Molecular Pathways Controlling Pancreas induction (original) (raw)
. Author manuscript; available in PMC: 2013 Aug 1.
Published in final edited form as: Semin Cell Dev Biol. 2012 Jun 26;23(6):656–662. doi: 10.1016/j.semcdb.2012.06.009
Abstract
Recent advances in generating pancreatic cell types from human pluripotent stem cells has depended on our knowledge of the developmental processes that regulate pancreas development in vivo. The developmental events between gastrulation and formation of the embryonic pancreatic primordia are both rapid and dynamic and studies in frog, fish, chick, and mouse have identified the molecular basis of how the pancreas develops from multipotent endoderm progenitors. Here, we review the current status of our understanding of molecular mechanisms that control endoderm formation, endoderm patterning, and pancreas specification and highlight how these discoveries have allowed for the development of robust methods to generate pancreatic cells from human pluripotent stem cells.
Introduction
The endocrine, exocrine and ductal components of the pancreas derive from the definitive endoderm germ layer. After gastrulation, the endoderm expresses several markers in broad anterior and posterior (A-P) domains. Subsequently, a series of inductive events progressively refines these broad A-P patterns into restricted gene expression domains along the A-P and dorsal-ventral (D-V) axes that define where specific organs such as the pancreas will form. Therefore, these early stages of pancreas development are critical as they involve the separation of the pancreatic lineage from the other endodermally derived organs including the thyroid, thymus, lungs, liver, biliary system and digestive tract.
This review will focus on the early developmental processes and molecular pathways that culminate in the induction of the pancreas. We will discuss how specific signaling pathways play highly conserved roles in early stages of vertebrate pancreas development and because of this have been effectively used to generate human pancreatic cells from pluripotent stem cells (Spence and Wells, 2007; Nostro and Keller, 2012, this Seminars Cell and Dev. Biol issue).
Endoderm Formation
At the morphologic level, the segregation of the endoderm, mesoderm and ectoderm germ layers that occurs during gastrulation is strikingly different in frogs, fish, chicken and mice. However, molecular studies have revealed an evolutionarily conserved molecular pathway that regulates endoderm formation across these vertebrate species. At the top of this pathway is the Nodal-signaling pathway, which is necessary and sufficient to initiate endoderm and mesoderm development, and is required for proper gastrulation and axial patterning in all species studied. Nodal is a member of the TGFβ family of secreted growth factors. There are 5 nodal-related genes in Xenopus (xnr1,2,4,5,6), two in zebra fish (squint and cyclops), and one in mouse (Nodal), and these ligands signal through a receptor complex containing type I and type II transmembrane serine-threonine kinase receptors as well as an EGF-CFC family co-receptor (Reviewed in de Caestecker, 2004). Nodal signaling plays two key roles in endoderm formation. First, Nodal initiates gastrulation and the formation of a bipotent mesendoderm precursor; second, a Nodal-signaling gradient segregates endoderm (high Nodal) and mesoderm (low Nodal) lineages. This was experimentally demonstrated in model organisms including Xenopus (Green and Smith, 1990; Clements et al., 1999) and zebrafish (Shen, 2007), and experiments using embryonic stem cell cultures indicate that a Nodal gradient plays a similar role in mice and humans (Kubo et al., 2004; D'Amour et al., 2005).
Receptor-mediated activation of Nodal signaling results in phosphorylation and nuclear translocation of Smad2/Smad3 proteins that form transcriptional complexes with co-factors (Foxh1/FAST1 or Mxl1) to regulate the transcription of target genes (Zorn and Wells, 2007). In the case of the endoderm lineage, these target genes include transcription factors that act in a network to regulate the endoderm transcriptional program. Again, the endoderm transcriptional network appears to be highly conserved from frogs (Sinner et al., 2006) to humans (Spence et al., 2011; Spence and Wells, 2007) and includes HMG-box factors (Sox), Forkhead factors (Fox), Homeodomain factors (Mix-like) and Zinc finger (Gata) factors.
Establishing anterior-posterior and dorsal-ventral pattern in the endoderm
By the end of gastrulation, endoderm cells have already acquired A-P character at the level of gene expression. For example, anterior endoderm cells express genes such as Hhex, Dkk1, and Cerberus and posterior endoderm cells express markers such as Cdx2. Evidence from cell lineage tracing experiments in mouse and chick suggest that initial A-P identity is linked to the timing of endoderm cell specification during gastrulation, specifically anterior endoderm cells are specified earlier than posterior endoderm cells (Lawson and Pedersen, 1987; Lawson and Schoenwolf, 2003; Kimura et al., 2006). In higher vertebrates including chicks, mice and humans, the location of endoderm cells within the primitive streak is another important factor that may impact AP endoderm fate. The primitive streak is a transient structure in the posterior of the embryo that expresses a number of growth factor ligands including Nodal, FGFs, WNTs, and BMPs, which differentially impact nascent endoderm cells. For example, endoderm cells in the high nodal environment of the anterior primitive streak become anterior whereas endoderm cells derived from more posterior regions of the streak are exposed to the posteriorizing effects of FGFs, BMPs and WNTs. However, experiments done in mice and frogs indicate that endoderm cells remain plastic after gastrulation. For example, pre-somitic stage endoderm that is recombined with mesoderm from a more posterior region will adopt a posterior fate and vice versa (Horb and Slack, 2001; Wells and Melton, 2000). Human definitive endoderm (DE) derived from pluripotent stem cells (PSCs) is also plastic and can be patterned along the A-P axis by manipulation of patterning pathways such as WNT and FGF (Spence et al., 2011; Maehr et al., 2011).
After gastrulation and the establishment of the A-P axis, a series of morphogenetic movements transforms the naïve endoderm into a 3-dimensional primitive gut tube, thus establishing the dorsal-ventral (D-V) axis. The resulting gut tube is loosely subdivided into foregut, midgut and hindgut domains that are also defined by expression of a specific set of genetic markers including Sox2 in the foregut, Pdx1 in the posterior foregut/midgut, and Cdx2 in the mid/hindgut. These broad domains become further subdivided into smaller domains that establish molecular territories from which specific organ primordium will form. For example, the primitive foregut tube expresses markers including Pax9, Nkx2.1, Sox2, Hhex, Sox17 and Pdx1 in restricted domains that give rise to the esophagus, trachea, stomach, lungs, thyroid, liver, extrahepatobiliary system, pancreas and duodenum. The establishment of a patterned foregut tube is controlled by a series of reciprocal interactions with nearby mesoderm tissues and is mediated by several key signaling pathways.
Molecular basis of A-P endoderm patterning
Although endoderm acquires initial A-P identity during gastrulation, the endoderm remains competent to respond to signals secreted from the adjacent mesodermal tissues. Based on a series of tissue recombination experiments using chick and mouse embryos, it was shown that gastrulation stage endoderm remains plastic along its A-P axis such that posterior endoderm moved to the anterior will adopt an anterior fate and vice versa for posterior mesoderm (Wells and Melton, 2000). However, by early somite stages posterior endoderm has lost the ability to become anteriorized (Kumar, 2003), suggesting the possibility of a “posterior dominance” effect, in which lateral plate mesoderm (LPM) can posteriorize endoderm taken from more anterior regions but it cannot anteriorize endoderm taken from more posterior regions. These data suggest that potent posteriorizing factors specify posterior endoderm between late gastrula and early somite stages. Moreover these data suggest that anterior mesoderm does not have potent anteriorizing activity, and perhaps the anterior fate requires the absence or inhibition of posterior cues. Consistent with this, when posterior endoderm isolated from 5-somite chick embryos was cultured in the absence of mesoderm, neither Pdx1 nor Cdx expression was observed. In the past few years a number of signaling pathways have been shown to play a central role in endoderm patterning, including WNT, FGF, and BMP, and these all exert a posteriorizing effect.
WNT Signaling
The canonical WNT signaling pathway (WNT/β-catenin) has an evolutionarily conserved role in endoderm patterning in vertebrates. Studies in Xenopus embryos showed that suppression of WNT/β-catenin is required for expression of the anterior endoderm marker Hhex, and ectopic activation of WNT/β-catenin in patterning stage embryos results in loss of anterior pattern and foregut-derived structures, including the pancreas and the liver (McLin et al., 2007). It was further demonstrated that Sfrp5, a secreted WNT signaling antagonist, is expressed in anterior endoderm and is required to maintain a low WNT/β-catenin signaling environment that is required for foregut development (Li et al., 2008). Recent work in mouse and human PSC cultures also demonstrated that low WNT/β-catenin signaling is required for anterior endoderm development and high signaling promotes development of posterior endoderm expressing Cdx2 (Maehr et al., 2011; Spence et al., 2011).
Although the above data clearly demonstrate a role for WNT/β-catenin signaling in patterning endoderm between gastrula and early somite stages, a role for this pathway in patterning pre-pancreatic endoderm remains unclear. Data from Xenopus demonstrates that Wnt8 over-expression inhibits pancreas specification (McLin et al., 2007) whereas in mouse, ectopic β-catenin activation in anterior endoderm does not profoundly impact early pancreas development, although activation of β-catenin was mosaic and may not have been sufficient to cause a more severe phenotype (Maehr et al., 2011). Additionally, it is yet to be determined whether a complete absence of WNT/β-catenin is required to specify the foregut or if a low-to-high gradient of WNT/β-catenin signaling activity patterns the endoderm along its A-P axis.
FGF Signaling
Like the WNT/β-Catenin pathway, FGF signaling induces posterior and represses anterior gene expression and foregut development. Experiments in chick and mouse endoderm have invoked a morphogen-like role for FGF4, as high doses of this factor promote hindgut and inhibit foregut/midgut gene expression, whereas intermediate levels of FGF4 can robustly induce Pdx1 with minimal effects on Cdx (Wells and Melton, 2000; Dessimoz et al., 2006). Moreover, FGF signaling has cell-autonomous effects on endoderm, since expression of activated FGF receptor in anterior endoderm can induce ectopic expression of Pdx1. Consistent with this, intermediate levels of FGF2 can induce the formation of Pdx1-positive cells in human PSC-derived definitive endoderm cultures (Ameri et al., 2009). It is important to distinguish the role of FGF signaling on posterior foregut development during endoderm patterning stage of development from its later roles in pancreas specification (described below) and in regulating proliferation of pancreatic progenitors (Bhushan et al., 2001; Serup 2012, this Seminars Cell and Dev. Biol issue). Thus, the timing of FGF manipulations in PSC cultures will have very different effects on pancreas development as they might affect endoderm patterning, pancreas specification, or proliferation of _Pdx1_-expressing progenitors.
BMP Signaling
The BMP signaling pathway has also been observed to have a posteriorizing effect on endoderm in several model organisms. In chick embryonic explants, BMP ligands were sufficient to induce Pdx1 and Cdx in anterior endoderm, but only in the presence of mesoderm. Moreover, the BMP signaling antagonist Noggin was able to abolish the ability of posterior LPM to induce Pdx1 and Cdx expression in anterior endoderm (Kumar, 2003). BMP signaling is also required for posterior gene expression in zebrafish and Xenopus endoderm (Tiso et al., 2002; Wills et al., 2008; Rankin et al., 2011). Similar to FGFs, it is difficult to distinguish an early patterning function of BMP signaling from a later one in pancreas specification (discussed below). These data suggest that BMP signaling is required for Pdx1 expression but may act in combination with other signaling pathways.
Signaling Pathway Crosstalk
There is now strong evidence that WNT, FGF, BMP and retinoic acid (RA) signaling pathways (discussed below) all act to regulate A-P patterning of endoderm. However, despite intensive study, the mechanisms by which each of these pathways interact with one another to affect region-specific gene expression is largely unknown. Specifically, it has yet to be determined whether WNT, FGF, BMP and retinoic acid (RA) signaling pathways act in parallel or in an epistatic manner. Moreover, these pathways similarly pattern mesoderm sometimes making it difficult to identify the endoderm cell-autonomous effects versus the secondary effects via mesoderm. Uncovering the molecular details by which these signals cooperate will be essential to understand the complete mechanism by which the endoderm is patterned.
Although WNT, BMP, FGF, and RA are known to have direct effects on endoderm in the context of the embryo or in the presence of mesoderm (Rankin et al., 2011; Maehr et al., 2011; Dessimoz et al., 2006), they have been shown to have limited patterning activity on isolated endoderm suggesting that other signals from mesoderm act synergistically. Several reports have begun to address how these posteriorizing signals act together to pattern endoderm. Using human definitive endoderm cultures as a model system, Spence and collaborators showed that while neither WNT3A nor FGF4 alone is sufficient to robustly induce posterior fate, the two factors act synergistically to specify posterior, _Cdx2_-positive endoderm (Spence et al., 2011). Similarly, experiments performed on chick embryos suggest that FGF may also act in parallel or synergize with RA to induce hindgut (Bayha et al., 2009; Kumar, 2003). One study in Xenopus has identified a molecular mechanism by which WNT and BMP synergize to promote posterior fate at the transcriptional level; where downstream effectors of WNT and BMP signaling converge on the promoter of Vent2, a master regulator of posterior identity in Xenopus endoderm (Rankin et al., 2011).
Despite the mounting evidence for the importance of A-P and D-V patterning in endoderm organogenesis, there is little attention paid to endoderm patterning in the context of pancreas directed differentiation of PSCs. Given that the pancreas develops from a very stereotypic region of the dorsal and ventral foregut, the use of patterning cues to direct PSC-derived definitive endoderm specifically into dorsal or ventral pancreas might improve both efficiency and functionality of PSC-derived pancreatic cell types.
Ventral Pancreas Specification
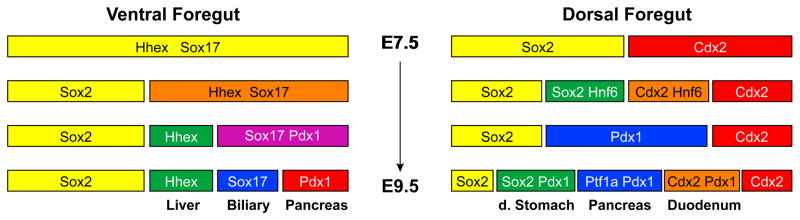
The posterior segment of the ventral foregut endoderm gives rise to the liver, extrahepatic biliary system, and ventral pancreas. Experiments done in zebrafish and mouse explants support the notion that liver and pancreas are derived from a common progenitor cell (Chung et al., 2008; Tremblay and Zaret, 2005; Deutsch et al., 2001), while genetic lineage tracing in mouse embryos has confirmed that the ventral pancreas and extrahepatic biliary system develop from a population of cells that co-express Pdx1 and Sox17 (Spence et al., 2009). The overlay of these findings suggests a model whereby multipotent progenitors in the ventral foregut are progressively segregated toward distinct organ lineages: first, hepatic precursors are specified followed by the separation of the extrahepatobiliary and ventral pancreatic lineages (summarized in figure 1).
Figure 1.
Schematic of lineage segregation in the ventral and dorsal endoderm. In the ventral foregut, hepatoblasts (Hhex-positive) are first specified from a pool of multipotent progenitors. Shortly after, bipotential pancreato-biliary progenitors (Pdx1/Sox17 double-positive) are segregated into the extrahepatic biliary and ventral pancreatic lineages. In dorsal endoderm, Pdx1 expression is initiated at the junction of the Cdx2-positive intestinal hindgut and the Sox2-positive foregut endoderm. Within the Pdx1-positive domain, cells that will contribute the future pancreas initiate expression of Ptf1a, while Pdx1-positive/Ptf1a-negative cells will contribute to parts of the distal stomach and the duodenum.
Very little is known regarding the extrinsic factors that govern the initial induction of the ventral pancreas. Work from a number of labs has identified pathways that may be involved in segregating hepatic and pancreatic fates (figure 2); however, most of these appear to promote liver and repress pancreas development. These findings suggest that pancreas may be the default fate of ventral foregut endoderm, for example cultures of isolated mouse ventral foregut endoderm will begin to express high levels of Pdx1, whereas liver marker expression requires signals from cardiac mesenchyme (Deutsch et al., 2001). The idea of pancreas as a default state is supported by a recent study, which found that a pre-existing pattern of chromatin modifications biases ventral foregut endoderm to become pancreas (Xu et al., 2011).
Figure 2.
Overview of pancreas-inducing signals in the dorsal and ventral mouse foregut. In the ventral foregut, BMP and FGF signaling activity promote liver (Hhex) and inhibit pancreas (Pdx1) specification. The Notch signaling effector Hes1 in involved in pancreato-biliary segregation, and it acts to prevent ectopic pancreas development in adjacent tissues. In dorsal endoderm, RA is required to promote pancreas specification, while Shh signaling is inhibitory. Hedgehog signaling is inhibited in the prepancreatic dorsal endoderm by Activin- and/or FGF2-mediated repression of Shh transcription.
Tissue Interactions
The extensive and coordinated movements that occur during foregut morphogenesis brings the ventral foregut endoderm into close proximity with the cardiac mesoderm and septum transversum mesenchyme, and it has been shown that these tissues can induce liver fate at the expense of the pancreas (Deutsch et al., 2001; Rossi et al., 2001). In vivo, it is hypothesized that Pdx1 expression initiates in the lip of the ventral foregut after it has extended beyond the cardiac mesoderm and thus escaped the influence of hepatic-inducing signals. In support of this, _Hhex_-null embryos fail to specify a ventral pancreas, although _Hhex_-null endoderm cultured in vitro is fully capable of expressing Pdx1 (Bort et al., 2004). The authors of this study concluded that a reduced proliferation rate in the ventral foregut of these embryos prevented presumptive pancreatic endoderm from being displaced from the cardiac mesoderm and thus prevented ventral pancreas specification. These results emphasize that in vivo, proliferation and morphogenesis in the early endoderm are tightly linked to organ development, and a highly orchestrated set of events must be preserved for pancreas specification to occur normally.
FGF Signaling
Several studies support the idea that the hepatic-inducing signals from the cardiac mesoderm include FGF signaling ligands (Jung et al., 1999). Data from mouse foregut explants identified that exogenous FGF ligands were sufficient to inhibit Pdx1 and activate liver-specific gene expression in isolated ventral foregut endoderm (Deutsch et al., 2001). These studies specifically implicate FGF1 and FGF2 as those responsible for patterning the ventral foregut. However, no liver or pancreatic defects are found in FGF1/2 double-knockout mice (Miller et al., 2000). This is likely due to compensation from other FGFs, as there are 22 FGF family members in humans (Böttcher and Niehrs, 2005). The level of functional redundancy between ligands, as well as the 4 FGF receptors, makes it a very difficult pathway to study using genetic loss-of-function approaches. Several studies used dominant-negative approaches to demonstrate that the FGF/MAPK pathway is required for hepatic specification in zebrafish (Shin et al., 2007) and mouse (Calmont et al., 2006) embryos. However, these in vivo studies did not reveal an increase in pancreas progenitors when liver fate was blocked, which suggests that other signaling pathways may act to regulate ventral pancreas specification in vivo.
BMP Signaling
Like the FGF pathway, the BMP signaling pathway is required to specify hepatoblasts and inhibit pancreas specification in isolated ventral foregut endoderm (Rossi et al., 2001), although it is not known if FGF and BMP act in an epistatic fashion or if both pathways act in parallel to inhibit pancreas specification. Moreover previous studies suggested but did not definitively show that liver and pancreas arose form a common progenitor. The definitive evidence for this came through single cell labeling experiments in zebrafish, in which it was shown that the BMP pathway controls the liver versus pancreas fate decision in zebrafish, largely through Bmp2 and its receptor Alk8 (Chung et al., 2008). In this case ectopic BMP signaling induces a pancreas-to-liver fate switch in cells that do not normally contribute to the liver. Moreover, embryos lacking Alk8 exhibited an expanded pancreatic domain and a reduced field of hepatic progenitors. In mouse, endoderm-specific deletion of Smad4, which is an obligatory effector of the BMP signaling pathway (Wandzioch and Zaret, 2009) resulted in reduced liver induction suggesting that this factor is required endoderm-cell autonomously. Interestingly, the pancreatic domain was not expanded in these embryos as would be expected based on zebrafish data. In fact, ventral Pdx1 expression was severely diminished at E9.5. However, these results are complicated by the fact that two different signaling pathways were disrupted in these embryos, as Smad4 also acts downstream of the TGFβ pathway. A possible explanation of the Smad4 data comes from studies in Xenopus, where TGFβ-induced factor 2 (TGIF2) was shown to be required for pancreas specification and it acts by cell-autonomously antagonizing BMP/Smad1 signaling (Spagnoli and Brivanlou, 2008). In summary, there is strong support from experimental data in several model organisms that suppression of BMP signaling is required for ventral foregut cells to adopt a pancreatic fate and that TGFβ may act in this capacity through regulation of BMP suppressors.
Notch Signaling
The Notch pathway has a critical function in determining lineage commitment within the developing pancreas (reviewed in (Gittes, 2009); Serup 2012, this Seminars Cell and Dev. Biol issue). Studies in mice deficient in Hes1, a downstream Notch effector, suggest that this pathway may be involved in pancreas specification in the ventral foregut as well. In _Hes1_−/− mice, ectopic pancreatic tissue is found in the extrahepatic biliary system, posterior stomach, and proximal duodenum (Sumazaki et al., 2004; Fukuda et al., 2006). Subsequent studies found that Hes1 and Sox17, an HMG-box transcription factor that is also required to prevent ectopic pancreatic development in the extrahepatic biliary system, form part of a complex regulatory network that controls organ cell fate commitment of pancreatobiliary progenitors in the ventral foregut (Spence et al., 2009). Although Hes1 is generally considered a Notch effector, it can also function independent of active Notch signaling. Therefore, further investigation is required to determine the action of Notch in ventral foregut lineage segregation.
Dorsal Pancreas Specification
Unlike the ventral foregut, which gives rise to several organs, the dorsal pancreas is the only budding organ that develops from the dorsal endoderm. Thus, dorsal pancreas specification seems more a matter of simply inducing pancreatic fate within the endoderm gut tube, rather than segregating multiple organ lineages (figure 1). Although the mechanisms that regulate the later stages of development in the ventral and dorsal pancreas are quite similar, the pathways that control their specification have some notable differences (as shown in figure 2). An important disparity may be in the endoderm itself; in stark contrast to ventral foregut endoderm, presumptive dorsal pancreatic endoderm does not activate pancreatic gene expression when cultured in isolation (Kim et al., 1997). This demonstrates that pancreas is not the default fate of dorsal endoderm. There are several molecular differences between dorsal and ventral prepancreatic endoderm. For example, expression of the gene Hlxb9 is unique to the dorsal endoderm and genetic knockout of this factor results in dorsal pancreatic agenesis (Harrison et al., 1999; Li et al., 1999). As discussed below there are also differences in tissue interaction and signaling pathways that appear to be unique to dorsal pancreas induction.
Tissue Interactions
As with the ventral pancreas, dorsal pancreas development is impacted by interactions with neighboring tissues. In mouse and chick, the notochord lies in close contact with the dorsal endoderm until it is displaced by the midline fusion of the dorsal aortae and both of these structures are involved in dorsal pancreas induction (Kim et al., 1997; Lammert et al., 2001). For example removal of the notochord from early chick embryos is sufficient to prevent dorsal pancreas specification, and Kim et. al. further demonstrated that it provides a permissive environment for Pdx1 expression in the context of the embryo (Kim et al., 1997). Later, the dorsal endoderm becomes surrounded by mesenchyme, which is known to be critical in later stages of pancreas development. These cells also have an important function in pancreas specification, evidenced by the fact that loss of either Islet1 or N-cadherin in the dorsal mesenchyme results in failure to induce Pdx1 in the adjacent endoderm (Ahlgren et al., 1997; Esni, 2001). The mechanism by which these genes regulate mesenchymal-to-epithelial signaling is unknown.
Retinoic Acid
Thus far, retinoid signaling has been found to affect pancreas specification in mouse, chicken, zebrafish, and Xenopus. In general, perturbations in RA signaling have a much greater impact on dorsal, rather than ventral, pancreas development. Initial experiments in zebrafish, in which embryos were cultured in either RA or an inhibitor of RA signaling, revealed that RA is required for the development of both the pancreas and the liver (Stafford and Prince, 2002). A later study employed endoderm-specific dominant-negative RA receptors to determine that only the dorsal pancreas has a cell-autonomous requirement for RA signaling (Stafford et al., 2006). The lack of reported defects in the ventral pancreas and liver in the latter study suggest that RA may act indirectly in endoderm patterning and ventral foregut specification. Similar results were obtained in experiments performed on Xenopus embryos (Chen et al., 2004). The dorsal-specific requirement for RA is conserved in mammalian pancreas development. Deficiency of Raldh2, the enzyme required for generating the majority of endogenous RA in the mouse embryo, prevents Pdx1 expression in the dorsal endoderm and causes agenesis of the dorsal pancreas (Molotkov et al., 2005; Martín et al., 2005). Specification of the ventral pancreas and liver were unaffected in RA-deficient embryos at early stages.
Hedgehog Signaling
In the early chick embryo, it was observed that Shh was broadly expressed in the developing endoderm but excluded from the dorsal pre-pancreatic endoderm, which is in close contact with the notochord (Hebrok et al., 1998). This led to the hypothesis that the notochord is required for dorsal pancreas induction through repression of Shh expression. In support of this, ectopic engraftment of notochord could repress Shh expression in the lateral and ventral endoderm and Shh neutralizing antibodies were sufficient to induce pancreas gene expression in dorsal endoderm explants. The effect of Shh antibodies was limited to the posterior foregut/prepancreatic endoderm, however, which indicates that suppression of hedgehog signaling is permissive rather than instructive for a pancreas fate. Accordingly, ectopic pancreatic tissue is not observed in the endoderm of _Shh_−/− embryos (Hebrok et al., 2000). The inhibitory effect Shh on pancreas development was further demonstrated in _Ptc1_-deficient mice, in which hedgehog signaling is constitutively active. Although these embryos arrest early (E9.5), Pdx1- and glucagon-expressing cells were absent in the pancreatic region of the endoderm at E9.0 (Hebrok et al., 2000). While these studies provide compelling data that hedgehog signaling represses pancreas specification in the dorsal endoderm of mouse and chick, this mechanism is not conserved in all model organisms. The converse relationship actually exists in zebrafish endoderm, where ectopic Shh signaling expands the _Pdx1_-positive pancreas domain and hedgehog inhibition results in pancreatic agenesis (Roy et al., 2001; diIorio et al., 2002). With respect to the pancreas specification stage, this example is the only major discrepancy observed among the common model organisms.
TGFβ Signaling
The notochord secretes many growth factors throughout the developing embryo. One such factor is the TGFβ signaling molecule Activin-βB, which is able to repress of Shh and induce Pdx1 expression in dorsal endoderm in a dose-dependent manner (Hebrok et al., 1998). In vivo, mouse embryos with compound mutations in the activin receptor subunits (ActRIIA+/−B−/−) have ectopic Shh expression in the Pdx1 domain and a reduction in pancreatic markers including Islet1 (Kim et al., 2000). In this case, Shh expression was shifted posteriorly, suggesting that Activin may play a role in A-P patterning, which has secondary affects on pancreas development. Interestingly, the dorsal pancreas is specified normally in endoderm-specific Smad4 mutants (Wandzioch and Zaret, 2009), demonstrating the need for additional studies to clarify the role of TGFβ family members in endoderm patterning and pancreas specification.
Summary of Pancreas Specification Signals
Although the functions and cell types comprising the dorsal and ventral pancreas are indistinguishable, all available evidence suggests that they are specified from early posterior foregut endoderm as two entirely distinct organs (summarized in figure 2). Whereas RA is essential for dorsal pancreas specification, it appears to be dispensable in the early ventral foregut. Similarly, suppression of BMP signaling is critical for ventral pancreas specification, but a role for BMP in the dorsal foregut has not been described. FGF signaling represses pancreatic fate in the ventral foregut whereas low levels of FGF2 from notochord are hypothesized to have a positive effect on specification of the dorsal pancreas (Hebrok et al., 1998). These distinct and sometimes contradictory mechanisms may be the basis for the diverse array of methods that have been used to successfully differentiate hPSCs to Pdx1-positive endoderm (reviewed in Nostro and Keller, 2012, this Seminars Cell and Dev. Biol issue).
Highlights.
- Patterning of the gut tube along the anterior-posterior and dorsal-ventral axis is essential for pancreas induction
- Significant differences exists in the mechanisms that regulate dorsal and ventral pancreas induction
- The core molecular pathways that regulate endoderm and pancreas induction are highly conserved across vertebrate species
- The molecular pathways that regulate pancreas induction have been successfully used to generate pancreatic progenitors from human pluripotent stem cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Ahlgren U, Pfaff SL, Jessell TM, Edlund T, Edlund H. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- Ameri J, Ståhlberg A, Pedersen J, Johansson JK, Johannesson MM, Artner I, Semb H. FGF2 Specifies hESC-Derived Definitive Endoderm into Foregut/Midgut Cell Lineages in a Concentration-Dependent Manner. Stem Cells. 2009 doi: 10.1002/stem.249. N/A–N/A. [DOI] [PubMed] [Google Scholar]
- Bayha E, Jørgensen MC, Serup P, Grapin-Botton A. Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS ONE. 2009;4:e5845. doi: 10.1371/journal.pone.0005845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhushan A, Itoh N, Kato S, Thiery JP, Czernichow P, Bellusci S, Scharfmann R. Fgf10 is essential for maintaining the proliferative capacity of epithelial progenitor cells during early pancreatic organogenesis. Development. 2001;128:5109–5117. doi: 10.1242/dev.128.24.5109. [DOI] [PubMed] [Google Scholar]
- Bort R, Martinez-Barbera JP, Beddington RSP, Zaret KS. Hex homeobox gene-dependent tissue positioning is required for organogenesis of the ventral pancreas. Development. 2004;131:797–806. doi: 10.1242/dev.00965. [DOI] [PubMed] [Google Scholar]
- Böttcher RT, Niehrs C. Fibroblast growth factor signaling during early vertebrate development. Endocr Rev. 2005;26:63–77. doi: 10.1210/er.2003-0040. [DOI] [PubMed] [Google Scholar]
- Calmont A, Wandzioch E, Tremblay KD, Minowada G, Kaestner KH, Martin GR, Zaret KS. An FGF Response Pathway that Mediates Hepatic Gene Induction in Embryonic Endoderm Cells. Developmental Cell. 2006;11:339–348. doi: 10.1016/j.devcel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Chen Y, Pan FC, Brandes N, Afelik S, Sölter M, Pieler T. Retinoic acid signaling is essential for pancreas development and promotes endocrine at the expense of exocrine cell differentiation in Xenopus. Developmental Biology. 2004;271:144–160. doi: 10.1016/j.ydbio.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Chung WS, Shin CH, Stainier DYR. Bmp2 Signaling Regulates the Hepatic versus Pancreatic Fate Decision. Developmental Cell. 2008;15:738–748. doi: 10.1016/j.devcel.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements D, Friday RV, Woodland HR. Mode of action of VegT in mesoderm and endoderm formation. Development. 1999;126:4903–4911. doi: 10.1242/dev.126.21.4903. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004;15:1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM. FGF signaling is necessary for establishing gut tube domains alongthe anterior–posterior axis in vivo. Mechanisms of Development. 2006;123:42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Deutsch G, Jung J, Zheng M, Lóra J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–881. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- diIorio PJ, Moss JB, Sbrogna JL, Karlstrom RO, Moss LG. Sonic hedgehog is required early in pancreatic islet development. Developmental Biology. 2002;244:75–84. doi: 10.1006/dbio.2002.0573. [DOI] [PubMed] [Google Scholar]
- Esni F. Dorsal Pancreas Agenesis in N-Cadherin- Deficient Mice. Developmental Biology. 2001;238:202–212. doi: 10.1006/dbio.2001.0405. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Kawaguchi Y, Furuyama K, Kodama S, Horiguchi M, Kuhara T, Koizumi M, Boyer DF, Fujimoto K, Doi R, et al. Ectopic pancreas formation in Hes1 -knockout mice reveals plasticity of endodermal progenitors of the gut, bile duct, and pancreas. J Clin Invest. 2006;116:1484–1493. doi: 10.1172/JCI27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittes GK. Developmental biology of the pancreas: A comprehensive review. Developmental Biology. 2009;326:4–35. doi: 10.1016/j.ydbio.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Green JB, Smith JC. Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature. 1990;347:391–394. doi: 10.1038/347391a0. [DOI] [PubMed] [Google Scholar]
- Harrison KA, Thaler J, Pfaff SL, Gu H, Kehrl JH. Pancreas dorsal lobe agenesis and abnormal islets of Langerhans in Hlxb9-deficient mice. Nat Genet. 1999;23:71–75. doi: 10.1038/12674. [DOI] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, Melton DA. Notochord repression of endodermal Sonic hedgehog permits pancreas development. Genes & Development. 1998;12:1705–1713. doi: 10.1101/gad.12.11.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebrok M, Kim SK, St Jacques B, McMahon AP, Melton DA. Regulation of pancreas development by hedgehog signaling. Development. 2000;127:4905–4913. doi: 10.1242/dev.127.22.4905. [DOI] [PubMed] [Google Scholar]
- Horb ME, Slack JM. Endoderm specification and differentiation in Xenopus embryos. Developmental Biology. 2001;236:330–343. doi: 10.1006/dbio.2001.0347. [DOI] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Melton DA. Notochord to endoderm signaling is required for pancreas development. Development. 1997;124:4243–4252. doi: 10.1242/dev.124.21.4243. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hebrok M, Li E, Oh SP, Schrewe H, Harmon EB, Lee JS, Melton DA. Activin receptor patterning of foregut organogenesis. Genes & Development. 2000;14:1866–1871. [PMC free article] [PubMed] [Google Scholar]
- Kimura W, Yasugi S, Stern CD, Fukuda K. Fate and plasticity of the endoderm in the early chick embryo. Developmental Biology. 2006;289:283–295. doi: 10.1016/j.ydbio.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, Keller G. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- Kumar M. Signals from lateral plate mesoderm instruct endoderm toward a pancreatic fate. Developmental Biology. 2003;259:109–122. doi: 10.1016/s0012-1606(03)00183-0. [DOI] [PubMed] [Google Scholar]
- Lammert E, Cleaver O, Melton D. Induction of pancreatic differentiation by signals from blood vessels. Science. 2001;294:564–567. doi: 10.1126/science.1064344. [DOI] [PubMed] [Google Scholar]
- Lawson A, Schoenwolf GC. Epiblast and primitive-streak origins of the endoderm in the gastrulating chick embryo. Development. 2003;130:3491–3501. doi: 10.1242/dev.00579. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Pedersen RA. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development. 1987;101:627–652. doi: 10.1242/dev.101.3.627. [DOI] [PubMed] [Google Scholar]
- Li H, Arber S, Jessell TM, Edlund H. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat Genet. 1999;23:67–70. doi: 10.1038/12669. [DOI] [PubMed] [Google Scholar]
- Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes & Development. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehr R, Mazzoni EO, Melton DA, Sherwood RI. Wnt signaling specifies and patterns intestinal endoderm. Mechanisms of Development. 2011:1–14. doi: 10.1016/j.mod.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín M, Gallego-Llamas J, Ribes V, Kedinger M, Niederreither K, Chambon P, Dollé P, Gradwohl G. Dorsal pancreas agenesis in retinoic acid-deficient Raldh2 mutant mice. Developmental Biology. 2005;284:399–411. doi: 10.1016/j.ydbio.2005.05.035. [DOI] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/ -catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Molecular and Cellular Biology. 2000;20:2260–2268. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid generated byRaldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;232:950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- Rankin SA, Kormish J, Kofron M, Jegga A, Zorn AM. A gene regulatory network controlling hhex transcription in the anterior endoderm of the organizer. Developmental Biology. 2011;351:297–310. doi: 10.1016/j.ydbio.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JM, Dunn NR, Hogan BL, Zaret KS. Distinct mesodermal signals, including BMPs from the septum transversum mesenchyme, are required in combination for hepatogenesis from the endoderm. Genes & Development. 2001;15:1998–2009. doi: 10.1101/gad.904601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Qiao T, Wolff C, Ingham PW. Hedgehog signaling pathway is essential for pancreas specification in the zebrafish embryo. Curr Biol. 2001;11:1358–1363. doi: 10.1016/s0960-9822(01)00402-x. [DOI] [PubMed] [Google Scholar]
- Shen MM. Nodal signaling: developmental roles and regulation. Development. 2007;134:1023–1034. doi: 10.1242/dev.000166. [DOI] [PubMed] [Google Scholar]
- Shin D, Shin CH, Tucker J, Ober EA, Rentzsch F, Poss KD, Hammerschmidt M, Mullins MC, Stainier DYR. Bmp and Fgf signaling are essential for liver specification in zebrafish. Development. 2007;134:2041–2050. doi: 10.1242/dev.000281. [DOI] [PubMed] [Google Scholar]
- Sinner D, Kirilenko P, Rankin S, Wei E, Howard L, Kofron M, Heasman J, Woodland HR, Zorn AM. Global analysis of the transcriptional network controlling Xenopus endoderm formation. Development. 2006;133:1955–1966. doi: 10.1242/dev.02358. [DOI] [PubMed] [Google Scholar]
- Spagnoli FM, Brivanlou AH. The Gata5 target, TGIF2, defines the pancreatic region by modulating BMP signals within the endoderm. Development. 2008;135:451–461. doi: 10.1242/dev.008458. [DOI] [PubMed] [Google Scholar]
- Spence JR, Wells JM. Translational embryology: Using embryonic principles to generate pancreatic endocrine cells from embryonic stem cells. Dev Dyn. 2007;236:3218–3227. doi: 10.1002/dvdy.21366. [DOI] [PubMed] [Google Scholar]
- Spence JR, Lange AW, Lin SCJ, Kaestner KH, Lowy AM, Kim I, Whitsett JA, Wells JM. Sox17 Regulates Organ Lineage Segregation of Ventral Foregut Progenitor Cells. Developmental Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;469:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D, Prince VE. Retinoic acid signaling is required for a critical early step in zebrafish pancreatic development. Curr Biol. 2002;12:1215–1220. doi: 10.1016/s0960-9822(02)00929-6. [DOI] [PubMed] [Google Scholar]
- Stafford D, White RJ, Kinkel MD, Linville A, Schilling TF, Prince VE. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949–956. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- Sumazaki R, Shiojiri N, Isoyama S, Masu M, Keino-Masu K, Osawa M, Nakauchi H, Kageyama R, Matsui A. Conversion of biliary system to pancreatic tissue in Hes1-deficient mice. Nat Genet. 2004;36:83–87. doi: 10.1038/ng1273. [DOI] [PubMed] [Google Scholar]
- Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mechanisms of Development. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Zaret KS. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Developmental Biology. 2005;280:87–99. doi: 10.1016/j.ydbio.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic Signaling Network for the Specification of Embryonic Pancreas and Liver Progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- Wills A, Dickinson K, Khokha M, Baker JC. Bmp signaling is necessary and sufficient for ventrolateral endoderm specification in Xenopus. Dev Dyn. 2008;237:2177–2186. doi: 10.1002/dvdy.21631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CR, Cole PA, Meyers DJ, Kormish J, Dent S, Zaret KS. Chromatin “Prepattern” and Histone Modifiers in a Fate Choice for Liver and Pancreas. Science. 2011;332:963–966. doi: 10.1126/science.1202845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Molecular basis of vertebrate endoderm development. Int Rev Cytol. 2007;259:49–111. doi: 10.1016/S0074-7696(06)59002-3. [DOI] [PubMed] [Google Scholar]