Association with Nitric Oxide Synthase on Insulin Secretory Granules Regulates Glucokinase Protein Levels (original) (raw)
Abstract
Glucokinase (GCK) association with insulin-secretory granules is controlled by interaction with nitric oxide synthase (NOS) and is reversed by GCK S-nitrosylation. Nonetheless, the function of GCK sequestration on secretory granules is unknown. Here we report that the S-nitrosylation blocking V367M mutation prevents GCK accumulation on secretory granules by inhibiting association with NOS. Expression of this mutant is reduced compared with a second S-nitrosylation blocking GCK mutant (C371S) that accumulates to secretory granules and is expressed at levels greater than wild type. Even so, the rate of degradation for wild type and mutant GCK proteins were not significantly different from one another, and neither mutation disrupted the ability of GCK to be ubiquitinated. Furthermore, gene silencing of NOS reduced endogenous GCK content but did not affect β-actin content. Treatment of GCK(C371S) expressing cells with short interfering RNA specific for NOS also blocked accumulation of this protein to secretory granules and reduced expression levels to that of GCK(V367M). Conversely, cotransfection of catalytically inactive NOS increased GCK-mCherry levels. Expression of GCK(C371S) in βTC3 cells enhanced glucose metabolism compared with untransfected cells and cells expressing wild type GCK, even though this mutant has slightly reduced enzymatic activity in vitro. Finally, molecular dynamics simulations revealed that V367M induces conformational changes in GCK that are similar to S-nitrosylated GCK, thereby suggesting a mechanism for V367M-inhibition of NOS association. Our findings suggest that sequestration of GCK on secretory granules regulates cellular GCK protein content, and thus cellular GCK activity, by acting as a storage pool for GCK proteins.
Insulin secretion from pancreatic β-cells is triggered by a rise in the cellular glucose concentration. This glucose dependency of secretion is largely due to limitations placed on glycolysis by glucokinase (GCK) (1, 2), which has a low binding affinity for glucose and half-maximal activity at approximately 8 mm. At glucose concentrations less than about 5 mm, metabolic flux through glycolysis slows to the point where insufficient quantities of ATP are generated by mitochondria to close ATP-sensitive K+ channels on the plasma membrane and stimulate secretion. The notion that GCK activity is rate limiting for insulin secretion has been firmly established by exogenous expression systems (3, 4) and more powerfully by molecular genetics. Activating mutations in GCK lead to hyperinsulimena (5–7), whereas deactivating mutations lead to high blood sugar and diabetes (5, 8, 9). Thus, GCK activity is an important control point for regulating glucose-stimulated insulin secretion.
Regulation of GCK generally involves sequestration to a biological compartment. In liver cells, nuclear localization of GCK functions as a reserve pool of GCK molecules. Elevated glucose levels cause translocation to the cytoplasm, where enhanced glucose phosphorylation leads to glycogen production (10). In pancreatic β-cells, association of GCK with secretory granules has been reported (11, 12), but the function of GCK localization is unknown. Translocation to the cytoplasm does not occur in response to glucose (13); however, disassociation from secretory granules can be triggered by activation of the neuronal-type nitric oxide synthase (NOS) (14) or dissociation of NOS dimers (15). S-nitrosylation of Cys371 on GCK leads to translocation to the cytoplasm and enhanced cellular activity. Although acute regulation of GCK activity by NOS can serve as a hormonal-regulatory mechanism for glucose metabolism (16, 17), this does not explain the functional importance of GCK sequestration to granules.
Here we have investigated the function of GCK association with granules using two mutations known to block GCK S-nitrosylation and describe differences in their ability to associate with NOS. Furthermore, we present data showing that manipulation of GCK-NOS association by expression of mutant GCK and NOS proteins impacts GCK protein levels. These results suggest that GCK accumulation on insulin-secretory granules regulates GCK content within pancreatic β-cells.
Materials and Methods
Reagents
For molecular biology reagents, primers were from Integrated DNA Technologies (Coralville, IA), enzymes were from New England Biolabs, (Ipswich, MA), and DNA preparation kits were from Qiagen Sciences (Germantown, MD). Cell culture media were from Mediatech (Manassas, VA), and serum was obtained from Life Technologies (Carlsbad, CA). Gene silencing reagents were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), as were GCK antibodies. Antibodies specific for NOS, _Aequorea_-derived fluorescent proteins, and β-actin were obtained from Abcam (Cambridge, MA), and Western blots were performed using the Fast Western Blot kit (Thermo Scientific, Rockford, IL). Transfections were performed using FuGENE6 (Roche Diagnostics, Indianapolis, IN) or LipoD293 (SignaGen Laboratories, Rockville, MD) as indicated. All other chemicals and reagents were from Sigma-Aldrich (St. Louis, MO), unless otherwise noted.
Vector preparation
Plasmids encoding mCherry (18) and mVenus (19) tagged wild-type (WT) and GCK(V367M) mutants were described previously (20). GCK(C371S) was subcloned from a previously described vector (14) into mCherry and mVenus N3 plasmids using _Nhe_I and _Bam_HI restriction sites. GCK-PAmCherry constructs were made using the same strategy. Vesicle-associated membrane protein 2 (VAMP2) was amplified by PCR from a pHluorin-VAMP2 vector (21) (sense primer, 5′-TTTTGCTAGCGCCACCATGTCGGCTACCGCTGCC-3′; antisense, 5′-TTTTACCGGTCCCCCGCCGCTTCCGCC-3′, and cloned into the mCerulean3-N3 vector (22) using _Nhe_I and _Age_I restriction sites to create VAMP2-mCerulean3. For NOS-mCerulean3 studies, mCerulean3 from the pmCerulean3-N1 vector was substituted for an earlier generation cyan fluorescent protein (CFP) using _Nhe_I and _Age_I restriction. Mutations were introduced into the NOS sequence using the QuikChange mutagenesis method, with the following primers; NOS E592A-sense (5′-GTACATGGGCACAGCGATCGGCGTCCGTG-3′; antisense, 5′-CACGGACGCCGATCGCTGTGCCCATGTAC-3′), NOS(G671A) (sense, 5′-CTGCAGAGGGGCCTGCCCCGCCG-3′; antisense, 5′-CGGCGGGGCAGGCCCCTCTGCAG-3′).
Cell culture
Pancreatic βTC3 cells were cultured as previously described (11). Media were exchanged 3–4 h before experimentation for the glucose-free and serum-free experimental buffer [125 mm NaCl, 5.7 mm KCl, 2.5 mm CaCl2, 1.2 mm MgCl2, 10 mm HEPES (pH 7.4), 0.1% BSA].
Biochemical experiments
For GCK-mVenus Western blots, βTC3 cells were plated on 60-mm dishes and transfected using 5 μg of plasmid with LipoD293 per the manufacturer's protocol. Transfection was confirmed 48 h later before cell lysis using a TS100 Eclipse fluorescence microscope (Nikon Instruments, Melville, NY). Before media exchange with a glucose-free experimental buffer, images of transfected cells were collected using phase contrast and fluorescence illuminations. A Coolsnap EZ charge-coupled device camera (Photometrics, Tucson, AZ) was used to collect images using μManager software (www.micro-manager.org). Observation was with a 20×/ 0.45 numerical aperture Plan Fluar objective lens (Nikon) containing a Ph1 phase ring, with a matching ring in the transmitted light illuminator. For fluorescence, mVenus fluorescence was captured using the Endow green fluorescent protein HQ filter cube (Chroma Technology, Bellows Falls, VT). Magnification scale was determined with a stage micrometer.
For Western blots, glucose-starved cells were washed in cold PBS three times and collected in 500 μl cold lysis buffer [50 mm Tris (pH 7.5), 150 mm NaCl, 2 mm EDTA, 0.1% Triton X-100] by scraping. Cells were then mechanically lysed by passage through a 25.5-gauge needle several times. Lysates were then incubated at 4 C under constant agitation for 30 min and cleared by centrifugation (4 C, 10,000 × g, 2 min) before analysis of the supernatants by SDS-PAGE and Western blot using rabbit antifluorescent protein or anti-β-actin primary antibodies, and peroxidase-conjugated donkey antirabbit secondary antibodies (Jackson Immunoresearch, Rockville, MD). The ECL PLUS (GE Healthcare, Laurel, MD) substrate was applied per the manufacturer's instructions, and blot images were collected using a ChemiDoc XRS system (Bio-Rad Laboratories, Hercules, CA). GK-mVenus immunoprecipitation experiments were performed on cell lysates using a biotinylated antifluorescent protein antibody and neutravidin-agarose (Thermo Scientific). Ubiquitin immunoprecipitation experiments were performed using agarose-conjugated antiubiquitin antibodies (Santa Cruz Biotechnology).
For gene knockdown studies, βTC3 cells were seeded on 60-mm dishes and transfected with 0.75 μg of the control short interfering RNA (siRNA)-A (sc-37007), or siRNA specific for murine neuronal NOS (sc-36091). The siRNA Reagent System was used for transfection (1:1 ratio) according to the manufacturer's protocol. Fresh media were exchanged 24 h after infection, and cells were harvested 40 h later. Cell lysates were performed as described above, and Western blots were used to detect NOS, GCK, or β-actin.
Deconvolution microscopy
βTC3 cells were seeded on the flame-sterilized high performance no. 1.5 coverslips (Carl Zeiss Microimaging, Thornwood, NY) and transfected the next day with VAMP2-mCerulean3 and either the wild-type (WT), V367M, or C371S variants of GCK-mVenus using LipoD293 transfection reagent (1:1 ratio, 1 μg DNA total, as per manufacturer's recommendations). Glucose-starved cells were fixed 48 h after transfection with a cold 4% paraformaldehyde solution containing 4% sucrose and PBS for 20 min. Cells were washed three times in PBS and mounted on glass slides using Pro-long gold antifade (Life Technologies). Specimens were cured for 48 h before sealing with nail polish and storage at −20 C. Imaging was performed with a Zeiss AxioObserver microscope with motorized focusing. Specimens were observed using 1.4 NA/63× oil-immersion Plan-apochromat. Images were acquired using Zeiss Axiovision software using a 400-nm light-emitting diode (LED) for excitation together with a high-efficiency 455-nm dichroic beamsplitter and a high-efficiency 480/40 bandpass filter for collection (Zeiss) of mCerulean3 fluorescence. For mVenus fluorescence, a 515-nm LED was used along with a high-efficiency yellow fluorescent protein (YFP) filter cube [500/25 nm bandpass exciter, 515 nm dichroic, and 535/30 nm emission filter (Zeiss)]. Z-stacks were collected at 0.2-μm intervals for 120–200 sections to capture detectable out of focus light. An Orca-R2 (Hamamatsu Photonics, Shizuoka, Japan) was used to capture fluorescence (0.1024 μm/pixel lateral resolution). For deconvolution, Volocity software (PerkinElmer, Waltham, MA) was used to perform constrained, iterative deconvolution using theoretical point spread functions for the mCerulean3 CFP (400-nm excitation, 475-nm emission, 0.05-μm axial resolution, 0.067-μm lateral resolution) and the mVenus YFP (515-nm excitation, 528-nm emission, same resolution as CFP). Forty iterations were performed with photobleaching correction enabled. For quantification of VAMP2-colocalized structures, the densitometric mean from five regions of interest each of VAMP2-colocalized structures and noncolocalized structures was sampled for each cell in unprocessed wide-field images collected from living cells (glucose-starved, 100×/1.45 NA Plan-Fluar lens, CFP and YFP collection filters with 455 nm and 505 nm LED illumination). The mean value from these regions was calculated and used in the analysis described in Fig. 1B.
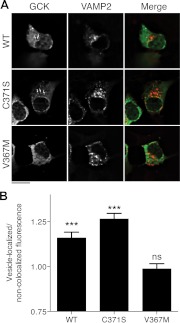
Fig. 1.
Accumulation of GCK(C371S), but not GCK(V367M), to insulin-secretory granules. A, βTC3 cells were cotransfected with plasmids encoding WT, C371S or V367M GCK-mVenus, and VAMP2-mCerulean3 to label insulin-secretory granules. Cells were fixed and imaged using wide-field fluorescence microscopy. Z-stacks were deconvolved using the constrained iterative algorithm to generate optical slices. Images show a single optical slice, and the scale bar indicates 10 μm. For the merged image, the GCK image was colored green, and VAMP2 image colored red. The arrows indicate granule-localized structures that are brighter than cytosolic GCK-mVenus fluorescence. B, GCK-mVenus fluorescence was quantified in VAMP2 colocalized regions and normalized to regions lacking VAMP2 fluorescence for cells expressing WT and mutant GCK-mVenus and VAMP2-mCerulean3. Statistical significance from unity was assessed by t test (n = 10 cells analyzed as described in Materials and Methods, mean ± sem; ***, P < 0.001; ns, _P_ > 0.05).
Quantitative live-cell imaging of GCK-mCherry expression
Cells were seeded on 35-mm glass-bottom dishes containing no. 1.5 coverglass (MatTek, Ashland, MA) and transfected with GCK-mCherry constructs. Glucose-starved cells were imaged 48 h after transfection at 37 C with a Zeiss AxioObserver microscope (20×/0.75 NA Plan-apochromat lens, Zeiss incubation system, Orca-R2 charge-coupled device for detection). Image capture conditions were kept constant for all collected images (200-msec exposure, constant analog gain). A 530-nm LED (40% power; 400 μW/cm2 at the objective) was used for illumination with a high-efficiency dsRed filter cube [no. 42 HE dsRed (Zeiss), BP 550/25 exciter, FT 570 HE dichroic, BP 605/70 emitter]. For cotransfections with NOS, filter collection conditions for CFP were used in conjunction with mCherry collection.
Gene silencing
For gene silencing experiments on cells expressing GCK-mCherry mutants, siRNA transfections were performed as described above on cells seeded in 35-mm glass-bottomed Mat-tek dishes. Approximately 30 min after addition of siRNA, plasmid DNA-LipoD complexes were added to the dishes. Plasmid DNA encoding GCK-mCherry and VAMP2-mCerulean3 were mixed at a 1:1 ratio with a total of 1 μg DNA. Fluorescence imaging was performed approximately 48 h after transfection, using conditions described above for mCherry and mCerulean3 fluorescence. A ×100, 1.45 oil α-Plan-Fluar lens was used to capture high-resolution images.
Förster resonance energy transfer (FRET) microscopy
For detection of protein-protein interactions, the anisotropy-based method was used as previously described (23). Cells were seeded on 35-mm glass bottom dishes with no. 1.5 coverslips and transfected with plasmids encoding NOS-mCerulean and GCK-mVenus with FuGENE 6. Glucose-starved cells were examined 48 h after transfection by two-photon microscopy as previously described (23). polarized excitation (820 nm) was delivered by a Zeiss LSM510/NLO confocal microscope through a ×40, 1.3 NA Plan-NeoFluar lens. Emitted light was first filtered through CFP (480/40 nm) or YFP (525/50 nm) emission filters. Vertical and horizontal polarizations were separated using a broadband polarizing beam splitter (Newport, Irving, CA), and appropriately oriented 25-mm polarizing filters (Chroma) with detection via separate non-descanned detectors. Experiments were performed at 32 C. Expression of mCerulean- and mVenus-labeled proteins was confirmed by spectral imaging. Images were acquired using Zeiss AIM software. Anisotropies were calculated from the mean fluorescence of specified regions of interests in CFP and FRET images as previously described (23).
Photoactivation experiments
Cells were cotransfected using GCK-PAmCherry and either VAMP2-mCerulean3 or mCerulean3-C1 for identification of transfected cells. Cells were placed in the experimental buffer before observation with a Zeiss AxioObserver microscope. CFP images were captured under 455-nm LED illumination and the CFP high-efficiency filter set, and PAmCherry images were captured using the DsRed filter cube and 530-nm LED illumination. For quantification over time, PAmCherry fluorescence was captured using a ×40 objective lens once every 3 min (40 images total). Cells were maintained at 37 C, and the Zeiss Definite Focus system was used to maintain a consistent focal depth throughout the experiment.
NAD(P)H autofluorescence imaging
NAD(P)H imaging was performed as previously described (20) using two-photon excitation microscopy with 710-nm illumination, a ×40/1.3 NA Plan-NeoFluar lens, and collection through a BP 390–465 filter. A Zeiss LSM510/NLO was used to perform the experiment, with Zeiss AIM software. Experiments were performed at 32 C.
Molecular dynamics simulations
Structures for molecular dynamics simulations were prepared from 1v4t.pdb (24) using the YASARA Structure software package (www.yasara.com). Simulations were performed at 298 K using the YASARA2 forcefield (25, 26), and trajectories were analyzed after 5000 psec using the YASARA software package to calculate average structures and root mean square deviations (RMSD). Minimized averaged structures (27) were superposed together using the Theseus algorithm (28). Graphics for presentation were also prepared using YASARA.
Image analysis and figure preparation
Band intensities from gel images were quantified with NIH ImageJ 1.44. Wide-field images were quantified using Zeiss Axiovision 4.7 software, and for two-photon microscopy experiments, fluorescence intensities were quantified using Zeiss AIM 3.5 software. Data were analyzed with Microsoft Excel 12. Prism 5 software (GraphPad, La Jolla, CA) was used to create graphs and perform statistical analyses. Photoshop CS5.1 and Illustrator CS5.1 (Adobe Systems, San Jose, CA) software were used to prepare figure graphics.
Results
Localization of mutant GCK proteins
Given that S-nitrosylation controls GCK association with secretory granules (14), we investigated whether mutations that prevent GCK S-nitrosylation [C371S (14), and V367M (20)] affect its subcellular localization. Immortalized βTC3 insulinoma cells were used in this study because they show the same glucose responsiveness and capacity for posttranslational GCK regulation as primary islet β-cells (16) and, unlike primary islets, can be manipulated by transfection. βTC3 cells were cotransfected with WT or mutant GCK proteins fused to mVenus (19) and mCerulean3 (22) targeted to secretory granules via fusion with VAMP2 (29, 30). Cells were then fixed and observed using wide-field illumination. Constrained-iterative deconvolution was used to obtain optical sections (Fig. 1A). WT and C371S GCK proteins (14) colocalized with VAMP2-labeled structures with distinguishably greater fluorescence than the cytosol, as indicated by the white arrows. GCK(V367M), however, did not obviously accumulate to secretory granule structures, suggesting a defect in the ability of this mutant to associate with secretory granules. To quantify this, the brightness of GCK-mVenus was compared for regions colocalized with VAMP2 to those that did not (Fig. 1B). In agreement with our qualitative assessment of GCK localization, colocalized structures were significantly brighter than non-colocalized cytosolic regions for WT and C371S GCK proteins, but not GCK(V367M)-mVenus. These results point to a weakened association of this mutant with secretory granules.
Impact of S-nitrosylation blocking mutations on GCK-NOS interaction
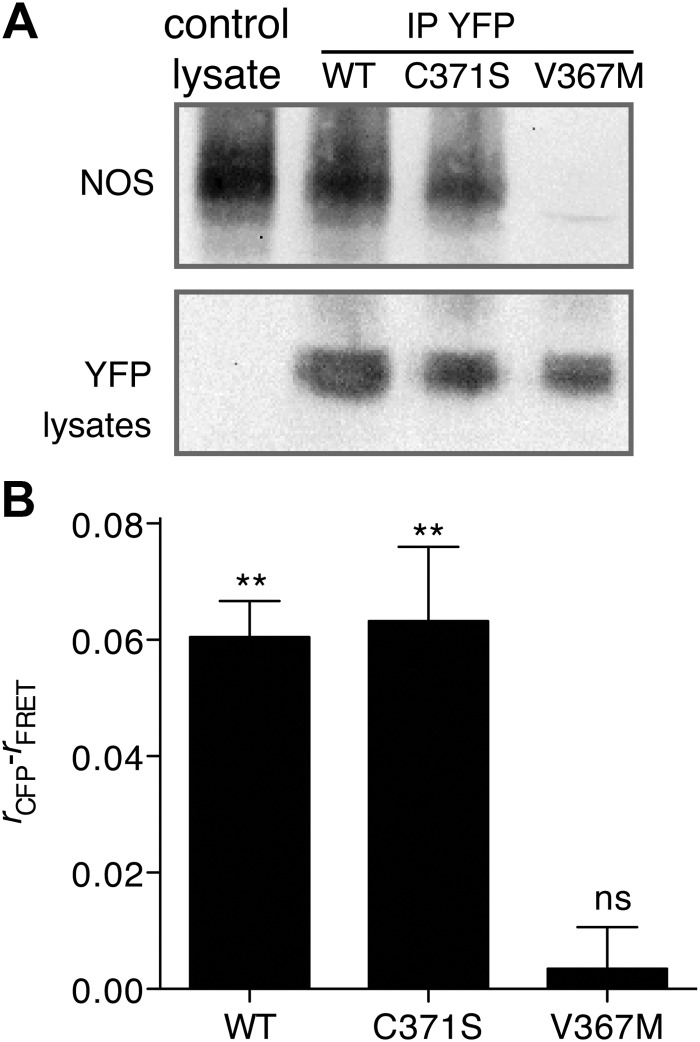
We have previously shown that GCK associates with secretory granules via interaction with NOS (14), and additional studies have shown that disruption of NOS dimerization is sufficient to disrupt GCK association with secretory granules (15). Given the essential role of NOS in regulating GCK localization, we investigated the impact of C371S and V367M mutations on GCK-NOS association. Association of mVenus-tagged WT and GCK(C371S) with endogenous NOS was detected by immunoprecipitation of fluorescent protein-tagged GCK and Western blot using anti-NOS antibodies (Fig. 2A). NOS was not detected in GCK(V367M)-mVenus precipitates, suggesting a defect in NOS-GCK interactions. To test whether the mutations inhibit GCK-NOS interactions in living cells, we cotransfected βTC3 cells with GCK-mVenus constructs and a NOS construct fused to mCerulean (31) and looked for FRET between the two proteins (14). The polarized FRET-based method was used (32). Depolarized anisotropies in the FRET channel were detected in cells containing WT or C371S GCK proteins relative to the polarized anisotropy of the NOS-mCerulean channel (Fig. 2B), indicating FRET and interaction between the two proteins. No change in polarization was observed in cells containing GCK(V367M)-mVenus, indicating a lack of FRET with mCerulean-labeled NOS. Taken together, these results suggest that the V367M mutation inhibits association with NOS.
Fig. 2.
GCK(V367M) inhibits association with NOS. A, βTC3 cells were left untransfected as a control or transfected with WT, C371S, and V367M GCK-mVenus as indicated. GCK proteins were immunoprecipitated from transfected cells using antifluorescent protein antibodies (YFP) and blotted for NOS as indicated (top). WT and mutant GCK expression from preimmunopreciptiated lysates was confirmed by Western blot using antifluorescent protein antibodies (bottom). B, FRET between GCK-mVenus and NOS-mCerulean in βTC3 cells was determined using the anisotropy FRET microscopy method (23). Fluorescence anisotropies (r) under CFP illumination conditions and FRET illumination conditions (CFP excitation, YFP collection) were calculated. FRET is indicated by a statistically significant reduction in the FRET anisotropy compared with CFP anisotropy (n = 5, mean ± sem, two-tailed t test, difference from zero; **, P < 0.01; ns, _P_ > 0.05). IP, Immunoprecipitation.
Impact of S-nitrosylation blocking mutations on GCK protein levels
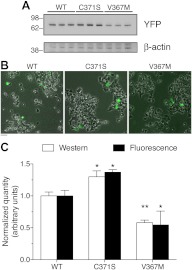
We hypothesized that accumulation of GCK on secretory granules could regulate GCK protein levels. Therefore, we examined protein levels of WT and mutant GCK-mVenus proteins in transfected βTC3 cells by Western blot. The granule-associated mutant, GCK(C371S)-mVenus was expressed at higher levels compared with WT and V367M GCK proteins (Fig. 3A). Western blots of GCK(V367M) showed decreased levels compared with WT, whereas β-actin levels did not significantly vary between groups (P = 0.7 across all groups by ANOVA; P > 0.05 for all possible comparisons of mean band density by Tukey). Even so, the transfection efficiency of the βTC3 cells was quite low (Fig. 3B), raising concerns that the standard β-actin loading control might be insufficiently sensitive to small differences in transfection efficiency that could contribute to the differences in protein expression observed by Western blot. Thus, we investigated whether similar differences in WT and mutant GCK-mCherry proteins could also be observed by fluorescence microscopy. The sum fluorescence from 50 cells agreed well with quantification of the Western blot data (Fig. 3C), indicating that differences in transfection efficiency do not explain differences in expression.
Fig. 3.
Differences in protein content between WT and mutant GCK proteins. A, βTC3 cells were transfected with plasmid DNA encoding WT, C371S, or V367M GCK-mVenus as indicated. Protein content 48 h after transfection, and after a 3-h glucose starvation, was assessed by Western blot using antibodies to the fluorescent protein tag (YFP) or β-actin as indicated. B, Transfection efficiency of the samples in panel A was assessed using phase contrast and fluorescence imaging. Fluorescence images of WT or mutant GCK-mVenus were pseudocolored green and overlaid with the transmitted light image. Scale bar, 20 μm. C, Band density was quantified from the Western blots performed as in panel A (n = 3) and compared with the total fluorescence of cells expressing WT or mutant GCK-mCherry fusions treated identically. Bars represent the sum fluorescence from 50 cells (n = 3), and error bars indicate sd. Values were normalized to the average WT quantity, and statistical significance was tested by ANOVA for the experiments separately (*, P < 0.05; **, _P_ < 0.01 for mutants _vs._ WT). A two-way ANOVA did not find significant differences between the assays (_P_ > 0.05).
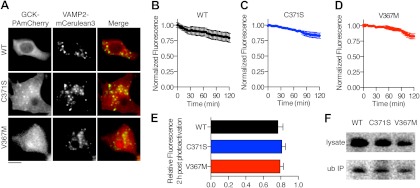
Studies on the thermostability of recombinantly generated GCK proteins have revealed that the stability of recombinant WT, C371S, and V367M GCK proteins are similar (8). However, differences in cytoplasmic degradation of mutant and WT GCK proteins could explain the relative differences in expression level observed for these proteins. To examine this possibility, we fused WT and mutant GCK to a photoactivatable variant of mCherry (PAmCherry) (33). Photoactivatable proteins can be used to quantify the rate of protein degradation (34), because irreversible photoactivation can specifically label a select population of proteins in a manner similar to a pulse chase experiment (35). Cells were cotransfected with GCK-PAmCherry expression plasmids and VAMP2-mCerulean3 to label secretory granules, and GCK-PAmCherry fluorescence was activated by illumination at 400 nm (Fig. 4A). Accumulation of WT and C371S GCK-PAmCherry to VAMP2-mCerulean3 colocalized structures was observed in the control group, but not with GCK(V367M)-PAmCherry. This result is consistent with the data obtained using GCK-mVenus (Fig. 1).
Fig. 4.
Clearance of GCK proteins from the cytoplasm is not affected by C371S or V367M. A, βTC3 cells were transfected with GCK proteins fused to a PAmCherry protein (red in Merge) and VAMP2-mCerulean3 (green in Merge). Red mCherry fluorescence was induced by illumination with a 400-nm LED. Scale bar, 10 μm. B–D, Decay of WT (B), C371S (C), and V367M (D) GCK-PAmCherry fluorescence was quantified over a 2-h period (n >7 cells). E, Remaining fluorescence after 2 h was not significantly different for WT and mutant GCK-PAmCherry, or for the two mutants (ANOVA, P > 0.05; Tukey's multiple comparison test for all possible group pairings). F, Ubiquitinated WT and mutant GCK-Venus were detected by immuoprecipitation (IP) with agarose-conjugated ubiquitin antibodies and Western blot for the fluorescent protein tag. Cell lysates and immuoprecipitates are indicated. ub, Ubiquitin.
Depletion of fluorescence was tracked over 2 h using illumination conditions that minimized fluorescence photobleaching. For PA-mCherry-expressing cells, a significant decrease was not observed after 60 measurements (mean fluorescence after 60 exposures = 99.5 ± 0.9% (sem) of initial intensity; P = 0.6; t test vs. original fluorescence, n = 4), indicating that fluorescence loss over time is a function of cellular protein degradation, rather than photodestruction of PA-mCherry fluorescence. The rate of fluorescence loss over time was similar for WT (Fig. 4B), GCK(C371S) (Fig. 4C), and GCK (V367M)(Fig. 4D). Fluorescence intensities remaining 2 h after photoactivation were not significantly different for each protein (Fig. 4E; P > 0.05, ANOVA, Tukey multiple comparison test). Because GCK is degraded by the proteosome (36), which is a cytoplasmic process (37), we examined whether V367M and C371S could prevent ubiquitination. Consistent with similar rates of cytoplasmic protein clearance, we were able to detect ubiquitinated WT, C371S, and V367M GCK-mVenus proteins by immunoprecipitation with an agarose-conjugated ubiquitin antibody (Fig. 4F). Thus, GCK S-nitrosylation mutants do not cause general defects in the cellular stability of GCK.
Manipulation of NOS expression alters cellular GCK levels
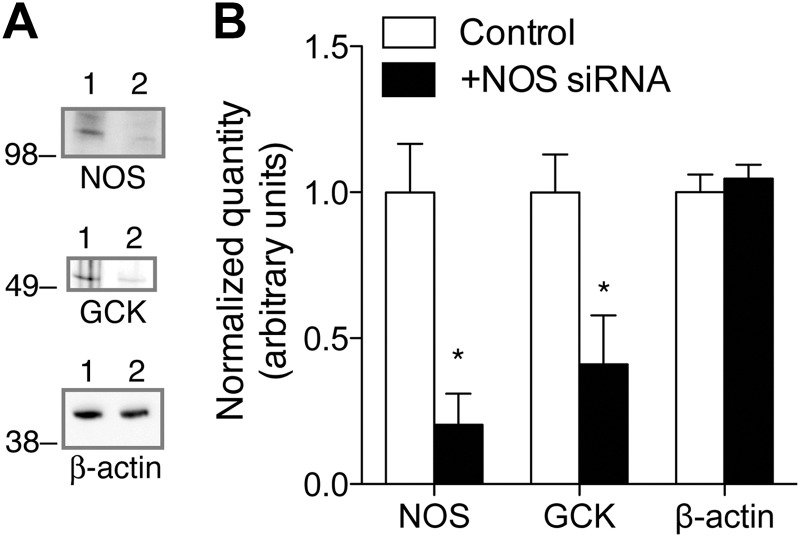
To examine whether differences in NOS association are directly related to differences in GCK protein levels, we examined the effect of NOS gene silencing on endogenous GCK content. siRNA specific for NOS reduced both NOS and GCK protein levels compared with cells transfected with a scrambled nucleotide siRNA control (Fig. 5, A and B). β-Actin levels were not affected by transfection with the NOS siRNA, indicating that differences in GCK expression were not a result of a generalized defect in protein expression.
Fig. 5.
Regulation of GCK protein levels by NOS. A, βTC3 cells were transfected with control, scrambled nucleotide siRNA (1) or siRNA for NOS (2) and examined for NOS, GCK, and β-actin expression via Western blot 40 h after transfection. B, Band densities for control and NOS siRNA-treated cells were quantified. Bar indicates mean ± sem (n = 3). Statistical significance (t test) between control and NOS siRNA-treated groups is indicated by *, P < 0.05.
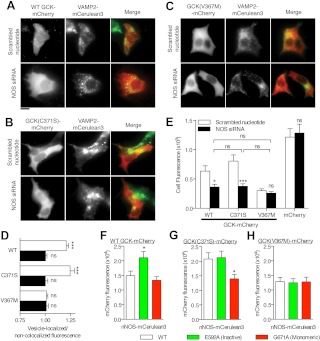
Gene knockdown had additional affects on the localization and expression levels of WT and C371S GCK-mCherry proteins (Fig. 6, A–E). Accumulation to VAMP2-mCerulean3 colocalized structures was not observed in cells transfected with NOS siRNA and WT (Fig. 6A) or C371S GCK-mCherry (Fig. 6B) compared with scrambled nucleotide siRNA cotransfection. Furthermore, localization (Fig. 6C, quantification in Fig. 6D) and mean cellular fluorescence (Fig. 6E) of GCK(V367M)-mCherry were not affected by transfection with NOS siRNA, consistent with decreased affinity of this mutant for NOS. In contrast, gene knockdown of NOS reduced the mean cellular fluorescence of WT and C371S GCK-mCherry proteins to the level of GCK(V367M)-mCherry. Expression of mCherry alone was not affected by gene knockdown of NOS, compared with transfection with a scrambled nucleotide control (Fig. 6E), indicating that alterations of protein levels by gene knockdown is specifically associated with WT and GCK(C371S) proteins.
Fig. 6.
Modulating NOS protein levels alters GCK protein levels. A–C, Scrambled nucleotide or NOS-specific siRNA was cotransfected with VAMP2-mCerulean3 and either WT (A), C371S (B), or V367M (C) GCK-mCherry and examined for colocalization by fluorescence microscopy. VAMP2-mCerulean3 was colored green and GCK-mCherry images were colored red in the Merge panels. Identifiable accumulation of GCK and to VAMP2-colocalized structures was only observable in WT and C371S-expressing cells cotransfected with control siRNA. Scale bar, 10 μm. D, GCK-mCherry fluorescence was quantified in VAMP2-colocalized regions and normalized to fluorescence from regions lacking VAMP2 fluorescence in cells expressing the indicated constructs and control (white bars) or NOS-specific siRNA (black bars). Statistical significance from unity was assessed by t test (n =10, mean ± sem; ***, P < 0.001; ns, _P_ > 0.05). E, The mean cellular fluorescence of cells (n = 100) cotransfected with WT and mutant GCK-mCherry and either control or NOS-specific siRNA is shown. Error bars indicate sem. Non-GCK-containing mCherry plasmids were used as a control. Statistical significance was determined by ANOVA (Tukey multiple comparison test for GCK proteins; ns, P > 0.05; *, P < 0.05; ***, _P_ < 0.001); mCherry control was tested separately using a _t_ test (two-tailed; n >100 cells; P > 0.05). F–H, Cellular fluorescence of WT (F), GCK(C371S) (G), and GCK(V367M)-mCherry (H) was quantified in cells coexpressing WT NOS, NOS(E592A), or NOS(G671A). Statistical significance was determined by ANOVA (bars indicate mean ± sem; n >50 cells; Tukey multiple comparison test compared with WT-NOS expression, *, P < 0.05).
If reduction of NOS protein levels can reduce GCK protein content, is the converse also true? A direct test of this is complicated by NOS activity itself, because S-nitrosylation of GCK reduces binding to secretory granules and NOS (14). Thus, we tested the ability of WT NOS to affect GCK-mCherry protein levels alongside two mutants; the E592A mutant (38), which inhibits arginine binding and is expected to bind GCK but not S-nitrosylate it, and the G671A mutant, which inhibits dimerization (38) and is not expected to associate with GCK (15). Cotransfection of GCK-mCherry with the WT, active NOS did not significantly increase GCK-mCherry fluorescence compared with he G671A negative control; however, coexpression with the catalytically inactive E592A mutant increased expression (Fig. 6F). To test whether these results were consistent with the state of GCK nitrosylation, we performed the same experiment with GCK(C371S). Coexpression of WT NOS raised GCK(C371S) protein levels to levels observed with NOS(E592A) and were significantly greater than observed with NOS(G671A) (Fig. 6G). Furthermore, coexpression of NOS(E592A) failed to raise the protein levels of GCK(V367M)-mCherry (Fig. 6H). Taken together, these results are consistent with GCK-NOS interaction acting as mechanism to raise GCK levels.
Effect of increased GCK(C371S) expression on glucose metabolism
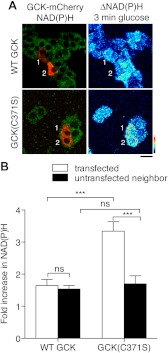
To test whether enhanced GCK levels observed for GCK(C371S) can functionally affect glucose metabolism, βTC3 cells were transfected with either WT or C371S GCK-mCherry, and glucose-stimulated changes to reduced nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide phosphate [collectively referred to as NAD(P)H] autofluorescence, were quantified using two-photon excitation microscopy (Fig. 7A). Expression of WT GCK did not alter the glucose responsiveness of NAD(P)H fluorescence with glucose alone compared with neighboring cells lacking detectable mCherry fluorescence (Fig. 7B) or compared with βTC3 cells alone (20). In cells expressing GCK(C371S)-mCherry, the glucose-stimulated increase in NAD(P)H autofluorescence was potentiated compared with WT GCK-mCherry cells and neighboring untransfected cells (Fig. 7B). Because GCK(C371S) has reduced activity compared with WT GCK in vitro (39), these data indicate that the enhanced levels of GCK(C371S), resulting from increased association with NOS, can enhance glucose metabolism in pancreatic β-cells.
Fig. 7.
Enhanced glucose metabolism in cells expressing GCK(C371S). βTC3 cells were transfected with WT and C371S GCK-mCherry vectors. NAD(P)H autofluorescence was captured by two-photon fluorescence microscopy. A, Merged image of NAD(P)H autofluorescence (green) and mCherry fluorescence (red) (left panels). The right panels indicate the change in NAD(P)H autofluorescence after a 3-min stimulation with 5 mm glucose. Transfected cells in the NAD(P)H image are indicated by number. Scale bar, 20 μm. The color profile of the pseudocolor LUT for the change in NAD(P)H fluorescence is indicated from low (bottom) to high (top). B, Cells expressing GCK(C371S) show enhanced NAD(P)H autofluorescence after glucose stimulation, represented as a fold increase compared with prestimulated condition (0 mm glucose, 3 h), compared with both neighboring cells lacking mCherry fluorescence, and also cells transfected with WT GCK-mCherry. Statistical significance is indicated by *** (P < 0.001, ANOVA; Tukey multiple comparison test, n = 7 cells for WT, n = 6 for C371S; ns, _P_ > 0.05).
V367M induces conformational changes in GCK similar to S-nitrosylated Cys371
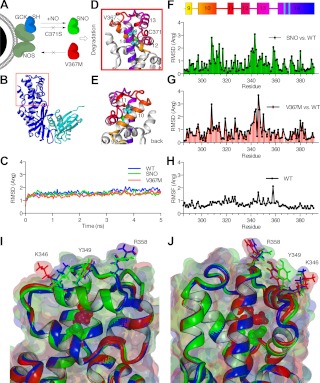
Our data suggest a model in which GCK mutations can prevent S-nitrosylation via two distinct mechanisms (Fig. 8A). C371S prevents S-nitrosylation by removing the requisite cysteine from the S-nitrosylation reaction, thus preventing the conformational change necessary for SNO-triggered dissociation of the GCK-NOS complex. The mechanism behind V367M-inhibition of GCK S-nitrosylation is less obvious. Even so, the fact that the V367M mutation inhibits association with NOS in a manner similar to S-nitrosylated GCK (GCK-SNO) suggests the possibility of conformational similarities between GCK(V367M) and GCK-SNO. To explore this possibility we performed molecular dynamics simulations of WT, GCK(V367M), and GCK-SNO.
Fig. 8.
Conformational changes induced by V367M are similar to those induced by GCK S-nitrosylation. A, association of GCK with NOS is represented schematically. S-nitrosylation of GCK triggers dissociation, and is blocked by direct mutation of the nitrosylated cysteine (C371S). The V367M mutant, however, does not associate with NOS, suggesting conformational similarities to S-nitrosylated GCK (GCK-SNO). B, the S-nitrosylation site is located in the distal end (red box) of the large domain (blue) of GCK. The smaller domain is colored cyan [1v4t.pdb (24)]. C, Backbone RMSD for the simulations are shown for WT, SNO, and V367M structures as indicated. D and E, The boxed region of panel B is shown for the WT structure in two views. Val367 and Cys371 are represented as space-filling balls, and α helixes are colored as indicated in panel F. F and G, RMSD were calculated for individual residues from SNO (F) and V367M (G) structures compared with WT. H, The RMSF of residues in the WT structure during the simulation are represented. I and J, overlays of WT (blue), SNO (green), and V367M (red) are shown from two vantage points. Molecular surfaces are shown in addition to secondary structures depicted by ribbons. V367M and S-nitrosylated Cys371 are represented by space-filling balls. Residues of interest on the exterior surface of GCK are also graphically represented.
GCK consists of two structural domains (Fig. 8B) separated by a hinge region that contains the active site. Residues Val367 and Cys371 reside on the inner face of α-helix 14 in the large domain of GCK, positioned one turn away from one another. Molecular dynamics simulations were conducted using the 1v4t.pdb WT GCK structure (24) and on modified structures containing either S-nitrosylated Cys371 or V367M. Simulations did indeed converge on stable structures, and simulations were run for 5 nsec (Fig. 8C). We focused our analysis on secondary structures that directly surround Val367 and Cys371 (Fig. 8, D and E, show alternative views of the boxed region in Fig. 8B). Residues Val367 and Cys371 are indicated by spherical representations, and α-helixes 9–14 are colored coded as indicated by the schematic in Fig. 8F. After simulations, average structures were calculated and energy minimized. S-nitrosylated and V367M structures were superimposed on the WT structure, and RMSD values were calculated for residues in the modified structures compared with WT GCK. S-nitrosylation of Cys371 resulted in displacement of residues primarily in α-helix 10 and in the turn between helixes 12 and 13 (Fig. 8F). Displacement of residues is these regions was also observed in GCK(V367M) (Fig. 8G). Residue fluctuations in the WT simulation in these regions were similar to neighboring residues (Fig. 8H), indicating that these regions are not particularly flexible. This suggests that the conformational changes observed in GCK(SNO) and GCK(V367M) structures were likely induced by the introduced modifications. Two views of the overlaid structures are shown in Fig. 8, I and J. Furthermore, Met367 of the GCK(V367M) structure fills the internal cavity surrounding Cys371 similarly to the S-nitrocysteine of GCK(SNO). The increased bulk of these residues notably produces a twist in α-helix 12 and alters the positioning of solvent-accessible residues, Tyr349 and Arg358 in GCK-SNO, and Lys346 in GCK(V367M). Taken together with our biochemical evidence, these simulations support the hypothesis that conformational changes induced by the V367M mutation mimic those induced by S-nitrosylation of Cys371 to reduce GCK affinity for the NOS complex.
Discussion
Acute regulation of β-cell GCK activity by hormonal factors has been well described (11, 16, 17, 40) and can proceed through S-nitrosylation-mediated potentiation of GCK activity (14, 16). Even though the specific activity of GCK is a strong contributor to regulation of insulin secretion, mathematical models have shown that metabolic flux is proportional not only to the enzymatic activity, but the concentration as well (1). Overexpression of GCK has been previously shown to enable insulin secretion at glucose concentrations that are normally subthreshold (4). Conversely, mutations in GCK that destabilize the protein and reduce protein expression are associated with diabetes. For a mutant with known in vitro temperature instability issues, GCK(E300K) (41), virus-transduced protein expression levels were reported to be approximately 10% of WT GCK levels (42), despite an equivalent amount of message. Such stability issues have been theorized to raise the threshold for glucose-stimulated insulin secretion in islets containing these mutants, even with heterozygotic expression (1). Thus, a precise quantity of GCK proteins in the β-cell is required for appropriate glucose-stimulated insulin secretion, and our data suggest a mechanism for precisely regulating GCK levels via GCK-NOS interaction.
First, our data show that expression of GCK and NOS mutants that promote interaction universally increase GCK content, whereas inhibition of interaction universally decreases GCK protein levels. Further, conditions that increase GCK protein content are correlated with accumulation of GCK on secretory granules to levels greater than the cytoplasm, whereas conditions that diminish interaction with NOS and GCK content are correlated with a reduced accumulation to secretory granules. Interpretation of these data can be tricky, because secretory granules are smaller than the optical resolution of light, and GCK and NOS are bound to the exterior surface of secretory granules, thus giving access of the enzyme to the cytoplasm. Taken together with our previous findings that GCK S-nitrosylation is a critical trigger for both translocation and activation, an argument can be made that GCK S-nitrosylation is dual functional: acute regulation of glucose-stimulated insulin secretion brought upon by enhanced glucose phosphorylating kinetics in the minutes after GCK activation (16), whereas release of activated GCK to the cytoplasm could facilitate signal termination by making GCK accessible to proteosomal degradation.
Does GCK activation require such tight constraints to necessitate both positive and negative regulatory signals in pancreatic β-cells? This pattern is consistent with physiological potentiation of insulin secretion. Incretin signals known to activate GCK, such as glucagon-like peptide-1, are cleared from the blood with a half-time on the order of minutes (43), and the signals initiated by glucagon-like peptide-1 receptors are rapidly terminated (44). Given that the physiological set point for blood glucose (∼5 mm) is positioned closely to levels considered dangerously hypoglycemic (∼3 mm), constraint of GCK activation is likely to be an important safety mechanism for limiting the impact of hormone-induced potentiation of GCK activity. Proteosomal degradation of S-nitrosylated GCK may be particularly important for β-cells, given that antioxidant proteins are poorly expressed (45). Rigorous examination of this hypothesis in more complex physiological systems will undoubtedly be required to determine the extent that S-nitrosylation-triggered degradation of GCK confers physiological safety against hypoglycemia. Nonetheless, this idea is consistent with GCK's power over the rate of glucose-stimulated insulin secretion (1).
Indeed, these ideas are consistent with the enhancement of glucose metabolism in cells expressing GCK(C371S), which is most likely explained by increased protein content. Even though the C371S mutation does confer a small amount of improved temperature stability to GCK over WT (39), this mutation also has small defects in glucose-phosphorylating ability (14, 39) that likely offset the positive effects on protein stability. Further, overexpression of either GCK (4) or hexokinase (3) has been shown to increase glucose-stimulated insulin secretion from pancreatic β-cells. This supports the conclusion that increased GCK protein levels enhance glucose metabolism in pancreatic β-cells.
Although the mechanism behind C371S appears to be a fairly straightforward disruption of nitrosative chemistry by removal of requisite cysteine, more surprising is that V367M not only inhibits association with NOS but also disrupts S-nitrosylation. Given the close proximity of positions Val367 and Cys371 in the GCK structure, induction to the NOS-free conformation by the V367M substitution, and S-nitrosylation is broadly supported by a number of experiments presented here, including co-immunoprecipitation experiments, FRET, microscopic observation, and molecular dynamics simulations. Even so, it is less clear whether interaction with NOS is an absolute requirement for GCK nitrosylation, given the diffusibility of nitric oxide. The existence of NOS adapter proteins, such as CAPON (46), suggests that proximity to the NOS complex is advantageous. Nonetheless, direct inhibition of the nitrosylation reaction via alteration of the delicate acid-base chemistry (47) by V367M has not been ruled out.
The V367M mutation has similar thermostability as WT GCK in recombinant protein preparations (8), making it unlikely that structural effects resulting from the mutation explain the observed decrease in protein content. Indeed, examination of GCK(V367M)-mCherry expression at an earlier 24-h time point did not show significant differences from WT, unlike a mutant with known stability issues (20), and similar rates of degradation in our PAmCherry experiments support this. We have previously shown that expression of this mutant does not alter glucose metabolism compared with expression of WT proteins and also neighboring untransfected cells (16). This is likely due to the presence of endogenous GCK in the βTC3 cells, which may provide supplemental GCK activity.
Clearance of GCK proceeds through the ubiquitin-proteosome pathway (36) and is largely cytoplasmic (37). We were able to detect ubiquitinated GCK in WT and mutant constructs, and this observation was consistent with similar rates of clearance for all GCK proteins examined in this study. Thus, accumulation on secretory granules functions to remove GCK proteins from the cytoplasm, which results in a net increase in total GCK levels. Destruction of GCK by secretory granule autophagy is an alternative possibility for removal of granule-bound GCK. However, this is an unlikely contributor to the rate of GCK protein clearance, given the slow rate of autophagy in pancreatic β-cells (48). Our data also show that experimental manipulations that enhance GCK accumulation to granules correlate with increased protein levels, and this supports the idea that GCK degradation proceeds primarily through the cytoplasmic pool (Fig. 8A).
It is also unknown whether S-nitrosylation affects interaction with other known binding partners of GCK in the β-cell, such as 6-phosphofructose kinase/fructose-2,6, bisphosphatase (PFK-2/FBPase) (49, 50). Even so, interaction with PFK-2/FBPase is glucose dependent (50), suggesting involvement of GCK domains in either the small domain or hinge regions, which can undergo profound conformational changes upon binding glucose (24). Intriguingly, the interaction between GCK and PFK-2/FBPase is strengthened at superphysiological glucose concentrations, suggesting interaction with GCK conformational states achieved at glucose concentrations exceeding 20 mm (51). Nonetheless, the regions of GCK affected by S-nitrosylation are distal to the active site and hinge region. Furthermore, glucose itself does not trigger changes in GCK localization (11, 13), suggesting that regulation of GCK by nitric oxide and PFK-2/FBPase are likely to be distinct mechanisms. Future work will undoubtedly shed more light on the subject.
Finally, the mechanism described here may have important implications to diabetes. Prevention of NOS-GCK interactions is the only known defect for GCK(V367M) (20), and this provides two mechanisms that can impair normal glucose-stimulated insulin secretion. First, it prevents S-nitrosylation of GCK, leading to diminished hormone-potentiated secretion (16, 20). Second, as suggested here, it leads to depletion of the secretory granule-bound GCK pool, which can lead to reduced GCK levels in β-islet cells and produce a phenotype similar to GCK mutations that destabilize the protein. Reduced GCK levels would then lead to an increase in the overall secretory threshold (52). This may contribute to the diabetes-associated phenotype of GCK(V367M) (9).
Acknowledgments
This work was supported by National Institutes of Health (NIH) grant R01DK077140 (to M.A.R), and from NIH-supported centers P60-DK079637 and P30-DK072488.
Disclosure Summary: The authors do not have any conflicts of interest to disclose.
Footnotes
Abbreviations:
CFP
Cyan fluorescent protein
FRET
Förster resonance energy transfer
GCK
glucokinase
LED
light-emitting diode
NAD(P)H
reduced nicotinamide adenine dinucleotide and reduced nicotinamide adenine dinucleotide phosphate
NOS
nitric oxide synthase
RMSD
root mean square deviation
siRNA
short interfering RNA
SNO
S-nitrosylated
VAMP2
vesicle-associated membrane protein 2
WT
wild type
YFP
yellow fluorescent protein.
References
- 1.Gloyn AL, Odili S, Buettger C, Njolstad PR, Shiota C, Magnuson MA, Matschinsky FM. 2004. Glucokinase and the regulation of blood sugar. In: Matschinsky FM, Magnuson MA, eds. Glucokinase and glycemic disease: from basics to novel therapeutics. Basel, Karger; 92–109 [Google Scholar]
- 2.Matschinsky FM. 2002. Regulation of pancreatic β-cell glucokinase: from basics to therapeutics. Diabetes 51( Suppl 3):S394–S404 [DOI] [PubMed] [Google Scholar]
- 3.German MS. 1993. Glucose sensing in pancreatic islet β cells: the key role of glucokinase and the glycolytic intermediates. Proc Natl Acad Sci USA 90:1781–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Iynedjian PB. 1997. Modulation of glucose responsiveness of insulinoma β-cells by graded overexpression of glucokinase. Proc Natl Acad Sci USA 94:4372–4377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis EA, Cuesta-Muñoz A, Raoul M, Buettger C, Sweet I, Moates M, Magnuson MA, Matschinsky FM. 1999. Mutants of glucokinase cause hypoglycaemia- and hyperglycaemia syndromes and their analysis illuminates fundamental quantitative concepts of glucose homeostasis. Diabetologia 42:1175–1186 [DOI] [PubMed] [Google Scholar]
- 6.Glaser B, Kesavan P, Heyman M, Davis E, Cuesta A, Buchs A, Stanley CA, Thornton PS, Permutt MA, Matschinsky FM, Herold KC. 1998. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med 338:226–230 [DOI] [PubMed] [Google Scholar]
- 7.Gloyn AL. 2003. Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum Mutat 22:353–362 [DOI] [PubMed] [Google Scholar]
- 8.Miller SP, Anand GR, Karschnia EJ, Bell GI, LaPorte DC, Lange AJ. 1999. Characterization of glucokinase mutations associated with maturity-onset diabetes of the young type 2 (MODY-2): different glucokinase defects lead to a common phenotype. Diabetes 48:1645–1651 [DOI] [PubMed] [Google Scholar]
- 9.Velho G, Blanché H, Vaxillaire M, Bellanné-Chantelot C, Pardini VC, Timsit J, Passa P, Deschamps I, Robert JJ, Weber IT, Marotta D, Pilkis SJ, Lipkind GM, Bell GI, Froguel P. 1997. Identification of 14 new glucokinase mutations and description of the clinical profile of 42 MODY-2 families. Diabetologia 40:217–224 [DOI] [PubMed] [Google Scholar]
- 10.Agius L. 2008. Glucokinase and molecular aspects of liver glycogen metabolism. Biochem J 414:1–18 [DOI] [PubMed] [Google Scholar]
- 11.Rizzo MA, Magnuson MA, Drain PF, Piston DW. 2002. A functional link between glucokinase binding to insulin granules and conformational alterations in response to glucose and insulin. J Biol Chem 277:34168–34175 [DOI] [PubMed] [Google Scholar]
- 12.Stubbs M, Aiston S, Agius L. 2000. Subcellular localization, mobility, and kinetic activity of glucokinase in glucose-responsive insulin-secreting cells. Diabetes 49:2048–2055 [DOI] [PubMed] [Google Scholar]
- 13.Arden C, Harbottle A, Baltrusch S, Tiedge M, Agius L. 2004. Glucokinase is an integral component of the insulin granules in glucose-responsive insulin secretory cells and does not translocate during glucose stimulation. Diabetes 53:2346–2352 [DOI] [PubMed] [Google Scholar]
- 14.Rizzo MA, Piston DW. 2003. Regulation of β cell glucokinase by S-nitrosylation and association with nitric oxide synthase. J Cell Biol 161:243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. 2007. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic β-cell dysfunction. Diabetes 56:2328–2338 [DOI] [PubMed] [Google Scholar]
- 16.Ding SY, Nkobena A, Kraft CA, Markwardt ML, Rizzo MA. 2011. Glucagon-like peptide 1 stimulates post-translational activation of glucokinase in pancreatic β cells. J Biol Chem 286:16768–16774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park JH, Kim SJ, Park SH, Son DG, Bae JH, Kim HK, Han J, Song DK. 2012. Glucagon-like peptide-1 enhances glucokinase activity in pancreatic β-cells through the association of Epac2 with Rim2 and Rab3A. Endocrinology 153:574–582 [DOI] [PubMed] [Google Scholar]
- 18.Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22:1567–1572 [DOI] [PubMed] [Google Scholar]
- 19.Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20:87–90 [DOI] [PubMed] [Google Scholar]
- 20.Ding SY, Tribble ND, Kraft CA, Markwardt M, Gloyn AL, Rizzo MA. 2010. Naturally occurring glucokinase mutations are associated with defects in posttranslational S-nitrosylation. Mol Endocrinol 24:171–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miesenböck G, De Angelis DA, Rothman JE. 1998. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394:192–195 [DOI] [PubMed] [Google Scholar]
- 22.Markwardt ML, Kremers GJ, Kraft CA, Ray K, Cranfill PJ, Wilson KA, Day RN, Wachter RM, Davidson MW, Rizzo MA. 2011. An improved cerulean fluorescent protein with enhanced brightness and reduced reversible photoswitching. PLoS ONE 6:e17896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piston DW, Rizzo MA. 2008. FRET by fluorescence polarization microscopy. Methods Cell Biol 85:415–430 [DOI] [PubMed] [Google Scholar]
- 24.Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. 2004. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure 12:429–438 [DOI] [PubMed] [Google Scholar]
- 25.Krieger E, Darden T, Nabuurs SB, Finkelstein A, Vriend G. 2004. Making optimal use of empirical energy functions: force-field parameterization in crystal space. Proteins 57:678–683 [DOI] [PubMed] [Google Scholar]
- 26.Krieger E, Joo K, Lee J, Lee J, Raman S, Thompson J, Tyka M, Baker D, Karplus K. 2009. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 77 (Suppl 9):114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krieger E, Koraimann G, Vriend G. 2002. Increasing the precision of comparative models with YASARA NOVA–a self-parameterizing force field. Proteins 47:393–402 [DOI] [PubMed] [Google Scholar]
- 28.Theobald DL, Wuttke DS. 2006. THESEUS: maximum likelihood superpositioning and analysis of macromolecular structures. Bioinformatics 22:2171–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobsson G, Bean AJ, Scheller RH, Juntti-Berggren L, Deeney JT, Berggren PO, Meister B. 1994. Identification of synaptic proteins and their isoform mRNAs in compartments of pancreatic endocrine cells. Proc Natl Acad Sci USA 91:12487–12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regazzi R, Wollheim CB, Lang J, Theler JM, Rossetto O, Montecucco C, Sadoul K, Weller U, Palmer M, Thorens B. 1995. VAMP-2 and cellubrevin are expressed in pancreatic β-cells and are essential for Ca(2+)-but not for GTP γ S-induced insulin secretion. EMBO J 14:2723–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizzo MA, Springer GH, Granada B, Piston DW. 2004. An improved cyan fluorescent protein variant useful for FRET. Nat Biotechnol 22:445–449 [DOI] [PubMed] [Google Scholar]
- 32.Rizzo MA, Piston DW. 2005. High-contrast imaging of fluorescent protein FRET by fluorescence polarization microscopy. Biophys J 88:L14–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subach FV, Patterson GH, Manley S, Gillette JM, Lippincott-Schwartz J, Verkhusha VV. 2009. Photoactivatable mCherry for high-resolution two-color fluorescence microscopy. Nat Methods 6:153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan D, Guo L, Wang Y. 2006. Requirement of dendritic Akt degradation by the ubiquitin-proteasome system for neuronal polarity. J Cell Biol 174:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lippincott-Schwartz J, Patterson GH. 2003. Development and use of fluorescent protein markers in living cells. Science 300:87–91 [DOI] [PubMed] [Google Scholar]
- 36.Bjørkhaug L, Molnes J, Søvik O, Njølstad PR, Flatmark T. 2007. Allosteric activation of human glucokinase by free polyubiquitin chains and its ubiquitin-dependent cotranslational proteasomal degradation. J Biol Chem 282:22757–22764 [DOI] [PubMed] [Google Scholar]
- 37.Wójcik C, DeMartino GN. 2003. Intracellular localization of proteasomes. Int J Biochem Cell Biol 35:579–589 [DOI] [PubMed] [Google Scholar]
- 38.Panda K, Ghosh S, Stuehr DJ. 2001. Calmodulin activates intersubunit electron transfer in the neuronal nitric-oxide synthase dimer. J Biol Chem 276:23349–23356 [DOI] [PubMed] [Google Scholar]
- 39.Tiedge M, Richter T, Lenzen S. 2000. Importance of cysteine residues for the stability and catalytic activity of human pancreatic β cell glucokinase. Arch Biochem Biophys 375:251–260 [DOI] [PubMed] [Google Scholar]
- 40.Dhanesha N, Joharapurkar A, Shah G, Dhote V, Kshirsagar S, Bahekar R, Jain M. 23 February 2012. Exendin-4 activates glucokinase. J Diabetes10.1111/j. 1753–0407.2012.00193.x [DOI] [PubMed] [Google Scholar]
- 41.Kesavan P, Wang L, Davis E, Cuesta A, Sweet I, Niswender K, Magnuson MA, Matschinsky FM. 1997. Structural instability of mutant β-cell glucokinase: implications for the molecular pathogenesis of maturity-onset diabetes of the young (type-2). Biochem J 322:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burke CV, Buettger CW, Davis EA, McClane SJ, Matschinsky FM, Raper SE. 1999. Cell-biological assessment of human glucokinase mutants causing maturity-onset diabetes of the young type 2 (MODY-2) or glucokinase-linked hyperinsulinaemia (GK-HI). Biochem J 342:345–352 [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen L, Deacon CF, Orskov C, Holst JJ. 1999. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology 140:5356–5363 [DOI] [PubMed] [Google Scholar]
- 44.Syme CA, Zhang L, Bisello A. 2006. Caveolin-1 regulates cellular trafficking and function of the glucagon-like peptide 1 receptor. Mol Endocrinol 20:3400–3411 [DOI] [PubMed] [Google Scholar]
- 45.Lenzen S. 2008. Oxidative stress: the vulnerable β-cell. Biochem Soc Trans 36:343–347 [DOI] [PubMed] [Google Scholar]
- 46.Jaffrey SR, Benfenati F, Snowman AM, Czernik AJ, Snyder SH. 2002. Neuronal nitric-oxide synthase localization mediated by a ternary complex with synapsin and CAPON. Proc Natl Acad Sci USA 99:3199–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Simplicio P, Franconi F, Frosalí S, Di Giuseppe D. 2003. Thiolation and nitrosation of cysteines in biological fluids and cells. Amino Acids 25:323–339 [DOI] [PubMed] [Google Scholar]
- 48.Marsh BJ, Soden C, Alarcón C, Wicksteed BL, Yaekura K, Costin AJ, Morgan GP, Rhodes CJ. 2007. Regulated autophagy controls hormone content in secretory-deficient pancreatic endocrine β-cells. Mol Endocrinol 21:2255–2269 [DOI] [PubMed] [Google Scholar]
- 49.Baltrusch S, Langer S, Massa L, Tiedge M, Lenzen S. 2006. Improved metabolic stimulus for glucose-induced insulin secretion through GK and PFK-2/FBPase-2 coexpression in insulin-producing RINm5F cells. Endocrinology 147:5768–5776 [DOI] [PubMed] [Google Scholar]
- 50.Langer S, Kaminski MT, Lenzen S, Baltrusch S. 2010. Endogenous activation of glucokinase by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase is glucose dependent. Mol Endocrinol 24:1988–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zelent B, Odili S, Buettger C, Shiota C, Grimsby J, Taub R, Magnuson MA, Vanderkooi JM, Matschinsky FM. 2008. Sugar binding to recombinant wild-type and mutant glucokinase monitored by kinetic measurement and tryptophan fluorescence. Biochem J 413:269–280 [DOI] [PubMed] [Google Scholar]
- 52.Gloyn AL, Noordam K, Willemsen MA, Ellard S, Lam WW, Campbell IW, Midgley P, Shiota C, Buettger C, Magnuson MA, Matschinsky FM, Hattersley AT. 2003. Insights into the biochemical and genetic basis of glucokinase activation from naturally occurring hypoglycemia mutations. Diabetes 52:2433–2440 [DOI] [PubMed] [Google Scholar]