to GSH Sepharose and incubated with CSF extract at 23°C for 20 min. The amounts of bound APC/C were analysed by immunoblotting. 50% input (lane 1) and APC/C bound to mock beads (lane 6) are also shown. The asterisk indicates a non-specific band. (C) Apc6 binds dephosphorylated Cdc20. Purified GST-N159-5A or 5E was bound to GSH Sepharose and incubated with Apc6 (left panel) or Apc8 (right panel)-expressing insect cell lysates. Co-purified Apc6 or Apc8 with GSH Sepharose-absorbed fusion proteins were monitored by immunoblotting. (D) Apc8 binds the C-box. Purified GST-N159 WT, ΔCb, 5A or 5A-ΔCb was incubated with Flag–Apc8-overexpressed insect cell lysates and Apc8 was isolated by anti-Flag IP. Co-purified N159 was monitored by immunoblotting. (E) A point mutation in Apc8 is sufficient to reduce the interaction with the C-box. Same as (D), but insect cell lysates expressing Flag–Apc8 WT, N321A mutation or mock (vector) were used. Flag–Apc8 was isolated and bound proteins were investigated. (F) The C-box is essential to activate the APC/C. Purified N159 WT or its mutant (ΔCb, 5A or 5A-ΔCb) was added to Cdc20-depleted CSF extracts and destruction of Nek2A and cyclin B (fission yeast Cdc13) was examined. Samples were taken at the indicated time points after addition of CaCl2 and analysed by SDS–PAGE and autoradiography.
We further examined how the affinity between Cdc20 and APC/C reflects the ability to activate the APC/C by monitoring Nek2A destruction in Cdc20-depleted CSF extracts. WT or 5A could degrade Nek2A whereas ΔCb or 5A-ΔCb could not (Figure 6F). Cyclin B served as a negative control for N159-dependent destruction of APC/C substrates. Since 5A-ΔCb clearly has a higher affinity for the APC/C than WT (Figure 6B) but fails to degrade Nek2A, this result suggests that the C-box is involved in subsequent activation after association with the APC/C.
Discussion
Despite the importance of CDK in the activation of the APC/C-ubiquitin system in mitosis, the mechanism controlling APC/C–Cdc20 interaction and activation has remained obscure for over a decade. The study presented here demonstrates how the activation role of Cdc20 is regulated by CDK and phosphatases, both of which control the phosphorylation status around the C-box in the N-terminal domain. We have identified three key phosphorylation sites (T64/T68/T79) and have shown that phosphatase(s) responsible for these sites, including PP2A, are active in mitosis. Dephosphorylation reinforces the interaction between the N-terminal domain of Cdc20 and the APC/C, allowing C-box-dependent activation of the APC/C. Moreover, we have shown that the TPR subunits Apc6 and Apc8 are involved in Cdc20-dependent activation of the APC/C. There are several lines of evidence that phosphorylation of APC/C subunits by CDK is essential for Cdc20 activation of the APC/C (Fang et al, 1998; Shteinberg et al, 1999; Kramer et al, 2000; Rudner and Murray, 2000; Yudkovsky et al, 2000), but we surmize that phosphorylation of APC/C may be for the ‘priming’ of activation and dephosphorylation of Cdc20 provides a second level of control in order to ensure that the APC/C is only active at the right time. Both phosphorylation and dephosphorylation are critical for the activation of the APC/C (Figure 7).
Figure 7.
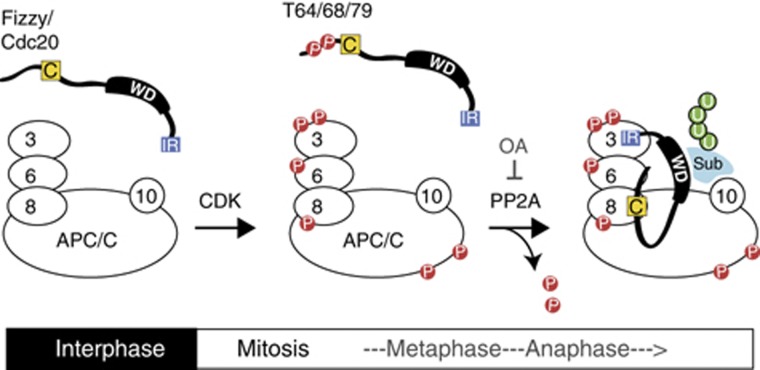
A model for phosphorylation-regulated APC/C activation. In interphase, both Cdc20 and the APC/C are unphosphorylated, and the APC/C is barely active. Upon entry into mitosis, CDK phosphorylates both the APC/C and Cdc20. Phosphorylation of APC/C increases its affinity for Cdc20 whereas phosphorylation of Cdc20 at T64/T68/T79 decreases its affinity to the APC/C. Thus, N-terminal phosphorylation of Cdc20 must be kept constantly in check during mitosis by phosphatase(s). At the metaphase–anaphase transition, T64/68/79 dephosphorylation of Cdc20 induces its binding to Apc6 subunit that ensures the association between the C-box and Apc8 and the subsequent activation of the APC/C. If PP2A is inhibited by OA, then the hyper-phosphorylated Cdc20 has little or no affinity for the APC/C and thus the C-box fails to activate APC/C-mediated ubiquitylation. Both CDK and PP2A are required for the activation of Cdc20-APC/C. For details, see text.
Our results are consistent with previous observations that after phosphorylation human Cdc20 is less active (Kramer et al, 2000), and binding to Xenopus APC/C is reduced (D'Angiolella et al, 2003). It has also been reported that phosphorylation of Cdc20 reduces its binding to the APC/C and negatively regulates APC/C activity in HeLa cells (Yudkovsky et al, 2000; D'Angiolella et al, 2003). Yet, in both cases, the APC/C was immunoprecipitated from cells in which the SAC had been activated with nocodazole. We show here that the negative effect of Cdc20 phosphorylation on its activation of the APC/C is observed under normal conditions and is not dependent upon the SAC as it is not active in Xenopus egg extracts. Our results are also in agreement with a mathematical modelling for cell-cycle oscillations during Xenopus early development. CDK activates the APC/C and simultaneously antagonizes Cdc20, which allows cycling as long as Cdc20 is phosphorylated and dephosphorylated faster than the APC/C (Ciliberto et al, 2005). We believe this is ensured by phosphatase(s) that specifically target Cdc20 even while CDK is active (Figure 7). Once the APC/C becomes active, degradation of cyclin B lowers CDK activity, which in turn decreases phosphorylation of Cdc20 and this positive feedback loop allows degradation of numerous APC/C substrates and exit from mitosis.
Since the phosphorylation sites in the N terminus are well conserved between Xenopus and humans (Supplementary Figure S2), the phosphatase regulation demonstrated here may be conserved among vertebrates. Indeed in HeLa cells phosphorylation negatively regulates Cdc20 so it is plausible that a Cdc20 phosphatase would be needed to ensure activation of the APC/C. Although we have not investigated the SAC involvement in this study, it is also possible that the SAC inhibits the activation of the APC/C through a regulation of Cdc20 dephosphorylation. For example, phosphatase activity for Cdc20 may be downregulated while the SAC is active either directly or indirectly or possibly activated only after the SAC is satisfied. However, it is also conceivable that our findings in Xenopus egg extracts may not be fully applicable to somatic and/or mitotic cells, because cell-free extracts prepared from unfertilized Xenopus eggs retain a number of meiotic proteins and lack some of the key somatic proteins such as Cdh1.
What is the phosphatase(s) responsible for Cdc20 dephosphorylation? When calcium is added to CSF extract (upon exit from meiotic metaphase II), PP2B/calcineurin partly dephosphorylates all CDK sites on Cdc20 except S114, but OA-sensitive phosphatase(s) seems to contribute more to the dephosphorylation of CDK sites on Cdc20 in Xenopus early cell cycles (Figure 3). The half-maximal inhibition by OA was observed at around 200–400 nM. Immunodepletion experiments imply that PP2A is a key phosphatase responsible for T64/79 dephosphorylation in anaphase, but it is likely that PP1 or other phosphatases are also involved in Cdc20 dephosphorylation. PP2A is composed of three subunits, a catalytic subunit (C), a scaffolding subunit (A) and one of several variable regulatory subunits (B) (Janssens et al, 2008). There are at least three subfamilies of B subunit in Xenopus each containing several isoforms: B (also known as B55 or PR55), B’ (B56 or PR61) and B’’ (PR48). Since we do not have antibodies against B subunits to specifically immunodeplete PP2A holocomplexes containing different B-subunits, at the moment, we do not know which B-subunit associated PP2A is responsible for T64/68/79 dephosphorylation. However, PP2A-B55δ, that has been shown to dephosphorylate S50, is unlikely to dephosphorylate those sites since it is inactive in mitosis (Mochida et al, 2009; Mochida et al, 2010; Gharbi-Ayachi et al, 2011).
We have shown that even within a single protein, Cdc20, different PP2A phosphatases are involved, depending on the sites modified (T64/T68/T79 versus S50). We postulate that phosphatase(s) targeting the APC/C and Cdc20 may also be different and thus APC/C and Cdc20 can be dephosphorylated at different times. To facilitate the formation of active APC/C-Cdc20 complex, phosphatases responsible for APC/C should be inactive or less active than Cdc20 phosphatase(s) in mitosis (Figure 7). One such responsible phosphatase targeting APC/C may be PP2A-B55δ, a CDK-counteracting phosphatase, which is inhibited during mitosis by Greatwall (Mochida et al, 2009; Mochida et al, 2010; Gharbi-Ayachi et al, 2011). Since PP2A-B55δ can only be activated upon CDK inactivation, this might explain how APC/C is more slowly dephosphorylated than Cdc20 in anaphase (Ciliberto et al, 2005).
Which APC/C subunit(s) have to be phosphorylated in order to bind Cdc20 in mitosis? This remains to be elucidated. We observed that the N-terminal Cdc20 (N159) was able to bind the APC/C in egg extracts in the presence of roscovitine (Figure 2) where the majority of APC/C subunits are likely to be dephosphorylated. However, N159-driven Nek2A destruction was much faster in mitotic extracts than in interphase extracts (data not shown). It is possible that phosphorylation of the APC/C may be required for a subsequent step after its binding to Cdc20 such as C-box-dependent activation. Alternatively, during the formation of the APC/C–Cdc20 complex, phosphorylation of APC/C subunit(s) might be preferentially monitored by the C terminus of Cdc20, rather than the N terminus. Identification of the responsible phosphorylation sites within the APC/C is an important topic for future investigation.
How is Cdc20 dephosphorylation involved in the activation of the APC/C? Our results suggest that the dephosphorylation-driven binding domain in the N terminus functions as a third APC/C binding site together with the C-box (Schwab et al, 2001) and the C-terminal IR dipeptide motif (Passmore et al, 2003; Vodermaier et al, 2003). Non-phosphorylatable N159-5A, but not the same fragment with phosphor-mimic residues (5E), binds Apc6. In contrast, N159 interacts with Apc8 in a C-box-dependent manner. These results may have implications for the mechanism of APC/C activation. For instance, since Apc8 is localized next to Apc6 in the TPR-containing subcomplex in the architectural map and cryo-EM structural analysis of the APC/C (Thornton et al, 2006; Schreiber et al, 2011), it is tempting to speculate that dephosphorylation-dependent engagement of Cdc20 with Apc6 ensures the adjacent C-box interacts with Apc8 (Figure 7). The C-box has been proven to be more important than the IR motif in vivo and in vitro for APC/C-dependent ubiquitylation (Thornton et al, 2006; Kimata et al, 2008; Matyskiela and Morgan, 2009). We speculate that the interaction between the C-box and Apc8 might provoke conformational changes and/or catalysis within the APC/C. In agreement with this, the Pines lab has recently shown that Apc8 plays a crucial role in activating the APC/C in the presence or absence of the SAC (Izawa and Pines, 2011). However, in budding yeast, APC/C from _apc2_Δ strains has been shown to cause a 10-fold reduction in Cdh1 binding (Thornton et al, 2006) and recent three-dimensional EM structural analysis of the APC/C suggests that the C-box motif may contact with Apc2 (Schreiber et al, 2011). We therefore investigated the interaction between N159 and Apc2 in our binding assay but could not detect any C-box-dependent signals (data not shown). Yet, from the structure of the APC/C, it is still possible for the C-box to interact with both Apc8 and Apc2, although the order or dependency of these interactions remains to be determined. Alternatively, the N-terminal domain of Cdc20 or Cdh1 might preferentially bind different APC/C subunits. Further work will be required to gain a full appreciation of how the C-box activates the APC/C.
Finally, it is worth noting that the regulation of the two co-activators, Cdc20 and Cdh1 might be more similar than was first envisaged. CDK phosphorylation of both co-activators has a negative effect on their APC/C activation and as such their dephosphorylation is required for APC/C activation. However, one of the major differences in regulation may stem from their phosphatase sensitivity. As we have shown in this study, Cdc20 is kept sufficiently dephosphorylated during mitosis, to allow APC/C activation, whereas Cdh1 appears to be only dephosphorylated at the end of mitosis and during interphase. In budding yeast, Cdc14 is clearly the key phosphatase responsible for the completion of mitosis (Visintin et al, 1998; Jaspersen et al, 1999; Shou et al, 1999). Since Cdh1 can activate both mitotic and interphase APC/C, it might be crucial to restrict activation of Cdh1 until the end of mitosis. In addition, it is highly probable that spatial regulation of phosphatases in vivo contributes to the timely activation of the APC/C. The interplay between CDK and the counteracting phosphatases controlling APC/C and its co-activators is of great interest, and it will be important to understand the mechanism of APC/C activation and catalysis in vivo.
Materials and methods
Xenopus egg extracts
Meiotic metaphase II-arrested (CSF) Xenopus laevis egg extracts were prepared as described (Murray, 1991). Interphase ‘cycling’ extracts were prepared following the protocol described in Ohsumi et al (2006). Anaphase extracts were made by adding non-degradable GST-fused Xenopus cyclinBΔ167 (a truncated cyclin B lacking the first N-terminal 167 amino acids) to interphase extracts and incubating for 30 min at 23°C. The anaphase induced in this fashion corresponds to the first mitotic anaphase.
Plasmid/bacmid construction and mutagenesis
To make a GST-fused series of peptides covering the whole of Cdc20, the corresponding double-stranded oligonucleotides were synthesized and subcloned into pGEX vector. The 19 peptides used in Figure 1 contain amino-acid numbers of Xenopus Cdc20 as follows: Peptide 1 (1–27), Peptide 2 (28–54), Peptide 3 (55–75), Peptide 4 (76–102), Peptide 5 (103–129), Peptide 6 (130–156), Peptide 7 (157–183), Peptide 8 (184–210), Peptide 9 (211–237), Peptide 10 (238–264), Peptide 11 (265–291), Peptide 12 (292–318), Peptide 13 (319–345), Peptide 14 (346–372), Peptide 15 (373–399), Peptide 16 (400–426), Peptide 17 (427–453), Peptide 18 (454–480) and Peptide 19 (481–507). Mutants and truncations of Xenopus Cdc20 constructs were generated by PCR-based mutagenesis and subcloned into pGEX and pET16b-NGST vectors (Kimata et al, 2008). Full-length Cdc20 constructs were subcloned into pHY22 (Yamano et al, 1996). Bacmids expressing APC/C subunits were constructed with the Bac-to-Bac system (Invitrogen, UK). Mutations were introduced using the QuickChange Site-directed mutagenesis kit (Stratagene, UK). All constructs were confirmed by DNA sequencing (Cogenics, UK and UCL in-house).
Recombinant proteins
GST-fused proteins were expressed in BL21-CodonPlus (DE3) bacteria overnight at 20°C in the presence of 0.1 mM IPTG. The cells were lysed by 1 mg/ml lysozyme followed by sonication in lysis buffer (50 mM Tris–HCl pH 7.5, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 0.5% NP-40) containing protease inhibitor cocktail (Sigma-Aldrich, UK). The proteins were purified from clarified lysates on Glutathione Sepharose 4B beads (GE Healthcare, UK). 6His-fused proteins were expressed in BL21-CodonPlus (DE3) bacteria at 37°C for 3 h in the presence of 0.1 mM IPTG. The cells were lysed by 2 mg/ml lysozyme followed by sonication in IMAC-5 (20 mM Tris–HCl pH 8, 0.5 M NaCl, 5 mM imidazole) containing 5 mM EGTA, 1 mM PMSF and protease inhibitor cocktail (Sigma-Aldrich). The proteins were purified from clarified lysates on Ni-NTA agarose beads (Qiagen, UK). Full-length Cdc20 proteins (WT and 5A mutant) were translated from the corresponding mRNA for 4 h at 23°C in a mixture containing 80% Xenopus interphase extract and 20% rabbit reticulocyte lysate (Promega, UK). Before mixing, the interphase extracts were first depleted of endogenous Cdc20 and then treated with RNase A followed by the addition of RNase A inhibitor RNasin (Promega) in order to degrade endogenous mRNA and protect added mRNAs from degradation, allowing maximum translation.
Kinase assays
GST-fused Cdc20 short peptides (2 μg) or Cdc20-N159 proteins (1 μg) were incubated for 20 min at room temperature in 10 μl of kinase buffer (20 mM HEPES pH 7.8, 10 mM MgCl2, 15 mM KCl, 1 mM EGTA, 5 mM NaF, 20 mM β-glycerophosphate) containing 0.1 mM ATP, [γ-32P]ATP (Hartmann Analytic, UK) and recombinant CDK2/cyclin A, CDK2/cyclin B or 2 μl of Xenopus egg extract (Figure 1B and C). Reactions were stopped with SDS sample buffer and analysed by SDS–PAGE. To prepare phosphorylated Cdc20-N159 (Figures 1E and 4), purified Cdc20-N159 proteins were phosphorylated in 10 μl of kinase buffer containing recombinant CDK2/cyclin A and 0.1 mM ATP. The non-phosphorylated Cdc20-N159 proteins were mock treated in the same conditions before their addition to the extracts.
APC/C-dependent ubiquitylation and destruction assays
Destruction assays were performed essentially as described previously (Yamano et al, 2009). Substrates were labelled with [35S]methionine (Hartmann Analytic) in a coupled in-vitro transcription-translation system (Promega). _In vitro_-translated Cdc20 constructs or recombinant GST-Cdc20-N159 proteins were supplemented into Cdc20-depleted egg extracts and after 5 min incubation at 23°C, the APC/C substrates were mixed. To analyse the effects of specific phosphorylation of Cdc20 (Figure 4), destructions assays were carried out in anaphase extracts with increased CDK activity and reduced phosphatase activity. Cdc20-depleted interphase extracts were incubated for 1 h with 1 μM GST-fused Xenopus cyclinBΔ167. After a further 10 min incubation with 2.5 μM OA, [35S]-labelled Nek2A was mixed to the extract and finally the pre-phosphorylated Cdc20-N159 constructs were added to trigger destruction of Nek2A. The samples were taken at the indicated time points and analysed by SDS–PAGE and autoradiography. The images were analysed using ImageQuant TL (GE Healthcare) and the statistical analysis was performed using Prism GraphPad Software (CA, USA). For ubiquitylation assays, Xenopus APC/C was immunoprecipitated from 15 μl of Cdc20-depleted mitotic extracts using monoclonal anti-Apc3 antibody (mAB, AF3.1) immobilized on Dynabeads Protein A (Invitrogen, UK). Reactions were performed at 23°C in 10 μl of ubiquitylation buffer (20 mM Tris–HCl pH 7.5, 100 mM KCl, 2.5 mM MgCl2, 2 mM ATP, 0.3 mM DTT) containing 0.1 mg/ml E1, Xenopus Ubc4 and Xenopus UbcX, 1.5 mg/ml ubiquitin, 1 μM ubiquitin-aldehyde, 150 μM MG132 and 1 μl of _in vitro_-translated Cdc20 or purified GST-N159 protein. Reactions were stopped at the indicated time points with SDS sample buffer and analysed by SDS–PAGE.
Binding assays
Purified GST-Cdc20-N159-6His protein or its derivatives were incubated with GSH-Sepharose beads for 2 h at 4°C. The beads were washed with PBSN (PBS containing 0.01% NP-40), XBcsf (10 mM Hepes pH 7.8, 50 mM sucrose, 100 mM KCl, 2 mM MgCl2, 5 mM EGTA) containing 0.01% NP-40 and XBcsf, and then incubated with CSF extract treated with 150 μM MG132. The beads were separated from the extract on Micro Bio-Spin columns (Bio-Rad, UK), washed three times in XB buffer (10 mM Hepes pH 7.8, 50 mM sucrose) and boiled in SDS sample buffer to elute bound proteins. For the binding assay using recombinant Xenopus APC/C subunit proteins (Figure 6), Sf9 insect cells were infected with the viruses bearing Myc–Apc6, Flag–Apc8 or Flag–Apc8N321A. After infection, the cells were incubated at 27°C for 72 h and harvested by centrifugation. The cell pellets were resuspended in Sf9 lysis buffer (50 mM Hepes pH 7.9, 150 mM NaCl, 1 mM EDTA, 0.1% NP-40, 1 mM DTT and 10 μg/ml LPC protease inhibitors) and rotated at 4°C for 30 min. Lysates were separated by centrifugation at 15 000 g for 15 min and then incubated with GSH beads bound to GST-Cdc20-N159-6His or its derivatives on ice for 20 min. The beads were separated from the lysate on Micro Bio-Spin columns (Bio-Rad), and washed three times in Sf9 lysis buffer. The bound proteins were eluted in SDS sample buffer. For the binding assay using Flag-M2 beads (Sigma-Aldrich; Figure 6D and E), Flag beads bound to Flag–Apc8, Flag–Apc8N321A or mock were incubated with purified GST-Cdc20-N159-6His or its derivatives on ice for 20 min and then beads were analysed as above.
Phosphatase assays
Phosphatase assays were performed essentially as described previously (Mackintosh and Moorhead, 1999). GST-fused N159-4A mutant proteins (one out of five possible sites is open for phosphorylation) (Supplementary Figure S4) were prepared and phosphorylated using CDK2/cyclin A and [γ-32P]ATP, and the radioactivity released, after addition to extracts, was measured by liquid scintillation counting (Beckman Coulter, UK). The statistic analysis was performed using Prism GraphPad Software. The same phosphorylated substrates were also incubated in Xenopus anaphase extract to investigate the dephosphorylation kinetics of individual phosphorylation sites (Supplementary Figure S7). In all, 1.5 μl of the [γ-32P]-labelled Cdc20-N159-4A was added to 12 μl of water or anaphase extract. Samples (2 μl) were taken at the indicated time points and analysed by SDS–PAGE and autoradiography. The resulting images were analysed using ImageQuant TL (GE Healthcare) and Prism GraphPad Software.
PP2A immunodepletion
Immunodepletion of PP2A from Xenopus egg extracts was carried out using protein-G Sepharose beads (Sigma-Aldrich) conjugated with rat monoclonal antibody 6F9, anti-A-alpha subunit of PP2A (a gift from Drs G Walter and R Ruediger). In all, 600 μg of 6F9 was bound to 60 μl of Protein G Sepharose beads and used for depleting PP2A holoenzyme from 40 μl of egg extracts. As described previously the extracts were kept as concentrated as possible during the immunodepletion procedure to minimize any increase in non-physiological phosphatase activity (Mochida et al, 2009).
Antibodies
To make phosphor-specific monoclonal antibody of Threonine 79 on Cdc20, a phospho-peptide GPKMQGpTPSRAGC was coupled to KLH and injected into Balb/c mice. Hybridoma cells were produced by fusing the spleen cells from a hyperimmune mouse to Sp/0 myeloma cells using standard procedures. Positive clones were identified using immunoadsorbent assays and immunoblotting of Xenopus egg extracts. The specificity of mAb BT2.1 to phospho-T79 was confirmed by further experiments (see Supplementary Figure S6). Antibodies were used as follows: anti-Apc3/Cdc27 (1:100; BD Transduction Laboratories), Apc6 (rbAB 3446, 1:100), Apc8 (rbAB 3492, 1:500), Cdc20 (mAb BA8, 1:100), Cdc20 phospho-T79 (mAb BT2.1, 1:20), Cdc20-N (mAb BG16.1 1:5000), PP2A-C (1:200; Cell Signaling Technology, UK), anti-cyclin B2 (mAb X121), anti-PSTAIRE (1:5000; a gift from Dr Y Nagahama, Japan).
Supplementary Material
Supplementary Data
Review Process File
Acknowledgments
We would like to thank members of the Yamano laboratory for helpful discussions and critical reading of the manuscript. We would also like to thank Drs Gernot Walter and Ralf Ruediger for providing the hybridoma producing 6F9 antibody; Yoshitaka Nagahama for anti-PSTAIRE antibody; Satoru Mochida for helpful advice on phosphatase assays; Bela Novak for thoughtful discussion; Tim Hunt and Hiro Mahbubani for access to the Cancer Research UK Clare Hall Laboratories Xenopus colony in the early stages of this project; the staff at the UCL Biological Services Unit for taking care of the Xenopus colony in UCL. This work was supported by Marie Curie Cancer Care and the Association for International Cancer Research (AICR).
Author contributions: HL, KF and HY conceived and designed experiments. HL, KF and NSB expressed and purified the proteins. TT and KF made baculoviruses. HL, KF, JG and HY performed the experiments and analysed the data. HL and HY wrote the paper. KF and JG commented on the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Barford D (2010) Structure, function and mechanism of the anaphase promoting complex (APC/C). Q Rev Biophys 44: 1–38 [DOI] [PubMed] [Google Scholar]
- Burton JL, Tsakraklides V, Solomon MJ (2005) Assembly of an APC-Cdh1-substrate complex is stimulated by engagement of a destruction box. Mol Cell 18: 533–542 [DOI] [PubMed] [Google Scholar]
- Buschhorn BA, Petzold G, Galova M, Dube P, Kraft C, Herzog F, Stark H, Peters JM (2011) Substrate binding on the APC/C occurs between the coactivator Cdh1 and the processivity factor Doc1. Nat Struct Mol Biol 18: 6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung E, Chen RH (2003) Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat Cell Biol 5: 748–753 [DOI] [PubMed] [Google Scholar]
- Ciliberto A, Lukacs A, Toth A, Tyson JJ, Novak B (2005) Rewiring the exit from mitosis. Cell Cycle 4: 1107–1112 [PubMed] [Google Scholar]
- D'Angiolella V, Mari C, Nocera D, Rametti L, Grieco D (2003) The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev 17: 2520–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Fonseca PC, Kong EH, Zhang Z, Schreiber A, Williams MA, Morris EP, Barford D (2011) Structures of APC/C(Cdh1) with substrates identify Cdh1 and Apc10 as the D-box co-receptor. Nature 470: 274–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore B, Pines J (2007) Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J Cell Biol 177: 425–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang G, Yu H, Kirschner MW (1998) Direct binding of CDC20 protein family members activates the anaphase-promoting complex in mitosis and G1. Mol Cell 2: 163–171 [DOI] [PubMed] [Google Scholar]
- Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, Lorca T (2011) The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330: 1673–1677 [DOI] [PubMed] [Google Scholar]
- Gmachl M, Gieffers C, Podtelejnikov AV, Mann M, Peters JM (2000) The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc Natl Acad Sci USA 97: 8973–8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskortenhaus R, Sprenger F (2002) Rca1 inhibits APC-Cdh1(Fzr) and is required to prevent cyclin degradation in G2. Dev Cell 2: 29–40 [DOI] [PubMed] [Google Scholar]
- Harper JW, Burton JL, Solomon MJ (2002) The anaphase-promoting complex: it's not just for mitosis any more. Genes Dev 16: 2179–2206 [DOI] [PubMed] [Google Scholar]
- Izawa D, Pines J (2011) How APC/C-Cdc20 changes its substrate specificity in mitosis. Nat Cell Biol 13: 223–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Longin S, Goris J (2008) PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem Sci 33: 113–121 [DOI] [PubMed] [Google Scholar]
- Jaspersen SL, Charles JF, Morgan DO (1999) Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr Biol 9: 227–236 [DOI] [PubMed] [Google Scholar]
- Kimata Y, Baxter JE, Fry AM, Yamano H (2008) A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol Cell 32: 576–583 [DOI] [PubMed] [Google Scholar]
- Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM (2005) The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol Cell 18: 543–553 [DOI] [PubMed] [Google Scholar]
- Kramer ER, Scheuringer N, Podtelejnikov AV, Mann M, Peters JM (2000) Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol Biol Cell 11: 1555–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremmer E, Ohst K, Kiefer J, Brewis N, Walter G (1997) Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol Cell Biol 17: 1692–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverson JD, Joazeiro CA, Page AM, Huang H, Hieter P, Hunter T (2000) The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol Biol Cell 11: 2315–2325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Maller JL (2005) Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol 15: 1458–1468 [DOI] [PubMed] [Google Scholar]
- Mackintosh C, Moorhead DG (1999) In Protein Phosphorylation: A Practical Approach, Hardie DG (ed.) pp 153–181. Oxford University Press: Oxford, UK [Google Scholar]
- Matyskiela ME, Morgan DO (2009) Analysis of activator-binding sites on the APC/C supports a cooperative substrate-binding mechanism. Mol Cell 34: 68–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Hunt T (2007) Calcineurin is required to release Xenopus egg extracts from meiotic M phase. Nature 449: 336–340 [DOI] [PubMed] [Google Scholar]
- Mochida S, Ikeo S, Gannon J, Hunt T (2009) Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J 28: 2777–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochida S, Maslen SL, Skehel M, Hunt T (2010) Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330: 1670–1673 [DOI] [PubMed] [Google Scholar]
- Murray AW (1991) Cell cycle extracts. Methods Cell Biol 36: 581–605 [PubMed] [Google Scholar]
- Ohsumi K, Yamamoto TM, Iwabuchi M (2006) Oocyte extracts for the study of meiotic M-M transition. Methods Mol Biol 322: 445–458 [DOI] [PubMed] [Google Scholar]
- Passmore LA, McCormack EA, Au SW, Paul A, Willison KR, Harper JW, Barford D (2003) Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. EMBO J 22: 786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesin JA, Orr-Weaver TL (2008) Regulation of APC/C activators in mitosis and meiosis. Annu Rev Cell Dev Biol 24: 475–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM (2006) The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7: 644–656 [DOI] [PubMed] [Google Scholar]
- Rauh NR, Schmidt A, Bormann J, Nigg EA, Mayer TU (2005) Calcium triggers exit from meiosis II by targeting the APC/C inhibitor XErp1 for degradation. Nature 437: 1048–1052 [DOI] [PubMed] [Google Scholar]
- Rudner AD, Murray AW (2000) Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J Cell Biol 149: 1377–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A, Stengel F, Zhang Z, Enchev RI, Kong EH, Morris EP, Robinson CV, da Fonseca PC, Barford D (2011) Structural basis for the subunit assembly of the anaphase-promoting complex. Nature 470: 227–232 [DOI] [PubMed] [Google Scholar]
- Schwab M, Neutzner M, Mocker D, Seufert W (2001) Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. EMBO J 20: 5165–5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou W, Seol JH, Shevchenko A, Baskerville C, Moazed D, Chen ZW, Jang J, Charbonneau H, Deshaies RJ (1999) Exit from mitosis is triggered by Tem1-dependent release of the protein phosphatase Cdc14 from nucleolar RENT complex. Cell 97: 233–244 [DOI] [PubMed] [Google Scholar]
- Shteinberg M, Protopopov Y, Listovsky T, Brandeis M, Hershko A (1999) Phosphorylation of the cyclosome is required for its stimulation by Fizzy/cdc20. Biochem Biophys Res Commun 260: 193–198 [DOI] [PubMed] [Google Scholar]
- Tang Z, Li B, Bharadwaj R, Zhu H, Ozkan E, Hakala K, Deisenhofer J, Yu H (2001) APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol Biol Cell 12: 3839–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton BR, Ng TM, Matyskiela ME, Carroll CW, Morgan DO, Toczyski DP (2006) An architectural map of the anaphase-promoting complex. Genes Dev 20: 449–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton BR, Toczyski DP (2006) Precise destruction: an emerging picture of the APC. Genes Dev 20: 3069–3078 [DOI] [PubMed] [Google Scholar]
- Tung JJ, Hansen DV, Ban KH, Loktev AV, Summers MK, Adler JR 3rd, Jackson PK (2005) A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc Natl Acad Sci USA 102: 4318–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintin R, Craig K, Hwang ES, Prinz S, Tyers M, Amon A (1998) The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell 2: 709–718 [DOI] [PubMed] [Google Scholar]
- Vodermaier HC, Gieffers C, Maurer-Stroh S, Eisenhaber F, Peters JM (2003) TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr Biol 13: 1459–1468 [DOI] [PubMed] [Google Scholar]
- Yamano H, Gannon J, Hunt T (1996) The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J 15: 5268–5279 [PMC free article] [PubMed] [Google Scholar]
- Yamano H, Trickey M, Grimaldi M, Kimata Y (2009) In vitro assays for the anaphase-promoting complex/cyclosome (APC/C) in Xenopus egg extracts. Methods Mol Biol 545: 287–300 [DOI] [PubMed] [Google Scholar]
- Yu H (2007) Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell 27: 3–16 [DOI] [PubMed] [Google Scholar]
- Yudkovsky Y, Shteinberg M, Listovsky T, Brandeis M, Hershko A (2000) Phosphorylation of Cdc20/fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem Biophys Res Commun 271: 299–304 [DOI] [PubMed] [Google Scholar]
- Zachariae W, Schwab M, Nasmyth K, Seufert W (1998) Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science 282: 1721–1724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data
Review Process File