Translating Metabolomics to Cardiovascular Biomarkers (original) (raw)
. Author manuscript; available in PMC: 2013 Jul 1.
Published in final edited form as: Prog Cardiovasc Dis. 2012 Jul;55(1):70–76. doi: 10.1016/j.pcad.2012.06.004
Abstract
Metabolomics is the systematic study of the unique chemical fingerprints of small-molecules, or metabolite profiles, that are related to a variety of cellular metabolic processes in a cell, organ, or organism. While mRNA gene expression data and proteomic analyses do not tell the whole story of what might be happening in a cell, metabolic profiling provides direct and indirect physiologic insights that can potentially be detectable in a wide range of biospecimens. Although not specific to cardiac conditions, translating metabolomics to cardiovascular biomarkers has followed the traditional path of biomarker discovery from identification and confirmation to clinical validation and bedside testing. With technological advances in metabolomic tools (such as nuclear magnetic resonance spectroscopy and mass spectrometry) and more sophisticated bioinformatics and analytical techniques, the ability to measure low-molecular-weight metabolites in biospecimens provides a unique insight into established and novel metabolic pathways. Systemic metabolomics may provide physiologic understanding of cardiovascular disease states beyond traditional profiling, and may involve descriptions of metabolic responses of an individual or population to therapeutic interventions or environmental exposures.
Introduction
Metabolism encompasses complex sets of chemical reactions that sustain life, often involving the conversion of food stores and nutrients (including sugars, amino acids, organic acids, nucleotides, and lipids) into energy or signals or the breakdown of noxious compounds for elimination. This occurs through a multitude of enzymatic reactions that are highly conserved across species and between cell types. For decades, there has been an emerging concept that dynamic alterations in such specific metabolites may reflect underlying disease states. The “metabolome” (a complete set of small-molecule metabolites such as intermediates, hormones, and other signaling molecules) therefore represents the collection of all low-molecular weight (often <1 kDa) metabolites in a biological cell, tissue, organ, or organism, which are the end products of these enzymatic cellular processes under a particular set of environmental conditions. It may be tightly controlled by the host genome (so-called “primary metabolome”) or may dependent on a consortium of micro-organisms that live commensally or symbiotically with the host (so-called “secondary metabolome”). The systematic study of such unique chemical finger-prints that reflect small changes in specific cellular processes has been referred to as “metabolomics”1, 2 (some referred to as “metabonomics”3).
Over the past decade, enhanced use of metabolomics tools in medicine has allowed for an unbiased approach to study altered metabolite profiles observed in disease state. The rapid expansion in the study of metabolomics has largely depended upon advances in analytical chemical techniques that allow one to precisely determine ever larger growing arrays of metabolites in a timely and accurate manner. Equally important in the rapid advances in analytical techniques, are the parallel expansion of computational and bioinformatic tools to integrate the vast information generated by these analyses. Companion areas of expansion to metabolomic research are new areas of research in the study of lipids (lipomics) and proteins (proteomics or peptidomics).
In metabolomics, the investigation of lipids, sugars, organic acids, amino acids and other small molecule analytes that are substrates and byproducts in myriad metabolic pathways can be used to investigate medical conditions, such as cardiovascular disease. This is a major feat given the shear number, concentration, and differences in polarity of these metabolites and the intrinsic limitations of any analytical tool to assimilate such large quantities of data. Indeed, the Human Metabolome Project was established to identify, quantify, and store data on discovered metabolites from human tissue or fluid in concentrations greater than 1 micromolar. To date over 8,500 metabolites have been identified in the Human Metabolome Database.4, 5 Currently, metabolomics is used widely as part of understanding of systems biology.6, 7 This has major advantages over traditional cardiovascular biomarkers as they are not restricted to known pathophysiologic pathways or single biomarker measurements that reflect only a specific aspect of the disease process of interest. Indeed, metabolomic research has been one of the most promising biomarker discovery approaches, particularly when analytical chemistry laboratories are increasingly applying these techniques to everyday clinical laboratory analyses.
Tools in Metabolomics
The analytical tools that have been most widely used in biomedical applications are nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry. The latter is typically coupled with a method for separation of analytes within a biospecimen prior to entry into the mass spectrometer, using either liquid chromatography (LC-MS) or gas chromatography (GC-MS). Both techniques (LC-MS and GC-MS) have been available to researchers for decades, but more recently have been increasingly applied to measurement of metabolites for potential clinical applications. Both techniques have similar challenges in terms of their dynamic range and the labile nature of many species that are part of ongoing metabolic processes. They also suffer from alterations in results often observed with subsets of analytes based on variabilities in sample collection and handling, and thus require stringent quality assurance and quality control protocols.
NMR spectroscopy relies on the property of magnetic spin of nuclei (paramagnetic) to determine the chemical entities in a molecule. The most commonly used paramagnetic elements for analysis are H1, C13, and P31. The advantages of NMR are that it requires relatively little sample preparation (i.e. no separation techniques are needed), is non-destructive, and provides information about the precise structure of the metabolite.8 However, NMR is limited based of magnet strength to detect chemical shifts for metabolite identification, throughput, and sensitivity. As a result, only the most abundant metabolites can be unambiguously detected since there is limited sensitivity for lower concentration compounds. While longer data acquisition times provide enhanced sensitivity, in practical terms, for rapid analyses (e.g. minute acquisition times) NMR requires concentrations of 100 nmol/L to 1umol/L or higher.
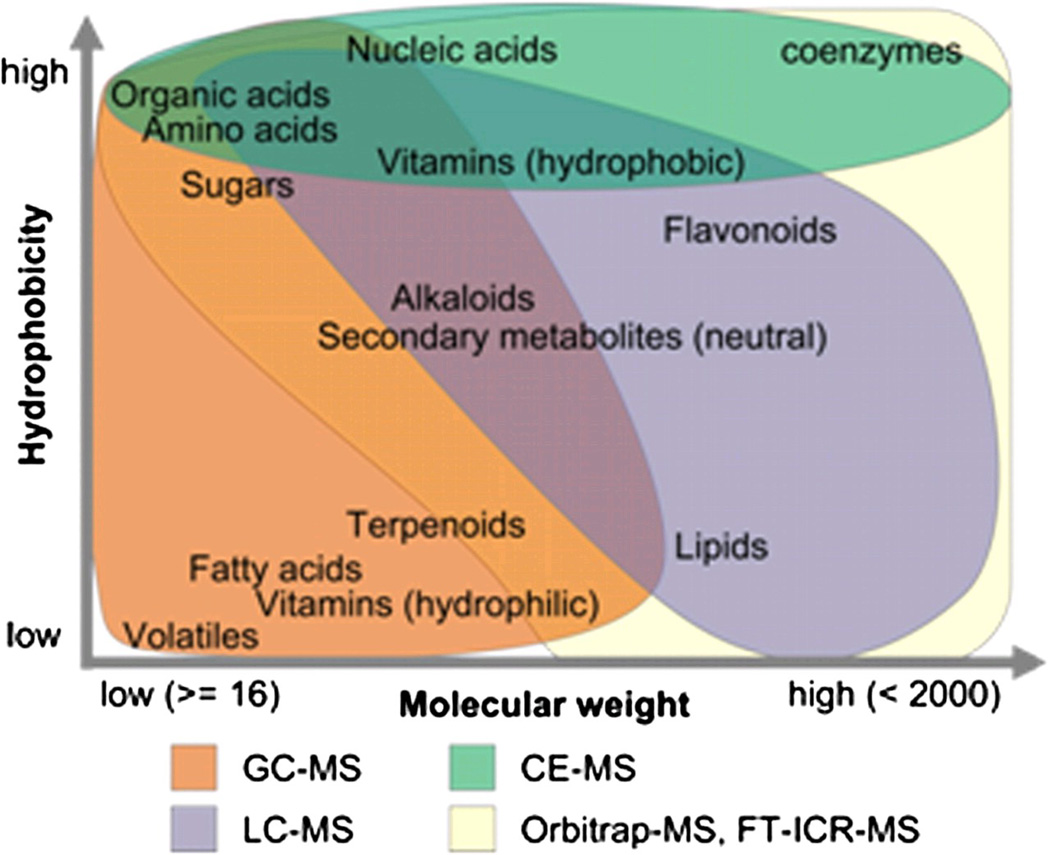
In contrast, mass spectrometry uses mass/charge (m/z) to distinguish metabolites. When coupled with separation methods, this powerful tool can analyze a vast array of metabolites with enhanced sensitivity. Metabolites separation upfront typically uses either gas chromatography (for volatile, nonpolar metabolites) or liquid chromatography (for nonvolatile metabolites in solution) as the two main strategies. The specific techniques suitable for quantifying different metabolites are primarily based on their hypdrophobicity and molecular weights (Figure 1), and various ionization methods can be used depending upon the type of mass spectrometer (e.g. “time-of-flight” to detect flight time, triple quadrupole with filters for m/z, ion trap with trapping frequencies) that are beyond the scope of this review. Optimally, quantification of natural analytes is achieved through addition of known quantities of isotopic standards for improved absolute quantification. Mass spectrometry-based techniques have much better sensitivity than NMR based approaches, allowing a large number of metabolite peaks to be quantified. Thus, LC-MS and GC-MS have been used to resolve compounds that are present in much smaller quantities allowing for identification and quantification well into the attomole to picomole/liter range.9
Figure 1.
Types of metabolites detectable by mass spectrometry techniques based on degree of hydrophobicity and molecular weights.
Approaches in Metabolomic Profiling
Metabolomics to date has been used as either an “open” or “closed” profile. Open profiling refers to an untargeted analysis resulting in raw data. This can lead to unbiased “patterns” of a disease without knowing the represented metabolites. This untargeted approach leads the way to previously unrecognized or new ideas about disease processes, and opens the doorway to new ways of diagnosis, possibly even before such conditions become clinically evident. However, there are potential problems with reproducibility in an untargeted profiling mode as the metabolic profiles may not be specific to a condition, and confounders may not be accounted for (depending on the condition of the sampling as well as the stability and reproducibility of the analytes of interest). This may lead to problems with understanding disease pathways, and have led some to contest that the patterns may just be noise rather than signal. Furthermore, pattern recognition may allow for the identification of a disease state or process, but may miss the precise underlying etiology of the disease.
In contrast, “closed” or targeted analysis refers to a targeted metabolomic approach to a limited set of metabolites, lending to a more purposeful investigation. Closed profiling can also be used to validate a biomarker after it has been discovered, and such an approach allows for both greater understanding and reproducibility of the underlying disease process if the precise metabolites are known and detectable.7
Beyond quantification, the major challenge in assimilating the vast amounts of measurements generated from these metabolomic studies is the ability to curate a reliable database that can match the phenotype(s) of interest, and to use appropriate statistical comparisons between groups in order to identify metabolites of interest. Since a large majority of these compounds are not specific to a disease process, the need for specialized bioinformatic support and analytic capabilities are necessary to explore known (and unknown) metabolic pathways and to determine their relevance in various disease states.
Metabolomics in Cardiovascular Research
Metabolomics has been used to study various forms of cardivascular risk factors such as diabetes mellitus, obesity, and metabolic syndrome10–12. At present it has focused predominantly on describing the natural history and identifying markers associated with various common pathologies in cardiovascular diseases. This has ranged from markers for myocardial ischemia13–15 and cardiogenic shock16, risk of developing atherosclerosis or future cardiovascular events17–19, risk of developing diabetes mellitus12, atrial fibrillation20, chemotherapy-induced cardiotoxicity21, and pulmonary hypertension related to advanced heart failure22. These are only some examples in the literature, but may illustrate the broad application of metabolomic data in the management of cardiovascular diseases.
Myocardial Ischemia, Infarction, and Cardiogenic Shock
At present only biomarkers for the consequences of ischemia (i.e. myocardial necrosis) are available. The potential detection of metabolic derangements that may occur during or even preceding myocardial ischemia may become a promising diagnostic tool. Sabatine and colleaugues used an untargeted approach LC-MS methodology to explore metabolic profiles of myocardial ischemia15. They collected serum samples before and after exercise stress testing in patients who did and did not demonstrate myocardial ischemia based on clinical indications. This allowed them to analyze discordance in metabolites before and after stress testing to identify potential novel biomarkers of coronary ischemia. In thier cohort, changes in levels of six metabolites were identifed with inducible ischemia, and were able to be validated with a risk score. Abnormal levels were found for gama aminobutyric acid, uric acid, citric acid, and several yet to be identified metabolites. They concluded that metabolomic studies will add to the diagnostic armamentarium and ultimately identify new targets for therapeutic intervention, although prospective validation of this diagnostic risk score has not been reported, particularly as to whether it provides incremental predictive value to existing clinical parameters. Clearly, exercise itself produces cardiovascular metabolic pertubations that can be detected with serial blood sampling. In a mechanistic study, “normal” exercise-induced changes in metabolites in major metabolic pathways of energy production (glucose-6-phosphate, succinate, malate, fumarate, and glycerol) can be demonstrated with the subjects as his/her own control. In fact, sampling from different sites simultaneously also demonstrates the production and release of glycerol generated from exercising muscles – illustrating the potential physiologic insights yielded from metabolomic profiling13.
To extend beyond ischemia, Lewis and colleagues have utilized an artificial infarction human model in the setting of alcohol septal ablation for obstructive hypertrophic cardiomyopathy to examine the temporal changes in metabolomic profiling during the course of myocardial infarction14. Interestingly, the plasma signature of aconitic acid, hypoxanthine, trimethylamine-N-oxide (TMAO), and threonine differentiated with high diagnostic accuracy between infarcted subjects and patients undergoing coronary angiography.
The pattern of metabolic derangements may also provide insights into disease severity. Acute myocardial infarction (AMI) complicated by cardiogenic shock is associated with significant morbidity and mortality rates despite aggressive therapy, including early revascularization.23, 24 Nitric oxide (NO) has been shown to have many beneficial affects including coronary vasodilation and other vascular bed relaxation. It is also known to have several deleterious effects. NO is known to depress cardiac contractility, and thus may play a key role in the hypotension and depressed cardiac function that defines cardiogenic shock. Based on the concepts that inflammatory stimuli can induce isoforms of NO synthase, it has been speculated that NO could play a role in the pathogenesis of cardiogenic shock.25 LC-MS was used in a targeted, closed analysis of metabolites in the NO metabolic pathway to study this concept. The formation of NO involves arginine and its metabolites (ornithine and citrulline) along with enzymes that induce or inhibit NO synthase. Nicholls and colleagues studied differences in the metobolic profiles of patients after AMI complicated by cardiogenic shock compared to patients with coronary artery disease and normal left ventricular systolic function16. They discovered that elevated level of the NO synthase inhibitor, asymmetric dimethylarginine (ADMA) serves as an independent predictor of mortality. However in a follow up study, Hochman and colleauges were unable to show a mortality benefit with therapeutic NO synthase inhibition by L-NG-monomethyl arginine26. Hence, metabolomic insights of dysregulated NO metabolism link to a more advanced disease phenotype, but in this scenario therapeutic interdiction of NO metabolic pathways have yet to modify the natural history of cardiogenic shock.
Anthracycline-induced Cardiotoxicity
The potential for metabolomic profiling to monitor the propensity to develop cardiotoxicity is attractive as early interventions may prevent the development of heart failure. A good example is the early risk stratification to prevent anthracycline-induced cardiomyopathy. Anthracycline-based chemotherapy is commonly used in treating breast and other forms of cancer. The use of anthracycline-based regimen is known to be limited by dose-dependent cardiotoxicity.27 The basis for anthracycline-induced cardiotoxicity is free-radical injury producing genetic damage and altering cardiac energy production.28, 29 Andreadou and colleaugues used 1H-NMR spectrometry in an untargeted approach to determing the metabolomic basis for the free-radical injury from anthracyclines.21 In rat hearts exposed to anthracyclines they were able to show elevated levels of acetate and succinate, with depressed levels of branched-chain amino acids. This correlated to anthracycline-induced nonenzymatic conversion of pyruvate to lactate, and α-ketoglutarate to succinate via free radicals. They also demonstrated restoration of acetate and succinate levels with the administration of the antioxidant oleuropein. Although this is yet to be demonstrated in humans, the potential to guide therapeutic intervention using metabolomic biomarkers to identify those at risk for developing anthracycline-induced cardiotoxicity is promising.
Atherosclerosis
One of the most unique opportunities for metabolomic research is to uncover metabolic pathways that are operative in the pathogenesis of cardiovascualr diseases. Our group and others have explored the use of metabolomics to identify risk in patients with atherosclerosis and its associated adverse events. These have ranged from studies of phosphatidylcholine and choline metabolism to dysregulation of the arginine-NO metabolic pathways. Our group has utilized an LC-MS untargeted approach to investigate stable patients undergoing elective coronary angiography who subsequently suffered a major adverse cardiac event (death, nonfatal AMI, or stroke) and compared the metabolic profiles of these patients to age- and sex-matched subjects in a stepwise validation process.18 In a “reverse-engineering” manner, we identified three metabolites that had consistent and significant associations with the development of major adverse cardiac events that also correlated with one another, and we hypothesized that these metabolites were linked via a common biochemical pathway. These metabolites were subsequentally identified through a variety of chemical approaches and isotope tracer studies as TMAO, choline, and betaine – all being involved in phosphatidylcholine (choline) metabolism. We subsequently determined that oral ingestion of phosphatidylcholine was directly linked to systemic levels of TMAO and choline, and that intestinal microflora were intricately involved in the production of TMAO with direct causal association with the development of atherosclerosis in animal models. Systemic levels of TMAO were also shown to be higher in patients that are at increased risk of developing coronary artery disease, such as those with diabetes mellitus and with renal insufficiency. Interestingly, phosphatidylcholine is predominatley found in eggs, milk, fish, shell fish, poultry, red meat, and liver – many food groups abundant in fat and cholesterol, and that physicians have long been advising their patients to avoid in reducing cardiovascular risks. Also, we were able to demonstrate in a larger cohort of patients undergoing coronary angiography that elevated levels of TMAO, choline, and betaine all had a dose-dependent association with both the presence of cardiovascular disease and the angiographic extent of coronary atherosclerotic heart disease. The ability for quantifying intestinal microflora-derived choline metabolites in humans provides a unique opportunity to investigate a variety of dietary and pharmacologic treatment strategies to prevent the development of atherosclerotic coronary artery disease. By identifying the potential involvement of intestinal microflora as an obligatory participant in this unique atherosclerotic process, this translational work illustrates the power of metabolomics as a discovery tool for identifying novel pathways involved in disease processes. Metabolomics studies, while they can provide insights into biochemical pathways, are still exercises in quantification of association between analytes and disease/phenotype. It is thus of critical importance that subsequent investigations examine the cause of the associations, and the mechanistic underpinnings of the specific metabolomic profiles observed in clinical phenotyes in order to uncover novel disease mechanisms.
As noted above, another area where metabolomics studues have proven fruitful in cardiovascular disease is in more targetted studies surrounfing pathways linked to NO, a critical molecule for vascular health. NO is synthesized from arginine via NO synthases in a multistep reaction that produces both NO and the amino acid citrulline. NO promotes beneficial effects in the vasculature producing vasodilitation, inhibiting platelet aggregation, leukocyte adhesion, endothelial generation, and smooth muscle cell proliferation.30 Methylated arginine species (such as ADMA) act as inhibitors to NO synthase, thus diminishing vasoreactivity and promoting endothelial dysfunction and vasculopathy.31 Our group and others have used targetted LC-MS approaches to study analytes involved in the arginine-NO pathway as they relate to CVD risk in patients undergoing cardiac evaluation17, 19 as well as those in the general population from the Framingham Heart Study32, 33. Of interest, a measure of diminished global arginine bioavailability (defined as the ratio of substrates [arginine] and by-products [ornithine + cirulline] of NO production) may represent patients with low NO synthetic capacity and increased risk. Citrulline levels alone also proved to be a strong and indepednent indicator of incident major adverse cardiac risks17. In patients undergoing cardiac evaluation, low global arginine bioavailability ratio was associated increased risk of obstructive coronary artery disease in addition to incident major adverse cardiac events, even independent of ADMA, and may represent patients who would benefit from aggressive risk reduction, or possibly arginine supplementation.
Atrial Fibrillation
It is estimated that 5 million Americans are affected with atrial fibrillation, and data suggests that as the population ages the incidence will contintinue to increase.34 Persistent atrial fibrillation shortens the atrial refractory period. Mayr and colleagues used an untargeted NMR-based approach to identify metabolic changes in patients with persistent atrial fibrillation, and in those undergoing cardiac surgery to potentially identify patients at risk for post-operative atrial fibrillation. Atrial fibrillation denotes a hypermetabolic state with prominent up-regulation of transcripts involved in metabolic activities of several glycolytic enzymes, suggesting a switch to glucose metabolism.35 Elevated levels of beta-hydroxybutyrate, ketogenic amino acids (tyrosine and leucine), and glycine were found in atrial tissue of patints with a history of persistent atrial fibrillation as compared with controls with no prior history of atrial fibrillation. This suggested an importance of ketone bodies as an energy source to perpetuate the arrythmia. The group then tried to identify differences in patients who developed post-operative atrial fibrillation. They noted a significantly lower ratio of glucose to acetate in the post-operative atrial fibrillation patients than in those that maintaned sinus rhythm. This suggested that metabolic alterations of impaired glucose metabolism may help facilitate the arrythmia, and not ischemia or inflammation as previously thought.36 Identifying patients at risk for post-operative atrial fibrillation after undergoing cardiac surgery could pave the way for preventative therapy in the pre or early post-operative state.
Conclusion
Metabolomics is a new and emerging area of cardiovascular biomarker research. It uses analytical chemistry based approaches to determine metabolic properties that underly varying cardiovascular disease states. Researchers have used both unbiased and targetted approaches to identify, describe, and verify metabolic differences between disease and non-disease conditions, including those at risk for incident MI, stroke or death indepenent of traditional risk factors. At present, biomarkers are identified in biospecimens primarily based on quantifying protein-based end-products rather than characterizing metobolomic profiles that represent the disease states. Metabolomics could open up a new avenue to both identify and diagnose patients before they have a major adverse cardiac event. This may further expand new treatments (diet-based) and medications (biochemical-based) to treat our patients.
Acknowledgments
FUNDING SOURCE
This work is supported by 1R01HL103866, 1R01HL103931, P01HL076491 and 1P20HL113452-01.
Dr. Tang has previously received research grant support from Abbott Laboratories. Dr. Hazen reports being listed as co-inventor on pending and issued patents held by the Cleveland Clinic relating to cardiovascular diagnostics. Dr. Hazen reports having been paid as a consultant or speaker for the following companies: Abbott, Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., Merck & Co., Inc., and Pfizer Inc. Dr. Hazen reports receiving research funds from Abbott, Cleveland Heart Lab, Liposcience Inc., and Pfizer Inc. Dr. Hazen reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from the companies shown below: Abbott, Cleveland Heart Lab., Esperion, Frantz Biomarkers, LLC, Liposcience Inc., and Siemens.
List of Abbreviations
AMI
Acute myocardial infarction
ADMA
Asymmetric dimethylarginine
GC-MS
Gas chromatography mass spectrometry
LC-MS
Liquid chromatography mass spectrometry
NO
Nitric oxide (nitrogen monoxide)
NMR
Nuclear magnetic resonance
TMAO
trimethylamine N-oxide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
Dr. Senn has no relationships to disclose.
REFERENCES
- 1.Oliver SG, Winson MK, Kell DB, Baganz F. Systematic functional analysis of the yeast genome. Trends Biotechnol. 1998;16:373–378. doi: 10.1016/s0167-7799(98)01214-1. [DOI] [PubMed] [Google Scholar]
- 2.Tweeddale H, Notley-McRobb L, Ferenci T. Effect of slow growth on metabolism of Escherichia coli, as revealed by global metabolite pool ("metabolome") analysis. J Bacteriol. 1998;180:5109–5116. doi: 10.1128/jb.180.19.5109-5116.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson JK, Lindon JC, Holmes E. 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–1189. doi: 10.1080/004982599238047. [DOI] [PubMed] [Google Scholar]
- 4.Wishart DS, Tzur D, Knox C, et al. HMDB: the Human Metabolome Database. Nucleic Acids Res. 2007;35:D521–D526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsythe IJ, Wishart DS. Exploring human metabolites using the human metabolome database. Curr Protoc Bioinformatics. 2009;Chapter 14(Unit14):8. doi: 10.1002/0471250953.bi1408s25. [DOI] [PubMed] [Google Scholar]
- 6.Griffin JL, Atherton H, Shockcor J, Atzori L. Metabolomics as a tool for cardiac research. Nat Rev Cardiol. 2011;8:630–643. doi: 10.1038/nrcardio.2011.138. [DOI] [PubMed] [Google Scholar]
- 7.Lewis GD, Asnani A, Gerszten RE. Application of metabolomics to cardiovascular biomarker and pathway discovery. J Am Coll Cardiol. 2008;52:117–123. doi: 10.1016/j.jacc.2008.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raamsdonk LM, Teusink B, Broadhurst D, et al. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations. Nat Biotechnol. 2001;19:45–50. doi: 10.1038/83496. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Wei D, Yap Y, Li L, Guo S, Chen F. Mass spectrometry-based"omics" technologies in cancer diagnostics. Mass Spectrom Rev. 2007;26:403–431. doi: 10.1002/mas.20132. [DOI] [PubMed] [Google Scholar]
- 10.Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faber JH, Malmodin D, Toft H, et al. Metabonomics in diabetes research. J Diabetes Sci Technol. 2007;1:549–557. doi: 10.1177/193229680700100413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis GD, Farrell L, Wood MJ, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med. 2010;2:33ra7. doi: 10.1126/scitranslmed.3001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis GD, Wei R, Liu E, et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J Clin Invest. 2008;118:3503–3512. doi: 10.1172/JCI35111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabatine MS, Liu E, Morrow DA, et al. Metabolomic identification of novel biomarkers of myocardial ischemia. Circulation. 2005;112:3868–3875. doi: 10.1161/CIRCULATIONAHA.105.569137. [DOI] [PubMed] [Google Scholar]
- 16.Nicholls SJ, Wang Z, Koeth R, et al. Metabolic profiling of arginine and nitric oxide pathways predicts hemodynamic abnormalities and mortality in patients with cardiogenic shock after acute myocardial infarction. Circulation. 2007;116:2315–2324. doi: 10.1161/CIRCULATIONAHA.107.693986. [DOI] [PubMed] [Google Scholar]
- 17.Tang WH, Wang Z, Cho L, Brennan DM, Hazen SL. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J Am Coll Cardiol. 2009;53:2061–2067. doi: 10.1016/j.jacc.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Tang WH, Cho L, Brennan DM, Hazen SL. Targeted metabolomic evaluation of arginine methylation and cardiovascular risks: potential mechanisms beyond nitric oxide synthase inhibition. Arterioscler Thromb Vasc Biol. 2009;29:1383–1391. doi: 10.1161/ATVBAHA.109.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayr M, Yusuf S, Weir G, et al. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol. 2008;51:585–594. doi: 10.1016/j.jacc.2007.09.055. [DOI] [PubMed] [Google Scholar]
- 21.Andreadou I, Papaefthimiou M, Zira A, et al. Metabonomic identification of novel biomarkers in doxorubicin cardiotoxicity and protective effect of the natural antioxidant oleuropein. NMR Biomed. 2009;22:585–592. doi: 10.1002/nbm.1370. [DOI] [PubMed] [Google Scholar]
- 22.Shao Z, Wang Z, Shrestha K, et al. Pulmonary hypertension associated with advanced systolic heart failure: dysregulated arginine metabolism and importance of compensatory dimethylarginine dimethylaminohydrolase-1. J Am Coll Cardiol. 2012;59:1150–1158. doi: 10.1016/j.jacc.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 24.Hochman JS, Sleeper LA, White HD, et al. One-year survival following early revascularization for cardiogenic shock. JAMA. 2001;285:190–192. doi: 10.1001/jama.285.2.190. [DOI] [PubMed] [Google Scholar]
- 25.Hochman JS. Cardiogenic shock complicating acute myocardial infarction: expanding the paradigm. Circulation. 2003;107:2998–3002. doi: 10.1161/01.CIR.0000075927.67673.F2. [DOI] [PubMed] [Google Scholar]
- 26.Alexander JH, Reynolds HR, Stebbins AL, et al. Effect of tilarginine acetate in patients with acute myocardial infarction and cardiogenic shock: the TRIUMPH randomized controlled trial. JAMA. 2007;297:1657–1666. doi: 10.1001/jama.297.15.joc70035. [DOI] [PubMed] [Google Scholar]
- 27.Cole MP, Chaiswing L, Oberley TD, et al. The protective roles of nitric oxide and superoxide dismutase in adriamycin-induced cardiotoxicity. Cardiovasc Res. 2006;69:186–197. doi: 10.1016/j.cardiores.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 28.Kawasaki N, Lee JD, Shimizu H, Ishii Y, Ueda T. Cardiac energy metabolism at several stages of adriamycin-induced heart failure in rats. Int J Cardiol. 1996;55:217–225. doi: 10.1016/0167-5273(96)02672-1. [DOI] [PubMed] [Google Scholar]
- 29.Yen HC, Oberley TD, Vichitbandha S, Ho YS, St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest. 1996;98:1253–1260. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bian K, Doursout MF, Murad F. Vascular system: role of nitric oxide in cardiovascular diseases. J Clin Hypertens (Greenwich) 2008;10:304–310. doi: 10.1111/j.1751-7176.2008.06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juonala M, Viikari JS, Alfthan G, et al. Brachial artery flow-mediated dilation and asymmetrical dimethylarginine in the cardiovascular risk in young Finns study. Circulation. 2007;116:1367–1373. doi: 10.1161/CIRCULATIONAHA.107.690016. [DOI] [PubMed] [Google Scholar]
- 32.Lieb W, Benndorf RA, Benjamin EJ, et al. Plasma asymmetric dimethylarginine, L-arginine and left ventricular structure and function in a community-based sample. Atherosclerosis. 2009;204:282–287. doi: 10.1016/j.atherosclerosis.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boger RH, Sullivan LM, Schwedhelm E, et al. Plasma asymmetric dimethylarginine and incidence of cardiovascular disease and death in the community. Circulation. 2009;119:1592–1600. doi: 10.1161/CIRCULATIONAHA.108.838268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 35.Barth AS, Merk S, Arnoldi E, et al. Reprogramming of the human atrial transcriptome in permanent atrial fibrillation: expression of a ventricular-like genomic signature. Circ Res. 2005;96:1022–1029. doi: 10.1161/01.RES.0000165480.82737.33. [DOI] [PubMed] [Google Scholar]
- 36.Ono N, Hayashi H, Kawase A, et al. Spontaneous atrial fibrillation initiated by triggered activity near the pulmonary veins in aged rats subjected to glycolytic inhibition. Am J Physiol Heart Circ Physiol. 2007;292:H639–H648. doi: 10.1152/ajpheart.00445.2006. [DOI] [PubMed] [Google Scholar]