WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing (original) (raw)
. Author manuscript; available in PMC: 2013 Feb 18.
Published in final edited form as: Cancer Cell. 2011 Dec 13;20(6):768–780. doi: 10.1016/j.ccr.2011.10.016
Summary
Angiogenesis is regulated by the balance of pro-angiogenic VEGF165 and anti-angiogenic VEGF165b splice isoforms. Mutations in WT1, the Wilms’ tumour suppressor gene, suppress VEGF165b and cause abnormal gonadogenesis, renal failure and Wilms’ tumours. In WT1 mutant cells, reduced VEGF165b was due to lack of WT1 mediated transcriptional repression of the splicing factor kinase SRPK1. WT1 bound to the SRPK1 promoter, and repressed expression through a specific WT1 binding-site. In WT1 mutant cells SRPK1-mediated hyperphosphorylation of the oncogenic RNA binding protein SRSF1 regulated splicing of VEGF, and rendered WT1 mutant cells pro-angiogenic. Altered VEGF splicing was reversed by wildtype WT1, knockdown of SRSF1 or SRPK1 and inhibition of SRPK1, which prevented in vitro and in vivo angiogenesis and associated tumour growth.
INTRODUCTION
Tumour growth requires new vessel formation, and this is driven predominantly by VEGF, the most potent angiogenic molecule known and the principal target for anti-angiogenic therapy(Hurwitz et al., 2004). VEGF is alternatively spliced to form two families, one by splicing to a proximal 3′ splice site in exon 8(Houck et al., 1991), and a second by splicing to a distal 3′ splice site in exon 8(Bates et al., 2002; Cebe Suarez et al., 2006; Woolard et al., 2004). Whereas proximal splice site (PSS) selection results in angiogenic isoforms of VEGF including VEGF165, distal splice site (DSS) selection results in a family with anti-angiogenic properties (e.g. VEGF165b, see figure S1A). VEGF165b inhibits VEGFR2 signalling by inducing differential phosphorylation(Kawamura et al., 2008) and intracellular trafficking(Ballmer-Hofer et al., 2011), and blocks angiogenesis in vivo in the mouse dorsal skin, and chick chorioallantoic membrane(Cebe Suarez et al., 2006), rabbit cornea and rat mesentery(Woolard et al., 2004), developing rat ovary(Artac et al., 2009) and testis(Baltes-Breitwisch et al., 2010), melanoma, prostate, renal, and colon cancer(Varey et al., 2008), sarcoma, and metastatic melanoma(Rennel et al., 2008), and in the mouse retina and choroid(Hua et al., 2010; Konopatskaya et al., 2006). The second member of the family so far investigated, VEGF121b is also anti-angiogenic in the retina and in colon cancer(Rennel et al., 2009). The role of the anti-angiogenic family has not yet been investigated in as much detail as the angiogenic family, although it appears to be relatively highly expressed in non-angiogenic tissues(Woolard et al., 2009), and is downregulated during angiogenesis(Bates et al., 2002; Perrin et al., 2005; Varey et al., 2008). VEGF165b is downregulated in the mammary gland during pregnancy, when vascular remodelling and angiogenesis are required for epithelial gland formation. Over-expression of VEGF165b in the mammary gland during pregnancy inhibits duct formation, resulting in reduced milk formation and pup starvation(Qiu et al., 2008), and inhibition of endogenous VEGF165b in the ovary results in abnormal angiogenesis and increased follicle progression(Artac et al., 2009). In the kidney, expression of VEGF165b in the podocyte controls permeability in the kidney and maintains normal glomerular filtration rates by regulating fenestral formation(Qiu et al., 2010).
As VEGF165b and VEGF165 are generated from the same transcript, the relative amount of the pro-angiogenic versus anti-angiogenic isoforms is dependent upon splicing to either the proximal splice site (PSS, angiogenic VEGF165) or distal splice site (DSS, antiangiogenic VEGF165b),(Harper and Bates, 2008). The control of this splicing is poorly understood, but recent studies have identified the role of three key serine –arginine rich (SR) proteins, SRSF6 (SRp55)(Manetti et al., 2011; Nowak et al., 2008), SRSF1 (ASF/SF2)(Nowak et al., 2010) and SRSF2 (SC35)(Merdzhanova et al., 2010) in the control of the terminal splice site selection. SRSF1 has been implicated in proximal splice site selection, induced by IGF-1, and binding to the region around the proximal splice site. SRSF6 has been implicated in distal splice site selection as it binds around the distal splice site and is upregulated by TGFβ1 in systemic sclerosis, resulting in increased VEGF165b expression and inhibition of angiogenesis(Manetti et al., 2011). A key study by Schumacher et al in 2007 identified a lack of the anti-angiogenic isoform in laser dissected glomeruli of Denys Drash Syndrome (DDS) patients with a genetic mutation in the Wilms’ tumour suppressor gene WT1(Schumacher et al., 2007). These patients have increased risk of Wilms’ tumours, intersex disorders and renal failure(Denys et al., 1967; Drash et al., 1970). Wilms’ tumours are highly vascularised tumours with high VEGF levels(Blann et al., 2001; Skoldenberg et al., 2001), and WT1 mutations or altered expression are also found in other highly vascularised tumours such as prostate cancer, haematological cancers and colorectal, breast, desmoid and brain tumours(Hohenstein and Hastie, 2006). WT1 is also expressed as different isoforms by alternative splicing(Haber et al., 1991). The most widely studied isoforms are the inclusion or exclusion of exon 5 and an alternative splice donor site in exon 9, which encodes three amino acids, KTS. The -KTS isoforms interact preferentially with DNA. Thus WT1 can be exon5+ or exon 5− and KTS+ or KTS− and all four isoforms are expressed in several tissues(Morrison et al., 2008). As DDS causing mutations in WT1 can alter VEGF splicing(Schumacher et al., 2007), we have investigated this link between WT1 and splicing of the VEGF transcript, testing the hypothesis that WT1 mutations regulate splicing through splice factor specific mechanisms.
RESULTS
DDS cells have reduced Distal Splice Site selection in VEGF resulting in less VEGF165b
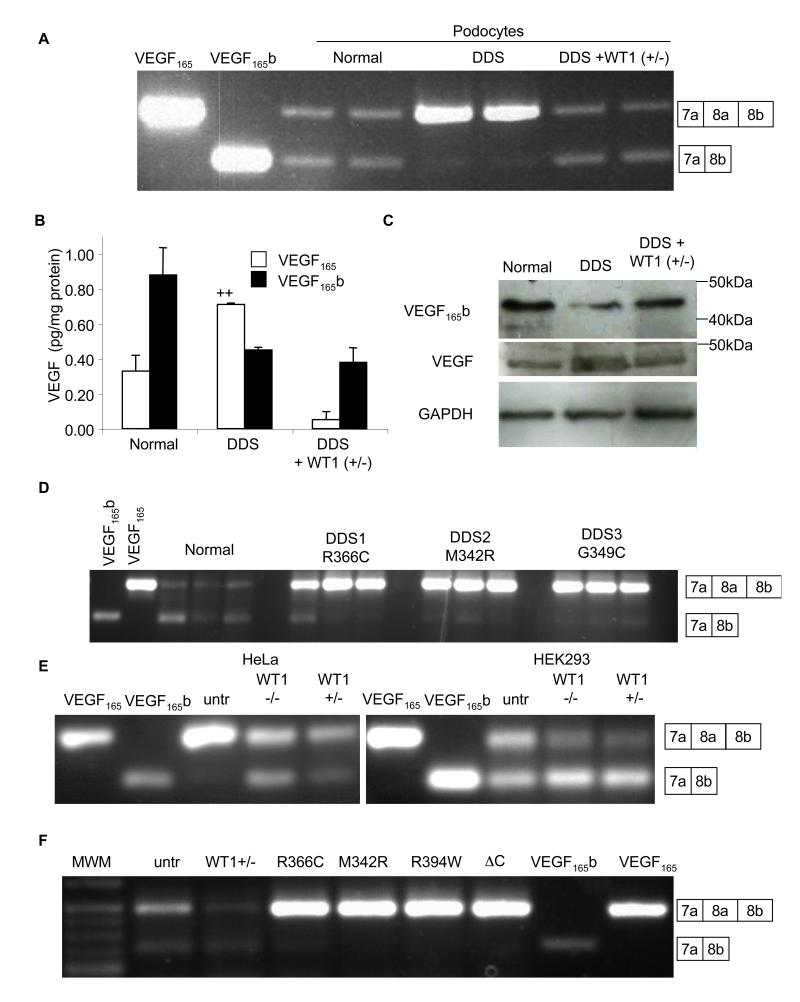
Schumacher et al have shown that laser dissected glomeruli from patients with DDS lack VEGF165b(Schumacher et al., 2007). To determine whether this was reproduced in an in vitro cell system, we used the previously described conditionally immortalised cell line from a patient with a C1096T mutation in WT1(Viney et al., 2007). This results in a single amino acid substitution at amino acid 366 from an arginine to a cysteine in the third zinc finger, critical for DNA binding. Figure 1A shows that whereas normal podocytes express both isoforms approximately equally, in the DDS podocytes the VEGF165 isoform was dominant. To ensure that this was due to a lack of a functional WT1 gene, a stable DDS cell line was generated expressing wild type WT1 (+exon5/−KTS; “rescued” DDS). This restored expression of VEGF165b. To determine whether altered splicing resulted in altered VEGF165b protein levels, cells were lysed, protein extracted and subjected to VEGF ELISA, using a pan-VEGF capture antibody and then either a pan-VEGF or a VEGF165b specific detection antibody. Figure 1B shows that DDS cells had increased VEGF165, but decreased VEGF165b. Rescued DDS cells restored overall VEGF expression and splicing towards normal podocyte levels. Q-PCR indicated no change in total VEGF mRNA levels between DDS (0.10±0.01% of GAPDH) and wild type (0.15±0.06% of GAPDH) cell lines, and over-expression of wild type WT1 (+/−) also resulted in no change in total VEGF expression (0.13±0.01%). To confirm that this was VEGF165b and VEGF165 that were being expressed, proteins were subjected to immunoblot. Figure 1C shows that it was the 165 amino acid isoform (VEGF165b) that was altered. To determine whether podocytes from DDS patients with other mutations in WT1 also had altered VEGF165b expression, conditionally immortalised podocytes generated from patients with M342R or G349C mutations were used. Figure 1D shows that cells derived from both additional patients had reduced VEGF165b expression relative to normal podocytes. To determine whether WT1 was capable of altering expression in other cell types, HeLa cells (which normally express low levels of VEGF165b), and HEK293T cells (which do express VEGF165b) were examined. Figure 1E shows that transfection of HeLa cells with either WT1−/− (−exon5/−KTS) or WT1+/− (+exon5/−KTS) resulted in VEGF165b expression and reduced VEGF165 expression, and in HEK293 cells this transfection resulted in increased VEGF165b and reduced VEGF165. The presence or absence of WT1’s exon 5 did not alter the effect, but the +KTS isoforms (WT1−/+ and WT1+/+) were unable to switch splicing to increase VEGF165b expression in either HeLa (figure S1B) or HEK cells (figure S1C). Splice site selection in exon 9 (+KTS) of WT1 itself was not altered in DDS cells (figure S1D). Thus, surprisingly, the splicing effect is limited to isoforms that lack the KTS sequence, isoforms that are mostly associated with stable DNA binding of its GC rich target sequence, suggesting it was not a direct RNA binding effect of WT1, but due to a transcriptional target of WT1. To determine whether other DDS mutations could alter splicing in HEK cells we transfected HEK cells with WT1 containing the R366C, M342R, or R394W DDS mutations and a C-terminal deletion(Bor et al., 2006). All four mutations reduced VEGF165b expression relative to untransfected cells, whereas wild type WT1(+/−) transfection increased the ratio of VEGF165b to VEGF165. (figure 1F).
Figure 1. Wild type WT1 induces anti-angiogenic VEGF165b expression.
A. RT-PCR of mRNA extracted from normal or DDS podocytes using primers that detect both proximal and distal splice isoforms of VEGF. VEGF165 and VEGF165b cDNAs are used as positive controls. Transfection of DDS podocytes with WT1 (+exon 5/−KTS) restored VEGF165b splicing. Boxes indicate exon usage represented by the bands. B. ELISAs for VEGF165b or VEGF165 protein using a pan-VEGF capture antibody and specific detection antibodies. VEGF165 was calculated from the difference between pan-VEGF and VEGF165b. C. Immunoblot of protein extracted from normal, DDS podocytes, or DDS cells transfected with wild type WT1(+/−) using antibodies to VEGF165b, total VEGF or GAPDH. D. RT-PCR of podocyte cell lines (three replicates) from three different DDS patients with different WT1 mutations show reduced VEGF165b expression. E. RT-PCR of mRNA from HeLa and HEK293 cells show expression of VEGF165b in HEK293 but not HeLa cells (untr=untransfected), but both cell types have increased VEGF165b expression in the presence of WT1 (−/−), and WT1 (+/−). F. Transfection of HEK293 cells with plasmids containing _DDS_-causing WT1 mutations abolished VEGF165b expression. Wild type WT1(+/−) over-expression increases distal splicing relative to proximally spliced isoforms. Bar charts are mean±SEM. See also figure S1.
The splice factor SRSF1 is nuclear in DDS podocytes and regulates VEGF splicing
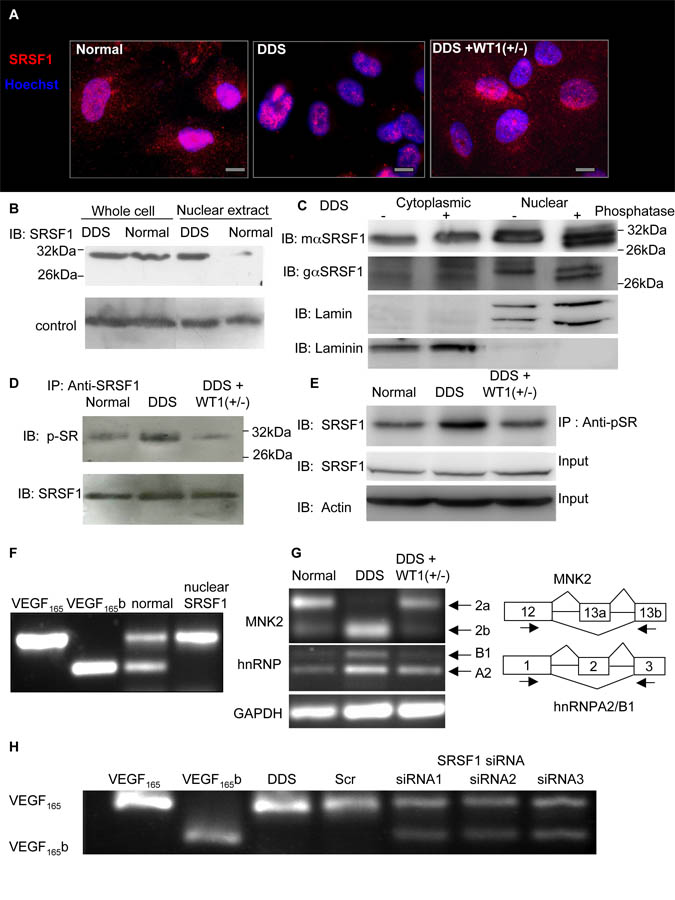
We have previously shown that over-expression of splicing factor, arginine/serine-rich 1 (SRSF1, also called SFRS1, ASF, SF2, SF2/ASF or ASF/SF2) results in increased VEGF165 but not VEGF165b(Nowak et al., 2008). To determine whether there was any alteration in SRSF1 expression, podocytes were stained with a goat polyclonal anti-SRSF1 antibody. In normal podocytes SRSF1 was found in both the nucleus and the cytoplasm whereas in DDS cells it was localised predominantly in the nucleus. In rescued DDS cells cytoplasmic localisation of SRSF1 was restored (Figure 2A). To confirm this, cells were stained with a different mouse monoclonal SRSF1 antibody and similar staining was seen (figure S2A). Staining of HeLa cells (no VEGF165b expression) indicated nuclear staining of SRSF1, but HEK293 cells (VEGF165b and VEGF165 expression) showed both nuclear and cytoplasmic staining (figure S2B), consistent with nuclear localisation of SRSF1 predicting reduced VEGF165b expression. To examine relative nuclear localisation, a nuclear extract or whole cell lysate from normal or DDS podocytes were subjected to immunoblotting with an anti-SRSF1 antibody. Figure 2B shows that whereas total SRSF1 protein expression (whole cell lysate) is the same in DDS and normal podocytes, it is weakly localised in the nucleus in normal cells (intensity was 18±6% of whole cell lysate) but strongly localised in the nucleus of DDS cells (95±5%, N=3). Q-PCR was carried out to confirm that there was no overall change in SRSF1 expression, which was not significantly different in DDS podocytes (0.12±0.02% of GAPDH) compared with wild-type (0.23±0.12%) or rescued (0.12±0.01%) cells. SRSF1 is known to shuttle to the nucleus when phosphorylated(Sanford et al., 2008; Sanford et al., 2005). To explore whether nuclear SRSF1 was phosphorylated in DDS podocytes a high-resolution immunoblot of nuclear or cytoplasmic protein untreated, or treated with phosphatase was carried out using two different SRSF1 antibodies. Figure 2C shows that whereas cytoplasmic SRSF1 was unaffected by phosphatase treatment, nuclear lysate treatment with phosphatase resulted in a lower molecular weight band. To then determine the phosphorylation status of SRSF1 after WT1 mutation, we immunoprecipitated protein from whole cell lysate with an anti-SRSF1 antibody, then probed with a pan-phospho-SR protein antibody. Compared with normal podocytes (77±2.5% of SRSF1 intensity) there was stronger phosphorylation of SRSF1 in DDS podocytes (87±1.6%), and this was inhibited in the rescued DDS cells (71±1%, figure 2D). This was also seen when protein was immunoprecipitated with a phospho-SR protein and probed for SRSF1 (Normal 181±7% of SRSF, DDS 244±13%, rescued 163±11%, figure 2E). To determine whether nuclear SRSF1 was required for the inhibition of VEGF distal splicing a vector encoding nuclear-targeted SRSF1 (that fails to shuttle to the cytoplasm) was transfected into the podocytes. This resulted in complete inhibition of distal VEGF splicing (figure 2F).
Figure 2. Mutant WT1 induces phosphorylation and nuclear localisation of SRSF1, which regulates VEGF splicing.
A. SRSF1 is absent from the cytoplasm when WT1 is mutated. Immunofluorescence staining of SRSF1 (red) of normal, DDS (R366C) and WT1 rescued DDS (DDS+WT1(+/−)) podocytes. Nuclei counterstained blue. Scale bar = 10μm. B. SRSF1 is more nuclearly localised in DDS than in normal podocytes. Protein was extracted from whole cells or from nuclear extracts of podocytes and subjected to immunoblotting for SRSF1. C. Nuclear SRSF1 is phosphorylated. Protein was extracted from flasks of DDS podocytes either as a cytoplasmic or a nuclear extract, and half treated with phosphatase and subjected to high resolution SDS PAGE and immunoblotting using mouse monoclonal (mαSRSF1), or goat polyclonal (gαSRSF1) antibodies to SRSF1, the cytoplasmic protein Laminin, and the nuclear protein Lamin. D SRSF1 is hyperphosphorylated in DDS. Protein was extracted from podocytes, immunoprecipitated with an SRSF1 antibody and immunoblotted with a panphospho SR antibody (top) or SRSF1 antibody (bottom). E. Protein was extracted from podocytes, and half immunoprecipitated with an anti-phosphoSR protein antibody. Both the IP and the crude protein extract were immunoblotted with a SRSF1 antibody and Actin as a loading control. F. Nuclear localisation of SRSF1 inhibits distal splicing. Normal podocytes were transfected with a vector encoding SRSF1 that fails to shuttle into the cytoplasm (nuclear specific), and RNA extracted and amplified for VEGF expression. G. Additional SRSF1 targets are alternatively spliced in DDS podocytes. RNA from normal or DDS podocytes was subjected to RT-PCR using exon spanning primers for the MNK2 cDNA (top) or hnRNPA2/B1 (middle) or GAPDH (bottom). H. Knockdown of SRSF1 induced VEGF165b expression in DDS podocytes. DDS podocytes were transfected with scrambled siRNA (scr) or three different siRNAs to SRSF1 in the 3′UTR (siRNA1), in exons 2-3 (siRNA2) or exon 3 (siRNA3). See also figure S2
To determine whether SRSF1 was exhibiting greater splicing activity in DDS than normal podocytes, known targets of SRSF1 were investigated. Mitogen-Activated Protein Kinase Signal-Integrating Kinase 2 (MNK2) is expressed as two splice isoforms, and SRSF1 over-expression has been shown to increase splicing of the MNK2b isoform(Karni et al., 2007). hnRNPA2/B1 is also alternatively spliced by SRSF1 with increased SRSF1 activity favouring inclusion of exon 2 to result in hnRNPB1 expression. MNK2 and hnRNPA2/B1 mRNA expression were investigated by RT-PCR. Figure 2G shows that DDS podocytes expressed the MNK2b isoform whereas normal podocytes express MNK2a. The expression of MNK2b in rescued DDS cells once again reflected its splicing pattern in wild type cells. Equally in DDS podocytes hnRNPB1 was increased and this was restored by rescue with WT1. In contrast Rac1b, another SRSF1 identified target(Goncalves et al., 2009) was not altered in DDS (figure S2C)
To determine whether SRSF1 was required for preferential proximal splicing of VEGF, DDS podocytes were transfected with three different siRNAs to SRSF1. Q-PCR for SRSF1 showed that this resulted in a reduction to 8.5±4%, 15±9.3% and 15.8±8.8% of SRSF1 mRNA expression in the scrambled siRNA transfected cells for siRNA1, 2 and 3 respectively. Figure 2H shows that all three siRNAs resulted in VEGF165b expression. To ensure that this was not an off target effect cells were transfected with an SRSF1 construct resistant to siRNA1. This abolished the effect of siRNA1, again inhibiting VEGF165b expression (figure S2D). Over-expression of splice factors SRSF1, 2, 4-7 had minimal effect on VEGF165b expression in HeLa cell, and over-expression of SRSF1-6 and SAM68 had no effect on DDS cells (figure S2E).
SRPK1 expression is elevated in Denys Drash Syndrome
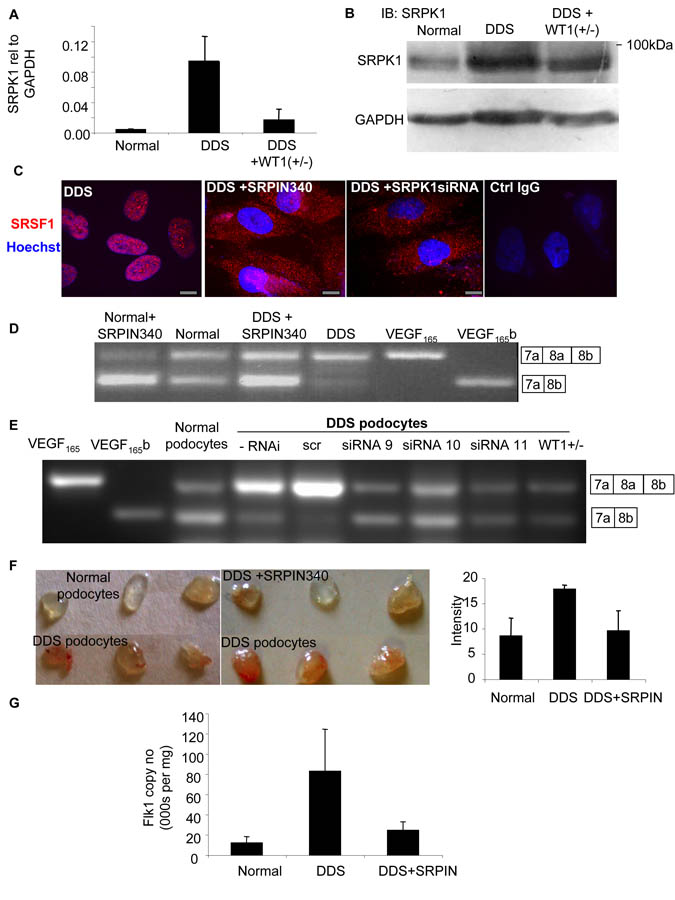
SRSF1 nuclear localisation is brought about by phosphorylation by a number of splicing factors including SR protein kinase 1 (SRPK1)(Zhong et al., 2009). To investigate whether SRPK1 might be involved we measured SRPK1 mRNA levels in podocytes by QPCR. DDS podocytes showed a 15-fold increase in SRPK1 mRNA compared with normal podocytes (figure 3A). This increase was significantly reduced in rescued DDS cells (p<0.01, ANOVA). To identify whether SRPK1 protein levels were also altered we subjected protein extracts from podocytes to immunoblotting using a SRPK1 antibody. Figure 3B shows that SRPK1 protein expression was increased in DDS cells (89±23% of GAPDH) compared with normal podocytes (68±22%), but not in rescued DDS cells (79±23%). To determine whether SRPK1 activation could be affecting SRSF1 localisation we used a small molecule inhibitor of SRPK1, _N_-[2-(1-piperidinyl)-5-(trifluoromethyl)phenyl] isonicotinamide (SRPIN340) (Fukuhara et al., 2006). Treatment of DDS cells with 10μM SRPIN340 or siRNA to SRPK1 resulted in relocalisation of the SRSF1 to the cytoplasm (figure 3C) as measured using a polyclonal goat antibody to SRSF1. This was confirmed with a monoclonal antibody to SRSF1 (figure S3A).
Figure 3. Increased SRPK1 expression increases SRSF1 nuclear localisation and VEGF proximal splicing.
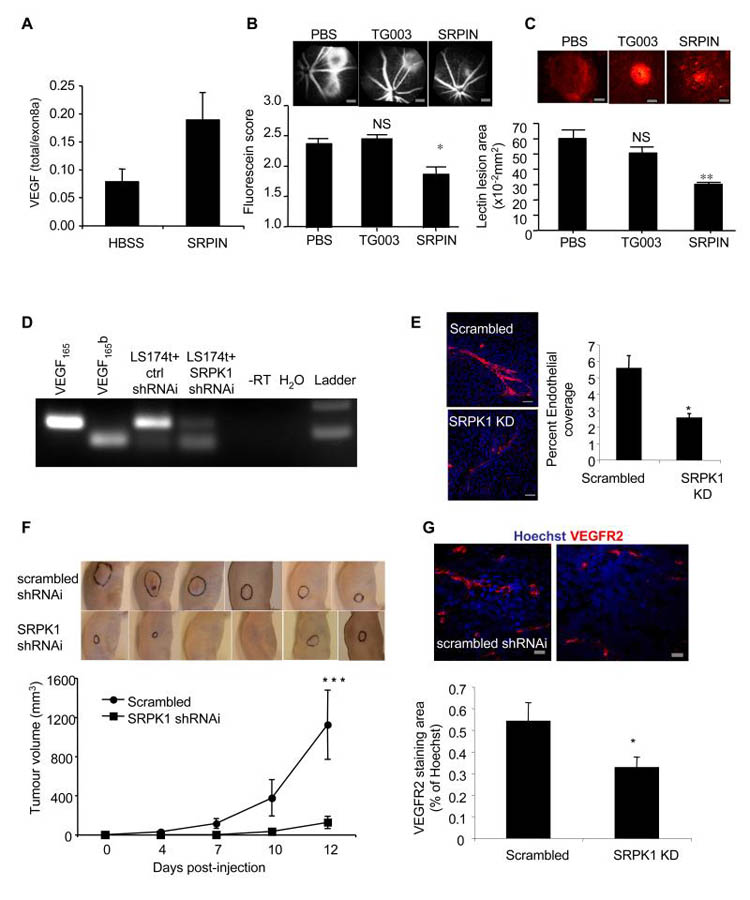
A. SRPK1 is upregulated in DDS podocytes. mRNA was extracted from normal, DDS or rescued podocytes and subjected to Q-PCR using primers specific for SRPK1. B. SRPK1 protein is upregulated in DDS podocytes. Immunoblot (IB) using SRPK1 antibody in protein extracted from DDS podocytes, normal podocytes, and wild type WT1(+/−) over-expressing podocytes. C. SRSF1 localisation is SRPK1 dependent. Immunofluorescence for SRSF1 (red). DDS cells were treated with 10μM of the SRPK1 inhibitor SRPIN340 or by knockdown of SRPK1 in DDS podocytes. Scale bar = 10μm D. SRPK1 inhibition switches splicing to VEGF165b. RT-PCR of mRNA extracted from normal or DDS podocytes untreated or treated with the SRPK1 inhibitor SRPIN340 using primers for VEGF165 (top band) or VEGF165b (lower band). E. Knockdown of SRPK1 switches VEGF splicing. RT-PCR of RNA extracted from DDS podocytes transfected with three different siRNAs to SRPK1, or scrambled siRNA (scr). VEGF165b or VEGF165 cDNA is used as a positive control. F. SRPK1 inhibition is anti-angiogenic. Normal or DDS cells were suspended in matrigel with or without SRPIN340 and implanted into nude mice. Matrigel was excised and photographed. The intensity of red haemoglobin was measured. G. RNA was extracted from the matrigel plugs and endothelial content estimated from VEGFR2 expression level assessed by qRT-PCR. Bar charts are mean±SEM. See also figure S3.
SR protein kinase inhibition restores the expression of anti-angiogenic VEGF
To identify whether SRPIN340 could affect alternative splicing of VEGF, cells were treated with 10μM SRPIN340 and mRNA extracted and subjected to RT-PCR. Figure 3D shows that normal podocytes treated with SRPIN340 increased their VEGF165b isoform expression. Treatment of DDS podocytes with SRPIN340 resulted in splicing to the distal splice site (VEGF165b expression). SRPIN340 exerts 80% inhibition of SRPK2 at 10μM, but inhibits only one other of 140 serine threonine kinases at that dose by more than 50% (ALK, 71%)(Fukuhara et al., 2006). To confirm that the switch in splicing was due to an effect on SRPK1, three different siRNAs specific for SRPK1 were used. Q-PCR showed that these inhibited SRPK1 expression to 35±6, 34±11 and 31±16% of scrambled siRNA for siRNA 9, 10 and 11 respectively. This was confirmed by western blotting (figure S3B). This compares with 20.5% SRPK1 mRNA expression in the WT1 transfected DDS podocytes. Figure 3E shows that all three siRNAs resulted in VEGF165b expression in the DDS podocytes. To ensure that the siRNAs were not acting through off-target effects, an SRPK1 construct was generated that was insensitive to siRNA9. Transfection of this into the siRNA9 transfected cells restored DDS VEGF expression, inhibiting VEGF165b and favouring proximal splicing (figure S3C). QPCR of SRPK2, a related splice factor kinase, showed no change in DDS cells compared with normal podocytes, and was not affected by SRPK1 knockdown. To determine whether the SRPK1 inhibition resulted in a functional alteration of VEGF production, podocytes were used in a matrigel angiogenesis assay (figure 3F). When implanted into nude mice normal podocytes induced little angiogenesis, as seen by mostly clear matrigel plugs. In contrast DDS cells resulted in vascularised plugs (3F lower panel). When DDS cells were treated with SRPIN340 in contrast, the vascularisation was inhibited. To quantify vascularisation, QPCR for mouse VEGFR2 was carried out and quantitated relative to a standard curve (figure S3D). Figure 3G shows that DDS podocytes induced more endothelialisation of the plugs than normal podocytes, and this was reversed by SRPIN340 treatment.
WT1 binds the SRPK1 promoter
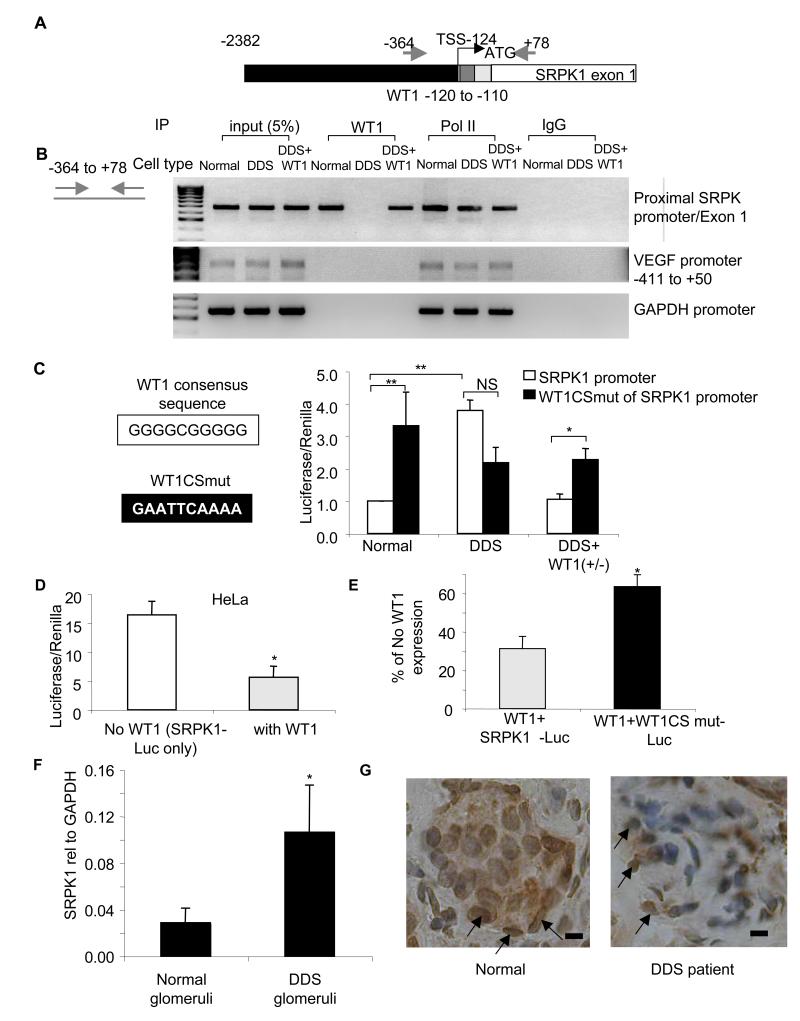
To identify whether WT1 interacted directly with the SRPK1 promoter, we used chromatin immunoprecipitation (ChIP) to determine whether WT1 could bind to the promoter region of the SRPK1 gene. Figure 4A shows a diagram of the SRPK1 gene, and a putative WT1 binding site (similar to the GC rich EGR consensus) close to the putative transcription start site. Podocytes were subjected to nuclear extraction of the chromatin/protein complex, followed by shearing of the DNA with sonication to generate fragments 200-1000bp long. This was then subjected to immunoprecipitation with an anti-WT1 antibody, and the precipitate subjected to PCR using primers across the transcriptional start. In LNCaP prostate cancer cells over-expressing WT1, binding of WT1 to the VEGF promoter has been seen in vitro(Graham et al., 2006). To determine whether, in podocytes, WT1 also bound the VEGF promoter, the precipitate was subjected to amplification of the VEGF promoter in the same region. Primers to the GAPDH promoter were used as a negative control. As a positive control chromatin was immunoprecipitated with an antibody to RNA polymerase II. Figure 4B shows that the SRPK1 promoter sequence around the transcriptional start site was precipitated with WT1 in normal podocytes and rescued podocytes but not with DDS podocytes. In contrast WT1 did not bind either the VEGF or the GAPDH promoters in either DDS or normal podocytes. To determine whether the putative WT1 binding site was functional in the SRPK1 promoter, a 1661 bp genomic fragment containing the proposed transcriptional start site, was cloned into a Luciferase/Renilla reporter gene. The Renilla is under control of the hTK promoter and acts as an expression control. Figure 4C (open bars) shows that the ratio of Luciferase to Renilla was almost four times greater in DDS podocytes than normal podocytes but this ratio was reduced in the rescued DDS podocytes. Figure S4A shows that luciferase expression was increased in all three DDS cell lines, R336C, M342R and G349C compared with normal podocytes. To identify whether the site of repression was located at the 10bp WT1-binding consensus sequence, this was mutated (figure 4C). This increased luciferase expression in the normal and rescued podocytes, but did not significantly affect expression in the DDS podocytes (figure 4C), of any type (figure S4A), as the mutant WT1 protein in these cells is unable to repress SRPK1 expression. However, there was a trend in three DDS cell lines to reduced expression in the mutated reporters than in the wild type. Therefore, to exclude the possibility of other transcription factor binding sites affecting expression, a shorter (484bp) sequence was used (figure S4B). Again DDS cells had a higher luciferase expression than normal and rescued cells, and when the WT1 binding site was mutated luciferase expression in normal and rescued cells was again increased although not to levels seen in DDS cells (figure S4C). We therefore cannot rule out additional regulators in the promoter region that interact with mutated DDS protein, but it is clear that the mutant WT1 protein results in elevated SRPK1 expression, at least in part, through lack of binding to the WT1 consensus binding sequence. To determine whether the SRPK1 promoter was inhibited by exogenous WT1, HeLa cells were transfected with the luciferase reporter gene with or without wild type WT1. Figure 4D shows that transfection of HeLa cells with wild type WT1 resulted in a significant inhibition of luciferase expression to approximately 30% of that in cells not transfected with WT1. Figure 4E shows that this repression of the normal SRPK1 promoter by wild type WT1 to 31±6% was significantly lifted by the use of the WT1 binding site mutant SRPK1 promoter (WTBSmut, 63±6%), but again, not to 100% so additional regulators may be active.
Figure 4. WT1 represses SRPK1 expression by binding the SRPK1 promoter region.
A. SRPK1 gene structure. Black=promoter region. Grey=WT1 binding site, silver=5′UTR, white=coding sequence of exon 1. Arrows=primer locations. Numbers are bp relative to start codon. B. WT1 binds the SRPK1 promoter. Nuclear extracts from podocytes were sheared to fragment DNA, immunoprecipitated with polymerase II antibody, human IgG or a WT1 antibody and subjected to PCR using primers to detect the region around the SRPK1 transcriptional start site (TSS), or the VEGF or GAPDH promoters. C. DDS mutations relieve SRPK1 repression. Podocytes were transfected with a SRPK1 promoter-luciferase reporter vector (white bars), or the same promoter with a 10base pair mutation of the WT1 consensus sequence (WT1CSmut, black bars). D. WT1 represses SRPK1 expression. HeLa cells were transfected with the wild type promoter reporter gene alone (open bars) or also with WT1 (silver bars), and bioluminescence measured. E. The 10bp consensus sequence adjacent to the TSS is responsible for part of the repression . HeLa cells transfected with WT1 and the wild type SRPK1-promoter (silver bars) or WT1 and the mutated SRPK1-promoter (black bars). Luciferase/Renilla is expressed as a percentage of bioluminescence in the absence of WT1 (white bar, in D) F. Q-PCR of SRPK1 RNA from histologically normal kidney (n=5) or children with DDS (n=5, p<0.05). Bar charts are mean±SEM. G. Immunohistochemistry for SRSF1 of kidney biopsies of DDS (right) compared with normal (left). Podocytes shown by arrows. Scale bar = 15μm, See also figure S4.
SRPK1 is upregulated and SRSF1 nuclear localised in human DDS
To determine whether the upregulation of SRPK1 was also seen in human DDS patients in vivo, RNA was extracted from paraffin embedded sections of renal cortex from five patients with Denys Drash Syndrome or five histologically normal kidney sections. Figure 4F shows that SRPK1 was significantly greater (4.3±1.2 fold, p<0.01 one sample t test), relative to GAPDH in the DDS patients. To determine whether SRSF1 was relocated from the cytoplasm, sections were stained with SRSF1 antibody. While there was no difference in tubular staining of SRSF1, in the glomeruli in the normal kidneys there was substantial podocyte cytoplasmic staining (figure 4G left panel, arrows), whereas in the DDS staining appeared to be more localised to the nuclei of the podocytes (right panel, arrows).
SRPK1 inhibition inhibits angiogenesis
To determine whether the pro-angiogenic VEGF isoform expression due to SRPK1 is necessary for angiogenesis we employed a widely used model of choroidal neovascularisation, that is amenable to local administration of pharmacological tools. Treatment of epithelial cells with 10μM SRPIN340 resulted in an increase in VEGF165b expression(Nowak et al., 2010). In a mouse model of choroidal neovascularisation three days after treatment with SRPIN340 (10pmol/eye) the ratio of total VEGF (containing exon2/3) to pro-angiogenic isoforms (containing exon 8a) was significantly greater than in saline treated controls (figure 5A) as determined by QPCR. Two weeks after SRPIN340 treatment, there was a substantial and significant inhibition of fluorescein leakage (figure 5B), and lesion size (figure 5C), compared with both saline injected and eyes injected with an inhibitor of a closely related kinase Clk1/4 (TG003). The inhibition of angiogenesis was similar to that seen with recombinant VEGF165b protein(Hua et al., 2010). To determine whether the inhibition of splicing to VEGF165b was sufficient to inhibit tumour growth and angiogenesis, LS174t colorectal tumour cells, the growth of which has previously been shown to be inhibited by the anti-angiogenic actions of VEGF165b, were stably transfected with an SRPK1-shRNAi lentiviral vector. Stable cell lines (figure S5A) were generated and SRPK1 levels measured by QPCR. These were knocked down by 96% (figure S5B). SRPK1 knockdown cells grew at the same rate as the scrambled shRNAi cells. To determine whether SRPK1 knockdown affected VEGF splicing, RNA and protein was extracted and subjected to RT-PCR and ELISA respectively. SRPK1 shRNAi expressing cells showed stronger expression of VEGF165b mRNA (figure 5D), protein in the cell (figure S5C), and secreted into the media (figure S5D). To determine whether the SRPK1 knockdown resulted in a functional alteration of VEGF, cells were transfected with SRPK1 shRNAi lentivirus, and media collected for in vitro angiogenesis assays. Media from untransfected cells stimulated endothelial cell tube formation on fibroblasts, but media from SRPK1 shRNAi transfected cells had no effect (figure 5E). These tumour cells were then implanted into nude mice and tumour growth rate measured. The SRPK1 shRNAi cells formed smaller tumours than scrambled shRNAi control and the tumours grew more slowly (figure 5F). Staining of these cells for microvascular density by VEGFR2 immunofluorescence showed significantly lower density for SRPK1 shRNAi than scrambled control (figure 5G).
Figure 5. SRPK1 inhibition is anti-angiogenic.
A. Mice underwent laser photocoagulation and were treated with SRPIN340 (inhibits SRPK1, n=24), TG003 (inhibits Clk1/4, n=18) or with vehicle (n=24). 2-3 days later six HBSS treated and six SRPIN340 treated mice were killed, eyes enucleated and RNA extracted and subjected to RT-qPCR for total VEGF (primers in exon 3) or exon 8a containing VEGF (e.g. VEGF164). B. The remaining mice were treated again seven days later with the compounds and then after another seven days subjected to fluorescein angiography. Lesion size was scored blind. Scale bar =300μm C. These mice were then killed and the retinal membranes flat mounted and stained for lectin to identify choroidal angiogenesis (red). The size of the lesions was measured. **=p<0.01, *=p<0.05 compared with PBS, ANOVA. Scale bar =100μm D. LS174t colon cancer cells were infected with lentivirus containing SRPK1-shRNAi or scrambled siRNA (ctrl) and selected with puromycin. Stable cell lines were subjected to RT-PCR for VEGF165b and VEGF165 levels. E. Knockdown of SRPK1 reduces the angiogenic potential of media. Cells were infected with SRPK1 shRNAi and the media collected. Media was used in an in vitro co-culture assay of endothelial cells and fibroblasts, and cells stained for CD31 (red, endothelial) and Hoechst (nuclei). Spread of endothelial cells was determined by measuring the ratio of area covered by CD31 staining relative to Hoechst staining. **=p<0.01. Scale bar = 200μm. F. Tumour cells were implanted into nude mice and tumours allowed to grow for 12 days. Tumours outlined in black. Tumour diameters were measured by callipers. ***=p<0.001 by two way ANOVA. G. Tumours were excised and stained for VEGFR2 (red) and Hoechst (blue) and vessel density determined by VEGFR2 staining relative to Hoechst. , *=p<0.05 compared with scrambled siRNA, t test. Scale bar =20μm. Bar charts are mean±SEM. See also figure S5.
DISCUSSION
These results indicate a link between the physiological role of an important tumour suppressor gene (WT1) and regulation of the angiogenic or anti-angiogenic properties of VEGF and other genes by alternative splicing. There have been many studies identifying alternative splicing as a process that regulates oncogenesis. For instance a splice variant of p53 (p47) is an N-terminally deleted form of p53 and inhibits its tumour suppressor activity(Ghosh et al., 2004), and a correlation was seen between inactive p53 isoforms and VEGF165b splicing in colorectal cancers(Diaz et al., 2008). However in this case as in many others, it is the tumour suppressor gene itself that is alternatively spliced to give different isoforms. It is less common to find a link between a tumour suppressor gene and a tumour regulatory process that involves alternative splice site regulation. One such example is the regulation of alternative Fas splicing by RBM5, whereby RBM5 binds to U2AF65 and prevents inclusion of exon 6 of Fas. Thus the tumour suppressor gene acts by directly interacting with the spliceosome(Bonnal et al., 2008). Other tumour mediated pathways have also been implicated, for instance, Ras increases alternative splicing of KLF6, a tumour suppressor gene in colorectal cancer, through PI3K and Akt, and through a mechanism that is also SRSF1 mediated, suggesting that Akt mediated phosphorylation of SRSF1 is also sufficient to alter splicing of the tumour suppressor gene itself(Yea et al., 2008). The data presented here goes one step further and demonstrates the mechanism by which a mutation in a known tumour suppressor gene causes a change in a splicing process that results in alternative splicing of other genes that contribute to tumour progression (in this case by angiogenesis). As WT1 is implicated in renal disease, gonadal development, and in several cancers, the mechanistic link between WT1 expression (including WT1 splice variants), SRPK1 expression, and SRSF1 activity will be of significant interest. It had previously been shown that WT1 could modify splicing and interact directly with splicing proteins, for instance by binding U2AF65, but this interaction required the KTS sequence(Davies et al., 1998). The finding that only the isoforms lacking the KTS domain (and hence non-RNA binding) provides another mechanism through which WT1 can alter RNA processing – transcriptional repression of a splicing factor kinase. Thus one of the consequences of mis-expression of WT1 in tumours is an altered pattern of alternative splicing of key cancer-associated genes (including VEGF), not only through direct interaction with splicing factors(Davies et al., 1999), but also through controlled expression of splice factor kinases.
The upregulation of SRPK1 expression by WT1 mutants (and hence suppression of SRPK1 by wild type WT1) indicates that SRPK1 upregulation may be a common cancer related event. It has been shown that SRPK1 is upregulated in pancreatic, breast and colon carcinomas(Hayes et al., 2007), but reduced in neuroblastoma (and this correlates with drug resistance(Krishnakumar et al., 2008)). This is consistent with studies showing that in tumour cells the target for SRPK1, SRSF1, is phosphorylated and located in the nucleus(Sanford et al., 2005), as seen in this study in HeLa cells, but not in HEK293T cells (an epithelial cell line that is not tumorigenic). Thus nuclear SRSF1 may be more common in cultured cancer cells than differentiated cells, a hypothesis that needs testing. It indicates that phosphorylated, nuclear SRSF1 is indicative of an angiogenic phenotype, in that it results in proximal splicing and production of angiogenic VEGF isoforms. This is supported by work showing that IGF mediated PKC activation induces binding of phosphorylated SRSF1 to the VEGF pre-mRNA(Nowak et al., 2010). However, this is evidence that upregulation of SRPK1 is by a specific mechanism. It also identifies a link between WT1 and a putative mechanism for the glomerular dysfunction of DDS, proposed by Schumacher et al to be mediated by alternative VEGF splicing(Schumacher et al., 2007), and for which there is indirect evidence from transgenic mice over-expressing WT1 mutants, which have abnormal glomerular capillaries postulated to be caused by altered VEGF function(Natoli et al., 2002).
We recently showed that neonatal retinal neovascularisation induced by hyperoxia is reduced by SRPIN340 treatment(Nowak et al., 2010). We have now extended these findings by showing that SRPK1 inhibition is effective in tumour models of cancers that are treated by anti-VEGF therapy. The substantial inhibition of angiogenesis coupled with reduced lesion size, demonstrates that interfering with VEGF splicing mechanisms is a rational target for anti-angiogenic therapy in tumours. In particular it suggest that angiogenesis may be driven in tumours that lack functional WT1 (exemplified by LS174t cells), or have enhanced SRPK1 expression(Koesters et al., 2004), by control of VEGF splicing rather than (or as well as) control of VEGF expression. This has implications for the rational use of anti-VEGF agents, as VEGF inhibitors have been shown to be ameliorated in the presence of increased VEGF165b expression(Varey et al., 2008).
These results provide a potential mechanism for a different anti-angiogenic therapeutic approach (specifically the inhibition of SRPK1), and also identify SRSF1 localisation as a possible rational diagnostic marker for WT1 dysfunction in the context of angiogenesis. Alternative splicing of several other cancer-associated genes may also be affected through WT1’s ability to regulate SRPK1.
EXPERIMENTAL PROCEDURES
Human samples and ethics
All human samples were anonymised and experiments were approved by the North Bristol Ethics committee (Southmead Hostpital) number 07/H0102/45.
Cell culture and transfection
Specific details of the experimental procedures are given in the online supplement
Conditionally immortalized podocytes were used from a healthy individual and from three patients suffering Denys Drash Syndrome (R336C, M342R, and G349C). After growing to required confluence, the podocytes were thermoswitched at 37°C for 14 days to ensure growth arrest and differentiation. Both podocyte cell types (DDS and normal) were used at the same stage of differentiation (14 days thermoswitched).
Protein studies – immunoblotting and immunoprecipitation
Both whole cell lysate (nuclear and cytoplasmic) protein extraction and nuclear protein extracts were used as described in the online supplement. The extracts were then immunoblotted using either mouse anti-SRPK1 (anti-SRPK1; BD 611072; 1:1000), rabbit anti-panVEGF (Santa Cruz A20 sc-152; 1:500), mouse anti-VEGFxxxb (MAB3045; R&D; 1:500), goat anti-SRSF1 (SC10255; 1:500), mouse anti-SRSF1 (AK96) (Santa Cruz SC-33562) or rabbit anti-GAPDH (Sigma G9545, 1:2000). For immunoprecipitation phospho-SRSF1 studies, cell lysates were incubated with mouse anti-SRSF1 (Santa Cruz SC-33562) or anti-Panphospho-SR antibody (Santa Cruz, SC-13509) and Protein G Dynabeads (Invitrogen). To detect phosphorylated SRSF1, the eluent was immunoblotted with either anti-SRSF1 or the anti-Pan-phospho-SR antibody (1:500).
ELISA
A pan-VEGF capture antibody (Duoset VEGF ELISA DY-293; R&D systems) and recombinant human VEGF165 or VEGF165b standards were used. Biotinylated goat anti-human VEGF (0.025 μg/ml; R&D systems) or mouse anti-human VEGF165b (clone 264610/1, 0.025 μg/ml, R&D systems) were used as detection antibodies. Details are given in the online supplement.
Semi quantitative PCR and qPCR
mRNA was extracted from differentiated podocytes using standard methodology, given in the online supplement. To extract RNA from paraffin embedded DDS kidneys (n=5,) and paraffin embedded non-WT1 child kidneys (n=5), 2 sections of 6μm thickness were used from each. RNA was then extracted using RNeasy FFPE kit (Qiagen). RT-PCR was performed to differentiate between exon 8a and exon 8b-containing isoforms within the same sample in the same reaction using primers specific to exon 7a and the 3′ untranslated region of the VEGF gene(Nowak et al., 2008). For qPCR, 1% of the cDNA from the cell lines were used per reaction while 5% of the cDNA from paraffin embedded sections were used. The qPCR reaction was set up using Roche SyBr Green and run in an ABI 7000. Validated primers specific to SRPK1, SRSF1 and GAPDH were used for the qPCR.
Chromatin Immunoprecipitation (ChIP)
ChIP was performed on the differentiated podocytes, using the Imprint ChIP Kit (Sigma). Cells were incubated with 1% formaldehyde to crosslink DNA to protein. The cells were then lysed and DNA sheared to about 1000bp using a sonicator. The DNA-protein mixture was incubated with WT1, Polymerase II antibody or non specific IgG, immunoprecipitated, the cross linked DNA-WT1 complex released using Proteinase K, the DNA was cleaned up and eluted using GeneElute Binding Colum (Sigma) and the eluted DNA subjected to PCR to detect SRPK1, VEGF and GAPDH promoter regions.
Luciferase Assays
A 1661 bp fragment and a 484bp fragment containing the upstream promoter sequence of SRPK1 was amplified from genomic DNA and cloned into pGL3 as described in the online supplement to generate the wild type SRPK1 promoter. The SRPK1 promoter sequence was verified by restriction analysis and sequencing. The putative WT1 binding site located -119 bp to -110 bp upstream of the SRPK1 start codon was mutated from (GGGGCGGGGG) to GAATTCAAAA as described in the online supplement
Animal experiments
All animal experiments were approved by the University of Southern California (USC) Animal Use Committee (ocular experiments), or under license by the UK Home Office and in accordance with the University of Bristol ethical review panel (tumour experiments).
Induction of choroidal neovascularization (CNV)
Detials are given in the online supplement. C56Bl/6J mice were anesthetized by intraperitoneal injection of ketamine and xylazine hydrochloride. Four photocoagulation lesions were delivered with a diode green laser between the retinal vessels 1-2 disc-diameters apart in each eye. Two μl of saline, 10ng rhVEGF-A165b, 100pmol SRPIN340, or 100pmol TG003 was injected into the vitreous immediately after photocoagulation and seven days later. 14 days after laser treatment, fluorescein angiography of the fundi was carried out and the animals were sacrificed after imaging. Eyes were enucleated, and fixed. Three animals given SRPIN340 or saline were sacrificed 3 days after photocoagulation, and RNA extracted for Q-PCR.
Tumour studies
LS174t colorectal tumour cells were infected with lentiviral SRPK1 shRNAi or standard and selected with puromycin. Cells were checked for positive expression by GFP (figure S5A). SRPK1 knockdown was assessed by Q-PCR as above. VEGF was assessed as above using a VEGF165b specific ELISA (see supplementary methods), and a pan VEGF ELISA. The difference was used to calculate VEGF165.
Supplementary Material
Supplemetary data
Significance.
Regulation of angiogenesis is critical for tumour growth. This is regulated by the pro-angiogenic VEGF isoforms. Anti-angiogenic isoforms, formed by alternative splicing, are found in normal, healthy tissues and are down regulated in many tumours. Here we identify a link between mutations in a tumour suppressor gene and regulators of angiogenesis. This link, through transcriptional regulation of a splicing factor kinase indicates a) that WT1 regulates splicing through repression of SRPK1, b) that SRPK1 may be a target for anti-angiogenic therapy, c) that alternative splicing in tumours under genetic as well as environmental control can regulate tumour growth. These results provide a pathway linking two mechanisms known to be altered in cancer – angiogenesis and alternative splicing.
Highlights.
- SR Protein Kinase 1 is a target for the Wilms Tumour suppressor gene-1
- WT1 mutations lead to altered VEGF splicing by hyperphosphorylated SRSF1
- SRPK1 over-expression regulates VEGF splicing and angiogenesis
- SRPK1 inhibition is anti-angiogenic in tumours in vivo.
ACKNOWLEDGMENTS
We would like to thank David Corry for technical help with the confocal microscopy, E Bunn and M Yates for help with transfections and PCRs. This work was supported by a UWE PhD Studentship (EMA), the British Heart Foundation (FS06/003 - DOB, PG08/022/21636, PG/11/20/28792-SO, FS04/09), the Wellcome Trust (079736-JH), MRC (G10002073-SJH, GR0600920-DOB), and NIH Core grant EY03040 and the Arnold and Mabel Beckman Foundation (DRH, SK), AICR (07-0605, MHZ), Cancer Research UK (C11392/A10484, KS and CF11392/A8451, MHZ), Fight for Sight (ESR), the Skin Cancer Research Fund (MG), and the Richard Bright VEGF Research Trust (SJH).
REFERENCES
- Artac RA, McFee RM, Smith RA, Baltes-Breitwisch MM, Clopton DT, Cupp AS. Neutralization of vascular endothelial growth factor antiangiogenic isoforms is more effective than treatment with proangiogenic isoforms in stimulating vascular development and follicle progression in the perinatal rat ovary. Biol Reprod. 2009;81:978–988. doi: 10.1095/biolreprod.109.078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmer-Hofer K, Andersson AE, Ratcliffe LE, Berger P. Neuropilin-1 promotes VEGFR-2 trafficking through Rab11 vesicles thereby specifying signal output. Blood. 2011 doi: 10.1182/blood-2011-01-328773. [DOI] [PubMed] [Google Scholar]
- Baltes-Breitwisch MM, Artac RA, Bott RC, McFee RM, Kerl JG, Clopton DT, Cupp AS. Neutralization of vascular endothelial growth factor antiangiogenic isoforms or administration of proangiogenic isoforms stimulates vascular development in the rat testis. Reproduction. 2010;140:319–329. doi: 10.1530/REP-09-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD, Peat D, Gillatt D, Harper SJ. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002;62:4123–4131. [PubMed] [Google Scholar]
- Blann AD, Li JL, Li C, Kumar S. Increased serum VEGF in 13 children with Wilms’ tumour falls after surgery but rising levels predict poor prognosis. Cancer Lett. 2001;173:183–186. doi: 10.1016/s0304-3835(01)00666-8. [DOI] [PubMed] [Google Scholar]
- Bonnal S, Martinez C, Forch P, Bachi A, Wilm M, Valcarcel J. RBM5/Luca-15/H37 regulates Fas alternative splice site pairing after exon definition. Mol Cell. 2008;32:81–95. doi: 10.1016/j.molcel.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Bor YC, Swartz J, Morrison A, Rekosh D, Ladomery M, Hammarskjold ML. The Wilms’ tumor 1 (WT1) gene (+KTS isoform) functions with a CTE to enhance translation from an unspliced RNA with a retained intron. Genes Dev. 2006;20:1597–1608. doi: 10.1101/gad.1402306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebe Suarez S, Pieren M, Cariolato L, Arn S, Hoffmann U, Bogucki A, Manlius C, Wood J, Ballmer-Hofer K. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol Life Sci. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R, Moore A, Schedl A, Bratt E, Miyahawa K, Ladomery M, Miles C, Menke A, van Heyningen V, Hastie N. Multiple roles for the Wilms’ tumor suppressor, WT1. Cancer Res. 1999;59:1747s–1750s. discussion 1751s. [PubMed] [Google Scholar]
- Davies RC, Calvio C, Bratt E, Larsson SH, Lamond AI, Hastie ND. WT1 interacts with the splicing factor U2AF65 in an isoform-dependent manner and can be incorporated into spliceosomes. Genes Dev. 1998;12:3217–3225. doi: 10.1101/gad.12.20.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denys P, Malvaux P, Van Den Berghe H, Tanghe W, Proesmans W. [Association of an anatomo-pathological syndrome of male pseudohermaphroditism, Wilms’ tumor, parenchymatous nephropathy and XX/XY mosaicism] Arch Fr Pediatr. 1967;24:729–739. [PubMed] [Google Scholar]
- Diaz R, Pena C, Silva J, Lorenzo Y, Garcia V, Garcia JM, Sanchez A, Espinosa P, Yuste R, Bonilla F, Dominguez G. p73 Isoforms affect VEGF, VEGF165b and PEDF expression in human colorectal tumors: VEGF165b downregulation as a marker of poor prognosis. Int J Cancer. 2008;123:1060–1067. doi: 10.1002/ijc.23619. [DOI] [PubMed] [Google Scholar]
- Drash A, Sherman F, Hartmann WH, Blizzard RM. A syndrome of pseudohermaphroditism, Wilms’ tumor, hypertension, and degenerative renal disease. J Pediatr. 1970;76:585–593. doi: 10.1016/s0022-3476(70)80409-7. [DOI] [PubMed] [Google Scholar]
- Fukuhara T, Hosoya T, Shimizu S, Sumi K, Oshiro T, Yoshinaka Y, Suzuki M, Yamamoto N, Herzenberg LA, Herzenberg LA, Hagiwara M. Utilization of host SR protein kinases and RNA-splicing machinery during viral replication. Proc Natl Acad Sci U S A. 2006;103:11329–11333. doi: 10.1073/pnas.0604616103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Stewart D, Matlashewski G. Regulation of human p53 activity and cell localization by alternative splicing. Mol Cell Biol. 2004;24:7987–7997. doi: 10.1128/MCB.24.18.7987-7997.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves V, Matos P, Jordan P. Antagonistic SR proteins regulate alternative splicing of tumor-related Rac1b downstream of the PI3-kinase and Wnt pathways. Hum Mol Genet. 2009;18:3696–3707. doi: 10.1093/hmg/ddp317. [DOI] [PubMed] [Google Scholar]
- Graham K, Li W, Williams BR, Fraizer G. Vascular endothelial growth factor (VEGF) is suppressed in WT1-transfected LNCaP cells. Gene Expr. 2006;13:1–14. doi: 10.3727/000000006783991953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber DA, Sohn RL, Buckler AJ, Pelletier J, Call KM, Housman DE. Alternative splicing and genomic structure of the Wilms tumor gene WT1. Proc Natl Acad Sci U S A. 1991;88:9618–9622. doi: 10.1073/pnas.88.21.9618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat Rev Cancer. 2008;8:880–887. doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes GM, Carrigan PE, Miller LJ. Serine-arginine protein kinase 1 overexpression is associated with tumorigenic imbalance in mitogen-activated protein kinase pathways in breast, colonic, and pancreatic carcinomas. Cancer Res. 2007;67:2072–2080. doi: 10.1158/0008-5472.CAN-06-2969. [DOI] [PubMed] [Google Scholar]
- Hohenstein P, Hastie ND. The many facets of the Wilms’ tumour gene, WT1. Hum Mol Genet. 2006;15(Spec No 2):R196–201. doi: 10.1093/hmg/ddl196. [DOI] [PubMed] [Google Scholar]
- Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Hua J, Spee C, Kase S, Rennel ES, Magnussen AL, Qiu Y, Varey A, Dhayade S, Churchill AJ, Harper SJ, et al. Recombinant human VEGF165b inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2010;51:4282–4288. doi: 10.1167/iovs.09-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Karni R, de Stanchina E, Lowe SW, Sinha R, Mu D, Krainer AR. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007;14:185–193. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer Res. 2008;68:4683–4692. doi: 10.1158/0008-5472.CAN-07-6577. [DOI] [PubMed] [Google Scholar]
- Koesters R, Linnebacher M, Coy JF, Germann A, Schwitalle Y, Findeisen P, von Knebel Doeberitz M. WT1 is a tumor-associated antigen in colon cancer that can be recognized by in vitro stimulated cytotoxic T cells. Int J Cancer. 2004;109:385–392. doi: 10.1002/ijc.11721. [DOI] [PubMed] [Google Scholar]
- Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol Vis. 2006;12:626–632. [PubMed] [Google Scholar]
- Krishnakumar S, Mohan A, Kandalam M, Ramkumar HL, Venkatesan N, Das RR. SRPK1: a cisplatin sensitive protein expressed in retinoblastoma. Pediatr Blood Cancer. 2008;50:402–406. doi: 10.1002/pbc.21088. [DOI] [PubMed] [Google Scholar]
- Manetti M, Guiducci S, Romano E, Ceccarelli C, Bellando-Randone S, Conforti ML, Ibba-Manneschi L, Matucci-Cerinic M. Overexpression of VEGF165b, an Inhibitory Splice Variant of Vascular Endothelial Growth Factor, Leads to Insufficient Angiogenesis in Patients With Systemic Sclerosis. Circ Res. 2011;109:e14–26. doi: 10.1161/CIRCRESAHA.111.242057. [DOI] [PubMed] [Google Scholar]
- Merdzhanova G, Gout S, Keramidas M, Edmond V, Coll JL, Brambilla C, Brambilla E, Gazzeri S, Eymin B. The transcription factor E2F1 and the SR protein SC35 control the ratio of pro-angiogenic versus antiangiogenic isoforms of vascular endothelial growth factor-A to inhibit neovascularization in vivo. Oncogene. 2010;29:5392–5403. doi: 10.1038/onc.2010.281. [DOI] [PubMed] [Google Scholar]
- Morrison AA, Viney RL, Ladomery MR. The post-transcriptional roles of WT1, a multifunctional zinc-finger protein. Biochim Biophys Acta. 2008;1785:55–62. doi: 10.1016/j.bbcan.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Natoli TA, Liu J, Eremina V, Hodgens K, Li C, Hamano Y, Mundel P, Kalluri R, Miner JH, Quaggin SE, Kreidberg JA. A mutant form of the Wilms’ tumor suppressor gene WT1 observed in Denys-Drash syndrome interferes with glomerular capillary development. J Am Soc Nephrol. 2002;13:2058–2067. doi: 10.1097/01.asn.0000022420.48110.4b. [DOI] [PubMed] [Google Scholar]
- Nowak DG, Amin EM, Rennel ES, Hoareau-Aveilla C, Gammons M, Damodoran G, Hagiwara M, Harper SJ, Woolard J, Ladomery MR, Bates DO. Regulation of vascular endothelial growth factor (VEGF) splicing from pro-angiogenic to antiangiogenic isoforms: a novel therapeutic strategy for angiogenesis. J Biol Chem. 2010;285:5532–5540. doi: 10.1074/jbc.M109.074930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak DG, Woolard J, Amin EM, Konopatskaya O, Saleem MA, Churchill AJ, Ladomery MR, Harper SJ, Bates DO. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by known splicing and growth factors. J Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422–2427. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Bevan H, Weeraperuma S, Wratting D, Murphy D, Neal CR, Bates DO, Harper SJ. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. Faseb J. 2008;22:1104–1112. doi: 10.1096/fj.07-9718com. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Ferguson J, Oltean S, Neal CR, Kaura A, Bevan H, Wood E, Sage LM, Lanati S, Nowak DG, et al. Overexpression of VEGF165b in podocytes reduces glomerular permeability. J Am Soc Nephrol. 2010;21:1498–1509. doi: 10.1681/ASN.2009060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennel E, Varey AHR, Churchill AJ, Wheatley ER, Stewart L, Mather S, Bates DO, Harper SJ. VEGF121b, a new member of the VEGFxxxb family of VEGF-A splice isoforms, inhibits neovascularisation and tumour growth in vivo. Br J Cancer. 2009;101:1250–1257. doi: 10.1038/sj.bjc.6605249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennel E, Waine E, Guan H, Schuler Y, Leenders W, Woolard J, Sugiono M, Gillatt D, Kleinerman E, Bates D, Harper S. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br J Cancer. 2008;98:1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JR, Coutinho P, Hackett JA, Wang X, Ranahan W, Caceres JF. Identification of nuclear and cytoplasmic mRNA targets for the shuttling protein SF2/ASF. PLoS ONE. 2008;3:e3369. doi: 10.1371/journal.pone.0003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford JR, Ellis JD, Cazalla D, Caceres JF. Reversible phosphorylation differentially affects nuclear and cytoplasmic functions of splicing factor 2/alternative splicing factor. Proc Natl Acad Sci U S A. 2005;102:15042–15047. doi: 10.1073/pnas.0507827102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher VA, Jeruschke S, Eitner F, Becker JU, Pitschke G, Ince Y, Miner JH, Leuschner I, Engers R, Everding AS, et al. Impaired glomerular maturation and lack of VEGF165b in Denys-Drash syndrome. J Am Soc Nephrol. 2007;18:719–729. doi: 10.1681/ASN.2006020124. [DOI] [PubMed] [Google Scholar]
- Skoldenberg EG, Christiansson J, Sandstedt B, Larsson A, Lackgren G, Christofferson R. Angiogenesis and angiogenic growth factors in Wilms tumor. J Urol. 2001;165:2274–2279. doi: 10.1016/S0022-5347(05)66183-6. [DOI] [PubMed] [Google Scholar]
- Varey AH, Rennel ES, Qiu Y, Bevan HS, Perrin RM, Raffy S, Dixon AR, Paraskeva C, Zaccheo O, Hassan AB, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br J Cancer. 2008;98:1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney RL, Morrison AA, van den Heuvel LP, Ni L, Mathieson PW, Saleem MA, Ladomery MR. A proteomic investigation of glomerular podocytes from a Denys-Drash syndrome patient with a mutation in the Wilms tumour suppressor gene WT1. Proteomics. 2007;7:804–815. doi: 10.1002/pmic.200600666. [DOI] [PubMed] [Google Scholar]
- Woolard J, Bevan HS, Harper SJ, Bates DO. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirculation. 2009;16:572–592. doi: 10.1080/10739680902997333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolard J, Wang WY, Bevan HS, Qiu Y, Morbidelli L, Pritchard-Jones RO, Cui TG, Sugiono M, Waine E, Perrin R, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- Yea S, Narla G, Zhao X, Garg R, Tal-Kremer S, Hod E, Villanueva A, Loke J, Tarocchi M, Akita K, et al. Ras promotes growth by alternative splicing-mediated inactivation of the KLF6 tumor suppressor in hepatocellular carcinoma. Gastroenterology. 2008;134:1521–1531. doi: 10.1053/j.gastro.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XY, Ding JH, Adams JA, Ghosh G, Fu XD. Regulation of SR protein phosphorylation and alternative splicing by modulating kinetic interactions of SRPK1 with molecular chaperones. Genes Dev. 2009;23:482–495. doi: 10.1101/gad.1752109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemetary data