Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells (original) (raw)
Abstract
Persistent Jak/Stat3 signal transduction plays a crucial role in tumorigenesis and immune development. Activated Jak/Stat3 signaling has been validated as a promising molecular target for cancer therapeutics discovery and development. Berbamine (BBM), a natural bis‐benzylisoquinoline alkaloid, was identified from the traditional Chinese herbal medicine Berberis amurensis used for treatment of cancer patients. While BBM has been shown to have potent antitumor activities with low toxicity in various cancer types, the molecular mechanism of action of BBM remains largely unknown. Here, we determine the antitumor activities of 13 synthetic berbamine derivatives (BBMDs) against human solid tumor cells. BBMD3, which is the most potent in this series of novel BBMDs, exhibits over 6‐fold increase in biological activity compared to natural BBM. Moreover, BBMD3, directly inhibits Jak2 autophosphorylation kinase activity in vitro with IC500.69μM. Autophosphorylation of Jak2 kinase at Tyr1007/1008 sites also was strongly inhibited in the range of 15μM of BBMD3 in human melanoma cells at 4h after treatment. Following inhibition of autophosphorylation of Jak2, BBMD3 blocked constitutive activation of downstream Stat3 signaling in melanoma cells. BBMD3 also down‐regulated expression of the Stat3 target proteins Mcl‐1and Bcl‐xL, associated with induction of apoptosis. In sum, our findings demonstrate that the novel berbamine derivative BBMD3 is an inhibitor of the Jak2/Stat3 signaling pathway, providing evidence for a molecular mechanism whereby BBMD3 exerts at least in part the apoptosis of human melanoma cells. In addition, BBMD3 represents a promising lead compound for development of new therapeutics for cancer treatment.
Keywords: Berbamine derivatives (BBMDs), Jak2, Stat3, Apoptosis
Highlights
- Novel berbamine derivatives (BBMDs) inhibit Jak2/Stat3 signaling in melanoma cells.
- Blockade of Jak2/Stat3 signaling is associated with induction of apoptosis.
- The findings suggest important pharmacological mechanism of action of berbamine.
- BBMD3 has potential as a novel chemotherapeutic agent for solid tumor treatment.
Abbreviations
BBMDs
berbamine derivatives
STAT
signal transducer and activator of transcription
JAKs
Janus kinases
SFKs
Src family kinases
Tyk2
tyrosine kinase 2
CML
chronic myelogenous leukemia
1. Introduction
Signal Transducer and Activator of Transcription (STAT) proteins play an important role in oncogenic signaling pathways (Bromberg et al., 1999; Darnell, 2002; Yu and Jove, 2004; Yu et al., 2009). Constitutive activation of STATs, including Stat3 and Stat5 are frequently observed in human tumor cells and tissues (Levy and Darnell, 2002; Yu and Jove, 2004; Yu et al., 2009). STATs have essential functions in regulating cell proliferation, survival, angiogenesis and immune function (Levy and Darnell, 2002; Parsons and Parsons, 2004; Yu and Jove, 2004; Yu et al., 2009). One of the seven different STAT family members, Stat3, is persistently activated by non‐receptor tyrosine kinases such as Janus kinases (JAKs) or Src family kinases (SFKs) (Levy and Darnell, 2002; O'Shea et al., 2004; Yu and Jove, 2004; Yu et al., 2009). Constitutive activation of Stat3 has a critical role in cell growth and survival in human solid tumor malignancies (Bromberg et al., 1999; Yu and Jove, 2004; Yu et al., 2009). In Stat3 signaling, two phosphorylated Stat3 monomers dimerize through reciprocal phosphotyrosyl‐SH2 domain interactions. The phosphorylated Stat3 dimers then translocate to the nucleus and bind to the promoters of specific Stat3 responsive genes (Darnell, 2002; Yu and Jove, 2004; Yu et al., 2009). Constitutively activated Stat3 up‐regulates the expression of anti‐apoptotic genes encoding Mcl‐1 and Bcl‐xL proteins in human cancer cells (Bromberg et al., 1999; Epling‐Burnette et al., 2001; Yu and Jove, 2004). In contrast, blockade of Stat3 signaling down‐regulates these downstream target genes of Stat3, associated with induction of apoptosis in human cancer cells (Darnell, 2002; Yu and Jove, 2004; Yu et al., 2009).
The JAK family proteins, which consist of Jak1, Jak2, Jak3 and tyrosine kinase 2 (Tyk2), display similar structures and functions to each other (O'Shea et al., 2004; Rane and Reddy, 2000; Yu et al., 2009). Cytokine receptors, which lack intrinsic kinase activity, lead to activation of JAKs through their association by dimerization (O'Shea et al., 2004; Yu et al., 2009). JAKs are also activated by interferons and growth hormones (Rane and Reddy, 2000; Yu et al., 2009). Activation of JAKs is critical in cytokine receptor signaling (Rane and Reddy, 2000; Yu et al., 2009). Constitutively activated JAKs phosphorylate critical cellular substrates such STAT family members, including Stat3, associated with oncogenic signaling pathways (Darnell, 2002; Yu et al., 2009). Activated JAK/STAT signaling has been extensively validated as a new molecular target pathway for human solid tumor treatment (Levine and Gilliland, 2008; Luo and Laaja, 2004; O'Shea et al., 2004; Yu et al., 2009).
Recently, it has been founded that a Jak2 mutation (JAK2V617F) in the JH2 domain, which could lead to constitutive activation of Jak2, was very often identified in myeloproliferative disorders (Baxter et al., 2005; Kralovics et al., 2005; Levine et al., 2005). The discovery of the Jak2 mutation accelerated to develop small‐molecule therapeutic agents targeting inhibition of Jak2 kinase activity (Hedvat et al., 2009; Levine and Gilliland, 2008).
BBBM, a natural bis‐benzylisoquinoline alkaloid, was identified from the traditional Chinese herbal medicine Berberis amurensis used for treatment of human cancers and inflammation (Liang et al., 2009; Wei et al., 2009). BBM has been shown to have potent antitumor activities with low toxicity in various cancer types, including hepatoma, breast cancer and imatinib‐resistant chronic myelogenous leukemia (CML) (Wang et al., 2009; Xie et al., 2009). BBM has also been largely used to enhance low levels of white blood cells in China (Wang et al., 2009). Recently, it was reported that BBM inhibits NF‐kappa B and Bcr‐Abl signaling in blood cancers (Liang et al., 2009). However, the mechanism of action of BBM in human cancers remains largely unknown.
In the present study, we examined the antitumor activities of thirteen synthetic BBMDs against human solid tumor cells. This is the first report of a novel BBMD, that is an inhibitor of Jak2/Stat3 signaling in human melanoma cells. BBMD3 exhibits inhibition of Jak2 autophosphorylation kinase activity in vitro. In addition, BBMD3 strongly inhibits autophosphorylation of Jak2 kinase in human melanoma cells. Following inhibition of autophosphorylation of Jak2, BBMD3 blocks constitutive activation of downstream Stat3 signaling in melanoma cells, associated with down‐regulation of expression of Stat3 target proteins Mcl‐1and Bcl‐xL and induction of apoptosis of human melanoma cells. Our findings demonstrate that the novel berbamine derivative BBMD3 is an inhibitor of the Jak2/Stat3 signaling pathway, suggesting that the antitumor activity of BBMDs is at least partially due to inhibition of this pathway in human melanoma cells. Thus, BBMD3 represents a promising lead compound for development of new therapeutics for melanoma treatment.
2. Materials and methods
2.1. Cell lines, reagents and BBMDs
Human A2058, A375, G361, SK‐MEL‐28 and SK‐MEL‐5 melanoma, and DU145 prostate cancer cells were obtained from the American Type Culture Collection (ATCC). Cells were cultured in RPMI‐1640 media containing 10% (v/v) fetal bovine serum (FBS). Normal Human Dermal Fibroblast (NHDF) cells were purchased from Lonza (Basel, Switzerland). Cells were cultured as described in the supplier's instructions. Polyclonal antibodies to p‐Jak2 (Tyr1007/1008), Jak2, p‐Src family (Tyr419), Src, p‐Stat3 (Tyr705), Stat3, p‐Tyk2 (Tyr1054/1055), Tyk2, p‐Erk1/2 (Thr202/Tyr204), Erk1/2, p‐Stat5 (Tyr694) and Stat5 were from Cell Signaling Technologies (Cambridge, MA). Monoclonal antibody to β‐actin was obtained from Sigma (St. Louis, MO). Non‐activated recombinant Jak2 purified protein–protein A‐agarose conjugate expressed by baculovirus in SF9 insect cells was obtained from Millipore (Billerica, MA). Human IL‐6 was purchased from R&D Systems (Minneapolis, MN) and reconstituted in sterilized PBS containing 0.1% (w/v) BSA. BBMDs were synthesized in Synthetic Chemistry Core Facility, City of Hope. BBMDs were synthesized by reaction of natural BBM with various NH2‐containing substituents. Structures and purities of all derivatives were analyzed using 1HNMR spectroscopy. All BBMDs displayed over 98% purities in NMR analysis.
2.2. Western blot analyses
Western analyses were performed as described previously with minor modification (Nam et al., 2005a). Briefly, human A2058, A375, G361 or SK‐MEL‐5 melanoma cells were treated with or without BBMD3 when cells were approximately 50% confluent. DMSO was used as vehicle control. Whole‐cell lysates (40μg) were resolved by SDS‐PAGE and immunoblotted with specific antibodies. Primary phospho‐specific antibodies were incubated in TBS (pH 7.5) with 0.1% (v/v) Tween‐20 and 5% (w/v) BSA with gentle agitation overnight at 4 °C. Other specific antibodies were diluted in PBS (pH 7.5) with 5% (w/v) nonfat milk and 0.1% (v/v) Tween‐20 over night at 4 °C. Horseradish peroxidase‐conjugated secondary antibodies were incubated in TBS (pH 7.5) with 5% (w/v) nonfat milk and 0.1% (v/v) Tween‐20 at a 1:2000 dilution for 1h at room temperature. Positive immuno‐reactive proteins were detected using the ECL system (Pierce, Rockford, IL).
2.3. Jak2 autophosphorylation kinase activity in vitro
Jak2 autophosphorylation kinase assay in vitro was performed as described in the supplier (Millipore, Billerica, MA) with modifications (Nam et al., 2005, 2005). Forty μl of mixture containing 10μl of non‐activated Jak2‐agarose conjugates and additional 30μl of protein A/G PLUS‐agarose was added to each tube. Each Jak2‐agarose was washed twice with 1ml of kinase assay buffer (10mM HEPES, pH 7.4, 50mM NaCl, 5mM MgCl2, 0.1mM Na3VO4). The Jak2‐agarose was suspended in 30μl of kinase assay buffer. DMSO, BBMD3, AG490 JAK inhibitor, or AZD01 Jak2 inhibitor was preincubated for 10min at room temperature. ATP (20μM) was added to each reaction tube and the reaction mixture was incubated with gentle agitation for 30min at room temperature. The reaction was terminated by washing the Jak2‐agarose three times with 1ml of storage buffer (50mM Tris–HCl, pH 8.0, 150mM NaCl, 10% (v/v) glycerol, 0.1mM EDTA, 0.1mM Na3VO4, 50mM NaF, 0.5% (v/v) NP‐40). Reaction mixtures were boiled with SDS‐PAGE sample buffer for 5min and resolved on 8% (w/v) SDS‐PAGE gels. Then, samples were immunoblotted with specific antibody to p‐Jak2 (Tyr1007/1008) and reblotted with specific antibody to Jak2. Briefly, primary phospho‐specific antibody to Jak2 was incubated in TBS (pH 7.5) with 0.1% (v/v) Tween‐20 and 5% (w/v) BSA with gentle agitation overnight at 4 °C. Horseradish peroxidase‐conjugated secondary antibodies were incubated in TBS (pH 7.5) with 5% (w/v) nonfat milk and 0.1% (v/v) Tween‐20 at a 1:2000 dilution for 1h at room temperature. Positive immuno‐reactive proteins were detected using the ECL system (Pierce, Rockford, IL).
2.4. Viability and apoptosis assays
MTS assays were performed for cell viability as described by the supplier (Promega, Madison, WI). Human A2058, A375, G361, SK‐MEL‐28 and SK‐MEL‐5 melanoma, and DU145 prostate cancer cells were seeded in 96‐well plates (5000/well), incubated overnight at 37 °C in 5% (v/v) CO2, and exposed to BBMDs for the indicated times. For effects of BBMD3 on viabilities of NHDFs, cells (3000/well) were seeded in 96‐well plates. Cells were treated with BBMD3 in a dose‐dependent manner for 48h. DMSO was used as the vehicle control. Viable cell numbers were determined by tetrazolium conversion to its formazan dye and absorbance was measured at 490nm using an automated ELISA plate reader.
Apoptosis assays of human A2058 melanoma cells based on loss of membrane integrity were carried out using Annexin V‐FITC as described by the supplier (BD Biosciences Pharmingen, San Diego, CA). Cells were analyzed using a FACScan flow cytometer to quantify fluorescence. Apoptotic cells were defined as Annexin V‐FITC positive.
3. Results
3.1. BBMDs inhibit human tumor cell viabilities
Thirteen novel BBMDs (Figure 1A) were synthesized from natural BBM. For primary screening, MTS cell viability assays were performed to determine whether they reduce cell viabilities in human A2058 melanoma and DU145 prostate cancer cells. All BBMDs showed antitumor activities with approximate IC50 values3μM–15μM in both cell lines, whereas natural BBM displayed over 20μM IC50 value (Figure 1B). Interestingly, BBMD3, BBMD6 and BBMD10, which demonstrate similar activities in this series of novel BBMDs, exhibited over 6‐fold increase in biological activity compared to natural BBM, with IC50 values between 2.9μM and 3.7μM (Figure 1B). These data suggest that BBMD3, BBMD6 and BBMD10 have potential as promising chemotherapeutic agents for human solid tumor treatment. Synthesis of BBMD3 requires less synthetic steps than that of BBMD6 or BBMD10, so BBMD3 was selected to further investigate in human melanoma cells.
Figure 1.
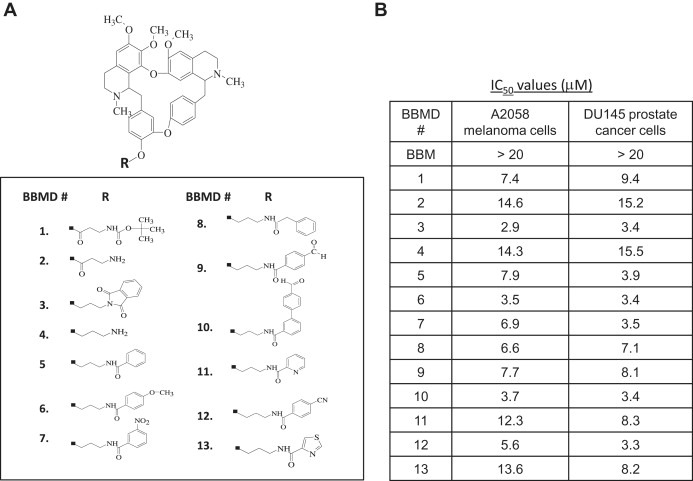
(A) Structures of BBMDs. (B) BBMDs inhibit human cancer cell viabilities. Cell viability was determined using MTS assays as described in Section 2. To determine IC50 values of BBMDs against human A2058 melanoma and DU145 prostate cancer cells, cells were treated with BBMDs in a dose‐dependent manner for 48h. Each experiment was performed in quadruplicate. Data are mean.
3.2. Effects of BBMD3 on various melanoma cells
Melanoma is the most dangerous malignancy among skin cancer types. The occurrence of melanoma persistently rises in Western countries (Gray‐Schopfer et al., 2007). The FDA has first approved Dacarbazine for treatment of metastatic melanoma and it responses to melanoma patients at a 15% rate (De Nicolo et al., 2009). Melanoma is highly metastatic, aggressive and resistant to existing chemotherapy (Gray‐Schopfer et al., 2007). Metastatic malignant melanoma is very often refractory to current therapies with very poor prognosis (Cummins et al., 2006). Therefore, new therapy, including molecular targeted therapeutic agents, will be beneficial to melanoma treatment. Importantly, BBMDs had antitumor activities in aggressive A2058 melanoma cells (Figure 1B). Next, to assess whether BBMD3 also displays antitumor activity against other human melanoma cells, including A375, G361, SK‐MEL‐28 and SK‐MEL‐5, cell viability assays were carried using MTS assays. BBMD3 remarkably decreased cell viability in the range of 1μM–5μM concentration in all melanoma cells (Figure 2A). IC50 values of BBMD3 were 2.9μM, 4.4μM, 1.8μM, 3.7μM and 3.6μM against A2058, A375, G361, SK‐MEL‐28 and SK‐MEL‐5 cells, respectively, indicating that BBMD3 is the most potent in G361 melanoma cells (Figure 2C). However, BBMD3 required higher concentrations with IC507.6μM in NHDF cells, which is 4‐fold higher than IC50 value in G361 cells (Figure 2B and C). The IC50 value of BBMD3 in NHDF cells was approximately 2.5‐fold less potent in comparison to the IC50 value in A2058 cells (Figure 2B and C). Interestingly, constitutively activated Jak2 was not detected in NHDF cells (Supplementary Figure 1A). This result suggests that BBMD3 might be less sensitive to melanoma cells that express no basal or low levels of activated Jak2, implying that inhibition of Jak2/Stat3 signaling by BBMD3 could be involved in loss of cell viability on melanoma cells. Taken together, these findings suggest BBMD3 could be a lead compound to develop anticancer therapeutic agents for human aggressive malignant melanoma treatment.
Figure 2.
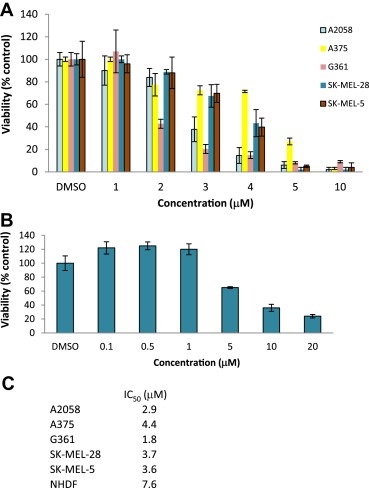
Effects of BBMD3 on various melanoma cells. Human A2058, A375, G361, SK‐MEL‐28 and SK‐MEL‐5 melanoma (A), and NHDF (B) cells were treated with BBMD3 in a dose‐dependent manner for 48h. Cell viability was determined using MTS assays as described in Section 2. (C) IC50 values of BBMD3 were determined in these cell lines. Each experiment was performed in quadruplicate. Data are mean.
3.3. BBMD3 inhibits Jak2/Stat3 signaling in cells
Numerous studies demonstrated that activation of JAKs is through by either their autophosphorylation or transphosphorylation by other JAKs, associated with cytokine receptors (Feng et al., 1997; Reddy et al., 2000; Yu et al., 2009). The function of JAKs is associated with activation of STAT signaling pathway (Rane and Reddy, 2000). While all cell lines except SK‐MEL‐28 express constitutively activated Stat3 (Figure 3A), there is heterogeneity among these cell lines in that they display different levels of JAK/Stat3 signaling. Thus, the four cell lines tested here may represent a cross section of melanoma heterogeneity. To evaluate the effects of BBMD3 on phosphorylation of Jak2 in melanoma cells, Western blot analysis was performed with specific antibodies to p‐Jak2 (Tyr1007/1008). As shown in Figure 3A, BBMD3 reduced levels of p‐Jak2 (Tyr1007/1008) at 10μM 4h after treatment in A2058, A375, G361 and SK‐MEL‐5 melanoma cells. In addition, consistent with reduction of cell viabilities, BBMD3 inhibited phosphorylation of Jak2 at autophosphorylation sites (Tyr1007/1008) in the range of 1μM–5μM concentration in A2058 cells, whereas total Jak2 protein levels remained unchanged (Figure 3B). Time course studies also indicate that 10μM BBMD3 dramatically blocked phosphorylation of Jak2 as early as 30min after treatment in melanoma cells (Figure 3C right panel). However, BBMD3 does not inhibit phosphorylation of Jak2 at 1μM concentration, whereas 1μM of BBMD3 slightly reduced level of p‐Stat3 24h after treatment (Figure 3C left panel). This observation suggests that BBMD3 does not block Jak2/Stat3 signaling at 1μM concentration. In addition, BBMD3 blocked autophosphorylation of activated mutant Jak2 at the Tyr1007/1008 sites in human erythroleukemia HEL Jak2V617F mutation cells (Supplementary Figure 1C). Similarly, BBMD3 inhibited phosphorylation of Src (Tyr 419) in a dose‐ or time‐dependent manner (Figure 3B and C). Next, to address whether BBMD3 consistently inhibits tyrosyl phosphorylation of endogenous Stat3 in cells, Western blot analysis with specific antibody to p‐Stat3 (Tyr705) was performed using whole‐cell lysates from melanoma cells treated with BBMD3. Indeed, BBMD3 inhibited phosphorylation of Stat3 in A2058, A375, G361 and SK‐MEL‐5 cells (Figure 3A). BBMD3 also blocked phosphorylation of Stat3 in the range of 1μM–5μM concentration or 30min after treatments (Figure 3B and C right panel). However, BBMD3 did not inhibit phosphorylation of Erk1/2, implicating that BBMD3 apparently has no effects on MAPK signaling pathway in melanoma cells (Figure 3B). These observations support that BBMD3 inhibits autophosphorylation of Jak2 in cells, which is essential for activation of crucial signal transduction pathways, including the JAK/Stat3 signaling pathway.
Figure 3.
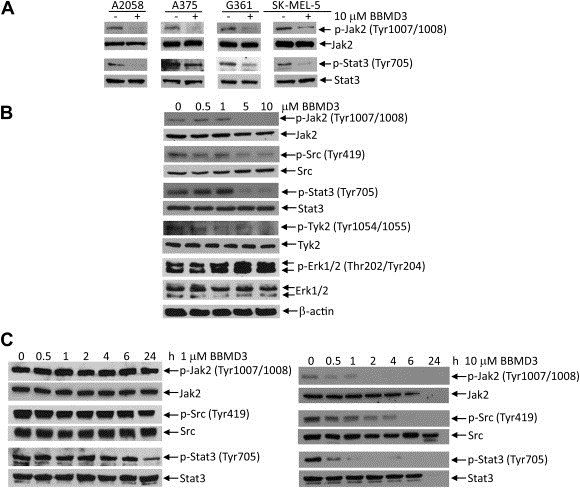
BBMD3 inhibits Jak2/Stat3 signaling in cells. (A) A2058, A375, G361 and SK‐MEL‐5 cells were treated with 10μM of BBMD3 for 4h. Whole‐cell lysates were resolved by SDS‐PAGE and immunoblotted with specific antibodies to p‐Jak2 (Tyr1007/1008), Jak2, p‐Stat3 and Stat3. (B) A2058 melanoma cells were treated with BBMD3 in a dose‐dependent manner for 4h. Whole‐cell lysates were immunoblotted with specific antibodies to p‐Jak2 (Tyr1007/1008), Jak2, p‐src family (Tyr419), Src, p‐Stat3 (Tyr705), Stat3, p‐Tyk2 (Tyr1054/1055), Tyk2, p‐Erk1/2 (Thr202/Tyr204), Erk1/2 and β‐actin as performed in A. (C). A2058 cells were treated with 1μM or 10μM BBMD3 in a time‐dependent manner. Whole‐cell lysates were resolved by SDS‐PAGE and immunoblotted with specific antibodies to p‐Jak2 (Tyr1007/1008), Jak2, p‐Src family (Tyr419), Src, p‐Stat3 (Tyr705) and Stat3.
To address whether inhibition of Jak2/Stat3 signaling by BBMD3 results in the loss of cell viability or whether the impairment of cell viability causes reduction of phosphorylation of proteins, western blot analysis and cell viability were performed using A2058 cells. Cells were treated with BBMD3 in a dose‐dependent manner for 4h, followed by IL‐6 stimulation for 10min. BBMD3 substantially inhibited IL‐6‐induced phosphorylation of Jak2 and Stat3 at 3μM of concentration (Figure 4A). This result consistently correlates with IC50 value of BBMD3 against A2058 cell viability (Figure 2C). Next, intact A2058 cells were treated with 5μM of BBMD3 for 1h (Figure 4B). Western blot analysis was carried using whole‐cell lysates, followed by cell viability assay. Levels of p‐Jak2 and p‐Stat3 were largely decreased (Figure 4B top panel), but cells were still viable (Figure 4B bottom panel). Similarly, 10μM of BBMD3 inhibited phosphorylation of Jak2 and Stat3 30min after treatment while cell viability remained unchanged (Figure 3C right panel and Supplementary Figure 2). Taken together, these observations suggest that blockade of Jak2/Stat3 signaling by BBMD3 is not due to the loss of cell viability in melanoma cells.
Figure 4.
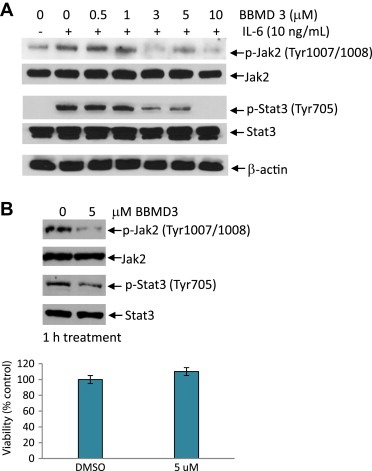
Inhibition of Jak2/Stat3 signaling by BBMD3 is associated with loss of cell viability. (A) A2058 cells were incubated in RPMI‐1640 medium containing 5% charcoal‐stripped serum overnight. Cells were treated with BBMD3 in a dose‐dependent manner for 4h, followed by IL‐6 stimulation for 10min. Whole‐cell lysates were resolved by SDS‐PAGE and immunoblotted with specific antibodies to p‐Jak2 (Tyr1007/1008), Jak2, p‐Stat3 (Tyr705), Stat3 and β‐actin. B. A2058 cells were treated with 5μM of BBMD3 for 1h. Whole‐cell lysates were resolved by SDS‐PAGE and immunoblotted with specific antibodies to p‐Jak2 (Tyr1007/1008), Jak2, p‐Stat3 (Tyr705) and Stat3. Viabilities of cells treated with 5μM of BBMD3 for 1h were determined using MTS assays. Each experiment was performed in quadruplicate. Data are mean±SD.
3.4. BBMD3 inhibits Jak2 autophosphorylation kinase activity in vitro
Autophosphorylation of Jak2 at Tyr1007 plays a critical role in Jak2 activation, function and regulation (Feng et al., 1997; Leonard and O'Shea, 1998). Blockade of autophosphorylation of Jak2 at Tyr1007 inhibits tyrosyl phosphorylation of downstream STAT signaling (Feng et al., 1997; Leonard and O'Shea, 1998). Western blot analysis indicated that BBMD3 blocks autophosphorylation of Jak2 at this site in cells (Figure 3). Next, to assess whether BBMD3 directly inhibits Jak2 autophosphorylation kinase activity, kinase assays in vitro were performed with non‐activated recombinant Jak2 protein in the absence of substrates. Indeed, BBMD3 displayed an inhibitory activity in vitro against Jak2 autophosphorylation kinase with an IC500.69μM (Figure 5). These results correlate with inhibition of autophosphorylation of Jak2 in cells as shown in Figure 3. Our data suggest that BBMD3 could directly interact with Jak2 at the Tyr1007 site, resulting in inhibition of activation of Jak2.
Figure 5.
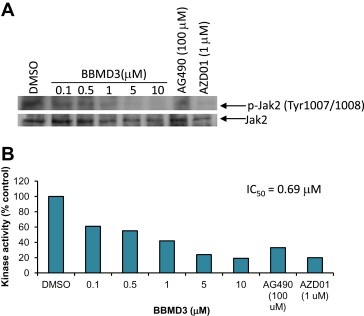
BBMD3 inhibits Jak2 autophosphorylation kinase activity in vitro. (A) Jak2 autophosphorylation kinase assay in vitro was performed as described in Section 2. The kinase assay in vitro was carried with non‐activated recombinant Jak2 protein in the absence of substrates. DMSO, BBMD3, AG490 JAK inhibitor, or AZD01 Jak2 inhibitor was preincubated for 10min at room temperature. ATP (20μM) was added to each reaction tube. Reaction mixtures were boiled with SDS‐PAGE sample buffer for 5min and resolved on SDS‐PAGE gels. Then, samples were immunoblotted with specific antibody to p‐Jak2 (Tyr1007/1008) and reblotted with specific antibody to Jak2. Positive immuno‐reactive proteins were detected using the ECL system. (B). To determine the IC50 value of BBMD3 against Jak2 autophosphorylation kinase activity in vitro shown in (A), levels of positive immuno‐reactive proteins were quantified with ImageQuant software (molecular Dynamics). Protein levels of p‐Jak2 were normalized to total Jak2.
3.5. BBMD3 down‐regulates Mcl‐1 and Bcl‐xL
Inhibition of Stat3 signaling down‐regulates expression of Stat3 downstream gene products such as the anti‐apoptotic proteins, Mcl‐1, Bcl‐xL and survivin, associated with induction of apoptosis (Yu and Jove, 2004; Yu et al., 2009). Down‐regulation of Mcl‐1 by siRNA dramatically reduces cell viability and induces apoptosis in cancer cells (Boisvert‐Adamo et al., 2009; Nagata et al., 2009; Zhang et al., 2011). To determine the effects of BBMD3 on the anti‐apoptotic proteins such as Mcl‐1 and Bcl‐xL, A2058 melanoma cells were treated with BBMD3 in a dose‐dependent manner for 24h before extensive apoptosis. Western blot analysis was carried out using whole‐cell lysates and specific antibodies to Mcl‐1 and Bcl‐xL proteins. Consistent with down‐regulation of p‐Stat3, expression of the anti‐apoptotic proteins Mcl‐1 and Bcl‐xL was reduced at 5μM (Figure 6A). In addition, down‐regulation of these anti‐apoptotic proteins correlates with inhibition of p‐Jak2 and p‐Stat3 as shown in Figure 3, associated with induction of apoptosis by BBMD3 in A2058 melanoma cells (Figure 6B). The observation of Mcl‐1 down‐regulation by BBMD3 implicates Mcl‐1 as a molecular therapeutic target for treatment of melanoma.
Figure 6.
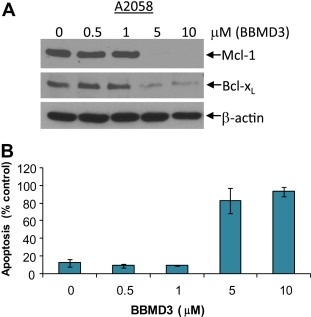
BBMD3 down‐regulates Mcl‐1 and Bcl‐xL, associated with induction of apoptosis. (A) A2058 melanoma cells were treated with BBMD3 in a dose‐dependent manner for 24h. Whole‐cell lysates were immunoblotted with specific antibodies to Mcl‐1, Bcl‐xL and β‐actin. (B) BBMD3 induces apoptosis. A2058 melanoma cells were treated with BBMD3 in a dose‐dependent manner for 48h. Cells were labeled with Annexin V‐PE. Cells were analyzed using a FACScan flow cytometer to quantify fluorescence. Apoptotic cells were defined as Annexin V‐PE positive. Each experiment was performed in quadruplicate. Data are mean±SD.
3.6. BBMD3 induces apoptosis
BBMD3 directly targeted autophosphorylation of Jak2 in vitro and in cells, and inhibited constitutive activation of Stat3, followed by down‐regulation of survival proteins such as Mcl‐1 and Bcl‐xL in human melanoma cells (3, 5, 6). In addition, MTS assay data revealed that BBMD3 reduced cell viability in various human melanoma and erythroleukemia HEL Jak2V617F mutation cells (Figure 2A and Supplementary Figure 1B). To further investigate the biological effects of BBMD3 on A2058 melanoma cells, apoptotic assays were performed with the early marker of apoptosis Annexin V. As expected, we observed that BBMD3 induced over 80% apoptosis at 5μM 48h after treatment in melanoma (Figure 6B). These apoptosis outcomes of BBMD3 treatment correlate well with inactivation of Jak2/Stat3 signaling, supporting a role of BBMD3 in apoptosis of A2058 melanoma cells.
4. Discussion
Since only a small number of BBM derivatives have been reported in the literature, little is known about BBM's structure activity relationship. We focused on modifying the phenolic hydroxyl group since this group is readily amenable to modification and in principle could serve as a useful point of attachment for other functionalities such as affinity column probes. Compounds 1–13 represent new derivatives of BBM. Based on the limited SAR data obtained, it appears that modification of the phenolic hydroxyl position by either acyl or alkyl substituents increases potency relative to BBM. The greatest effect is seen with BBM3. The relatively tight range, however, suggests that this position may not be the primary region of interaction between the substrate and the protein binding site. This increase efficacy may be a result from stabilizing the electron rich ring, which could undergo relatively facile oxidation. Additional analogs and/or X‐ray crystallographic analysis will be needed to fully delineate the structural role of BBM and its derivatives.
It has been suggested that inhibiting upstream tyrosine kinases of Stat3 by small‐molecule inhibitors may significantly provide benefit in the clinic (Yu and Jove, 2004). JAK/Stat3 signaling pathway plays a crucial role in melanoma malignancy (Kong et al., 2008). Stat3 is constitutively activated in human melanoma cells and tissues (Kortylewski et al., 2005; Niu et al., 2002). Inactivation of Stat3 by overexpression of dominant negative Stat3 suppresses tumor growth and invasion and metastasis of cancer cells in nude mouse study (Kong et al., 2008). Therefore, Stat3 is a promising target to develop anticancer drugs to melanoma treatment (Bill et al., 2010; Kortylewski et al., 2005; Niu et al., 2002). Several small‐molecule inhibitors induce apoptosis in melanoma cells, associated with inactivation of Stat3 (Bill et al., 2010; Kong et al., 2008; Liu et al., 2011). Particularly, it was reported that WP1066, a Jak2/Stat3 inhibitor, has potential for metastatic melanoma treatment in the preclinical study (Kong et al., 2008). They showed that Jak2/Stat3 is a promising molecular target for developing anticancer therapeutic agents for metastatic melanoma treatment. IC50 values of WP1066 against human and murine melanoma cell lines are in the low micromolar range. Despite different classes of inhibitors, BBMD3 with low micromolar IC50 values exhibits promising cytotoxic effects on melanoma cells. BBMD3 displays relatively less toxicity with IC507.6μM against NHDF cells, which is 4‐fold higher than IC50 value in G361 cells (Figure 2B and C). Moreover, our findings demonstrate that novel BBMD3, a natural herbal product derivative, inhibits tyrosyl phosphorylation of Stat3 through inactivation of upstream Jak2 in cells. This pharmacological mechanism of action of BBMD3 could be important for melanoma therapy. Thus, our data suggest that BBMD3 should be further evaluated in preclinical and clinical studies. Previous study showed that cucurbitacin I, a traditional herbal medicine, selectively blocks JAK/Stat3 signaling in solid tumor cells (Blaskovich et al., 2003). Similarly, the novel compound BBMD3 targets Jak2/Stat3 signaling in human melanoma cells (Figure 3). Together, these findings suggest that BBMD3 may have promising therapeutic value for melanoma treatment.
We showed the main molecular target of BBMD3 is an inhibition of Jak2 autophosphorylation. A kinase screening in vitro was further performed with ten recombinant kinases, including c‐Met, AKT, EGFR, ABL, FGFR1, PDGFRα, BRAF, PI3K, IKKβ and VEGFR2. Among these kinase proteins, at least EGFR, FGFR1 and PDGFRα are involved in Stat3 signaling (Brantley and Benveniste, 2008; Yu and Jove, 2004). The data demonstrate that BBMD3 does not inhibit kinase activities of these proteins in vitro (Supplementary Figure 3A). Together, these findings indicate that BBMD3 selectively reduces Jak2 autophosphorylation kinase activity in vitro and is associated with inhibition of downstream Stat3 signaling, implicating a dominant mechanistic action of BBMD3 in melanoma cells.
BBMD3 inhibited phosphorylation of Tyk2 in melanoma cells, which is one of the JAK family proteins (Figure 3B middle panel). One possible explanation is that BBMD3 may inhibit autophosphorylation of Jak2, followed by inhibition of transphosphorylation of Tyk2 by Jak2. Many studies demonstrate that activation of specific JAKs is mediated by either its autophosphorylation or transphosphorylation by other JAKs (Feng et al., 1997; Reddy et al., 2000; Yu et al., 2009). This result indicates that JAKs reciprocally transphosphosphorylate each other to regulate activation of other JAKs.
We observed reduction of levels of p‐Src after BBMD3 treatment (Figure 3B and C right panel). In a Src kinase assay in vitro using recombinant Src protein, BBMD3 did not inhibit Src kinase activity in vitro, indicating BBMD3 does not directly interact with Src kinase (Supplementary Figure 3B). Recently, we found that 6‐BIO indirubin, a JAKs inhibitor, reduced levels of p‐Src in human melanoma cells without inhibition of Src kinase in vitro (Liu et al., 2011). Previous studies demonstrated that JAKs and SFKs cooperate to constitutively activate Stat3 signaling (Campbell et al., 1997; Garcia et al., 2001; Nam et al., 2005a; Sen et al., 2009; Zhang et al., 2000). JAKs and SFKs might be cross‐regulated in the signaling events (Rane and Reddy, 2002). In addition, it was reported that Jak2 inhibitors or Jak2 siRNA lead to deactivation of Lyn in CML cells, which is one of the SFKs (Samanta et al., 2009). Taken together, our data suggest that BBMD3 could inhibit cooperation or cross‐regulation of Jak2 and Src, resulting in inhibition of tyrosyl phosphorylation of Stat3 in melanoma cells.
In summary, our findings demonstrate that the novel BBMD3 compound is an inhibitor of autophosphorylation of Jak2, resulting in inhibition of constitutively activated Stat3 signaling. BBMD3 induces apoptosis of human melanoma cells, associated with down‐regulation of the Stat3 downstream gene products Mcl‐1 and Bcl‐xL. These results suggest that the antitumor activity of BBMDs is at least partially due to inhibition of Jak2/Stat3 signaling in human melanoma cells. Thus, these findings suggest a mechanism of pharmacological action of BBMDs in human cancer cells that have important implications for solid tumor treatment. In particular, BBMD3 represents a promising lead compound for development of new therapeutics for cancer treatment.
Supporting information
The following are the Supplementary data related to this article:
Supplementary Figure 1 (A) Levels of constitutively activated Jak2 in A2058 melanoma and NHDF cells. Whole‐cell lysates were resolved by SDS‐PAGE and immunoblotted with specific antibodies to p‐Jak2 (Tyr1007/1008) and Jak2. (B) Effects of BBMD3 on viabilities of human erythroleukemia (HEL) cells. HEL cell line, which is positive for homozygous Jak2V617F mutation, was purchase from ATCC. Cells were cultured in RPMI‐1640 media containing 10% fetal bovine serum (FBS). Cell viability was determined using MTS assays as described in Section 2. HEL cells were treated with BBMD3 in a dose‐dependent manner for 48h. Each experiment was performed in quadruplicate. Data are mean. (C) BBMD3 inhibits Jak2/Stat5 signaling in HEL cells. HEL cells were treated with BBMD3 in a dose‐dependent manner for 4h. Whole‐cell lysates were resolved by SDS‐PAGE and immunoblotted with specific antibodies to p‐Jak2 (Tyr1007/1008), Jak2, p‐Stat5 (Tyr694) and Stat5.
Supplementary Figure 2 Human A2058 melanoma cells were treated with 10μM of BBMD3 for 30min. Cell viability was determined using MTS assays. Each experiment was performed in quadruplicate. Data are meanSD.
Supplementary Figure 3 (A) Kinase assays in vitro. The kinase assays were performed with recombinant c‐Met, AKT, EGFR, ABL, FGFR1, PDGFRα, BRAF, PI3K, IKKβ and VEGFR2 proteins using the HotSpot protocol (Reaction Biology Corp., Malvern, PA). Briefly, proteins, freshly prepared substrates and 33P‐ATP (specific activity 0.01μCi/μl final) were mixed in reaction buffer (20mM HEPES pH 7.5, 10mM MgCl2, 1mM EGTA, 0.02% Brij35, 0.02mg/ml BSA, 0.1mM Na3VO4, 2mM DTT, 1% DMSO) in the presence of DMSO as control or BBMD3. The mixtures were reacted for 120min at room temperature. Samples were transferred onto P81 ion exchange paper and filters were extensively washed with 0.75% phosphoric acid. The radioactivities were monitored. B. Src kinase assay in vitro. The kinase assays were performed using recombinant Src protein for 20min in a final volume of 25μl (SignalChem, Richmond, Canada). Briefly, approximately 20nM of Src protein (final concentration was mixed with 1μg of Src peptide substrate in the presence of BBMD3 or 10% DMSO in kinase assay buffer. The assay was initiated with the addition of 5μl of 33P‐ATP (250μM of stock solution, 0.8μCi). Then, the reaction mixture was incubated at ambient temperature for 20min. Ten μl of reaction mixture was spotted on Mutiscreen phosphocellulose P81 plate. The radioactivities on the P81 plate were monitored using a scintillation counter.
Acknowledgments
We thank AstraZeneca for generously providing AZD01. We thank our lab colleagues for helpful technical suggestions and critically discussing our data. We thank Analytical Cytometry Core of City of Hope for apoptosis analysis. We also thank Synthetic and Biopolymer Chemistry Core of City of Hope for synthesis of BBMDs. This study was supported by NIH grant R01 CA115674‐05 to RJ.
Appendix A. Supplementary data 1.
Supplementary data related to this article can be found online at doi:10.1016/j.molonc.2012.05.002
Nam Sangkil, Xie Jun, Perkins Angela, Ma Yuelong, Yang Fan, Wu Jun, Wang Yan, Xu Rong-zhen, Huang Wendong, Horne David A. and Jove Richard, (2012), Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells, Molecular Oncology, 6, doi: 10.1016/j.molonc.2012.05.002.
Contributor Information
Sangkil Nam, Email: snam@coh.org.
Richard Jove, Email: rjove@coh.org.
References
- Baxter, E.J. , Scott, L.M. , Campbell, P.J. , East, C. , Fourouclas, N. , Swanton, S. , Vassiliou, G.S. , Bench, A.J. , Boyd, E.M. , Curtin, N. , Scott, M.A. , Erber, W.N. , Green, A.R. , 2005. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 365, 1054–1061. [DOI] [PubMed] [Google Scholar]
- Bill, M.A. , Fuchs, J.R. , Li, C. , Yui, J. , Bakan, C. , Benson, D.M. , Schwartz, E.B. , Abdelhamid, D. , Lin, J. , Hoyt, D.G. , Fossey, S.L. , Young, G.S. , Carson, W.E. , Li, P.K. , Lesinski, G.B. , 2010. The small molecule curcumin analog FLLL32 induces apoptosis in melanoma cells via STAT3 inhibition and retains the cellular response to cytokines with anti-tumor activity. Mol. Cancer. 9, 165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaskovich, M.A. , Sun, J. , Cantor, A. , Turkson, J. , Jove, R. , Sebti, S.M. , 2003. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res.. 63, 1270–1279. [PubMed] [Google Scholar]
- Boisvert-Adamo, K. , Longmate, W. , Abel, E.V. , Aplin, A.E. , 2009. Mcl-1 is required for melanoma cell resistance to anoikis. Mol. Cancer Res.. 7, 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley, E.C. , Benveniste, E.N. , 2008. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol. Cancer Res.. 6, 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg, J.F. , Wrzeszczynska, M.H. , Devgan, G. , Zhao, Y. , Pestell, R.G. , Albanese, C. , Darnell, J.E. , 1999. Stat3 as an oncogene. Cell. 98, 295–303. [DOI] [PubMed] [Google Scholar]
- Campbell, G.S. , Yu, C.L. , Jove, R. , Carter-Su, C. , 1997. Constitutive activation of JAK1 in Src-transformed cells. J. Biol. Chem.. 272, 2591–2594. [DOI] [PubMed] [Google Scholar]
- Cummins, D.L. , Cummins, J.M. , Pantle, H. , Silverman, M.A. , Leonard, A.L. , Chanmugam, A. , 2006. Cutaneous malignant melanoma. Mayo Clin. Proc.. 81, 500–507. [DOI] [PubMed] [Google Scholar]
- Darnell, J.E. , 2002. Transcription factors as targets for cancer therapy. Nat. Rev. Cancer. 2, 740–749. [DOI] [PubMed] [Google Scholar]
- De Nicolo, A. , Parisini, E. , Zhong, Q. , Dalla Palma, M. , Stoeckert, K.A. , Domchek, S.M. , Nathanson, K.L. , Caligo, M.A. , Vidal, M. , Cusick, M.E. , Garber, J.E. , 2009. Multimodal assessment of protein functional deficiency supports pathogenicity of BRCA1 p.V1688del. Cancer Res.. 69, 7030–7037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epling-Burnette, P.K. , Liu, J.H. , Catlett-Falcone, R. , Turkson, J. , Oshiro, M. , Kothapalli, R. , Li, Y. , Wang, J.M. , Yang-Yen, H.F. , Karras, J. , Jove, R. , Loughran, T.P. , 2001. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J. Clin. Invest.. 107, 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, J. , Witthuhn, B.A. , Matsuda, T. , Kohlhuber, F. , Kerr, I.M. , Ihle, J.N. , 1997. Activation of Jak2 catalytic activity requires phosphorylation of Y1007 in the kinase activation loop. Mol. Cell Biol.. 17, 2497–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, R. , Bowman, T.L. , Niu, G. , Yu, H. , Minton, S. , Muro-Cacho, C.A. , Cox, C.E. , Falcone, R. , Fairclough, R. , Parsons, S. , Laudano, A. , Gazit, A. , Levitzki, A. , Kraker, A. , Jove, R. , 2001. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 20, 2499–2513. [DOI] [PubMed] [Google Scholar]
- Gray-Schopfer, V. , Wellbrock, C. , Marais, R. , 2007. Melanoma biology and new targeted therapy. Nature. 445, 851–857. [DOI] [PubMed] [Google Scholar]
- Hedvat, M. , Huszar, D. , Herrmann, A. , Gozgit, J.M. , Schroeder, A. , Sheehy, A. , Buettner, R. , Proia, D. , Kowolik, C.M. , Xin, H. , Armstrong, B. , Bebernitz, G. , Weng, S. , Wang, L. , Ye, M. , McEachern, K. , Chen, H. , Morosini, D. , Bell, K. , Alimzhanov, M. , Ioannidis, S. , McCoon, P. , Cao, Z.A. , Yu, H. , Jove, R. , Zinda, M. , 2009. The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and oncogenesis in solid tumors. Cancer Cell. 16, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L.Y. , Abou-Ghazal, M.K. , Wei, J. , Chakraborty, A. , Sun, W. , Qiao, W. , Fuller, G.N. , Fokt, I. , Grimm, E.A. , Schmittling, R.J. , Archer, G.E. , Sampson, J.H. , Priebe, W. , Heimberger, A.B. , 2008. A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells. Clin. Cancer Res.. 14, 5759–5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortylewski, M. , Jove, R. , Yu, H. , 2005. Targeting STAT3 affects melanoma on multiple fronts. Cancer Metastasis Rev.. 24, 315–327. [DOI] [PubMed] [Google Scholar]
- Kralovics, R. , Passamonti, F. , Buser, A.S. , Teo, S.S. , Tiedt, R. , Passweg, J.R. , Tichelli, A. , Cazzola, M. , Skoda, R.C. , 2005. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med.. 352, 1779–1790. [DOI] [PubMed] [Google Scholar]
- Leonard, W.J. , O'Shea, J.J. , 1998. Jaks and STATs: biological implications. Annu. Rev. Immunol.. 16, 293–322. [DOI] [PubMed] [Google Scholar]
- Levine, R.L. , Gilliland, D.G. , 2008. Myeloproliferative disorders. Blood. 112, 2190–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, R.L. , Wadleigh, M. , Cools, J. , Ebert, B.L. , Wernig, G. , Huntly, B.J. , Boggon, T.J. , Wlodarska, I. , Clark, J.J. , Moore, S. , Adelsperger, J. , Koo, S. , Lee, J.C. , Gabriel, S. , Mercher, T. , D'Andrea, A. , Frohling, S. , Dohner, K. , Marynen, P. , Vandenberghe, P. , Mesa, R.A. , Tefferi, A. , Griffin, J.D. , Eck, M.J. , Sellers, W.R. , Meyerson, M. , Golub, T.R. , Lee, S.J. , Gilliland, D.G. , 2005. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 7, 387–397. [DOI] [PubMed] [Google Scholar]
- Levy, D.E. , Darnell, J.E. , 2002. Stats: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol.. 3, 651–662. [DOI] [PubMed] [Google Scholar]
- Liang, Y. , Xu, R.Z. , Zhang, L. , Zhao, X.Y. , 2009. Berbamine, a novel nuclear factor kappaB inhibitor, inhibits growth and induces apoptosis in human myeloma cells. Acta Pharmacol. Sin.. 30, 1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Nam, S. , Tian, Y. , Yang, F. , Wu, J. , Wang, Y. , Scuto, A. , Polychronopoulos, P. , Magiatis, P. , Skaltsounis, L. , Jove, R. , 2011. 6-Bromoindirubin-3’-oxime inhibits JAK/STAT3 signaling and induces apoptosis of human melanoma cells. Cancer Res.. 71, 3972–3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, C. , Laaja, P. , 2004. Inhibitors of JAKs/STATs and the kinases: a possible new cluster of drugs. Drug Discov. Today. 9, 268–275. [DOI] [PubMed] [Google Scholar]
- Nagata, M. , Wada, K. , Nakajima, A. , Nakajima, N. , Kusayama, M. , Masuda, T. , Iida, S. , Okura, M. , Kogo, M. , Kamisaki, Y. , 2009. Role of myeloid cell leukemia-1 in cell growth of squamous cell carcinoma. J. Pharmacol. Sci.. 110, 344–353. [DOI] [PubMed] [Google Scholar]
- Nam, S. , Buettner, R. , Turkson, J. , Kim, D. , Cheng, J.Q. , Muehlbeyer, S. , Hippe, F. , Vatter, S. , Merz, K.H. , Eisenbrand, G. , Jove, R. , 2005. Indirubin derivatives inhibit Stat3 signaling and induce apoptosis in human cancer cells. Proc. Natl. Acad. Sci. U S A. 102, 5998–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, S. , Kim, D. , Cheng, J.Q. , Zhang, S. , Lee, J.H. , Buettner, R. , Mirosevich, J. , Lee, F.Y. , Jove, R. , 2005. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res.. 65, 9185–9189. [DOI] [PubMed] [Google Scholar]
- Niu, G. , Bowman, T. , Huang, M. , Shivers, S. , Reintgen, D. , Daud, A. , Chang, A. , Kraker, A. , Jove, R. , Yu, H. , 2002. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 21, 7001–7010. [DOI] [PubMed] [Google Scholar]
- O'Shea, J.J. , Gadina, M. , Schreiber, R.D. , 2002. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 109, (Suppl.) S121–S131. [DOI] [PubMed] [Google Scholar]
- O'Shea, J.J. , Pesu, M. , Borie, D.C. , Changelian, P.S. , 2004. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat. Rev. Drug Discov.. 3, 555–564. [DOI] [PubMed] [Google Scholar]
- Parsons, S.J. , Parsons, J.T. , 2004. Src family kinases, key regulators of signal transduction. Oncogene. 23, 7906–7909. [DOI] [PubMed] [Google Scholar]
- Rane, S.G. , Reddy, E.P. , 2000. Janus kinases: components of multiple signaling pathways. Oncogene. 19, 5662–5679. [DOI] [PubMed] [Google Scholar]
- Rane, S.G. , Reddy, E.P. , 2002. JAKs, STATs and Src kinases in hematopoiesis. Oncogene. 21, 3334–3358. [DOI] [PubMed] [Google Scholar]
- Reddy, E.P. , Korapati, A. , Chaturvedi, P. , Rane, S. , 2000. IL-3 signaling and the role of Src kinases, JAKs and STATs: a covert liaison unveiled. Oncogene. 19, 2532–2547. [DOI] [PubMed] [Google Scholar]
- Samanta, A.K. , Chakraborty, S.N. , Wang, Y. , Kantarjian, H. , Sun, X. , Hood, J. , Perrotti, D. , Arlinghaus, R.B. , 2009. Jak2 inhibition deactivates Lyn kinase through the SET-PP2A-SHP1 pathway, causing apoptosis in drug-resistant cells from chronic myelogenous leukemia patients. Oncogene. 28, 1669–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, B. , Saigal, B. , Parikh, N. , Gallick, G. , Johnson, F.M. , 2009. Sustained Src inhibition results in signal transducer and activator of transcription 3 (STAT3) activation and cancer cell survival via altered Janus-activated kinase-STAT3 binding. Cancer Res.. 69, 1958–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S. , Liu, Q. , Zhang, Y. , Liu, K. , Yu, P. , Luan, J. , Duan, H. , Lu, Z. , Wang, F. , Wu, E. , Yagasaki, K. , Zhang, G. , 2009. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol. Cancer. 8, 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, Y.L. , Xu, L. , Liang, Y. , Xu, X.H. , Zhao, X.Y. , 2009. Berbamine exhibits potent antitumor effects on imatinib-resistant CML cells in vitro and in vivo. Acta Pharmacol. Sin.. 30, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, J. , Ma, T. , Gu, Y. , Zhang, X. , Qiu, X. , Zhang, L. , Xu, R. , Yu, Y. , 2009. Berbamine derivatives: a novel class of compounds for anti-leukemia activity. Eur. J. Med. Chem.. 44, 3293–3298. [DOI] [PubMed] [Google Scholar]
- Yu, H. , Jove, R. , 2004. The STATs of cancer – new molecular targets come of age. Nat. Rev. Cancer. 4, 97–105. [DOI] [PubMed] [Google Scholar]
- Yu, H. , Pardoll, D. , Jove, R. , 2009. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer. 9, 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Turkson, J. , Carter-Su, C. , Smithgall, T. , Levitzki, A. , Kraker, A. , Krolewski, J.J. , Medveczky, P. , Jove, R. , 2000. Activation of Stat3 in v-Src-transformed fibroblasts requires cooperation of Jak1 kinase activity. J. Biol. Chem.. 275, 24935–24944. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Guttikonda, S. , Roberts, L. , Uziel, T. , Semizarov, D. , Elmore, S.W. , Leverson, J.D. , Lam, L.T. , 2011. Mcl-1 is critical for survival in a subgroup of non-small-cell lung cancer cell lines. Oncogene. 30, 1963–1968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following are the Supplementary data related to this article:
Supplementary Figure 1 (A) Levels of constitutively activated Jak2 in A2058 melanoma and NHDF cells. Whole‐cell lysates were resolved by SDS‐PAGE and immunoblotted with specific antibodies to p‐Jak2 (Tyr1007/1008) and Jak2. (B) Effects of BBMD3 on viabilities of human erythroleukemia (HEL) cells. HEL cell line, which is positive for homozygous Jak2V617F mutation, was purchase from ATCC. Cells were cultured in RPMI‐1640 media containing 10% fetal bovine serum (FBS). Cell viability was determined using MTS assays as described in Section 2. HEL cells were treated with BBMD3 in a dose‐dependent manner for 48h. Each experiment was performed in quadruplicate. Data are mean. (C) BBMD3 inhibits Jak2/Stat5 signaling in HEL cells. HEL cells were treated with BBMD3 in a dose‐dependent manner for 4h. Whole‐cell lysates were resolved by SDS‐PAGE and immunoblotted with specific antibodies to p‐Jak2 (Tyr1007/1008), Jak2, p‐Stat5 (Tyr694) and Stat5.
Supplementary Figure 2 Human A2058 melanoma cells were treated with 10μM of BBMD3 for 30min. Cell viability was determined using MTS assays. Each experiment was performed in quadruplicate. Data are meanSD.
Supplementary Figure 3 (A) Kinase assays in vitro. The kinase assays were performed with recombinant c‐Met, AKT, EGFR, ABL, FGFR1, PDGFRα, BRAF, PI3K, IKKβ and VEGFR2 proteins using the HotSpot protocol (Reaction Biology Corp., Malvern, PA). Briefly, proteins, freshly prepared substrates and 33P‐ATP (specific activity 0.01μCi/μl final) were mixed in reaction buffer (20mM HEPES pH 7.5, 10mM MgCl2, 1mM EGTA, 0.02% Brij35, 0.02mg/ml BSA, 0.1mM Na3VO4, 2mM DTT, 1% DMSO) in the presence of DMSO as control or BBMD3. The mixtures were reacted for 120min at room temperature. Samples were transferred onto P81 ion exchange paper and filters were extensively washed with 0.75% phosphoric acid. The radioactivities were monitored. B. Src kinase assay in vitro. The kinase assays were performed using recombinant Src protein for 20min in a final volume of 25μl (SignalChem, Richmond, Canada). Briefly, approximately 20nM of Src protein (final concentration was mixed with 1μg of Src peptide substrate in the presence of BBMD3 or 10% DMSO in kinase assay buffer. The assay was initiated with the addition of 5μl of 33P‐ATP (250μM of stock solution, 0.8μCi). Then, the reaction mixture was incubated at ambient temperature for 20min. Ten μl of reaction mixture was spotted on Mutiscreen phosphocellulose P81 plate. The radioactivities on the P81 plate were monitored using a scintillation counter.