Dynamic Education of Macrophages in Different Areas of Human Tumors (original) (raw)
Abstract
Human tumor tissues can often be anatomically classified into areas of cancer nest, invading edge, and peritumoral stroma, each with distinct compositions and functional properties. Macrophages (Mφ) constitute a major component of the leukocyte infiltrate in tumors. These cells are derived from circulating monocytes, and in response to environmental signals, they exhibit distinct phenotypes with diverse functions. Soluble factors derived from cancer cells can alter the normal developmental process of Mφ that is intended to trigger transient early activation of monocytes in the peritumoral region, which in turn induces formation of suppressive Mφ in cancer nests. The activated monocytes in the peritumoral region attenuated the T-cell response by expressing B7-H1, and were superior to the suppressive tumor Mφ in inducing Th17 expansion, and thus repurpose the inflammatory response away from anti-tumor immunity (the sword) and towards tissue remodeling and proangiogenic pathways (a plowshare). In contrast, the suppressive Mφ can induce the production of Tregs in cancer nest. Accordingly, angiogenesis was most active at the invading edge, which was situated close to the peritumoral stroma with activated Mφ and the density of these activated monocytes is selectively associated with vascular invasion and metastasis in patients with hepatocellular carcinoma. These data reveal an intriguing mechanism in which human Th17 cells are generated and regulated by a fine-tuned collaborative action between different types of immune cells in distinct tumor microenvironments. These results give important new insights into the distinct role of macrophages in human tumor progression which would be helpful for the rational design of novel immune-based anticancer therapies.
Keywords: Macrophages (Mφ), Microenvironment, Hepatocellular carcinoma (HCC), Inflammation, Immune-editing
Introduction
In the early twentieth century, tumorigenesis became recognized as a multistep process during which cancer cells accumulate multiple and consecutive genetic alterations [1, 2]. However, this cancer-cell-centered model largely ignored the heterogeneous and structurally complex nature of the tissue environment. According to Paget’s Seed and Soil hypothesis, tumor progression was the product of an evolving crosstalk between different cell types within the tumor and its surrounding supporting tissue, or tumor stroma [1]. In a manner similar to the development and function of normal organs, which occurs through reciprocal communication between different cell types, the interaction between cancer cells and their microenvironment can largely determine the phenotype of the tumor [3]. Thus, increasing efforts have been made to identify molecules/pathways involved in the interplay between tumor cells and their stroma, in hopes of providing new cues for cancer therapies.
Although the amount of stroma and its composition vary considerably from tumor to tumor, it’s well recognized that tumor stroma includes not only a specific type of extracellular matrix, but also large amount of immune and inflammatory cells [1, 4]. Instead of combating cancer cells, the immune cells in tumor tissues have many tumor-promoting effects. It aids the proliferation and survival of malignant cells, promotes angiogenesis and metastasis, subverts adaptive immune responses, and alters responses to hormones and chemotherapeutic agents [5]. Experimental and clinical evidences have shown that many of the tumor infiltrating inflammatory components can be educated by local environments and rerouted from tumor surveillance to a tumor-promoting direction [6–8]. One of these components we will focus in this review is the tumor-associated monocytes/macrophages (TAM).
Macrophages (Mφ) are essential components of host defense and act as both antigen presenting cells (APC) and effector cells. TAM markedly outnumber other APC in tissues [9, 10]. Mφ are derived almost entirely from circulating monocytes, and, in response to environmental signals, they acquire special phenotypic characteristics with diverse functions [9, 11, 12]. In contrast to mouse models, human solid tumors can often be anatomically classified into areas of cancer nest, invading edge, and peritumoral stroma, each with distinct compositions and functional properties [13, 14]. Accordingly, TAM exhibit different phenotypes and functions at these distinct tumor sites (Fig. 1). Mφ in normal or inflamed tissues exhibit spontaneous antitumor activity, whereas TAM could be oriented towards promoting tumor growth, remodeling tissues, promoting angiogenesis and suppressing adaptive immunity [9, 15–17]. Deciphering the distinct role of Mφ in different human tumor areas would be helpful for the rational design of effective immune-based anticancer therapies. Here we summarized recent knowledge of the infiltration, phenotypes and functions of TAM as well as their underlying regulatory mechanisms, paying particular attention to their distinct micro-location in tumors. Since Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies in Asia with poor prognosis and limited therapeutic options [18–20], data discussed in this review are mainly focused on HCC, unless otherwise specified.
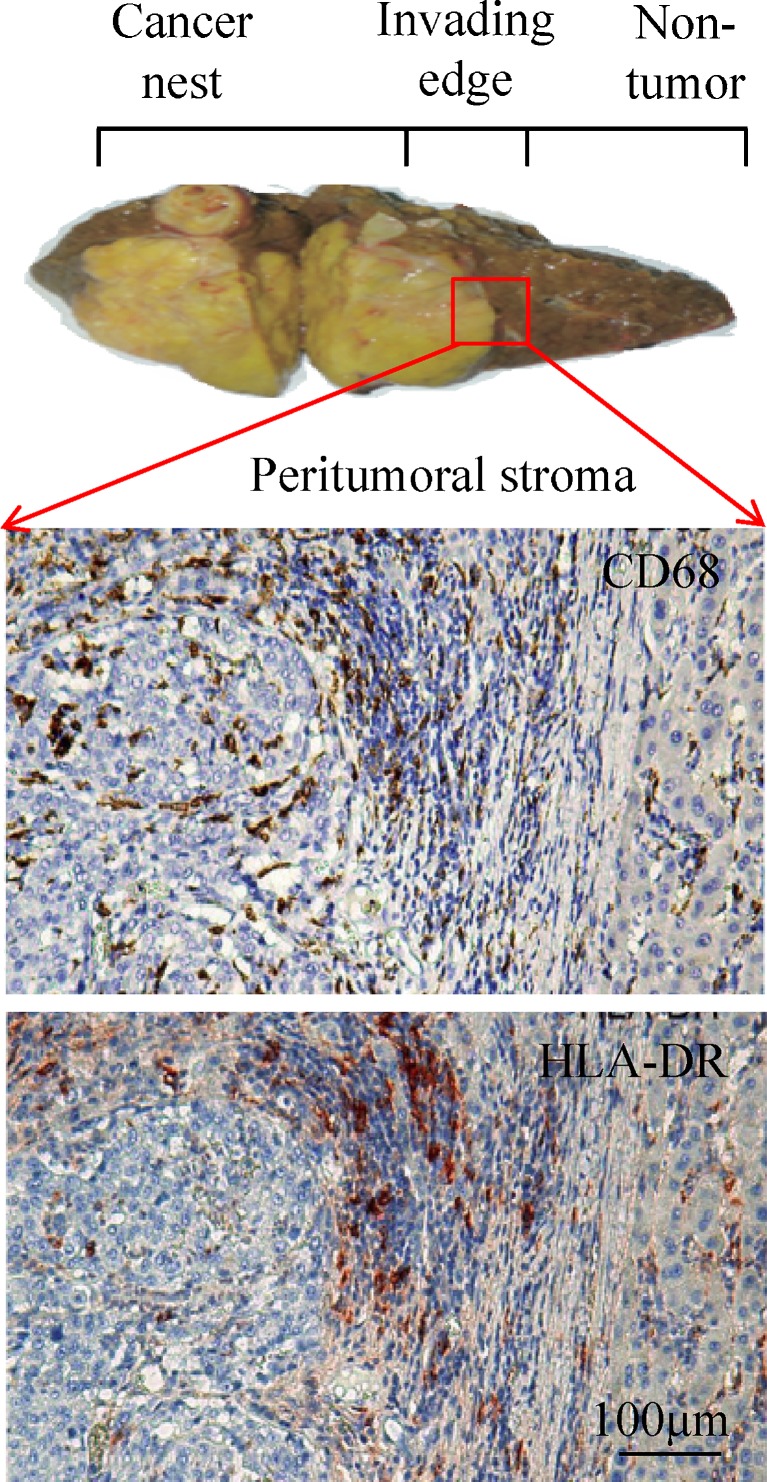
Fig. 1.
Human HCC tumor tissue can be anatomically classified into areas of cancer nest, invading edge and peritumoral stroma. The enlarged micrographs show adjacent sections of paraffin-embedded HCC samples stained with anti-CD68 or anti-HLA-DR. Most CD68-positive cells show high expression of HLA-DR in the peritumoral stroma region, but not in the cancer nest
Infiltration, Phenotype and Dynamic Education of TAM
Mφ originates from CD34+ bone marrow progenitors which enter circulation upon sensing signals from infection/inflammation and differentiate into monocytes [21, 22]. Monocytes migrated into the tissue throughout the life span of tumors, and a number of tumor-derived chemoattractants are thought to ensure this ongoing recruitment, including colony-stimulating factor-1 (CSF-1 also known as M-CSF), the CC chemokines, CCL2, CCL3, CCL4, CCL5, and CCL8, and vascular endothelial growth factor (VEGF) [9]. After extravasating into tumor tissues, monocytes are educated by local environments and differentiate into “resident” Mφ with specific phenotypes [10, 23].
Mφ isolated from established metastatic mouse and human tumors generally have a relatively immature phenotype, as detailed in many other excellent reviews [9, 11, 16]. However, with the hallmark of remarkable plasticity and diversity, the phenotype of Mφ varies from tumor to tumor or within different areas of the same tumor [9, 11]. In human HCC and lung cancer tissues, most Mφ (CD68-positive cells) are smaller with high expression of HLA-DR in the peritumoral stromal region, suggesting an activated state, whereas they exhibit a HLA-DRlowIL10high phenotype in the cancer nest, which is in accordance with a general view of tolerance/suppressive phenotype of TAM [24].
The stark contrast between Mφ phenotypes in different tumor area suggests that these cells react to and might be regulated by distinct set/combination of signals from distinct tissue microenvironments. In support, cytokines such as IL-4, IL-13, IL-10 and TGF-β were found to polarize Mφ to an M2-like phenotype, which supported tumor metastasis; whereas local over-expression of IFN-γ and IL-12 could induce Mφ to adopt an activated phenotype [11, 25, 26]. We have recently shown that soluble tumor-derived factors could promote the development of immunosuppressive Mφ in cancer nests by triggering a transient early activation of monocytes in peritumoral stromas, thus bridging distinct Mφ phenotypes in different tumor areas with a single and continuous mechanism [24].
According to this model, two opposing functional stages exist in the TAM life cycle: monocytes are rapidly activated during a narrow time window after their first exposure to tumor derived soluble factors, and afterward the same cells become exhausted and their production of cytokines is extinguished, with the exception of IL-10. Because TAM are derived from circulating monocytes, this dynamic regulation of monocyte activity may represent a novel escape mechanism by which tumors co-opt the normal development of Mφ to educate the recruited monocytes to adopt specific phenotypes in different niches in a lesion. More precisely, this means that during their first exposure to the tumor microenvironment, the newly recruited monocytes may be transiently activated while they are approaching the stroma surrounding the tumor, with the aim of minimizing their potential to damage tumor cells. Thereafter, when these Mφ are in close proximity to the tumor cells, they become exhausted and thus fail to mount an effective antitumor immune response. During this dynamic regulatory process, hyaluronan (HA) fragments were found to constitute a common factor produced by a variety of human tumors to induce such preactivation-exhaustion of Mφ [24]. Accordingly, HA concentrations are usually higher in malignant tumors than in corresponding benign or normal tissues and upregulation of HA synthase II in tumor cells is correlated with their ability to cause Mφ dysfunction [24].
Given the paramount importance of the tumor microenvironment in regulating Mφ phenotype, substantial efforts have been made to identify the key regulators and signaling pathways involved in this process. A range of mediators, including HA, transforming growth factor-β1, prostaglandin E2 and IL-10 have been shown to regulate the functional activities of Mφ, but the precise underlying intracellular signaling mechanisms are still largely unknown [7, 9, 11]. It was reported that components such as IL-4Rα, transcription factor STAT6 and C/EBPβ [11, 27] might be involved in regulating TAM differentiation by endowing them with distinct cytokine profiles. Ongoing investigations were clearly required to further dissect the molecular mechanisms that determined TAM phenotypes in distinct tumor areas.
Distinct Roles of TAM in Different Tumor Areas
TAM influence nearly all steps of carcinogenesis and tumor progression. These include: contribution to genetic alterations and instability; promotion of angiogenesis and lymphangiogenesis; regulation of senescence; interaction with and remodeling of the extracellular matrix; suppression of adaptive immunity; and promotion of invasion and metastasis [9, 11, 28, 29]. Such diverse impacts of TAM may reflect the distinct nature of different Mφ subsets under various tissue conditions. For example, a subset of monocytes that express the angiopoietin receptor Tie-2 was identified as important inducers for angiogenesis in both spontaneous and orthotopic tumors [5, 30]. CCL-2 produced by both the tumor and the tissue stroma at target-sites can selectively recruit CCR2-expressing Mφ to promote the extravasation, seeding and persistent growth of breast cancer cells at metastatic sites [31]. On another hand, Mφ could be dynamically educated by distinct microenvironments, as illustrated above, to adopt specific phenotypes and perform diverse functions in different tumor areas. We will discuss TAM functions below with particular attention paid to their micro-locations.
Immunosuppressive Mφ in Cancer Nest
Several studies have shown that increased Treg cells in tumor tissue was associated with poor survival of patients with HCC, but the source of these cells remained unclear [32–34]. We found that FoxP3+ Treg cells are preferentially gathered in the HCC cancer nests, where their prevalence was associated with the density of Mφ. Depletion of tissue Mφ attenuated the increase of liver FoxP3+ Treg cell frequency, while Mφ exposed to tumor culture supernatants from hepatoma-derived cell lines increased Treg frequency in vitro. Moreover, this increase was partially blocked by anti-IL-10 antibody [35]. Therefore, TAM may trigger a rise of the intratumoral FoxP3+ Treg population in an IL-10-dependent manner, which in turn promote HCC progression.
Besides immunosuppressive cell populations, molecules with inhibitory properties were also accumulated in tumor tissues [7, 36]. IDO is a rate-limiting enzyme for tryptophan catabolism. In both humans and mice, IDO inhibits Ag-specific T cell proliferation in vitro and suppresses T cell responses to fetal alloantigens during pregnancy [37]. Expression of IDO is often induced or maintained by many inflammatory cytokines, of which IFN-γ is the most potent [37]. In addition to being expressed in APC, most human cancers also express high levels of IDO protein, which correlates with poor prognosis in some cases [36]. In contrast, low or rare IDO expression is observed in most mouse and human tumor cell lines, possibly due to the lack of a complete cancer microenvironment in cell lines in vitro [38]. We have recently observed that IDO is selectively expressed at high levels in Mφ in situ in several types of human solid tumors, but not in tumor-educated suppressive Mφ generated in vitro. The expression pattern of IDO coincided with the infiltration of CD69+ T cells in situ tumor. Accordingly, the CD69+ T cells isolated from HCC tissues induced significant levels of IDO in autologous monocytes in vitro by releasing IFN-γ. IL-12 derived from tumor Mφ was required for early T cell activation and subsequent IDO expression [39]. These data indicate that, upon encountering autologous T cells, tumor Mφ produce IL-12 to activate T cells, which in turn lead to IDO expression in Mφ, and in that way creates favorable conditions for tumor growth. Therefore, activated CD69+ T cell-mediated Mφ IDO expression may represent a novel mechanism by which the adaptive activation is linked to immune tolerance in the tumor milieu. Such an active induction of immune-tolerance should be considered for the rational design of effective immune-based anti-cancer therapies.
Activated Monocytes in Peritumoral Areas
In contrast to intratumoral areas which usually contain abundant immunosuppressive molecules and cells, the peritumoral stroma areas in most tumors comprise a significant amount of leukocyte infiltrate which was long assumed to represent the host response to tumors [5, 7, 40]. We found that tumors can alter the normal developmental process of Mφ to trigger transient activation of monocytes in the peritumoral stroma [24]. Notably, the density of these activated monocytes is selectively associated with vascular invasion and metastasis in HCC patients [41]. To unveil the tumor-promoting effects of these activated monocytes in HCC, we investigated their impact on anti-tumor immunity and angiogenesis/tissue remodeling [40, 42–44].
Activated Monocytes Suppress Tumor-Specific T Cell Immunity Through B7-H1
B7-H1 is a cell-surface glycoprotein belonging to the B7 family of co-signaling molecules with a profound regulatory effect on T cell responses [45, 46]. Studies in mouse models have revealed that expression of B7-H1 helped dormant tumor cells to evade cytotoxic T cell responses [7]. Although expression of B7-H1 protein is often found on activated cells and various human carcinomas, the regulatory mechanisms of human B7-H1 remain to be defined. We found that a fraction of monocytes/Mφ in peritumoral stroma, but not in cancer nests, expresses surface B7-H1 molecules. These cells strongly express B7-H1 proteins with kinetics similar to their activation status, and significant correlations were found between the levels of B7-H1+ and HLA-DRhigh on tumor infiltrating monocytes. Autocrine tumor necrosis factor and IL-10 released from activated monocytes stimulated their expression of B7-H1, and these B7-H1+ monocytes effectively suppressed tumor-specific T cell immunity and contributed to the growth of human tumors in vivo; the effect could be reversed by blocking B7-H1 on those monocytes. Moreover, B7-H1 expression on tumor-infiltrating monocytes was increased with HCC progression, and the intensity of the protein was associated with high mortality and reduced survival of HCC patients [43]. Thus, expression of B7-H1 on activated monocytes/Mφ may represent a novel mechanism that links the proinflammatory response to immune tolerance in the tumor milieu.
Activated Monocytes Promote Angiogenesis Through the Regulation of IL-17-Producing Cells
Angiogenesis is essential for the growth and spread of malignant tumors, and the magnitude and quality of this process is ultimately determined by the sum of pro- and anti-angiogenic signals or, more specifically, their unique activities on multiple cell types [47, 48]. Considerable evidence indicates an important role of TAM in regulating angiogenesis. These cells accumulate in the hypoxic/necrotic areas in tumor tissues, where they release potent proangiogenic cytokines and growth factors [9, 49, 50]. For human HCC, angiogenesis was most active at the invading edge, which was situated close to the peritumoral stroma with activated monocytes, and the density of these activated monocytes is selectively associated with vascular invasion and metastasis [41]. We found that there is a fine-tuned collaborative action between cancer cells and different types of immune cells in distinct tumor microenvironments, which reroutes the inflammatory response into a tumor-promoting direction [40, 42, 44].
Th17 cell was a recently discovered novel IL-17-producing CD4+ T helper cell subset with potent pro-inflammatory properties [51, 52]. These cells were highly enriched in HCC and their levels were positively correlated with microvessel density in tissues and poor survival in HCC patients [53]. In contrast to the classical Th17 cells that hardly express IFN-γ, almost half of the IL-17-producing CD4+ T cells isolated from HCC tissues were able to simultaneously produce IFN-γ, suggesting that the tumor microenvironment can profoundly determine the phenotype of such cells [40].
These IL-17-producing cells were enriched predominantly in peritumoral stroma, and their levels were well correlated with the density of monocytes/Mφ in the same area. Most of these CD68+ cells exhibited an activated phenotype, and, accordingly, tumor-activated monocytes effectively promoted in vitro expansion of Th17 cells with phenotypic features similar to those isolated from HCC. Proliferation of Th17 cells from circulating memory T cells was triggered by a set of key proinflammatory cytokines (IL-1β, IL-6, or IL-23) secreted by tumor-activated monocytes, and inhibition of monocytes/Mφ-mediated inflammation in liver markedly reduced the level of tumor infiltrating Th17 cells and tumor growth in vivo. Therefore, activation of monocytes in tumors may represent a novel route to promote Th17 expansion in human cancer. This concept is supported by studies showing that activated APC are involved in the differentiation and expansion of Th17 cells and thereby also in Th17-mediated chronic inflammation [40]. It should be noted that, in addition to the local expansion of Th17 cells, migration from blood is also a potential source for the increased Th17 cells in tumors. In accordance with this assumption, CCR6 was found expressed in the majority of Th17 cells and that CCL20, the ligand for CCR6, was significantly increased in HCC tissues [41, 53, 54].
Besides Th17, IL-17-producing CD8+ T cells (Tc17 cells) constitute another remarkable portion of IL-17-producing cells in human HCC [55, 56]. Just like Th17, Tc17 cells were also enriched predominantly in the invading tumor edge and regulated by tumor activated monocytes in the same areas by mechanisms similar to that of Th17 [42]. These data reveal that in human HCC, IL-17 is generated by a fine-tuned collaborative action between different types of immune cells in distinct tumor microenvironments.
However, caution should be taken when interpreting the role of IL-17 under distinct experimental backgrounds. Some studies have shown that IL-17 impairs immune surveillance and promotes de novo carcinogenesis and neovascularization, while others have reported the anti-tumor activity of IL-17 or Th17-polarized cells [11, 57–59]. In human HCC, activated monocytes in peritumoral areas induced the expansion of IL-17-producing cells. These IL-17+ cells were capable of stimulating epithelial cells to produce CXC chemokines that induced neutrophil trafficking to tumors [44]. Neutrophils are the cells known to have short lifespan, but tumor derived soluble factors such as HA could delay their apoptosis by activating neutrophils through the TLR4/PI3K/Akt signaling pathways [60, 61]. These newly recruited and activated neutrophils in the peritumoral stroma are the major sources of MMP-9 in situ tumors [44]. MMP-9 has a distinct role in tumor angiogenesis, mainly regulating the bioavailability of vascular endothelial growth factor (VEGF), the most potent inducer of tumor angiogenesis and a major therapeutic target [62–64]. Consistent with these results, high neutrophil infiltration was correlated with increased VEGF expression and sinusoidal vasculature in HCC tissues, and the deletion of neutrophils markedly inhibited tumor growth and angiogenesis in mice. In addition, exposure to neutrophils upregulated the expression of several neutrophil chemoattractants in tumor cells, which could lead to a positive-feedback loop to recruit more neutrophils into the tumor microenvironments [44]. Since both IL-17 and neutrophil infiltration are positively correlated with angiogenesis progression in peritumoral areas of HCC patients, the above data provide an interesting mechanistic link between these two clinical associations and indicate new therapeutic targets for treating human HCC.
IL-17 Activates Monocytes to Express B7-H1
TNF-α and IL-10 released from tumor-activated monocytes up-regulate B7-H1 on the surface of these cells to inhibit tumor-specific T-cell immunity, whereas IL-1β, IL-6, and IL-23 released from the same cells promote Th17-mediated inflammation in peritumoral stroma [40, 43]. In that way, activated monocytes repurpose the inflammatory response away from antitumor immunity (the sword) and toward tissue remodeling and proangiogenic pathways (a plowshare). Interestingly, our recent study indicate a potential link between IL-17 and the expression of B7-H1 on monocytes [65]. IL-17-producing cells positively correlated with B7-H1-expressing monocytes in the peritumoral areas of HCC, and IL-17 stimulated monocytes to express B7-H1 in a dose-dependent manner. Although culture supernatants derived from hepatoma cells also induced B7-H1 expression on monocytes, IL-17 additionally increased hepatoma-mediated B7-H1 expression [65]. These data reveal a delicate and inter-twisted collaborative action between different stromal cells to counteract T-cell responses in tumors.
Concluding Remarks
TAM play an important role in various key steps of tumor progression. With the recognized hallmarks of plasticity and diversity, Mφ carry out specific functions in support of tumor cell requirements through responding to different environmental cues. Recent studies have made great progress in defining the surface phenotype, activating signals and molecular pathways associated with specific Mφ located in distinct tumor areas. As discussed in this review, despite the generalized immunosuppressive status in cancer patients, soluble factors derived from cancer cells can dynamically regulate the developmental process of Mφ that is intended to trigger transient early activation of monocytes in the peritumoral region, which in turn induces formation of suppressive Mφ in cancer nests. Activated monocytes in the peritumoral region attenuated T-cell response and fostered angiogenesis, whereas suppressive Mφ in the cancer nests induced immune tolerance.
Such sequential preactivation and exhaustion of Mφ is reminiscent of a well-known phenomenon that has been described as LPS tolerance in APC. As pointed out in several previous reviews [7, 46, 52], tumors can mimic some of the existing pathways of the immune system to propagate conditions that favor their progression. Although “black and white” phenotypes are commonly used to define tumor-infiltrating Mφ, it is clear that interaction between TAM and their neighbors (including tumor and other immune cells) in different micro-location of tissues would be best represented by 3-D action with a multitude of colors. In this regard, we need rigorous methods and models to define the complicated in situ and dynamic network. A better understanding of these contexts would be helpful to “restore” the spontaneous anti-tumor activity of normal macrophages.
Acknowledgments
This work was supported by project grants from the National Natural Science Foundation of China (91029737), and the “973” Program (2010CB529904 and 2011CB811305).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Zijl F, Mair M, Csiszar A, et al. Hepatic tumor-stroma crosstalk guides epithelial to mesenchymal transition at the tumor edge. Oncogene. 2009;28:4022–4033. doi: 10.1038/onc.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 8.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–835. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 10.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 11.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2011;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 12.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 13.Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nat Rev Immunol. 2004;4:641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 15.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2011;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 17.Gao B, Jeong WI, Tian Z. Liver: an organ with predominant innate immunity. Hepatology. 2008;47:729–736. doi: 10.1002/hep.22034. [DOI] [PubMed] [Google Scholar]
- 18.Liu H, Pan Z, Li A, et al. Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol. 2008;5:373–378. doi: 10.1038/cmi.2008.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Ding T, He Q et al (2012) In situ molecular signature predict early recurrence in Hepatitis B virus-related hepatocellular carcinoma. J Hepatol, in press [DOI] [PubMed]
- 21.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor PR, Martinez-Pomares L, Stacey M, et al. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. doi: 10.1146/annurev.immunol.23.021704.115816. [DOI] [PubMed] [Google Scholar]
- 23.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 24.Kuang DM, Wu Y, Chen N, et al. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110:587–595. doi: 10.1182/blood-2007-01-068031. [DOI] [PubMed] [Google Scholar]
- 25.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 26.Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 27.He M, Xu Z, Ding T, Kuang DM, Zheng L. MicroRNA-155 regulates inflammatory cytokine production in tumor-associated macrophages via targeting C/EBPbeta. Cell Mol Immunol. 2009;6:343–352. doi: 10.1038/cmi.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng J, Huo DH, Kuang DM, et al. Human macrophages promote the motility and invasiveness of osteopontin-knockdown tumor cells. Cancer Res. 2007;67:5141–5147. doi: 10.1158/0008-5472.CAN-06-4763. [DOI] [PubMed] [Google Scholar]
- 29.Yang M, Chen J, Su F, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis CE, Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 31.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. doi: 10.1053/j.gastro.2007.03.102. [DOI] [PubMed] [Google Scholar]
- 33.Gao Q, Qiu SJ, Fan J, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 34.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 35.Zhou J, Ding T, Pan W, et al. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125:1640–1648. doi: 10.1002/ijc.24556. [DOI] [PubMed] [Google Scholar]
- 36.Lob S, Konigsrainer A, Rammensee HG, Opelz G, Terness P. Inhibitors of indoleamine-2,3-dioxygenase for cancer therapy: can we see the wood for the trees? Nat Rev Cancer. 2009;9:445–452. doi: 10.1038/nrc2639. [DOI] [PubMed] [Google Scholar]
- 37.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 38.Godin-Ethier J, Pelletier S, Hanafi LA, et al. Human activated T lymphocytes modulate IDO expression in tumors through Th1/Th2 balance. J Immunol. 2009;183:7752–7760. doi: 10.4049/jimmunol.0901004. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Q, Kuang DM, Wu Y, et al. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J Immunol. 2012;188:1117–1124. doi: 10.4049/jimmunol.1100164. [DOI] [PubMed] [Google Scholar]
- 40.Kuang DM, Peng C, Zhao Q, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010;51:154–164. doi: 10.1002/hep.23291. [DOI] [PubMed] [Google Scholar]
- 41.Ding T, Xu J, Wang F, et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40:381–389. doi: 10.1016/j.humpath.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Kuang DM, Peng C, Zhao Q, et al. Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J Immunol. 2010;185:1544–1549. doi: 10.4049/jimmunol.0904094. [DOI] [PubMed] [Google Scholar]
- 43.Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuang DM, Zhao Q, Wu Y, et al. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948–955. doi: 10.1016/j.jhep.2010.08.041. [DOI] [PubMed] [Google Scholar]
- 45.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 46.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 47.Hou J, Tian L, Wei Y. Cancer immunotherapy of targeting angiogenesis. Cell Mol Immunol. 2004;1:161–166. [PubMed] [Google Scholar]
- 48.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 49.Lewis CE, Hughes R. Inflammation and breast cancer. Microenvironmental factors regulating macrophage function in breast tumours: hypoxia and angiopoietin-2. Breast Cancer Res. 2007;9:209. doi: 10.1186/bcr1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu S, Cao X. Interleukin-17 and its expanding biological functions. Cell Mol Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 54.Su X, Ye J, Hsueh EC, et al. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 55.Yen HR, Harris TJ, Wada S, et al. Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol. 2009;183:7161–7168. doi: 10.4049/jimmunol.0900368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li J, Huang ZF, Xiong G, et al. Distribution, characterization, and induction of CD8 regulatory T cells and IL-17-producing CD8 T cells in nasopharyngeal carcinoma. J Transl Med. 2011;9:189. doi: 10.1186/1479-5876-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang L, Yi T, Kortylewski M, et al. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kryczek I, Wei S, Zou L, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 59.Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 61.Wu Y, Zhao Q, Peng C, et al. Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol. 2011;225:438–447. doi: 10.1002/path.2947. [DOI] [PubMed] [Google Scholar]
- 62.Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147:992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 63.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2011;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi GM, Ke AW, Zhou J, et al. CD151 modulates expression of matrix metalloproteinase 9 and promotes neoangiogenesis and progression of hepatocellular carcinoma. Hepatology. 2010;52:183–196. doi: 10.1002/hep.23661. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Q, Xiao X, Wu Y, et al. Interleukin-17-educated monocytes suppress cytotoxic T-cell function through B7-H1 in hepatocellular carcinoma patients. Eur J Immunol. 2011;41:2314–2322. doi: 10.1002/eji.201041282. [DOI] [PubMed] [Google Scholar]