A Novel Target of IscS in Escherichia coli: Participating in DNA Phosphorothioation (original) (raw)
Abstract
Many bacterial species modify their DNA with the addition of sulfur to phosphate groups, a modification known as DNA phosphorothioation. DndA is known to act as a cysteine desulfurase, catalyzing a key biochemical step in phosphorothioation. However, bioinformatic analysis revealed that 19 out of the 31 known dnd gene clusters, contain only four genes (dndB-E), lacking a key cysteine desulfurase corresponding gene. There are multiple cysteine desulfurase genes in Escherichia coli, but which one of them participates into DNA phosphorothioation is unknown. Here, by employing heterologous expression of the Salmonella enterica dnd gene cluster named dptBCDE in three E. coli mutants, each of which lacked a different cysteine desulfurase gene, we show that IscS is the only cysteine desulfurase that collaborates with _dpt_B-E, resulting in DNA phosphorothioation. Using a bacterial two-hybrid system, protein interactions between IscS and DptC, and IscS and DptE were identified. Our findings revealed IscS as a key participant in DNA phosphorothioation and lay the basis for in-depth analysis of the DNA phosphorothioation biochemical pathway.
Introduction
Sequence and stereo specific physiological DNA phosphorothioation occurs in many bacteria [1]–[4]. In Streptomyces lividans 1326, a five-gene cluster, dndA–E, determines the modification [1]. Orthologs of these genes were found in 30 bacterial species and one Archaea [2]. The dnd genes are usually located on genomic islands that were probably acquired by horizontal gene transfer [3].
Several of these gene clusters contain dndB-E homologues, but lack a dndA homologue [2], [3]. In-frame deletion of dndA in S. lividans showed that the gene is essential for DNA phosphorothioation [1], [4]. DndA was then shown to be a cysteine desulfurase involved in the iron-sulfur cluster assembly for apo-Fe DndC [5].
Salmonella. enterica serovar cerro 87 contains dndB-E orthologs that are called dptB-E [6]. There is, however, no dndA ortholog in the entire 20 kb genomic island that contains the dpt genes (Fig. 1A) [2]. Heterologous expression of dptB-E in E. coli DH10B [7] resulted in DNA phosphorothioation [8]. Since DndA is essential for DNA phosphorothioation in S. lividans, we hypothesized that there should be one or more genes in the E. coli genome that could provide the cysteine desulfurase activity known to be necessary for the modification. Searching for a putative dndA orthologue in E. coli BW25113 was easier than in S. enterica because of the availability of a comprehensive library of knockout mutants of all nonessential genes [9]. In E.coli, there are at least three different cysteine desulfurases: IscS, SufS and CsdA [10], . Here we show that only one of them, IscS, supports DNA phosphorothioation in E. coli expressing the S. enterica dptB-E gene cluster. Protein interactions, which are likely necessary for DNA phosphorothioation, were detected between IscS and both DptC and DptE.
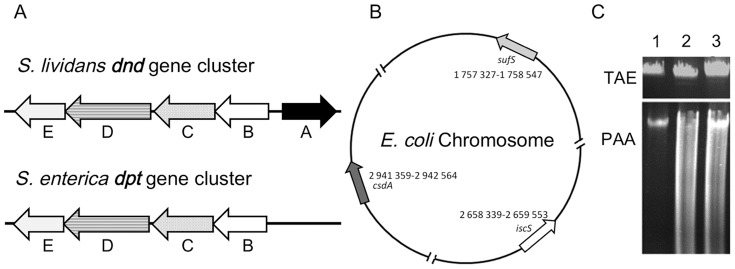
Figure 1. Heterologous expression of the S. enterica serovar cerro 87 dptBCDE genes in E. coli BW25113.
A. Orthologous DNA phosphorothioation gene clusters from S. lividans (dndABCDE) and S. enterica (dptBCDE). The cysteine desulfurase gene dndA of S. lividans is required for DNA phosphorothioation. The S. enterica dptBCDE gene cluster lacks a dndA ortholog. The dndA function may be performed by an unknown, unlinked gene in S. enterica and also in E. coli expressing dptBCDE. B. The three cysteine desulfurases in the E. coli genome. C. E. coli BW25113 DNA becomes phosphorothioated when expressing dptBCDE of S. enterica. Ethidium bromide-stained agarose gels containing total genomic DNA, separated in Tris-acetate EDTA (TAE) buffer. TAE (top panel), untreated samples; PAA (bottom panel), identical DNA samples after incubation in TAE containing 1% per-acetic acid (PAA). Lane 1, E. coli BW25113 (wild-type, not S-modified); lane 2, S. enterica serovar 87 (wild-type, containing phosphorothioate DNA); lane 3, E. coli BW25113 expressing the S. enterica serovar cerro 87 dptBCDE gene cluster. The fluorescent smear in lanes 2 and 3 of the lower gel indicates that the DNA was phosphorothioate modified.
Materials and Methods
Bacterial strains, plasmids and primers
Bacterial strains, plasmids, and primers are listed in Table 1, 2 and 3.
Table 1. Strains that are used in this study.
| STRAINS | CHARACTERISTICS | REFERENCE |
|---|---|---|
| Salmonella enterica Cerro 87 | Strain containing naturally S-modified DNA, source of the dptB-E gene cluster | [6] |
| E. coli DH10B | Non-restricting host strain for gene cloning | [7] |
| E. coli BW25113 | acI q rrnB T14 ΔlacZ WJ16 hsdR514 ΔaraBAD AH33 _Δ_rhaBADLD78, strain used for creating gene knockouts | [12] |
| BL21(DE3)pLysS | Lacks lon and ompT proteases Cmlr | Novagen |
| JW2514-4 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, ΔiscS776::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW1670-1 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, ΔsufS755::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW2781-1 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, ΔcsdA738::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW2513-1 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, ΔiscU775::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW3955-2 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, rph-1, Δ(rhaD-rhaB)568, ΔthiS762::kan, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW3956-1 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, rph-1, Δ(rhaD-rhaB)568, ΔthiF763::kan, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW2512-1 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, ΔiscA774::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW2508-1 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, ΔiscX770::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW0810-2 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, ΔmoeB726::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW3779-3 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, rph-1, ΔcyaY752::kan, Δ(rhaD-rhaB)568, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW3435-1 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, ΔyhhP(tusA)725::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Yale Coli Genetic Stock Center [9] |
| JW0413-1 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), ΔthiI780::kan, λ-, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Yale Coli Genetic Stock Center [9] |
| AXH034 | E.coli F-, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), &lambda−, ΔiscS1191::kan, rph-1, Δ(rhaD-rhaB)568, hsdR514 | This study |
| E. coli XL1-Blue MR | Host strain for propagating pBT and pTRG recombinants Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96; relA1 lac | BacterioMatch II Kit (Agilent) |
| E. coli XL1-Blue MRF′ Kan | Derivative of XL1-Blue MR. Reporter strain for two-hybird test using pBT and pTRG derivatives Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 endA1 supE44 thi-1 recA1 gyrA96 relA1 lac [F′ proAB lacI q ZΔM15 Tn5 (Kan r)] | BacterioMatch II Kit (Agilent) |
Table 2. Plasmids that are used in this study.
| PLASMIDS | CHARACTERISTICS | REFERENCE |
|---|---|---|
| pKD46 | amp repts (30° for replication, 42° for curing) | [7] |
| pJTU3510 | dptB-E from S. enterica Cerro 87, p15A origin of replication, Cmlr | This study |
| pJTU3523 | dptC from S. enterica Cerro 87, cloned in pSJ7 expression vector | This study |
| pJTU3525 | dptE from S. enterica Cerro 87, cloned in pSJ7 expression vector | This study |
| pBT | Bait plasmid, λcI Cmlr, cloning between _Not_I and _Xho_I | bacterioMatch II Two-Hybrid System Vector Kit (Agilent) |
| pTRG | Target plasmid, Tetr, cloning between _Bam_HI and _Xho_I | bacterioMatch II Two-Hybrid System Vector Kit (Agilent) |
| pBT-LGF2 | Control plasmid λcI LGF2 Cmlr | bacterioMatch II Two-Hybrid System Vector Kit (Agilent) |
| pTRG-GAL11P | Control plasmid RNAP-α GAL11Pr | bacterioMatch II Two-Hybrid System Vector Kit (Agilent) |
| pJTU3609 | dptB cloned in pTRG with site _Bam_HI and _Xho_I | This study |
| pJTU3610 | dptC cloned in pTRG with site _Bam_HI and _Xho_I | This study |
| pJTU3611 | _dptD_cloned in pTRG with site _Bam_HI and _Xho_I | This study |
| pJTU3612 | dptE cloned in pTRG with site _Bam_HI and _Xho_I | This study |
| pJTU3618 | iscS cloned in pBT with site _Not_I and _Xho_I | This study |
| pET15b | Expression vector with His6-tag Ampr | Novagen |
| pJTU3619 | Expressing E. coli iscS (amplified using primers iscS exU/exD) in pET15b _Nde_I and _Bam_HI | This study |
| pJTU3625 | pJTU3619 derivative site mutant with C111A | This study |
| pJTU3626 | pJTU3619 derivative site mutant with C170A | This study |
| pJTU3627 | pJTU3619 derivative site mutant with C328A | This study |
| pJTU3622 | dptC with TEV site insert into pGEX-6P-1 between _Sma_I and _Xho_I | This study |
| pJTU3624 | dptE with TEV site insert into pGEX-6P-1 between _Sma_I and _Xho_I | This study |
Table 3. Primers that are used in this study.
| PRIMERS | SEQUENCE | USE |
|---|---|---|
| P1 | ATTCCGGGGATCCGTCGACC | Amplification of neo FRT |
| P2 | TGTAGGCTGGAGCTGCTTC | Amplification of neo FRT |
| H1P1 | GGTAGCCTGATTCCTTGCATTGAGTGATGTACGGAGTTTATAGAGCAATGATTCCGGGGATCCGTCGACC | Replacement of iscS |
| H2P2 | ATTATAAATTCTCCTGATTCCGATACCGATTAATGATGAGCCCATTCGATTGTAGGCTGGAGCTGCTTC | Replacement of iscS |
| U | AAGTGCTGGATGTGTCTG | Verification of iscS deletion |
| D | GACGTTCTCGTCGTTGTT | Verification of iscS deletion |
| iscS exU | GGGAATTCCATATGAAATTACCGATTTATC | To clone iscS with _Nde_I site |
| iscS exD | CCGGGATCCAGCCATTATAAATTCTCC | To clone iscS with _Bam_HI site |
| GST-dptC F | TGATTACGATATCCCAACGAC | To clone dptC |
| GST-dptC R | CCGCTCGAGTGTAATACCAGTTG | To clone dptC with _Xho_I site |
| GST-dptE F | TGATTACGATATCCCAACGAC | To clone dptE |
| GST-dptE R | CCGCTCGAGGTTGATGCTGCCGT | To clone dptC with _Xho_I site |
| C111A F | GCGGTACTGGATACCGCACGTCAGCTGGAGCGC | Mutated site in IscS |
| C111A R | GCGCTCCAGCTGACGTGCGGTATCCAGTACCGC | Mutated site in IscS |
| C170A F | GCTATCGGCGAAATGGCACGTGCTCGTGGCATT | Mutated site in IscS |
| C170A R | AATGCCACGAGCACGTGCCATTTCGCCGATAGC | Mutated site in IscS |
| C328A F | TCTTCAGGTTCCGCCGCAACGTCAGCAAGCCTC | Mutated site in IscS |
| C328A R | GAGGCTTGCTGACGTTGCGGCGGAACCTGAAGA | Mutated site in IscS |
| IscS-CMu F | ATCTGACAACCTGGCGATCA | To verify IscS mutantions |
| IscS-CMu R | CTTCAGTAGTAAAACGACCT | To verify IscS mutantions |
| _dptB_TRG U | CCGGGATCCATGGCTAGTGTTGATGCAG | To clone dptB to pTRG with _Bam_HI |
| _dptB_TRG D | CCGCTCGAGAAATCGTAGGCCTGAACT | To clone dptB to pTRG with _Xho_I |
| _dptC_TRG U | CCGGGATCCATGAGTAAATTAGTTCAGG | To clone dptC to pTRG with _Bam_HI |
| _dptC_TRG D | CCGCTCGAGTTATGTAATACCAGTTGC | To clone dptC to pTRG with _Xho_I |
| _dptD_TRG U | CCGGGATCCATGCGGGCGAATCGTCTG | To clone dptD to pTRG with _Bam_HI |
| _dptD_TRG D | CCGCTCGAGCCATTCGATTCGGGAGCA | To clone dptD to pTRG with _Xho_I |
| _dptE_TRG U | CCGGGATCCATGCTCCCGAATCGAATG | To clone _dptE_to pTRG with _Bam_HI |
| _dptE_TRG D | CCGCTCGAGTTGATGCTGCCGTAAAAG | To clone dptE to pTRG with _Xho_I |
| iscS BT U | ATAAGAATGCGGCCGCAATGAAATTACCGATTTAT | To clone iscS to pBT with _Not_I |
| iscS BT D | CCGCTCGAGCCATTATAAATTCTCC | To clone iscS to pBT with _Xho_I |
The E. coli BW25113 gene replacement mutants listed in Table 1 were obtained from Yale Coli Genetic Stock Center [9]. Among these, the iscS mutant JW2514 was not viable, and was recreated by using the gene knockout method described by Datsenko [12]. For this, the neo-FRT (FLP, recombinase recognition target) cassette was amplified using primer P1 and P2, then H1P1 and H2P2. Successful iscS deletion was confirmed by PCR using the flanking primers U and D ( Fig. 2A ).
Figure 2. E.coli iscS is required for DNA phosphorothioation.
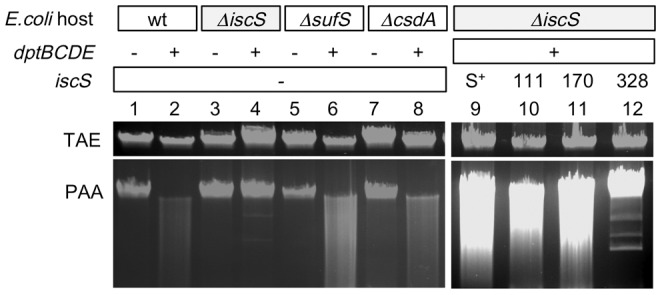
Ethidium bromide-stained agarose gels containing E. coli total genomic DNA, separated in Tris-acetate EDTA (TAE) buffer. Top gel (TAE), untreated samples; bottom gel (PAA), identical DNA samples after incubation in TAE containing 1% per-acetic acid (PAA). A fluorescent smear in the lower gel indicates that the DNA was S-modified. Lanes 1–8, Dnd (DNA degradation) phenotypes of E. coli cysteine desulfurase deletion mutants (ΔiscS, ΔsufS, ΔcsdA) containing the S. enterica dptBCDE gene cluster cloned on pJTU3510 (lane 1–8); lanes 9–12, trans complementation of the chromosomal ΔiscS mutation by pJTU3619 containing mutant derivatives of iscS (lanes 9–12). E. coli hosts: wt, wild type. The mutations ΔiscS, ΔsufS and ΔcsdA are in the E. coli chromosome. pJTU3510: −, no plasmid; +, pJTU3510 expressing dptBCDE. pJTU3619 (compatible with pJTU3510) containing the following genes: S+, wild-type E. coli iscS; 111, 170, 328, mutant iscS genes containing the aa changes Cys111Ala, Cys170Ala or Cys328Ala, respectively. −, no plasmid. TAE, gel running buffer; PAA, TAE containing per-acetic acid.
Detection of DNA phoshorothioation
Phosphorothioate DNA is sensitive to double-strand cleavage by Tris-peracetic acid (TPA) [13]. The phosphorothioation was detected by incubating DNA samples for 30 min at 25°C in TAE buffer (40 mM Tris, 20 mM sodium acetate, 0.8 mM EDTA pH 7.5) supplemented with 1.0% peracetic acid. Phosphorothioate DNA, but not normal DNA, shows Dnd phenotype, producing a smear of DNA fragments in an agarose gel. To prevent DNA degradation during electrophoresis, 50 mM thiourea was added to the TAE electrophoresis buffer [13], [14].
Bacterial two-hybrid analysis
Protein-protein interactions were investigated using the BacterioMatch II two-hybrid system (Stratagene), according to the manual [15] with some modifications. The system features a HIS3-aadA reporter cassette, whose expression allows E. coli growth in the presence of 3-AT (3-amino-1,2,4-triazole), which is a competitive inhibitor of His3 (imidazoleglycerol-phosphate dehydratase), and in the presence of streptomycin.
To test protein-protein interactions, in-frame gene fusions were created in the pBT (bait) or pTRG (target) vectors. PCR primers with suitable restriction sites were constructed and are listed in Table 1. IscS was fused with a bait protein, generating pBT-IscS; DndB-E were fused with target protein, generating pTRG-DptB, pTRG-DptC, pTRG-DptD and pTRG-DptE respectively. The resulting bait and target clones were co-transformed into the reporter strain E. coli XL1-Blue MRF′ Kan (Stratagene/Agilent) and selected on LB agar containing 25 µg/ml chloramphenicol (to select for pBT derivatives), 12.5 µg/ml tetracycline (to select for pTRG derivatives), and 50 µg/ml kanamycin (to maintain F′proAB lacI q ZΔM15 Tn5).
To test for resistance to 3-AT, single colonies were inoculated into 1 mL LB containing the three above antibiotics, and kept shaking overnight at 30°C. 500 µl of this overnight culture was then inoculated into 5 mL SOC medium and incubated for 90 min at 37°C. The cells were then spun down at 3500 rpm for 5 min at room temperature, and the supernatant was carefully removed. The cells were then re-suspended in 2 ml M9+ His-drop out broth, collected by centrifugation as described above, and re-suspended in 3 mL M9+ His-drop out broth [15]. After incubation for 2 hours at 37°C, three parallel ten-fold dilutions 10−1–10−7 were prepared and plated 100, 10−1, 10−2 and 10−3 on Selective Screening Medium (SSM) containing 5 mM 3-AT and 10−4, 10−5, 10−6 and 10−7 on Nonselective Screening Medium (NSM) without 3-AT. Colonies were counted after 24 h incubation at 37°C. If there were no visible colonies, the plates were incubated in dark at 25°C for another 16 hours.
Putative positive interactions were verified using Dual Selective Screening Medium containing 5 mM 3-AT + 12.5 µg mL−1 streptomycin.
Strep and GST Pull-down
Ten milliliters of E. coli BL21 (DE3) strain (harboring Strep-iscS, or GST-DptC, or GST-DptE) was inoculated to 1 L and grow at 37°C for 3 hours with shaking (220 rpm). IPTG was then added to a final concentration of 0.2 mM (from 1000 folds stock). The culture was then moved to 16°C and grew for another 24 hours. The cells were collected by centrifuge for 10 minutes at 4°C.
Cell pellet was re-suspended using Buffer S (25 mM Hepes pH7.6, 100 mM KCl, 10% glycerol, 1 to 10 folds (w/w)) and sonicated for 30 minutes. Cell debris was removed by centrifugation at 15000 g for 20 minutes. Equal volume of the extract was mixed (IscS-GSTDptC or IscS-GSTDptE). Two milliliter of the mixture was incubated with 0.1 ml Streptactin resin (Qiagen) or GST resin (Qiagen) pre-equilibrated using Buffer S. After 1 hour, the resin was spin down (400 g, 3 minutes). Supernatant was removed. The resin was wash 5 times using 2 ml Buffer S. The protein was eluted using 0.3 ml Buffer S supplemented with 2.5 mM Desthiobiotin or 20 mM Glutathione. Western blot was done using antibodies from Abcam (ab58626 for GST, or ab76949 for StreptagII).
Results
Expression of S. enterica dptB-E in E. coli BW25113 results in DNA phosphorothioation
Owing to the observation that in Streptomyces lividans, dndA is essential for DNA phosphorothioation, we sought to find the cysteine desulfurase gene in E. coli. The E.coli genome was searched for orthologs of a cysteine desulfurase gene.
Fig. 1B shows that there are at least three cysteine desulfurase genes in the E. coli genome [10], [11].
Fig. 1C shows that introducing pJTU3510 carrying dptB-E four genes, a low-copy plasmid, into E. coli BW25113 resulted in DNA S-modification (lane 3). We speculated that dptB-E, in cooperation with one or more E. coli desulfurase gene, leads to DNA phosphorothioation.
_Isc_S is responsible for the DNA phosphorothioation in E.coli
For DNA phosphorothioation in E.coli BW25113, it seemed likely that a protein similar to the cysteine desulfurase DndA was needed in addition to the S. enterica dptB-E gene cluster. Individual E.coli BW25113 knockout mutants, ΔiscS, ΔsufS and ΔcsdA were available from the Yale Coli Genetic Stock Center. The iscS mutant did not survive the transport and was reconstructed (Fig. S1).
E. coli BW25113 and the three cysteine desulfurase mutants were transformed with pJTU3510 expressing dptB-E, and tested for the phosphorothioation status by Dnd phenotypic assay (DNA smear, an indicator of DNA phosphorothioate modification) (Fig. 2). Only the iscS mutant failed to modify its DNA (lane 4), suggesting that only E. coli IscS, but not SufS or CsdA, was responsible for DNA phosphorothioation in E. coli.
To confirm that iscS is responsible for DNA phosphorothioation, iscS was cloned into pET15b, and co-transformed with a dpt gene cluster harboring low copy number plasmid pJTU3510 into the E. coli iscS deletion mutant. Fig. 2 lane 9 shows that DNA phosphorothioation was restored in the strain, proving that IscS, in cooperation with DptB-E, restored DNA phosphorothioation in E. coli.
Involvement of IscS in DNA phosphorothioation in E. coli was further confirmed by site-directed mutagenesis. Three conserved cysteine residues in IscS, were mutated to Ala, generating three iscS cysteine mutants (C111A, C170A, and C328A). These mutants were again co-transformed with pJTU3510 (harboring the dpt gene cluster) into the iscS deletion mutant. Fig. 2 lane 10–12 shows that only C328A abolished DNA phosphorothioation.
IscS might participate in DNA phosphorothioation directly
The cysteine desulfurase IscS is a highly conserved master enzyme initiating sulfur transfer via persulfide to a range of acceptor proteins. IscS is involved in various physiological processes, including Fe-S cluster assembly, tRNA modification, and sulfur-containing cofactor biosynthesis. IscS-interacting partners, including IscU, TusA, ThiI, ThiF and MoeB are sulfur acceptors. Other proteins, such as CyaY, IscA and IscX, also bind to IscS, but their functional roles are not directly related to sulfur transfer [16].
Mutants of cyaY, iscA, iscU, iscX, moeB, tusA, thiF, thiI and thiS, proteins known to interact with IscS in E. coli, were tested for their possibility to participate into DNA phosphorothioation. Fig. 3 shows that none of these genes was required for the modification, as assayed by Dnd phenotype. This suggested that IscS in E. coli might participate directly into the modification process.
Figure 3. IscS might participate DNA phosphorothioation directly.
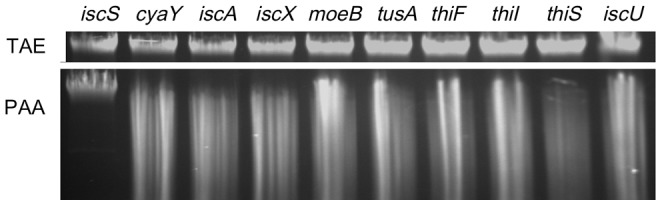
Ethidium bromide-stained agarose gels. TAE (top gel), samples run in normal TAE buffer; PAA (bottom gel), samples run in TAE containing PAA. Expression of S. enterica dptB-E resulted in DNA S-modification and a fluorescent smear in all samples, except for E. coli ΔiscS. IscS was therefore the only gene that was required for DNA S-modification among the tested deletions.
Protein-protein interactions between IscS and Dpt proteins
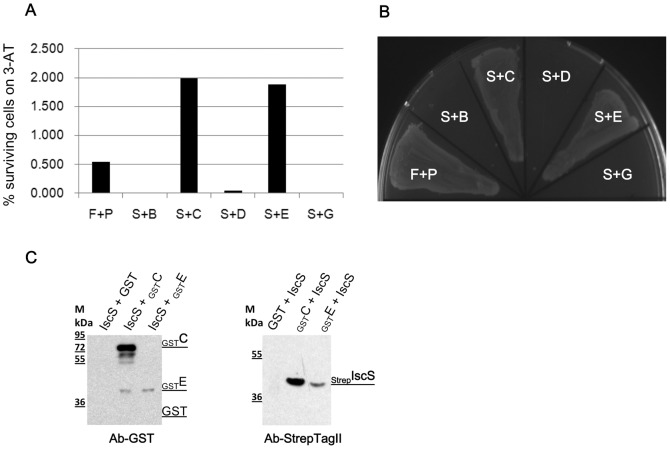
The bacterial two-hybrid system was used to detect interactions between E. coli IscS and DptB, C, D and E. IscS was fused with the bait protein, while DptB, C, D, and E were fused with the target protein.
Strong protein-protein interactions were immediately detected between IscS and DptC (2% surviving cells on 3AT), IscS and DptE (2% surviving cells on 3AT), but not between IscS and DptB and DptD (Fig. 4A). These protein interactions were confirmed further by plating the co-transformed strains on medium containing streptomycin (Fig. 4B).
Figure 4. Protein interactions between IscS and Dpt proteins.
A.The bar graph shows protein interactions that enable the E. coli cells to survive on medium containing 3AT (3-amino-1,2,4-triazole). F, pBT-LGF2; P, pTRG-Gal11P; S, pBT-IscS; B, pTRG-DptB; C, pTRG-DptC; D, pTRG-DptD; E, pTRG-DptE; G, pTRG only. F and P were co-expressed as positive control; S and G were co-expressed as negative control. E. coli can grow on 3-AT selective screening medium only when there is a binding interaction between the fusion proteins expressed from the bait and target plasmids. B. Dual selection plate containing 3-amino-1,2,4-triazole and streptomycin. F+P, LGF2+GallP (growth, positive control); S+B, IscS+DptB (no growth, no interaction); S+C, IscS+DptC (growth indicating protein interaction); S+D, IscS+DptD (no growth, no interaction); S+E, IscS+DptE (growth indicating protein interaction); S+G, IscS+pTRG (no growth, negative control). C. Interactions between IscS and DptC as well as IscS and DptE confirmed by pull-down experiments. Left panel: IscS (N terminus Strep tagged) extraction was mixed with GSTDptC or GSTDptE extraction and then purified by Streptactin affinity purification. Western blot was done using antibody against GST. Right panel: the mixture was purified by GST affinity purification. Western blotting was done using antibody against StreptagII.
Protein-protein interaction between IscS and DptC as well as IscS and DptE were further confirmed by pull-down experiments. Fig. 4C shows that Strep tagged IscS can pull-down both GST tagged DptC and DptE. Reciprocally, GST tagged DptC and DptE can also pull-down Strep tagged IscS.
Discussion
IscS is a highly conserved, but functionally versatile pyridoxal-5′-phosphate (PLP)-dependent enzyme. It delivers sulfur to players within various metabolic pathways, including iron-sulfur cluster assembly, thiamine and biotin synthesis, tRNA modifications, and molybdopterin biosynthesis [16], [17]. We show here that IscS can also participate in DNA phosphorothioation.
The involvement of IscS in DNA phosphorothioation could be direct or indirect. By analyzing the Dnd phenotype and the mutants (Fig. 3), we were able to rule out the possibility that IscS participates indirectly via other pathways. We hypothesized that if IscS is involved in the DNA phosphorothioation process directly, we might be able to detect protein-protein interaction between IscS and the Dnd proteins. In keeping with this hypothesis, protein interaction between IscS and DndE and DndC were detected using the bacterial two hybrid system.
There are two potential functions of IscS in the process of DNA phosphorothioation. One is Fe-S cluster assembly for the DndC protein. It is known that DndA can catalyze apo-Fe DndC to its Fe-S cluster form [5]. Another function might be to transfer sulfur from cysteine to the target DNA via protein interactions with the Dnd proteins, which is reminiscent of tRNA modification [18], [19]. These hypothesises are currently under intensive investigation.
Supporting Information
Figure S1
Disruption of _isc_S gene. A. Replacement of iscS by PCR targeting using a neo cassette flanked by 50 bp homologous E. coli sequences. B. Ethidium bromide-stained agarose gel showing PCR products obtained from _E. coli Δ_iscS and wild-type E. coli, using flanking primers.
(TIF)
Acknowledgments
We are grateful to Tobias Kieser and Dr. Shirali Pandya in UC Berkeley for editing the manuscript, and to Pro. Linquan. Bai, Dr. Tingting Huang and Jun Yin for their helpful advice.
Funding Statement
The authors wish to thank the National Science Foundation of China, the Ministry of Science and Technology (973 and 863 programs), the Ministry of Education of China, the Shanghai Municipal Council of Science and Technology and Shanghai Leading Academic Discipline Project B203, the State Key Laboratory of Bio-Organic and Natural Products Chemistry (CAS) and the National Program of Development of Transgenic New Species of China for research support. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zhou XF, He XY, Liang JD, Li AY, Xu TG, et al. (2005) A novel DNA modification by sulphur. Molecular Microbiology 57: 1428–1438. [DOI] [PubMed] [Google Scholar]
- 2.Ou HY, He X, Shao Y, Tai C, Rajakumar K, et al. (2009) dndDB: a database focused on phosphorothioation of the DNA backbone. PLoS One 4: e5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He X, Ou HY, Yu Q, Zhou X, Wu J, et al. (2007) Analysis of a genomic island housing genes for DNA S-modification system in Streptomyces lividans 66 and its counterparts in other distantly related bacteria. Molecular Microbiology 65: 1034–1048. [DOI] [PubMed] [Google Scholar]
- 4.Xu T, Liang J, Chen S, Wang L, He X, et al. (2009) DNA phosphorothioation in Streptomyces lividans: mutational analysis of the dnd locus. BMC Microbiol 9: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You DL, Wang LR, Yao F, Zhou XF, Deng ZX (2007) A novel DNA modification by sulfur: DndA is a NifS-like cysteine desulfurase capable of assembling DndC as an iron-sulfur cluster protein in Streptomyces lividans. Biochemistry 46: 6126–6133. [DOI] [PubMed] [Google Scholar]
- 6.Xu TG, Yao F, Zhou XF, Deng ZX, You DL (2010) A novel host-specific restriction system associated with DNA backbone S-modification in Salmonella. Nucleic Acids Research 38: 7133–7141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durfee T, Nelson R, Baldwin S, Plunkett G, Burland V, et al. (2008) The complete genome sequence of Escherichia coli DH10B: Insights into the biology of a laboratory workhorse. Journal of Bacteriology 190: 2597–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang LR, Chen S, Xu TG, Taghizadeh K, Wishnok JS, et al. (2007) Phosphorothioation of DNA in bacteria by dnd genes. Nature Chemical Biology 3: 709–710. [DOI] [PubMed] [Google Scholar]
- 9.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, et al. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2: 2006 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihara H, Kurihara T, Yoshimura T, Soda K, Esaki N (1997) Cysteine sulfinate desulfinase, a NIFS-like protein of Escherichia coli with selenocysteine lyase and cysteine desulfurase activities - Gene cloning, purification, and characterization of a novel pyridoxal enzyme. Journal of Biological Chemistry 272: 22417–22424. [DOI] [PubMed] [Google Scholar]
- 11.Mihara H, Maeda M, Fujii T, Kurihara T, Hata Y, et al. (1999) A nifS-like gene, csdB, encodes an Escherichia coli counterpart of mammalian selenocysteine lyase - Gene cloning, purification, characterization and preliminary x-ray crystallographic studies. Journal of Biological Chemistry 274: 14768–14772. [DOI] [PubMed] [Google Scholar]
- 12.Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray T, Weaden J, Dyson P (1992) Tris-dependent site-specific cleavage of Streptomyces lividans DNA. Federation of European Microbiological Societies 96: 6. [DOI] [PubMed] [Google Scholar]
- 14.Liang J, Wang Z, He X, Li J, Zhou X, et al. (2007) DNA modification by sulfur: analysis of the sequence recognition specificity surrounding the modification sites. Nucleic Acids Res 35: 2944–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stratagene (2008) BacterioMatch II Two-Hybrid System Vector Kit. Stratagene [Google Scholar]
- 16.Shi R, Proteau A, Villarroya M, Moukadiri I, Zhang L, et al. (2010) Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol 8: e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang WJ, Urban A, Mihara H, Leimkuhler S, Kurihara T, et al. (2010) IscS Functions as a Primary Sulfur-donating Enzyme by Interacting Specifically with MoeB and MoaD in the Biosynthesis of Molybdopterin in Escherichia coli. Journal of Biological Chemistry 285: 2302–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjork GR (1995) Genetic dissection of synthesis and function of modified nucleosides in bacterial transfer RNA. Prog Nucleic Acid Res Mol Biol 50: 263–338. [DOI] [PubMed] [Google Scholar]
- 19.Bjork GR, Ericson JU, Gustafsson CE, Hagervall TG, Jonsson YH, et al. (1987) Transfer RNA modification. Annu Rev Biochem 56: 263–287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Disruption of _isc_S gene. A. Replacement of iscS by PCR targeting using a neo cassette flanked by 50 bp homologous E. coli sequences. B. Ethidium bromide-stained agarose gel showing PCR products obtained from _E. coli Δ_iscS and wild-type E. coli, using flanking primers.
(TIF)