Pharmacological inhibition of βIIPKC is cardioprotective in late-stage hypertrophy (original) (raw)
. Author manuscript; available in PMC: 2012 Dec 1.
Published in final edited form as: J Mol Cell Cardiol. 2011 Sep 2;51(6):980–987. doi: 10.1016/j.yjmcc.2011.08.025
Abstract
We previously found that in the hearts of hypertensive Dahl salt-sensitive rats, βIIPKC levels increase during the transition from compensated cardiac hypertrophy to cardiac dysfunction. Here we showed that a six-week treatment of these hypertensive rats with a βIIPKC-specific inhibitor, βIIV5-3, prolonged their survival by at least six weeks, suppressed myocardial fibrosis and inflammation, and delayed the transition from compensated hypertrophy to cardiac dysfunction. In addition, changes in the levels of the Ca2+-handling proteins, SERCA2 and the Na+/Ca2+ exchanger, as well as troponin I phosphorylation, seen in the control-treated hypertensive rats were not observed in the βIIPKC-treated rats, suggesting that βIIPKC contributes to the regulation of calcium levels in the myocardium. In contrast, treatment with the selective inhibitor of βIPKC, an alternative spliced form of βIIPKC, had no beneficial effects in these rats. We also found that βIIV5-3, but not βIV5-3, improved calcium handling in isolated rat cardiomyocytes and enhanced contractility in isolated rat hearts. In conclusion, our data using an in vivo model of cardiac dysfunction (late-phase hypertrophy), suggest that βIIPKC contributes to the pathology associated with heart failure and thus an inhibitor of βIIPKC may be a potential treatment for this disease.
1. Introduction
Despite the advances in pharmacological interventions, improvements in cardiac devices and in heart transplantation, mortality associated with heart failure continues to increase [1]. Therefore, the identification of novel therapeutic targets for the treatment of heart failure remains a major priority. Because there was an increase in β protein kinase C (βPKC) levels in failing human hearts [2, 3] and in a rat model of hypertension-induced cardiac dysfunction by high-salt diet [4], we set out to determine the role of βPKC isozymes, βI and βIIPKC in cardiac dysfunction model using Dahl rats fed high-salt diet.
We used isozyme-selective βI- and βIIPKC inhibitors, βIV5-3 and βIIV5-3, which were previously developed in our lab [5-7]. These six amino acid peptide inhibitors were derived from the least homologous sequence in the only divergent region in these alternatively spliced forms of βPKC[5]. These isozyme-specific peptide inhibitors are linked to membrane permeable peptides, TAT47-57 [8], to enable their effective delivery into cells and make them useful pharmacological tools. We tested here the possibility that selective pharmacological inhibition of βI- or βIIPKC could inhibit the progression of cardiac dysfunction in these hypertensive rats.
2. Materials and Methods
2.1. Peptide synthesis
βIV5-3 (βIPKC inhibitor, corresponding to amino acids 646-651 [KLFIMN]) and βIIV5-3 (βIIPKC inhibitor, amino acids 645-650 [QEVIRN] [5], were synthesized and conjugated to TAT carrier peptide (amino acids 47–57 [YGRKKRRQRRR]) via a disulfide bond between Cys residues at the N-terminus of each peptide [9] by American Peptide, Inc. (Sunnyvale, CA).
2.2 Hypertension-induced rat model of cardiac dysfunction
Male Dahl rats were obtained from Harlan Sprague-Dawley (Indianapolis, Indiana). Rats were fed with an 8% NaCl-containing diet (high salt diet) or with a 0.3% NaCl-containing diet (low salt diet; control) from the age of 6 weeks onward, as described.[4] Using osmotic pumps implanted subcutaneously and replaced every two weeks, Dahl rats were treated between the ages of 11 and 17 weeks with the selective βIPKC inhibitor peptide, TAT47-57-βIV5-3 (n=13, 3mg/kg/day), the selective βIIPKC inhibiting peptide, TAT47-57-βIIV5-3 (n=11, 3mg/kg/day), an equimolar concentration of TAT47-57 carrier peptide alone (n=13, 1.6mg/kg/day) or with saline (n=13) (Fig. 1A). Subcutaneous pump implantation was performed in 4% vaporized isoflurane-anesthetized rats. This concentration provided deep anaesthesia, allowing mini-pumps implantation without any clinical sign of pain, such as withdrawal reflex. Pump was inserted in the back of animals after making a sub-scapular incision.
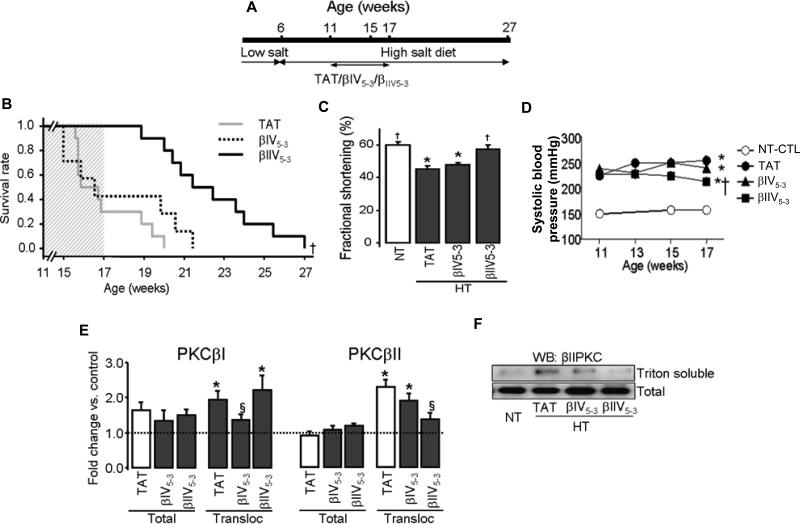
Figure 1. βIIV5-3, but not βIV5-3, slowed down the progression of cardiac dysfunction.
(A): Schematic panel of pharmacological treatment in Dahl rats. (B): Survival rate of hypertensive Dahl rats after six weeks with either TAT carrier peptide (TAT, n=13) or the βPKC inhibitors, βIV5-3 (n=13) or βIIV5-3 (n=11). The shaded area represents the duration of treatment. (C): Fractional shortening. (D): Systolic blood pressure measured at the age of 11, 13, 15 and 17 weeks (n=6-17 per group). (E): Presented are total βIPKC and βIIPKC levels and their relative levels in the particulate fraction (expressed as the ratio of levels in Triton-soluble over total fraction) in 17-week old rats (n=6 per group). The level of cellular PKC distribution between the total and particulate fractions was normalized using GAPDH and Gαo, respectively. Note that βIPKC translocation changed significantly in hypertensive hearts. However, its sustained inhibition had no effect on survival and cardiac function. (F): Representative blot showing the levels and subcellular distribution of βIIPKC in 17-week old rats (n=6 per group). *P<0.05 vs. normotensive control (NT-C). §P<0.05 vs. hypertensive TAT-treated animals. †P<0.05 vs. hypertensive TAT and βIV5-3-treated animal. Survival was analyzed by the standard Kaplan-Meier analysis. Fractional shortening, total βIPKC and βIIPKC levels and translocations were analyzed by the one-way analysis of variance (ANOVA) with post-hoc testing by Fisher. NT-CTL, normotensive rats; HT-TAT, hypertensive rats; HT-βIV5-3 and HT-βIIV5-3, hypertensive rats treated with specific βIPKC and βIIPKC inhibitor, respectively.
Cardiac functions and blood pressure were similar between groups of animals before any drug treatment was initiated. Survival rate, fractional shortening, lung weight/body weight, LV weight/body weight and blood pressure were evaluated in these groups. Note that 50% of the hypertensive Dahl rats die from hypertensive encephalopathy no later than week 15 of the study [10] whereas cardiac dysfunction-induced death occurs by week 20-22 [11]. None of the PKC inhibitors except for the delta PKC inhibitor inhibited hypertensive encephalopathy and death [10]. Further, the delta PKC inhibitor had no effect on cardiac dysfunction [11]. Further, examination of the brains of the animals that remained alive beyond 15 weeks did not show any pathology, there was only modest renal pathology in the hypertensive rats and there were no differences between the various treatment groups [not shown]. All animal protocols were approved by the Stanford University Institutional Animal Care and Use Committee.
2.3. Cardiovascular measurements
Systolic blood pressure was determined non-invasively using a computerized tail-cuff system (BP-2000, Visitech System, Apex, NC). Non-invasive cardiac function was assessed by two-dimensional guided M-mode echocardiography, in 1% vaporized isoflurane-anesthetized rats after the experimental period. Transthoracic echocardiography was performed using a GE Vivid 7 echocardiograph equipped with a 14 MHz linear transducer.
2.4. Myocardial fibrosis, cardiomyocyte hypertrophy and infiltration of inflammatory cells
Paraffin embedded mid-ventricular sections were deparaffinized, rehydrated and further stained with Masson's trichrome stain. Collagen deposition, an index of myocardial fibrosis, was observed and quantified by measuring the blue stain in the extracellular space (Adobe Photoshop). At least 15 fields (400X) per slide were counted for each group. Photomicrographs of hematoxylin-eosin stained sections (400X) were used to measure the diameter of cardiomyocytes. The myocytes which had the nucleus at their center have been selected for the measurement. At least 150 such myocytes were scored for each group. To evaluate infiltration of inflammatory cells, we again used photomicrographs of hematoxylin-eosin stained sections (400X) and at least 15 fields were counted from each heart.
2.5. Isolated perfused rat heart model
Dahl rats (17-19 weeks old) were injected with heparin (2000U/kg IP) and anesthesia was induced with sodium pentobarbital (100mg/kg IP). The hearts were rapidly excised and then perfused with an oxygenated Krebs-Henseleit solution containing NaCl 120mM, KCl 5.8mM, NaHCO3 25mM, NaH2PO4 1.2mM, MgSO4 1.2mM, CaCl2 1.0mM, and dextrose 10mM, pH 7.4, at 37°C in a Langendorff coronary perfusion system at a constant flow rate of 10mL/min. A thin latex balloon filled with water was inserted into the left ventricle (LV) and connected to a pressure transducer (PowerLab/8SP, AD Instruments) for measurement of the isovolumic LV pressure. The LV volume was adjusted initially at an end-diastolic pressure (EDP) of 5mmHg. All peptides were administered five minutes following a ten minutes equilibration period and the measurements were taken before and after 5 minutes of peptide delivery.
2.6. Isolation of adult rat cardiomyocytes
After the measurement of LV pressure as described above, the control non-treated hearts were further perfused with 2mM EGTA containing low-Ca2+ solution 1 (100mM NaCl, 10 mM KCl, 1.2 mM KH2PO4, 5 mM MgSO4, 20 mM glucose, 50 mM taurine, 10 mM HEPES and 100μM CaCl2), then with low-Ca2+ solution 2 (low-Ca2+ solution 1 containing pronase E (8mg/100ml; Sigma), proteinase K (1.7mg/100ml; Sigma), bovine albumin (100mg/100ml, fraction V; Sigma) and 200μM CaCl2). Following digestion, the ventricles were cut into fragments (2-5 mm3) in low-Ca2+ solution 1 at room temperature. Tissue fragments were then transferred to a 50ml beaker containing low Ca2+ digestion solution 2 supplemented with collagenase (0.5mg/ml; Sigma) and cells were isolated by stirring the tissue for 10 minutes at 37°C. The cell suspension was then filtered through a nylon sieve and centrifuged for 1 min (at 300–400×g). Cell pellets were washed 3 times in low-Ca2+ solution 1.
2.7. Calcium transient measurements
Myocytes were loaded with 0.5μM Fura2-acetoxymethyl ester (Molecular Probes, Eugene, Oregon) for 15min, washed and allowed to rest for an additional 40 min to allow the de-esterification of the dye. Myocytes were stimulated at 0.5 Hz and superfused with a HEPES-buffered solution at 25°C. Cells were continuously alternatively excited at 340 and 380nm, at rates as high as 250 pairs/sec using a HyperSwitch system (IonOptix, Milton, MA). Background-corrected Fura2 ratios, reflecting intracellular calcium concentration [12, 13] were collected at 510nm.
2.8. Western blot analysis
At the end of the treatment protocols, the levels and translocation of βI and βIIPKC in LV tissues from 17-week-old Dahl rats were determined by Western blot analysis (n=6/group). The whole tissue lysate, soluble fractions (cytosolic) and particulate fractions (Triton soluble) were prepared, as previously described [14]. Samples were separated on SDS-polyacrylamide gels by electrophoresis and the proteins were transferred to immobilon-P membranes (Millipore, Billerica, MA). Anti-βIPKC, βIIPKC and Gαo rabbit polyclonal antibodies, anti-Collagen-I goat antibodies, anti-GAPDH mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA), anti-troponin I and anti-phospho ser23/24 troponin I polyclonal antibodies (Cell Signaling, Danvers, MA), anti-SERCA2 and anti-NCX mouse monoclonal antibodies (Affinity BioReagents, Golden, CO) were used for immunoblotting followed by secondary probing with HRP-conjugated goat anti-rabbit, mouse or goat IgG antibody. All data were normalized to internal controls of GAPDH or Gαo.
2.9. Statistics
Data are expressed as the mean ± SEM. Statistical analysis was assessed by one-way analysis of variance (ANOVA) with post-hoc testing by Fisher, two-way repeated measures analysis of variance (ANOVA) with post-hoc testing by Fisher or Student's _t_-test (one-tailed distribution/two-sample equal variance) when appropriate. Survival was analyzed by the standard Kaplan-Meier analysis with log-rank test. A value of P<0.05 was considered significant. Note that comparisons conducted at each time point after the start of treatment include only those rats which are still alive at those time points. Therefore, there is inherent survivorship bias in these comparisons.
3. Results
3.1. Inhibition of cardiac hypertrophy, suppression of myocardial fibrosis and inflammation, improvement of cardiac function and prolongation of survival by pharmacological inhibition of βIIPKC in a hypertension-induced heart failure rat model
Since the expression and activity of βPKC increases during heart failure in humans as well as in hypertension-induced cardiac dysfunction in Dahl rats [2-4], we determined the effect of βPKC inhibition on the development of cardiac dysfunction in hypertensive Dahl rats. When placed on a high-salt diet from the age of 6 weeks, the hypertensive Dahl rats (230 vs. 160 mmHg) [4] develop compensatory left ventricular hypertrophy by the age of 11 weeks, and die from cardiac dysfunction between 16 to 21 weeks [15]. Using Alzet pump, we delivered PKC peptide inhibitors in a sustained fashion at 3mg/kg/day. Since there were no differences between TAT and saline control-treated groups [11], analyses were done only with the hypertensive TAT-treated group. Delivery of βIIV5-3 between weeks 11-17 prolonged the survival of hypertensive Dahl rats by about 6 weeks (Fig. 1A and B), diminished end-systolic diameter (Table 1), prevented the decrease in fractional shortening (Fig. 1C) and reduced systolic blood pressure from 254 to 213mmHg at the end of the treatment (17 weeks; Fig. 1D). As expected, βIIV5-3 treatment inhibited βIIPKC activity, but had no effect on βIPKC in these rats (Fig. 1E and F). Conversely, βIV5-3 treatment almost abolished βIPKC activation, but did not significantly affect βIIPKC activation (Fig. 1E and F). In vivo sustained treatment with either βIV5-3 or βIIV5-3 did not affect βIPKC and βIIPKC total levels (Fig. 1F) or translocation of other PKC isozymes (Supplement Fig. S1).
Table 1.
Body weight and in vivo echocardiographic data in Dahl rats.
| Group | n | BW (g) | LVW/BW (mg/g) | PWT (mm) | EDD (mm) | ESD (mm) | SWS (g/cm2) |
|---|---|---|---|---|---|---|---|
| NT-CTL | 28 | 424±5 | 2.3±0.1† | 1.6±0.0 | 7.0±0.1 | 2.8±0.1† | 29.1±3.3† |
| HT-TAT | 9 | 358±18* | 4.5±0.2* | 1.6±0.0 | 7.6±0.2* | 4.2±0.2* | 82.7±18.5* |
| HT-βIV5-3 | 4 | 373±17* | 3.9±0.1* | 1.6±0.0 | 7.4±0.5* | 4.0±0.9* | 86.8±14.4* |
| HT-βIIV5-3 | 9 | 391±13* | 3.5±0.1*† | 1.6±0.1 | 7.6±0.1* | 3.3±0.3† | 46.1±8.1† |
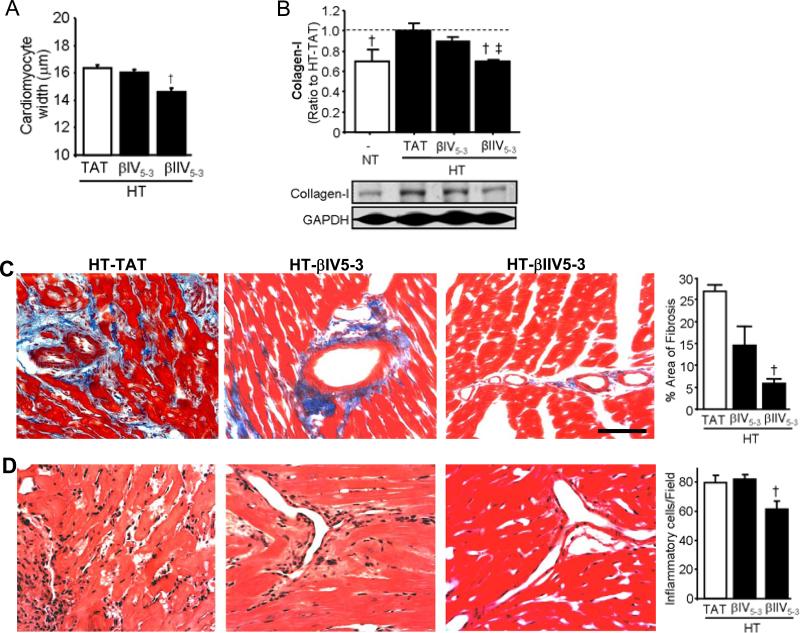
βIIV5-3 treatment also significantly decreased LVW/BW ratio groups (Table 1), cardiomyocytes diameter (Fig. 2A), cardiac collagen-I levels (Fig. 2B), area of fibrosis groups (Fig. 2C) and infiltration of inflammatory cells (Fig. 2D) at week 17 relative to hypertensive TAT-treated animals. In contrast, βIV5-3 did not significantly affect any of these parameters (Fig. 2A-D). Therefore, treatment of hypertensive Dahl rats with βIIV5-3 improved systolic function and increased survival probably due to diminished βIIPKC activation (Fig. 1B and C).
Figure 2. Treatment with βIIV5-3, but not with βIV5-3, reduced cardiomyocyte hypertrophy, fibrosis and infiltration of inflammatory cells.
(A): Presented are data on averaged cardiomyocyte diameters. Myocytes whose nuclei were at the center of the cells were selected for the measurement. At least 150 such myocytes from each group were measured. †P<0.05 vs. HT-TAT. (B): Quantification of collagen-I levels in hearts treated with either βPKC inhibitors were compared with hearts control peptide (100%). Protein levels are normalized to an internal loading control (GAPDH). Representative blots are presented in the lower panels. †P<0.05 vs. HT-TAT; ‡P<0.05 vs. HT-βIV5-3. (C): Representative photomicrographs (bar=200μm) and average of collagen deposition by Masson's trichrome stain. (D): Infiltration of inflammatory cells at the perivascular region was assessed using Hematoxylin-eosin stain (400X). The number of infiltrating cells was quantified. †P<0.05 vs. HT-TAT. One-way analysis of variance (ANOVA) with post-hoc testing by Fisher was used in those analyses. NT, normotensive rats; HT-TAT, hypertensive rats; HT-βIV5-3 and HT-βIIV5-3, hypertensive rats treated with specific βIPKC and βIIPKC inhibitor, respectively.
3.2. Sustained βIIPKC-inhibition corrects changes in calcium handling proteins and contractile proteins in hypertensive Dahl rats
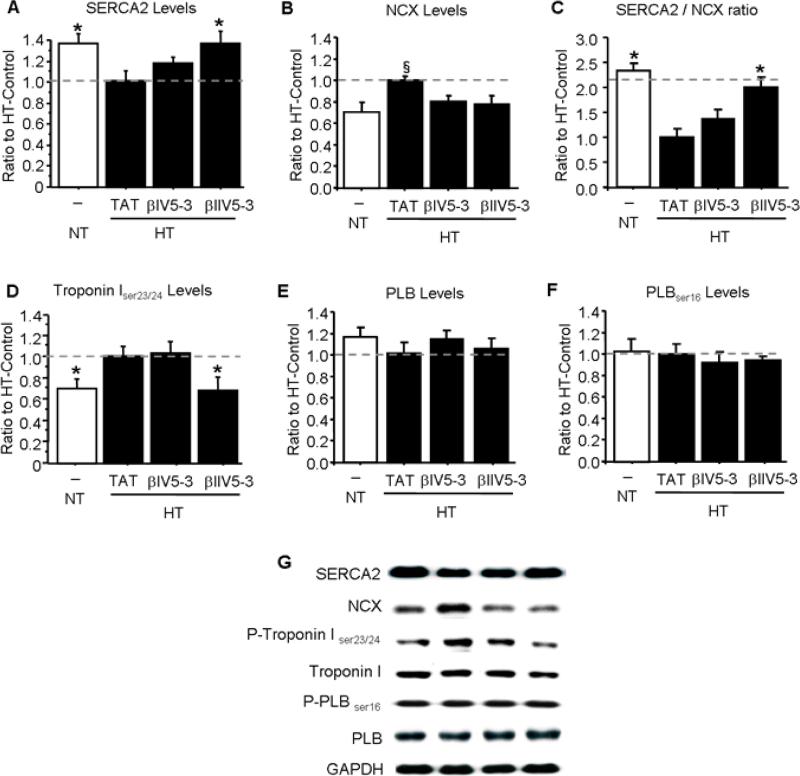
Previous studies showed that SERCA2 sequesters Ca2+ from the cytosol into the sarcomeric reticulum and is down-regulated during heart failure.[16] SERCA2 levels decreased also in the hypertensive rats and treatment with βIIV5-3 in vivo restored SERCA2 levels to normal (Fig. 3A). Up-regulation of the Na+/Ca2+ exchanger (NCX), which causes sarcoplasmic reticulum calcium depletion in myocytes during heart failure [17-19], was found in the hypertensive hearts and decreased with either βIIV5-3 or βIV5-3 treatments (Fig. 3B). As the sarcoplasmic Ca2+ content depends on Ca2+ reuptake by SERCA2 relative to transsarcolemmal Ca2+ elimination by NCX, we calculated the SERCA2: NCX ratio for all rats studied (Fig. 3C). SERCA2:NCX ratio decreased in the hypertensive rats and treatment with βIIV5-3 in vivo restored SERCA2: NCX ratio to normal levels. Cardiac troponin I blocks the contractile function of myofilaments during increased Ca2+ transients, which is enhanced through a mechanism involving phosphorylation of Ser23/24 [20, 21]. Troponin phosphorylation in 17 week old hypertensive Dahl rats also increased and selective inhibition of βIIPKC by βIIV5-3 treatment blocked this phosphorylation (Fig. 3D). Therefore, selective inhibition of βIIPKC in hypertensive Dahl rats during the six week period of transition to cardiac failure corrected hypertension-induced chances in the calcium handling protein SERCA2, NCX and troponin I. No changes were observed in either phospholamban levels or its phosphorylated form at serine 16 (Fig. 3E and F).
Figure 3. Phosphorylation and levels of proteins involved in cardiac contractility are regulated by βIIV5-3 treatment.
(A): SERCA2, (B): Na+/Ca2+ exchanger, (C) SERCA2: Na+/Ca2+ exchanger ratio, (D): phosphorylated troponin I, (E) phospholamban and (F) phosphorylated phopsholamban levels in normotensive (NT) and hypertensive (HT) rats are presented after normalization with GAPDH or total troponin I level. (G) Representative blots. *P<0.05 vs. HT-TAT, §P<0.05 vs. NT-CTR, HT-βIV5-3 and HT-βIIV5-3. One-way analysis of variance (ANOVA) with post-hoc testing by Fisher was used in those analyses. NT-CTL, normotensive rats; HT-TAT, hypertensive rats; HT-βIV5-3 and HT-βIIV5-3, hypertensive rats treated with specific βIPKC and βIIPKC inhibitor, respectively.
3.3. Improved contractility of isolated ex vivo perfused hearts and a more efficient calcium handling in isolated cardiomyocytes following acute inhibition of βIIPKC
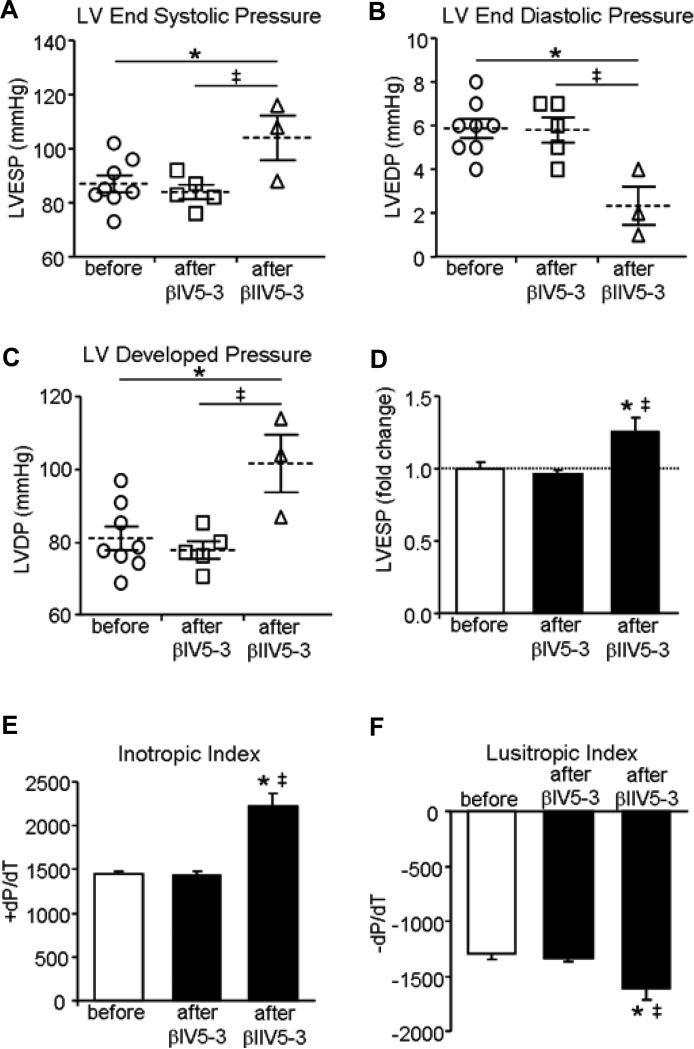
Impaired Ca2+ handling has been previously reported in an ex vivo Dahl rat heart failure model [16]. Here we found that acute treatment of isolated normotensive Dahl rat hearts in Langendorff mode with βIIV5-3 increased LV end systolic pressure. LV end diastolic pressure decreased, thus resulting in a 30% increase in LV developed pressure relative to the non-treated group (Fig. 4A-F). Importantly, selective inhibition of βIPKC (by treating with βIV5-3 for the same duration) had no effect on contractility of isolated ex vivo perfused hearts (Fig. 4).
Figure 4. Treatment with βIIV5-3, but not with βIV5-3, caused better contractility in isolated ex vivo perfused heart.
Isolated hearts from normotensive Dahl rats were perfused on a Langendorff apparatus with Kreb's buffer. 1μM of either βIV5-3 or βIIV5-3 was perfused in the buffer for ten minutes, Left ventricular (LV) end systolic pressure (LVESP, A) and LV end diastolic pressure (LVEDP, B) were measured. LV developed pressure (LVDP, C) was measured by subtracting LV end diastolic pressure from LV end systolic pressure. Comparison of LVDP from the non-treated independent control and βIIV5-3 treated hearts is shown in D. Squares, triangles and circles represent the values for individual hearts. +dP/dT (E) and - dP/dT (F) values were recorded from the same experiment as described above. *P<0.05 vs. before treatment. ‡P<0.05 vs. βIV5-3 treatment. One-way analysis of variance (ANOVA) with post-hoc testing by Fisher was used to compare the effect of βIPKC or βIIPKC on LVESP, LVEDP, LVDP, positive and negative left ventricular dP/dT.
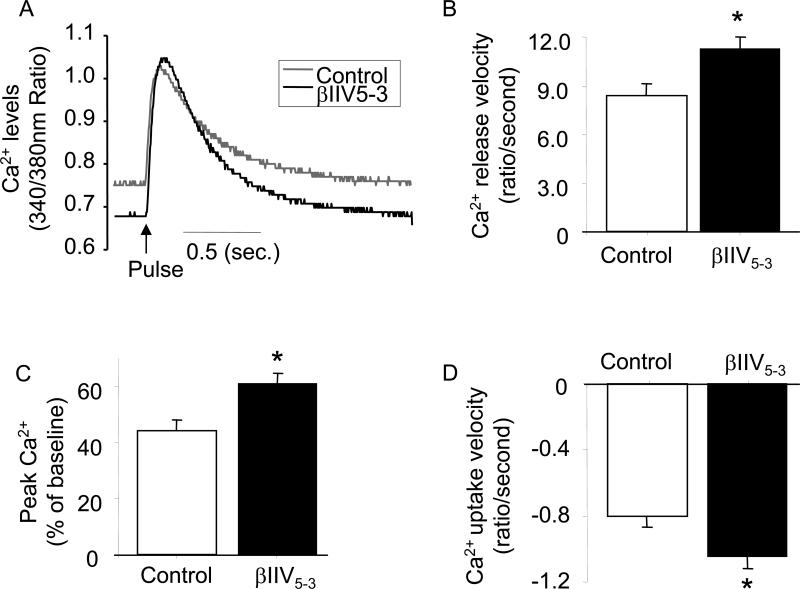
Since βIIV5-3 increased cardiac performance in an ex vivo model (Fig. 4) and in vivo (Fig. 1), we tested the hypothesis that this PKC isozyme may contribute to the impairment of cardiac contractility by alterations in calcium handling. Cytosolic Ca2+ transients were measured in cardiomyocytes isolated from normotensive rats and treated with or without the βIIPKC-specific inhibitor, βIIV5-3. In vitro, βIIV5-3 treatment resulted in an increase in the total amount of released Ca2+ (Ca2+ release velocity and peak Ca2+ of baseline, respectively; Fig. 5A and B) and the velocity of calcium uptake (Fig. 5C). In contrast, selective inhibition of βIPKC had no effect on Ca2+ transient measurements (Supplement Fig. S2). We did not measure Ca2+ transients in failed hearts, since the integrity of the cardiomyocytes after isolation was lost. Nevertheless, improvement of cardiac Ca2+ handling in hearts isolated from normotensive rats and treated with the βIIPKC-specific inhibitor, βIIV5-3, supports its contribution to reversing Ca2+ handling dysfunction in HF.
Figure 5. Treatment with βIIV5-3, resulted in better Ca2+ handling in isolated myocytes.
Intracellular Ca+2 transient in isolated ventricular myocytes. Ventricular cardiomyocytes were isolated from normotensive Dahl rats (17-19 weeks old). 1μM of βIIV5-3 was perfused in the buffer for ten minutes. Ca2+ trace (340/380nm ratio; A), Ca2+ release velocity (ratio/second; B), peak Ca2+ (% of baseline; C) and Ca2+ uptake velocity (ratio/second; D) *P<0.05 vs. control. One-way analysis of variance (ANOVA) with post-hoc testing by Fisher was used to compare the effect of βIIPKC on Ca2+ release velocity, peak Ca2+ and Ca2+ uptake velocity.
4. Discussion
Using an in vivo model of cardiac dysfunction (late-phase hypertrophy), we demonstrate that sustained treatment with a βIIPKC-specific peptide inhibitor, βIIV5-3, improved survival and cardiac contractility (Fig. 1 and Table 1), and reduced myocyte hypertrophy, cardiac fibrosis and inflammation (Fig. 2) in a rat model of hypertension-induced cardiac dysfunction. Cardiac hypertrophy is associated with impaired Ca2+ handling [16, 22, 23]. As expected, we found a decrease in SERCA levels and an increase in levels of the Na/Ca exchanger in this model of cardiac dysfunction and βIIPKC inhibition corrected these changes (Fig. 3). Furthermore, we found that acute treatment with the βIIPKC inhibitor in vitro increased Ca2+ release and uptake (Fig. 5). This effect on calcium handling may be due to βIIPKC-mediated regulation of the L-type Ca2+ channel [24] and/or the ryanodine receptor to control Ca2 release and SERCA2 and/or NCX to control Ca2 uptake [17, 18]. There is an alternatively-spliced form of βIIPKC in the hearts that differs only in the last 50 amino acids of the 750 amino acid enzymes. Yet, selective inhibition of βIPKC had no effect on any of these parameters. These data suggest a selective role for βIIPKC in hypertension-induced cardiac dysfunction.
PKC has been previously shown to regulate the sensitivity of myofilaments to calcium through phosphorylation of troponin I at three different sites (Ser43, Ser45 and Thr144), resulting in diminished contractility of the sarcomeric filament [25, 26]. Thus, βIIPKC and εPKC has been demonstrated to phosphorylate troponin I on Ser23/24, which are well-known PKA-sensitive sites [20, 21, 25]. Using the βIPKC and βIIPKC-selective inhibitors, we showed that treatment of the hypertensive Dahl rats with βIIV5-3 (and not with the βIPKC inhibitor) significantly decreased troponin I phosphorylation on Ser23/24 (Fig. 3C), suggesting that the improved cardiac function and contractility associated with βIIV5-3 treatment may be due to both the partial correction of the heart failure-induced impaired Ca2+ handling and to an increase in Ca2+ sensitivity of the cardiac myofilaments. However, the direct and/or indirect contribution of βIIPKC to troponin I phosphorylation at the different PKC-sensitive sites as well as the precise mechanism of PKC-mediated troponin I phosphorylation remain to be fully established. A role for βIIPKC (and perhaps also βIPKC) in regulating the levels of SERCA2 and NCX has also been shown in this hypertension-induced cardiac dysfunction model (Fig. 3A and B).
Cardiac fibrosis is considered the major determinant of diastolic dysfunction and failure in hypertension [27], since it leads to passive stiffness, impairment of relaxation and diastolic filling. Continuous accumulation of collagen interferes in cardiomyocytes contractility whereas alteration in collagen metabolism significantly contributes to ventricular dysfunction [27-29]. Similar to human failing heart findings [30], in the present study we found a pronounced increase in fibrosis and collagen type-1 level in the myocardium from hypertensive rats. Further, treatment with βIIV5-3 attenuated perivascular collagen deposition (Fig. 2C) and collagen-1 levels in the myocardium. In general, attenuation of perivascular fibrosis improves oxygen supply and removal of waste metabolites and ultimately the cardiac function. In vitro studies indicated that different PKC isozymes participate in different stages of fibrotic [31]. This in vivo study reaffirms that suppression of cardiac fibrosis by specifically inhibiting βIIPKC from hypertrophic stage is an important mechanism to prevent myocardial dysfunction and failure in hypertensive heart failure. The cellular mechanisms underlying the decrease in cardiac fibrosis mediated by βIIPKC inhibition are out of the scope of the present study. Another important contributing factor for heart failure pathogenesis is inflammation. We and others have shown that PKC isozymes are involved in this phenomenon [31]. However, further studies on the role of PKC isozymes in the activation of specific inflammatory cells during cardiac remodeling are needed.
Further, it is clear that βIIPKC phosphorylates multiple protein substrates, each of which may have different role in heart failure development, perhaps even at different time-point of the disease. However, the available pharmacological tools cannot individually inhibit the regulation of each of these substrates. Inhibitors that will prevent βIIPKC-mediated phosphorylation of each protein substrates, so called separation-of-function inhibitors, will identify which of the many substrates of βIIPKC is critical in a given response and at what time during the development of heart failure.
Importantly, although the heart expresses several PKC isozymes, βIIPKC seems to be the key effector isozyme in the failing human hearts [2]. These human data are in agreement with the data we described here in rats. However, it should be noted that there is a controversy about the role of βPKC in the mouse heart. Roman et al. (2001) [32] showed no cardiac phenotype in βPKC KO mice, whereas overexpression of βIIPKC induced cardiac hypertrophy [33] and contractility dysfunction [34]. These studies, examining the role of βPKC, were done in mice and therefore may reflect species differences. Further, benefits of βPKC inhibition in diabetes-induced cardiomyopathy in rats were reported by several groups [35, 36], suggesting perhaps difference between mice and rats regarding the role of PKC isozymes in cardiac pathology.
In the current study, we selected βI- and βIIPKC, because in our previous studies we found that their levels were elevated in the end stage failed hearts from hypertensive Dahl rats [4]. In addition to the current study, α-, βII- and εPKCs have been evaluated as targets for heart failure drugs by chronic pharmacological methods [11, 37, 38]. Using the same model, we previously reported that εPKC activation occurs early during the compensatory stage (11-17 weeks) and sustained inhibition of εPKC attenuated the development of cardiac fibrosis and dysfunction in hypertension-induced heart failure [11]. αPKC has also been suggested as a therapeutic candidate for the treatment of heart failure based on studies in mice [11, 37, 39]. However, the inhibitors used in those studies may inhibit several or all of the four classical PKC isozymes [39, 40].
5. Conclusion
In conclusion, an isozyme-specific inhibitor for βIIPKC, βIIV5-3, improved cardiac contractility ex vivo in normal and failing hearts and in vivo in failing hearts. Further, whereas βIPKC inhibition in vivo was without significant effects, the selective inhibitor of βIIPKC attenuated cardiac hypertrophy, myocardial fibrosis, cardiac inflammation, slowed down the progression of cardiac dysfunction and prolonged survival of rats. Together, we found that the βIIPKC-mediated pathological effects may result, in part, by inducing calcium handling defect, increasing fibrosis and increasing inflammation in the failing heart. Considering that PKCβII phosphorylates multiple protein substrates, other cellular and molecular events may also contribute to βIIPKC-mediated role in failed hearts at different time of the disease development. Further, PKCβII over-activation may contribute indirectly to some the pathologies associated with heart failure. Finally, an important question not addressed in the present study is related to the time course of PKCβII activation following the onset of hypertension, which can help identify the optimal time window for drug therapy with a selective βIIPKC inhibitor. Further, whether our findings in these hypertensive rats are translatable to other models of heart failure have yet to be determined. Because similar to this rat model of cardiac dysfunction, failed human hearts also have a selective increase in βPKC [2, 3], our data suggest that a selective inhibitor of βIIIIPKC function may have a therapeutic potential in the treatment of human heart failure.
Supplementary Material
01
Highlights.
- Selective βIIPKC inhibition improves cardiac function and survival in heart failure.
- βIIPKC inhibition reverses pathological cardiac remodeling.
- βIIV5-3 peptide suppresses myocardial fibrosis and inflammation.
- βIIPKC inhibition improves calcium handling and myocardial contractility.
Acknowledgements
This study was supported by National Institute of Health Grant HL076675 and HL52141 to DMR. J.C.B.F. holds a post-doctoral fellowship from Fundação de Amparo a Pesquisa do Estado de São Paulo - Brasil (FAPESP 2009/03143-1).
Footnotes
Conflict of interest statement
DM-R is the founder of KAI Pharmaceuticals, Inc, a company that plans to bring PKC regulators to the clinic. However, none of the work described in this study is based on or supported by the company. Other authors have no disclosure.
References
- 1.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Bowling N, Walsh RA, Song G, Estridge T, Sandusky GE, Fouts RL, et al. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99:384–91. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 3.Simonis G, Briem SK, Schoen SP, Bock M, Marquetant R, Strasser RH. Protein kinase C in the human heart: differential regulation of the isoforms in aortic stenosis or dilated cardiomyopathy. Mol Cell Biochem. 2007;305:103–11. doi: 10.1007/s11010-007-9533-3. [DOI] [PubMed] [Google Scholar]
- 4.Inagaki K, Iwanaga Y, Sarai N, Onozawa Y, Takenaka H, Mochly-Rosen D, et al. Tissue angiotensin II during progression or ventricular hypertrophy to heart failure in hypertensive rats; differential effects on PKC epsilon and PKC beta. J Mol Cell Cardiol. 2002;34:1377–85. doi: 10.1006/jmcc.2002.2089. [DOI] [PubMed] [Google Scholar]
- 5.Stebbins EG, Mochly-Rosen D. Binding specificity for RACK1 resides in the V5 region of beta II protein kinase C. Journal of Biological Chemistry. 2001;276:29644–50. doi: 10.1074/jbc.M101044200. [DOI] [PubMed] [Google Scholar]
- 6.Souroujon MC, Mochly-Rosen D. Peptide modulators of protein-protein interactions in intracellular signaling. Nat Biotechnol. 1998;16:919–24. doi: 10.1038/nbt1098-919. [DOI] [PubMed] [Google Scholar]
- 7.Churchill E, Budas G, Vallentin A, Koyanagi T, Mochly-Rosen D. PKC Isozymes in Chronic Cardiac Disease: Possible Therapeutic Targets? Annu Rev Pharmacol Toxicol. 2007 doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 8.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–72. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Wright LR, Chen CH, Oliver SF, Wender PA, Mochly-Rosen D. Molecular transporters for peptides: delivery of a cardioprotective epsilonPKC agonist peptide into cells and intact ischemic heart using a transport system, R(7). Chem Biol. 2001;8:1123–9. doi: 10.1016/s1074-5521(01)00076-x. [DOI] [PubMed] [Google Scholar]
- 10.Qi X, Inagaki K, Sobel RA, Mochly-Rosen D. Sustained pharmacological inhibition of deltaPKC protects against hypertensive encephalopathy through prevention of blood-brain barrier breakdown in rats. J Clin Invest. 2008;118:173–82. doi: 10.1172/JCI32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palaniyandi SS, Inagaki K, Mochly-Rosen D. Mast cells and epsilonPKC: a role in cardiac remodeling in hypertension-induced heart failure. J Mol Cell Cardiol. 2008;45:779–86. doi: 10.1016/j.yjmcc.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagata K, Liao R, Eberli FR, Satoh N, Chevalier B, Apstein CS, et al. Early changes in excitation-contraction coupling: transition from compensated hypertrophy to failure in Dahl salt-sensitive rat myocytes. Cardiovasc Res. 1998;37:467–77. doi: 10.1016/s0008-6363(97)00278-2. [DOI] [PubMed] [Google Scholar]
- 13.Silver RB. Ratio imaging: measuring intracellular Ca++ and pH in living cells. Methods Cell Biol. 2003;72:369–87. doi: 10.1016/s0091-679x(03)72018-4. [DOI] [PubMed] [Google Scholar]
- 14.Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltaPKC during reperfusion. Cardiovasc Res. 2010;85:385–94. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwanaga Y, Kihara Y, Hasegawa K, Inagaki K, Yoneda T, Kaburagi S, et al. Cardiac endothelin-1 plays a critical role in the functional deterioration of left ventricles during the transition from compensatory hypertrophy to congestive heart failure in salt-sensitive hypertensive rats. Circulation. 1998;98:2065–73. doi: 10.1161/01.cir.98.19.2065. [DOI] [PubMed] [Google Scholar]
- 16.Seki S, Nagai M, Takeda H, Onodera T, Okazaki F, Taniguchi M, et al. Impaired Ca2+ handling in perfused hypertrophic hearts from Dahl salt-sensitive rats. Hypertension Research - Clinical & Experimental. 2003;26:643–53. doi: 10.1291/hypres.26.643. [DOI] [PubMed] [Google Scholar]
- 17.Houser SR, Piacentino V, 3rd, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. Journal of Molecular & Cellular Cardiology. 2000;32:1595–607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- 18.Hoshijima M. Gene therapy targeted at calcium handling as an approach to the treatment of heart failure. Pharmacology & Therapeutics. 2005;105:211–28. doi: 10.1016/j.pharmthera.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Bartholomeu JB, Vanzelli AS, Rolim NP, Ferreira JC, Bechara LR, Tanaka LY, et al. Intracellular mechanisms of specific beta-adrenoceptor antagonists involved in improved cardiac function and survival in a genetic model of heart failure. J Mol Cell Cardiol. 2008;45:240–9. doi: 10.1016/j.yjmcc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi T, Yang X, Walker LA, Van Breemen RB, Solaro RJ. A non-equilibrium isoelectric focusing method to determine states of phosphorylation of cardiac troponin I: identification of Ser-23 and Ser-24 as significant sites of phosphorylation by protein kinase C. J Mol Cell Cardiol. 2005;38:213–8. doi: 10.1016/j.yjmcc.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Itoh S, Ding B, Bains CP, Wang N, Takeishi Y, Jalili T, et al. Role of p90 ribosomal S6 kinase (p90RSK) in reactive oxygen species and protein kinase C beta (PKC-beta)-mediated cardiac troponin I phosphorylation. J Biol Chem. 2005;280:24135–42. doi: 10.1074/jbc.M413015200. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira JC, Moreira JB, Campos JC, Pereira MG, Mattos KC, Coelho MA, et al. Angiotensin receptor blockade improves the net balance of cardiac Ca(2+) handling-related proteins in sympathetic hyperactivity-induced heart failure. Life Sci. 2011;88:578–85. doi: 10.1016/j.lfs.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Pereira MG, Ferreira JC, Bueno CR, Jr., Mattos KC, Rosa KT, Irigoyen MC, et al. Exercise training reduces cardiac angiotensin II levels and prevents cardiac dysfunction in a genetic model of sympathetic hyperactivity-induced heart failure in mice. Eur J Appl Physiol. 2009;105:843–50. doi: 10.1007/s00421-008-0967-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhang ZH, Johnson JA, Chen L, El-Sherif N, Mochly-Rosen D, Boutjdir M. C2 region-derived peptides of beta-protein kinase C regulate cardiac Ca2+ channels. Circ Res. 1997;80:720–9. doi: 10.1161/01.res.80.5.720. [DOI] [PubMed] [Google Scholar]
- 25.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Grant JE, Doede CM, Sadayappan S, Robbins J, Walker JW. PKC-betaII sensitizes cardiac myofilaments to Ca2+ by phosphorylating troponin I on threonine-144. J Mol Cell Cardiol. 2006;41:823–33. doi: 10.1016/j.yjmcc.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27:92–8. doi: 10.1007/s00059-002-2354-y. [DOI] [PubMed] [Google Scholar]
- 28.Diez J, Lopez B, Gonzalez A, Querejeta R. Clinical aspects of hypertensive myocardial fibrosis. Curr Opin Cardiol. 2001;16:328–35. doi: 10.1097/00001573-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Plaksej R, Kosmala W, Frantz S, Herrmann S, Niemann M, Stork S, et al. Relation of circulating markers of fibrosis and progression of left and right ventricular dysfunction in hypertensive patients with heart failure. J Hypertens. 2009;27:2483–91. doi: 10.1097/HJH.0b013e3283316c4d. [DOI] [PubMed] [Google Scholar]
- 30.Querejeta R, Lopez B, Gonzalez A, Sanchez E, Larman M, Martinez Ubago JL, et al. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation. 2004;110:1263–8. doi: 10.1161/01.CIR.0000140973.60992.9A. [DOI] [PubMed] [Google Scholar]
- 31.Palaniyandi SS, Sun L, Ferreira JC, Mochly-Rosen D. Protein kinase C in heart failure: a therapeutic target? Cardiovasc Res. 2009;82:229–39. doi: 10.1093/cvr/cvp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roman BB, Geenen DL, Leitges M, Buttrick PM. PKC-beta is not necessary for cardiac hypertrophy. American journal of physiology. 2001;280:H2264–70. doi: 10.1152/ajpheart.2001.280.5.H2264. [DOI] [PubMed] [Google Scholar]
- 33.Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, et al. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci U S A. 1997;94:9320–5. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowman JC, Steinberg SF, Jiang T, Geenen DL, Fishman GI, Buttrick PM. Expression of protein kinase C beta in the heart causes hypertrophy in adult mice and sudden death in neonates. J Clin Invest. 1997;100:2189–95. doi: 10.1172/JCI119755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arikawa E, Ma RC, Isshiki K, Luptak I, He Z, Yasuda Y, et al. Effects of insulin replacements, inhibitors of angiotensin, and PKCbeta's actions to normalize cardiac gene expression and fuel metabolism in diabetic rats. Diabetes. 2007;56:1410–20. doi: 10.2337/db06-0655. [DOI] [PubMed] [Google Scholar]
- 36.Connelly KA, Kelly DJ, Zhang Y, Prior DL, Advani A, Cox AJ, et al. Inhibition of protein kinase C-beta by ruboxistaurin preserves cardiac function and reduces extracellular matrix production in diabetic cardiomyopathy. Circ Heart Fail. 2009;2:129–37. doi: 10.1161/CIRCHEARTFAILURE.108.765750. [DOI] [PubMed] [Google Scholar]
- 37.Hambleton M, Hahn H, Pleger ST, Kuhn MC, Klevitsky R, Carr AN, et al. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114:574–82. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palaniyandi SS, Ferreira JC, Brum PC, Mochly-Rosen D. PKCbetaII inhibition attenuates myocardial infarction induced heart failure and is associated with a reduction of fibrosis and pro-inflammatory responses. J Cell Mol Med. 2011;15:1769–77. doi: 10.1111/j.1582-4934.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hahn HS, Marreez Y, Odley A, Sterbling A, Yussman MG, Hilty KC, et al. Protein kinase Calpha negatively regulates systolic and diastolic function in pathological hypertrophy. Circ Res. 2003;93:1111–9. doi: 10.1161/01.RES.0000105087.79373.17. [DOI] [PubMed] [Google Scholar]
- 40.Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294(Pt 2):335–7. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwanaga Y, Kihara Y, Inagaki K, Onozawa Y, Yoneda T, Kataoka K, et al. Differential effects of angiotensin II versus endothelin-1 inhibitions in hypertrophic left ventricular myocardium during transition to heart failure. Circulation. 2001;104:606–12. doi: 10.1161/hc3101.092201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01