Multimolecular signaling complexes enable Syk-mediated signaling of CD36 internalization (original) (raw)
. Author manuscript; available in PMC: 2014 Feb 25.
Summary
CD36 is a versatile receptor known to play a central role in the development of atherosclerosis, the pathogenesis of malaria, and in the removal of apoptotic cells. Remarkably, the short cytosolically-exposed regions of CD36 lack identifiable motifs, which has hampered elucidation of its mode of signaling. Using a combination of phosphoprotein isolation, mass spectrometry, super-resolution imaging and gene silencing we have determined that the receptor induces ligand internalization through a heteromeric complex consisting of CD36, β1 and/or β2 integrins, and the tetraspanins CD9 and/or CD81. This receptor complex serves to link CD36 to the adaptor FcRγ, which bears an immunoreceptor tyrosine activation motif. By coupling to FcRγ, CD36 is able to engage Src-family kinases and Syk, which in turn drives the internalization of CD36 and its bound ligands.
Introduction
CD36, a highly glycosylated transmembrane protein belonging to the class B scavenger receptor family, is expressed by several cell types such as adipocytes, erythrocytes, platelets, as well as endothelial and myeloid cells. It binds a broad range of endogenous ligands, including proteins like amyloid-β (Coraci et al., 2002), collagen (Tandon et al., 1989) and thrombospondin (Asch et al., 1987), and lipids that include oxidized low-density lipoprotein (oxLDL; Endemann et al., 1993), intact and oxidized phosphatidylserine (Rigotti et al., 1995; Greenberg et al., 2006) and long-chain fatty acids (Abumrad et al., 1993). In addition, CD36 also recognizes exogenous proteins like the Plasmodium falciparum protein PfEMP1 (Baruch et al., 1996), and lipids such as bacterial diacylglycerides (Hoebe et al., 2005). By interacting with such ligands the scavenger receptor plays a critical role in the removal of apoptotic bodies and of _Plasmodium_-infected red blood cells (McGilvray et al., 2000; Patel et al., 2004), and its association with thrombospondin is thought to have anti-metastatic effects (Clezardin et al., 1993; Uray et al., 2004). However, not all of these interactions are beneficial: CD36 contributes to the retention of macrophages at atherosclerotic lesions and their subsequent conversion into foam cells (Febbraio et al., 2000), contributes to macrophage apoptosis and to the sterile inflammation induced by oxLDL by interacting with toll-like receptors (TLR) 2, 4 and/or 6 (Manning-Tobin et al. 2009; Seimon et al., 2010; Stewart et al., 2010), and is also implicated in the progression of Alzheimer’s dementia through its pro-inflammatory engagement of amyloid-β (El Khoury et al., 2003; Coraci et al., 2002).
The ligands recognized by CD36 are generally multivalent and can therefore engage multiple receptors simultaneously. The resulting formation of CD36 clusters initiates signal transduction and internalization of receptor-ligand complexes. Remarkably, despite the broad biological importance of CD36, the signaling pathways that direct its internalization remain to be identified. Elucidation of the mode of action of the receptor has been stymied by the absence of discernible signaling motifs in its short intracellular domains (Armesilla and Vega, 1994). Together with the varied chemical nature of its ligands and the heterogeneous cellular responses they elicit, this suggests that CD36 signals in conjunction with co-receptors or accessory molecules. Indeed, several proteins have been implicated as CD36 co-receptors, including toll-like receptors (TLR) 2, 4 and 6, as well as the α3β1 and αvβ5 integrins (Stewart et al., 2010; Calzada et al., 2004; Chang and Finnemann, 2007). The dependency on co-receptor signaling is strongly ligand-specific. For example, signaling via TLR4/6 and their downstream adaptors MyD88 and TRIF is required for pro-inflammatory cytokine production after engagement of oxLDL and fibrillar amyloid-β (Stewart et al., 2010). In contrast, neither TLR receptors nor their downstream signaling components are required for the production of pro-inflammatory cytokines following the CD36-dependent uptake of malaria-infected erythrocytes (Erdman et al., 2009). Of note, none of the signaling pathways identified to date dictate the internalization of CD36, and instead seem to regulate downstream events such as gene transcription.
There is currently disagreement whether CD36 is internalized via caveolae (Uittenbogaard et al., 2000; Ring et al., 2006; Truong et al., 2006), macropinocytosis (Boyanovsky et al., 2009), clathrin-mediated endocytosis (Shamsul et al., 2010) or an actin-dependent process distinct from macropinocytosis (Collins et al., 2009). Recent evidence suggests that CD36 uptake requires Src-family kinases, Jnk and Rho-family GTPases, but the pathway(s) leading to their activation remain unclear. We used a combination of immunoprecipitation, mass spectrometry and gene silencing or deletion to demonstrate that CD36 exists in heterogeneous membrane complexes containing β1 and/or β2 integrins, and the tetraspanins CD9 and CD81, and that these ancillary molecules bridge CD36 to the immunoreceptor tyrosine activation motif (ITAM)-bearing adaptor FcRγ (FcER1G), which is required for the CD36-dependent activation of Syk. By coupling it to an ITAM adaptor, this complex allows CD36 to engage Src and Syk kinases, thus triggering the internalization of CD36 and its bound ligands.
Results
Identification of proteins phosphorylated in response to CD36 cross-linking
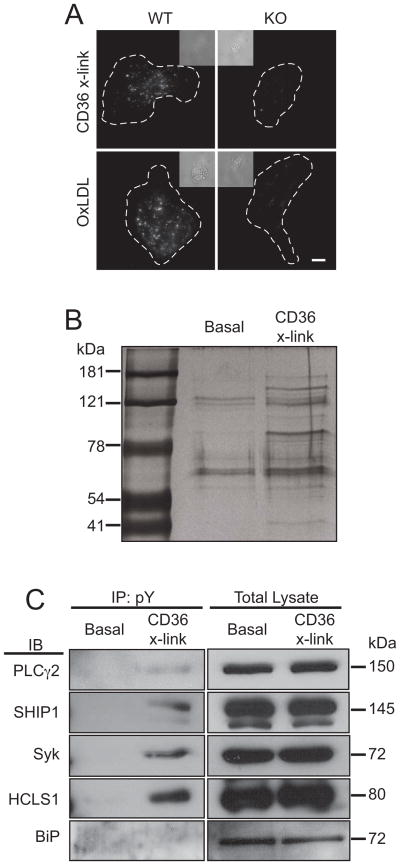
The comparatively large particles that are the physiological targets of phagocytic receptors expose a multiplicity of ligands that can engage a variety of different receptors. To selectively stimulate CD36 in RAW264.7 cells (called RAW hereafter) while avoiding spurious stimulation of Fcγ receptors, which are abundant on the surface of macrophages, we employed a primary anti-CD36 IgA, followed by a secondary F(ab′)2. The specificity of these antibodies was initially demonstrated comparing macrophages isolated from wildtype (WT) and CD36-deficient mice. As shown in Figure 1A, when anti-CD36 IgA was allowed to bind and cross-linked using Cy3-tagged F(ab′)2 the complexes were formed and internalized by WT, but no labeling or internalization was detectable in knockout cells. Moreover, non-immune IgA or IgG were unable to bind or induce internalization. Figure 1A also illustrates that CD36 is the predominant pathway underlying the uptake of oxLDL in murine macrophages.
Figure 1. Activation of CD36 and immunoprecipitation of tyrosine-phosphorylated proteins.
A) Internalization of CD36 and uptake of oxLDL by murine macrophages. Wild-type (WT) or CD36−/− (KO) mouse peritoneal macrophages were incubated with either anti-CD36 IgA that was then cross-linked using Cy3-tagged F(ab′)2 (top panels) or with DiI-labeled oxLDL; after incubation at 37°C for 15 min non-internalized ligand was removed by acid washing and the cells were imaged. B) Silver-stained SDS-PAGE gel of phosphotyrosine immunoprecipitates from unstimulated (Basal) and CD36 cross-linked (CD36 X-link) cells. Representative of 4 similar experiments. C) Phosphotyrosine (pY) immunoprecipitates were prepared from unstimulated (Basal) or CD36 cross-linked cells (CD36 X-link). The precipitates were then subjected to SDS-PAGE and immunoblotted with antibodies to PLCγ2, SHIP1, Syk, HCLS1 or BiP, as indicated. Immunoprecipitates (IP) shown to the left and or samples of the total lysates to the right. Each blot is representative of 3–4 independent experiments. See also Figure S1.
To identify components of the signaling pathway activated by CD36, we sought to detect proteins that become tyrosine phosphorylated in response to receptor stimulation. Immunoprecipitation with phosphotyrosine antibodies covalently coupled to beads, and a gentle elution protocol using phenylphosphate, were used to minimize contamination with immunoglobulins or non-specifically bound proteins. When eluates prepared from untreated and cross-linked cells were compared by silver staining, it was apparent that stimulation of CD36 induced phosphorylation of numerous proteins and increased the degree of phosphorylation of other proteins that are visibly phosphorylated in the resting state (Figure 1B). Bands corresponding to proteins that displayed a reproducible increase in phosphorylation were excised and subjected to mass spectrometry. This analysis identified 36 proteins that were consistently tyrosine-phosphorylated in response to CD36 cross-linking (Table 1).
Table 1.
Proteins phosphorylated in response to CD36 cross-linking, as identified by mass spectrometry of phosphotyrosine immunoprecipitates prepared from CD36 cross-linked cells. “Protein” is the protein symbol, and “Uniprot” is the proteins accession number, as listed in the Uniprot database (www.uniprot.org). “Spectra” refers to the number of unique peptides identified in the mass spectrometry screens. Data represents the aggregate results of four separate experiments. Proteins in bold have been previously associated with ITAM-dependent signalling pathways.
Significantly, 13 of these proteins (Table 1) have previously been identified as components of ITAM-dependent signaling pathways, suggesting that CD36 may signal via an ITAM-bearing adaptor or co-receptor. The identity of some of the proteins detected by mass spectrometry was verified by immunoblotting eluates of the phosphotyrosine immunoprecipitate. Four candidates were selected based on their previously identified roles in either ITAM-dependent signaling or phagocytosis, and on the availability of antibodies. As illustrated in Figure 1C, immunoblotting confirmed the presence of PLCγ2, SHIP1, Syk and HCLS-1 in the immunoprecipitates. This conclusion was verified independently by immunoprecipitating either PLCγ2 or SHIP1 and blotting the precipitates for phosphotyrosine (Figure S1; the Syk and HCLS-1 antibodies tested were not suitable for immunoprecipitation). By contrast, BiP was not immunoprecipitated under comparable conditions (Figure 1C), suggesting that this abundant protein was a non-specific contaminant detected by the mass spectrometer in the phosphotyrosine immunoprecipitates.
Src and Syk are required for CD36 internalization
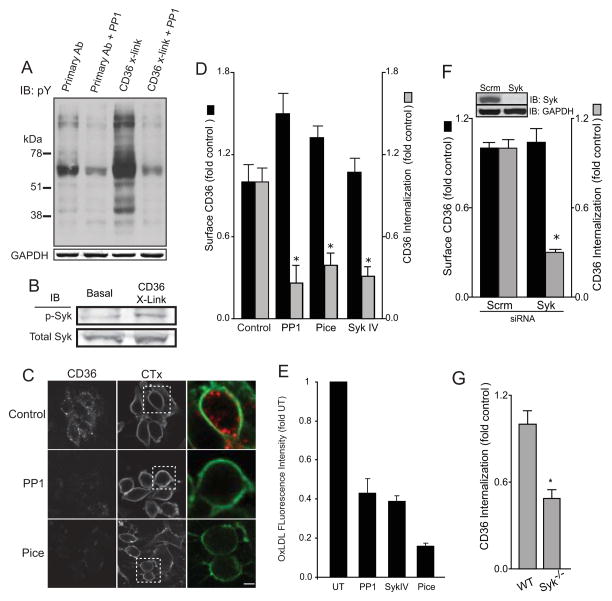
Of particular interest was the identification of Syk, a tyrosine kinase known to be a central mediator of ITAM-dependent signaling and phagocytosis. We therefore tested the role of Syk and its upstream activator, the Src-family kinases, in CD36-dependent signaling and CD36 internalization. As illustrated in Figure 2A, the marked phosphorylation induced by CD36 cross-linking could be inhibited by addition of the Src-family kinase inhibitor PP1. Phospho-specific antibodies revealed that stimulation of CD36 promoted phosphorylation of Syk on Y525/Y526 (Figure 2B); phosphorylation of these sites is indicative of Syk activation (Kurosaki et al., 1994). The phosphorylation –and hence the activation– of Syk could be inhibited by PP1 (not shown), implying that Src-family kinases were required for the process, as is the case in other canonical ITAM-dependent pathways (Johnson et al., 1995; Schmitz et al., 1996; Zoller et al., 1997).
Figure 2. Src-family and Syk kinases are required for CD36 internalization.
A) Phosphotyrosine immunoblot of identical amounts of cell lysates from vehicle control or PP1-treated (PP1) cells that were treated with anti-CD36 IgA alone (Primary Ab) or subjected to CD36-cross-linking (CD36 X-link) by subsequent incubation at 37°C with F(ab′)2. Blot is representative of 4 similar experiments. GAPDH was immunoblotted (below) to ensure equal loading. B) Immunoblot of active (phosphorylated) Syk of cell lysates from vehicle control or PP1-treated (PP1) cells that were either otherwise untreated (Basal) or subjected to CD36-cross-linking (CD36 X-Link). C) Assessment of internalization of cross-linked CD36 in vehicle control, PP1-treated (PP1) or piceatannol-treated (Pice) cells. Fluorescent cholera toxin subunit B (CTx) was used to demarcate the plasma membrane. Confocal images are representative of multiple fields from 5 independent experiments. D) Quantification of the level of surface CD36 prior to cross-linking (black bars) and of its internalization in response to cross-linking (gray bars) in RAW cells treated with vehicle control only, PP1 (PP1), piceatannol (Pice) or Syk inhibitor IV (Syk IV). E) Quantification of the internalization of oxLDL by RAW cells treated with vehicle control only (UT), PP1 (PP1), Syk inhibitor IV (Syk IV) or piceatannol (Pice). Data were normalized to untreated control and are presented as means ± SE of at least 200 cells for each condition, from 3 separate experiments of each kind. F) Quantification of the level of surface CD36 prior to cross-linking (black bars) and of its internalization in response to cross-linking (gray bars) in cells treated with scrambled (Scrm) or Syk-specific siRNA. The top lanes in the insert show a typical immunoblot validating the effectiveness of the siRNA-induced knockdown of Syk, while the bottom lanes show that loading, assessed by GAPDH blotting, was comparable. G) Internalization of cross-linked CD36 in bone-marrow derived macrophage from C57Bl/6 (WT) or Syk-deficient (Syk−/−) mice. Data in D-F were normalized to untreated control and are presented as means ± SE of at least 3 separate experiments of each kind. The asterisks denote p<0.05 compared to untreated, scrambled siRNA-treated or wild-type cells, respectively, calculated using ANOVA with Bonferroni correction (D), or Student’s _t_-test (E-F).
We proceeded to test whether the Src/Syk signaling pathway is required for the internalization of ligated CD36. Pharmacological inhibition of Src-family kinases (using PP1) or Syk (using either piceatannol or compound SykIV) markedly depressed internalization of cross-linked CD36 (Figure 2C-D), without reducing the surface exposure of CD36. Endocytosis of CD36 induced by the (patho)physiological ligand oxLDL was also markedly inhibited by Src-family or Syk inhibitors (Figure 2E). To circumvent possible non-specific effects of the inhibitors, the role of Syk in receptor endocytosis was also tested in Syk siRNA-treated RAW macrophages (Figure 2F) and in bone-marrow-derived macrophages isolated from Syk-deficient mice [Figure 2G, (Crowley et al., 1997)]. As with the pharmacological inhibitors, depletion or knockout of Syk clearly reduced CD36 internalization without altering the expression of CD36 at the cell surface. Together these results demonstrate that optimal CD36 internalization requires Src and Syk, and that the Src-family kinases act upstream of Syk.
CD36 associates with β1 and β2 integrins
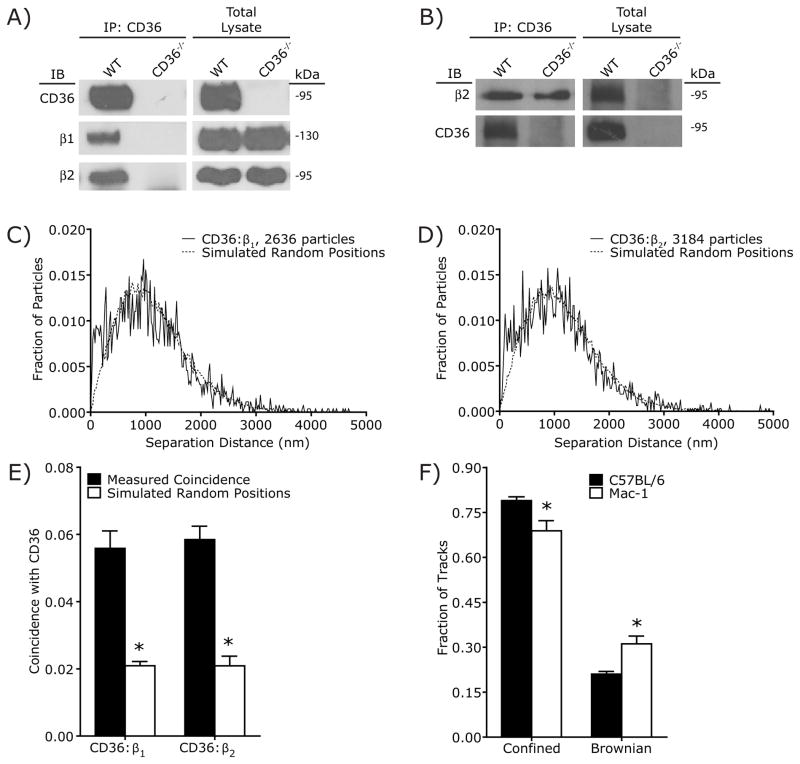
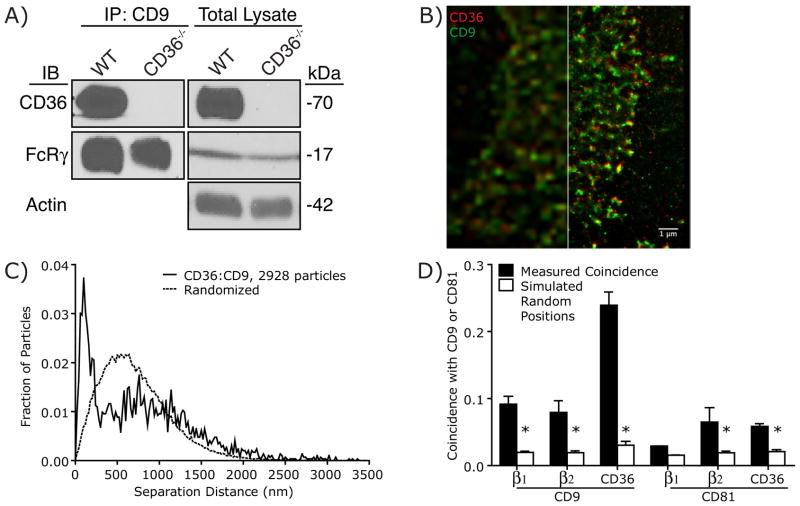
While the previous experiments demonstrated that CD36 internalization requires Src and Syk, it is unclear how CD36 engages these signaling molecules. Canonically, Src phosphorylates ITAM motifs, resulting in the recruitment of Syk via its tandem SH2 domains. Src then phosphorylates Syk, inducing downstream signaling. However, CD36 contains no motifs known to interact with Syk, nor is CD36 known to interact with any ITAM-bearing adaptors or co-receptors. We therefore hypothesized that CD36 induces Syk signaling through a heretofore unknown ancillary molecule, which we attempted to identify by immunoprecipitation followed by mass spectrometry. Anti-CD36 IgA was used to precipitate the receptor from lysates of peritoneal macrophages derived from wild-type C57BL/6 mice or, as a control for specificity, from CD36 knockout (CD36−/−) mice. Immunoprecipitated proteins were analyzed by SDS-PAGE and silver staining and bands that were unique to the wild-type sample were subjected to mass spectrometry. In addition to a prominent band corresponding to CD36, both the alpha and beta subunits of the integrin Mac-1 (αmβ2) were detected solely in the wild-type precipitate. The association between the β2 subunit of Mac-1 and CD36 was confirmed independently by immunoblotting, which demonstrated the presence of the integrin in immunoprecipitates from wild-type, but not CD36 knockout cells (Figure 3A). The same approach confirmed the previously reported association between CD36 and β1 integrin [Figure 3A; (Miao et al., 2001)]. The association between β2 and CD36 was validated independently by immunoprecipitating the integrin subunit, followed by immunoblotting of CD36 (Figure 3B).
Figure 3. CD36 is associated with β1 and β2 integrins.
A) Co-precipitation of β1 and β2 integrins with CD36. CD36 immunoprecipitates were prepared from macrophages obtained from wild-type C57Bl/6 (WT) or CD36-deficient (CD36−/−) mice. After SDS-PAGE the immunoprecipitated material was blotted with antibodies to CD36 (top), the integrin β1 chain (middle) or the integrin β2 chain (bottom). Samples of the total lysates are shown to the right, validating comparable loading and the absence of CD36 in CD36−/− macrophages. Representative of 3 experiments. B) Co-precipitation of CD36 with β2 integrins. β2 integrin immunoprecipitates were prepared from macrophages obtained from wild-type C57Bl/6 (WT) or CD36-deficient (CD36−/−) mice. After SDS-PAGE the immunoprecipitated material was blotted with antibodies to β2 integrins (top), or CD36 (bottom). Samples of the total lysates are shown to the right, validating comparable loading. Representative of 3 experiments. C,D) Analysis of the spatial association of CD36 and β1 integrin (C), or CD36 and β2 integrin (D) in the plasma membrane of RAW macrophages (solid line) compared to the predicted separation based on randomized particle positions (Simulated Particle Positions; dotted line). Representative of 5 experiments, each combining data from 20 fields. E) Comparison of the spatial association between CD36 and the β1/β2 integrins measured experimentally (Measured Coincidence) with the predicted coincidence based on randomized particle positions (Simulated Random Positions). Data are means ± SE of measurements from 100 cells in 5 independent experiments. Asterisk denotes p < 0.05 compared to Measured Coincidence, paired _t_-test. F) Knockout of the β2 integrin Mac-1 reduces the proportion of CD36 undergoing confined diffusion. * p < 0.05 compared to C57BL/6, Student’s _t_-test, n = 3. See also Figure S2.
Because incomplete solubilization or spurious association following solubilization can lead to non-specific co-immunoprecipitation of transmembrane proteins, we assessed the association of CD36 with the β1 and β2 integrins using a super-resolution microscopy approach (see Supplemental Text and Figure S2 for details and validation). Cells were fixed and dual-labeled with distinct secondary antibodies was used to detect the position of CD36 and the integrins. The relative distances measured with sub-diffraction resolution were compared to a theoretical distribution generated assuming that the two types of molecules are distributed randomly on the cell surface. Validation of this approach, termed spatial apposition analysis (SAA) hereafter, is provided in Supplementary Figure 3 and the corresponding Supplemental Text. Quantification of the fraction of CD36:integrin pairs separated by less than the co-localization distance criterion (CDC; see Experimental Procedures for definition) revealed a considerable (≈3-fold) enrichment in closely apposed CD36 and β1, compared to what would be predicted from random distribution (Figure 3C,E). Analysis of CD36 and β2 integrin revealed a similarly significant association (Figure 3D,E). Moreover, genetic deletion of the β2 integrin Mac-1 increased the fraction of CD36 undergoing free diffusion (Figure 3F), confirming the association between CD36 and β2 integrins. Together, these findings indicate that CD36 and the β1 and β2 integrins are associated in situ.
The ITAM-bearing adaptor FcRγ is required for CD36 internalization
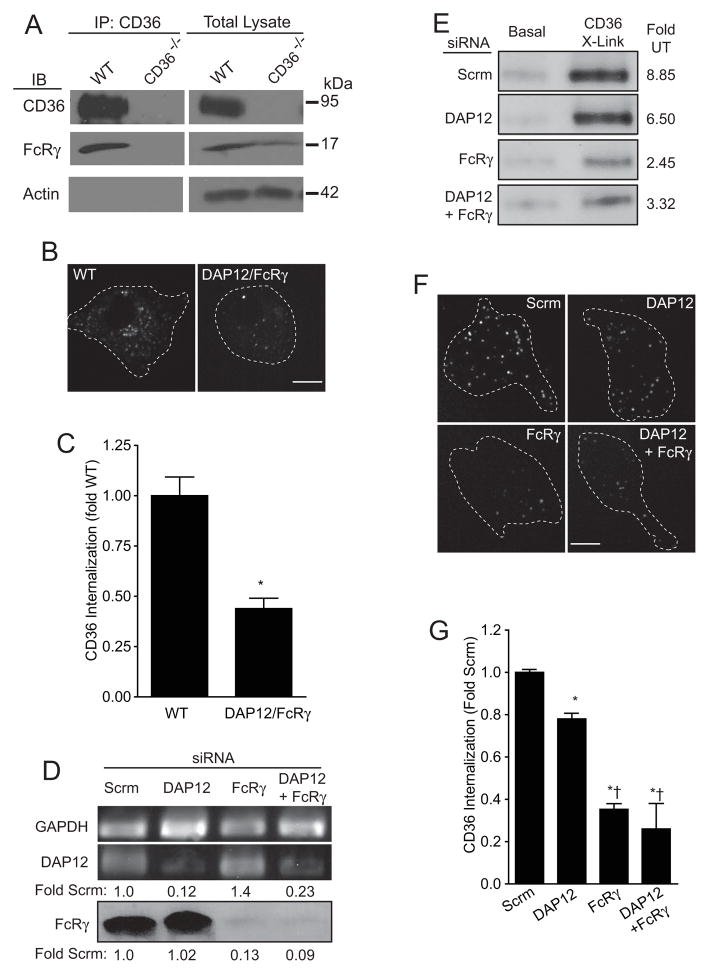
While the previous experiments confirmed the association between CD36 and the β1 and β2 integrins, they did not reveal the mechanism whereby CD36 engages the Src/Syk signaling pathway. However, integrin activation is associated with stimulation of Src-family kinases and, despite lacking an ITAM or other Syk-interacting motifs, integrins are known to engage Syk (Fujii et al., 1994; Yan et al., 1997). It is therefore likely that CD36 and integrins utilize a similar pathway to activate Syk. Integrin activation of Syk requires the ITAM-bearing adaptors DAP12 and FcRγ (Mócsai et al., 2006), small (12 kDa and 9 kDa, respectively) transmembrane proteins that exist in the macrophage plasma membrane as disulfide-linked homodimers (Küster et al., 1990; Tomasello et al., 1998). We therefore tested whether these adaptors associate with and are required for signaling by CD36 complexes. Though not detected in our mass spectrometry screens, we found FcRγ to co-immunoprecipitate with CD36 in peritoneal macrophages (Figure 4A). The specificity of the interaction is suggested by the absence of FcRγ from immunoprecipitates obtained from CD36-deficient macrophages (Figure 4A). We were unable to perform equivalent experiments for DAP12, due to the lack of suitable antibodies.
Figure 4. CD36 internalization requires the ITAM-bearing adaptor FcRγ.
A) Co-precipitation of FcRγ with CD36. CD36 immunoprecipitates were prepared from macrophages obtained from wild-type C57Bl/6 (WT) or CD36-deficient (CD36−/−) mice. After SDS-PAGE the immunoprecipitated material was blotted with antibodies to CD36 (top), FcRγ chain (middle) or actin (bottom), an abundant protein used to rule out nonspecific precipitation (left) and to normalize loading in total lysates (right). Blots are representative of 3 experiments. B–C) CD36 internalization was measured in bone marrow macrophages from wild-type C57Bl/6 (WT) and in DAP12/FcRγ-deficient (DAP12/FcRγ−/−) mice. Panel B illustrates a typical confocal image, obtained after removal of surface-exposed antibodies by an acid-wash. Panel C summarizes the quantitation of 120 cells from 3 independent experiments, obtained by integration of the fluorescence of 3-dimensional projections of the entire cell. Asterisk indicates p < 0.05 compared to wild-type using Student’s _t_-test. D) RAW macrophages were treated with scrambled (Scrm) siRNA, with siRNA targeting either DAP12 or FcRγ, or both. In the top lanes, the effect of these treatments on the DAP12 mRNA content is compared to that of GAPDH, measured by RT-PCR. The numbers at the bottom indicate the expression levels relative to the Scrm sample, normalized to GAPDH content. In the bottom lane the FcRγ protein level was assessed by immunoblotting. The numbers at the bottom indicate the expression levels relative to the Scrm sample. Data are representative of 3 experiments of each type. E) RAW macrophages were treated with scrambled (Scrm) siRNA, with siRNA targeting either DAP12 or FcRγ, or both. The cells were then left untreated (UT) or stimulated by CD36 cross-linking. Identical amounts of cell lysates were then subjected to SDS-PAGE followed by immunoblotting for phosphorylated (activated) Syk. Blot is representative of 4 experiments. F–G) RAW macrophages were treated with scrambled (Scrm) siRNA, with siRNA targeting either DAP12 or FcRγ, or both. CD36 internalization was then measured as in B–C. Representative images are illustrated in F and quantifications performed as above are summarized in G. Data are means ± SE of 150 cells for each condition, from 3 independent experiments. Asterisks indicate p < 0.05 compared to Scrm; † denotes p < 0.05 compared to DAP12, calculated by ANOVA with Bonferroni correction.
We next tested the possible role of the ITAM-bearing adaptors in CD36-initiated signaling and function. As illustrated in Figure 4B–C, CD36 internalization was impaired in bone marrow-derived macrophages from DAP12/FcRγ double-knockout mice. The decrease was of similar magnitude to that observed in Syk-deficient macrophages (Figure 2F), leading us to hypothesize that DAP12 and/or FcRγ were required for CD36-mediated Syk activation. The individual contribution of each one of the adaptors was investigated using siRNA in RAW cells. Silencing of FcRγ expression was validated by immunoblotting, while RT-PCR was used for DAP12, due to the unavailability of antibodies (Figure 4D). The effect of adaptor silencing on the phosphorylation (and hence activation) of Syk is documented in Figure 4E. Whereas reducing the expression of DAP12 had only a modest effect on Syk phosphorylation, silencing FcRγ had a profound effect and a similar degree of inhibition was observed when both DAP12 and FcRγ were simultaneously silenced (Figure 4E). In accordance with our previous observations (Figure 2), the decreased activation of Syk associated with down-regulation of FcRγ impaired the internalization of cross-linked CD36 (Figures 4F-G). Jointly, these observations suggest that phosphorylation of FcRγ by Src-family kinases is required for optimal internalization of activated CD36.
CD36 and FcRγ associate with tetraspanins
The tetraspanins CD9 and CD81 have previously been found to associate with CD36, as well as with integrins (Huang et al., 2011; Chang and Finnemann, 2007; Miao et al., 2001; Gutierrez-Lopez et al., 2003; Thorne et al., 2000). Because tetraspanins are thought to establish and stabilize interactions between transmembrane proteins (Hemler, 2005), we hypothesized that CD9 and/or CD81 may mediate the interactions between CD36, integrins and FcRγ, leading to the formation of higher-order complexes. Consistent with this hypothesis, FcRγ and CD36 co-precipitate with CD9 (Figure 5A). Importantly, FcRγ also immunoprecipitated with CD9 in macrophages derived from CD36-knockout mice (Figure 5A), suggesting that CD9 may act as a scaffold between CD36 and FcRγ.
Figure 5. CD36 and integrins form higher order complexes with CD9 and CD81.
A) CD36 and FcRγ co-precipitate with CD9. CD9 immunoprecipitates were prepared from macrophages obtained from wild-type C57Bl/6 (WT) or CD36-deficient (CD36−/−) mice. After SDS-PAGE the immunoprecipitated material was blotted with antibodies to CD36 (top), FcRγ (bottom) or actin (bottom), an abundant protein used to normalize loading in total lysates (right). Representative blots of 3 experiments. B) Representative wide-field (left) and GSD (right) image of CD9 (green) and CD36 (red), in the plasma membrane of the same RAW macrophage. C) Representative SAA trace of CD36 and CD9 in RAW macrophages (solid line) compared to the predicted separation based on randomized particle positions (Randomized; dotted line). Representative of 3 experiments, each combining data from 25 fields. D) Comparison of the spatial association between CD9 or CD81 with CD36, β1 and β2 measured experimentally (black bars) with the predicted coincidence based on randomized particle positions (white bars). Data are mean ± SE of measurements from 60 cells in 3 independent experiments. Asterisks denote p <0.05 compared to measured coincidence. See also Figure S3.
Wide-field and ground state depletion imaging (GSD; Figure 5B), followed by SAA (Figures 5C,D and S3) were used to establish that both CD9 and CD81 associate with CD36 and with β1 and β2 integrins (Figure 5D) in the membranes of macrophages. Interestingly, CD81 displayed significant association only with the β2-integrin (Figures 5D, S3D–E), raising the possibility that preferential association between CD36 and specific tetraspanins may generate molecularly distinct signaling platforms.
Tetraspanins scaffold CD36-integrin complexes
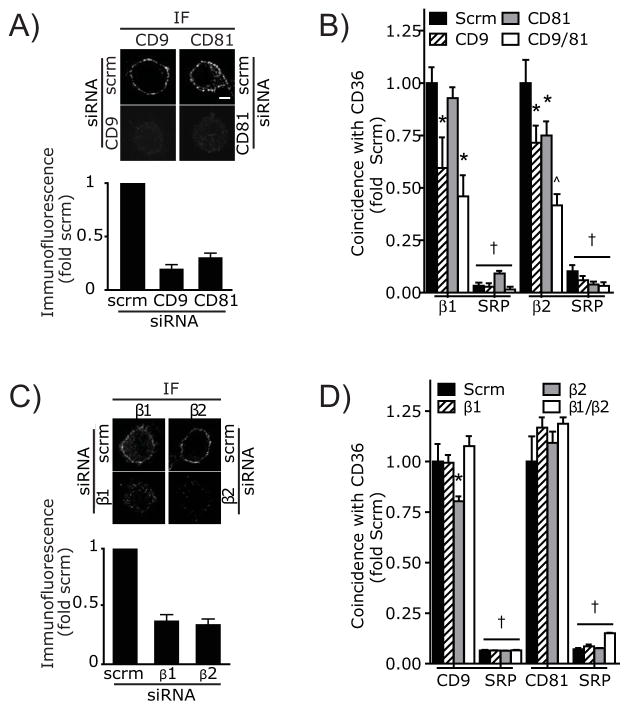
The preceding experiments suggest the existence of tripartite CD36/integrin/tetraspanin complexes. We analyzed if CD36 associates separately with integrins and tetraspanins, or whether the interaction of integrins and tetraspanins with CD36 is interdependent. To this end, we used siRNA to silence CD9, CD81 and the integrin β1 and β2 chains separately and in combination, followed by measurement of the association of CD36 with the remaining components using SAA. As illustrated in Figure 6A, expression of CD9 and CD81 on the surface of RAW cells could be effectively silenced using siRNA. Down-regulation of CD9 resulted in a significant decrease in the association between CD36 and both integrins (Figure 6B). Silencing CD81 expression similarly depressed association of CD36 with β2 integrins, but the decrease in β1 association was not significant. Concomitant silencing of both tetraspanins had an additive effect (Figure 6B).
Figure 6. Tetraspanins Scaffold CD36-Integrin Complexes.
A) Silencing of CD9 or CD81 gene expression using siRNA. RAW macrophages were treated with irrelevant, scrambled (scrm) siRNA, or with siRNA targeting CD9 or CD81. After 48 hrs cells were immunostained to detect surface CD9 or CD81. Representative immunofluorescence images are shown at the top and the quantification of at least 200 cells from 3 independent experiments is illustrated below. Fluorescence intensity is expressed relative to the corresponding control, i.e. cells treated with scrambled siRNA and stained with the appropriate antibody. B) Interactions of CD36 with β1 and β2 integrins as quantified using SAA analysis in RAW macrophages 48 h after treatment with scrambled (scrm) siRNA, with a pool of four oligonucleotides targeting CD9 or with a single oligonucleotide targeting CD81. C) Silencing of the β1 or β2 integrin subunits using siRNA. Macrophages were treated with irrelevant, scrambled (scrm) siRNA or with siRNA targeting either β1 or β2 integrin, as indicated. After 48 hrs cells were immunostained to detect surface integrins. Representative immunofluorescence images are shown at the top and the quantification of at least 200 cells from 3 independent experiments is illustrated below. Fluorescence intensity is expressed relative to the corresponding control, i.e. cells treated with scrambled siRNA and stained with the appropriate antibody. D) Interactions of CD36 with CD9 or CD81 as quantified using SAA analysis in RAW macrophages 48 hrs after treatment with scrambled (scrm) siRNA or with a single siRNA targeting either β1 or β2. SRP = simulated random positions. Data are means ± SE of 3 similar experiments; Asterisk denotes p <0.05 compared to Measured Coincidence, † denotes p<0.05 compared to equivalent siRNA treatment in non-randomized sample, ^ denotes p<0.05 compared to CD9 or CD81 siRNA alone, ANOVA with Bonferroni correction.
The expression of integrins on the surface of RAW cells could also be effectively depressed using siRNA (Figure 6C). A single siRNA was used in the experiments of Figures 6C–D to minimize off-target effects. Of note, decreasing the expression of the integrins –whether separately or jointly– had little effect on tetraspanin association with CD36 (Figure 6D); only a small change in CD9 apposition to CD36 was noted when β2 was silenced. Jointly, these findings indicate that, while the integrins are dispensable for tetraspanin binding to CD36, the tetraspanins are required to establish tripartite complexes. These findings support the notion that tetraspanins serve as molecular scaffolds that stabilize signaling complexes at the membrane. Moreover our data imply that CD9 and CD81 form distinct complexes, potentially creating different, ligand-specific signaling platforms.
Integrins and tetraspanins are required for CD36 internalization
The physical association of CD36 with integrins and tetraspanins noted in Figures 3, 5 and 6 is suggestive of a functional interaction. We therefore tested whether the presence of CD9, CD81 and/or of the β1 and β2 integrins is required for the function of CD36, assessed by measuring endocytosis of oxLDL. As discussed above in the context of Figure 1, CD36 provides the major route for oxLDL internalization. Treatment with CD9 or CD81 siRNA resulted in a sizable, though incomplete inhibition of oxLDL uptake (Figure 7A). Two lines of evidence suggest that this is caused by silencing of the intended tetraspanins and not to off-target effects: first, the inhibition could be reversed by heterologous expression of the human CD9 or CD81 orthologues, respectively. Indeed, overexpression of the tetraspanins potentiated oxLDL uptake beyond the levels recorded in control cells (Figure 7A). Secondly, very similar effects were obtained when using any one of four individual siRNAs, but not with irrelevant, scrambled siRNA (Figure S4). Silencing of β1 or β2 was also accompanied by a very significant reduction in the ability of the cells to internalize oxLDL (Figure 7B). The partial inhibition obtained in each case most likely reflects the incomplete silencing of the integrins and/or functional redundancy of the different integrin species. The specificity of the gene silencing strategy was tested using four distinct targeted siRNAs, all of which depressed integrin expression, while irrelevant scrambled siRNA was without effect (Figure S4). A single siRNA was used in the experiments of Figure 7B to minimize off-target effects. Jointly, these results imply that both tetraspanins and integrins are required for normal internalization of CD36 following engagement by ligands.
Figure 7. Tetraspanins and Integrins are Required for CD36 Function.
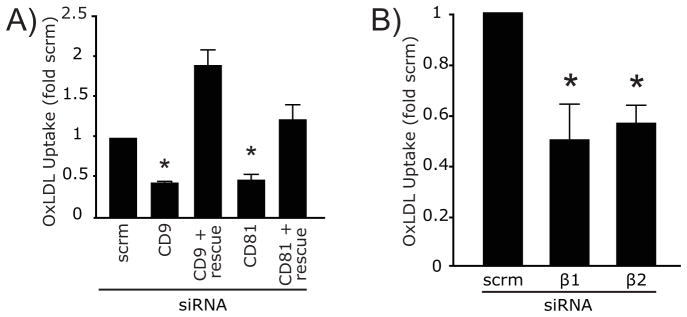
A) oxLDL uptake was quantified in RAW macrophages 48 h after treatment with scrambled (scrm) siRNA, with a pool of four oligonucleotides targeting CD9 or with a single oligonucleotide targeting CD81. Where indicated (rescue), the cells were co-transfected with GFP and plasmids encoding the human orthologues of CD9 or CD81, which were expressed on the surface of the cells despite the presence of the siRNAs targeting the murine forms (not illustrated). B) oxLDL uptake was quantified in RAW macrophages 48 h after treatment with irrelevant, scrambled (scrm) siRNA or with siRNA targeting either β1 or β2 integrin. Asterisk denotes p <0.05 compared to scrm, ANOVA with Bonferroni correction. See also Figure S4.
Discussion
Our findings point to the existence of multimolecular complexes of transmembrane proteins that include CD36, integrins, tetraspanins and ITAM-bearing adaptors that enable the transmission of signals via Syk. These observations, together with earlier findings that CD36 can associate with and activate Src-family kinases (Huang et al., 1991), provide a defined pathway whereby this receptor triggers internalization of important ligands like modified LDL that can result in foam-cell and atheroma formation. Earlier studies determined that the internalization of CD36 occurs through a pathway distinct from macropinocytosis and canonical endocytosis (Collins et al., 2009). This unconventional internalization mechanism shares some similarities with the Fcγ receptor-mediated phagocytosis of IgG-opsonized particles, notably the requirement for Src-family kinases, Rho-family GTPases and actin polymerization (Hackam et al., 1997; Coppolino et al., 2001; Hall et al., 2006). The current study has reinforced these similarities by identifying a role for Syk in the uptake of CD36, akin to that played by this tyrosine kinase during phagocytosis (Kiefer et al., 1998). Phosphorylation of the scaffold proteins Fyb and LCP2 (Coppolino et al., 2001) is also a common consequence of the activation of CD36 and Fcγ receptors. The identification of these adaptor molecules is of particular interest, as they provide a possible link between Src/Syk activation, integrin signaling, actin reorganization and Jnk signaling (Mizuno et al., 2005; Wang et al., 2009; Barda-Saad et al., 2010) – all processes known to be critical for the uptake of CD36. Lipid-signaling enzymes known to regulate phagocytosis were also identified in our screen, notably PLC 2 –which is known to be activated by Src-family kinases during phagocytosis (Makranz et al., 2004; Palmbos et al., 2002) – and SHIP1, a negative regulator of phosphatidylinositol 3,4,5-_tris_phosphate signaling, which regulates Fc receptor-mediated phagocytosis. Of note, SHIP1 can be recruited to integrins by DOK1 (Horan et al., 2007; Sattler et al., 2001), which was also identified in our mass spectrometry screen (Table 1).
CD36 is a receptor for a broad range of ligands, which can be of proteinacious (thrombospondin, fibronectin, collectin, amyloid-β, PfEMP1) or lipidic nature (oxLDL, oxidized phospholipids, bacterial diacylglycerides). Remarkably, the cellular responses to these ligands vary, ranging from pro-inflammatory to anti-inflammatory (Stewart et al., 2010; Chung et al., 2007; Girkontaite et al., 2007). The recognition of such a broad spectrum of ligands, and the diversity of the resultant cellular responses, suggests the existence of multiple types of co-receptors that confer to the complexes distinct ligand-identification and signaling properties. Indeed, previous studies have identified several ligand-specific CD36 complexes: interaction of TLR4 or TLR6 with CD36 is required for pro-inflammatory signaling in response to amyloid-β or oxLDL (Stewart et al., 2010); by contrast the recognition of P. falciparum PfEMP1 by CD36 is independent of TLR receptors and their downstream signaling components (Baruch et al., 1996; Erdman et al., 2009). Integrin-containing complexes have also been reported; recognition of apoptotic retinal pigment epithelial cells appears to require association of CD36 with CD81 and αvβ5 integrin (Chang and Finnemann, 2007), and thrombospondin is bound by α3β1 integrin in conjunction with CD36 (Krutzsch et al., 1999; Li et al., 1993).
The ability of CD36 to signal the uptake of oxLDL in macrophages is similarly dependent on its association with other transmembrane proteins. Huang et al. (2011) reported that CD9 not only associates with CD36 in murine macrophages, but is partially required for the internalization of oxLDL and the formation of foam cells. Our findings extend these observations, indicating that another tetraspanin, CD81, is also associated with CD36 and required for its optimal function, as are β1 and β2 integrins. Our data also suggest that the tetraspanins mediate the association between CD36 and the integrins, and that different tetraspanins may function in the formation of distinct, possibly ligand-specific CD36 receptor complexes.
It is unlikely that CD36 and all of these co-receptors exist in a single, stable molecular entity. Instead, it seems more probable that CD36 is part of several distinct signalosomes, which need not be stable over time. Some of these may be cell-type specific, but the evidence collected here and elsewhere suggests that significant heterogeneity exists in the membrane of individual macrophages and likely other cells as well. Further evidence of heterogeneity comes from our studies of endocytosis. Pharmacological inhibition or knockout/knockdown of Src, Syk or FcRγ all had partial inhibitory effects on CD36 internalization, implying that in every instance studied at least one additional internalization pathway must exist. One potential route is the caveolae-dependent pathway used by TLR receptors (Shuto et al., 2005). This would be consistent with studies demonstrating that caveolin-1 is required for CD36-dependent signaling under some conditions (Tsai et al., 2011; Ring et al., 2006).
While the exact nature of the CD36 complexes remains to be defined, the salient feature of our findings is the presence of ITAM-containing adaptors in a significant fraction of these. We envisage FcRγ to be attached to the signaling complex via the tetraspanins CD9 and CD81, which also link to integrins that can serve as co-receptors. This assembly enables certain CD36 ligands to signal via Syk and its downstream effectors, which in other systems can promote actin remodeling. Other CD36 complexes, such as those involving TLR receptors, may use different means of signaling and internalization pathways. The co-existence of heterogeneous signalosomes would in this manner explain the multiplicity of entry routes and the ability to trigger either inflammatory or anti-inflammatory responses, depending on the nature of the ligand.
Experimental Procedures
Cell isolation and culture
RAW macrophages were maintained in DMEM with 10% FBS at 37°C under 5% CO2. Peritoneal murine macrophages were isolated as described (Patel et al., 2004). Bone marrow-derived macrophages were grown in whole-marrow cultures. Mice were euthanized, the femurs and tibias removed, and the bone marrow removed by perfusion with PBS. The marrow was passed 15 times through a 21-gauge needle to produce a single-cell suspension, which was plated in DMEM with 10% FBS, 100 ng/mL murine M-CSF and penicillin/streptomycin/gentamicin. Cultures were maintained for 5 days, and after removal of non-adherent cells, the adherent cells were cultured in fresh medium for an additional 2–3 days to complete maturation.
siRNA treatment
DAP12 and FcRγ siRNA was transfected into RAW macrophages by electroporation using an Amaxa apparatus and a Nucleofect V kit, as per manufacturer’s instructions. Cells were then plated at ≈50% confluence and incubated for 24–32 h. Knockdown was confirmed by immunoblotting or RT-PCR. Knockdown experiments were performed using a Mirus kit and an Amaxa electroporation apparatus. Knockdown was confirmed by immunoblotting. Human CD9, CD81 and β2 integrin plasmids were obtained from Addgene. Human β1 integrin cDNA was from the Hospital for Sick Children’s Signaling and Degradation Network (SIDNET) collection. Rescue experiments were performed by co-electroporating the relevant mouse siRNA, human rescue and GFP plasmids, and incubating for 48 h.
Oxidized LDL uptake
Cells were treated with 1 μg/mL DiI-labeled oxidized LDL for 20 min. Non-internalized oxLDL was stripped off the cell surface by brief treatment with trypsin.
Phosphotyrosine immunoprecipitation and mass spectrometry
RAW macrophages were grown to ≈80% confluence on 100 mm diameter plates. Cells were serum-starved for 3 h and cooled on ice. CD36 was cross-linked by incubation with anti-CD36 IgA (1:100) for 20 min, followed by 20 min with a cross-linking secondary F(ab′)2 (1:100). The cells were washed 3 times in cold PBS and then scraped into immunoprecipitation buffer (20 mM Tris-HCl, 0.15 M NaCl, 2 μM EDTA, 1% NP40, 10% glycerol and phosphatase/protease inhibitors). The resulting suspensions were rotated for 40 min at 4°C, and centrifuged at 16000g for 15 min to remove nuclei and large debris. Forty μL of anti-phosphotyrosine agarose slurry was added to 1 mL of cleared lysate and incubated under constant mixing for 12 h at 4°C. Bound proteins were recovered by centrifugation at 16000g for 30 sec at 4°C. The agarose beads were washed 5 times with cold PBS and bound proteins were next eluted by incubating the pellet in 70 μL PBS containing 25 mg/mL phenylphosphate for 20 min at 4°C, mixing constantly. Eluted proteins were separated on a 10% SDS-PAGE gel and visualized using silver staining. Bands of interest were excised and the proteins identified via mass spectrometry at the Advanced Protein Technology Centre of the Hospital for Sick Children. Silver stain was removed using a 15 min potassium ferricyanide (9.8 mg/mL) and 100 mM sodium thiosulfate wash, the gel dehydrated in 50% acetonitrile/25 mM ammonium bicarbonate, reduced for 30 min with 37.5% β-mercaptoethanol, alkylated using 100 mM iodoacetamide, and dehydrated a second time. In-gel digestion was performed for 3 hrs at 37°C with 13 ng/L trypsin. Peptides were recovered by extracting the gel 3 times with 25 mM ammonium bicarbonate and 5% formic acid. The peptides were separated on an Agilent 1100 Capillary LC system equipped with a C18 pre-column and a μLC analytical column that also served as a μESI emitter. Peptides were identified with LTQ mass spectrometer in data-dependent mode automatically cycling through acquisition of a full-scan mass spectrum and three MS/MS spectra recorded sequentially on the three most abundant ions present in the initial MS scan. All MS/MS spectra searched against the NCBInr database.
Immunoprecipitation of membrane proteins
Peritoneal macrophages were lysed for 2–3 h at 4°C in a buffer containing 1% Brij 99 (polyoxy ethylene (20)-oleylether), 150 mM NaCl, 5 mM MgCl2 and 25 mM Hepes (pH 7.5), supplemented with protease inhibitors (Miao et al., 2001; Thorne et al., 2000). Insoluble material was removed by centrifugation for 10 min at 13,000g at 4°C. Protein G- or protein L-agarose beads (60 μL) were preincubated overnight with 10 μg of the indicated antibodies. Antibody-coated beads were washed with Brij 99 lysis buffer, and incubated with 2 mL of cleared lysate overnight at 4°C. The beads were washed 4 times with lysis buffer, and the immunoprecipitated proteins eluted in 50 μL of 2x-concentrated Laemmli buffer containing 100 mM dithiothreitol by boiling for 5 min. Eluted samples were separated by SDS-PAGE and transferred onto PVDF membrane for immunoblotting.
CD36 internalization assay
Macrophages were serum starved for 3 h, transferred to medium RPMI-1640 without serum, and cooled to 10°C to prevent endocytosis during labeling. Anti-CD36 IgA (1:500) was added to the cells for 20 min, the cells washed 3 times in RPMI, and a cross-linking Cy3-tagged F(ab′)2 added to cross-link CD36. Twenty min after the addition of the F(ab′)2 the cells were washed 3-fold in RPMI and warmed to 37°C for 15 min to allow for internalization of CD36. Internalization was then stopped by addition of RPMI at 10°C, and surface-bound antibodies that were not internalized were removed by a 1.5 min acid wash (0.9% NaCl, 89 mM citric acid, 11 mM Na2HPO4, pH 2.5, 10°C). Cells were then fixed for 30 min in 4% paraformaldehyde at 10°C. Z-stacks (0.5 μm/slice) of the labeled cells were acquired using a Zeiss Axiovert 200/Quorum spinning-disc confocal microscope equipped with a 63x/1.4 NA and 100x/1.4 NA oil immersion objectives and a heated stage. The number of CD36-positive endosomes and the fluorescence per endosome was then quantified using customized Matlab scripts.
Detection of Single Molecules
Detection of single molecules was performed as described previously (Jaqaman et al., 2008; Jaqaman et al., 2011). See Supplemental Experimental Procedures for details of labeling, single-particle tracking and for description and validation of the spatial apposition analysis (SAA).
Statistics
All ANOVA and _t-_test statistics were preformed using Graphpad Prism software. All other statistical and mathematical analyses were preformed using custom scripts written in Mathworks Matlab software. Data are presented as mean ± SE unless otherwise noted.
Supplementary Material
01
HIGHLIGHTS.
- CD36 forms complexes with β1 and β2 integrins and the tetraspanins CD9/CD81
- These complexes engage immunoreceptor tyrosine activation motif (ITAM) adaptor FcRγ
- FcRγ links CD36 complexes to Src-family kinases, Syk, and endocytosis
- These interactions control internalization of CD36 ligands such as oxidized LDL
Acknowledgments
Mac-1 deficient mouse bones were kindly provided by Dr. P. Kubes (University of Calgary). This study was funded in part by a Canadian Institutes of Health Research (CIHR) Team Grant in Malaria CTP-79842 (KCK, SG), CIHR grant MOP-102474 (SG), CIHR grant MOP-13721 (KCK), and a CIHR Canada Research Chair (KCK). BH was funded by post-doctoral fellowships from the Heart and Stroke Society of Canada and from the Canadian Institutes of Health Research. HK was supported by the CIHR Team Grant. GC was funded by a post-doctoral fellowship from Consejo Nacional de Ciencia y Tecnología, Mexico. DC was funded by a scholarship from COLCIENCIAS, Colombia. SG is the recipient of the Pitblado Chair in Cell Biology. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
The authors have no financial or other conflicts of interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- Armesilla AL, Vega MA. Structural organization of the gene for human CD36 glycoprotein. J Biol Chem. 1994;269:18985–18991. [PubMed] [Google Scholar]
- Asch AS, Barnwell J, Silverstein RL, Nachman RL. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barda-Saad M, Shirasu N, Pauker MH, Hassan N, Perl O, Balbo A, Yamaguchi H, Houtman JCD, Appella E, Schuck P, et al. Cooperative interactions at the SLP-76 complex are critical for actin polymerization. EMBO J. 2010;29:2315–2328. doi: 10.1038/emboj.2010.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch DI, Gormely JA, Ma C, Howard RJ, Pasloske BL. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyanovsky BB, Shridas P, Simons M, van der Westhuyzen DR, Webb NR. Syndecan-4 mediates macrophage uptake of group V secretory phospholipase A2-modified LDL. J Lipid Res. 2009;50:641–650. doi: 10.1194/jlr.M800450-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzada MJ, Annis DS, Zeng B, Marcinkiewicz C, Banas B, Lawler J, Mosher DF, Roberts DD. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J Biol Chem. 2004;279:41734–41743. doi: 10.1074/jbc.M406267200. [DOI] [PubMed] [Google Scholar]
- Chang Y, Finnemann SC. Tetraspanin CD81 is required for the alpha v beta5-integrin-dependent particle-binding step of RPE phagocytosis. J Cell Sci. 2007;120:3053–3063. doi: 10.1242/jcs.006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clezardin P, Frappart L, Clerget M, Pechoux C, Delmas PD. Expression of thrombospondin (TSP1) and its receptors (CD36 and CD51) in normal, hyperplastic, and neoplastic human breast. Cancer Res. 1993;53:1421–1430. [PubMed] [Google Scholar]
- Collins RF, Touret N, Kuwata H, Tandon NN, Grinstein S, Trimble WS. Uptake of oxidized low density lipoprotein by CD36 occurs by an actin-dependent pathway distinct from macropinocytosis. J Biol Chem. 2009;284:30288–30297. doi: 10.1074/jbc.M109.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppolino MG, Krause M, Hagendorff P, Monner DA, Trimble W, Grinstein S, Wehland J, Sechi AS. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J Cell Sci. 2001;114:4307–4318. doi: 10.1242/jcs.114.23.4307. [DOI] [PubMed] [Google Scholar]
- Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El-Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley MT, Costello PS, Fitzer-Attas CJ, Turner M, Meng F, Lowell C, Tybulewicz VL, DeFranco AL. A critical role for Syk in signal transduction and phagocytosis mediated by Fcgamma receptors on macrophages. J Exp Med. 1997;186:1027–1039. doi: 10.1084/jem.186.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- Erdman LK, Cosio G, Helmers AJ, Gowda DC, Grinstein S, Kain KC. CD36 and TLR interactions in inflammation and phagocytosis: implications for malaria. J Immunol. 2009;183:6452–6459. doi: 10.4049/jimmunol.0901374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF, Sharma K, Silverstein RL. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii C, Yanagi S, Sada K, Nagai K, Taniguchi T, Yamamura H. Involvement of protein-tyrosine kinase p72syk in collagen-induced signal transduction in platelets. Eur J Biochem. 1994;226:243–248. doi: 10.1111/j.1432-1033.1994.tb20047.x. [DOI] [PubMed] [Google Scholar]
- Girkontaite I, Urbonaviciute V, Maseda D, Neubert K, Herrmann M, Voll RE. Apoptotic cells selectively suppress the Th1 cytokine interferon gamma in stimulated human peripheral blood mononuclear cells and shift the Th1/Th2 balance towards Th2. Autoimmunity. 2007;40:327–330. doi: 10.1080/08916930701356846. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006;203:2613–2625. doi: 10.1084/jem.20060370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Lopez MD, Ovalle S, Yanez-Mo M, Sanchez-Sanchez N, Rubinstein E, Olmo N, Lizarbe MA, Sanchez-Madrid F, Cabanas C. A functionally relevant conformational epitope on the CD9 tetraspanin depends on the association with activated beta1 integrin. J Biol Chem. 2003;278:208–218. doi: 10.1074/jbc.M207805200. [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Schreiber A, Zhang Wj, Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcgamma receptors in macrophages. J Exp Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AB, Gakidis MAM, Glogauer M, Wilsbacher JL, Gao S, Swat W, Brugge JS. Requirements for Vav guanine nucleotide exchange factors and Rho GTPases in FcgammaR- and complement-mediated phagocytosis. Immunity. 2006;24:305–316. doi: 10.1016/j.immuni.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zähringer U, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- Horan KA, Watanabe KI, Kong AM, Bailey CG, Rasko JEJ, Sasaki T, Mitchell CA. Regulation of FcgammaR-stimulated phagocytosis by the 72-kDa inositol polyphosphate 5-phosphatase: SHIP1, but not the 72-kDa 5-phosphatase, regulates complement receptor 3 mediated phagocytosis by differential recruitment of these 5-phosphatases to the phagocytic cup. Blood. 2007;110:4480–4491. doi: 10.1182/blood-2007-02-073874. [DOI] [PubMed] [Google Scholar]
- Huang MM, Bolen JB, Barnwell JW, Shattil SJ, Brugge JS. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci USA. 1991;88:7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Febbraio M, Silverstein RL. CD9 tetraspanin interacts with CD36 on the surface of macrophages: a possible regulatory influence on uptake of oxidized low density lipoprotein. PLoS One. 2011;6:e29092. doi: 10.1371/journal.pone.0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaqaman K, Loerke D, Mettlen M, Kuwata H, Grinstein S, Schmid SL, Danuser G. Robust single-particle tracking in live-cell time-lapse sequences. Nat Methods. 2008;5:695–702. doi: 10.1038/nmeth.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaqaman K, Kuwata H, Touret N, Collins R, Trimble WS, Danuser G, Grinstein S. Cytoskeletal control of CD36 diffusion promotes its receptor and signaling function. Cell. 2011;1(46):593–606. doi: 10.1016/j.cell.2011.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Pleiman CM, Pao L, Schneringer J, Hippen K, Cambier JC. Phosphorylated immunoreceptor signaling motifs (ITAMs) exhibit unique abilities to bind and activate Lyn and Syk tyrosine kinases. J Immunol. 1995;155:4596–4603. [PubMed] [Google Scholar]
- Kiefer F, Brumell J, Al-Alawi N, Latour S, Cheng A, Veillette A, Grinstein S, Pawson T. The Syk protein tyrosine kinase is essential for Fcgamma receptor signaling in macrophages and neutrophils. Mol Cell Biol. 1998;18:4209–4220. doi: 10.1128/mcb.18.7.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutzsch HC, Choe BJ, Sipes JM, Guo Nh, Roberts DD. Identification of an alpha(3)beta(1) integrin recognition sequence in thrombospondin-1. J Biol Chem. 1999;274:24080–24086. doi: 10.1074/jbc.274.34.24080. [DOI] [PubMed] [Google Scholar]
- Kurosaki T, Takata M, Yamanashi Y, Inazu T, Taniguchi T, Yamamoto T, Yamamura H. Syk activation by the Src-family tyrosine kinase in the B cell receptor signaling. J Exp Med. 1994;179:1725–1729. doi: 10.1084/jem.179.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küster H, Thompson H, Kinet JP. Characterization and expression of the gene for the human Fc receptor gamma subunit. Definition of a new gene family. J Biol Chem. 1990;265:6448–6452. [PubMed] [Google Scholar]
- Li WX, Howard RJ, Leung LL. Identification of SVTCG in thrombospondin as the conformation-dependent, high affinity binding site for its receptor, CD36. J Biol Chem. 1993;268:16179–16184. [PubMed] [Google Scholar]
- Makranz C, Cohen G, Baron A, Levidor L, Kodama T, Reichert F, Rotshenker S. Phosphatidylinositol 3-kinase, phosphoinositide-specific phospholipase-Cgamma and protein kinase-C signal myelin phagocytosis mediated by complement receptor-3 alone and combined with scavenger receptor-AI/II in macrophages. Neurobiol Dis. 2004;15:279–286. doi: 10.1016/j.nbd.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, Alvarez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood. 2000;96:3231–3240. [PubMed] [Google Scholar]
- Miao WM, Vasile E, Lane WS, Lawler J. CD36 associates with CD9 and integrins on human blood platelets. Blood. 2001;97:1689–1696. doi: 10.1182/blood.v97.6.1689. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Tagawa Y, Watanabe N, Ogimoto M, Yakura H. SLP-76 is recruited to CD22 and dephosphorylated by SHP-1, thereby regulating B cell receptor-induced c-Jun N-terminal kinase activation. Eur J Immunol. 2005;35:644–654. doi: 10.1002/eji.200425465. [DOI] [PubMed] [Google Scholar]
- Mócsai A, Abram CL, Jakus Z, Hu Y, Lanier LL, Lowell CA. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–1333. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas Metropolis and Stanley Ulam. The Monte Carlo method. Journal of the American Statistical Association. 1949;44:335–341. doi: 10.1080/01621459.1949.10483310. [DOI] [PubMed] [Google Scholar]
- Palmbos PL, Sytsma MJ, DeHeer DH, Bonnema JD. Macrophage exposure to particulate titanium induces phosphorylation of the protein tyrosine kinase lyn and the phospholipases Cgamma-1 and Cgamma-2. J Orthop Res. 2002;20:483–489. doi: 10.1016/S0736-0266(01)00147-4. [DOI] [PubMed] [Google Scholar]
- Patel SN, Serghides L, Smith TG, Febbraio M, Silverstein RL, Kurtz TW, Pravenec M, Kain KC. CD36 mediates the phagocytosis of Plasmodium falciparum-infected erythrocytes by rodent macrophages. J Infect Dis. 2004;189:204–213. doi: 10.1086/380764. [DOI] [PubMed] [Google Scholar]
- Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- Ring A, Le Lay S, Pohl J, Verkade P, Stremmel W. Caveolin-1 is required for fatty acid translocase (FAT/CD36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochim Biophys Acta. 2006;1761:416–423. doi: 10.1016/j.bbalip.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Sattler M, Verma S, Pride YB, Salgia R, Rohrschneider LR, Griffin JD. SHIP1, an SH2 domain containing polyinositol-5-phosphatase, regulates migration through two critical tyrosine residues and forms a novel signaling complex with DOK1 and CRKL. J Biol Chem. 2001;276:2451–2458. doi: 10.1074/jbc.M006250200. [DOI] [PubMed] [Google Scholar]
- Schmitz R, Baumann G, Gram H. Catalytic specificity of phosphotyrosine kinases Blk, Lyn, c-Src and Syk as assessed by phage display. J Mol Biol. 1996;260:664–677. doi: 10.1006/jmbi.1996.0429. [DOI] [PubMed] [Google Scholar]
- Seimon TA, Nadolski MJ, Liao X, Magallon J, Nguyen M, Feric NT, Koschinsky ML, Harkewicz R, Witztum JL, Tsimikas S, Golenbock D, Moore KJ, Tabas I. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–82. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsul HM, Hasebe A, Iyori M, Ohtani M, Kiura K, Zhang D, Totsuka Y, Shibata K-ichiro. The Toll-like receptor 2 (TLR2) ligand FSL-1 is internalized via the clathrin-dependent endocytic pathway triggered by CD14 and CD36 but not by TLR2. Immunology. 2010;130:262–272. doi: 10.1111/j.1365-2567.2009.03232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto T, Kato K, Mori Y, Viriyakosol S, Oba M, Furuta T, Okiyoneda T, Arima H, Suico MA, Kai H. Membrane-anchored CD14 is required for LPS-induced TLR4 endocytosis in TLR4/MD-2/CD14 overexpressing CHO cells. Biochem Biophys Res Commun. 2005;338:1402–1409. doi: 10.1016/j.bbrc.2005.10.102. [DOI] [PubMed] [Google Scholar]
- Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon NN, Kralisz U, Jamieson GA. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989;264:7576–7583. [PubMed] [Google Scholar]
- Thorne RF, Marshall JF, Shafren DR, Gibson PG, Hart IR, Burns GF. The integrins alpha3beta1 and alpha6beta1 physically and functionally associate with CD36 in human melanoma cells. Requirement for the extracellular domain OF CD36. J Biol Chem. 2000;275:35264–35275. doi: 10.1074/jbc.M003969200. [DOI] [PubMed] [Google Scholar]
- Tomasello E, Olcese L, Vély F, Geourgeon C, Bléry M, Moqrich A, Gautheret D, Djabali M, Mattei MG, Vivier E. Gene structure, expression pattern, and biological activity of mouse killer cell activating receptor-associated protein (KARAP)/DAP-12. J Biol Chem. 1998;273:34115–34119. doi: 10.1074/jbc.273.51.34115. [DOI] [PubMed] [Google Scholar]
- Truong TQ, Aubin D, Bourgeois P, Falstrault L, Brissette L. Opposite effect of caveolin-1 in the metabolism of high-density and low-density lipoproteins. Biochim Biophys Acta. 2006;1761:24–36. doi: 10.1016/j.bbalip.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Tsai TH, Chen SF, Huang TY, Tzeng CF, Chiang AS, Kou YR, Lee TS, Shyue SK. Impaired Cd14 and Cd36 expression, bacterial clearance, and Toll-like receptor 4-Myd88 signaling in caveolin-1-deleted macrophages and mice. Shock. 2011;35:92–99. doi: 10.1097/SHK.0b013e3181ea45ca. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard A, Shaul PW, Yuhanna IS, Blair A, Smart EJ. High density lipoprotein prevents oxidized low density lipoprotein-induced inhibition of endothelial nitric-oxide synthase localization and activation in caveolae. J Biol Chem. 2000;275:11278–11283. doi: 10.1074/jbc.275.15.11278. [DOI] [PubMed] [Google Scholar]
- Uray IP, Liang Y, Hyder SM. Estradiol down-regulates CD36 expression in human breast cancer cells. Cancer Lett. 2004;207:101–107. doi: 10.1016/j.canlet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Wang H, Wei B, Bismuth G, Rudd CE. SLP-76-ADAP adaptor module regulates LFA-1 mediated costimulation and T cell motility. Proc Natl Acad Sci USA. 2009;106:12436–12441. doi: 10.1073/pnas.0900510106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SR, Huang M, Berton G. Signaling by adhesion in human neutrophils: activation of the p72syk tyrosine kinase and formation of protein complexes containing p72syk and Src family kinases in neutrophils spreading over fibrinogen. J Immunol. 1997;158:1902–1910. [PubMed] [Google Scholar]
- Zoller KE, MacNeil IA, Brugge JS. Protein tyrosine kinases Syk and ZAP-70 display distinct requirements for Src family kinases in immune response receptor signal transduction. J Immunol. 1997;158:1650–1659. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01