RNA-binding protein nucleolin in disease (original) (raw)
Abstract
Nucleolin is a multifunctional protein localized primarily in the nucleolus, but also found in the nucleoplasm, cytoplasm and cell membrane. It is involved in several aspects of DNA metabolism, and participates extensively in RNA regulatory mechanisms, including transcription, ribosome assembly, mRNA stability and translation, and microRNA processing. Nucleolin’s implication in disease is linked to its ability to associate with target RNAs via its four RNA-binding domains and its arginine/glycin-rich domain. By modulating the post-transcriptional fate of target mRNAs, which typically bear AU-rich and/or G-rich elements, nucleolin has been linked to cellular events that influence disease, notably cell proliferation and protection against apoptotic death. Through its diverse RNA functions, nucleolin is increasingly implicated in pathological processes, particularly cancer and viral infection. Here, we review the RNA-binding activities of nucleolin, its influence on gene expression patterns, and its impact upon diseases. We also discuss the rising interest in targeting nucleolin therapeutically.
Keywords: AU-rich elements, G-rich sequences, mRNA stability, mRNA translation, post-transcriptional gene regulation, therapy, transcriptome
Introduction
In coordination with transcription, post-transcriptional mechanisms elicit robust, efficient, and dynamic changes to the patterns of expressed proteins, enabling cells to respond to intracellular and environmental stimuli. Transcriptional and post-transcriptional processes are tightly interconnected. Post-transcriptional events are mainly governed by RNA-binding proteins (RBPs) and by noncoding (nc)RNAs, which jointly regulate steps such as mRNA splicing, maturation, transport, editing, stability and translation.1,2 However, some post-transcriptional regulators perform many functions; for example, the RBP family of hnRNPs (heterogeneous nuclear ribonucleoproteins) is capable of modulating DNA transcription, as well as replication, telomere maintenance, transcription, and repair.3,4
First described almost four decades ago,5 nucleolin is one of the most multifunctional RBPs known to-date. It is most abundant in the nucleolus, but is also found in other nuclear regions, as well as in the cytoplasm and the plasma membrane.6-8 Nucleolin plays key functions in processes such as chromatin remodeling, transcription of ribosomal (r)RNA, rRNA maturation, ribosome assembly, nucleocytoplasmic transport, and ribosome biogenesis.7-10 It binds DNA and RNA, functions as a DNA and RNA helicase, and has self-cleaving activity.10,11 The multiple functions and subcellular localization of nucleolin reflect its complex structure. Its acidic N-terminal region contains multiple phosphorylation sites, participates in the transcription of rRNA, and interacts with components of the pre-rRNA processing complex.12 The central region of nucleolin contains four RNA-recognition motifs (RRMs) and mediates the interaction with mRNAs and pre-rRNA.13,14 The C-terminal region of nucleolin contains an arginine/glycine-rich domain (RGG), through which nucleolin can interact with target mRNAs as well as with other proteins, including ribosomal proteins.13,15,16
Nucleolin has been extensively implicated in DNA metabolism. It affects DNA replication, telomere maintenance, and DNA repair and recombination.17-22 At the level of transcription, it represses the function of RNA polymerase I, thereby lowering rRNA transcription.7 Nucleolin also modulates the transcription of mRNAs in several ways. It represses RNA polymerase II function and it can affect mRNA transcription both positively and negatively through its interaction with chromatin components like histone H1,23,24 and chromatin remodeling enzymes such as SWI/SNF.25 In addition, nucleolin can affect mRNA transcription directly by interacting with DNA by interacting with DNA.26
Nucleolin’s influence on ribosome biogenesis has also been studied in detail. Nucleolin binds to a stem-loop structure found in nascent pre-rRNA and plays a chaperone function by facilitating the proper folding of pre-rRNA.14,27 Nucleolin also catalyzes the first processing step of pre-rRNA occurring in the 5′ external transcribed spacer (5′-ETS) resulting in cleavage of the precursor transcript of rRNA.28,29 The affinity of nucleolin for ribosomal proteins suggests that it may also help assemble ribosomal subunits in the nucleus,15 and likely participates in the transport of these newly assembled ribosomal subunits into the cytoplasm.30 Through its involvement in rDNA transcription, its association with nascent pre-rRNA, and its assembly and transport of ribosomal particles, nucleolin is believed to assist with every step of ribosome biosynthesis.31
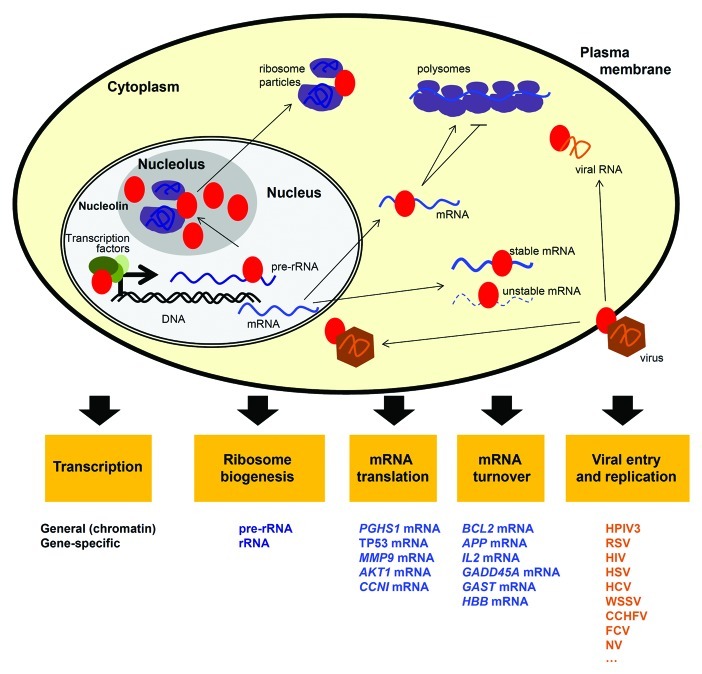
The transcriptional and ribosome assembly functions of nucleolin have been known for many years. However, the past decade has uncovered many other functions linked to the binding of nucleolin to mRNAs (Fig. 1). This review will focus mainly on this relatively new activity of nucleolin, given its direct implication in disease. We will describe the subsets of mRNAs with which nucleolin interacts, the influence of nucleolin on their expression, and the impact of the encoded proteins on pathologies, mainly cancer and viral infections. Finally, we will discuss emerging efforts to target nucleolin for therapeutic intervention.
Figure 1. Regulation of mRNA by nucleolin. Schematic representation of nucleolin (red oval) and its influence on the post-transcriptional regulation of mRNA fate (mRNA turnover and translation) in the context of its multiple cellular functions (yellow boxes) and its presence in the nucleolus, nucleoplasm, cytoplasma, and cell membrane. The target mRNAs whose regulation by nucleolin has been characterized are indicated under ‘mRNA translation’ and ‘mRNA turnover.’
Nucleolin Target Transcripts
Nucleolin affects the stability and/or translation of target mRNAs in multiple ways. Many of nucleolin target mRNAs bear AU-rich elements (AREs), typically present in their 3′-untranslated region (UTR), and/or G-rich sequences distributed in the 5′UTR, coding region (CR), and 3′UTR.13,32 In this section, we will discuss those nucleolin target mRNAs which are best characterized, and we will highlight the impact of the encoded proteins in disease processes (Table 1).
Table 1. mRNA targets of nucleolin.
| mRNA (protein) | Interaction with Nucleolin | Consequenceson mRNA | Other factors involved | Implication in disease | References |
|---|---|---|---|---|---|
| BCL2 (Bcl-2) | 3′UTR ARE | Stabilization | HuR cooperatesAUF1 antagonizes | Cancer (anti-apoptotic) | [32–36] |
| APP (APP) | 3′UTR | Decay | hnRNP C antagonizes | Alzheimer’s Disease | [38–41] |
| IL2 (IL-2) | 5′UTR | Stabilization | YB-1 cooperates | Cancer (immunosurveillance) | [43] |
| GADD45A (Gadd45α) | 3′UTR | Stabilization | TIAR antagonizes(?)AUF1 antagonizes(?) | Cancer (stress response) | [44,45] |
| GAST (Gastrin) | 3′UTR | Stabilization | hnRNP C cooperatesPCBP1 cooperates | Cancer (inflammation) | [46,47] |
| HBB (β-globin) | 3′UTR | Stabilization | hnRNP E cooperates | Thalassemia | [48,49] |
| PGHS1 (Pghs-1) | 5′UTR NRE | Translation repression | NF45, NF90 cooperate(?) | Inflammation | [50,51] |
| TP53 (p53) | 5′UTR | Translation repression | RPL26 antagonizes | Cancer | [52] |
| MMP9 (MMP9) | 3′UTR | Translation enhancement | HuR cooperates(?) | Cancer (invasion) | [53–55] |
| AKT1 (Akt1) | 5′UTR,CR,3′UTR | Translation enhancement | - | Cancer (survival) | [13] |
| CCNI (Cyclin I) | 5′UTR,CR,3′UTR | Translation enhancement | - | Cancer (anti-apoptotic) | [13] |
Nucleolin effect on mRNA turnover
Nucleolin affects mRNA turnover, both increasing and decreasing mRNA half-life, by interacting with the UTRs of target mRNAs. As described below, the regulation of mRNA turnover by nucleolin is often functionally linked to the association of other turnover-regulatory RBPs.
BCL2 mRNA
Nucleolin associates with the 3′UTR ARE of the BCL2 mRNA, enhances its stability, and thus augments expression of the anti-apoptotic, pro-oncogenic protein B-cell lymphoma 2 (Bcl-2).32 Accordingly, elevated expression of nucleolin was linked to the ARE-dependent stabilization of BCL2 mRNA and increased Bcl-2 levels in leukemia cells.32,33 The positive effect of nucleolin on Bcl-2 expression agrees with the finding that nucleolin inhibits oxidative stress-induced apoptosis in human umbilical vascular endothelial cells.34 In HL-60 leukemia cells, nucleolin and the RBP HuR bound the BCL2 ARE concurrently and had a synergistic effect on Bcl-2 expression, as both RBPs stabilized the BCL2 mRNA, while HuR also enhanced its translation;35 by contrast, AUF1 antagonized nucleolin binding to the BCL2 ARE and triggered BCL2 mRNA decay.36 Treatments with all-trans retinoic acid, taxol or okadaic acid enhanced nucleolin degradation and promoted BCL2 mRNA decay.32,37
APP mRNA
The amyloid precursor protein (APP) is linked to Alzheimer’s disease. The 3′UTR of APP mRNA was found to be a substrate for association by nucleolin, as well as by the RBPs hnRNP C and fragile X mental retardation protein (FMRP).38-41 The helicase activity of nucleolin was proposed to accelerate APP mRNA degradation in response to stress, an effect that was counteracted by the stabilizing influence of hnRNP C.41
IL2 mRNA
Interleukin (IL)-2 is a major cytokine implicated in the response against microbial infection and is used in certain cancer therapies.42 Unlike nucleolin’s binding to the 3′UTR of BCL2 and APP mRNAs, nucleolin binds the 5′UTR of IL2 mRNA, and this interaction is required for JNK-mediated IL2 mRNA stabilization during T-cell activation, likely in cooperation with the Y box-binding protein-1 (YB-1).43
GADD45A mRNA
The growth arrest- and DNA damage-inducible 45 protein (Gadd45α) is induced under stress conditions.44 The stress agent arsenite enhances the association of nucleolin with GADD45A mRNA, leading to transcript stabilization.45 Interestingly, GADD45A mRNA is also a target of the translational suppressor TIAR and the decay-promoting protein AUF1, two RBPs that dissociate after treatment with the alkylating agent methyl methanesulfonate (MMS).44 These findings suggest a functional interplay between nucleolin, TIAR, and AUF1 to promote the expression of Gadd45α under stress conditions.
GAST mRNA
The gastrointestinal hormone gastrin, responsible for gastric acid secretion, is highly expressed in malignancies such as pancreatic and colorectal cancers.46 GAST mRNA is co-regulated by nucleolin, PCBP1 [poly(C)-binding protein 1] and hnRNP K, as the three RBPs are required for stabilizing GAST mRNA in cells treated with epidermal growth factor (EGF).47
HBB mRNA
Dysregulation of β-globin, encoded by HBB mRNA, can result in pathologies such as thalassemia.48 Nucleolin binds the HBB 3′UTR mRNA and enhances its stability, likely facilitating the binding of hnRNP E.49
Nucleolin influence on mRNA translation
Nucleolin can also affect the translation of a subset of associated mRNAs. As described below, binding to the 5′UTR often suppresses translation, while binding to the 3′UTR enhances mRNA translation.
PGHS1 mRNA
Binding of nucleolin to the 5′UTR nucleolin response element (NRE) of the prostaglandin endoperoxide H synthase-1 (PGHS1) mRNA reduced translation of the encoded proinflammatory enzyme Pghs-1 in megakaryoblastic cells.50 Interestingly, NF45 and NF90 also associated with the 5′UTR of PGHS1 mRNA and repressed its translation,51 although the possible functional links among these RBPs to control Pghs1 production have not been reported.
TP53 mRNA
Nucleolin and ribosomal protein L26 (RPL26) were found to bind the 5′UTR of the TP53 mRNA, which encodes the tumor suppressor p53. While RPL26 enhanced the translation of TP53 mRNA in response to DNA damage, nucleolin suppressed TP53 mRNA translation and prevented the induction of p53 after DNA damage.52
MMP9 mRNA
Degradation of extracellular matrix (ECM) components by matrix metalloproteinases is essential for processes like wound healing and angiogenesis and is central to pathophysiological conditions such as cancer progression and metastasis.53 Nucleolin binds the 3′UTR of MMP9 mRNA and enhances its translation.54 HuR also binds the 3′UTR of MMP9 mRNA and increases its stability,55 although it is not yet known if HuR and nucleolin may regulate MMP9 expression competitively or cooperatively.
AKT1_,_ CCNI and other G-rich mRNAs
Recently, nucleolin was reported to enhance translation of a subset of target mRNAs by interacting with G-rich elements present in the CR and UTRs. These mRNAs included those that encode the prosurvival proteins Akt1 and cyclin I.13,56,57 Akt is one of the most active kinases in cancer cells, while the anti-apoptotic protein cyclin I is highly expressed in breast cancer and its increased level in serum is associated with pancreatic cancer.56,58,59
Nucleolin and microRNA processing
MicroRNAs (miRNAs) are short RNA molecules (~22-nt long) that potently regulate gene expression.60,61 Primary miRNAs are transcribed and processed into precursor miRNAs in the nucleus; after their export to the cytoplasm, they undergo maturation and are assembled into an RNP silencing complex (RISC) that directs the miRNA to a specific mRNA in order to influence mRNA stability and translation.62 Altered levels of miRNAs have been implicated in a myriad of physiologic and pathologic processes.63-65 Recently, nucleolin was reported to interact with the microprocessor complex and thereby regulated the biogenesis of the pro-tumorigenic microRNAs miR-15a and miR-16.66 It is likely that nucleolin influences the processing of other miRNAs in a similar manner, although this possibility remains to be investigated.
Nucleolin function on viral RNA
Nucleolin influences multiple aspects of viral infection, including association of the virus with the host cell, insertion of the viral genetic material, and utilization of the host cell to produce viral proteins. Nucleolin was shown to be essential for entry of the human parainfluenza virus type 3 (HPIV3) during infection of lung epithelial cells, and functioned as a receptor for the respiratory syncytial virus (RSV).67,68 The synthetic peptide HB-19, a specific antagonist that binds the C-terminal RGG domain of nucleolin, inhibited the attachment of human immunodeficiency virus (HIV) to cells, indicating that this domain of nucleolin could be functional during the process of HIV anchorage.69 Nucleolin is also believed to participate in the infection of Hepatitis C virus (HCV), herpes simplex virus (HSV) type 1, white-spot syndrome virus (WSSV), and the Crimean-Congo hemorrhagic fever virus (CCHFV).70-73 Interestingly, nucleolin forms RNP complexes with the 3′ UTR of feline calicivirus (FCV) and Norwalk virus (NV); in this regard, lowering nucleolin reduced viral protein synthesis and FCV replication, while the mobilization of nucleolin from the nucleolus to the perinuclear region was proposed to enhance viral mRNA translation.74 The association of nucleolin with the 5′UTR IRES of rhinovirus and poliovirus stimulated the translation of viral proteins in vitro and in vivo.75 In sum, nucleolin is important for viral entry to the host cell and for assisting viral RNA translation and viral replication (Fig. 1).
Regulation of Nucleolin Function, Implication in Disease
Regulation of nucleolin abundance
It is well established that nucleolin promotes cell proliferation and survival linked to disease processes like carcinogenesis, but the mechanisms that control nucleolin abundance are not well understood. In phorbol ester-treated peripheral blood mononuclear treated cells, the levels of NCL mRNA, which encodes nucleolin, increased transcriptionally via the activity of extracellular-regulated kinase (ERK).40 At the post-transcriptional level, HuR was found to interact with the 3′UTR of NCL mRNA and promoted its translation, while miR-494 competed with HuR and repressed nucleolin translation.13 Post-translationally, nucleolin abundance was shown to be controlled by proteolysis. Oxidative stress increased the cleavage of nucleolin and lowered the levels of intact nucleolin; in laryngeal squamous cell carcinoma (LSCC), the 70-kDa heat shock protein (HSP70) inhibited the cleavage and degradation of nucleolin, likely impacting on LSCC radiotherapy resistance, and in lung cancer cells, HSP90 protected nucleolin stability during mitosis.76,77 The self-cleaving activity of nucleolin was significantly decreased in dividing T lymphocytes, likely due to the increased concentration of proteolytic inhibitors in the nuclei of these cells.78 LYAR, a zinc finger nucleolar oncoprotein that is highly expressed in undifferentiated embryonic stem cells (ESCs), associated with nucleolin, prevented its self-cleavage, and elevated nucleolin concentration.79 It is not yet known if the truncated nucleolin is functional.
Control of nucleolin localization
Nucleolin is ubiquitously expressed in proliferating tissues and is primarily localized in the nucleolus. Although it contains a specific bipartite nuclear localization signal, its accumulation in the nucleolus is thought to depend on its affinity for nucleolar components.7 Phosphorylation by Cdc2 (also known as Cdk1) promotes the cytoplasmic localization of nucleolin, while dephosphorylated nucleolin resides in the nucleus.80 Under stress conditions such as heat shock, ionizing radiation, and treatment with camptothecin, nucleolin is mobilized from the nucleolus to the nucleoplasm in a p53-dependent manner, likely exerting a transient influence on DNA replication and repair.81
Although in lesser abundance than in the nucleolus, nucleolin is also localized in the cytoplasm and on the cell membrane. In the cytoplasm, nucleolin binds mRNAs to influence their stability and translation, as described above. However, the function of nucleolin on the plasma membrane is less clear. Perhaps nucleolin transports RNA molecules to the cell membrane in order to affect cell fate, is mobilized to the cell membrane for transient repression of its nuclear and cytoplasmic functions, or functions as a receptor for extracellular RNA that needs to be internalized. The latter putative activity is reminiscent of the actions of the glucocorticoid receptor, which associates with a subset of cytoplasmic mRNAs and regulates their turnover rates.82 It also resembles the function of the transmembrane receptor DCC (‘deleted in colorectal carcinoma’), which recruits translational components and retains them in an inactive state; in neurons, association of netrin-1 with DCC led the the release of the translation initiation machinery and promoted translation.83
The cell surface localization of nucleolin has received considerable attention in the past few years. Its high expression on the surface of cancer cells is believed to facilitate processes that affect the fate of the cancer cell.84,85 In this regard, reducing the levels or activity of cell-surface nucleolin inhibited the growth of cultured hepatocellular carcinoma cells and triggered endothelial cell apoptosis.86,87 Cell-surface nucleolin also mediated signaling events such as calcium entry and calcium-regulated pathways, lipopolysaccharide-stimulated expression and secretion of TNFα and IL-1β, and P-selectin-induced pathways that regulate cell adhesion and spreading.88-90 Nucleolin was found to be the receptor of endostatin and peptides F3 and N6L.91-93
Besides tumorigenesis, angiogenesis, and signaling pathways, cell-surface nucleolin also participates in viral and bacterial infection. For example, HIV inhibitors such as pleiotrophin (PTN), the pseudopeptide HB-19, and the cytokine midkine bind specifically to cell surface-expressed nucleolin and inhibit HIV entry, indicating that nucleolin facilitates viral entry to the host cell.94 In addition, nucleolin plays an important ligand-binding role in Francisella tularensis bacterial infection, facilitating host tissue invasion.95
Regulation of nucleolin by phosphorylation
Nucleolin is phosphorylated by numerous kinases, notably Cdc2, casein kinase II (CKII), protein kinase C (PKC)ζ, and the stress-activated protein kinase (SAPK) p38;8,10,96-98 additional kinases include the nucleolar-type NII protein kinase, casein kinase-like ectoprotein kinase, phosphatidylinositol 3-kinase (PI3K), Akt, and Rho-associated kinase. A number of these phosphorylation events were linked to cellular processes and diseases associated with changes in the subcellular localization of nucleolin, as well as to the interaction of nucleolin with several target mRNAs.
Cdc2
Nucleolin is phosphorylated by Cdc2 during mitosis and associates with metaphase chromosomes; in addition, Cdc2-phosphorylated nucleolin is enriched in cytoplasmic neurofibrillary tangles found in neurons from Alzheimer’s disease patients, suggesting that Cdc2-phosphorylated nucleolin may link two distinct processes, mitosis and neurodegeneration.21 In Xenopus laevis, phosphorylation by Cdc2 promotes the cytoplasmic localization of nucleolin, while unphosphorylated nucleolin was preferentially nuclear;99 an opposite scheme was seen for HuR, whose phosphorylation by Cdc2 promoted its nuclear localization, affecting its RNA-binding activity and its anti-apoptotic function.100,101
p38
Phosphorylation by p38 increased the RNA-binding activity of nucleolin following exposure to stresses like UV light (UV) and ionizing radiation, leading to enhanced interaction of nucleolin with a subset of mRNAs encoding stress-response proteins (e.g., glutathione peroxidase, peroxiredoxin 1, and HSP90).21 This regulation also recapitulates the increased affinity of HuR for CDKN1A mRNA (encoding the cdk inhibitor p21) after phosphorylation by p38 following ionizing radiation.102
CKII
Phosphorylation by CKII and cdc2 promoted nucleolin’s helicase activity on different double-stranded nucleic acids, including RNA-RNA and RNA-DNA duplexes. This activity was linked to nucleolin’s influence on cell cycle progression, embryonic development, and tumorigenesis.103-105 The proteolytic cleavage of nucleolin into 30- and 72-kDa peptides was also enhanced by CKII.106
PKC
Phosphorylation of nucleolin by PKCζ reportedly mediated the effects of nerve growth factor (NGF) on rat pheochromocytoma PC12 cells, transmitting NGF signaling from the cell membrane to the inside of the cell.107 Activation of PKC leading to nucleolin phosphorylation was proposed to mediate the transcriptional activation of the cytosolic phospholipase A2 (cPLA2) by transcription factors c-Jun and Sp1 and to affect NF-κB transcriptional activity.107-109
Other post-translational modifications of nucleolin
Nucleolin is also post-translationally modified via methylation, glycosylation and ADP-ribosylation. With approximately one-third of its arginine residues methylated, nucleolin is one of the most highly methylated nuclear proteins.110 Recently, the RIO kinase 1 was shown to recruit nucleolin to the arginine methyltransferase (PRMT5) complex, suggesting that PRMT5 could be responsible for methylating nucleolin.111 Nucleolin methylation appeared to affect its interaction with DNA and RNA,112,113 but its influence upon mRNA stabilization or translation has not yet been examined. Nucleolin is glycosylated in Chinese hamster ovary cells (CHO) and in the lymphoma-derived cell line U937.114,115 Although N- and O-glycosylation of nucleolin promotes its localization and function on the plasma membrane,116 it also remains to be studied whether it affects its RNA-binding activity. Nucleolin is modified by ADP-ribosylation,117 but the consequence of this modification upon nucleolin’s RNA-binding function is unknown.
The mechanisms that mediate nucleolin glycosylation and recruitment to the plasma membrane are still ill-defined. In this regard, nucleolin does not have a signal peptide sequence that would target it to the endoplasmic reticulum, where glycosylation and protein targeting to the membrane are initiated. It is possible that nucleolin overexpression in pathologic states results in its localization in unusual sites like the plasma membrane.
Nucleolin and the Hallmarks of Cancer
Nucleolin is highly expressed in proliferating cells, including stem cells and cancer cells. The oncogenic effect of nucleolin appears to be multifactorial, in keeping with the many functions of this protein. Cells acquire a number of features in order to become malignant, including the abilities to grow and proliferate, overcome senescence, evade apoptosis and the immune system, invade and metastasize other tissues, and promote angiogenesis.118,119 Nucleolin can enable these traits through its RNA-binding activity, as elaborated below.
Toward implementing an anti-apoptotic phenotype, nucleolin modulates the expression of several proteins that influence the survival of malignant cells during cell damage. As explained above, nucleolin binds to BCL2 mRNA and promotes the expression of proto-oncogene Bcl-2 (explained above), which blocks apoptosis in cancer cells,120 and it binds to AKT1 mRNA and enhances translation of Akt1, another potent pro-survival protein.56 Additionally, nucleolin associates with the 5′UTR of TP53 mRNA and reduces the translation of the pro-apoptotic tumor suppressor p53,52 further promoting an anti-apoptotic cell phenotype.
Another protein under positive regulation by nucleolin, gastrin, is highly expressed in gastrointestinal cancers and functions as a pro-survival factor that enhances cancer cell proliferation, angiogenesis, and migration.46,47 Indeed, enhanced expression of gastrin by nucleolin is directly relevant to the tumorigenicity of human colon cancer cells and upregulates cyclooxygenase-2 (COX-2), which in turn catalyzes prostaglandin E2 production and promotes tumor cell proliferation.121,122 Nucleolin may also assist cancer cells in overcoming cellular senescence, as it associates with the enzyme telomerase (TERT) and its RNA template (TERC) in the nucleoplasm. Through these interactions, nucleolin may assist in the localization, assembly, and/or maintenance of telomeres,123 whose compromised integrity can trigger senescence. In keeping with this broad influence on cancer gene expression programs, a subset of nucleolin-associated mRNAs encoding tumor-related proteins were recently identified in mitotic cells.21,77
Degradation of the extracellular matrix (ECM) is essential for angiogenesis and metastasis.124,125 Since nucleolin associates with MMP9 mRNA and enhances its translation, it may help cancer cells to invade and metastasize. This effect on the ECM could be further amplified by nucleolin’s influence on gastrin, itself an inducer of MMP9 expression.126
Nucleolin can also enhance oncogenesis in ways that are independent of its mRNA-binding activity. For instance, nucleolin transcriptionally promotes expression of the vascular endothelial growth factor (VEGF) and the interferon regulatory factor-2 (IRF-2), other proteins that are highly expressed in cancers and are potent regulators of cancer cell growth.127-129 In addition, as explained above, cell-surface nucleolin functions as a receptor for tumorigenic factors like the carcinogenic factor released from Helicobacter pylori Tipα (the TNFα-inducing protein); nucleolin shuttles Tipα from the cell membrane to the cytosol and the nucleus, where it induces the expression of TNF-α and chemokines, and promotes inflammation.130
Targeting Nucleolin for Therapy
Nucleolin is an attractive target for therapy in cancer and viral infection given its high abundance, its multifaceted influence on oncogenesis, and its selective presence on the plasma membrane of cancer cells, but not untransformed, normal cells. A number of therapeutic efforts have been directed at controlling nucleolin levels and activity.
Nucleolin-directed anticancer aptamers
Aptamers are short, stable DNA or RNA molecules that can target specific cellular and extracellular targets with high affinity.131,132 The aptamer AS1411, also known as AGRO100, a 26-mer unmodified guanosine (G)-rich oligonucleotide [5′-d(GGTGGTGGTGGTTGTGGTGGTGGTGG)-3′], is among the aptamers most extensively studied and used. Nucleolin has high affinity for AS1411, in agreement with the strong binding of nucleolin to G-quadruplex DNA sequences.133,134 AS1411 may directly inhibit the RNA-binding activity of nucleolin, as AS1411 reduced nucleolin binding to BCL2 mRNA resulting in BCL2 mRNA destabilization, decreased Bcl-2 protein levels, apoptotic death of cultured human breast cancer cells, and inhibition of cancer cell growth in xenografts.135,136 Other effects of AS1411 include the inhibition of NF-κB via the formation of a complex containing nucleolin and the NF-κB essential modulator (NEMO).137 Cell-surface nucleolin mediates the effects of this aptamer through binding and internalization leading to inhibition of cell growth. The promising effects of this aptamer have led to several clinical trials in patients with advanced solid tumors.138
Nucleolin-directed anticancer peptides
As explained above, the synthetic peptide HB-19 specifically antagonizes the C-terminal RGG domain of nucleolin and inhibits cell membrane-associated nucleolin function, thereby preventing the growth, angiogenesis, and tumorigenicity of numerous cancer cells using different assays.139,140 The V3 loop-mimicking pseudopeptide 5[Kpsi(CH2N)PR]-TASP binds to the cell-surface nucleolin in monocyte-derived macrophages and synergizes with the inhibitory effects of β-chemokines on HIV infection.141 Another synthetic ligand targeting cell-surface nucleolin, N6L, also displays potent anti-tumor activities, enhances apoptosis, blocks angiogenesis, and prevents tumor growth.92 Cell-surface nucleolin was found to be the receptor of a specific tumor-homing peptide, F3, which is internalized by vascular endothelial cells and by some tumor cells.91 Although these ligands can impair nucleolin binding to target mRNAs, it is not yet known if their antitumor actions are explained by nucleolin’s altered RNA-binding activity.
Nucleolin ligand endostatin
The anticancer drug endostatin potently reduces endothelial function and lowers angiogenesis in tumor tissues, in part by inhibiting the action of VEGF.142 Nucleolin expressed on the surface of lymphangiogenic endothelial cells functions as a receptor of endostatin and mediates its anti-metastatic actions and its inhibitory influence on lymphatic angiogenesis.143 Interestingly, VEGF itself stimulates the mobilization of nucleolin to the cell surface via the action of the cytoskeletal motor MyH9 (nonmuscle myosin heavy chain 9), suggesting a possible negative feedback mechanism.144
Nucleolin-directed siRNA and microRNA therapy
MicroRNAs play important roles in gene regulation through binding and regulating target mRNAs and thus influencing protein levels. The fact that many miRNAs are dysregulated in cancer and elicit tumor suppressive or oncogenic functions has stimulated efforts toward miRNA-based therapy.145,146 Recently, miR-494 was found to bind the NCL 3′UTR and repressed nucleolin expression, an effect that was competed by HuR, which also bound the NCL 3′UTR and instead elevated nucleolin abundance. Similar to AS1411 and N6L, miR-494 significantly reduced the survival of cancer cells.147 Future studies will determine if miR-494 might have therapeutic value by targeting nucleolin in cancer cells. Small interfering (si)RNA approaches aimed at reducing nucleolin levels are also emerging; for example, treatment of nasopharyngeal carcinoma (NPC) cells with antisense phosphorothioate-modified oligodeoxynucleotides (S-ODNs) directed at NCL mRNA triggered NPC apoptosis, while administration of NCL S-ODNs to mice suppressed the growth of NPC tumor xenografts.148
Nucleolin-based viral therapy
HIV inhibitors such as pleiotrophin (PTN), the pseudopeptide HB-19,69 and the cytokine midkine bind specifically to cell surface-expressed nucleolin and inhibit HIV entry, indicating that nucleolin facilitates viral entry into host cells.94 A receptor for human RSV, nucleolin reduction by RNA interference (RNAi)-mediated knockdown lowered RSV infection in mice.68
Concluding Remarks
Through functions independent of sequence-specific RNA-binding, including transcriptional regulation and ribosome biogenesis, nucleolin is a long-recognized inducer of cell proliferation. However, over the past decade, nucleolin has established a solid presence as a sequence-specific RNA-binding protein with key roles in disease. Via the direct interaction of nucleolin with subsets of target mRNAs, typically bearing AU- or G-rich sequences, nucleolin modulates the stability or translation of transcripts encoding several cancer-related proteins, including Bcl-2, MMP-9, gastrin, PGHS1, p53, Akt1 and cyclin I. These associations have strengthened nucleolin’s pro-growth and pro-survival function. Nonetheless, it will be important to identify the complete set of nucleolin target RNAs, including coding and non-coding transcripts. The use of techniques such as PAR-CLIP (photoactivatable-ribonucleoside-enhanced crosslinking and immunoprecipitation)149 can provide a catalog of nucleolin-target RNAs and uncover a more complete spectrum of nucleolin actions.
As the analysis of nucleolin’s RNA-binding activity progresses, it will be particularly interesting to investigate if cell-surface nucleolin binds RNA. Positioned on the plasma membrane, nucleolin can serve as a receptor for extracellular RNA, perhaps RNA actively released by neighboring cells as extracellular RNPs or possibly RNA remnants from lysed cells. Thus, the selective identification of RNA present in cell-surface nucleolin may yield exciting new insight on nucleolin function.
Cell-membrane nucleolin serves as a receptor for a number of viruses and is a strong diagnostic marker of cancer cells, as described above. Thus, its accessible plasma membrane localization has been exploited in different ways. It is the target of aptamers, including the G-rich oligonucleotide AS1411 that is presently in cancer clinical trials; in this regard, perhaps other G- or AU-rich oligonucleotides could be identified with superior inhibitory actions. Additional anti-cancer and anti-viral approaches directed at cell-membrane nucleolin include artificial and endogenous peptides. Chemical inhibitors directed toward nucleolin or its many post-translational regulators (e.g., cdc2, CKII, p38) may also prove therapeutically viable. Moreover, targeting intracellular nucleolin through the use of RNAi, miRNA (particularly miR-494) or intracellularly delivered G-rich RNA, may also prove to be effective.
Besides cancer and viral infection, nucleolin inhibition could be beneficial for treating inflammation. Since nucleolin promotes expression of gastrin, an inducer of COX-2 expression, efforts to inhibit the RNA-binding activity of nucleolin might be particularly helpful in treating inflammatory diseases of the gastrointestinal system. Furthermore, nucleolin mediates the effects of Tipα on TNF-α, and thus modulates inflammation in the cancer microenvironment.130,150 Thus, the impact of the RNA-binding activity of nucleolin upon inflammation warrants future investigation. Finally, the association of nucleolin with the microprocessor and its influence on microRNA biosynthesis sets the stage for future work to investigate if some of nucleolin’s effects are elicited via microRNAs. In closing, despite the knowledge accumulated for this long-known multifunctional protein, many questions still remain, particularly regarding nucleolin’s impact on protein expression programs. As we strive to answer them, we will gain critical insight into nucleolin’s role in tumorigenesis, infection, and inflammatory diseases, and will be better able to target nucleolin therapeutically.
Acknowledgments
We thank Jennifer L. Martindale for critical reading of the article. This work was entirely supported by the Intramural Research Program of the National Institute on Aging, NIH.
Glossary
Abbreviations:
ARE
AU-rich element
CR
coding region
RBP
RNA-binding protein
RNP
ribonucleoprotein
UTR
untranslated region
Footnotes
References
- 1.Orphanides G, Reinberg D. A unified theory of gene expression. Cell. 2002;108:439–51. doi: 10.1016/S0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Tollervey D. mRNA stability in eukaryotes. Curr Opin Genet Dev. 2000;10:193–8. doi: 10.1016/S0959-437X(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 3.Krecic AM, Swanson MS. hnRNP complexes: composition, structure, and function. Curr Opin Cell Biol. 1999;11:363–71. doi: 10.1016/S0955-0674(99)80051-9. [DOI] [PubMed] [Google Scholar]
- 4.He Y, Smith R. Nuclear functions of heterogeneous nuclear ribonucleoproteins A/B. Cell Mol Life Sci. 2009;66:1239–56. doi: 10.1007/s00018-008-8532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orrick LR, Olson MO, Busch H. Comparison of nucleolar proteins of normal rat liver and Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1973;70:1316–20. doi: 10.1073/pnas.70.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56:379–90. doi: 10.1016/0092-8674(89)90241-9. [DOI] [PubMed] [Google Scholar]
- 7.Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J Cell Sci. 1999;112:761–72. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- 8.Tuteja R, Tuteja N. Nucleolin: a multifunctional major nucleolar phosphoprotein. Crit Rev Biochem Mol Biol. 1998;33:407–36. doi: 10.1080/10409239891204260. [DOI] [PubMed] [Google Scholar]
- 9.Mongelard F, Bouvet P. Nucleolin: a multiFACeTed protein. Trends Cell Biol. 2007;17:80–6. doi: 10.1016/j.tcb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Srivastava M, Pollard HB. Molecular dissection of nucleolin’s role in growth and cell proliferation: new insights. FASEB J. 1999;13:1911–22. [PubMed] [Google Scholar]
- 11.Tajrishi MM, Tuteja R, Tuteja N. Nucleolin: The most abundant multifunctional phosphoprotein of nucleolus. Commun Integr Biol. 2011;4:267–75. doi: 10.4161/cib.4.3.14884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roger B, Moisand A, Amalric F, Bouvet P. Nucleolin provides a link between RNA polymerase I transcription and pre-ribosome assembly. Chromosoma. 2003;111:399–407. doi: 10.1007/s00412-002-0221-5. [DOI] [PubMed] [Google Scholar]
- 13.Abdelmohsen K, Tominaga K, Lee EK, Srikantan S, Kang MJ, Kim MM, et al. Enhanced translation by Nucleolin via G-rich elements in coding and non-coding regions of target mRNAs. Nucleic Acids Res. 2011;39:8513–30. doi: 10.1093/nar/gkr488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serin G, Joseph G, Faucher C, Ghisolfi L, Bouche G, Amalric F, et al. Localization of nucleolin binding sites on human and mouse pre-ribosomal RNA. Biochimie. 1996;78:530–8. doi: 10.1016/0300-9084(96)84759-6. [DOI] [PubMed] [Google Scholar]
- 15.Bouvet P, Diaz JJ, Kindbeiter K, Madjar JJ, Amalric F. Nucleolin interacts with several ribosomal proteins through its RGG domain. J Biol Chem. 1998;273:19025–9. doi: 10.1074/jbc.273.30.19025. [DOI] [PubMed] [Google Scholar]
- 16.Ghisolfi L, Kharrat A, Joseph G, Amalric F, Erard M. Concerted activities of the RNA recognition and the glycine-rich C-terminal domains of nucleolin are required for efficient complex formation with pre-ribosomal RNA. Eur J Biochem. 1992;209:541–8. doi: 10.1111/j.1432-1033.1992.tb17318.x. [DOI] [PubMed] [Google Scholar]
- 17.Seinsoth S, Uhlmann-Schiffler H, Stahl H. Bidirectional DNA unwinding by a ternary complex of T antigen, nucleolin and topoisomerase I. EMBO Rep. 2003;4:263–8. doi: 10.1038/sj.embor.embor770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollice A, Zibella MP, Bilaud T, Laroche T, Pulitzer JF, Gilson E. In vitro binding of nucleolin to double-stranded telomeric DNA. Biochem Biophys Res Commun. 2000;268:909–15. doi: 10.1006/bbrc.2000.2237. [DOI] [PubMed] [Google Scholar]
- 19.Khurts S, Masutomi K, Delgermaa L, Arai K, Oishi N, Mizuno H, et al. Nucleolin interacts with telomerase. J Biol Chem. 2004;279:51508–15. doi: 10.1074/jbc.M407643200. [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Kim MS, Chakravarty D, Indig FE, Carrier F. Nucleolin Binds to the Proliferating Cell Nuclear Antigen and Inhibits Nucleotide Excision Repair. Mol Cell Pharmacol. 2009;1:130–7. doi: 10.4255/mcpharmacol.09.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C, Maiguel DA, Carrier F. Identification of nucleolin and nucleophosmin as genotoxic stress-responsive RNA-binding proteins. Nucleic Acids Res. 2002;30:2251–60. doi: 10.1093/nar/30.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Storck S, Shukla M, Dimitrov S, Bouvet P. Functions of the histone chaperone nucleolin in diseases. Subcell Biochem. 2007;41:125–44. doi: 10.1007/1-4020-5466-1_7. [DOI] [PubMed] [Google Scholar]
- 23.Yang TH, Tsai WH, Lee YM, Lei HY, Lai MY, Chen DS, et al. Purification and characterization of nucleolin and its identification as a transcription repressor. Mol Cell Biol. 1994;14:6068–74. doi: 10.1128/MCB.14.9.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erard MS, Belenguer P, Caizergues-Ferrer M, Pantaloni A, Amalric F. A major nucleolar protein, nucleolin, induces chromatin decondensation by binding to histone H1. Eur J Biochem. 1988;175:525–30. doi: 10.1111/j.1432-1033.1988.tb14224.x. [DOI] [PubMed] [Google Scholar]
- 25.Angelov D, Bondarenko VA, Almagro S, Menoni H, Mongélard F, Hans F, et al. Nucleolin is a histone chaperone with FACT-like activity and assists remodeling of nucleosomes. EMBO J. 2006;25:1669–79. doi: 10.1038/sj.emboj.7601046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uribe DJ, Guo K, Shin YJ, Sun D. Heterogeneous nuclear ribonucleoprotein K and nucleolin as transcriptional activators of the vascular endothelial growth factor promoter through interaction with secondary DNA structures. Biochemistry. 2011;50:3796–806. doi: 10.1021/bi101633b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allain FH, Bouvet P, Dieckmann T, Feigon J. Molecular basis of sequence-specific recognition of pre-ribosomal RNA by nucleolin. EMBO J. 2000;19:6870–81. doi: 10.1093/emboj/19.24.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ginisty H, Amalric F, Bouvet P. Nucleolin functions in the first step of ribosomal RNA processing. EMBO J. 1998;17:1476–86. doi: 10.1093/emboj/17.5.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ginisty H, Serin G, Ghisolfi-Nieto L, Roger B, Libante V, Amalric F, et al. Interaction of nucleolin with an evolutionarily conserved pre-ribosomal RNA sequence is required for the assembly of the primary processing complex. J Biol Chem. 2000;275:18845–50. doi: 10.1074/jbc.M002350200. [DOI] [PubMed] [Google Scholar]
- 30.Xue Z, Mélèse T. Nucleolar proteins that bind NLSs: a role in nuclear import or ribosome biogenesis? Trends Cell Biol. 1994;4:414–7. doi: 10.1016/0962-8924(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 31.Lo SJ, Lee CC, Lai HJ. The nucleolus: reviewing oldies to have new understandings. Cell Res. 2006;16:530–8. doi: 10.1038/sj.cr.7310070. [DOI] [PubMed] [Google Scholar]
- 32.Sengupta TK, Bandyopadhyay S, Fernandes DJ, Spicer EK. Identification of nucleolin as an AU-rich element binding protein involved in bcl-2 mRNA stabilization. J Biol Chem. 2004;279:10855–63. doi: 10.1074/jbc.M309111200. [DOI] [PubMed] [Google Scholar]
- 33.Willimott S, Wagner SD. Post-transcriptional and post-translational regulation of Bcl2. Biochem Soc Trans. 2010;38:1571–5. doi: 10.1042/BST0381571. [DOI] [PubMed] [Google Scholar]
- 34.Zhang B, Wang H, Jiang B, Liang P, Liu M, Deng G, et al. Nucleolin/C23 is a negative regulator of hydrogen peroxide-induced apoptosis in HUVECs. Cell Stress Chaperones. 2010;15:249–57. doi: 10.1007/s12192-009-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishimaru D, Ramalingam S, Sengupta TK, Bandyopadhyay S, Dellis S, Tholanikunnel BG, et al. Regulation of Bcl-2 expression by HuR in HL60 leukemia cells and A431 carcinoma cells. Mol Cancer Res. 2009;7:1354–66. doi: 10.1158/1541-7786.MCR-08-0476. [DOI] [PubMed] [Google Scholar]
- 36.Ishimaru D, Zuraw L, Ramalingam S, Sengupta TK, Bandyopadhyay S, Reuben A, et al. Mechanism of regulation of bcl-2 mRNA by nucleolin and A+U-rich element-binding factor 1 (AUF1) J Biol Chem. 2010;285:27182–91. doi: 10.1074/jbc.M109.098830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Otake Y, Sengupta TK, Bandyopadhyay S, Spicer EK, Fernandes DJ. Drug-induced destabilization of bcl-2 mRNA: a new approach for inducing apoptosis in tumor cells. Curr Opin Investig Drugs. 2004;5:616–22. [PubMed] [Google Scholar]
- 38.Zaidi SH, Malter JS. Nucleolin and heterogeneous nuclear ribonucleoprotein C proteins specifically interact with the 3′-untranslated region of amyloid protein precursor mRNA. J Biol Chem. 1995;270:17292–8. doi: 10.1074/jbc.270.29.17292. [DOI] [PubMed] [Google Scholar]
- 39.Rajagopalan LE, Westmark CJ, Jarzembowski JA, Malter JS. hnRNP C increases amyloid precursor protein (APP) production by stabilizing APP mRNA. Nucleic Acids Res. 1998;26:3418–23. doi: 10.1093/nar/26.14.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westmark CJ, Malter JS. Up-regulation of nucleolin mRNA and protein in peripheral blood mononuclear cells by extracellular-regulated kinase. J Biol Chem. 2001;276:1119–26. doi: 10.1074/jbc.M009435200. [DOI] [PubMed] [Google Scholar]
- 41.Westmark CJ, Malter JS. Extracellular-regulated kinase controls beta-amyloid precursor protein mRNA decay. Brain Res Mol Brain Res. 2001;90:193–201. doi: 10.1016/S0169-328X(01)00112-7. [DOI] [PubMed] [Google Scholar]
- 42.Dillman RO. Cancer immunotherapy. Cancer Biother Radiopharm. 2011;26:1–64. doi: 10.1089/cbr.2010.0902. [DOI] [PubMed] [Google Scholar]
- 43.Chen CY, Gherzi R, Andersen JS, Gaietta G, Jürchott K, Royer HD, et al. Nucleolin and YB-1 are required for JNK-mediated interleukin-2 mRNA stabilization during T-cell activation. Genes Dev. 2000;14:1236–48. [PMC free article] [PubMed] [Google Scholar]
- 44.Lal A, Abdelmohsen K, Pullmann R, Kawai T, Galban S, Yang X, et al. Posttranscriptional derepression of GADD45alpha by genotoxic stress. Mol Cell. 2006;22:117–28. doi: 10.1016/j.molcel.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Bhatia D, Xia H, Castranova V, Shi X, Chen F. Nucleolin links to arsenic-induced stabilization of GADD45alpha mRNA. Nucleic Acids Res. 2006;34:485–95. doi: 10.1093/nar/gkj459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ferrand A, Wang TC. Gastrin and cancer: a review. Cancer Lett. 2006;238:15–29. doi: 10.1016/j.canlet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 47.Lee PT, Liao PC, Chang WC, Tseng JT. Epidermal growth factor increases the interaction between nucleolin and heterogeneous nuclear ribonucleoprotein K/poly(C) binding protein 1 complex to regulate the gastrin mRNA turnover. Mol Biol Cell. 2007;18:5004–13. doi: 10.1091/mbc.E07-04-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peixeiro I, Silva AL, Romão L. Control of human beta-globin mRNA stability and its impact on beta-thalassemia phenotype. Haematologica. 2011;96:905–13. doi: 10.3324/haematol.2010.039206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Y, Xu XS, Russell JE. A nucleolin-binding 3′ untranslated region element stabilizes beta-globin mRNA in vivo. Mol Cell Biol. 2006;26:2419–29. doi: 10.1128/MCB.26.6.2419-2429.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bunimov N, Smith JE, Gosselin D, Laneuville O. Translational regulation of PGHS-1 mRNA: 5′ untranslated region and first two exons conferring negative regulation. Biochim Biophys Acta. 2007;1769:92–105. doi: 10.1016/j.bbaexp.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Bunimov N, Laneuville O. Characterization of proteins associating with 5′ terminus of PGHS-1 mRNA. Cell Mol Biol Lett. 2010;15:196–214. doi: 10.2478/s11658-010-0005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takagi M, Absalon MJ, McLure KG, Kastan MB. Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell. 2005;123:49–63. doi: 10.1016/j.cell.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 53.Krizkova S, Zitka O, Masarik M, Adam V, Stiborova M, Eckschlager T, et al. Clinical importance of matrix metalloproteinases. Bratisl Lek Listy. 2011;112:435–40. [PubMed] [Google Scholar]
- 54.Fähling M, Steege A, Perlewitz A, Nafz B, Mrowka R, Persson PB, et al. Role of nucleolin in posttranscriptional control of MMP-9 expression. Biochim Biophys Acta. 2005;1731:32–40. doi: 10.1016/j.bbaexp.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Huwiler A, Akool el-S, Aschrafi A, Hamada FM, Pfeilschifter J, Eberhardt W. ATP potentiates interleukin-1 beta-induced MMP-9 expression in mesangial cells via recruitment of the ELAV protein HuR. J Biol Chem. 2003;278:51758–69. doi: 10.1074/jbc.M305722200. [DOI] [PubMed] [Google Scholar]
- 56.Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cell Signal. 2011;23:1515–27. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Griffin SV, Olivier JP, Pippin JW, Roberts JM, Shankland SJ. Cyclin I protects podocytes from apoptosis. J Biol Chem. 2006;281:28048–57. doi: 10.1074/jbc.M513336200. [DOI] [PubMed] [Google Scholar]
- 58.Landberg G, Nilsson K, Jirström K, Rydén L, Kitching R, Burger AM, et al. Cyclin I is expressed in human breast cancer and closely associated with VEGF and KDR expression. Breast Cancer Res Treat. 2005;89:313–6. doi: 10.1007/s10549-004-2230-y. [DOI] [PubMed] [Google Scholar]
- 59.Sun ZL, Zhu Y, Wang FQ, Chen R, Peng T, Fan ZN, et al. Serum proteomic-based analysis of pancreatic carcinoma for the identification of potential cancer biomarkers. Biochim Biophys Acta. 2007;1774:764–71. doi: 10.1016/j.bbapap.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Chekulaeva M, Filipowicz W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr Opin Cell Biol. 2009;21:452–60. doi: 10.1016/j.ceb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Du T, Zamore PD. microPrimer: the biogenesis and function of microRNA. Development. 2005;132:4645–52. doi: 10.1242/dev.02070. [DOI] [PubMed] [Google Scholar]
- 63.Miller BH, Wahlestedt C. MicroRNA dysregulation in psychiatric disease. Brain Res. 2010;1338:89–99. doi: 10.1016/j.brainres.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol. 2009;21:461–9. doi: 10.1016/j.ceb.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li M, Li J, Ding X, He M, Cheng SY. microRNA and cancer. AAPS J. 2010;12:309–17. doi: 10.1208/s12248-010-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pickering BF, Yu D, Van Dyke MW. Nucleolin protein interacts with microprocessor complex to affect biogenesis of microRNAs 15a and 16. J Biol Chem. 2011;286:44095–103. doi: 10.1074/jbc.M111.265439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bose S, Basu M, Banerjee AK. Role of nucleolin in human parainfluenza virus type 3 infection of human lung epithelial cells. J Virol. 2004;78:8146–58. doi: 10.1128/JVI.78.15.8146-8158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tayyari F, Marchant D, Moraes TJ, Duan W, Mastrangelo P, Hegele RG. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat Med. 2011;17:1132–5. doi: 10.1038/nm.2444. [DOI] [PubMed] [Google Scholar]
- 69.Nisole S, Said EA, Mische C, Prevost MC, Krust B, Bouvet P, et al. The anti-HIV pentameric pseudopeptide HB-19 binds the C-terminal end of nucleolin and prevents anchorage of virus particles in the plasma membrane of target cells. J Biol Chem. 2002;277:20877–86. doi: 10.1074/jbc.M110024200. [DOI] [PubMed] [Google Scholar]
- 70.Xiao X, Feng Y, Zhu Z, Dimitrov DS. Identification of a putative Crimean-Congo hemorrhagic fever virus entry factor. Biochem Biophys Res Commun. 2011;411:253–8. doi: 10.1016/j.bbrc.2011.06.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirano M, Kaneko S, Yamashita T, Luo H, Qin W, Shirota Y, et al. Direct interaction between nucleolin and hepatitis C virus NS5B. J Biol Chem. 2003;278:5109–15. doi: 10.1074/jbc.M207629200. [DOI] [PubMed] [Google Scholar]
- 72.Xing Y, Shi Z. Nucleocapsid protein VP15 of White spot syndrome virus colocalizes with the nucleolar proteins nucleolin and fibrillarin. Can J Microbiol. 2011;57:759–64. doi: 10.1139/w11-061. [DOI] [PubMed] [Google Scholar]
- 73.Sagou K, Uema M, Kawaguchi Y. Nucleolin is required for efficient nuclear egress of herpes simplex virus type 1 nucleocapsids. J Virol. 2010;84:2110–21. doi: 10.1128/JVI.02007-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cancio-Lonches C, Yocupicio-Monroy M, Sandoval-Jaime C, Galvan-Mendoza I, Ureña L, Vashist S, et al. Nucleolin interacts with the feline calicivirus 3′ untranslated region and the protease-polymerase NS6 and NS7 proteins, playing a role in virus replication. J Virol. 2011;85:8056–68. doi: 10.1128/JVI.01878-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izumi RE, Valdez B, Banerjee R, Srivastava M, Dasgupta A. Nucleolin stimulates viral internal ribosome entry site-mediated translation. Virus Res. 2001;76:17–29. doi: 10.1016/S0168-1702(01)00240-4. [DOI] [PubMed] [Google Scholar]
- 76.Xu J, Wang K, Zhang X, Qiu Y, Huang D, Li W, et al. HSP70: a promising target for laryngeal carcinoma radiaotherapy by inhibiting cleavage and degradation of nucleolin. J Exp Clin Cancer Res. 2010;29:106. doi: 10.1186/1756-9966-29-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang SA, Li HY, Hsu TI, Chen SH, Wu CJ, Chang WC, et al. Heat shock protein 90 stabilizes nucleolin to increase mRNA stability in mitosis. J Biol Chem. 2011;286:43816–29. doi: 10.1074/jbc.M111.310979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen CM, Chiang SY, Yeh NH. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J Biol Chem. 1991;266:7754–8. [PubMed] [Google Scholar]
- 79.Li H, Wang B, Yang A, Lu R, Wang W, Zhou Y, et al. Ly-1 antibody reactive clone is an important nucleolar protein for control of self-renewal and differentiation in embryonic stem cells. Stem Cells. 2009;27:1244–54. doi: 10.1002/stem.55. [DOI] [PubMed] [Google Scholar]
- 80.Schwab MS, Dreyer C. Protein phosphorylation sites regulate the function of the bipartite NLS of nucleolin. Eur J Cell Biol. 1997;73:287–97. [PubMed] [Google Scholar]
- 81.Daniely Y, Dimitrova DD, Borowiec JA. Stress-dependent nucleolin mobilization mediated by p53-nucleolin complex formation. Mol Cell Biol. 2002;22:6014–22. doi: 10.1128/MCB.22.16.6014-6022.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishmael FT, Fang X, Houser KR, Pearce K, Abdelmohsen K, Zhan M, et al. The human glucocorticoid receptor as an RNA-binding protein: global analysis of glucocorticoid receptor-associated transcripts and identification of a target RNA motif. J Immunol. 2011;186:1189–98. doi: 10.4049/jimmunol.1001794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tcherkezian J, Brittis PA, Thomas F, Roux PP, Flanagan JG. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell. 2010;141:632–44. doi: 10.1016/j.cell.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watanabe T, Hirano K, Takahashi A, Yamaguchi K, Beppu M, Fujiki H, et al. Nucleolin on the cell surface as a new molecular target for gastric cancer treatment. Biol Pharm Bull. 2010;33:796–803. doi: 10.1248/bpb.33.796. [DOI] [PubMed] [Google Scholar]
- 85.Weidle UH, Maisel D, Klostermann S, Schiller C, Weiss EH. Intracellular proteins displayed on the surface of tumor cells as targets for therapeutic intervention with antibody-related agents. Cancer Genomics Proteomics. 2011;8:49–63. [PubMed] [Google Scholar]
- 86.Fogal V, Sugahara KN, Ruoslahti E, Christian S. Cell surface nucleolin antagonist causes endothelial cell apoptosis and normalization of tumor vasculature. Angiogenesis. 2009;12:91–100. doi: 10.1007/s10456-009-9137-5. [DOI] [PubMed] [Google Scholar]
- 87.Meng GZ, Xiao SJ, Zeng SE, Li YQ. [Downregulation of cell-surface-expressed nucleolin inhibits the growth of hepatocellular carcinoma cells in vitro] Zhonghua Zhong Liu Za Zhi. 2011;33:23–7. [PubMed] [Google Scholar]
- 88.Losfeld ME, Khoury DE, Mariot P, Carpentier M, Krust B, Briand JP, et al. The cell surface expressed nucleolin is a glycoprotein that triggers calcium entry into mammalian cells. Exp Cell Res. 2009;315:357–69. doi: 10.1016/j.yexcr.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 89.Fang L, Wang KK, Jiang L, Jiang BM, Wei X, Song L, et al. [Role of cell-surface nucleolin in lipopolysaccharide-stimulated expression and secretion of TNF-alpha and IL-1β] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2008;33:999–1004. [PubMed] [Google Scholar]
- 90.Reyes-Reyes EM, Akiyama SK. Cell-surface nucleolin is a signal transducing P-selectin binding protein for human colon carcinoma cells. Exp Cell Res. 2008;314:2212–23. doi: 10.1016/j.yexcr.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163:871–8. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Destouches D, Page N, Hamma-Kourbali Y, Machi V, Chaloin O, Frechault S, et al. A simple approach to cancer therapy afforded by multivalent pseudopeptides that target cell-surface nucleoproteins. Cancer Res. 2011;71:3296–305. doi: 10.1158/0008-5472.CAN-10-3459. [DOI] [PubMed] [Google Scholar]
- 93.Shi H, Huang Y, Zhou H, Song X, Yuan S, Fu Y, et al. Nucleolin is a receptor that mediates antiangiogenic and antitumor activity of endostatin. Blood. 2007;110:2899–906. doi: 10.1182/blood-2007-01-064428. [DOI] [PubMed] [Google Scholar]
- 94.Said EA, Courty J, Svab J, Delbé J, Krust B, Hovanessian AG. Pleiotrophin inhibits HIV infection by binding the cell surface-expressed nucleolin. FEBS J. 2005;272:4646–59. doi: 10.1111/j.1742-4658.2005.04870.x. [DOI] [PubMed] [Google Scholar]
- 95.Barel M, Meibom K, Charbit A. Nucleolin, a shuttle protein promoting infection of human monocytes by Francisella tularensis. PLoS One. 2010;5:e14193. doi: 10.1371/journal.pone.0014193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tediose T, Kolev M, Sivasankar B, Brennan P, Morgan BP, Donev R. Interplay between REST and nucleolin transcription factors: a key mechanism in the overexpression of genes upon increased phosphorylation. Nucleic Acids Res. 2010;38:2799–812. doi: 10.1093/nar/gkq013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Garcia MC, Williams J, Johnson K, Olden K, Roberts JD. Arachidonic acid stimulates formation of a novel complex containing nucleolin and RhoA. FEBS Lett. 2011;585:618–22. doi: 10.1016/j.febslet.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dranovsky A, Vincent I, Gregori L, Schwarzman A, Colflesh D, Enghild J, et al. Cdc2 phosphorylation of nucleolin demarcates mitotic stages and Alzheimer’s disease pathology. Neurobiol Aging. 2001;22:517–28. doi: 10.1016/S0197-4580(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 99.Schwab MS, Dreyer C. Protein phosphorylation sites regulate the function of the bipartite NLS of nucleolin. Eur J Cell Biol. 1997;73:287–97. [PubMed] [Google Scholar]
- 100.Kim HH, Abdelmohsen K, Gorospe M. Regulation of HuR by DNA Damage Response Kinases. J Nucleic Acids. 2010;2010:981487. doi: 10.4061/2010/981487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abdelmohsen K, Lal A, Kim HH, Gorospe M. Posttranscriptional orchestration of an anti-apoptotic program by HuR. Cell Cycle. 2007;6:1288–92. doi: 10.4161/cc.6.11.4299. [DOI] [PubMed] [Google Scholar]
- 102.Lafarga V, Cuadrado A, Lopez de Silanes I, Bengoechea R, Fernandez-Capetillo O, Nebreda AR. p38 Mitogen-activated protein kinase- and HuR-dependent stabilization of p21(Cip1) mRNA mediates the G(1)/S checkpoint. Mol Cell Biol. 2009;29:4341–51. doi: 10.1128/MCB.00210-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schneider HR, Reichert GH, Issinger OG. Enhanced casein kinase II activity during mouse embryogenesis. Identification of a 110-kDa phosphoprotein as the major phosphorylation product in mouse embryos and Krebs II mouse ascites tumor cells. Eur J Biochem. 1986;161:733–8. doi: 10.1111/j.1432-1033.1986.tb10501.x. [DOI] [PubMed] [Google Scholar]
- 104.Schneider HR, Issinger OG. Growth-dependent modulation of casein kinase II and its substrate nucleolin in primary human cell cultures and HeLa cells. Biochim Biophys Acta. 1989;1014:98–100. doi: 10.1016/0167-4889(89)90246-2. [DOI] [PubMed] [Google Scholar]
- 105.Tuteja N, Huang NW, Skopac D, Tuteja R, Hrvatic S, Zhang J, et al. Human DNA helicase IV is nucleolin, an RNA helicase modulated by phosphorylation. Gene. 1995;160:143–8. doi: 10.1016/0378-1119(95)00207-M. [DOI] [PubMed] [Google Scholar]
- 106.Warrener P, Petryshyn R. Phosphorylation and proteolytic degradation of nucleolin from 3T3-F442A cells. Biochem Biophys Res Commun. 1991;180:716–23. doi: 10.1016/S0006-291X(05)81124-6. [DOI] [PubMed] [Google Scholar]
- 107.Zhou G, Seibenhener ML, Wooten MW. Nucleolin is a protein kinase C-zeta substrate. Connection between cell surface signaling and nucleus in PC12 cells. J Biol Chem. 1997;272:31130–7. doi: 10.1074/jbc.272.49.31130. [DOI] [PubMed] [Google Scholar]
- 108.Tsou JH, Chang KY, Wang WC, Tseng JT, Su WC, Hung LY, et al. Nucleolin regulates c-Jun/Sp1-dependent transcriptional activation of cPLA2alpha in phorbol ester-treated non-small cell lung cancer A549 cells. Nucleic Acids Res. 2008;36:217–27. doi: 10.1093/nar/gkm1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leitges M, Sanz L, Martin P, Duran A, Braun U, García JF, et al. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell. 2001;8:771–80. doi: 10.1016/S1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- 110.Lischwe MA, Roberts KD, Yeoman LC, Busch H. Nucleolar specific acidic phosphoprotein C23 is highly methylated. J Biol Chem. 1982;257:14600–2. [PubMed] [Google Scholar]
- 111.Guderian G, Peter C, Wiesner J, Sickmann A, Schulze-Osthoff K, Fischer U, et al. RioK1, a new interactor of protein arginine methyltransferase 5 (PRMT5), competes with pICln for binding and modulates PRMT5 complex composition and substrate specificity. J Biol Chem. 2011;286:1976–86. doi: 10.1074/jbc.M110.148486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pellar GJ, DiMario PJ. Deletion and site-specific mutagenesis of nucleolin’s carboxy GAR domain. Chromosoma. 2003;111:461–9. doi: 10.1007/s00412-003-0231-y. [DOI] [PubMed] [Google Scholar]
- 113.Raman B, Guarnaccia C, Nadassy K, Zakhariev S, Pintar A, Zanuttin F, et al. N(omega)-arginine dimethylation modulates the interaction between a Gly/Arg-rich peptide from human nucleolin and nucleic acids. Nucleic Acids Res. 2001;29:3377–84. doi: 10.1093/nar/29.16.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lapeyre B, Bourbon H, Amalric F. Nucleolin, the major nucleolar protein of growing eukaryotic cells: an unusual protein structure revealed by the nucleotide sequence. Proc Natl Acad Sci U S A. 1987;84:1472–6. doi: 10.1073/pnas.84.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salazar R, Brandt R, Kellermann J, Krantz S. Purification and characterization of a 200 kDa fructosyllysine-specific binding protein from cell membranes of U937 cells. Glycoconj J. 2000;17:713–6. doi: 10.1023/A:1011074705615. [DOI] [PubMed] [Google Scholar]
- 116.Losfeld ME, Khoury DE, Mariot P, Carpentier M, Krust B, Briand JP, et al. The cell surface expressed nucleolin is a glycoprotein that triggers calcium entry into mammalian cells. Exp Cell Res. 2009;315:357–69. doi: 10.1016/j.yexcr.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 117.Leitinger N, Wesierska-Gadek J. ADP-ribosylation of nucleolar proteins in HeLa tumor cells. J Cell Biochem. 1993;52:153–8. doi: 10.1002/jcb.240520207. [DOI] [PubMed] [Google Scholar]
- 118.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 119.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–48. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 120.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 121.Singh P, Owlia A, Varro A, Dai B, Rajaraman S, Wood T. Gastrin gene expression is required for the proliferation and tumorigenicity of human colon cancer cells. Cancer Res. 1996;56:4111–5. [PubMed] [Google Scholar]
- 122.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–22. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khurts S, Masutomi K, Delgermaa L, Arai K, Oishi N, Mizuno H, et al. Nucleolin interacts with telomerase. J Biol Chem. 2004;279:51508–15. doi: 10.1074/jbc.M407643200. [DOI] [PubMed] [Google Scholar]
- 124.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–8. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 125.Campbell NE, Kellenberger L, Greenaway J, Moorehead RA, Linnerth-Petrik NM, Petrik J. Extracellular matrix proteins and tumor angiogenesis. J Oncol. 2010;2010:586905. doi: 10.1155/2010/586905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wroblewski LE, Pritchard DM, Carter S, Varro A. Gastrin-stimulated gastric epithelial cell invasion: the role and mechanism of increased matrix metalloproteinase 9 expression. Biochem J. 2002;365:873–9. doi: 10.1042/BJ20020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uribe DJ, Guo K, Shin YJ, Sun D. Heterogeneous nuclear ribonucleoprotein K and nucleolin as transcriptional activators of the vascular endothelial growth factor promoter through interaction with secondary DNA structures. Biochemistry. 2011;50:3796–806. doi: 10.1021/bi101633b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Masumi A, Fukazawa H, Shimazu T, Yoshida M, Ozato K, Komuro K, et al. Nucleolin is involved in interferon regulatory factor-2-dependent transcriptional activation. Oncogene. 2006;25:5113–24. doi: 10.1038/sj.onc.1209522. [DOI] [PubMed] [Google Scholar]
- 129.Cui L, Deng Y, Rong Y, Lou W, Mao Z, Feng Y, et al. IRF-2 is over-expressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumour Biol. 2012;33:247–55. doi: 10.1007/s13277-011-0273-3. [DOI] [PubMed] [Google Scholar]
- 130.Watanabe T, Tsuge H, Imagawa T, Kise D, Hirano K, Beppu M, et al. Nucleolin as cell surface receptor for tumor necrosis factor-alpha inducing protein: a carcinogenic factor of Helicobacter pylori. J Cancer Res Clin Oncol. 2010;136:911–21. doi: 10.1007/s00432-009-0733-y. [DOI] [PubMed] [Google Scholar]
- 131.Nimjee SM, Rusconi CP, Sullenger BA. Aptamers: an emerging class of therapeutics. Annu Rev Med. 2005;56:555–83. doi: 10.1146/annurev.med.56.062904.144915. [DOI] [PubMed] [Google Scholar]
- 132.Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006;5:2957–62. doi: 10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 133.Dempsey LA, Sun H, Hanakahi LA, Maizels N. G4 DNA binding by LR1 and its subunits, nucleolin and hnRNP D, A role for G-G pairing in immunoglobulin switch recombination. J Biol Chem. 1999;274:1066–71. doi: 10.1074/jbc.274.2.1066. [DOI] [PubMed] [Google Scholar]
- 134.Hanakahi LA, Sun H, Maizels N. High affinity interactions of nucleolin with G-G-paired rDNA. J Biol Chem. 1999;274:15908–12. doi: 10.1074/jbc.274.22.15908. [DOI] [PubMed] [Google Scholar]
- 135.Soundararajan S, Chen W, Spicer EK, Courtenay-Luck N, Fernandes DJ. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008;68:2358–65. doi: 10.1158/0008-5472.CAN-07-5723. [DOI] [PubMed] [Google Scholar]
- 136.Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006;5:2957–62. doi: 10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 137.Girvan AC, Teng Y, Casson LK, Thomas SD, Jüliger S, Ball MW, et al. AGRO100 inhibits activation of nuclear factor-kappaB (NF-kappaB) by forming a complex with NF-kappaB essential modulator (NEMO) and nucleolin. Mol Cancer Ther. 2006;5:1790–9. doi: 10.1158/1535-7163.MCT-05-0361. [DOI] [PubMed] [Google Scholar]
- 138.Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp Mol Pathol. 2009;86:151–64. doi: 10.1016/j.yexmp.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Destouches D, El Khoury D, Hamma-Kourbali Y, Krust B, Albanese P, Katsoris P, et al. Suppression of tumor growth and angiogenesis by a specific antagonist of the cell-surface expressed nucleolin. PLoS One. 2008;3:e2518. doi: 10.1371/journal.pone.0002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Krust B, El Khoury D, Soundaramourty C, Nondier I, Hovanessian AG. Suppression of tumorigenicity of rhabdoid tumor derived G401 cells by the multivalent HB-19 pseudopeptide that targets surface nucleolin. Biochimie. 2011;93:426–33. doi: 10.1016/j.biochi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 141.Seddiki N, Nisole S, Krust B, Callebaut C, Guichard G, Muller S, et al. The V3 loop-mimicking pseudopeptide 5[Kpsi(CH2N)PR]-TASP inhibits HIV infection in primary macrophage cultures. AIDS Res Hum Retroviruses. 1999;15:381–90. doi: 10.1089/088922299311358. [DOI] [PubMed] [Google Scholar]
- 142.Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res. 2006;312:594–607. doi: 10.1016/j.yexcr.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 143.Zhuo W, Luo C, Wang X, Song X, Fu Y, Luo Y. Endostatin inhibits tumour lymphangiogenesis and lymphatic metastasis via cell surface nucleolin on lymphangiogenic endothelial cells. J Pathol. 2010;222:249–60. doi: 10.1002/path.2760. [DOI] [PubMed] [Google Scholar]
- 144.Huang Y, Shi H, Zhou H, Song X, Yuan S, Luo Y. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood. 2006;107:3564–71. doi: 10.1182/blood-2005-07-2961. [DOI] [PubMed] [Google Scholar]
- 145.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–17. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Petrocca F, Lieberman J. Micromanipulating cancer: microRNA-based therapeutics? RNA Biol. 2009;6:335–40. doi: 10.4161/rna.6.3.9013. [DOI] [PubMed] [Google Scholar]
- 147.Tominaga K, Srikantan S, Lee EK, Subaran SS, Martindale JL, Abdelmohsen K, et al. Competitive regulation of nucleolin expression by HuR and miR-494. Mol Cell Biol. 2011;31:4219–31. doi: 10.1128/MCB.05955-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu CD, Chou HW, Kuo YS, Lu RM, Hwang YC, Wu HC, et al. Nucleolin antisense oligodeoxynucleotides induce apoptosis and may be used as a potential drug for nasopharyngeal carcinoma therapy. Oncol Rep. 2012;27:94–100. doi: 10.3892/or.2011.1476. [DOI] [PubMed] [Google Scholar]
- 149.Mukherjee N, Corcoran DL, Nusbaum JD, Reid DW, Georgiev S, Hafner M, et al. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol Cell. 2011;43:327–39. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]