Thymus–blood protein interactions are highly effective in negative selection and regulatory T cell induction (original) (raw)
. Author manuscript; available in PMC: 2012 Nov 27.
Published in final edited form as: J Immunol. 2009 Dec 15;183(12):7909–7918. doi: 10.4049/jimmunol.0902632
Abstract
Using hen egg-white lysozyme (HEL), the effect of blood proteins on CD4 thymic cells was examined. A small fraction of intravenously injected HEL rapidly entered the thymus into the medulla. There it was captured, and presented by dendritic cells (DCs) to thymocytes from two T-cell receptor transgenic mice, one directed to a dominant peptide, a second to a poorly displayed peptide, both presented by MHC class II molecules I-Ak. Presentation by DC led to negative selection and induction of regulatory T cells (Tregs), independent of epithelial cells. Presentation took place at very low levels, less than 100 peptide-MHC (pMHC) complexes per DC. Such low levels could induce negative selection, but even lower levels could induce Tregs. The anatomy of the thymus-blood barrier, the highly efficient presentation by DC, together with the high sensitivity of thymic T cells to pMHC complexes, results in blood protein antigens having a profound effect on thymic T cells.
INTRODUCTION
Presentation of self-antigens by thymic antigen presenting cells (APCs) results in the modulation of the autoreactive T cell repertoire through positive selection (1), negative selection (2) and/or, induction of Tregs (3). Thymic APCs induce tolerance to self-antigens of different origins, including constitutively expressed proteins derived from them and other thymic cells, tissue-restricted antigens expressed by medullary epithelial cells (mTECs) under the control of the AIRE transcriptional regulator (4, 5) and blood-borne antigens (6, 7). The role of thymic presenting cells in mediating tolerance has been extensively studied showing both specialized and overlapping functions among them (8-10).
The conditions that dictate whether antigen presentation by specific APC results in negative selection and/or, Treg differentiation are not entirely understood. Deciphering the role of APC type in such decisions needs to be examined in the context of blood proteins where thymic architecture modulates antigen accessibility to various cellular components. Accessibility and the different endocytic and antigen retention capacities of thymic APC subsets (11) could affect the density of pMHC complexes presented, thereby influencing negative selection and Treg development. Both processes require the same co-stimulatory molecules (12, 13), as well as high avidity interaction between the TCR and pMHC complex (14, 15). A long-standing hypothesis is that Tregs must interact with self-pMHC with an avidity intermediate between positive and negative selection (reviewed in (16)). But it has also been suggested that the APC type dictates whether TCR-pMHC interaction results in negative selection or Treg differentiation. This view is supported by studies in which selective expression of antigen by mTECs (15, 17) or restricted expression of class II MHC molecules or ligands to the cortical epithelium was sufficient for Treg differentiation (18-20). Moreover, in addition to their well-described role in negative selection (21), thymic DC have been implicated in Treg development (22-24).
The thymus is permeable to circulating proteins (25-27). Early permeability studies suggested the existence of a blood-thymus barrier, allowing access to low molecular weight tracers while mostly excluding high molecular weight particles (27-29). The anatomical studies of Raviola and Karnovsky (27), using peroxidase as a tracer, indicated that the venules at the cortico-medullary junction were the site of leakage for blood antigens, while the capillaries draining the cortex were largely impermeable. A perivascular system in the medulla which effectively traps small blood-borne molecules, has been described (30-32). In studies examining the presentation efficiency of different thymic APC, Kyewski et al showed that circulating protein antigens injected intravenously rapidly entered the thymus and were efficiently presented by thymic rosettes enriched for dendritic cells (28, 29). Entry and presentation was observed over a wide range of molecular weights, albeit with different efficiencies, and was time and dose dependent. Additionally, injection of peptides induced clonal deletion of thymocytes (33, 34) and naturally circulating antigens were shown to induce effective negative selection of TCR transgenic T-cells in several experimental models upon presentation by thymic APC (6, 7, 10, 35).
Here we examined the response of thymic CD4 T-cells from two different TCR transgenic mice to the protein HEL injected intravenously. One TCR transgenic T-cell (3A9) recognized a dominant long-lived peptide, while a second (LB11.3) recognized a weak and poorly displayed peptide (36, 37). HEL circulated briefly, entered the thymus and was rapidly processed and presented. This allowed us to establish ligand density and how it dictates the cell fate decisions of the two TCR transgenic T cells: a very low concentration of pMHC was tightly linked with negative selection and/or Treg induction.
MATERIALS AND METHODS
Mice
Mice were maintained under specific pathogen-free conditions in accordance with institutional animal care guidelines. B10.BR mice were purchased from Jackson Laboratory and maintained in our facility. 3A9 TCR transgenic mice against the major HEL48-62 peptide were a kind gift from Dr. Mark Davis (Stanford University, Stanford, CA) (38).
Generation of TCR transgenic mice against HEL20-35
The LB11.3 T cell clone reactive to the 20-35 segment of HEL was isolated from B10.BR mice immunized with HEL. After sub-cloning the cells, genomic DNA was amplified by PCR and cloned into a shuttle vector (38). Shuttle vectors containing the LB11.3 hybridoma alpha and beta-chains were linearized and microinjected into oocytes from C57BL/6 × B10.BR (F1) mice. Founders were screened by PCR for expression of the transgene. Sub-lines bearing a stable integration pattern were identified by Southern blot analysis (39) and a line, LB11.3, was chosen for subsequent mating. Positive founders were backcrossed to the B10.BR background. LB11.3 T-cells proliferated specifically to HEL20-35, but not to any other HEL peptides including HEL48-62.
In vivo induction of negative selection and Tregs
Six- to eight-week old mice were injected intravenously with the indicated amount of HEL. (HEL was purchased from Sigma Chemical Co. (St. Louis, MO) and further purified to remove endotoxins and other impurities. Sterile pyrogen free saline was used as a diluent and a control in all experiments.) Mice were sacrificed at three days post-injection and thymic cells were examined by flow cytometry to evaluate the effects of HEL on the various T-cells. All data were analyzed with GraphPad Prism software (GraphPad Software, San Diego, CA). Foxp3 induction and negative selection data were analyzed with the non-parametric Mann-Whitney test. Statistically significant differences are indicated in the graphs.
Flow cytometry
Flow-cytometry data were acquired using a FACSCalibur or LSRII flow-cytometers (BD, Franklin Lakes, NJ) and analyzed using the FlowJo software (TS Inc, Ashland, OR). Thymocytes were stained with anti-CD4 (L3T4) and anti-CD8 (Ly-2) mAbs (eBioscience, San Diego, CA or BD PharMingen, San Jose, CA). Transgenic T-cells were identified using anti-Vα2 (B20.1) (eBioscience, San Diego) for LB11.3 T-cells or 1G12 mAb (40) for 3A9 T-cells. To evaluate negative selection and Treg induction, single cell-suspensions of thymocytes were made and stained with anti-CD4 (L3T4), anti-CD8 (Ly-2), anti-Vα2 (B20.1) or 1G12 mAb. Intracellular staining with anti-Foxp3 (FKJ-16s) was performed according to manufacturer's protocol (eBioscience).
Thymic Uptake of HEL
HEL (1mg) was labeled with 1mCi of 125I (Amersham Biosciences, Piscataway, NJ) using the chloramine T method. Efficiency of labeling was determined by measuring radioactivity in the fractions of a Sephadex-G25 separation after trichloroacetic acid precipitation using a γ-counter (PerkinElmer, Waltham, MA). Six week-old B10.BR mice were injected i.v. with 45.4 μg 125I-HEL (37.6 ×106 CPM). Mice were sacrificed at different time-points (1, 8, 12 h) to harvest thymi and collect blood serum. Radioactivity was determined in thymi and in the trichloracetic acid precipitate from sera.
To localize HEL in the thymus, B10.BR mice were injected with 1mg biotin-HEL. At 15 min, thymi were fixed on ice for 1h in 3% formaldehyde. Organs were immersed in 30% sucrose at 4C overnight, flash frozen on dry ice in Tissue-tek O.C.T. compound (Sakura Finetek USA Inc, Torrance, CA) and sectioned. HEL was detected using a TSA biotin system kit according to the manufacturer's instructions (PerkinElmer LAS Inc., Waltham, MA). Alternatively, B10.BR mice were injected with 10 mg purified HEL. Thymi were harvested at 20 min and flash frozen on dry ice. Sections were permeabilized with Triton X-100 and HEL was detected using an anti-HEL mAb (F10.1.6.6) conjugated to mouse IgG1 Zenon Alexa fluor 488 (Invitrogen Co., Carlsbad, CA). Images were captured at 20X magnification using an Olympus BX51 microscope (Olympus, Center Valley, PA).
Isolation and testing of thymic APC
All sortings were performed using a Dako MoFlo Cell sorter (Dako North American Inc., CA) or a BD FACSAria cell sorter (BD, Franklin Lakes, NJ). All antibodies used for segregation and phenotyping of thymic APCs are listed in Table S1. DC were purified from the thymi of 5-8wk B10.BR injected with HEL and sacrificed 30 min or 36 hours post injection. DC from uninjected mice were also purified side by side. Briefly, thymic fragments were subjected to two rounds of 30-min digestion with 10μg/ml DNAse I (Sigma-Aldrich Co, St. Louis, MO) and 0.14 U/ml Liberase blendzyme 3 (Roche, Indianapolis, IN). T-cells were removed by magnetic bead isolation using anti-CD90.2 (Thy1.2) beads (Miltenyi Biotec Inc., CA). Further purification of APCs was performed by cell sorting. In some experiments, DC were isolated using a Nycodenz gradient (Greiner Bio-One North America Inc., NC) (41). mTECs and cTECs were enriched from the thymi of 5-8 wk B10.BR mice injected with or without HEL as previously described (42, 43).
In experiments evaluating the ability of thymic APC to present HEL, T-cells from LB11.3 and 3A9α-/- (or 3A9) spleens were cultured for 4 days with sorted APC from mice injected with or without HEL and the extent of proliferation determined by standard assays using 3H-Thymidine incorporation into the cell DNA. For hybridoma assays, 3A9 T-cell hybridoma were cultured with APCs for 24 hours and the supernatant were harvested and fed to CTLL-2 T-cell line to assay for IL-2 production. CTLL-2 proliferation was measured by pulsing the cells with 3H-Thymidine 6 hours prior to harvest with a β-counter (PerkinElmer, Waltham, MA)
Autoradiography of thymic DCs
Thymi were isolated from B10.BR mice (5-6wk) injected intravenously with 200μg 125I-HEL (1.3 ×109 cpm) and killed 1hr later. CD11c DC were enriched by Nycodenz gradient (Greiner Bio-One North America Inc.,) as described previously (41) followed by depletion of T-cells using anti-CD90.2 beads (Miltenyi Biotec, CA). The radioactivity of the enriched cells was determined using a γ counter (PerkinElmer, Waltham MA). For autoradiography, DC were spun unto slides which were coated with NTB liquid emulsion (Eastman Kodak, Rochester, NY), dried and exposed at 4C for variable periods of time. Development was performed with Kodak D19 developer followed by fixation (Eastman Kodak, Rochester NY). Cells were counterstained with the Hema3 stain set (Fisher Scientific, Pittsburg PA) and examined microscopically.
RESULTS
Generation and characterization of the LB11.3 TCR transgenic mouse
Processing of HEL by I-Ak expressing APC results in the presentation of four peptides with different numbers of pMHC complexes. Among these, the chemically dominant 48-62 peptide is represented at a molar ratio 250-fold greater than the minor 20-35 peptide (36). T-cells from the 3A9 TCR transgenic mice directed to HEL48-62 are available (38, 40). A second HEL TCR transgenic mouse (LB11.3) was generated using TCR genes from a T-cell hybridoma directed to HEL 20-35 peptide. LB11.3 mice had a normal thymic profile with efficient selection of T-cells that expressed the correct Vα and Vβ chains and proliferated specifically to HEL20-35 (Figure S1). About a 30-fold difference in the proliferative response of LB11.3 T-cells compared to 3A9 T-cells was noted when APCs were cultured with HEL, but both responded identically to peptide (Figure S2). Using an in vitro thymocyte assay, LB11.3 thymocytes in contrast to 3A9 thymocytes required more HEL to achieve a level of pMHC density sufficient for deletion of DP thymocytes and activation of CD4 single positive (CD4SP) (Figure S2). Therefore, the differential response to HEL was likely a function of the several hundred fold difference in the presentation of these two epitopes.
Uptake of HEL by the thymus
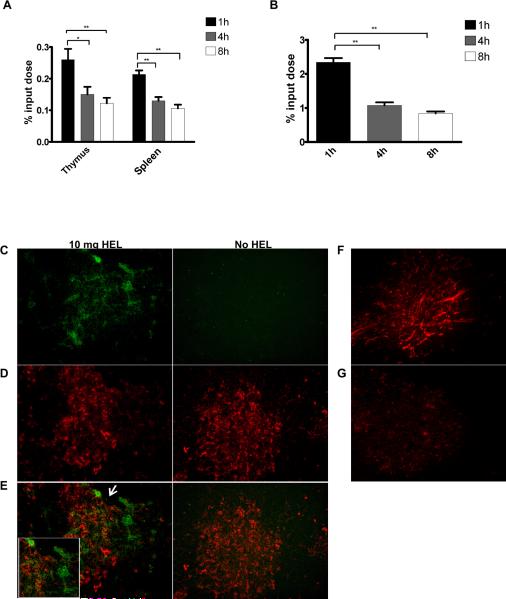
The response of 3A9 and LB11.3 thymocytes was examined after the intravenous injection of graded amounts of HEL. This approach allowed the precise control of HEL levels in the thymus. To determine the kinetics of HEL entry and uptake in the thymus, protein-bound radioactivity in the thymus, spleen and blood was measured following injection of 125I-HEL. HEL circulated briefly, most of it leaving the circulation in about 30 min (Figure 1A, B). By 24 h there was a negligible amount remaining in the thymus and the blood (data not shown). At 1 hour, only 0.25% of the injected HEL had entered the thymus such that with injection of 5μg, 1.25 ng (0.87 pmol) entered, corresponding to 5.2 ×1011 molecules (Table I). HEL staining of the thymus had a vascular-like pattern at the cortico-medullary junction and within the medulla itself (Figure 1, panels C-G). No HEL was localized to the cortex. Permeability was highly influenced by the size of the circulating protein with proteins below albumin being more permeable (Figure S3).
Figure 1. Limiting amounts of injected HEL enters the thymus and localizes to the medulla.
Protein bound radioactivity is shown in (A) the thymus and spleen and in (B) blood at different times post-injection with 45.4 μg 125I-HEL. Data is presented as % input dose recovered from the thymus and spleen and B, in the trichloroacetic precipitated fraction from serum. There are 5-6 mice in each group. Thymus, (*) indicates p< 0.05, (**) p=0.0087. Spleen, (**) indicates p=0.0043. Serum, (**) indicates p=0.0043. (C-G) Thymic sections from a B10.BR mice injected with 10mg purified HEL (C-E) or 1 mg HEL biotin (F-G). Mice were sacrificed at 20 min and 15 min respectively. In panels C-E, 6 μm sections were stained using monoclonal antibodies for (C) HEL (green) and (D) CD11c (red). Panels (E) shows overlay of HEL and CD11c staining. Inset in panel C shows region indicated by arrow. Panel (F) shows streptavidin staining of a thymic section from a B10.BR mouse injected with 1 mg HEL-biotin or (G) an uninjected B10.BR mouse.
Table I.
Autoradiography of CD11c+ DC isolated from B10.BR thymi
| Sample | CPM injected | Amount of protein injected (μg) | CPM DC enriched fraction | Total # DC counted | Total # grain (+) DC | % grain (+) DC |
|---|---|---|---|---|---|---|
| 125I-HEL | 1.3×109 | 220 | 1155 | 1996 | 726 | 36.4% |
| No HEL | - | 0 | 79 | 895 | 2 | 0.2% |
Density of pMHC modulates negative selection of thymocytes and induction of Tregs
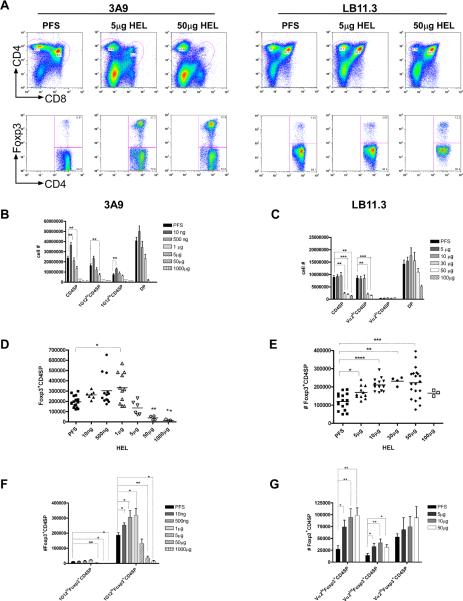
Thymi were harvested from 3A9 and LB11.3 mice three days after HEL injection, and the percentage and absolute number of CD4 single positive cells (CD4SP) and Foxp3+CD4SP was assessed by flow cytometry. CD4SP and Foxp3+CD4SP in 3A9 and LB11.3 thymocytes underwent negative selection, depending on the dose (Figure 2). 3A9 CD4 SP required 5μg HEL for complete negative selection (Figure 2A, B), but an effect was found with as little as 1μg. LB11.3 required 30-100μg for complete negative selection (Figure 2A, C): the 10μg dose was ineffective, but very abruptly the 30μg dose had a pronounced effect (Figure 2C). Thus, the threshold for negative selection for LB11.3 CD4SP thymocytes was higher, requiring more antigen to reach a pMHC density sufficient for deletion.
Figure 2. Negative selection and Foxp3 expression in LB11.3 and 3A9 mice injected with HEL.
(A) top. Representative CD4 and CD8 flow cytometry profiles of thymocytes from 3A9 and LB11.3 TCR transgenic mice injected with saline (PFS), 5, or 50 μg of HEL. Bottom shows corresponding Foxp3 expression gated on CD4SP. (B) Total number of thymocytes within CD4SP/DP from 3A9 mice injected with the indicated amounts of HEL. IG12 indicates thymocytes positive for a monoclonal antibody to the TCR of 3A9. Bars correspond to pooled data from 15 mice (PFS), 7 mice (10ng), 13 mice (500 ng), 11 mice (1μg), 6 mice (5μg), 4 mice (50μg) and 3 mice (1000μg). (C) Total number of thymocytes within CD4SP/DP sub-population from LB11.3 mice injected with titrated amounts of HEL. Bars represent pooled data from 17 mice (PFS), 13 mice (5μg), 15 mice (10μg), 4 mice (30μg), 20 mice (50μg) and 4 mice (100 μg) (D) Total number of Foxp3+CD4SP thymocytes in 3A9 mice shown in (B). (*) indicates p=0.0148. (E) Total number of Foxp3+CD4SP thymocytes in LB11.3 mice shown in (C). Each symbol represents an individual mouse. (F) Foxp3 expression within 1G12hi and 1G12lo CD4SP in 3A9 mice shown in figure D. (G) Foxp3 expression within different Vα2 expressing CD4SP subset in LB11.3 mice shown in figure E. Unless exact p values are provided, for all figures, (****) indicates p<0.0001, (***) indicates p<0.001, (**) indicates p<0.01 and (*) indicates p<0.05. All graphs are based on flow cytometry data gating on CD4SP cells.
Tregs cells were less sensitive to negative selection compared to conventional CD4SP (Figure 2D, E), confirming previous reports (44, 45). While the frequency of Foxp3+ compared to Foxp3- CD4SP increased with HEL dose (Figure 2A and S4), the greatest increase in their absolute number occurred at doses below that required for complete negative selection (compare Figures 2B and D, Figures 2C and E). For 3A9, Foxp3+CD4 SP thymocytes underwent significant depletion with 50μg HEL, although a similar effect was noted in about half the mice at the 5μg dose (Figure 2D). This was ten fold higher the 5μg dose that resulted in the complete deletion of Foxp3-CD4SP (Figure 2B). 3A9 mice injected with 1μg HEL had a mild but statistically significant increase in Foxp3+CD4SP cells (p=0.0148, Figure 2D): 4/11 mice had a two-fold or more increase compared to the PFS control, 3/11 had a 50% increase, and 4/11 showed no change. The increase in the number of Foxp3+ T-cells in 3A9 mice was largely restricted to CD4SP subset that expressed low levels of the 1G12 clonotype (Figure 2F). Interestingly, in mice that did not receive HEL, all Foxp3+ T-cells were in the clonotype low CD4SP population (Figure 2F, see PFS). This data suggest that cells that expressed high levels of the 3A9 TCR did not develop into Tregs. Modest deletion of Foxp3-CD4SP subsets also occurred with 1μg HEL (p=0.0007), indicating that this dose could mark the threshold for negative selection in 3A9 mice.
In comparison, LB11.3 Foxp3+CD4SP cells were not negatively selected at the 100μg dose that deleted almost all Foxp3-CD4SP (compare Figure 2E and 2C). LB11.3 mice had to receive as much as 10 folds that amount (1000μg) for effective deletion of Foxp3+CD4SP (data not shown). Foxp3+CD4SP induction occurred over a wide range of doses, in marked contrast to 3A9 (Figure 2E). The increase in Foxp3+CD4SP cells happened in the absence of overt negative selection (at the 5-10μg dose) or with significant deletion of Foxp3- CD4SP (at the 30-50μg dose) (compare Figures 2C and 2E). The response peaked in mice that received 30 and 50μg HEL, with the increase being more than two fold over the PFS control (Figure 2E). The change in the number of Foxp3+ T-cells was more robust in Vα2hiCD4SP cells, less so in cells that expressed intermediate Vα2 levels while there was no significant effect on the Vα2 negative population (Figure 2G). Notably, at the doses where Foxp3+CD4SP cells were induced in LB11.3, both Foxp3- and Foxp3+ CD4SP in 3A9 mice were negatively selected (compare Figures 2E and Figures 2B,D). In LB11.3 and 3A9 mice, Foxp3+ T-cells were enriched specifically within the CD4SP set, suggesting that these cells arose during the later stages of T-cell development (Figure S4). The enrichment in Foxp3+CD4SP cells was HEL specific. Wild Type B10.BR mice injected with the same amounts of HEL had no changes in total thymic cellularity or in the numbers Foxp3+CD4 SP (Figure S5).
In sum, pMHC density determined the outcome of TCR/pMHC interaction whether that outcome was negative selection or development of Treg precursors. The induction of Foxp3+CD4SP T-cells required and was initiated by TCR interaction with a specific pMHC complex presented at a particular density, irrespective of the final antigen-specificity of the Foxp3+ CD4SP T-cells. Thus, while TCR/pMHC interaction may influence the decision of a T-cell to become negatively selected or continue to develop as a Treg, it does not necessarily determine whether the T-cells that emerge retain the same antigen specificity. Other mechanisms such as receptor editing may influence the final specificity.
The efficiency of Foxp3+ CD4SP T cell induction in LB11.3 and 3A9 mice is dependent on alpha-rearrangement
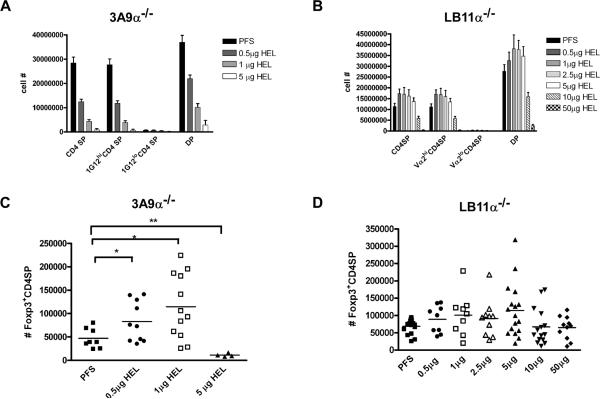
To assess the effects of TCR alpha-chain gene rearrangement in Foxp3+CD4SP induction and/or negative selection, mice crossed to TCR alpha deficient strain were injected with HEL and analyzed. 3A9 alpha-/- mice were more sensitive to negative selection with a little more than half the CD4SP deleted at the 0.5μg HEL, a dose that did not delete 3A9 T-cells (Figure 3A). LB11.3alpha-/-CD4SP were also more sensitive, with approximately half of the cells deleted at the 10μg dose, which had no effect on its LB11.3 counterpart (Figure 3B). These results suggest that at the population level, the loss of the alpha chain rearrangement likely increased the avidity of the TCR/pMHC interaction, decreasing the threshold of pMHC sufficient for negative selection.
Figure 3. Role of TCR alpha chain gene rearrangement in Foxp3+CD4SP Treg induction in mice injected with HEL.
(A) Total number of CD4SP and DP in 3A9 alpha-/- injected with HEL. Data are pooled from two independent experiments with individual bars corresponding to 8 mice (PFS), 10 mice (0.5μg), 12 mice (1μg) and 4 mice (5μg). (B) Total number of CD4SP and DP from LB11.3 alpha-/- mice injected with HEL. Data are pooled from five independent experiments with individual bars corresponding to 17 mice (PFS), 9 mice (0.5μg), 9 mice (1μg), 10 mice (2.5μg), 17 mice (5μg), 15 mice (10μg), and 11 mice (50μg). (C) Total number of Foxp3+CD4SP cells from 3A9 alpha-/- mice shown in (A). (D) Total number of Foxp3+CD4SP cells from LB11.3 alpha-/-shown in (B). All graphs are derived from flow cytometry data gating on CD4SP. Where indicated, (*) denotes p<0.05 and (**) denotes p<0.01.
Perturbation of endogenous TCR alpha rearrangement did not preclude Foxp3+ T-cell development in 3A9alpha-/- and LB11.3alpha-/- as there were still Foxp3+CD4SP cells that developed (see PFS controls), though in lesser numbers than in the wild type mice (Figure 3C, D). In 3A9 mice, there was a modest increase in the number of Foxp3+CD4SP (Figure 3C), but with much variation among the mice: 4/6 mice increased Foxp3+CD4SP at the 0.5μg dose (p=0.0434) and 6/12 mice at the 1ug amount (p<0.02). At a higher dose of HEL (5μg) there was negative selection of Foxp3+CD4SP instead of induction. The LB11.3alpha-/- mice showed no increase in the number of Foxp3+CD4SP cells with the 10μg and 50μg amounts that previously induced them in wild type LB11.3 (Figure 3D). Decreasing the dose of HEL below 10μg (0.5-5μg) showed a small level of induction, albeit with great mouse-to-mouse variability: a little less than half of the mice had Foxp3+ CD4SP T-cell numbers slightly above the control. (This effect was most striking at the 5μg dose where half the mice, 8/16, showed induction (≥100,000 Foxp3+CD4SP), but half had Foxp3+CD4SP T-cell numbers similar to the control.) This data indicates that TCR alpha gene rearrangement is not absolutely required for the generation of HEL dependent Tregs, although there is a noticeable effect on the efficiency of the process.
The enrichment of Foxp3+CD4SP cells could be due to proliferation rather than differentiation. Co-injection of HEL and BrdU into LB11.3 showed an increase in the number of Foxp3+CD4SP primarily within the BrdU negative population (Figure S4E). Thus, the induction of the Treg population observed was likely not a result of expansion, but rather, could be due to other mechanisms such as differentiation of Treg precursors.
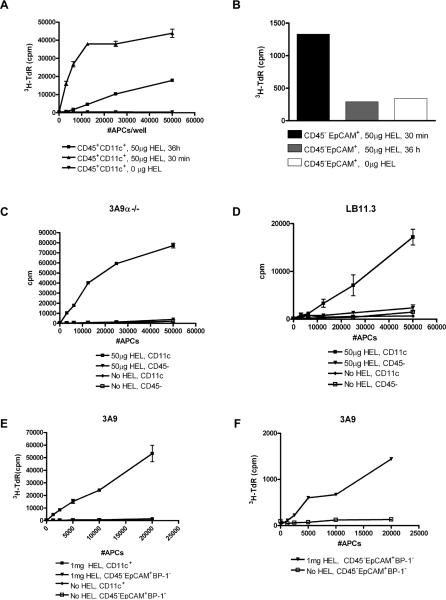
Injected HEL is presented by thymic DC and not TECs
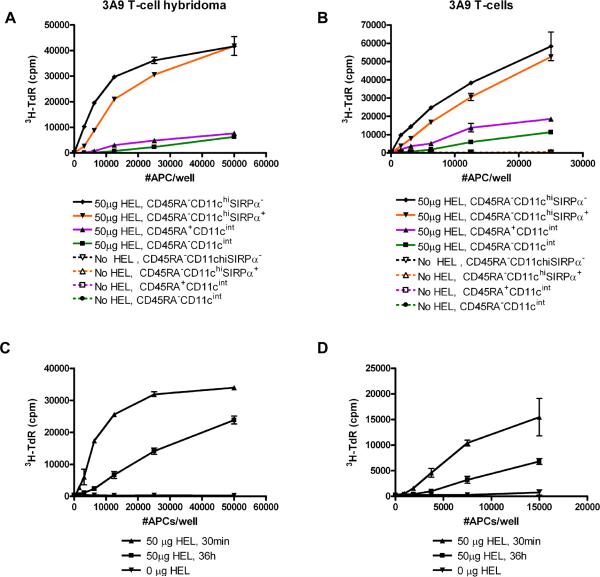
APC subsets were isolated from the thymi of B10.BR mice at 30 min and 36 h post-injection of 50μg HEL, a dose effective at inducing negative selection in 3A9 and LB11.3 mice, and Tregs in LB11.3. Robust presentation to 3A9 T-cell hybridoma occurred when they were cultured with different numbers of CD45+CD11chi DC isolated from mice injected with HEL, but not from uninjected mice (Figure 4A). DC presented HEL when isolated as early as 30 min post-injection, but presentation was significantly lower by 36 h (Figure 4A). In contrast, there was minimal presentation to 3A9 T-hybridoma at the 30 min time-point by CD45-EpCAM+ cells which comprise cTEC and mTEC (Figure 4B). By 36h, presentation by CD45-EpCAM+ cells from mice injected with HEL was no different from the control untreated mice (Figure 4B). The same results were obtained with primary CD4 T-cells isolated from 3A9α-/- and LB11.3 spleens (Figure 4C, D). Only the fractions enriched for CD11c+ DC induced proliferation of 3A9α-/-T-cells (Figure 4C) and LB11.3 T-cells (Figure 4D). Further, increasing the antigen dose did not change the weak presentation of HEL by TECs (Figure 4E, F): sorted CD45-EpCAM+BP-1- cells from mice injected with 1mg HEL still presented poorly compared to CD11c+DC from the same mice (Figure 4E, F). While there was a small level of 3A9 T-cell proliferation induced with sorted mTECs (Figure 4F), no proliferation was observed when 3A9 T-cells were similarly cultured with CD45-EpCAM+BP-1+ cTECs (data not shown). CD45- cells were not intrinsically deficient at presentation as they could still present HEL to 3A9 and LB11.3 T-cells when pulsed with soluble HEL ex vivo (data not shown). Given the low level presentation of soluble HEL by mTECs early after injection, it is possible that in vivo these cells are able to capture and present more circulating HEL than suggested by our ex vivo assays. However, our results suggest that presentation by TECs will occur with a significantly much lower efficiency when compared to DCs.
Figure 4. Presentation of blood-borne HEL by thymic DC and not TECs.
(A) Presentation of HEL by CD11c + DC isolated at 30 min and 36 h from B10.BR mice injected with 50μg HEL. (B) Corresponding presentation by 2-3×104 TECs isolated from the same mice and cultured as in (A). For panels A-B, APCs were cultured for 24 h with 5×104 3A9 T-cell hybridoma and IL-2 production was measured by CTLL proliferation indicated by 3H-Thymidine incorporation. Proliferation of primary (C) 3A9α-/- and (D) LB11.3 T-cells in response to presentation by CD11c+ DC and CD45- TECs from mice injected with 50μg HEL. (E-F) Proliferation of primary 3A9 T-cells in response to presentation by (E) CD11c+ DC or (E, F). Sorted CD45-EpCAM+BP-1- mTECs isolated from mice injected with 1mg HEL. For primary proliferation, APCs were co-cultured with T-cells for 4 days and 3H-Thymidine incorporation was measured. In Panels C-F, mice were sacrificed 30 min post-injection and APCs isolated from un-injected B10.BR mice were used as controls.
Thus, the CD11c+ cells were the primary early capture cell for HEL, bearing most of the available pMHC complexes, and thymic epithelial cells did not contribute much, if at all, to the presentation of blood-borne HEL. This observation is compatible with the tissue immunofluorescent staining, showing a close apposition of DC to medullary vessel areas that stained for HEL (Figure 1 panels C-G). The fast kinetics of presentation agree with a previous report showing that DC in thymic rosettes present injected myoglobin as early as 15 min post-injection (28). The finding that presentation of the 48-62 epitope, which has a half-time of dissociation from I-Ak of more than 96 hrs, wanes over time indicates turnover of thymic DC. (HEL was cleared from the blood within minutes after injection and supplies the thymic DC system briefly, see Figure 1.) Additionally, the fast kinetics of presentation rules out any contribution from a circulating APC internalizing HEL in the periphery and trafficking into the thymus (24, 46, 47).
Thymic DC population include both conventional (cDC) and plasmacytoid (pDC) DC (24, 48-50). While thymic pDC have been shown to migrate in from the periphery, thymic cDC are comprised of two populations, CD8α-Sirpα+ cDC that stem from the periphery and CD8α+Sirpα- cDC that originate in the thymus (47, 49). Additionally, we have observed a fourth CD11c subset that is CD11cintCD45RAlo, which exhibits intermediate levels of MHC II and co-stimulatory molecules compared to cDC and pDC populations, suggesting an immature DC phenotype (data not shown).
To identify further the antigen capturing DC, we sorted thymic DC from mice thirty minutes after injection of HEL into four sets: CD11chiCD8α+Sirpα- (intra-thymic cDC), CD11chiCD8α-Sirpα+ (extra-thymic cDC), CD11cintCD45RAhi (pDC) and CD11cintCD45RAlo (immature DC). Presentation to 3A9 T-cell hybridoma or primary 3A9 T-cells was assessed. Both cDC subsets captured and presented blood borne HEL very efficiently, as evidenced by high IL-2 production by 3A9 hybridoma and proliferation of primary 3A9 T-cells (Figure 5A, B). pDC were significantly less efficient at capturing and presenting to the T-cells, but were still superior when compared to the immature DC subset and TEC populations (Figure 5A, B and Figure 4C, D). Among the intra-thymic cDC, CD8α+ subsets were more efficient than CD8α- subsets at capturing and presenting HEL, both at 30 min and 36 h after injection of HEL (Figures 5C, D). We conclude from these experiments that cDC in the thymus (both Sirpα+ and Sirpα-) are the main APC that take up and primarily present blood-borne HEL.
Figure 5. HEL is efficiently presented by conventional thymic DC.
HEL presentation by dendritic cell subsets isolated 30 min post-injection and sorted based on differential expression of CD11c, CD45RA, Sirpα, and CD8α. APC were cultured with (A) 3A9 T-cell hybridoma or (B) primary 3A9 T-cells. (C) HEL Presentation to 3A9 T-cell hybridoma by sorted CD8α+ and (D) CD8α- CD11chi dendritic cells isolated 30 min and 36 h post-injection. For hybridoma assays, APCs were cultured for 24 h with 3A9 T-cell hybridoma and IL-2 production was measured indirectly by CTLL proliferation indicated by 3H-Thymidine incorporation. For primary proliferation, APCs were co-cultured with T-cells for 4 days. In all figures, APCs were isolated from mice injected with either 50μg HEL or no HEL: all results of the latter are on baseline.
To assay how many DCs took up blood-borne HEL, thymic DC were isolated one hour after injection of 125I-HEL into B10.BR mice. After the final step of enrichment only a fraction of the initial radioactivity was recovered (1155 cpm) indicating that only a small amount of the input HEL was cell associated (Table I). Radioactive grains contained per DC were enumerated by microscopy. 36.4% of DC counted were positive for 125I HEL grains compared to 0.2% in slides from un-injected mice (Table I). These results point to the efficacy of DC at taking up blood-borne antigens and indicate that HEL becomes concentrated in a few DCs that are able to effectively mediate negative selection and Treg induction.
DISCUSSION
This study reports on three findings. First, it confirms that the thymus is highly effective at taking up and presenting blood proteins as a result of its DC network surrounding a restricted anatomical area. Second, it presents data that the thymus-blood interaction influences negative selection and Treg development as a function of the level of protein in the blood resulting in variable pMHC density. Third, it indicates that the pMHC density required for negative selection and Treg development is limiting and different for each function, albeit overlapping.
We confirm here that entry into the thymus of circulating proteins and their presentation by thymic APC can result in tolerance. Several features were evident. First, the permeability of blood proteins was largely dependent on molecular size and on the anatomical region, the thymic medulla. The blood-thymic barrier restricted access of large molecular weight proteins, as others have examined, favoring entry of proteins below the molecular weight of albumin (Figure S3 and (27, 29)). In our experimental system, thymic DC, and not TECs, were particularly effective at capturing and presenting blood-derived HEL, agreeing with the early findings of Kyewski et al (28, 29). HEL was largely presented by both Sirpα positive and negative cDC subsets found in the medulla. Together the results demonstrate the effectiveness of presentation of blood proteins. The presentation of blood constituents may complement the function of the AIRE transcriptional regulator in presentation of tissue specific antigens. Indeed, some tissue-derived proteins and peptides are found in the circulation, although the extent of this representation still needs examination. This speculation is supported by the findings that AIRE deficiency shows restricted rather than broad patterns of autoimmunity, as would be expected if it would be the sole process for control of autoimmunity. Recent studies have also speculated in mechanisms outside the thymus that may maintain tolerance to tissue antigens (51, 52) (discussed in (53).
In examining the dose responses of the 3A9 and LB11.3 T cells (Figure 2), a consistent quantitative difference was observed between them in the HEL levels required for negative selection and Treg generation (Table II). There are notable differences between the two HEL pMHC complexes: the 48-62-Ak complex is distinguished by high binding to I-Ak molecules and a long persistence. In contrast, the 20-35-Ak complex shows a low degree of binding and a fast dissociation rate. Estimates on the presentation efficacy of these two peptides can be made by combining data from the (1) injection of titrating amounts of HEL, (2) previous studies on uptake and handling of HEL(54), and (3) data showing that the amount of HEL that entered the thymus was 0.25% of input (Fig 1 and Table II). While the efficiency of uptake and processing of HEL by thymic APC is unknown, previous data suggest this will be relatively low (54). Based on results in that study, it was found that 48-62-Ak complexes represented about 10% of the total class II molecules on a given APC reflecting only 1 per 1000 molecules of the internalized HEL ends as a pMHC complex for HEL48-62. Similar examination of presentation efficiency using FLT3 ligand generated splenic DC showed that HEL 48-62-Ak complexes represent a little less than 3% of the total I-Ak complexes (unpublished data). Thus, assuming 10 % uptake of HEL, we estimate a total of about 107 pMHC complexes distributed among about 105-106 thymic DC (Table II). The number of DC containing the pMHC has been difficult to obtain, but it is likely that as much as about 40% bear the complexes (Table I) leading to 25 to 250 pMHC of 48-62 per APC when 1μg HEL is injected (Table I and II). These calculations suppose that all thymic APCs have equal access to soluble HEL and present a uniform number of pMHC complexes which may not be the case, although microscopically there were no discernible differences in the number of grains among the positive cells. A point to note is that our estimate of the fraction of DC bearing HEL is based on autoradiography analysis of mice injected with high dose (200μg) HEL. It is more likely that at the small doses used in this study (0.01μg-100μg), this value could be significantly lower suggesting that the pMHC that mediate negative selection and Treg induction are likely in the single digits. (The predicted values for pMHC agree with in vitro studies showing that as few as 2-3 pMHC complexes on an APC were sufficient to induce apoptosis of immature DP thymocytes (55, 56)). Comparing the two epitopes, for LB11.3 T-cells to undergo complete negative selection to the same extent as 3A9, more than a 20-fold higher amount of HEL (1 mg HEL) had to be injected. Making calculations like those in Table 2, the range of pMHC complexes that supported negative selection and Treg induction for both 3A9 and LB11.3 T cells must be about the same. Regardless of the degree of error of these estimates, the amounts of pMHC per DC must be very low.
Table II.
Identifying the pMHC threshold for negative selection and regulatory T-cell generation
| Amounts injected: | 1 μg |
|---|---|
| % entering thymus: | 0.25% |
| apmol HEL in thymus: | 0.17 pmol |
| Molecules HEL in thymus: | 1011 molecules |
| bAssume 10% uptake: | 1010 molecules |
| cEfficiency of processing: | 0.1% of uptake |
| dNumber of pMHC: | 107 |
| eEstimated number of 48-62 complexes/DC: | 25-250 /DC |
| *f pMHC/DC | ||||
|---|---|---|---|---|
| T reg induction | Negative selection | |||
| Amts | pMHC/DC | Amts | pMHC/DC | |
| 3A9 | 1 μg | 25-250 | 5 μg | 125-1250 |
| gLB11 | 5 μg | 0.1-1 | 30 μg | 3-30 |
A second feature is that for each T cells there was a very narrow margin between the doses that resulted in the induction of negative selection (of both conventional CD4SP and Tregs) and those that induced Tregs. Although the lowest doses induced Tregs without a change in negative selection, there was also notable overlap, in both LB11.3 and 3A9 mice. This overlap may explain the wide range of responses found at critical doses of the injected HEL. Based on the numbers calculated for HEL, it is likely that the expression of most self-peptides falls close to the level required for negative selection or somewhere below it. For some tissue antigens (e.g. those influenced by AIRE), the pMHC density levels on TECs compared to DCs may be more compatible with Treg selection than negative selection. This view is supported by data showing that AIRE induces stochastic and heterogeneous expression of tissue restricted ectopically expressed antigens (57), such that at the single cell level, the extent to which any one pMHC will be represented on an individual mTEC will vary considerably. Hence, the sensitivity of both negative selection and Treg induction to pMHC levels ensures that central tolerance will be effective, regardless of the constraints imposed by the levels of antigen expression.
The induction of Tregs had several features. Their generation was not solely a result of selective survival (45), since both a relative and absolute enrichment in the number Foxp3+CD4SP cells was found even at doses that resulted in increased negative selection. Our results are consistent with a late stage DP→CD4SP differentiation as Foxp3+ cells accumulated within the CD4SP and not DP when mice were treated with an inductive dose of HEL. Additionally, generation of Tregs in response to HEL occurred independently of TCR alpha-chain gene rearrangement, although in its absence the induction was inefficient even at low HEL doses. Moreover, the absence of endogenous alpha rearrangement altered the kinetics of negative selection in both 3A9 and LB11.3, decreasing the threshold of HEL required. Our findings support an avidity model of Treg selection whereby TCR-dependent interaction of Treg precursors with antigen at the ‘right’ window of pMHC density induces the final maturational steps of Treg differentiation, as denoted by induction of Foxp3 expression.
The exquisite efficiency, sensitivity and stringency of central tolerance took place not only by the presentation abilities of the DC system, but importantly by the low threshold of activation of the T cells during their thymic sojourn. The ability of thymocytes to respond to a few pMHC ensures that autoreactive T-cells specific for rare antigens can be effectively removed from the T-cell repertoire. To note is that the required number of pMHC predicted for negative selection and Treg induction was about ten fold lower than those estimated for mature CD4 response (58-60). As others have discussed the threshold for responses of T cell changes during their differentiation (61-65). The findings that the pMHC threshold for CD4SP negative selection is much lower compared to that required to initiate an effector response in the periphery is the “biochemical margin of safety”, a process that ensures that T cells that escape negative selection remain dormant in the periphery (55, 66).
Note: While this manuscript was under review a report by Baba et al.(67) was published. This study reported that thymic Sirpα+ cDC entering the thymus from the periphery are a specialized APC for the induction of central tolerance to blood-borne antigens. We find the authors’ data showing the unique localization of Sirpα+ DC inside perivascular regions (PVR) of blood vessels in the cortex intriguing and this needs further examination in other experimental models. We are less convinced about data ascribing unique participation of these cells in tolerance induction to blood-borne antigens. The results examining negative selection in CCR2-/- mice that have decreased numbers of Sirpα+ DC showed a very modest reduction. This could suggest that other cell types such as Sirpα-CD8α+ cDC participate in the induction of tolerance to blood-borne antigens and that Sirpα+ DC are not required and may in fact have a minor role in this process. This view is supported by our own data showing that both Sirpα+ cDC and Sirpα- cDC presented HEL to a similar extent (Figure 5A, B) suggesting that both these cell types can efficiently acquire blood-borne HEL to mediate negative selection. Similarly to Baba et al., our results show that the fast kinetics of uptake of blood-borne antigen by thymic DC make it unlikely that antigen is being brought in by Sirpα+ DC that have captured antigen in the periphery. Therefore we concur that antigen uptake from the blood-stream is most likely a local event whereby DC localized in close proximity to the blood-vessels have access to antigen leaking from the blood. It remains to be examined exactly what the contribution of peripheral DC trafficking to the thymus is in mediating tolerance induction to blood-borne antigens at steady state.
Supplementary Material
Supp Figures
ACKNOWLEDGEMENTS
We thank Kathy Frederick, Shirley Petzold and Dr. Boris Calderon for their skillful technical assistance. We also thank members of the Unanue Laboratory particularly Beverly Strong for helpful discussions.
Footnotes
a
This work was supported by the National Institutes of Health grant (NIAID, AI02033).
b
The authors declare that they have no competing financial interest.
REFERENCES
- 1.Kisielow P, Teh H, Blüthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–733. doi: 10.1038/335730a0. [DOI] [PubMed] [Google Scholar]
- 2.Kappler J, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 4.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 5.Anderson M, Venanzi E, Klein L, Chen Z, Berzins S, Turley S, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 6.Zal T, Volkmann A, Stockinger B. Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med. 1994;180:2089–2099. doi: 10.1084/jem.180.6.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volkmann A, Zal T, Stockinger B. Antigen-presenting cells in the thymus that can negatively select MHC class II-restricted T cells recognizing a circulating self antigen. J Immunol. 1997;158:693–706. [PubMed] [Google Scholar]
- 8.Anderson G, Partington K, Jenkinson E. Differential effects of peptide diversity and stromal cell type in positive and negative selection in the thymus. J Immunol. 1998;161:6599–6603. [PubMed] [Google Scholar]
- 9.Klein L, Kyewski B. Self-antigen presentation by thymic stromal cells: a subtle division of labor. Curr Opin Immunol. 2000;12:179–186. doi: 10.1016/s0952-7915(99)00069-2. [DOI] [PubMed] [Google Scholar]
- 10.Klein L, Roettinger B, Kyewski B. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance. Eur J Immunol. 2001;31:2476–2486. doi: 10.1002/1521-4141(200108)31:8<2476::aid-immu2476>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Müller K, Schumacher J, Kyewski B. Half-life of antigen/major histocompatibility complex class II complexes in vivo: intra- and interorgan variations. Eur J Immunol. 1993;23:3203–3207. doi: 10.1002/eji.1830231224. [DOI] [PubMed] [Google Scholar]
- 12.Punt J, Osborne B, Takahama Y, Sharrow S, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 14.Ashton-Rickardt P, Tonegawa S. A differential-avidity model for T-cell selection. Immunol Today. 1994;15:362–366. doi: 10.1016/0167-5699(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 15.Jordan M, Boesteanu A, Reed A, Petrone A, Holenbeck A, Lerman M, Naji A, Caton A. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz R. Natural regulatory T cells and self-tolerance. Nat Immunol. 2005;6:327–330. doi: 10.1038/ni1184. [DOI] [PubMed] [Google Scholar]
- 17.Aschenbrenner K, D'Cruz L, Vollmann E, Hinterberger M, Emmerich J, Swee L, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 18.Bensinger S, Bandeira A, Jordan M, Caton A, Laufer T. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J Exp Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liston A, Nutsch K, Farr A, Lund J, Rasmussen J, Koni P, Rudensky A. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci U S A. 2008;105:11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ribot J, Enault G, Pilipenko S, Huchenq A, Calise M, Hudrisier D, Romagnoli P, van Meerwijk J. Shaping of the autoreactive regulatory T cell repertoire by thymic cortical positive selection. J Immunol. 2007;179:6741–6748. doi: 10.4049/jimmunol.179.10.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe N, Wang Y, Lee H, Ito T, Cao W, Liu Y. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 24.Proietto A, van Dommelen S, Zhou P, Rizzitelli A, D'Amico A, Steptoe R, Naik S, Lahoud M, Liu Y, Zheng P, Shortman K, Wu L. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci U S A. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green I, Bloch K. Uptake of Particulate Matter within the Thymus of Adult and New-Born mice. Nature. 1963;200:1099–1101. doi: 10.1038/2001099a0. [DOI] [PubMed] [Google Scholar]
- 26.Sainte-Marie G. Antigen Penetration into the Thymus. J Immunol. 1963;91:840–845. [PubMed] [Google Scholar]
- 27.Raviola E, Karnovsky M. Evidence for a blood-thymus barrier using electron-opaque tracers. J Exp Med. 1972;136:466–498. doi: 10.1084/jem.136.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyewski B, Fathman C, Kaplan H. Intrathymic presentation of circulating non-major histocompatibility complex antigens. Nature. 1984;308:196–199. doi: 10.1038/308196a0. [DOI] [PubMed] [Google Scholar]
- 29.Kyewski B, Fathman C, Rouse R. Intrathymic presentation of circulating non-MHC antigens by medullary dendritic cells. An antigen-dependent microenvironment for T cell differentiation. J Exp Med. 1986;163:231–246. doi: 10.1084/jem.163.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato S. Thymic microvascular system. Microsc Res Tech. 1997;38:287–299. doi: 10.1002/(SICI)1097-0029(19970801)38:3<287::AID-JEMT9>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 31.Drumea-Mirancea M, Wessels J, Müller C, Essl M, Eble J, Tolosa E, Koch M, Reinhardt D, Sixt M, Sorokin L, Stierhof Y, Schwarz H, Klein G. Characterization of a conduit system containing laminin-5 in the human thymus: a potential transport system for small molecules. J Cell Sci. 2006;119:1396–1405. doi: 10.1242/jcs.02840. [DOI] [PubMed] [Google Scholar]
- 32.Müller S, Stolt C, Terszowski G, Blum C, Amagai T, Kessaris N, Iannarelli P, Richardson W, Wegner M, Rodewald H. Neural crest origin of perivascular mesenchyme in the adult thymus. J Immunol. 2008;180:5344–5351. doi: 10.4049/jimmunol.180.8.5344. [DOI] [PubMed] [Google Scholar]
- 33.Murphy K, Heimberger A, Loh D. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 34.Liblau R, Tisch R, Shokat K, Yang X, Dumont N, Goodnow C, McDevitt H. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc Natl Acad Sci U S A. 1996;93:3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein L, Klein T, Rüther U, Kyewski B. CD4 T cell tolerance to human C-reactive protein, an inducible serum protein, is mediated by medullary thymic epithelium. J Exp Med. 1998;188:5–16. doi: 10.1084/jem.188.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Velazquez C, DiPaolo R, Unanue E. Quantitation of lysozyme peptides bound to class II MHC molecules indicates very large differences in levels of presentation. J Immunol. 2001;166:5488–5494. doi: 10.4049/jimmunol.166.9.5488. [DOI] [PubMed] [Google Scholar]
- 37.Velazquez C, Vidavsky I, van der Drift K, Gross M, Unanue E. Chemical identification of a low abundance lysozyme peptide family bound to I-Ak histocompatibility molecules. J Biol Chem. 2002;277:42514–42522. doi: 10.1074/jbc.M202316200. [DOI] [PubMed] [Google Scholar]
- 38.Ho W, Cooke M, Goodnow C, Davis M. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med. 1994;179:1539–1549. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yancopoulos G, Blackwell T, Suh H, Hood L, Alt F. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986;44:251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]
- 40.Peterson D, DiPaolo R, Kanagawa O, Unanue E. Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity. 1999;11:453–462. doi: 10.1016/s1074-7613(00)80120-x. [DOI] [PubMed] [Google Scholar]
- 41.Vremec D, Shortman K. The isolation and identification of murine dendritic cell populations from lymphoid tissues and their production in culture. Methods Mol Biol. 2008;415:163–178. doi: 10.1007/978-1-59745-570-1_10. [DOI] [PubMed] [Google Scholar]
- 42.Gray D, Chidgey A, Boyd R. Analysis of thymic stromal cell populations using flow cytometry. J Immunol Methods. 2002;260:15–28. doi: 10.1016/s0022-1759(01)00493-8. [DOI] [PubMed] [Google Scholar]
- 43.Gray D, Fletcher A, Hammett M, Seach N, Ueno T, Young L, Barbuto J, Boyd R, Chidgey A. Unbiased analysis, enrichment and purification of thymic stromal cells. J Immunol Methods. 2008;329:56–66. doi: 10.1016/j.jim.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Romagnoli P, Hudrisier D, van Meerwijk J. Preferential recognition of self antigens despite normal thymic deletion of CD4(+)CD25(+) regulatory T cells. J Immunol. 2002;168:1644–1648. doi: 10.4049/jimmunol.168.4.1644. [DOI] [PubMed] [Google Scholar]
- 45.van Santen H, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200:1221–1230. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonasio R, Scimone M, Schaerli P, Grabie N, Lichtman A, von Andrian U. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206:607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 49.Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semin Immunol. 2005;17:304–312. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 50.Lahoud M, Proietto A, Gartlan K, Kitsoulis S, Curtis J, Wettenhall J, Sofi M, Daunt C, O'keeffe M, Caminschi I, Satterley K, Rizzitelli A, Schnorrer P, Hinohara A, Yamaguchi Y, Wu L, Smyth G, Handman E, Shortman K, Wright M. Signal regulatory protein molecules are differentially expressed by CD8- dendritic cells. J Immunol. 2006;177:372–382. doi: 10.4049/jimmunol.177.1.372. [DOI] [PubMed] [Google Scholar]
- 51.Lee J, Epardaud M, Sun J, Becker J, Cheng A, Yonekura A, Heath J, Turley S. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–190. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 52.Gardner J, Devoss J, Friedman R, Wong D, Tan Y, Zhou X, Johannes K, Su M, Chang H, Krummel M, Anderson M. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kyewski B. Immunology. A breath of Aire for the periphery. Science. 2008;321:776–777. doi: 10.1126/science.1162966. [DOI] [PubMed] [Google Scholar]
- 54.Dadaglio G, Nelson C, Deck M, Petzold S, Unanue E. Characterization and quantitation of peptide-MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity. 1997;6:727–738. doi: 10.1016/s1074-7613(00)80448-3. [DOI] [PubMed] [Google Scholar]
- 55.Peterson D, DiPaolo R, Kanagawa O, Unanue E. Cutting edge: negative selection of immature thymocytes by a few peptide-MHC complexes: differential sensitivity of immature and mature T cells. J Immunol. 1999;162:3117–3120. [PubMed] [Google Scholar]
- 56.Ebert P, Ehrlich L, Davis M. Low ligand requirement for deletion and lack of synapses in positive selection enforce the gauntlet of thymic T cell maturation. Immunity. 2008;29:734–745. doi: 10.1016/j.immuni.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 58.Demotz S, Grey H, Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990;249:1028–1030. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- 59.Harding C, Unanue E. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- 60.Reay P, Matsui K, Haase K, Wulfing C, Chien Y, Davis M. Determination of the relationship between T cell responsiveness and the number of MHC-peptide complexes using specific monoclonal antibodies. J Immunol. 2000;164:5626–5634. doi: 10.4049/jimmunol.164.11.5626. [DOI] [PubMed] [Google Scholar]
- 61.Pircher H, Rohrer U, Moskophidis D, Zinkernagel R, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 62.Ericsson P, Orchansky P, Carlow D, Teh H. Differential activation of phospholipase C-gamma 1 and mitogen-activated protein kinase in naive and antigen-primed CD4 T cells by the peptide/MHC ligand. J Immunol. 1996;156:2045–2053. [PubMed] [Google Scholar]
- 63.Kimachi K, Croft M, Grey H. The minimal number of antigen-major histocompatibility complex class II complexes required for activation of naive and primed T cells. Eur J Immunol. 1997;27:3310–3317. doi: 10.1002/eji.1830271230. [DOI] [PubMed] [Google Scholar]
- 64.Davey G, Schober S, Endrizzi B, Dutcher A, Jameson S, Hogquist K. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J Exp Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Q, Chau J, Ebert P, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein L, Davis M, Chen C. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Yagi J, Janeway CJ. Ligand thresholds at different stages of T cell development. Int Immunol. 1990;2:83–89. doi: 10.1093/intimm/2.1.83. [DOI] [PubMed] [Google Scholar]
- 67.Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol. 2009;183:3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Figures