Double-stranded Endonuclease Activity in Bacillus halodurans Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)-associated Cas2 Protein (original) (raw)
Background: Cas2 is universally conserved and essential for new CRISPR spacer acquisition.
Results: Bha_Cas2 uses a single metal ion to cleave dsDNA and is likely activated by a pH-dependent conformational change. A method to classify Cas2 into ssRNase and dsDNase is proposed.
Conclusion: B. halodurans and T. thermophilus Cas2 are metal-dependent endonucleases.
Significance: dsDNase activity is consistent with the direct involvement of Cas2 in new spacer acquisition.
Keywords: DNase, Isothermal Titration Calorimetry, Mutagenesis, RNA Interference (RNAi), X-ray Crystallography, CRISPR, CRISPR Adaptation, CRISPR-associated Protein, Cas2, Endonuclease
Abstract
The CRISPR (clustered regularly interspaced short palindromic repeats) system is a prokaryotic RNA-based adaptive immune system against extrachromosomal genetic elements. Cas2 is a universally conserved core CRISPR-associated protein required for the acquisition of new spacers for CRISPR adaptation. It was previously characterized as an endoribonuclease with preference for single-stranded (ss)RNA. Here, we show using crystallography, mutagenesis, and isothermal titration calorimetry that the Bacillus halodurans Cas2 (Bha_Cas2) from the subtype I-C/Dvulg CRISPR instead possesses metal-dependent endonuclease activity against double-stranded (ds)DNA. This activity is consistent with its putative function in producing new spacers for insertion into the 5′-end of the CRISPR locus. Mutagenesis and isothermal titration calorimetry studies revealed that a single divalent metal ion (Mg2+ or Mn2+), coordinated by a symmetric Asp pair in the Bha_Cas2 dimer, is involved in the catalysis. We envision that a pH-dependent conformational change switches Cas2 into a metal-binding competent conformation for catalysis. We further propose that the distinct substrate preferences among Cas2 proteins may be determined by the sequence and structure in the β1–α1 loop.
Introduction
CRISPR (clusters of regularly interspaced short palindromic repeats) is a recently discovered RNA-based defense mechanism against invading foreign genetic elements (1–10). Together with the Cas (CRISPR-associated) proteins, CRISPR-Cas systems have been identified in 83% of archaeal genomes and 45% of bacterial genomes thus far sequenced. These organisms include many important human pathogens such as Campylobacter jejuni, Clostridium botulinum, Escherichia coli, Listeria monocytogenes, Mycobacterium tuberculosis, and Yersinia pestis (1–5, 7, 11, 12, 14). The CRISPR locus is composed of conserved repeat sequences (21–48 base pairs (bp)2) interspaced by variable spacer sequences (26–72 bp) and is transcribed into precursor CRISPR RNAs (4). Conserved CRISPR repeat sequences frequently encode a 6–7-bp GC-rich stem loop structure (15, 16), which bears recognition signals for further endoribonucleolytic processing into the mature CRISPR RNA by Cse3, Csy4, and Cas5d (11, 17–21, 48), whereas non-palindromic repeat sequences are processed by Cas6 (18, 21). The spacer sequences between CRISPR repeats appear to be derived from extrachromasomal DNA elements (bacteriophages and plasmids) through an active acquisition mechanism (22–24). These sequences in the processed CRISPR RNAs are actively used as “barcodes” to target foreign genetic elements for degradation (6, 11, 18, 25–30).
cas (CRISPR-associated) genes encode a set of conserved proteins found in the vicinity of the CRISPR loci (4, 6, 31). These proteins can be classified into a set of core Cas proteins (Cas1–6) as well as subtype-specific genes (32–34). Cas proteins support the completion of three molecular events: the acquisition of new spacers derived from the extrachromasomal elements into the CRISPR loci, processing of precursor CRISPR RNAs into the mature form, and mediating the degradation of the complementary nucleic acids, in most cases DNA, in a CRISPR RNA-specific fashion (2–4, 7).
cas1 and cas2 are two core cas genes universally present in all CRISPR-Cas subtypes, required in the new spacer acquisition step (7, 35). Cas1 proteins from Pseudomonas aeruginosa and E. coli were characterized as metal-dependent endonuclease (26, 36). A recent study further classified the cas2 genes among different subtypes into three different clades based on phylogenetic analysis (34). The crystal structures of Cas2 from Sulfolobus solfataricus (Sso_Cas2) and Desulfovibrio vulgaris (Dvu_Cas2) revealed that Cas2 contains a ferredoxin domain and assemblies into a symmetric dimer (27, 37) A conserved N-terminal aspartate residue in the S. solfataricus Cas2 was hypothesized to coordinate the catalytic divalent metal ion(s) in the Cas2 dimer. Th_e S. solfataricus_ Cas2 was further characterized as a metal-dependent single-strand (ss) endoribonuclease with preference for Uracil-rich ssRNA (27); however, this activity was not observed in the D. vulgaris Cas2 (37). Furthermore, the ssRNase activity of Cas2, combined with its essential function in spacer acquisition, may imply that new spacers could be derived from transcribed RNAs through a reverse transcription mechanism (4, 33, 38, 39). This is, however, inconsistent with the literature showing that new spacers can be derived from untranscribed regions.
In this study, we report the structural and biochemical characterization of the B. halodurans Cas2 protein (denoted as Bha_Cas2). Our assay revealed that Bha_Cas2 is a metal-dependent double-stranded (ds)DNA endonuclease instead of an RNase. This conclusion was further strengthened by the identification of dsDNase activities in the Thermus thermophilus Cas2 (Tth_Cas2) protein from another CRISPR subtype. We propose a way to classify Cas2 proteins into dsDNase and ssRNase based on sequence and structure features. Mutagenesis combined with isothermal titration calorimetry (ITC) revealed that the two conserved Asp8 residues in the Bha_Cas2 dimer coordinated one divalent metal ion for catalysis. The distance between these two aspartate residues in the crystal structure is, however, too far away to chelate one metal ion together. These together with the observation that the dsDNase activity and metal chelation in Bha_Cas2 are strongly correlated and steeply pH-dependent, point to the possibility that a pH-dependent conformational change enables the Bha_Cas2 protein to chelate a divalent metal ion in the active site, which in turn enables the protein to cleave dsDNA.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Cas2 (accession no. Q9KFX8; gene name, BH0342) was PCR-amplified from Bacillus halodurans C-125 genomic DNA and cloned into the pET28b vector (Novagen) via NdeI and XhoI sites. The protein containing an N-terminal His6 tag was expressed from E. coli BL21 Star (Novagen) at 18 °C for 18 h in Luria Bertani media after 1 mm isopropyl β-d-1-thiogalactopyranoside induction. Five grams of cell pellet was resuspended in ice-cold lysis buffer (50 mm Tris-HCl, pH 8.0, 0.1 m NaCl, 2 mm β-mercaptoethanol, and 0.2 mm phenylmethylsulfonyl fluoride) and disrupted by sonication. The supernatant after centrifugation was loaded onto a nickel-nitrilotriacetic acid column (Qiagen) and eluted with the lysis buffer supplemented with 300 mm imidazole. The N-terminal His tag was then removed by thrombin cleavage at 4 °C overnight. The protein was further purified on the heparin column (GE Healthcare), followed by the Superdex 200 10/300 column (GE Healthcare) purification using an elution buffer containing 10 mm Tris-HCl, pH 8.0, 100 mm NaCl, and 2 mm dithiothreitol. Bha_Cas2 mutants (D8N) were generated using a modified site-directed mutagenesis Phusion method (New England Biolabs) and verified using DNA sequencing. The SUMO-Cas2 construct was generated by subcloning the Bha_Cas2 gene into a modified pSUMO vector. The T. thermophilus cas2 gene was cloned into the vector pQE80 via BamHI and XhoI sites. These proteins were expressed and purified following the protocol for His6-tagged Bha_Cas2.
Nuclease Activity Assays
All nuclease activity assays were performed at 37 °C for 60–90 min with the exception of the time course study. Fluorescently labeled ssRNA (1 μm), ssDNA (10 μm), or dsDNA (1.19–2.38 μg of a pUC19 plasmid) substrates were incubated with the Bha_Cas2 (20 μm) in a reaction buffer containing 25 mm HEPES, pH 7.5, 200 mm KCl, and 2.5 mm MgCl2. Metal dependence was measured in the same buffer where Mg2+ was replaced with either 2.5 mm of Mn2+, Ca2+, Zn2+, Cu2+, or EDTA. In the pH dependence assays, the HEPES component in the reaction buffer was replaced with 50 mm of either sodium citrate (pH 3.0–5.0), MES (pH 6.0), Tris-HCl (pH 7.0–8.0), or CAPS (pH 9.0–11.0). The optimal salt concentration for the Bha_Cas2 nuclease activity was determined by increasing the KCl or NaCl concentration from 50 to 200 mm. Reaction products were separated by electrophoresis with either 0.7–2.0% (w/v) agarose or 15–18% (w/v) 8 m urea-PAGE gels and visualized using ethidium bromide staining or fluorescence scanning, respectively. The latter method involved scanning the urea-PAGE gel on a Typhoon 9400 (GE Healthcare) to detect fluorescent signals.
Crystallization, Data Collection, and Structure Determination
Bha_Cas2 (8 mg/ml) was crystallized using the hanging drop vapor diffusion method at 18 °C by mixing 2 μl of protein solution with 2 μl of one of the following three reservoir buffers: (i) data 1 (50 mm MES, pH 6.0, 2% (w/v) PEG MME 2000, and 10 mm MgSO4); (ii) data 2 (50 mm MES, pH 6.0, 2% (w/v) 2-methyl-2,4-pentanediol, and 10 mm MgSO4); (iii) data 3 (50 mm MES, pH 6.0, 4% (w/v) PEG 6000, and 10 mm MgSO4). Crystals were cryo-protected with the addition of 30% (v/v) ethylene glycol and flash-frozen in liquid nitrogen. X-ray diffraction data were collected at 100 K in beamline A1 at MACCHESS. Diffraction data were processed and scaled using the program HKL2000 (40). Initial phases were obtained using molecular replacement program MOLREP (41) using the deposited T. thermophilus Cas2 structure (gene name TT1823; Protein Data Bank code 1ZPW) as the search model. Interactive manual model building and refinement were carried out using the programs Coot (42) and Refmac5 (43) in the CCP4 package, respectively. Simulated annealing omit maps were systematically generated to check the quality of the model. The structure was analyzed using the programs CNS (44), PDBSUM (45), and MolProbity (46). All figures were prepared using Program PyMOL. The data processing and refinement statistics are shown in Table 1.
TABLE 1.
Data collection and refinement statistics
The highest resolution shell is shown in parentheses.
| Data I | Data II | Data III | |
|---|---|---|---|
| Data collection statistics | |||
| Space group | _P_21212 | _P_21212 | _P_21212 |
| Unit cell parameters (Å) | a = 65.887; b = 33.440; c = 39.093 | a = 66.078; b = 33.509; c = 39.163 | a = 52.619; b = 37.904; c = 44.914 |
| Resolution (Å) | 10.0-1.10 | 20.0-1.30 | 15.0-1.70 |
| (1.12-1.10) | (1.32-1.30) | (1.73-1.70) | |
| Completeness (%) | 96.9 (86.7) | 95.2 (83.9) | 92.8 (82.3) |
| Redundancy | 4.9 (2.9) | 7.8 (6.3) | 4.9 (3.0) |
| I/σ(I) | 44.35 (6.95) | 49.46 (5.66) | 33.00 (2.70) |
| _R_merge (%)a | 5.6 (13.5) | 4.7 (16.8) | 7.9 (26.5) |
| Refinement statistics | |||
| Resolution (Å) | 10.0-1.10 | 15.0-1.30 | 15.0-1.70 |
| _R_work/_R_free (%)b | 15.82/18.86 | 16.84/19.19 | 21.07/24.91 |
| B-factor (Averaged, Å2) | |||
| Protein | 11.666 | 20.191 | 25.695 |
| Water | 22.018 | 33.695 | 37.168 |
| R.m.s deviations | |||
| Bond lengths (Å) | 0.0094 | 0.0086 | 0.0133 |
| Bond angles | 1.407° | 1.407° | 1.402° |
| Ramachandran plot (%)c | |||
| Most favored | 100 | 100 | 100 |
Isothermal Titration Calorimetry
All ITC experiments were performed at 25 °C using a Nano-ITC instrument (TA Instrument). To remove non-specifically bound metal ions, the N-terminal His6 tag on Bha_Cas2 was removed by thrombin cleavage during purification, and the proteins were dialyzed first into an EDTA-containing buffer (10 mm Tris-HCl, pH 8.0, 100 mm NaCl, 2 mm DTT, and 2 mm EDTA) and then into the ITC buffer containing 20 mm HEPES, pH 7.5, and 50 mm NaCl. The pH-dependent ITC titration used a different buffer containing 25 mm MES, pH 6.0, and 50 mm NaCl. To determine the metal-binding stoichiometry, 2 μl of 20 mm Mg2+ or Mn2+ solution was titrated in 20 injections into the 190 μl of Bha_Cas2 protein (0.8–1 mm) in the same solution. Injections were administered with a 240-s interval with continuous stirring at 300 rpm. The base-line heat was measured by making identical injections in the absence of divalent metal ions. The identity of the monovalent cation (Na+ versus K+) did not produce appreciable differences in ITC experiments. Data analysis was performed using independent binding modes available in NanoAnalyze Software program (version 2.1.13, TA Instrument). Upward peaks in a NanoITC trace signify an exothermic reaction. This is the opposite from the convention by MicroCal.
RESULTS
Metal-dependent Double-stranded Nuclease Activity in B. halodurans Cas2
Non-homologous recombination in CRISPR-cas system has been suggested for new spacer acquisition (33). The source of the new spacers were suggested to be either from mRNAs presumably with the assistance of a putative reverse transcriptase that is occasionally present in vicinity of the cas operon, or more likely, from dsDNA (4, 33, 38, 39). A genetic study revealed that Cas1 or Cas2 proteins are required for the new spacer acquisition (8). In this study, we focused on the Bha_Cas2 protein to characterize its enzymatic activities.
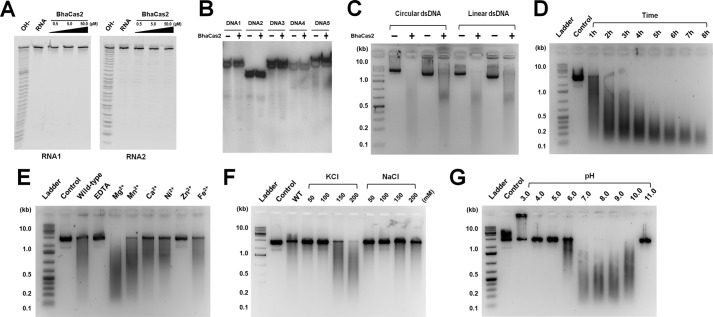
Various DNA and RNA substrates were incubated with the Bha_Cas2 protein. We were not able to detect cleavage activity in Bha_Cas2 for ssRNAs or ssDNAs (28–32 nucleotides) (Fig. 1, A and B, and supplemental Table S1). Lack of ssRNase activity in Bha_Cas2 is consistent with the D. vulgaris Cas2 study but not the S. sulfataricus Cas2 study (5, 27). Instead, robust dsDNase activity was detected from the Bha_Cas2 protein (Fig. 1C). The observation that Bha_Cas2 could actively degrade both circular and linear plasmid DNA pointed to endo- instead of exonuclease activity (Fig. 1C). The time course experiment further revealed that the end product after Bha_Cas2 processing was ∼120 bp in size (Fig. 1E). Divalent metal ions were required for this dsDNase activity, as addition of EDTA completely inhibited plasmid degradation (Fig. 1E). A survey of the metal dependence revealed that Bha_Cas2 activity could be supported to various extent by different divalent metal ions, in the descending order of Mg2+ ≫ Mn2+ > Fe2+ > Ni2+ > Ca2+ (Fig. 1E). Zn2+ on the other hand, could not support Bha_Cas2 activity. A monovalent cation dependence was also observed. It was found that the Bha_Cas2 protein was more active in higher salt concentrations (measured from 50–200 mm), and preferred K+ over Na+ in the assay condition (Fig. 1F). In the pH dependence experiments from pH 3.0 to 11.0, the dsDNase activity was more pronounced between pH 7.0 and 10.0 (Fig. 1G). The activity decreases sharply below pH 6.0 (<10% of that in pH 7.0). Taken together, our data strongly suggested that unlike S. solfataricus Cas2, B. halodurans Cas2 protein was a metal-dependent endonuclease targeting dsDNA for degradation.
FIGURE 1.
The dsDNase activity in Bha_Cas2. Two different chemically synthesized ssRNAs (A) and five different ssDNA substrates (B) were incubated with increasing concentrations of Bha_Cas2. No detectable RNA cleavage was observed. An alkaline hydrolysis ladder is loaded in the left lane of each gel. Compared with the robust dsDNase activity in Fig. 1, the ssDNase is at the base line. The sequences of the RNA and DNA used in the assays are documented in supplemental Table S1. C, Bha_Cas2 can cleave both circular and linearized dsDNA plasmid. Reactions were done in duplicates. D, time course of the nuclease activity. E, the nuclease activity in Bha_Cas2 is activated by divalent metal ions, whereas addition of EDTA or Zn2+ strongly inhibits the activity. F, effect of increasing monovalent metal ion concentration on nuclease activity, Bha_Cas2 is more active in higher concentrations of KCl. 2.5 mm Mg2+ is present in the solution. G, the nuclease activity in Bha_Cas2 is steeply pH-dependent. The substrate used in D–G is a linearized dsDNA plasmid.
Crystal Structure of Bha_Cas2
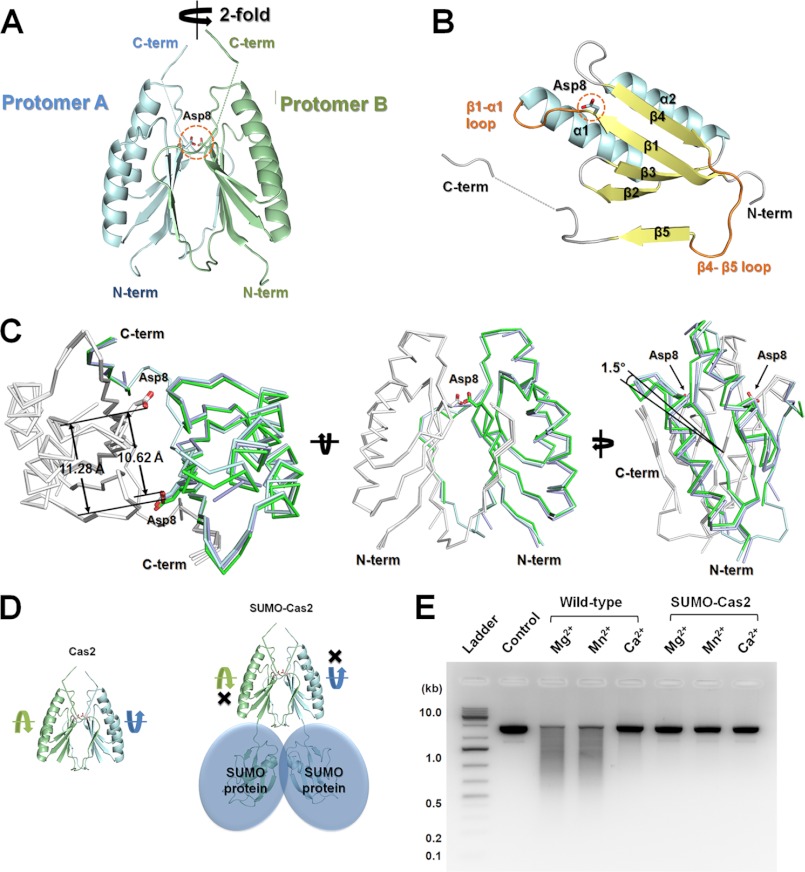
To gain insight into the distinct dsDNase activity in the Bha_Cas2, we determined the crystal structure of Bha_Cas2. The space group of the crystal structure of Bha_Cas2 was determined to be the orthorhombic _P_21212 space group, with one molecule occupying the asymmetric unit. Bha_Cas2 forms a symmetric dimer in the crystal lattice (Fig. 2A). This oligomerization state is consistent with the observed dimer formation in size-exclusion chromatography (supplemental Fig. S1). The _P_21212 space group could be obtained using PEG MME 2000, PEG 6000, or MPD as a precipitant. To investigate whether different precipitants may influence the Bha_Cas2 conformation, we determined structures from crystals grown from each precipitant at resolution of 1.10, 1.30, and 1.70 Å, respectively (Table 1). Comparison between these structures revealed minor global and local conformational changes. As much as 1.5 ° rigid-body rotation can be detected between each protomer in the Bha_Cas2 dimer (Fig. 2C), the distance between active site residues varies from 10.6–11.3, and the conformation of two surface loops are also different among these three Bha_Cas2 structures (supplemental Fig. S2). The electron density map allowed tracing of the entire molecule except three flexible regions: between β1–α1 (Ala9– Ala13), between β4–β5 (Ala73–Ala77), and the C-terminal tail (Ala85 C-terminal) (supplemental Fig. S2).
FIGURE 2.
Crystal structure of Cas2 from B. halodurans. A, dimer formation in Bha_Cas2. Two molecules of Cas2 form a non-crystallographic dimer in the asymmetric unit cell. The active site residue Asp8 pair (shown in stick representation) is located in β1 of each Cas2 protomer. B, each Bha_Cas2 protomer contains a ferredoxin fold and a C-terminal (C-term) β5-strand. C, different views of the superposition of the three Bha_Cas structures crystallized from different precipitants (one structure in green, the other two in silver). Results showed an ∼1.5 ° hinge motion between the two protomers and distance variations between the catalytic Asp8 pair. D, modeling exercise showing that the N-terminal (N-term) SUMO fusion may interfere with the hinge motion in Bha_Cas2 and cause an allosteric inhibition of the dsDNase activity. E, agarose gel showing that N-terminal SUMO fusion greatly reduced dsDNase activity in Bha_Cas2.
Each Bha_Cas2 protomer contains a ferredoxin fold (βαββαβ; Met1–Arg70) composed of two α-helices packing against the four-stranded antiparallel β-sheet (Fig. 2B). Following the ferredoxin fold, the C-terminal β5-strand (Glu80–Ile82) interacts with the β4-strand in the partner protomer to extend the existing anti-parallel β-sheet (supplemental Fig. S3). As shown later, our mutagenesis data suggested that this domain swapping interaction played an essential role in the dimer formation (Fig. 2, D and E).
The total buried surface area at the Bha_Cas2 dimer interface is ∼1430 Å2 (∼30% of the surface area of the protomer). Closer analysis revealed that the dimer interface was dominated by polar interactions (68% hydrophilic, 32% hydrophobic). Significant H-bond and salt-bridge interactions (<3.0Å) include the following residue pairs in β-sheets (β1–4): Asp8 (β1)–Asn36*(between β2 and β3) (2.90 Å), Gln35(β2)-Thr6*(β1) (2.71 Å), Gln35(β2)-Ser65*(β4) (2.71 Å), Glu40(β3)-Arg67(β4)* (2.87 Å), and Glu40(β3)-Tyr69*(β4) (2.58 Å) (supplemental Fig. S3; asterisk signifies residues from the interacting dimer).
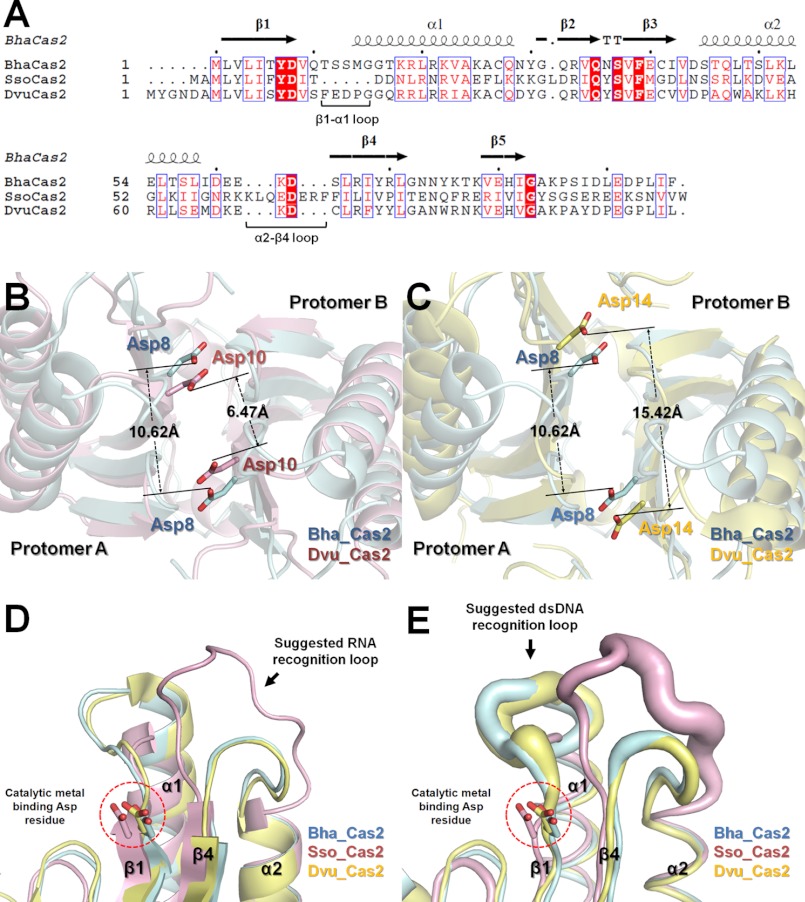
The Bha_Cas2 structure aligns well with S. solfataricus and D. vulgaris Cas2 structures (24 and 51% sequence identity, respectively), with root mean square deviations of 1.61 and 2.57 Å, respectively (Fig. 3A). The conserved structure features include the dimer formation, the β5-strand swapping at the dimer interface, and a conserved aspartate residue pair in the putative active site (Fig. 3A) (27, 37). Interestingly, the distance between this invariable Asp pair varied significantly among three structures, from 10.62 Å in Bha_Cas2, 6.5 Å in Sso_Cas2, to 15.42 Å in Dvu_Cas2, pointing to the possibility of substrate-induced conformational changes or diversity in the catalytic mechanism (Fig. 3, B and C). In addition, large conformational differences were observed in the α2-β4 and β1-α1 loops in the Cas2 structures. The long α2-β4 loop in the Sso_Cas2 structure, which was suggested to be responsible for recognizing the RNA substrates (27, 47), is much shorter and more rigid in the Bha_Cas2 and Dvu_Cas2 structures (Fig. 3, D and E). By contrast, the β1-α1 loop is larger and more flexible in these two structures. The net result is a deeper and narrower substrate binding groove in the Sso_Cas2, more appropriate for ssRNA binding, and a wider and shallower binding groove in the Dvu_Cas2 and Bha_Cas2, better tuned for dsDNA binding.
FIGURE 3.
Sequence and structure comparison of Cas2 proteins. A, amino acid sequence alignment of Bha_Cas2 (SWISS-PROT entry Q08638) with Sso_Cas2 (Q97YC2), and Dvu_Cas2 (Q72WF4). The absolutely conserved residues among three Cas2 proteins are boxed in red, and the highly conserved residues are in unfilled boxes and red letters. Superposition of Bha_Cas2 (B; cyan) with Dvu_Cas2 (magenta) and Bha_Cas2 with Sso_Cas2 (C; yellow) structures revealed differences in the distance of the catalytic Asp pair in the Cas2 dimer. D, superposition of all three structures revealing differences in the size and conformation of the two proposed substrate recognition loops (α2-β4, and β1-α1). E, same superposition rendered in B-factor representation. The diameter of the tube is proportional to the B factor of the individual residue.
Role of Asp8 in Coordinating the Catalytic Metal Ion
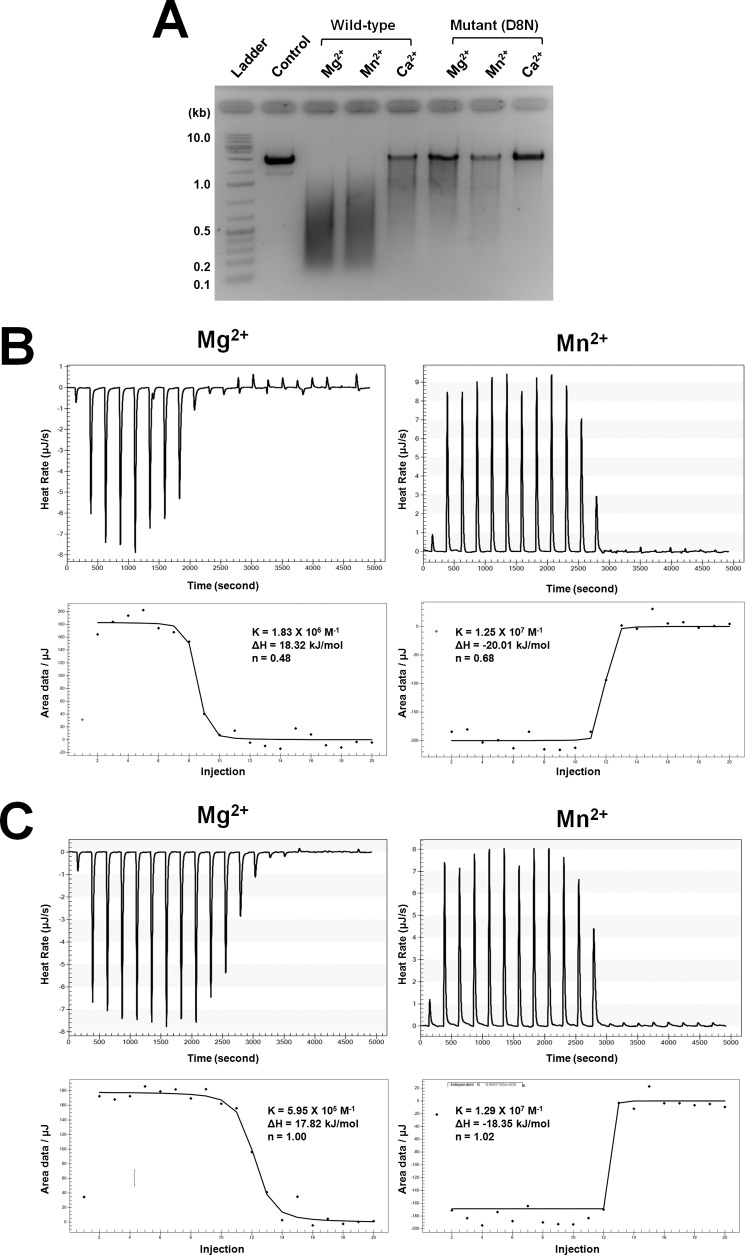
The Asp10 pair in the S. solfataricus Cas2 dimer has been shown to be critical for catalysis. Based on the structural and sequence alignments with the Sso_Cas2 and other Cas proteins (Fig. 3 and supplemental Fig. S4), the Asp8 residue in Bha_Cas2 was thought to play an equivalent role in possibly coordinating a catalytic divalent metal ion(s) for dsDNA cleavage. Indeed, the dsDNase activity in the D8N Bha_Cas2 mutant was drastically reduced as compared with the wild type in the presence of Mg2+, Mn2, or Ca2+ (Fig. 4A). However, none of the three apo-Cas2 structures revealed the binding of divalent metal ion(s) near the invariable Asp pair. Moreover, the distance between the invariable Asp pair varies significantly among the three Cas2 structures (Fig. 3, B and C), leaving open the question about whether one or two divalent metal ions are coordinated by the Asp pair in the Cas2 dimer.
FIGURE 4.
Characterizing the catalytic metal binding stoichiometry using mutagenesis and ITC. A, comparison of nuclease activity between wild type and mutant (D8N) Bha_Cas2 using agarose gel. The dsDNase activity is greatly reduced in the Bha_Cas2-D8N mutant. Representative calorimetric titrations of Mg2+ (left panel) or Mn2+ (right panel) into Bha_Cas2-WT (B) or D8N (C) solution. The upper panels contain the raw trace of heat changes accompanying each metal injection, and the lower panels show the data fitting of the integrated heat changes. ITC experiments for Mg2+ and Mn2+ titrations into Bha_Cas2 proteins were carried out at 25 °C in a solution containing 10 mm HEPES, pH 7.5, and 50 mm NaCl.
We used ITC to measure the metal-binding stoichiometry in the wild type and D8N mutant Bha_Cas2 proteins (Bha_Cas2-WT and Bha_Cas2-D8N). To prevent prebound metal ions from complicating the analysis, purified Bha_Cas2 proteins were first dialyzed into an EDTA-containing solution to strip residual metal ions before dialyzing into the ITC buffer. The thermodynamic parameters and the metal-binding stoichiometry can be derived from ITC analysis. Interestingly, the thermodynamic process of Bha_Cas2 binding to Mg2+ and Mn2+ was very different (Fig. 4 and Table 2). Titration of Mg2+ into the Bha_Cas2-WT protein solution was an endothermic process, with a binding enthalpy (Δ_H_°) of 18.3 kJ/mol and a binding free energy (Δ_G_°) of −5.8 ± 0.006 kJ/mol (Fig. 4B). Binding of Mn2+ by Bha_Cas2, on the other hand, was an exothermic process, with enthalpy values of Δ_H_° = −20.0 kJ/mol and a binding free energy (Δ_G_°) of −4.2 ± 0.005 kJ/mol (Fig. 4B). The affinity of Bha_Cas2-WT for Mg2+ (Kd = 54 μm) was 14 times higher than that for Mn2+ (Kd = 791 μm).
TABLE 2.
ITC of divalent metal ion binding to Bha_Cas2 protein
Values for n, Kd, and Δ_H_ are the averages of three independent titrations. N/A is indicated as the not available result (no measurable heat). Δ_G_ was obtained by following equation: Δ_G_ = −RT ln(KaC), where Δ_G_ is the change in Gibbs free energy (cal/mol), R is the universal gas constant, 8.314 J/mol K, T is the absolute temperature (K), and C is the concentration of standard state (1 m). T_Δ_S was obtained by the following equation: Δ_G_ = Δ_H_ − T Δ_S_, where Δ_H_ and Δ_S_ are the changes in enthalpy (cal/mol) and entropy (cal/mol/K), respectively.
| Bha_Cas2 | Metal | n | Kd | Δ_H_ | Δ_G_ | −T_Δ_S |
|---|---|---|---|---|---|---|
| μ_m_ | kcal/mol | kcal/mol | kcal/mol | |||
| WT | Mg2+ | 0.48 ± 0.001 | 54.1 ± 0.57 | 18.3 ± 0.0013 | −5.82 ± 0.0062 | 24.1 ± 0.0062 |
| pH 7.5 | Mn2+ | 0.69 ± 0.001 | 791 ± 6.4 | −20.0 ± 0.0013 | −4.23 ± 0.0050 | −15.9 ± 0.0050 |
| D8N | Mg2+ | 1.00 ± 0.001 | 1.68 ± 0.01 | 17.8 ± 0.001 | −7.88 ± 0.0035 | 25.7 ± 0.0035 |
| pH 7.5 | Mn2+ | 1.02 ± 0.001 | 773 ± 4.9 | −18.3 ± 0.001 | −4.25 ± 0.0022 | −14.1 ± 0.0022 |
| WT | Mg2+ | N/A | N/A | N/A | ||
| pH 6.0 | Mn2+ | N/A | N/A | N/A |
Titration of Mg2+ and Mn2+ into the Bha_Cas2-D8N mutant followed a similar trend to wild type (Fig. 4C). The binding of Mg2+ was an exothermic binding event, with Δ_H_° and Δ_G_° of 17.8 kJ/mol and −7.9 ± 0.004 kJ/mol, respectively. Mn2+ binding was an endothermic binding event, with Δ_H_° and Δ_G_° of −18.3 kJ/mol and −4.2 ± 0.002 kJ/mol, respectively. Notably, although the binding affinity of Bha_Cas2-D8N for Mn2+ ions (Kd = 773 μm) remained roughly the same as the Cas2-WT, its affinity for Mg2+ ions (Kd = 1.68 μm) was 32-fold tighter than that of the wild type protein, suggesting that the Mg2+ coordination is significantly altered in the D8N mutant.
Metal-binding stoichiometry could also be calculated from the same ITC data. The binding stoichiometry of Mg2+:Bha_Cas2-WT was ∼1:2 (n = 0.48 ± 0.001), or one Mg2+ per Bha_Cas2-WT dimer, presumably coordinated by the Asp8 pair (Fig. 4 and Table 2). This coordination would require the Asp8 pair to move much closer toward each other from the conformation in the crystal structure (10.7 Å apart), which may explain why Mg2+ binding was not captured in our crystal structure. The binding stoichiometry of Mn2+:Bha_Cas2 was ∼1.4:2 (n = 0.69 ± 0.001), which is consistent with a 50% mixture of one- and two-metal ion coordination. Assuming 1:2 stoichiometry leads to productive catalysis, this would explain why the DNase activity of Bha_Cas2 is weaker in the presence of Mn2+. On the other hand, the binding stoichiometry of both Mg2+ and Mn2+ for the Bha_Cas2-D8N mutant was changed to 1:1, suggesting that two metal ions were coordinated by the Asn8 pair in the D8N mutant. This change of divalent metal coordination could explain why the Cas2-D8N mutant displayed much weaker dsDNA nuclease activity. Taken together, our ITC data suggest that the Bha_Cas2 protein coordinated one metal ion in the active site to catalyze the endonucleolytic cleavage of dsDNA.
Binding of the Catalytic Metal Ion Was pH-dependent
A sharp pH dependence in nuclease activity was observed in the nuclease assay (Fig. 1D). In particular, the activity of Bha_Cas2 was shown to decrease steeply when the pH dropped below 6.0. This paralleled with the ITC observation that when the metal titration was carried out in pH 6.0 instead of 7.5 shown above, Bha_Cas2 no longer showed appreciable affinity for Mg2+ and Mn2+ (supplemental Fig. S5). The strong correlation suggests that the loss of dsDNase activity at low pH is very likely the result of the loss of the catalytic metal ion at the active site. This points to the possible existence of a pH-dependent conformational change that switches on Bha_Cas2 to chelate a metal ion for catalysis. Because Bha_Cas2 was only crystallizable at pH lower than 6.0, we were only able to reveal the “nonproductive” conformation in the crystal structure. Jumping the solution pH to 7.0 before crystal freezing did not result in significant conformational changes presumably due to crystal lattice trapping (data not shown).
Another piece of evidence pointing to the existence of a different conformational state in Bha_Cas2 during catalysis came from the observation that the N-terminal SUMO-tagged Bha_Cas2, although still capable of dimerization (supplemental Fig. S1), had drastically reduced DNase activity, despite the fact that the ∼11-kDa SUMO tag was located opposite from the active site, therefore unlikely to sterically interfere with DNA binding. Our interpretation is that this distal SUMO tag causes an allosteric inhibition to prevent the Bha_Cas2 from adopting the productive conformation for catalysis.
dsDNase Activity in Other Cas2 Proteins
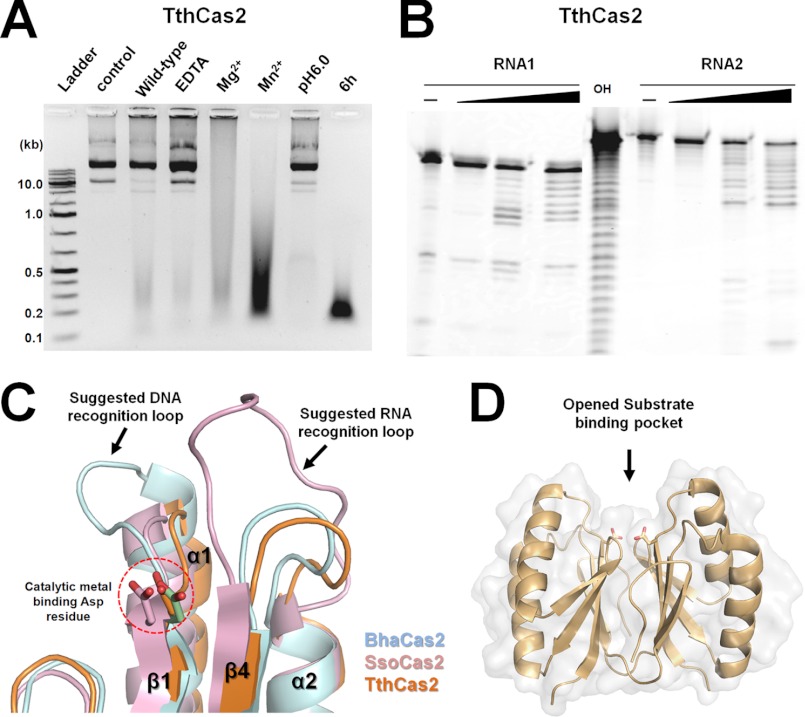
Cas2 protein was previously characterized as a ssRNase (27), whereas here we identified dsDNase activity in the Bha_Cas2 protein. To investigate whether dsDNase activity may be present in other Cas2 proteins, we carried out nuclease activity survey experiments on the T. thermophilus Cas2 (Tth_Cas2) protein (Fig. 5). Results showed predominant dsDNase activity in Tth_Cas2 but also rather weak ssRNase as well (Fig. 5, A and B). Similar to Bha_Cas2, activity in Tth_Cas2 is metal- and pH-dependent, although Mn2+ is slightly favored over Mg2+, and the final product size is slightly bigger, averaging ∼170 bp. These results suggest that dsDNase activity is present in Cas2 proteins from at least two different CRISPR subtypes.
FIGURE 5.
Tth_Cas2 contains both dsDNase and ssRNase activities. A, dsDNA plasmid is degraded by Tth_Cas2, and the final product is ∼170 bp in size (lane 8). Tth_Cas2 is more active in Mn2+ instead of Mg2+ (compare lane 5 with 6). The dsDNase activity is significantly reduced in the presence of metal ion chelator EDTA (lane 4) or acidic pH condition (lane 7). B, Tth_Cas2 also contains weak nonspecific endoribonuclease activity against ssRNA with a sequence-nonspecific manner. Increasing concentration of Tth_Cas2 caused increasing degradation of two different ssRNA substrate. Alkaline hydrolysis ladder for the second RNA substrate is shown in lane 5. C, comparison of the proposed substrate recognition loop from Bha_Cas2, Sso_Cas2, and Tth_Cas2, indicating that Tth_Cas2 lacks extensive substrate recognition loops found in Bha_Cas2 and Sso_Cas2, which may explain its promiscuous enzymatic activity (see “Discussion” for details). D, opened substrate binding pocket in Tth_Cas2.
DISCUSSION
Conserved Active Site Configuration and Envisioned Conformational Changes During Catalysis
Despite sequence variations and distinct enzymatic activities among Cas2 proteins, the three-dimensional structures of the two previously determined Cas2 structures agree well with the Bha_Cas2 structure reported here. It is clear that Cas2 proteins function as a dimer and contain a conserved symmetric Asp pair in the active site cleft. Our mutagenesis and ITC analyses provided the first direct evidence that the invariable Asp pair is involved in chelating a single divalent metal ion in the active site and that the Asp-to-Asn mutation perturbs the metal coordination scheme, allowing two Mg2+ ions into the active site, hence resulting in severe loss of dsDNase activity in Cas2.
Several observations, including the correlated strong pH dependence in the dsDNase activity and the divalent metal ion binding at the active site of Bha_Cas2, the non-optimal metal binding site in the low-pH Bha_Cas2 crystal structure, as well as the allosteric inhibition by SUMO tagging, all point to the possible presence of a pH-dependent conformational change that enables Bha_Cas2 to bind the active site metal to cleave dsDNA. The conformational change likely involves a rigid-body hinge motion between the two Cas2 protomers to bring the Asp8 pair closer to coordinate a single metal ion, and a hint of such motion has been observed when Bha_Cas2 is crystallized from different conditions (Fig. 2C). One of the future goals is to capture the productive Bha_Cas2 conformation from an alternative crystal form in neural or basic pH conditions and/or in the presence of its dsDNA substrate.
Rationalization of Different Enzymatic Activities among Cas2 Proteins and Their Functional Implications
Although the overall structures of Bha_Cas2 and Sso_Cas2 are quite similar, one is characterized as a dsDNase, and the other is characterized a an ssRNase. Localized structural differences in the α2-β4 and β1-α1 loops above the active site pocket may predetermine their different substrate preferences. The α2-β4 loop in the Sso_Cas2 structure, which contains important residues for ssRNA recognition (27, 47), is significantly longer and more flexible than in the Bha_Cas2 structure (Fig. 3D). By contrast, the β1-α1 loop is larger and more flexible in the Bha_Cas2 and Dvu_Cas2 than the Sso_Cas2. The net result is a deeper and narrower substrate binding groove in the Sso_Cas2, which is more appropriate for ssRNA binding, and a wider and shallower binding groove in the Dvu_Cas2 and Bha_Cas2, which is better tuned for dsDNA binding (Fig. 3E) (37). Using the length of the α2-β4 and β1-α1 loops as an indicator, the annotated Cas2 proteins can be roughly divided into two groups, presumably with different enzymatic activities. There are yet some Cas2 proteins that do not belong to either of the classes. For example, the T. thermophilus Cas2 (Tth_Cas2) structure contains an open cleft atop of the active site, lacking extensive structures in either of the substrate recognition loops (Fig. 5, C and D). Is the Tth-Cas2 a ssRNase, dsDNase, or both? Our results showed that its biochemical activity agreed more with the Bha_Cas2, but not the Sso_Cas, which is consistent with our observation that the active site conformation in Tth_Cas2 is more similar to the Bha_Cas2 (Fig. 5A).
The DNase activity in Cas2 agrees well with its essential function in the new spacer acquisition process, which involves the dicing of foreign dsDNA in short fragments (proto-pacers) and the insertion of these into the CRISPR loci as new spacers. This process minimally requires Cas1 and Cas2 proteins (9, 13), and both are metal-dependent dsDNases. Neither protein produces dsDNA fragments as short as found in the CRISPR spacers; therefore, it is imaginable that these two proteins may function in a concerted fashion to generate the right sized proto-spacers. This scenario agrees well with a recent study showing that combined action of Cas1 and Cas2 extends the CRISPR region in E. coli (35).
Acknowledgments
We thank the beam line staff at Macromolecular Diffraction at Cornell High Energy Synchrotron Source for assistance in data collection and Jason C. Grigg for helpful discussions and comments on the manuscript.
*
This work is supported in part by the Public Health Service Grants GM-086766 and GM-059604 (to A. K.) and by the National Research Foundation of Korea Grant from the Korean Ministry of Education, Science, and Technology NRF-2010-357-C00106 (to K. H. N.).
The atomic coordinates and structure factors (codes 4ES1, 4ES2, and 4ES3) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
2
The abbreviations used are:
bp
base pair(s)
ITC
isothermal titration calorimetry
Bha_Cas2
Bacillus halodurans Cas2
Sso_Cas2
Sulfolobus solfataricus Cas2
Dvu_Cas2
Desulfovibrio vulgaris Cas2
Tth_Cas2
Thermus thermophilus Cas2
ssRNA
single-stranded RNA
dsDNA
double-stranded DNA.
REFERENCES
- 1.Sorek R., Kunin V., Hugenholtz P. (2008) CRISPR–a widespread system that provides acquired resistance against phages in bacteria and archaea. Nat. Rev. Microbiol. 6, 181–186 [DOI] [PubMed] [Google Scholar]
- 2.van der Oost J., Jore M. M., Westra E. R., Lundgren M., Brouns S. J. (2009) CRISPR-based adaptive and heritable immunity in prokaryotes. Trends Biochem. Sci. 34, 401–407 [DOI] [PubMed] [Google Scholar]
- 3.Karginov F. V., Hannon G. J. (2010) The CRISPR system: small RNA-guided defense in bacteria and archaea. Mol. Cell 37, 7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deveau H., Garneau J. E., Moineau S. (2010) CRISPR/Cas system and its role in phage-bacteria interactions. Annu. Rev. Microbiol. 64, 475–493 [DOI] [PubMed] [Google Scholar]
- 5.Waters L. S., Storz G. (2009) Regulatory RNAs in bacteria. Cell 136, 615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marraffini L. A., Sontheimer E. J. (2010) CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea. Nat. Rev. Genet. 11, 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horvath P., Barrangou R. (2010) CRISPR/Cas, the immune system of bacteria and archaea. Science 327, 167–170 [DOI] [PubMed] [Google Scholar]
- 8.Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S., Romero D. A., Horvath P. (2007) CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 [DOI] [PubMed] [Google Scholar]
- 9.Bhaya D., Davison M., Barrangou R. (2011) CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 45, 273–297 [DOI] [PubMed] [Google Scholar]
- 10.Wiedenheft B., Sternberg S. H., Doudna J. A. (2012) RNA-guided genetic silencing systems in bacteria and archaea. Nature 482, 331–338 [DOI] [PubMed] [Google Scholar]
- 11.Brouns S. J., Jore M. M., Lundgren M., Westra E. R., Slijkhuis R. J., Snijders A. P., Dickman M. J., Makarova K. S., Koonin E. V., van der Oost J. (2008) Small CRISPR RNAs guide antiviral defense in prokaryotes. Science 321, 960–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grissa I., Vergnaud G., Pourcel C. (2007) CRISPRFinder: a web tool to identify clustered regularly interspaced short palindromic repeats. Nucleic Acids Res. 35, W52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sapranauskas R., Gasiunas G., Fremaux C., Barrangou R., Horvath P., Siksnys V. (2011) The Streptococcus thermophilus CRISPR/Cas system provides immunity in Escherichia coli. Nucleic Acids Res. 39, 9275–9282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nam K. H., Kurinov I., Ke A. (2011) Crystal structure of clustered regularly interspaced short palindromic repeats (CRISPR)-associated Csn2 protein revealed Ca2+-dependent double-stranded DNA binding activity. J. Biol. Chem. 286, 30759–30768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunin V., Sorek R., Hugenholtz P. (2007) Evolutionary conservation of sequence and secondary structures in CRISPR repeats. Genome Biol. 8, R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R., Preamplume G., Terns M. P., Terns R. M., Li H. (2011) Interaction of the Cas6 riboendonuclease with CRISPR RNAs: recognition and cleavage. Structure 19, 257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebihara A., Yao M., Masui R., Tanaka I., Yokoyama S., Kuramitsu S. (2006) Crystal structure of hypothetical protein TTHB192 from Thermus thermophilus HB8 reveals a new protein family with an RNA recognition motif-like domain. Protein Sci. 15, 1494–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carte J., Wang R., Li H., Terns R. M., Terns M. P. (2008) Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes. Genes Dev. 22, 3489–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haurwitz R. E., Jinek M., Wiedenheft B., Zhou K., Doudna J. A. (2010) Sequence- and structure-specific RNA processing by a CRISPR endonuclease. Science 329, 1355–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gesner E. M., Schellenberg M. J., Garside E. L., George M. M., Macmillan A. M. (2011) Recognition and maturation of effector RNAs in a CRISPR interference pathway. Nat. Struct. Mol. Biol. 18, 688–692 [DOI] [PubMed] [Google Scholar]
- 21.Lawrence C. M., White M. F. (2011) Recognition of archaeal CRISPR RNA: No P in the alindromic repeat? Structure 19, 142–144 [DOI] [PubMed] [Google Scholar]
- 22.Mojica F. J., Díez-Villaseñor C., García-Martínez J., Soria E. (2005) Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 60, 174–182 [DOI] [PubMed] [Google Scholar]
- 23.Pourcel C., Salvignol G., Vergnaud G. (2005) CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA and provide additional tools for evolutionary studies. Microbiology 151, 653–663 [DOI] [PubMed] [Google Scholar]
- 24.Bolotin A., Quinquis B., Sorokin A., Ehrlich S. D. (2005) Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology 151, 2551–2561 [DOI] [PubMed] [Google Scholar]
- 25.Hale C. R., Zhao P., Olson S., Duff M. O., Graveley B. R., Wells L., Terns R. M., Terns M. P. (2009) RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex. Cell 139, 945–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiedenheft B., Zhou K., Jinek M., Coyle S. M., Ma W., Doudna J. A. (2009) Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense. Structure 17, 904–912 [DOI] [PubMed] [Google Scholar]
- 27.Beloglazova N., Brown G., Zimmerman M. D., Proudfoot M., Makarova K. S., Kudritska M., Kochinyan S., Wang S., Chruszcz M., Minor W., Koonin E. V., Edwards A. M., Savchenko A., Yakunin A. F. (2008) A novel family of sequence-specific endoribonucleases associated with the clustered regularly interspaced short palindromic repeats. J. Biol. Chem. 283, 20361–20371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han D., Lehmann K., Krauss G. (2009) SSO1450–a CAS1 protein from Sulfolobus solfataricus P2 with high affinity for RNA and DNA. FEBS Lett. 583, 1928–1932 [DOI] [PubMed] [Google Scholar]
- 29.Marraffini L. A., Sontheimer E. J. (2008) CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 322, 1843–1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hale C., Kleppe K., Terns R. M., Terns M. P. (2008) Prokaryotic silencing (psi)RNAs in Pyrococcus furiosus. RNA 14, 2572–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen R., Embden J. D., Gaastra W., Schouls L. M. (2002) Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 43, 1565–1575 [DOI] [PubMed] [Google Scholar]
- 32.Haft D. H., Selengut J., Mongodin E. F., Nelson K. E. (2005) A guild of 45 CRISPR-associated (Cas) protein families and multiple CRISPR/Cas subtypes exist in prokaryotic genomes. PLoS Comput. Biol. 1, e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makarova K. S., Grishin N. V., Shabalina S. A., Wolf Y. I., Koonin E. V. (2006) A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action. Biol. Direct 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makarova K. S., Haft D. H., Barrangou R., Brouns S. J., Charpentier E., Horvath P., Moineau S., Mojica F. J., Wolf Y. I., Yakunin A. F., van der Oost J., Koonin E. V. (2011) Evolution and classification of the CRISPR-Cas systems. Nat. Rev. Microbiol. 9, 467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yosef I., Goren M. G., Qimron U. (2012) Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli. Nucleic Acids Res. 40, 5569–5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babu M., Beloglazova N., Flick R., Graham C., Skarina T., Nocek B., Gagarinova A., Pogoutse O., Brown G., Binkowski A., Phanse S., Joachimiak A., Koonin E. V., Savchenko A., Emili A., Greenblatt J., Edwards A. M., Yakunin A. F. (2011) A dual function of the CRISPR-Cas system in bacterial antivirus immunity and DNA repair. Mol. Microbiol. 79, 484–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samai P., Smith P., Shuman S. (2010) Structure of a CRISPR-associated protein Cas2 from Desulfovibrio vulgaris. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 66, 1552–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mojica F. J., Díez-Villaseñor C., García-Martínez J., Almendros C. (2009) Short motif sequences determine the targets of the prokaryotic CRISPR defense system. Microbiology 155, 733–740 [DOI] [PubMed] [Google Scholar]
- 39.Shah S. A., Hansen N. R., Garrett R. A. (2009) Distribution of CRISPR spacer matches in viruses and plasmids of crenarchaeal acidothermophiles and implications for their inhibitory mechanism. Biochem. Soc. Trans. 37, 23–28 [DOI] [PubMed] [Google Scholar]
- 40.Otwinowski Z., Minor W. (1997) Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 41.Vagin A., Teplyakov A. (2010) Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 66, 22–25 [DOI] [PubMed] [Google Scholar]
- 42.Emsley P., Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 43.Winn M. D., Murshudov G. N., Papiz M. Z. (2003) Macromolecular TLS refinement in REFMAC at moderate resolutions. Methods Enzymol. 374, 300–321 [DOI] [PubMed] [Google Scholar]
- 44.Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 45.Laskowski R. A. (2001) PDBsum: summaries and analyses of PDB structures. Nucleic Acids Res. 29, 221–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., 3rd, Snoeyink J., Richardson J. S., Richardson D. C. (2007) MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, W375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Callaghan A. J., Marcaida M. J., Stead J. A., McDowall K. J., Scott W. G., Luisi B. F. (2005) Structure of Escherichia coli RNase E catalytic domain and implications for RNA turnover. Nature 437, 1187–1191 [DOI] [PubMed] [Google Scholar]
- 48.Nam K. H., Haitjema C., Liu X., Ding F., Wang H., Delisa M. P., Ke A. (2012) Cas5d protein processes pre-crRNA and assembles into a cascade-like Interference complex in subtype I-C/Dvulg CRISPR-Cas System. Structure 20, 1574–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]